Preface
Based on literature evidence and expert consensus, the Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma1,2) (JCCRC) has been developed and sustained by the Japanese Society for Cancer of the Colon and Rectum (JSCCR) since 1977. Through defining detailed rules for evaluating and documenting clinical and pathological findings, and handling specimens of colorectal cancer, the JCCRC has contributed to the standardization and improvement of the diagnosis and treatment of colorectal cancer in Japan. Furthermore, the JSCCR guidelines for colorectal cancer treatment3), which have been revised several times since the first edition was issued in 2005, describe treatment algorithms conforming to the JCCRC; therefore, its importance is continuing to increase. Under these circumstances, the revisions have to maintain the classification's role to further improve treatment outcomes for colorectal cancer in Japan, which are already among the best in the world. At the same time, there is a focus on harmonizing with the eighth edition of the TNM classification4) and classification of cancers in other organs in Japan.
Stage grouping of colorectal cancer was compromised with that of the TNM classification. However, some differences were caused by the classification of lymph node metastasis. Expertise built up over several years emphasizes the importance of the main and lateral lymph nodes (N3) in Japan. Further, the definition of “extramural cancer deposits without lymph node structure (EX)” differs from that of “tumor deposits” in the TNM classifications. Meanwhile, because of the low prevalence of appendiceal and anal cancers arising from the anoderm in Japan, the current edition incorporates the TNM classification in these clinical entities.
1 Aims and Subjects
1.1 Aims
This classification presents the anatomical extent of colorectal cancer as a means to broadly share clinicopathological information on colorectal cancer and serves as the basis for improving treatment outcomes for colorectal cancer in Japan.
1.2 Subjects
This classification applies to primary carcinomas of the colon and rectum and does not apply to recurrence or metastasis. It is recommended that findings for primary colorectal tumors other than carcinomas be recorded according to this classification. The large intestine comprises the colon (cecum, ascending colon, transverse colon, descending colon, and sigmoid colon) and rectum (rectosigmoid junction, upper rectum, and lower rectum). The current edition also pertains to the appendix and anal canal; tumors occurring in these regions are tallied separately from those occurring in the colorectum.
2 General Principles of Description Methods
Findings are recorded using uppercase letters for the extent of primary tumor (T), lymph node metastasis (N), and distant metastasis (M). The degree of each finding is recorded in Arabic numerals following the designated letter. If the degree of findings needs to be subclassified, then it is recorded using lowercase letters placed after the Arabic numeral (e.g., T4a). “X” denotes the absence or uncertainty of assigning a given category (e.g., NX). Staging is recorded using Roman numerals, and subclassifications are recorded using lowercase letters (e.g., Stage IIIa). Staging for appendiceal and anal cancers originating from the squamous epithelium, anal glands, and anal gland ducts is recorded using Roman numerals and subdivision using uppercase alphabets (e.g., Stage IIIA).
2.1 Clinical, surgical, and pathological findings
Categories of findings, i.e., clinical, surgical, and pathological, are identified by placing the lowercase letters “c,” “s,” and “p,” respectively, in front of the designated finding.
Clinical findings: physical findings, diagnostic imaging findings, and biopsy/cytology as a preoperative diagnosis.
Surgical findings: surgical and diagnostic imaging findings during surgery.
Pathological findings: pathological findings of specimens obtained by endoscopic and/or surgical treatments, including intraoperative and rapid intraoperative cytology.
2.2 Findings following preoperative treatment
Findings following preoperative treatment are identified by the prefix “y.”
Clinical findings following preoperative treatment are identified by “yc,” and pathological findings following preoperative treatment are identified by “yp.”
2.3 Findings of recurrence
Findings of recurrence are identified by the prefix “r.”
Examples: Clinical findings rT0N0M1a (H); pathological findings rT0N0pM1a (H).
3 Recording of Findings
3.1 Primary tumors
3.1.1 Tumor location
The cancer location is recorded in accordance with the anatomical division of the large intestine. For rectal and anal cancers, the circumferential division of the intestinal wall is recorded.
3.1.2 Anatomical divisions of colon and rectum, vermiform appendix, and anal canal (Figure 1)
Figure 1.
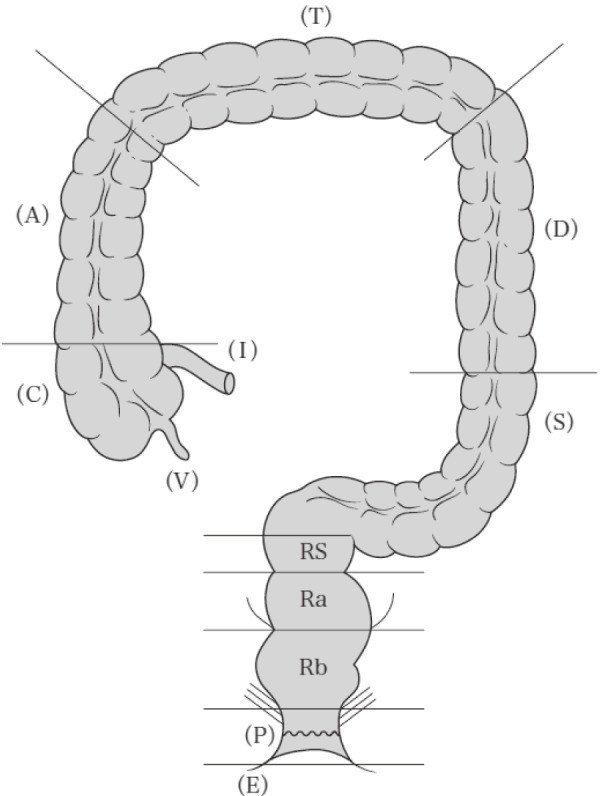
Anatomical divisions of the large intestine.
Reprinted with permission from the Japanese Society for Cancer of the Colon and Rectum and Kanehara & Co., Ltd.: Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma- the 3rd English edition, 2019.
Colon
C: Cecum
The pouch-like region extending caudal to the upper lip of the ileocecal valve. The boundary with the ascending colon is at the height of the upper lip of the ileocecal valve.
A: Ascending colon
The segment that extends from the cecum to the right colic flexure.
T: Transverse colon
The segment that extends between the left and right colic flexures.
D: Descending colon
The segment fixed to the retroperitoneum extending from the left colic flexure to the root of the sigmoid colon (approximately at the height of the iliac crest).
S: Sigmoid colon
The segment extending from the descending colon to the height of the sacral promontory.
Rectum
RS: Rectosigmoid
The segment from the height of the sacral promontory to the inferior border of the second sacral vertebra.
Ra: Upper rectum
The segment from the height of the inferior border of the second sacral vertebra to the peritoneal reflection.
Rb: Lower rectum
The segment from the peritoneal reflection to the superior border of the puborectal sling.
Note 1: The tubular portion corresponding to the ileocecal valve (transitional portion from the ileum to the cecum) is included in the cecum.
Note 2: It is not consistent, worldwide, whether the intestinal tract from the sacral promontory to the inferior border of the second sacral vertebra is included in either the colon or the rectum; however, in this classification, it is called RS and treated as the rectum.
Note 3: If more than one division is involved, then each division involved is recorded in the order of degree of involvement, starting with the division in which the bulk of the tumor is located.
e.g.: RS-Ra
Note 4: For rectal cancer, the distance between the lower edge of the tumor and the anal verge or dentate line is recorded. The anal verge is the connection between the anoderm and hair-bearing skin.
V: Vermiform appendix
Note: The TNM classification4) is used for the classification of tumors originating in the appendix.
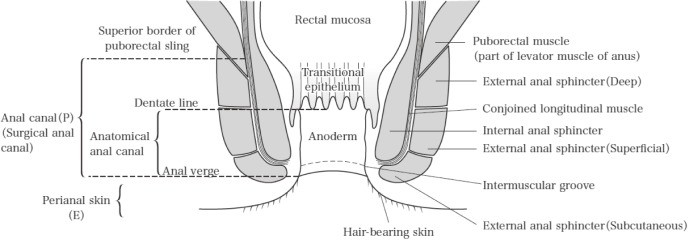
P: Anal canal
The “anal canal” can be described either as the surgical or anatomical anal canal. The surgical anal canal corresponds to the tubular structure extending from the superior border of the puborectal sling to the anal verge, whereas the anatomical anal canal corresponds to the tubular structure covered by the anoderm from the dentate line to the anal verge. The current JCCRC refers to the surgical anal canal (Figure 2).
Figure 2.
Anal canal.
Reprinted with permission from the Japanese Society for Cancer of the Colon and Rectum and Kanehara & Co., Ltd.: Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma- the 3rd English edition, 2019.
Note: The types of cancer that develop in the anal canal include those that originate from the mucosa of the rectal part of the anal canal (rectal type adenocarcinoma) and those that originate from the squamous epithelium of the anal canal, the anal gland, or a duct of it (squamous cell carcinoma, anal gland carcinoma, carcinoma associated with fistula). The former type, anal canal cancer, is described as colorectal cancer, and the TNM classifications4) is used to describe the latter type.
[Extra]
E: Perianal skin
Perianal skin is defined as hair-bearing skin within 5 cm of the anal verge (excluding the external genitalia).
3.1.3 Circumferential divisions of the wall of the rectum and anal canal (Figure 3)
Figure 3.
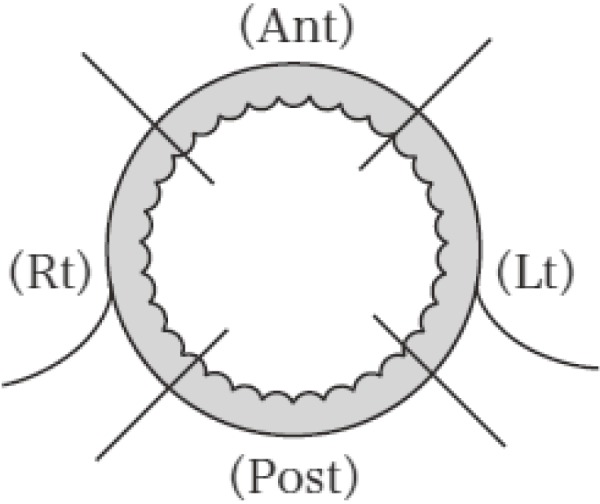
Proctorectal wall divisions.
Reprinted with permission from the Japanese Society for Cancer of the Colon and Rectum and Kanehara & Co., Ltd.: Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma- the 3rd English edition, 2019.
The circumference of the wall of the rectum and anal canal is anatomically divided into quadrants as anterior wall (Ant), posterior wall (Post), left wall (Lt), and right wall (Rt). Tumors located in the entire circumference are recorded as “Circ.”
Note: If the tumor occupies more than one quadrant, then the primary quadrant is recorded first, e.g., Ant-Lt
3.1.4 Number and sizes of lesions and proportion of the tumor in relation to the circumference of the bowel
The maximum dimension of the primary tumor, associated maximum perpendicular dimension, and proportion of the tumor in relation to the circumference of the bowel (the percentage of the maximum transverse diameter of the tumor occupying the circumference of the bowel) are recorded. All modalities used for these measurements (such as barium enema, colonoscopy, CT, MRI, ultrasonography, and palpation) are recorded. If the number and size cannot be assessed, then it is recorded as “unknown.”
In multiple tumors, the locations, sizes, proportions in relation to the bowel circumference, macroscopic types, and extent of primary tumor are recorded for each tumor. The primary lesion is defined as the lesion with the deepest wall invasion or that with the largest diameter when the depths of wall invasion are the same.
3.1.5 Macroscopic types
3.1.5.1 Main macroscopic types
Type 0: Superficial type
Type 1: Polypoid type
Type 2: Ulcerated type with clear margin
Type 3: Ulcerated type with infiltration
Type 4: Diffusely infiltrating type
Type 5: Unclassified type
3.1.5.2 Subtypes of macroscopic type 0
Type 0-I: Protruded type
0-Ip: Pedunculated type
0-Isp: Subpedunculated type
0-Is: Sessile type
Type 0-II: Superficial type
0-IIa: Elevated type
0-IIb: Flat type
0-IIc: Depressed type
Note 1: Lesions presumed to be Tis and T1 cancers are classified as superficial (type 0).
Note 2: Endoscopic findings are given priority when diagnosing superficial type lesions. The shape of the lesion is perceived as a whole image, irrespective of histogenesis and neoplastic or non-neoplastic difference.
Note 3: It is difficult to distinguish between adenomas and cancers based on macroscopic findings; therefore, the subclassification of superficial type is also used for the macroscopic classification of adenomatous lesions.
Note 4: If the tumor presents 2 different elements of superficial type, then the type occupying the largest area is recorded first, followed by “+,” e.g., 0-IIc+IIa.
Note 5: Laterally spreading tumors (LSTs) have grown (laterally) to more than 10 mm in diameter on the surface and are not included in the macroscopic classification. LSTs are divided according to morphology into granular (G) (homogenous and nodular mixed types) and non-granular (NG) types (flat elevated and pseudodepressed types).
Note 6: The macroscopic type must remain unchanged according to the histopathological examination results, e.g., superficial lesions remain type 0 even for histologically advanced cancers.
Note 7: Among anal tumors, those that originate from the anal glands or ducts of the anal canal wall and predominantly occupy the muscular layer or beyond are defined as “extra-canal type,” whereas those classified as either type 0, 1, 2, 3, 4, or 5 are defined as “intra-canal type.”
Note 8: In the event of chemotherapy or radiotherapy, macroscopic types before and after treatment are recorded.
3.1.6 Depth of tumor invasion (T)
TX: Primary tumor cannot be assessed
T0: No evidence of primary tumor
Tis: Tumor is confined to the mucosa (M) and does not invade the submucosa (SM)
T1: Tumor is confined to the SM and does not invade the muscularis propria (MP)
T1a: Tumor is confined to the SM, and invasion is within 1000 μm
T1b: Tumor is confined to the SM, and invasion is 1000 μm or more, but it does not extend to the MP
T2: Tumor invasion to, but not beyond, the MP
T3: Tumor invades beyond the MP. In sites with serosa, the tumor grows into the subserosa (SS). In sites with no serosa, the tumor grows into the adventitia (A)
T4: Tumor invades or perforates the serosa (SE) or directly invades other organs or structures (SI/AI)
T4a: Tumor invades or perforates the serosa (SE)
T4b: Tumor directly invades adjacent organs or structures (SI/AI)
Note 1: The extent of invasion of the primary tumor is recorded according to the T classification. Invasion into each layer of the bowel wall and into adjacent organs is denoted using the letters M, SM, MP, SS, A, and SI/AI. SI indicates invasion through the serosa into adjacent organs in sites with the serosa, and AI indicates invasion into adjacent organs in sites with no serosa.
Note 2: In the portion without the serosa, the adventitia (A) describes the pericolic/perirectal tissues equivalent to the SS of the portion with the serosa.
Note 3: The prefixes “c” (clinical findings) and “p” (pathological findings) are only used for the T classification and not for M-SI/AI (pathologically diagnosed mucosal cancer is recorded as pTis, and not pM).
Note 4: Tis conventionally refers to carcinoma in situ without invasion into the lamina propria; however, in colorectal cancer, Tis refers to cancer not extending beyond the lamina propria (i.e., intramucosal carcinoma) regardless of invasion.
Note 5: Regardless of the existence of metastasis, Tis and T1 are designated as “early cancers,” and cancers that have grown into the MP or beyond are designated as “advanced cancers.” The globally used terms “early stage colorectal cancer” and “advanced colorectal cancer” refer to stages I-III colorectal cancers and unresectable colorectal cancers, respectively, which define stages differently from T classification.
Note 6: For pT4b, the organ invaded is also noted, e.g., pT4b (prostate).
Note 7: The extent of histopathological depth is evaluated using the deepest area of cancer invasion. In case the deepest area is vascular/nerve invasion, it should be noted.
Example 1: If the cancer invasion is of the proper muscle layer and venous invasion is observed in the SS, then the correct designation is pT3 (V)-MP.
Example 2: If the cancer invasion is of the SM, the submucosal invasion distance is 1500 μm, and lymphatic vessel invasion is observed in the SS, then the correct designation is pT3 (Ly)-SM: 1500 μm.
Note 8: The definition of the extent of primary tumors has been determined to be consistent with that of other digestive system tumors.
Note 9: TNM classifications4) take no account of vascular invasion into T classification; consequently, the extent of primary tumor in the TNM classification and the current JCCRC may not agree in a low number of cases.
Note 10: The extent of primary tumor of rectal-type anal adenocarcinomas is defined as follows:
TX: Primary tumor cannot be assessed
T0: No evidence of primary tumor
Tis: Tumor is confined to the mucosa (M) and does not extend to the submucosa (SM)
T1: Tumor is confined to the SM and does not extend to the internal sphincter
T1a: Tumor is confined to the SM, and the distance of submucosal invasion is less than 1000 μm
T1b: Tumor is confined to the SM, and the distance of submucosal invasion is 1000 μm or greater
T2: Tumor extends to the internal anal sphincter but not to the conjoined longitudinal muscle
T3: Tumor has invaded beyond the conjoined longitudinal muscle
T4: Tumor has invaded the levator ani muscles or adjacent organs or structures
3.2 Metastasis
3.2.1 Lymph node metastasis
3.2.1.1 Lymph node groups and station numbers
Lymph node groups are classified and numbered according to their anatomical relationship to the superior mesenteric, inferior mesenteric, and iliac arteries as shown on Table 1, and Figure 4.
Table 1.
Lymph Node Groups and Station Numbers.
Reproduced with permission from the Japanese Society for Cancer of the Colon and Rectum and Kanehara & Co., Ltd.: Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma- the 3rd English edition, 2019.
| Superior mesenteric artery | Inferior mesenteric artery | Iliac artery | |
|---|---|---|---|
| Pericolic/perirectal lymph nodes | Lymph nodes along the marginal arteries and near the bowel wall • Pericolic lymph nodes (201, 211, 221) |
Lymph nodes along the marginal arteries, near the bowel wall, and along the terminal sigmoid artery • Pericolic lymph nodes (231, 241: 241-1, 241-2, 241-t) Lymph nodes along the superior rectal artery • Perirectal lymph nodes (251) |
Lymph nodes medial to the pelvic nerve plexus along the middle rectal artery. • Perirectal lymph nodes (251) |
| Intermediate lymph nodes | Lymph nodes along the ileocolic, right colic, and middle colic arteries. • Ileocolic nodes (202) • Right colic nodes (212) • Right middle colic nodes (222-rt) • Left middle colic nodes (222-lt) |
Lymph nodes along the left colic and sigmoid arteries and the inferior mesenteric artery between the origin of the left colic artery of the terminal sigmoid artery • Left colic nodes (232) • Sigmoid colic nodes (242: 242-1, 242-2) • Inferior mesenteric trunk nodes (252) |
|
| Main lymph nodes | Lymph nodes at the origin of the ileocolic, right colic, and middle colic arteries • Ileocolic root nodes (203) • Right colic root nodes (213) • Middle colic root nodes (223) |
Lymph nodes along the inferior mesenteric artery from the origin of the inferior mesenteric artery to that of the left colic artery • Inferior mesenteric root nodes (253) |
|
| Lateral lymph nodes | Lymph nodes along the internal iliac arteries and along the obturator vessels and nerves • Proximal internal iliac nodes (263P) • Distal internal iliac nodes (263D) • Obturator nodes (283) Lymph nodes along the common iliac external iliac, and median sacral arteries • Common iliac nodes (273) • External iliac nodes (293) • Lateral sacral nodes (260) • Median sacral nodes (270) • Aortic bifurcation nodes (280) |
||
| Downward lymph nodes | • Inguinal nodes (292) | ||
| Lymph nodes proximal to the main lymph nodes | Lymph nodes at the origin of the superior mesenteric artery and along the aorta • Superior mesenteric arterial root nodes (214) • Para-aortic nodes (216) |
Lymph nodes along the aorta • Para-aortic nodes (216) |
|
| Other lymph nodes | • Sub-pyloric nodes (206) • Gastroepiploic nodes (204) • Splenic hilar nodes (210) |
Note 1: The sigmoid artery commonly comprises the first, second, and terminal arteries, with pericolic lymph nodes recorded as 241-1, 242-2, and 241-t, respectively, and intermediate lymph nodes recorded as 242-1 and 242-2.
Note 2: Iliac arterial lymph nodes are recorded according to whether they are to the left or right (right = rt and left = lt); e.g., right distal internal iliac lymph nodes are recorded as rt263D.
Note 3: In anal cancer, 292 is treated as intermediate lymph nodes.
Figure 4.
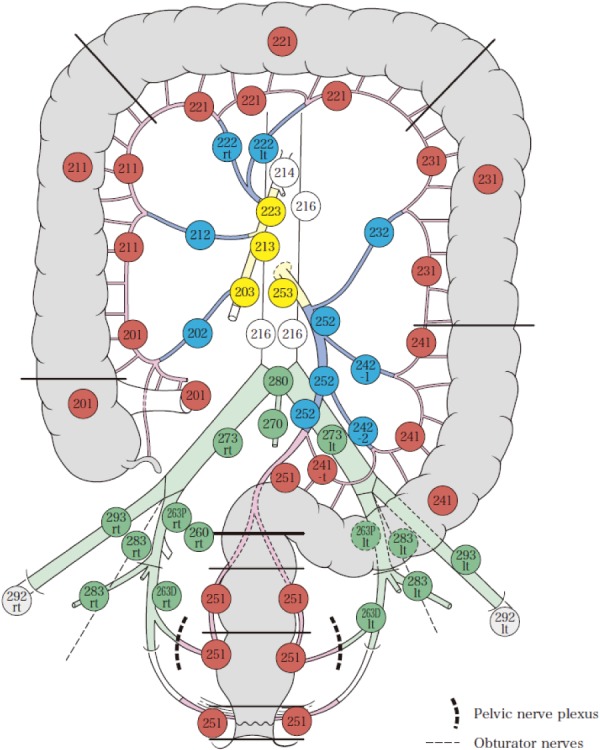
Lymph node groups and station numbers.
Red: Pericolic/perirectal lymph nodes
Blue: Intermediate lymph nodes
Yellow: Main lymph nodes
Green: Lateral lymph nodes
Gray: Downward lymph nodes
White: Lymph nodes proximal to the main lymph nodes
Reprinted with permission from the Japanese Society for Cancer of the Colon and Rectum and Kanehara & Co., Ltd.: Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma- the 3rd English edition, 2019.
3.2.1.2 Lymph node station number
The lymph node station of the large intestine is indicated with 3-digit numbers in the 200's.
For lymph nodes of the superior and inferior mesenteric arteries, the first digit represents the group, with pericolic lymph nodes denoted by “1,” intermediate lymph nodes by “2,” and main lymph nodes by “3.” The second digit represents the main artery, with the ileocolic artery denoted by “0,” right colic artery by “1,” middle colic artery by “2,” left colic artery by “3,” sigmoid artery by “4,” and inferior mesenteric artery, along with the superior rectal artery by “5.”
Internal iliac lymph nodes are denoted by “P” for central nodes and “D” for peripheral nodes.
Internal iliac lymph nodes are identified by “3” for the first digit indicating the group, with “rt” for the right side and “lt” for the left side. As an exception to this convention, lymph nodes in contact with the anterior surface of the sacrum are identified by “0,” and inguinal lymph nodes classified as intermediate lymph nodes in anal cancer are identified by “2.”
For consistency with the classification of gastric carcinoma, superior mesenteric, para-aortic, sub-pyloric, omental, and splenic hilar lymph nodes are numbered 214, 216, 206, 204, and 210, respectively.
3.2.1.3 Regional lymph nodes
Lymph nodes are divided into regional lymph nodes and others. The presence or absence of regional lymph node metastasis and the degree of metastasis are recorded using the classification N0-N3.
Regional lymph nodes are classified into 3 groups as pericolic, intermediate, and main lymph nodes. In addition, lateral lymph nodes are included in the lower rectum (Figure 5).
Figure 5.
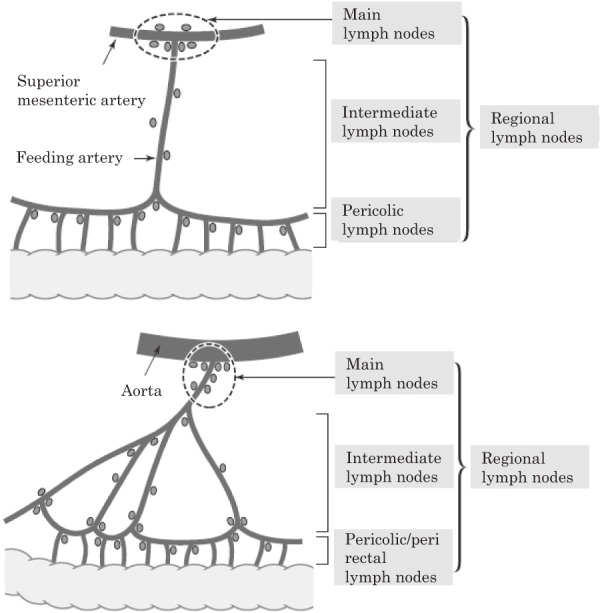
Basic principles of lymph node grouping.
Reprinted with permission from the Japanese Society for Cancer of the Colon and Rectum and Kanehara & Co., Ltd.: Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma- the 3rd English edition, 2019.
The specific range of regional lymph nodes is individually defined according to the anatomical relationship between the location of the tumor and its main feeding artery/arteries (Figure 6).
Figure 6.
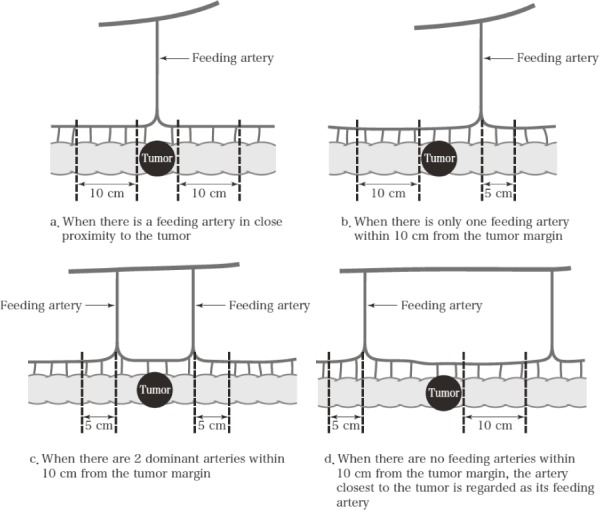
Pericolic lymph nodes.
Reprinted with permission from the Japanese Society for Cancer of the Colon and Rectum and Kanehara & Co., Ltd.: Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma- the 3rd English edition, 2019.
The main arteries of the colon are the ileocolic, right colic, middle colic (right and left branches), left colic, and sigmoid arteries. The range of pericolic lymph nodes in the colon can be classified into the following 4 types on the basis of the positional relationship with the tumor and feeding artery (Figure 6).
a: When the feeding artery is directly below the tumor, the area extends 10 cm in both oral and anal directions from the tumor margin.
b: When there is one feeding artery within 10 cm from the tumor margin, the area extends up to 5 cm beyond the arterial inflow site and the opposite side extends up to 10 cm from the tumor margin.
c: When there are 2 dominant arteries within 10 cm from the tumor margin, the area extends up to 5 cm beyond the arterial inflow site in both oral and anal sides.
d: When there are no feeding arteries within 10 cm from the tumor margin, the area extends up to 5 cm beyond the artery closest to the tumor margin and the opposite side extends up to 10 cm from the tumor margin.
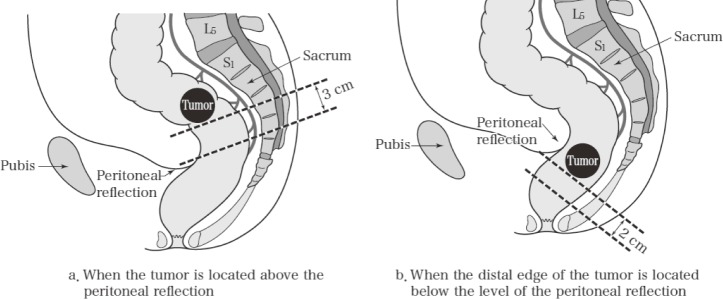
In the rectum, the main lymph node is number 253, and the intermediate lymph node is number 252. Pericolic lymph nodes include those between the lowest sigmoid artery inflow point and 3 cm (RS and Ra) or 2 cm (Rb) distal to the tumor margin. However, if the distance from the tumor margin to the lowest sigmoid artery inflow point is less than 10 cm, then pericolic lymph nodes include those in the area up to 10 cm (Figure 7).
Figure 7.
Perirectal lymph nodes.
Reprinted with permission from the Japanese Society for Cancer of the Colon and Rectum and Kanehara & Co., Ltd.: Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma- the 3rd English edition, 2019.
3.2.1.4 Lymph node metastasis (N)
NX: Lymph node metastasis cannot be assessed
N0: No evidence of lymph node metastasis
N1: Metastasis in 1-3 pericolic/perirectal or intermediate lymph nodes
N1a: Metastasis in 1 lymph node
N1b: Metastasis in 2-3 lymph nodes
N2: Metastasis in 4 or more pericolic/perirectal or intermediate lymph nodes
N2a: Metastasis in 4-6 lymph nodes
N2b: Metastasis in 7 or more lymph nodes
N3: Metastasis in the main lymph node (s). In the lower rectal cancer, metastasis in the main and/or lateral lymph node (s)
Note 1: Lymph node metastasis beyond the regional lymph nodes is classified as distant metastasis (M1).
Note 2: Of extramural cancer deposits without lymph node structure (EX), tumor deposits other than vascular/perineural invasion (tumor nodule: ND) are classified as metastatic lymph nodes.
Note 3: The number of dissected and metastatic lymph nodes is described according to the lymph node metastasis ratio (number of metastatic lymph nodes/number of dissected lymph nodes) for each station of the lymph node. The tumor of ND is integrated into that of dissected lymph nodes and the number of ND is described.
Example: If in the 251 area, 3 positive lymph nodes, 2 ND, 1 ND(Pn+), and 5 negative lymph nodes are present, then the designation is #251: 6/11 [ND 2, ND(Pn+) 1].
3.2.2 Distant metastasis (M)
M0: No distant metastasis
M1: Distant metastasis
M1a: Distant metastasis confined to one organ. Peritoneal metastasis not present
M1b: Distant metastasis in more than one organ. Peritoneal metastasis not present
M1c: Presence of peritoneal metastasis
M1c1: Metastasis to the peritoneum only
M1c2: Metastasis to the peritoneum with other distant metastasis
Note 1: All metastases (lymphogenous, hematogenous, and peritoneal), except for metastasis to the regional lymph nodes, are classified as M1.
Note 2: Ovarian metastasis is now classified as distant metastasis (M1).
Note 3: In the event of hepatic, pulmonary, and peritoneal metastases, the extent of metastasis noted in 3.2.2.1-3.2.2.3 is recorded.
Note 4: In the event of distant metastasis (M1), the site of metastasis is recorded in parentheses. When recording the site of metastasis, the following abbreviations can be used:
Liver: H, Peritoneum: P, Lung: PUL, Bone: OSS, Brain: BRA, Bone marrow: MAR, Adrenal glands: ADR, Skin: SKI, Pleura: PLE, Extraregional lymph nodes: LYM, Ovaries: OVA, Other: OTH
Example: M1a (H1), M1a (ADR), M1b (PUL1, H3).
Note 5: With regard to the pathological findings of distant metastasis (pM), “pM0” indicates the absence of distant metastasis confirmed by autopsy, and “pM1” indicates histologically confirmed distant metastasis. Accordingly, the diagnosis of distant metastasis based on clinical findings, intraoperative palpation, and/or image findings without histological confirmation are recorded as “cM0” and “cM1.” When the pathological findings of distant metastasis are unclear, “pMX” is not used.
3.2.2.1 Liver metastasis (H)
HX: Liver metastasis cannot be assessed
H0: No liver metastasis
H1: 1-4 metastatic tumors, all of which are 5 cm or less in maximum diameter
H2: Other than H1 or H3
H3: Five or more metastatic tumors, at least one of which is more than 5 cm in maximum diameter
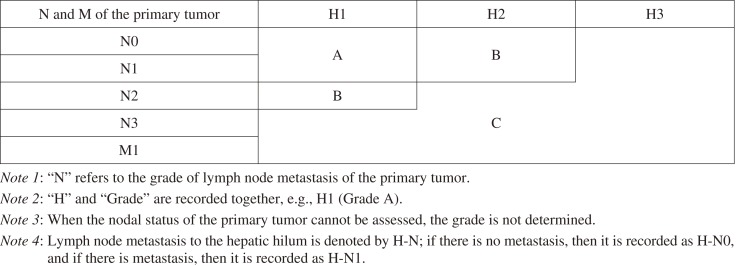
The prognostic grouping (Grade classification) of patients with liver metastasis is recorded as follows (Table 2):
Table 2.
Grade of Patients with Liver Metastases.
Reproduced with permission from the Japanese Society for Cancer of the Colon and Rectum and Kanehara & Co., Ltd.: Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma- the 3rd English edition, 2019.
Grade A: H1 N0/N1
Grade B: H1 N2 or H2 N0/N1
Grade C: Other than the above
3.2.2.2 Peritoneal metastasis (P)
PX: Peritoneal metastasis cannot be assessed
P0: No peritoneal metastasis
P1: Metastasis localized to adjacent peritoneum
P2: Limited metastasis to distant peritoneum
P3: Diffuse metastasis to distant peritoneum
Note 1: When ascites is present, cytological examination is recommended.
Note 2: Negative results of cytological examination are represented as Cy0 and positive results as Cy1. Cytological examination of ascites is I: negative, III: false-positive, and V: positive, with only positive (V) results defined as Cy1.
Note 3: The impact on the prognosis of Cy1 is currently unclear; therefore, Cy1 is not included as a factor to determine staging.
Note 4: The clinical significance of cytological examination of peritoneal lavage fluid has yet to be determined. If the examination is proved to be positive, then the result is recorded as such, but not as Cy1.
3.2.2.3 Pulmonary metastasis (PUL)
PULX: Pulmonary metastasis cannot be assessed
PUL0: No pulmonary metastasis
PUL1: 1 or 2 pulmonary metastases or 3 metastases or more on one side
PUL2: 3 or more pulmonary metastases bilaterally or carcinomatous lymphangitis, carcinomatous pleuritis, and lymph node metastasis in the pulmonary hilum and/or mediastinum
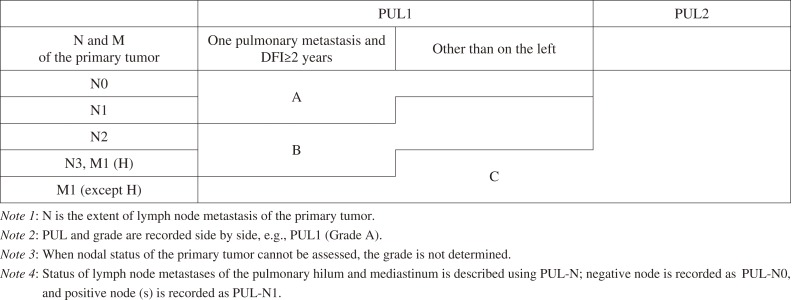
Prognostic grouping (Grade classification) of patients with pulmonary metastasis is recorded as follows (Table 3).
Table 3.
Grade Classification for Patients with Pulmonary Metastases.
Reproduced with permission from the Japanese Society for Cancer of the Colon and Rectum and Kanehara & Co., Ltd.: Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma- the 3rd English edition, 2019.
The grade of patients with pulmonary metastasis is determined according to the extent of lymph node metastasis of the primary tumor, distant metastasis, pulmonary metastasis, and disease-free interval (DFI).
Grade A: One pulmonary metastasis, DFI≥2 years, and N0/N1; one pulmonary metastasis, DFI<2 years, and N0; 2 pulmonary metastases or 3 or more pulmonary metastases on one side and N0.
Grade B: One pulmonary metastasis, DFI≥2 years, and N2/N3 or M1 (H); one pulmonary metastasis and DFI<2 years; 2 pulmonary metastases or 3 or more pulmonary metastases on one side and N1/N2.
Grade C: Other than the above
DFI is the period from the day of surgery of the primary tumor till the day when pulmonary metastasis is confirmed. For synchronous pulmonary metastasis, DFI is recorded as “0.”
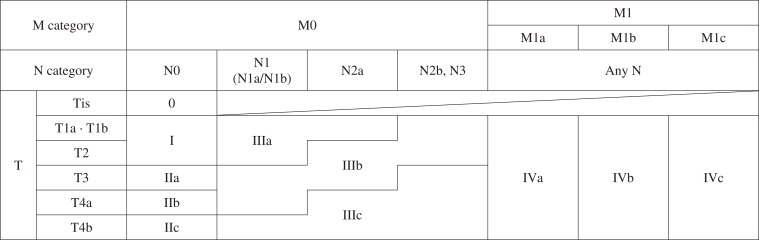
3.3 Stage grouping (Table 4)
Table 4.
Stage Grouping of Colorectal Cancer.
Reproduced with permission from the Japanese Society for Cancer of the Colon and Rectum and Kanehara & Co., Ltd.: Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma- the 3rd English edition, 2019.
3.3.1 Clinical and pathological classifications for stage grouping
Stage is divided into clinical and pathological classifications, which are denoted by the letters “c” and “p” placed before each respective staging (cStage and pStage).
cStage is based on pretreatment clinical findings and not on surgical findings.
pStage is based on pathological findings. However, clinical and/or surgical findings can be used to determine distant metastasis (M).
e.g., Resection of colon cancer without distant metastasis: pT3pN1M0, pStage IIIb
Resection of the primary tumor only in rectal cancer with pulmonary metastasis: pT3pN2M1 (PUL2), pStage IV
Stage grouping is possible in the following, even with TX and/or NX.
3.3.2 Stage grouping following preoperative treatment
Staging following preoperative treatment is designated by the prefix “y” placed before the Roman numeral. The clinical classification following preoperative treatment is designated by “yc,” and the pathological classification following preoperative treatment is designated by “yp,” e.g., ypT1N1M0 ypStage IIIa.
3.4 Multiple colorectal cancers, multiple primary cancers
In case of multiple colorectal cancers, the number of lesions is recorded.
In case of multiple primary cancers, the organs involved are recorded.
Note 1: Presence of Tis cancer is specified in multiple colorectal cancer.
Note 2: Whether cancers are synchronous or metachronous is recorded.
Multiple colorectal cancer is defined as the development of two or more primary tumors in the large intestine. Multiple primary cancer is defined as the development of malignant tumors in different organs.
Synchronous and metachronous tumors
Tumors diagnosed within 2 months are designated as synchronous.
Tumors diagnosed after 2 months or greater are designated as metachronous.
In the event of both synchronous and metachronous tumors, it is referred to as synchronous/metachronous tumor.
Note: The abovementioned criteria on synchronous and metachronous do not apply to cancer metastasis. Metachronous metastasis is described as metastatic lesions that are newly diagnosed after a series of examinations and/or treatments for the relevant cancer, regardless of the time period between diagnosis and examinations and/or treatments.
3.5 Family history and hereditary diseases
For all cancer prevalence in first-degree relatives (parents, children, or siblings) of the patient, the disease name, relationship, sex, and age at diagnosis are recorded. In the event that cancer is found in a first-degree relative of the patient, the disease name, relationship, sex, and age at diagnosis of that individual's first-degree relative are also recorded.
Cases of familial adenomatous polyposis or Lynch syndrome (hereditary non-polyposis colorectal cancer) should be noted.
4 Endoscopic and Surgical Treatments
4.1 Endoscopic treatment
4.1.1 Endoscopic treatment method
Snare polypectomy
Endoscopic mucosal resection (EMR)
Endoscopic submucosal dissection (ESD)
Note 1: Any other therapeutic procedure, if performed, is recorded.
Note 2: Whether the resection has been performed en bloc or by piecemeal is recorded.
Note 3: Resections that do not utilize high-frequency currents include cold forceps polypectomy and cold snare polypectomy.
Note 4: In the narrow sense, techniques that accomplish peeling without using a snare are termed as “ESD.” Techniques that implement snaring without any peeling of the submucosa after making an incision around the lesion using either an ESD knife or a snare tip are termed as “precutting EMR,” and techniques that involve peeling of the submucosa after making an incision around the lesion using either an ESD knife or a snare tip with ultimate snaring are termed as “hybrid ESD.”
4.2 Surgical treatment
For surgical treatment, the approach, surgical procedure, extent of lymph node dissection, anastomosis method (anastomosis form and procedure), and combined organ resection are recorded. In surgery for rectal cancer, the preservation of autonomic nerves is recorded.
4.2.1 Approach to the lesion
Transanal, trans-sphincter, trans-sacral, transabdominal (laparoscopic, open), and others
4.2.2 Surgical procedures
Polypectomy
Local excision
Appendectomy
Ileocecal resection
Partial colectomy
Right hemicolectomy
Left hemicolectomy
Sigmoidectomy
Subtotal colectomy
Total colectomy
Proctocolectomy
High anterior resection
Low anterior resection
Ultra-low anterior resection
Intersphincteric resection
Hartmann procedure
Abdominoperineal resection
Total pelvic exenteration
Other types of colectomy
Bypass surgery
Colostomy, ileostomy
Exploratory laparotomy
Other palliative procedures
Note 1: Surgical polypectomy is a surgical method used to resect polyps at the base.
Note 2: Local excision is divided into submucosal and full-thickness resections.
Note 3: Colectomy other than ileocecal resection, right hemicolectomy, left hemicolectomy, sigmoid colectomy, subtotal colectomy, and total colectomy are partial colectomies.
Note 4: In partial colectomy, the part of the resected colon is specified in parentheses, e.g., partial colectomy (ascending colon) or partial colectomy (transverse colon).
Note 5: High and low anterior resections are classified by the height of the resection line relative to the level of the peritoneal reflection.
Note 6: Ultra-low anterior resection is a surgical method performed via a transabdominal approach to remove the rectum en bloc near the attachment point at the puborectal muscle (either proximal or distal side); anastomosis of the proximal side of the intestinal tract and anal canal is performed.
Note 7: Intersphincteric resection (ISR) is a surgical method that involves both the transabdominal and anal approaches. It involves surgical dissection between the inner and outer sphincter, resecting the rectum en bloc together with the internal anal sphincter within the anatomical anal duct from directly above the dentate line to the intersphincteric groove, and anastomosing transanally the distal end of the sigmoid colon and the anus.
Note 8: Surgical methods for treating familial adenomatous polyposis include total colectomy with ileostomy, total colectomy with ileal pouch-anal canal anastomosis (IACA), total colectomy with ileal pouch-anal anastomosis (IAA), and total colectomy with ileorectal anastomosis (IRA).
4.2.3 Lymph node dissection
4.2.3.1 Extent of lymph node dissection (D)
DX: Extent of lymph node dissection cannot be assessed
D0: Incomplete pericolic/perirectal lymph node dissection
D1: Complete pericolic/perirectal lymph node dissection
D2: Complete pericolic/perirectal and intermediate lymph node dissection
D3: Pericolic/perirectal, intermediate, and main lymph nodes are dissected
Note 1: For lower rectal cancers or cancers that infiltrate into the lower rectum, the extent of lateral lymph node dissection (LD) should be determined as described below and recorded separately from the extent of dissection of the perirectal intermediate or main lymph nodes along the inferior mesenteric artery (D).
Note 2: The inguinal lymph nodes (292) in rectal-type anal canal adenocarcinoma are regarded as “intermediate lymph nodes.”
Note 3: The extent of lymph node dissection does not apply to anal squamous cell carcinomas and cancers arising from the anal gland and anal fistula.
4.2.3.2 Extent of lateral lymph node dissection (LD)
The extent of LD is classified as follows:
LDX: Extent of LD cannot be assessed
LD0: LD is not performed
LD1: LD does not satisfy LD2
LD2: Dissection of 263D, 263 P, and 283 is performed
LD3: Dissection of all lateral lymph nodes is performed
Note 1: Lateral lymph nodes are 263D, 263P, 283, 273, 293, 260, 270, and 280.
Note 2: If the extent of dissection is different on the left and right sides, then each side should be separately described and annotated using “rt-LD number” for the right and “lt-LD number” for the left.
Examples:
Right side dissections are 263D, 263P, 283, 273, 293, and 260, and left side dissections are 263D, 263P, and 283, with 270 and 280 also dissected: LD2 (rt-3/lt-2)
263D, 263P, and 283 are dissected on both left and right sides: LD2
263D, 263P, and 283 are dissected on the right side with no dissections on the left side: LD1 (rt-2/lt-0)
Note 3: For lower rectal cancers or cancer infiltrates into the lower rectum, the extent of lymph node dissection along the intestine and feeding artery (D) and the extent of LD should also be noted.
Example:
The extent of lymph node dissection along the intestine and feeding artery is D3, and the extent of LD is LD2: D3LD2.
4.2.4 Anastomosis
4.2.4.1 Types of anastomosis
End-to-end anastomosis
Side-to-end anastomosis
End-to-side anastomosis
Side-to-side anastomosis
Functional end-to-end anastomosis
Note: When an ileal or colonic pouch is constructed, it is recorded.
4.2.4.2 Methods of anastomosis
Hand-sewn anastomosis
Stapled anastomosis
Single stapling anastomosis
Double stapling anastomosis
Functional end-to-end anastomosis
4.2.5 Combined resection of adjacent organs and structures
All organs or structures resected due to cancer invasion/metastasis are recorded.
Note: For combined organ resection, whether the organ is completely or partially resected should be recorded.
4.2.6 Preservation of autonomic nerves (AN) (Figure 8)
Figure 8.
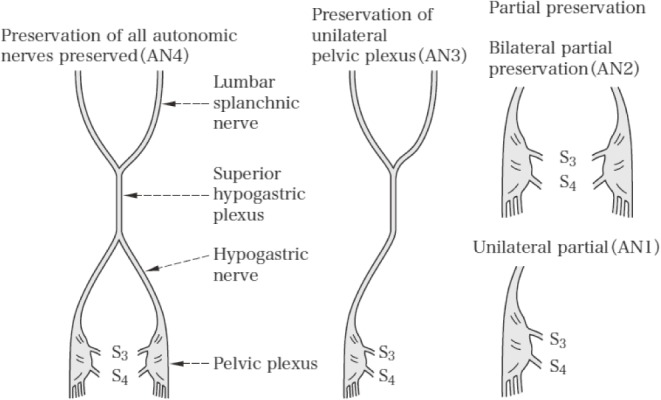
Preservation of autonomic nerves.
Reprinted with permission from the Japanese Society for Cancer of the Colon and Rectum and Kanehara & Co., Ltd.: Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma- the 3rd English edition, 2019.
ANX: Preservation of autonomic nerves cannot be assessed
AN0: All autonomic nerves are removed
AN1: Pelvic plexus on one side is preserved, and pelvic plexus on the other side and superior hypogastric plexus are removed
AN2: Pelvic plexuses on both sides are preserved, and superior hypogastric plexus is removed
AN3: Pelvic plexus on one side and superior hypogastric plexus are preserved, and pelvic plexus on the other side is removed
AN4: All autonomic nerves are preserved
Note 1: AN involved in surgery for rectal cancer include the lumbar splanchnic nerve, superior hypogastric plexus, hypogastric nerve (sympathetic nerve), pelvic splanchnic nerve (parasympathetic nerve), pelvic plexus, and visceral branch arising from the pelvic plexus.
Note 2: In AN1 and AN3, the side of the autonomic nerves preserved is recorded. e.g., AN3 rt, AN1 lt
Note 3: When partial preservation of pelvic plexus is performed, it is recorded. e.g., resection of the 3rd pelvic splanchnic nerve (S3).
5 Assessment of Cancer Involvement at Resected Margin, Residual Tumor, and Curability
5.1 Cancer involvement at resection margins
Tumor invasion in the resected margin is confirmed by histological examination.
5.1.1 Specimens obtained by endoscopic resection
5.1.1.1 Horizontal margin (lateral/mucosal margin) (HM)
HMX: Tumor involvement of the lateral margin cannot be assessed
HM0: No tumor identified at the lateral margin
HM1: Tumor identified at the lateral margin
Note 1: In HM0, the margin of clearance is measured and recorded.
Note 2: When the adenoma gland alone extends to the margin in a lesion that contains both carcinoma and adenoma component, it should be recorded as HM0 (adenoma component positive).
Note 3: Specify the margin even when the lesion comprises an adenoma alone.
5.1.1.2 Vertical margin (deep/intramural margin) (VM)
VMX: Tumor involvement of the deep margin cannot be assessed
VM0: No tumor identified at the deep margin
VM1: Tumor identified at the deep margin
Note 1: In VM0, the margin of clearance is measured and recorded.
Note 2: Occurrences of local recurrence were reported in tumors with the margin clearance < 500 μm.
5.1.2 Specimens obtained by surgical resection
5.1.2.1 Proximal margin (PM)
PMX: Tumor involvement of the proximal margin cannot be assessed
PM0: No tumor identified at the proximal margin
PM1: Tumor identified at the proximal margin
5.1.2.2 Distal margin (DM)
DMX: Tumor involvement of the distal margin cannot be assessed
DM0: No tumor identified at the distal margin
DM1: Tumor identified at the distal margin
5.1.2.3 Radial margin (circumferential resection margin) (RM)
RMX: Tumor involvement of the radial margin cannot be assessed
RM0: No tumor identified at the radial margin
RM1: Tumor identified at the radial margin
Note 1: In PM0, DM0 and RM0, the distance of proximal, distal, and radial margins of clearance are measured and recorded.
Note 2: The tumor is described as HRM0 when not exposed to the cut surface of the liver and as HRM1 when exposed.
5.2 Residual tumor
5.2.1 Residual tumor following endoscopic treatment (ER)
ERX: HMX or VMX
ER0: HM0 and VM0
ER1: HM1 and/or HM1
ER1a: HM1, VM0
ER1b: HM0, VM1, or HM1, VM1
ER2: Macroscopic residual tumor
Note 1: Residual tumor following endoscopic treatment is histologically classified as ERX-ER1.
Note 2: Resection with a macroscopic residual tumor is defined as ER2.
5.2.2 Residual tumor following surgical treatment (R)
RX: Presence of residual tumor cannot be assessed
R0: No residual tumor
R1: Microscopic residual tumor at resection lines or planes
R2: Macroscopic residual tumor
Note 1: In the event of distant metastasis (hepatic metastasis, pulmonary metastasis, and peritoneal dissemination), the residual tumor is assessed for both primary tumor and distant metastasis lesions, and the greater degree of residual tumor is recorded as the final R; for example, even when the residual tumor after the primary tumor resection is R0, if the residual tumor after metastatic liver tumor resection is R1, then the final R is recorded as R1.
Note 2: In the event of the two-stage resection of distant metastasis in a Stage IV tumor, the residual tumor of the primary tumor and distant metastatic tumor in the first and second stages of surgery are comprehensively assessed; for example, with synchronous pulmonary metastasis, when the primary tumor margin in the first stage of surgery is R0 (R2 in assessment of the first stage of surgery) and the margin of the pulmonary metastatic tumor in the second stage of surgery is R1, the final R is recorded as R1.
5.3 Curability
Curability with surgical treatment (Cur)
Curability X (CurX): Curability cannot be evaluated
Curability A (CurA): No distant metastasis (M0), and no residual tumor at both proximal/distal and radial margins (PM0, DM0, and RM0)
Curability B (CurB): Not corresponding to Curability A or C
Curability C (CurC): Macroscopic residual tumor
Note 1: Curability in surgical treatment is comprehensively assessed on the basis of clinical, surgical, and pathological findings.
Note 2: Excluding Tis cancers, designate the curability as unclear (CurX) for local resections without lymph node dissection.
6 Chemotherapy and Radiotherapy
6.1 Chemotherapy documentation
Name of regimen used
Duration (start date and completed date of administration)
Reason for discontinuing administration (completion, disease progression, adverse events, refusal, etc.)
Time relationship with surgery when used in combination with surgery (prior to surgery in resectable cases, conversion in unresectable cases, and postoperative adjuvant therapy)
The indicator of the general condition [performance status (PS)] is sequentially recorded.
Response to chemotherapy is evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST), and adverse events are evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE).
6.2 Radiotherapy documentation
6.2.1 Aims of radiotherapy
Radical, adjuvant (preoperative, intraoperative, postoperative, and combination), palliative
6.2.2 Methods of radiotherapy
Equipment (source)
Radiation quality
Energy
Radiation technique: fixed radiation, moving beam radiation, etc.
Number of beams
Treatment position
Field size
Dose per fraction (Gy)
Fractionation: number of fractions per day and per week
Treatment period
Total dose (Gy)
Whether concomitant therapy (chemotherapy, etc.) was performed and the details of it, if any
6.2.3 Radiation field
7 Evaluation of Resected Specimens
7.1 Macroscopic findings
7.1.1 Tumor location
7.1.2 Macroscopic types
7.1.3 Size
7.1.3.1 Tumor size
7.1.3.2 Size of the intramucosal component of the tumor
7.1.3.3 Size of the ulcerated area
7.1.4 Proportion of the tumor in relation to the circumference of the bowel
7.1.5 Distance from the lesion to the resection margin
7.1.6 Extent and properties of invasion and metastasis
7.1.7 Depth of tumor invasion
7.1.8 Lymph node metastasis and location
7.2 Histological findings
7.2.1 Histological types
A Colon and rectum
1 Benign epithelial tumors
1.1 Adenoma
1.1.1 Tubular adenoma
1.1.2 Tubulovillous adenoma
1.1.3 Villous adenoma
1.1.4 Traditional serrated adenoma
2 Malignant epithelial tumors
2.1 Adenocarcinoma
2.1.1 Papillary adenocarcinoma (pap)
2.1.2 Tubular adenocarcinoma (tub)
2.1.2.1 Well differentiated type (tub1)
2.1.2.2 Moderately differentiated type (tub2)
2.1.3 Poorly differentiated adenocarcinoma (por)
2.1.3.1 Solid type (por1)
2.1.3.2 Non-solid type (por2)
2.1.4 Mucinous adenocarcinoma (muc)
2.1.5 Signet-ring cell carcinoma (sig)
2.1.6 Medullary carcinoma (med)
2.2 Adenosquamous carcinoma (asc)
2.3 Squamous cell carcinoma (scc)
2.4 Carcinoid tumor
2.5 Endocrine cell carcinoma
2.6 Miscellaneous histological types of malignant epithelial tumors
3 Non-epithelial tumors
3.1 Myogenic tumor
3.2 Neurogenic tumor
3.3 GIST (Gastrointestinal stromal tumor)
3.4 Lipoma and lipomatosis
3.5 Vascular tumor
3.6 Miscellaneous tumor
4 Lymphoma
4.1 B-cell lymphoma
4.1.1 Mucosa-associated lymphoid tissue (MALT) lymphoma
4.1.2 Follicular lymphoma
4.1.3 Mantle cell lymphoma
4.1.4 Diffuse large B-cell lymphoma
4.1.5 Burkitt's lymphoma
4.1.6 Others
4.2 T-cell lymphoma
4.3 Hodgkin's lymphoma
5 Unclassified tumors
6 Metastatic tumors
7 Tumor-like lesions
7.1 Hyperplastic nodule
7.2 Hyperplastic (metaplastic) polyp
7.3 Sessile serrated adenoma/polyp (SSA/P)
7.4 Juvenile polyp
7.5 Inflammatory polyp and polyposis
7.6 Inflammatory fibroid polyp
7.7 Inflammatory myoglandular polyp
7.8 Hamartomatous polyp
7.9 Mucosal prolapse syndrome
7.10 Cap polyposis
7.11 Benign lymphoid polyp
7.12 Endometriosis
7.13 Others (Heterotopic gastric mucosa, elastofibromatous polyp, colonic muco-submucosal elongated polyp, etc.)
8 Hereditary tumors and gastrointestinal polyposis
8.1 Familial adenomatous polyposis
8.2 Lynch syndrome
8.3 Peutz-Jeghers syndrome
8.4 Serrated polyposis/hyperplastic polyposis
8.5 Cronkhite-Canada syndrome, Cronkhite-Canada polyp
8.6 Juvenile polyposis
8.7 Cowden syndrome, phosphate and tensin homolog (PTEN) hamartoma tumor syndrome
8.8 Others
B Vermiform appendix
1 Benign epithelial neoplasia
2 Low-grade appendiceal mucinous neoplasm
3 Malignant epithelial neoplasia
3.1 Adenocarcinoma
3.2 Goblet cell carcinoid
3.3 Carcinoid tumor
4 Mesenchymal tumor
5 Malignant lymphoma
6 Tumor-like lesions
7 Others
C Anal canal (including perianal skin)
1 Benign epithelial neoplasia
1.1 Adenoma
1.2 Serrated lesion
1.3 Condyloma acuminatum
1.4 Squamous cell papilloma
1.5 Hidradenoma papilliferum
1.6 Others
2 Squamous intraepithelial neoplasia
2.1 Low-grade intraepithelial neoplasia
2.2 High-grade intraepithelial neoplasia
2.3 Carcinoma in situ
2.4 Bowen's disease
2.5 Others
3 Malignant epithelial tumors
3.1 Adenocarcinoma
3.1.1 Rectal-type adenocarcinoma
3.1.2 Extramucosal (fistula-associated, perianal) adenocarcinoma
3.2 Squamous cell carcinoma
3.3 Adenosquamous carcinoma
3.4 Carcinoid tumor
3.5 Endocrine cell carcinoma
3.6 Others
4 Malignant melanoma
5 Extramammary Paget's disease
6 Mesenchymal neoplasia
7 Malignant lymphoma
8 Tumor-like lesion
9 Others
7.2.2 Infiltration pattern (INF)
The most predominant invasion growth types at the leading front of the tumor are classified into the following 3 types:
INFa (expansive type): The tumor shows expansive growth, and there is a distinct boundary between the tumor and the surrounding tissue.
INFb (intermediate type): Intermediate between INFa and INFc.
INFc (infiltrative type): The tumor exhibits infiltrative growth, and the boundary with the surrounding normal tissue is indistinct.
Note 1: Cancers that have grown to T1 or further are recorded.
Note 2: Judgement is made based on images acquired using either a pathology image of loupe device or low magnification.
7.2.3 Lymphovascular invasion
7.2.3.1 Lymphatic invasion (Ly)
Lymphatic invasion is the invasion of tumor cells into the lymphatic vessels.
LyX: Lymphatic invasion cannot be assessed
Ly0: No lymphatic invasion
Ly1: Lymphatic invasion identified
Ly1a: Minimal lymphatic invasion
Ly1b: Moderate lymphatic invasion
Ly1c: Severe lymphatic invasion
7.2.3.2 Venous invasion (V)
Venous invasion is the invasion of tumor cells into the blood vessels.
VX: Venous invasion cannot be assessed
V0: No venous invasion
V1: Microscopic venous invasion
V1a: Minimal venous invasion
V1b: Moderate venous invasion
V1c: Severe venous invasion
V2: Macroscopic venous invasion
Note 1: Invasion of the vascular system is assessed with pathological specimens prepared from the section of tumor at the largest tumor diameter.
Note 2: When immunostaining is used to examine for lymphatic invasion, it should be stated. e.g., Ly1a (D2-40)
Note 3: When elastic fiber staining is used to examine for venous invasion, it should be stated. e.g., V1a (VB) with Victoria blue staining, V1b (EVG) with Elastica van Gieson staining.
Note 4: When vascular invasion is present, but it is difficult to judge whether this is a lymphatic invasion or venous invasion, designate it as Ly/V.
Note 5: The level of the deepest vascular invasion should be recorded. e.g., V1a (SS) (EVG)
Note 6: When a tumor has an elastic plate confirmed at one-half or more of its circumference, it is considered “V,” and when D2-40-positive endothelial cells are confirmed at one-half or more of its circumference, it is considered “Ly”. Using this method, inconsistencies among individuals judging vascular invasions should be reduced.
Note 7: Subtyping of Ly1 and V1 is not needed for endoscopic resection specimens.
7.2.4 Tumor budding (BD)
“Tumor budding” is defined as a cancer cell nest consisting of one or fewer than five cells that infiltrate the interstitium at the invasive margin of the cancer.
On selecting the region where tumor budding is the greatest, the front of the tumor growth is observed at 200×magnification to count the number of tumor buds.
BDX: Budding cannot be assessed
BD1: 0-4 buds
BD2: 5-9 buds
BD3: 10 or more buds
Note: BD should be described in case of T1 cancer. In the cancers of T2 or greater, describe if possible.
7.2.5 Extramural cancer deposits without lymph node structure (EX) (Figure 9)
Figure 9.
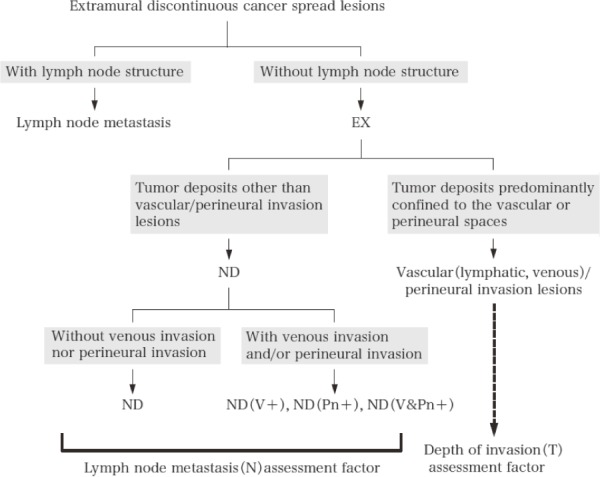
Extramural cancer deposits without lymph node structure.
Reprinted with permission from the Japanese Society for Cancer of the Colon and Rectum and Kanehara & Co., Ltd.: Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma- the 3rd English edition, 2019.
Extramural cancer deposits without lymph node structure (EX) within the regional lymph node area should be recorded. EX includes localized lesions comprising lymphatic invasion, venous invasion, perineural invasion (vascular/perineural invasion lesions), and other lesions (tumor nodule: ND).
Note 1: All tumor deposits located in the extramural fatty tissue are regarded as EX in tumors in which continuous spread was confined within the SM or MP. For tumors that directly penetrate the MP, tumor deposits located ≥5 mm from the leading edge of the primary tumor are designated as EX.
Note 2: ND is treated as lymph node metastasis.
Note 3: ND involving veins and/or neural fascicles at their growing front should be designated as ND(V+), ND(Pn+), or ND(V&Pn+) because these are associated with an extremely poor prognosis.
Note 4: When localized foci of lymphovascular or perineural invasion are identified, the number of foci should be described in parentheses per relevant lymph node station. e.g., if one focus of each lymphatic and venous invasion is identified in station 201, then it would be described as #201: Ly (1), V (1).
7.2.6 Perineural invasion (Pn)
PnX: Perineural invasion cannot be assessed
Pn0: No perineural invasion
Pn1: Presence of perineural invasion
Pn1a: Intramural perineural invasion only
Pn1b: Extramural perineural invasion
Note 1: A distinctive pattern of horizontal spread in a form to replace the myenteric (Auerbach's) plexus can be regarded as intramural perineural invasion, irrespective of the confirmation of perineural invasion.
Note 2: Extramural perineural invasion was histological finding of tumor cells invading or spreading along the nerve fascicles external to the MP. Extramural perineural invasion exists as an isolated lesion or within the body of the primary tumor. In diagnosing the latter, emphasis is placed on findings of tumor nests in direct contact with the nerve fascicle without being intervened by connective tissue.
7.3 Histological criteria for the assessment of response to chemotherapy/radiotherapy
Grade 0 (No effect): No tumor cell necrosis or degeneration in response to treatment is observed.
Grade 1 (Mild effect)
(a) Minimal effect: Tumor cell necrosis or degeneration is present in less than one-third of the entire lesion.
(b) Mild effect: Tumor cell necrosis, degeneration, and/or lytic change is present in more than one-third but less than two-thirds of the entire lesion.
Grade 2 (Moderate effect): Prominent tumor cell necrosis, degeneration, lytic change, and/or disappearance is present in more than two-thirds of the entire lesion, but viable tumor cells remain.
Grade 3 (Marked effect): Necrosis and/or lytic change is present throughout the entire lesion and is replaced by fibrosis with or without granulomatous change. No viable tumor cells are observed.
Note: Assessment is performed on as many pathological specimens as possible, including those prepared from the section of whole tumor at its largest diameter.
7.4 Histological assessment of biopsy specimens (Group classification)
Group X: Inadequate material for histological diagnosis
Group 1: Normal tissue or a non-neoplastic lesion
Group 2: Lesions in which it is difficult to determine whether the lesion is neoplastic or non-neoplastic
Group 3: Benign tumor
Group 4: Neoplastic lesion suspected of being carcinoma
Group 5: Carcinoma
7.5 Measurement of the depth of invasion
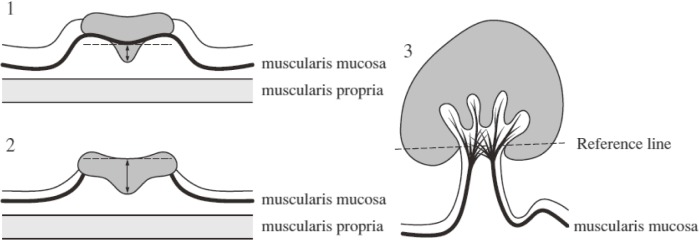
7.5.1 T1 cancer (Figure 10)
Figure 10.
Measurement of the depth of invasion in T1 cancers.
Reprinted with permission from the Japanese Society for Cancer of the Colon and Rectum and Kanehara & Co., Ltd.: Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma- the 3rd English edition, 2019.
Irrespective of the macroscopic type, when it is possible to identify or estimate the location of the muscularis mucosa, the depth of submucosal invasion is measured from the lower border of the muscularis mucosa (Figure 10-1); when this is not possible, the depth of invasion is measured from the surface of the lesion (Figure 10-2).
Note 1: In this description, the phrase “possible to identify or estimate the location” indicates that there is no “deformity” caused by submucosal invasion, i.e., muscularis mucosa without disarray, dissection, rupture, or fragmentation. When a deformed muscularis mucosa is used as the baseline of measurement, the depth of submucosal invasion can be underestimated. Although it is not necessarily easy to judge whether there is a “deformity,” if there is a desmoplastic reaction present around the muscularis mucosa, then it is assumed to be “deformed.”
Note 2: In pedunculated lesions with a tangled muscularis mucosa, it may not be possible to identify the muscularis mucosa to use as the baseline to measure invasion. In such instances, the depth of submucosal invasion is measured as the distance between the point of the deepest invasion and the reference line, which is defined as the neck (boundary between the tumor head and the stalk). Pedunculated lesions with a tangled muscularis mucosa, in which invasion is limited to within the head, are defined as “head invasion” (Figure 10-3).
Note 3: Differentiation is required for the findings of submucosal infiltration of tubular adenoma glands, i.e, pseudocarcinomatous invasion (submucosal misplacement and submucosal pseudoinvasion).
7.5.2 Tumor invading beyond the MP in sites without the serosa
The depth of extramural invasion is measured at the deepest part of the tumor.
The distance is measured at the site of continuous spread of the tumor.
Note 1: Lymphatic/venous/perineural invasion, which is not contiguous from the main lesion, is not included in the measurement site.
Note 2: When the MP is preserved, the distance from the lower border of the MP to the deepest part of the extramural invasion is measured.
Note 3: When the lower border of the MP is not entirely identifiable, the distance from the uppermost limit of the lower border of the MP to the deepest part of the extramural invasion is measured. When there is a right and left difference at the ruptured edge of the MP, the distance from the lower edge of break point of the MP closest to the tumor surface to the deepest part of the extramural invasion is measured.
8 Treatment Outcome Record
Following data are recorded for statistical analysis:
8.1 Number of patients
Total number of outpatients with colorectal cancer
Total number of inpatients with colorectal cancer
8.2 Multiple colorectal cancers, multiple primary cancers
8.3 Modalities of treatment and adjuvant therapy
Endoscopic treatment
Surgical treatment
Chemotherapy
Radiotherapy
Other non-operative treatment
No treatment
Note: Refer to Section 4.2. Surgical treatment when documenting surgical procedures.
8.4 The total number of colorectal cancer cases with treatment, and the number and rate of cases by treatment types
The number of cases and the proportion by treatment methods and types are described.
8.4.1 Resection rate
Resection rate=number of patients who underwent surgical resection of tumors/total number of surgical patients
Number and proportion of patients who underwent surgical resection of tumors with respect to each curability (A, B, and C).
Note: The number of resection surgeries includes polypectomy and local excision in addition to intestinal resection.
8.4.2 Endoscopic treatment
Patients treated by endoscopic procedure alone are distinguished from those who underwent surgical resection.
8.4.3 Chemotherapy and radiotherapy
The number and proportion of patients who underwent chemotherapy and radiation therapy are recorded according to the RECIST outcome.
8.5 Number and rate of operative mortality
Note 1: Operative mortality is defined as death within 30 days after surgery irrespective of whether the patient was in the hospital or discharged.
Note 2: Rate of operative mortality is defined as the ratio of the number of operative deaths to the total number of patients who underwent surgery.
8.6 Number and rate of hospital mortality following surgery
Note 1: Hospital mortality is defined as death while in hospital following any surgical treatment.
Note 2: The rate of hospital mortality is defined as the ratio of the number of hospital deaths to the total number of patients who underwent surgical treatment.
8.7 Survival analysis
The following data are recorded for survival analysis:
8.7.1 Survival
Alive: The most recent date of follow-up is recorded.
Dead: The date of death is recorded.
Unknown (lost to follow-up): The final date of follow-up is recorded.
Cause of death
Treatment-related death
Death due to colorectal cancer
Death due to other malignancy: the name of the malignancy is recorded
Death due to other diseases: the name of the disease is recorded (not including deaths from other malignant tumors).
Death due to accidents, including suicide
Death due to unknown cause.
8.7.2 Recurrence/metastasis; site(s) and mode
Presence or absence of recurrence
Date when recurrence is confirmed
Methods for confirming recurrence
Site(s) and mode of recurrence
Note: Site(s) and mode of each recurrence are recorded in the order in which they were confirmed.
Local recurrence
Anastomotic recurrence
Recurrence in regional lymph nodes
Other types of local recurrence
Lymph node metastasis (recurrence in non-regional lymph nodes)
Liver metastasis
Lung metastasis
Hematogenous metastasis (other than liver and lung metastases)
Peritoneal metastasis
Recurrence at unspecified sites
Note: Sites of recurrence are recorded using abbreviation demonstrated 3.2.2 Distant metastasis (M).
8.7.3 Survival analysis method
The following data are recorded for statistical analysis:
Target population (i.e., patients who underwent endoscopic treatment, surgical treatment, etc.)
Methods of estimating survival
Actuarial survival rate
Direct method
Cumulative survival rate: Life-table method, Kaplan-Meier method
Relative survival rate
Event classification
All deaths, colorectal tumor deaths (primary tumor death), recurrence, secondary cancer, progression, and treatment discontinuation
Test of significance for survival rates
Proportion of patients lost in follow-up
Footnote
This manuscript is reproduced from the first chapter of the third English edition of the Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma by permission of the Japanese Society for Cancer of the Colon and Rectum, the Japanese Society of Coloproctology, and Kanehara & Co., Ltd. with a few editorial changes. The second chapter and after (i.e., “Assessment of response to chemotherapy and radiotherapy,” “Explanation of pathological items and supplementary histology atlas,” and others) are omitted from this manuscript. The original version is available from https://www.kanehara-shuppan.co.jp/
Disclaimer
Yojiro Hashiguchi, Masaaki Ito, Shinji Tanaka, Hideki Ueno and Takashi Yao are the Associate Editors of Journal of the Anus, Rectum and Colon and on the journal's Editorial Board. They were not involved in the editorial evaluation or decision to accept this article for publication at all.
Conflicts of Interest
All committee members involved in producing the JCCRC submitted conflict-of-interest statements based on academic society rules, which are managed by the JSCCR. The JSCCR, the author of this manuscript, declares that there are no conflicts of interest.
English edition editors
Masafumi Inomata, Kenjiro Kotake, Yoichi Ajioka, Kazushige Kawai, Hideki Ueno, Takanori Goi, Kentaro Nakajima, Tomonori Akagi, Hideaki Yano, Kenichi Sugihara
Appendix
Members of the JCCRC Committee and the JCCRC Revision Committee.
The JCCRC Committee
Kenichi Sugihara (Chairman), Yoichi Ajioka, Tsutomu Chiba, Takahiro Fujimori, Hiroharu Isomoto, Shingo Ishiguro, Masaaki Ito, Akinori Iwashita, Yukihide Kanemitsu, Tomoyuki Kato, Tomoe Katsumata, Yusuke Kinugasa, Susumu Kodaira, Fumio Konishi, Kenjiro Kotake, Yasuo Koyama, Shinei Kudo, Ryoji Kushima, Masaki Mori, Takeo Mori, Yoshihiro Moriya, Kei Muro, Tetsuichiro Muto, Atsushi Ochiai, Kiyotaka Okuno, Masatoshi Ohya, Yutaka Saito, Yoshihiro Sakai, Yoshiharu Satake, Yasuhiro Shimada, Kazuo Shirouzu, Tamotsu Sugai, Akinori Takagane, Shinji Tanaka, Kazutaka Yamada, Takashi Yao, Masayuki Yasutomi
The JCCRC Revision Committee
Kenjiro Kotake (Chairman), Yoichi Ajioka, Hideki Ueno, Masazumi Okajima, Atsushi Ochiai, Yukihide Kanemitsu, Ryoji Kushima, Keiji Koda, Hirotoshi Kobayashi, Yutaka Saito, Yasuhiro Shimada, Shinji Tanaka, Yojiro Hashiguchi, Kazuo Hase, Tetsuya Hamaguchi, Kotaro Maeda, Takashi Yao, Kazutaka Yamada, Toshiaki Watanabe, Masahiko Watanabe
References
- 1.Japanese Society for Cancer of the Colon and Rectum. Japanese Classification of colorectal, appendiceal, and anal carcinoma. 9th ed. Tokyo: Kanehara & Co., Ltd.; 2018. 108p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Japanese Society for Cancer of the Colon and Rectum. Japanese Classification of colorectal, appendiceal, and anal carcinoma. 3rd English ed. Tokyo: Kanehara & Co., Ltd.; 2019. 123p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watanabe T, Muro K, Ajioka Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2018 Feb; 23(1): 1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brierley JD, Gospodarowicz MK, Wittekind C, et al. UICC TNM classification of malignant tumours. 8th ed. Oxford: Wiley-Blackwell, 2017. 272p. [Google Scholar]