Abstract
Objective: Skeletal muscle loss (sarcopenia) is a prognostic factor in patients undergoing gastrointestinal surgery. However, the influence of muscle quality on prognosis remains unclear. We retrospectively examined preoperative skeletal muscle quantity and quality impact on survival of elderly patients undergoing curative resection of colorectal cancer. Methods: We examined data from 142 patients aged ≥75 years who underwent curative resection of colorectal cancer between 2007 and 2012. We determined the size and quality of skeletal muscles, represented by the psoas muscle mass index (PMI) and intramuscular adipose tissue content (IMAC), respectively, using a preoperative computed tomography image. Overall survival (OS) and relapse-free survival (RFS) rates were determined according to values of PMI, IMAC, and other prognostic factors. Results: OS and RFS rates in patients with low PMI were lower than those in patients with normal PMI. The OS and RFS rates in patients with high IMAC were also lower than those in patients with normal IMAC. PMI and IMAC were independent prognostic factors for OS (hazard ratio [HR], 3.81, and 3.04, respectively); IMAC was an independent factor for RFS (hazard ratio [HR], 3.03). Conclusion: Preoperative sarcopenia, indicating low quality and size of skeletal muscle, predicts mortality after curative resection of colorectal cancer in the elderly.
Keywords: colorectal cancer, elderly patient, skeletal muscle mass, skeletal muscle quality
Introduction
Colorectal cancer (CRC) is the second most common cancer in Japan1) and the second leading cause of cancer deaths (data from Vital Statistics of the Japan Ministry of Health, Labour and Welfare). The incidence of colorectal cancer has been increasing, and approximately 40% of patients are older than 75-years1). The incidence is presumed to increase in the future with the aging population1).
Sarcopenia was originally reported by Rosenberg in 1989 as an age-related decline in muscle mass2). In 2010, the European Working Group on Sarcopenia in Older People made a recommendation stating that the definition of sarcopenia should include low muscle mass as well as low muscle strength or function3).
Reports have confirmed the significant association between sarcopenia and the poor outcomes of various kinds of cancer4-7). Based on a systemic review, preoperative low skeletal muscle mass identified before surgery using single-slice computed tomography (CT) was associated with poor prognosis in gastrointestinal and hepatopancreatobiliary malignancies8) as well as increased postoperative morbidity in patients with colorectal cancer with or without hepatic metastases9).
However, the oldest studies examined only skeletal muscle mass for defining sarcopenia, because muscle strength and function were difficult to estimate in those retrospective studies8).
To the best of our knowledge, there have been no published reports on the association between skeletal muscle quality and prognosis of elderly patients with colorectal cancer after curative resection.
Muscle steatosis (low skeletal muscle quality) has attracted much attention since the increased intramuscular adipose tissue with aging has been associated with decreased muscle strength and quality10). Loss of muscle strength depends on both decline in muscle mass and accumulation of intramuscular adipose tissue11). To define the quality of skeletal muscle, Kitajima et al. estimated the amount of skeletal muscle steatosis by measuring the intramuscular adipose tissue content (IMAC) using CT images and discovered that skeletal muscle steatosis was associated with the pathogenesis and severity of nonalcoholic steatohepatitis12,13).
In this study, we estimated the quality as well as the quantity of skeletal muscle by defining the IMAC and psoas muscle mass index (PMI), taking measurements on preoperative plain CT images. We also evaluated the impact of PMI and IMAC on the prognosis of elderly patients undergoing curative resection of colorectal cancer.
Methods
Patients
We performed a retrospective analysis of data from 978 consecutive patients with colorectal cancer who underwent primary tumor resections at Hiroshima City Hiroshima Citizens Hospital (Hiroshima, Japan) from January 2007 to December 2012. We included patients aged ≥75 years with histologically-confirmed colorectal adenocarcinoma who had undergone curative resection for stages 0-III from the cecum to the rectosigmoid.
Preoperative abdominal CT images without contrast medium (taken within 30 days of the operations) were used for measurements of 142 patients who were enrolled in this cohort study.
The Ethics Committee of Hiroshima City Hiroshima Citizens Hospital approved the study that was conducted in accordance with the Helsinki Declaration of 1996.
Methods
Image Analysis
We measured the cross-sectional areas of the bilateral psoas muscles as the quantity of skeletal muscle by manually tracing a line on the preoperative plain CT at the umbilical level. We then calculated the PMI as follows14):
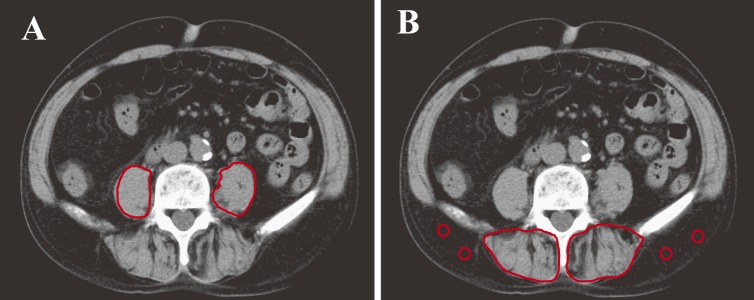
PMI = cross-sectional area of bilateral psoas muscle/height2 (cm2/m2) (Figure 1A)
Figure 1.
Cross-sectional computed tomographic images at the level of the umbilicus.
(A) The bilateral psoas muscle areas were measured by manual tracing. PMI = cross-sectional area of bilateral psoas muscle/height2 (cm2/m2).
(B) Subfascial muscular tissue in the multifidus muscle was precisely traced, with four circles placed on subcutaneous fat.
IMAC = mean CT value of ROI of multifidus muscle (HU)/mean CT value of ROI of subcutaneous fat (HU)
We considered the PMI measurement to reflect the muscle volume.
We manually traced the subfascial muscular tissue in the multifidus muscle at the same level on the preoperative plain CTs and measured the mean CT values (Hounsfield units [HU]) for these areas using the Aquarius NET server (TeraRecon, San Mateo, CA). We made four circles on areas of subcutaneous fat away from major vessels in CT images to show the region of interest (ROI) for subcutaneous fat assessment14).
We also measured the mean CT values (HU) for the ROI of subcutaneous fat. We calculated the IMAC by the ratio of these CT values, as previously reported by Kitajima et al12,15):
IMAC = mean CT value of ROI of multifidus muscle(HU)/mean CT value of ROI of subcutaneous fat (HU) (Figure 1B).
The skeletal muscle density decreases, and IMAC becomes higher with fat deposition increments. Therefore, we considered a high IMAC as a proxy for low muscle quality.
Cutoff Values for PMI and IMAC
Since PMI and IMAC differ significantly between men and women, we provided different cutoff values for each using gender-specific quartiles. The cutoff values for PMI in men and women were 5.71 and 4.32-cm2/m2, respectively (Figure 2). On the basis of these cutoff values, we divided patients in two groups: low PMI (n = 35) and normal PMI (n = 107). The cutoff values for IMAC in men and women were −0.036 and 0.135, respectively. We divided patients into two groups according to these cutoff values: high IMAC (n = 35) and normal IMAC (n = 107) (Figure 2).
Figure 2.
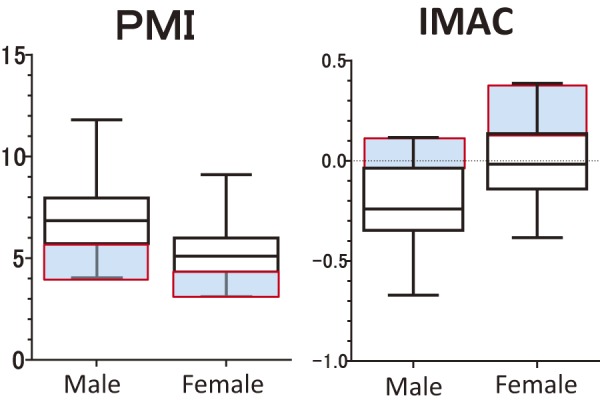
Sex distribution in PMI and IMAC. Those highlighted in blue indicate quartiles.
Parameters Analyzed
We analyzed the overall survival (OS) and relapse-free survival (RFS) rates after colectomy in patients classified according to PMI or IMAC. We investigated prognostic factors according to those rates using the following variables: skeletal muscle mass (low PMI vs. normal PMI), skeletal muscle quality (high IMAC vs. normal IMAC), age (<80-years vs. ≥80-years), gender (male vs. female), body mass index (BMI; ≤20.0 kg/m2 vs. >20.0 kg/m2), serum albumin (<3.5 g/dl vs. ≥3.5 g/dl), C-reactive protein (CRP) level (<0.5 mg/dL vs. ≥0.5 mg/dL), and prognostic nutritional index (PNI) (<40 vs. ≥40). We calculated the modified Glasgow Prognostic Score (GPS) as previously described16). To summarize, patients with both increased CRP (>0.5-mg/dL) and hypoalbuminemia (<3.5-g/dL) were allocated a score of 2 (mGPS 2). Patients with only one factor were allocated a score of 1 (mGPS 1), and patients with neither factor were allocated a score of 0 (mGPS 0). We calculated the PNI as 10×albumin (g/dL) +0.005×total lymphocyte count (/mL)17). Adjuvant chemotherapy was administered with capecitabine, S-1, UFT or UFT/LV, for a duration of 6 months.
Statistical Analysis
We expressed all data as medians showing minimum to maximum values in parentheses. We used the Mann-Whitney U and χ2 tests to compare groups and proportions between groups, respectively. We estimated survival curves using the Kaplan-Meier method and performed analyzes using the log-rank test. We built univariate Cox proportional hazard models of all potential baseline predictors to compute the hazard ratios with their 95% confidence intervals.
We analyzed continuous variables nonparametrically using the Mann-Whitney U test and categorical variables using a χ2 test or Fisher exact test as appropriate. We considered any variable with p< .10 in the univariate analysis to be a candidate for multivariate analysis using the Cox proportional hazard model. We calculated the cumulative OS and RFS rates using the Kaplan-Meier method and evaluated the differences between curves using a log-rank test. We considered a P value <.05 as significant. All statistical data were generated using the JMP 13 (SAS Institute, Cary, NC) and Prism 6 (GraphPad Software, La Jolla, CA).
Results
Patient Characteristics
From our database of 978 patients with CRC, only 142 patients (14.5%) were eligible for analysis. The baseline characteristics and laboratory data of the 142 patients are shown in Table 1. The median patient age was 80.5 years (range 75-96).
Table 1.
Clinical Characteristics of All Patients.
| Total, n = 142 | ||
|---|---|---|
| Age, median (range) | 80.5 (75-96) | |
| Sex, male/female | 63/79 | |
| BMI, median (range) | 22.6 (12.4-32.8) | |
| Hb, g/dl, median (range) | 11.4 (4.2-17.4) | |
| Albumin, g/dl, median (range) | 3.8 (2.0-5.0) | |
| CRP, mg/dl, median (range) | 0.14 (0.01-11.96) | |
| Creatinine, mg/dl, median (range) | 0.78 (0.46-7.47) | |
| Total lymphocyte count (/μL) | 1600 (600-3400) | |
| Prognostic nutritional index | 46.0 (25.5-61.0) | |
| Modified Glasgow prognostic score (0/1/2) | 87/33/20 | |
| pStage (0/I/II/IIIa/IIIb) | 1/36/60/30/15 | |
| Adjuvant chemotherapy | 9 (6.3) | |
| Surgical approach | Laparoscopic | 57 (40.1) |
| Open | 85 (59.9) | |
| Operative time, median (range) (min) | 211 (92-369) | |
| Blood loss, median (range) (g) | 45 (0-985) | |
| Lymph node dissection | D1 | 5 (3.5) |
| D2 | 32 (22.5) | |
| D3 | 105 (74.0) | |
| Tumor location | Rt side | 78 (54.9) |
| Lt side | 64 (45.1) | |
The median follow-ups for the RFS and OS were 48.3 months (range 0.4-99.7) and 56.4 months (range 0.4-99.7 months), respectively. During the follow-up, 16 patients (11.3%) developed recurrence, and 19 (16.5 %) died.
Executing rate of adjuvant chemotherapy was nine cases (6.3%).
Baseline Characteristics of Patients Classified According to PMI
The clinicopathological characteristics of patients with low and normal PMI are shown in Table 2.
Table 2.
Clinical Characteristics of Patients with Low PMI and Normal PMI.
| low PMI, n = 35 |
normal PMI, n = 107 |
P value | ||
|---|---|---|---|---|
| Age, median (range) | 82 (75-96) | 80 (75-93) | 0.049 | |
| Sex, male/female | 15/20 | 48/59 | 1.000 | |
| BMI, median (range) | 20.9 (12.4-29.9) | 23.1 (15.8-32.8) | 0.0001 | |
| Hb, g/dl, median (range) | 10.3 (4.2-14.1) | 11.8 (5.4-17.4) | 0.007 | |
| Albumin, g/dl, median (range) | 3.4 (2.2-4.5) | 3.8 (2.0-5.0) | 0.001 | |
| CRP, mg/dl, median (range) | 0.30 (0.02-11.96) | 0.11 (0.01-10.89) | 0.006 | |
| Creatinine, mg/dl, median (range) | 0.78 (0.51-3.11) | 0.76 (0.46-7.47) | 0.904 | |
| Total lymphocyte count (/μL) | 1500 (600-3100) | 1600 (600-3400) | 0.111 | |
| Prognostic nutritional index | 43.0 (29.0-57.5) | 46.5 (25.5-61.0) | 0.001 | |
| Modified Glasgow prognostic score (0/1/2) | 10/16/9 | 77/17/11 | 0.0001 | |
| pStage (0/I/II/IIIa/IIIb) | 0/3/15/10/7 | 1/33/45/20/8 | 0.008 | |
| Adjuvant chemotherapy, n (%) | 2 (5.7) | 7 (6.5) | 1.000 | |
| Surgical approach | Laparoscopic | 9 (25.7) | 48 (44.9) | 0.049 |
| Open | 26 (74.3) | 59 (55.1) | ||
| Operative time, median (range) (min) | 199 (108-305) | 214 (92-369) | 0.132 | |
| Blood loss, median (range) (g) | 35 (0-565) | 45 (0-985) | 0.596 | |
| Lymph node dissection | D1 | 3 (8.6) | 2 (1.9) | 0.176 |
| D2 | 7 (20.0) | 25 (23.3) | ||
| D3 | 25 (71.4) | 80 (74.8) | ||
| Tumor location | Rt side | 18 (51.4) | 60 (56.1) | 0.697 |
| Lt side | 17 (48.6) | 47 (43.9) | ||
Patients with low PMI had a lower BMI (p = .0001), hemoglobin, serum albumin, and PNI values than those with normal PMI, whereas their age (p = .049) and CRP were higher (p = .044). Patients with low PMI included significantly more cases of mGPS 1 and mGPS 2, and their pStages were significantly more advanced than those in patients with high PMI.
Baseline Characteristics of Patients Classified According to IMAC
The clinicopathological characteristics of patients with high and normal IMAC are shown in Table 3. We found no significant differences between the high and normal IMAC groups except in age.
Table 3.
Clinical Characteristics of Patients with Normal IMAC and High IMAC.
| normal IMAC, n = 107 |
high IMAC, n = 35 |
P value | ||
|---|---|---|---|---|
| Age, median (range) | 79 (75-93) | 82 (75-96) | 0.006 | |
| Sex, male/female | 47/60 | 16/19 | 1.000 | |
| BMI, median (range) | 22.5 (12.4-32.8) | 23.8 (14.7-28.0) | 0.460 | |
| Hb, g/dl, median (range) | 11.5 (4.2-17.4) | 10.5 (5.4-14.6) | 0.140 | |
| Albumin, g/dl, median (range) | 3.8 (2.0-5.0) | 3.7 (2.4-4.5) | 0.305 | |
| CRP, mg/dl, median (range) | 0.12 (0.01-10.89) | 0.19 (0.01-11.96) | 0.382 | |
| Creatinine, mg/dl, median (range) | 0.77 (0.46-7.47) | 0.78 (0.52-1.98) | 0.682 | |
| Total lymphocyte count (/μL) | 1700 (600-3400) | 1500 (600-2700) | 0.174 | |
| Prognostic nutritional index | 45 (29-57.5) | 46 (25.5-61) | 0.156 | |
| Modified Glasgow prognostic score (0/1/2) | 66/25/14 | 19/7/7 | 0.546 | |
| pStage (0/I/II/IIIa/IIIb) | 1/29/42/23/12 | 0/7/18/7/3 | 0.423 | |
| Adjuvant chemotherapy, n (%) | 1 (2.9) | 8 (7.5) | 0.452 | |
| Surgical approach | Laparoscopic | 41 (38.3) | 16 (45.7) | 0.553 |
| Open | 66 (61.7) | 19 (54.3) | ||
| Operative time, median (range) (min) | 208 (114-369) | 211 (92-346) | 0.356 | |
| Blood loss, median (range) (g) | 40 (0-985) | 70 (0-565) | 0.134 | |
| Lymph node dissection | D1 | 3 (2.8) | 2 (5.7) | 0.523 |
| D2 | 26 (24.3) | 6 (17.1) | ||
| D3 | 78 (72.9) | 27 (77.2) | ||
| Tumor location | Rt side | 59 (55.1) | 19 (54.3) | 1.000 |
| Lt side | 48 (44.9) | 16 (45.7) | ||
OS and RFS Rates After Curative Resection
After the Kaplan-Meier analysis, patients in the low PMI group experienced significantly shorter OS (5-year OS, 61% vs. 87%; log-rank p = .0007) and RFS (5-year RFS, 51% vs. 78%; log-rank p = .0014) than those in the normal PMI group (Figure 3).
Figure 3.
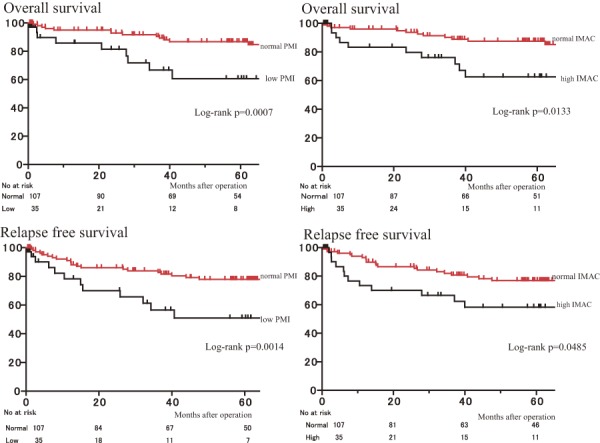
Overall survival rates and relapse-free survival after curative resection of colorectal cancer according to PMI and IMAC.
PMI psoas muscle mass index, IMAC intramuscular adipose tissue content
Similarly, the rates of OS and RFS for patients with high IMAC were significantly lower than those for patients with normal IMAC (p = .0133, and p = .0485, respectively) (Figure 3).
The patients with normal PMI were divided into two groups: those with high IMAC and those with normal IMAC.
The clinicopathological characteristics of patients with normal PMI/normal IMAC and normal PMI/high IMAC are shown in Table 4. We found no significant differences between the two groups.
Table 4.
Clinical Characteristics of Patients with Normal PMI/Normal IMAC and Normal PMI/High IMAC.
| normal PMI/ normal IMAC n = 82 |
normal PMI/ high IMAC n = 25 |
P value | ||
|---|---|---|---|---|
| Age, median (range) | 79 (75-93) | 81 (75-88) | 0.148 | |
| Sex, male/female | 36/46 | 12/13 | 0.819 | |
| BMI, median (range) | 22.7 (15.8-32.8) | 24.6 (17.9-28.0) | 0.052 | |
| Hb, g/dl, median (range) | 11.9 (5.6-17.4) | 11.1 (5.4-14.6) | 0.104 | |
| Albumin, g/dl, median (range) | 3.9 (2.0-5.0) | 3.8 (2.8-4.4) | 0.260 | |
| CRP, mg/dl, median (range) | 0.10 (0.01-10.9) | 0.12 (0.01-2.6) | 0.383 | |
| Creatinine, mg/dl, median (range) | 0.76 (0.46-7.47) | 0.78 (0.54-1.31) | 0.889 | |
| Total lymphocyte count (/μL) | 1700 (600-3400) | 1550 (700-2700) | 0.456 | |
| Prognostic nutritional index | 47.0 (25.5-61.0) | 46.0 (34.5-55.5) | 0.220 | |
| Modified Glasgow prognostic score (0/1/2) | 60/12/9 | 17/5/2 | 0.749 | |
| pStage (0/I/II/IIIa/IIIb) | 1/26/33/15/7 | 0/7/11/5/1 | 0.885 | |
| Surgical approach | Laparoscopic | 36 (43.9) | 12 (48.0) | 0.819 |
| Open | 46 (56.1) | 13 (52.0) | ||
| Operative time, median (range) (min) | 214 (92-346) | 218 (122-369) | 0.599 | |
| Blood loss, median (range) (g) | 45 (0-985) | 50 (5-495) | 0.256 | |
| Lymph node dissection | D1 | 1 (1.2) | 1 (4.0) | 0.434 |
| D2 | 21 (25.6) | 4 (16.0) | ||
| D3 | 60 (73.2) | 20 (80.0) | ||
| Tumor location | Rt side | 46 (56.1) | 14 (56.0) | 1.000 |
| Lt side | 36 (43.9) | 11 (44.0) | ||
The OS rate for patients with normal PMI/high IMAC was lower than that for those with normal PMI/normal IMAC (p = .0015) (Figure 4). Similarly, the RFS rate for patients with normal PMI/high IMAC was lower than that for patients with normal PMI/low IMAC (p = .0331) (Figure 4).
Figure 4.
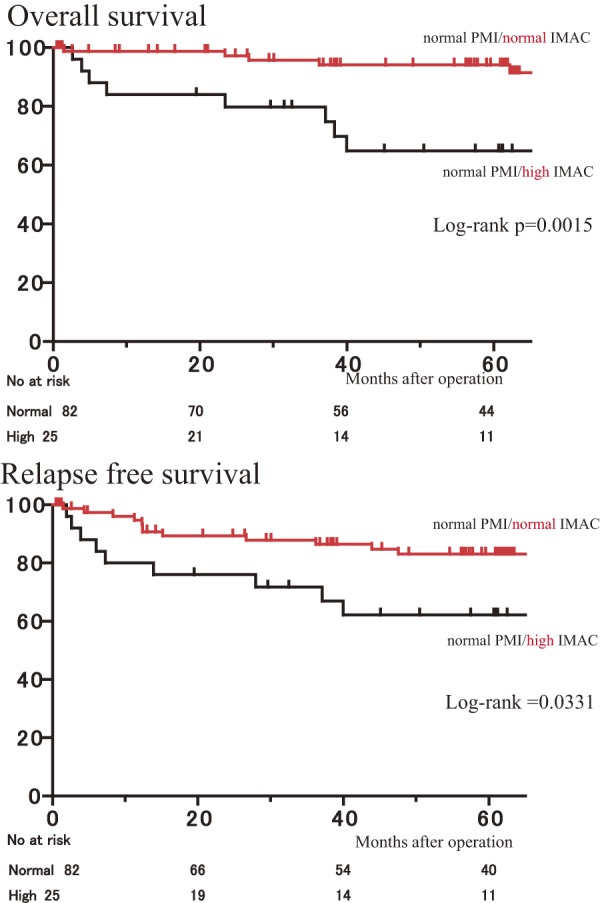
Overall survival rates and relapse-free survival after curative resection of colorectal cancer for patients with normal PMI classified according to IMAC.
Risk Factors for Poor Outcome in Patients Undergoing Colonic Curative Resection
Our univariate analysis found significant outcome differences between the groups in terms of PMI, IMAC, age, albumin level, PNI, and surgical approach (open surgery) for death after curative resection (Table 5).
Table 5.
Univariate and Multivariate Analyzes of Clinical Factors and Overall Survival after Resection of Colorectal Cancer.
| Variable | Univariate Hazard ratio (95% CI) |
P value | Multivariate Hazard ratio (95% CI) |
P value |
|---|---|---|---|---|
| Low PMI | 3.81 | 0.003 | 3.81 | 0.006 |
| (1.62-8.68) | (1.47-9.83) | |||
| High IMAC | 2.69 | 0.023 | 3.06 | 0.019 |
| (1.16-6.02) | (1.20-7.50) | |||
| Age (≥80 y) | 3.15 | 0.008 | 2.59 | 0.042 |
| (1.34-8.26) | (1.03-7.15) | |||
| Albumin (<3.5 g/dl) | 2.49 | 0.036 | ||
| (1.06-5.68) | ||||
| CRP (<0.5 mg/dl) | 1.76 | 0.233 | ||
| (0.67-4.13) | ||||
| PNI (<40) | 3.77 | 0.008 | ||
| (1.44-9.61) | ||||
| Operation approach (LAP) | 0.28 | 0.011 | 0.25 | 0.008 |
| (0.08-0.77) | (0.07-0.70) | |||
| Stage I/II/III | 0.170 |
Our multivariate analysis identified the following independent significant risk factors for death after curative colonic resection for colorectal cancer: low PMI (low skeletal muscle mass), high IMAC (low skeletal muscle quality), older age (≥80 years), and treatment with open surgery (Table 5).
The univariate analysis also revealed that low PMI, older age (≥80), low albumin (<3.5 g/dl), low PNI (<40), treatment with open surgery, and pStage are significant factors for recurrence (Table 6). Moreover, the multivariate analysis identified high IMAC, high age (≥80), and pStage as three independent risk factors for recurrence (Table 6).
Table 6.
Univariate and Multivariate Analyzes of Clinical Factors and Relapse-free Survival after Resection of Colorectal Cancer.
| Variable | Univariate Hazard ratio (95% CI) |
P value | Multivariate Hazard ratio (95% CI) |
P value |
|---|---|---|---|---|
| Low PMI | 3.12 | 0.003 | ||
| (1.50-6.27) | ||||
| High IMAC | 1.95 | 0.083 | 3.03 | 0.010 |
| (0.91-3.95) | (1.33-6.66) | |||
| Age (≥80 y) | 2.81 | 0.005 | 2.80 | 0.009 |
| (1.37-6.23) | (1.28-6.62) | |||
| Albumin (<3.5 g/dl) | 2.42 | 0.016 | ||
| (1.19-4.80) | ||||
| CRP (<0.5 mg/dl) | 1.99 | 0.075 | ||
| (0.93-4.03) | ||||
| PNI (<40) | 2.73 | 0.009 | ||
| (1.30-5.48) | ||||
| Operation approach (LAP) | 0.41 | 0.022 | ||
| (0.18-0.88) | ||||
| Stage I/II/III | 0.001 | 0.007 |
Discussion
The results of our retrospective study indicated that preoperative PMI and IMAC are significant prognostic factors for mortality after curative resection for colorectal cancer in the elderly. Other studies concerning the association between sarcopenia and colon cancer have focused only on skeletal muscle volume4,18).
To our knowledge, this is the first study to examine the effect of skeletal muscle quality on the survival after colectomy in the elderly.
The amount of muscle mass is correlated with muscle strength; thus the loss of muscle mass results in a loss of muscle strength. However, some patients display lower levels of muscle function and strength even though their muscular mass appears to be normal, probably because of declined muscle quality. We found that measuring the skeletal muscle area exclusively using CT imaging yielded inadequate results because we could not discriminate muscle from adipose tissue.
The muscle has been inversely correlated with the size of intramuscular adipose tissue19).
Therefore, a high IMAC represents a lower level of lean muscle mass as well as a large amount of intramuscular adipose tissue.
The loss of muscle tissue in sarcopenia has been associated with fatty infiltration, a condition known as myosteatosis11). We assessed the presence of sarcopenia using the measured intramuscular adipose tissue accumulation and the PMI.
In this study, although the patients with normal PMI appeared to have normal muscle volume, those with low muscle quality in this group had poorer prognosis than those with normal muscle quality. The OS and RFS rates for patients with normal PMI/normal IMAC were significantly better than those for patients with normal PMI/high IMAC (p = .0015 and p = .0331, respectively), which suggests that the prognosis is dependent on the skeletal muscle quality and that patients with low muscle quality have a poor prognosis even if their muscular volume is normal. Thus, estimation of muscle volume alone is not enough, and assessments of both the quality and the quantity of skeletal muscle are more accurate for determining prognosis.
We found that the prognosis after resection of colorectal cancer depended on several tumor-specific factors, including the tumor depth, lymph node metastasis presence, harvested lymph node number, and resection margin status.
However, most tumor-specific factors become evident only after surgery. Instead, sarcopenia assessments can be done before the operation. Studies have pointed to preoperative parameters, such as CRP, mGPS, and PNI, as potential prognostic markers for curative colonic cancer cases17,20,21).
In our study, the preoperative parameters such as CRP, PNI, and serum albumin were not independent prognostic factors after the multivariate analysis.
Accordingly, PMI and IMAC may represent more accurate preoperative patient-specific prognostic markers.
The mechanisms by which sarcopenia is associated with mortality are yet to be clearly understood.
The skeletal muscle and adipose tissue are important secretory endocrine organs. Skeletal muscle releases several different cytokines and peptides (myokines), such as IL-6, IL-15, and insulin-like growth factor-1. On the other hand, adipose tissue releases several adipocytokines (adipokines), such as adiponectin and leptin.
Myokines are thought to balance and counteract the action of adipokines. Moreover, this interaction between muscle and adipose tissue and the association between immunity and inflammation appear to explain at least partly how sarcopenia worsens patient survival.
Studies have indicated that preoperative rehabilitation can reduce the chance of postoperative complications in patients with lung22), colorectal18), and esophageal cancers23). However whether preoperative rehabilitation affects survival is not clear.
Okumura et al. published a study applying preoperative and postoperative nutritional and exercise therapeutic protocols for patients having living donor liver transplantation, especially for those with sarcopenia or poor nutrition24). The same supportive therapy focusing on nutrition and rehabilitation may be indicated for elderly patients with various kinds of cancer, but the evidence supporting the approach needs to be produced in a prospective study.
Preoperative PMI and IMAC may be considered reliable prognostic factors before surgery in elderly patients with colon cancer. Moreover, the sarcopenia risk assessment can be carried out easily prior to surgery using our approach. With this, the resulting risk assessment and scoring may be helpful for taking clinical decisions about treatment, especially the indications of surgery for high-risk elderly patients, although further research is necessary.
We are aware of the limitations in our study. First, we could not study the association between IMAC and skeletal muscle strength due to the retrospective nature of our study. However, studies have suggested that intramuscular fat accumulation leads to decreases in muscle strength and quality10,25). In addition, these findings support our results that IMAC is a possible new marker to evaluate sarcopenia to replace the measurement of muscle strength alone. We believe our choice of the psoas muscle for estimating skeletal muscle mass and the multifidus muscle for estimating skeletal muscle quality at the umbilical level in cross-sectional CT images is appropriate as they have been used already12,14,15).
Last, we cannot be sure that our cutoff values for PMI and IMAC are sufficient to identify sarcopenia. Other reports have used discrete definitions for sarcopenia, although no consensus or objective criteria to define the condition exist. In this study, we used gender-specific quartiles rather than standard deviations for setting cutoff values to define sarcopenia.
However the most common sarcopenia definition is an appendicular skeletal muscle index of more than two standard deviations below that of healthy adults (5.45-kg/m2 for females and 7.26-kg/m2 for males)26). These values were obtained from dual-energy X-ray scanning, but we got our data from CT images instead, a broadly available and accurate method for detecting sarcopenia27,28) that is performed before colorectal cancer surgery for cancer staging.
In addition, since the prevalence of sarcopenia in Japanese patients is unknown, we selected gender-specific quartiles. However, further investigations are necessary.
In conclusion, our findings show that preoperative sarcopenia, defined by both quality and quantity of skeletal muscle, was closely related to the postoperative survival (OS and RFS) of elderly patients undergoing curative resection of colon cancer. The estimation of sarcopenia using PMI and IMAC is easy. In addition to postoperative tumor-specific prognostic factors, preoperative evaluation of sarcopenia, including muscle quality and muscle quantity, may be helpful for risk assessment and clinical decision making for elderly patients with colon cancer.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Hori M, Matsuda T, Shibata A, et al. Cancer incidence and incidence rates in Japan in 2009: a study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jap J Clin Oncol. 2015 Sep; 45(9): 884-91. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg IH. Sarcopenia: origins and clinical relevance. Clin Geriatr Med. 2011 Aug; 27(3): 337-9. [DOI] [PubMed] [Google Scholar]
- 3.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010 Jul; 39(4): 412-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyamoto Y, Baba Y, Sakamoto Y, et al. Negative Impact of Skeletal Muscle Loss after Systemic Chemotherapy in Patients with Unresectable Colorectal Cancer. PLoS One. 2015 Jun; 10(6): e0129742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ida S, Watanabe M, Yoshida N, et al. Sarcopenia is a predictor of postoperative respiratory complications in patients with esophageal cancer. Ann Surg Oncol. 2015 Dec; 22(13): 4432-7. [DOI] [PubMed] [Google Scholar]
- 6.Fukushima H, Yokoyama M, Nakanishi Y, et al. Sarcopenia as a prognostic biomarker of advanced urothelial carcinoma. PLoS One. 2015 Jan; 10(1): e0115895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voron T, Tselikas L, Pietrasz D, et al. Sarcopenia impacts on short- and long-term results of hepatectomy for hepatocellular carcinoma. Ann Surg. 2015 Jun; 261(6): 1173-83. [DOI] [PubMed] [Google Scholar]
- 8.Levolger S, van Vugt JL, de Bruin RW, et al. Systematic review of sarcopenia in patients operated on for gastrointestinal and hepatopancreatobiliary malignancies. Br J Surg. 2015 Nov; 102(12): 1448-58. [DOI] [PubMed] [Google Scholar]
- 9.Richards CH, Roxburgh CS, MacMillan MT, et al. The relationships between body composition and the systemic inflammatory response in patients with primary operable colorectal cancer. PLoS One. 2012 Aug; 7(8): e41883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcus RL, Addison O, Kidde JP, et al. Skeletal muscle fat infiltration: impact of age, inactivity, and exercise. J Nutr Health Aging. 2010 May; 14(5): 362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zoico E, Corzato F, Bambace C, et al. Myosteatosis and myofibrosis: relationship with aging, inflammation and insulin resistance. Arch Gerontol Geriatr. 2013; 57: 411-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitajima Y, Hyogo H, Sumida Y, et al. Severity of non-alcoholic steatohepatitis is associated with substitution of adipose tissue in skeletal muscle. J Gastroenterol Hepatol. 2013 Sep; 28(9): 1507-14. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi A, Kaido T, Hamaguchi Y, et al. Impact of Visceral Adiposity as Well as Sarcopenic Factors on Outcomes in Patients Undergoing Liver Resection for Colorectal Liver Metastases. World J Surg. 2018 Apr; 42(4): 1180-91. [DOI] [PubMed] [Google Scholar]
- 14.Okumura S, Kaido T, Hamaguchi Y, et al. Impact of preoperative quality as well as quantity of skeletal muscle on survival after resection of pancreatic cancer. Surgery. 2015 Jun; 157(6): 1088-98. [DOI] [PubMed] [Google Scholar]
- 15.Kitajima Y, Eguchi Y, Ishibashi E, et al. Age-related fat deposition in multifidus muscle could be a marker for nonalcoholic fatty liver disease. J Gastroenterol. 2010 Feb; 45(2): 218-24. [DOI] [PubMed] [Google Scholar]
- 16.Toiyama Y, Miki C, Inoue Y, et al. Evaluation of an inflammation-based prognostic score for the identification of patients requiring postoperative adjuvant chemotherapy for stage II colorectal cancer. Exp Ther Med. 2011 Dec; 2(1): 95-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients (in Japanese with English abstract). Nihon Geka Gakkai zasshi. 1984 Sep; 85(9): 1001-5. [PubMed] [Google Scholar]
- 18.Mayo NE, Feldman L, Scott S, et al. Impact of preoperative change in physical function on postoperative recovery: argument supporting prehabilitation for colorectal surgery. Surgery. 2011 Sep; 150(3): 505-14. [DOI] [PubMed] [Google Scholar]
- 19.Akima H, Yoshiko A, Tomita A, et al. Relationship between quadriceps echo intensity and functional and morphological characteristics in older men and women. Arch Gerontol Geriatr. 2017 May; 70: 105-11. [DOI] [PubMed] [Google Scholar]
- 20.Allin KH, Nordestgaard BG. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit Rev Clin Lab Sci. 2011 Jul-Aug; 48(4): 155-70. [DOI] [PubMed] [Google Scholar]
- 21.McMillan DC, Canna K, McArdle CS. Systemic inflammatory response predicts survival following curative resection of colorectal cancer. Br J Surg. 2003 Feb; 90(2): 215-9. [DOI] [PubMed] [Google Scholar]
- 22.Benzo R, Wigle D, Novotny P, et al. Preoperative pulmonary rehabilitation before lung cancer resection: results from two randomized studies. Lung cancer. 2011 Dec; 74(3): 441-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoue J, Ono R, Makiura D, et al. Prevention of postoperative pulmonary complications through intensive preoperative respiratory rehabilitation in patients with esophageal cancer. Dise Esophagus. 2013 Jan; 26(1): 68-74. [DOI] [PubMed] [Google Scholar]
- 24.Okumura S, Kaido T, Hamaguchi Y, et al. Impact of the preoperative quantity and quality of skeletal muscle on outcomes after resection of extrahepatic biliary malignancies. Surgery. 2016 March; 159(3): 821-33. [DOI] [PubMed] [Google Scholar]
- 25.Delmonico MJ, Harris TB, Visser M, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009 Dec; 90(6): 1579-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998 Apr; 147: 755-63. [DOI] [PubMed] [Google Scholar]
- 27.Mourtzakis M, Prado CM, Lieffers JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008 Oct; 33(5): 997-1006. [DOI] [PubMed] [Google Scholar]
- 28.Ryan AM, Power DG, Daly L, et al. Cancer-associated malnutrition, cachexia and sarcopenia: the skeleton in the hospital closet 40 years later. Proc Nutr Soc. 2016 May; 75(2): 199-211. [DOI] [PubMed] [Google Scholar]