Abstract
Aim: Knowledge of subclinical plaque morphology and plaque distribution in the aorta in vivo remains unclear. This study aimed to increase the body of knowledge in this area.
Methods: We enrolled 37 consecutive patients with stable angina pectoris patients who underwent non-obstructive angioscopy for both the coronary artery and aorta immediately after percutaneous coronary intervention. We evaluated the presence of aortic plaques and the distribution of plaque instability. Patients were allocated into two groups according to the number of vulnerable plaques in whole aorta (a low [0–11] and high [≥ 12] group). We evaluated the relationships between the two groups in terms of cardiovascular risk factors.
Results: Aortic plaques were identified using non-obstructive angioscopy in all patients, and the greatest number of plaques was found at the infrarenal abdominal aorta (IAA) (the aortic arch, the descending thoracic aorta, the suprarenal abdominal aorta, the IAA, and common iliac artery; 65%, 76%, 65%, 95%, and 49%, respectively; p < 0.001). The maximum yellow grade, and the number of intense yellow plaques, ruptured plaques, and thrombi were highest at the IAA (p < 0.001). The prevalence of diabetes mellitus and peripheral arterial disease was higher in the high vulnerable plaque group (83.3% vs. 40.0%, p = 0.010, 50.0% vs. 8.0%, p = 0.005, respectively).
Conclusions: Aortic atherosclerosis was the most severe at the IAA, and aortic plaque vulnerability and distribution were associated with the prevalence of diabetes mellitus and peripheral artery disease in patients with stable angina pectoris. Non-obstructive angioscopy may identify patients at high risk of future aortic events.
Keywords: Aortic angioscopy, Aortic plaque distribution, Atheromatous plaque, Atherosclerotic risk factors, Plaque morphology
Introduction
Aortic atherosclerotic plaques are a crucial risk factor for cardiovascular death1). Aortic plaques can develop into critical diseases such as aortic aneurysm2), can develop because of aortic dissection caused by penetrating atherosclerotic ulcer3), or can cause aortogenic cerebral embolism4, 5). Thus, a detailed evaluation of aortic plaques in vivo is clinically important to elucidate the mechanisms behind these critical illnesses.
Previous studies have reported that intravascular imaging modalities are useful for the assessment of morphologies and plaque characteristics in coronary artery lesions. These devices can clearly detect atherosclerotic changes and can predict high risk of acute coronary syndrome6–8). In particular, non-obstructive angioscopy can produce real, visible, detailed images of the vessel lumen surface and is useful for detection of plaque vulnerability9).
Generally, computed tomography, magnetic resonance imaging, and transesophageal echocardiography have been used for the evaluation of aortic atherosclerosis. However, these imaging modalities may be limited in the assessment of aortic plaque instability9). Recently, using non-obstructive angioscopy for the aorta (Ao-angioscopy), several studies have reported a novel evaluation method for atherosclerotic changes in the aortic wall9–11). Ao-angioscopy may have higher reliability in the identification of aortic plaques and contribute to elucidating them, because of its higher spatial resolution than other imaging technologies9). On the contrary, plaque instability and distribution in the aorta have not been well detected in vivo, and the association between aortic plaque instability and atherosclerotic risk factors remains to be investigated.
In the present study, we evaluated aortic plaque morphology and instability using Ao-angioscopy and assessed their differences according to each segment of the aorta. Furthermore, we investigated the association between atherosclerotic plaque compositions detected by Ao-angioscopy and atherosclerotic risk factors.
Aims
The aims of our study were to evaluate aortic plaque morphology, instability, and distribution, and to assess the relationships between atherosclerotic plaque compositions and atherosclerotic risk factors using Ao-angioscopy.
Methods
We retrospectively reviewed 37 consecutive patients with stable angina pectoris who underwent non-obstructive angioscopy for both the coronary artery and the aorta immediately after percutaneous coronary intervention (PCI) using a femoral arterial approach for chronic total occlusion, between June 2014 and February 2016, at Yokosuka Kyosai Hospital, Kanagawa, Japan. Patients with acute aortic syndrome such as aortic dissection or rupture of the aortic aneurysm, congestive heart failure, left main trunk lesions, unstable hemodynamics, unsuccessful PCI, and insufficient angioscopic quality were excluded. Laboratory data were examined before PCI according to established procedures. The Institutional Review Committee of Yokosuka Kyosai Hospital approved this study, and all patients provided written informed consent.
Atherosclerotic Risk Factors
Hypertension was defined as blood pressure > 140/90 mmHg or use of antihypertensive drugs. Dyslipidemia was defined as total cholesterol level > 220 mg/dL, low-density lipoprotein cholesterol level > 140 mg/dL, high-density lipoprotein cholesterol < 40 mg/dL, triglyceride levels > 150 mg/dL, or use of drugs for dyslipidemia. Diabetes mellitus (DM) was defined as fasting blood glucose > 126 mg/dL and HbA1c > 6.5%, or use of an oral hypoglycemic agent (OHA) or insulin therapy. Peripheral artery disease (PAD) was defined as an ankle-brachial index value of < 0.9 and a Fontaine classification of II–IV.
Angioscopic Equipment and Procedures
Catheterization was performed via the femoral artery using a 6- or 7-F sheath and a catheter. Angioscopic examination of the coronary artery and the aorta was performed after PCI using a non-obstructive angioscopy system (FT-203F angioscope and VISIBLE fiber; Fiber Tech Co., Ltd., Tokyo, Japan), as previously reported9, 12). Observations were made while the blood was cleared away from the view using the dual infusion method to obtain a visual field, as previously reported9, 12). For evaluation of the coronary artery, the outer section of the 4-Fr probing catheter was used as a guide for inserting the imaging fiber into the chronic totally occluded lesion of the coronary artery12). Coronary angioscopic data were acquired before balloon dilatation after wire-crossing. If this was difficult, the data were collected after dilation with a small balloon (1.0–1.5 mm in diameter) to acquire the minimum diameter for crossing with the angioscopy catheter13). For evaluation of the aorta, we observed five segments: the aortic arch, the descending thoracic aorta, the suprarenal abdominal aorta, the infrarenal abdominal aorta (IAA), and the common iliac artery14). The ascending aorta was excluded because of poor angioscopic quality. Angioscopic observation of the aorta was performed continuously while a guiding catheter was slowly pulled back from the aortic arch to the common iliac artery and rotated for vessel-wide screening, as previously reported9, 14).
Definition and Analysis of Angioscopic Findings
Yellow color grade (yellow grade) was defined as 0 (white), 1 (light yellow), 2 (yellow), or 3 (intense yellow)12). Presence of thrombus and yellow plaque was assessed in the coronary artery before placing the stent. Presence of yellow plaques was defined as a maximum yellow color grade of 2 or 315). Aortic vulnerable plaque in this study was defined as intense yellow plaque, ruptured plaque, and thrombus. We evaluated the presence of aortic plaques, and the distribution of the maximum yellow grade and the number of intense yellow plaques, ruptured plaques, thrombi, and total number of vulnerable plaques in each segment of the aorta. All patients were allocated to two groups based on the total number of vulnerable plaques in the whole aorta: a low group (low and middle tertile: 0–11, n = 25) and a high group (high tertile: ≥ 12, n = 12). Differences in clinical characteristics, medications, laboratory data, and coronary findings were compared between the groups. Angioscopic evaluations were performed by two specialists in coronary intervention and angioscopy who were blinded to the patient's clinical status.
Statistical Analysis
JMP statistics 12.2.0 (SAS Institute, Cary, NC, USA) was used for all statistical analyses. Continuous variables were expressed as mean ± SD for normally distributed variables or as median (25–75th percentiles) for non-normally distributed variables, and were compared using a Student t-test and ANOVA, and a Mann–Whitney U-test. A Wilcoxon test was used for ordinal variables. Categorical variables were expressed as frequencies and analyzed using chi-square statistics or Fisher exact test. Multivariate logistic regression analysis was used to evaluate the relationships among the clinical characteristics and the high vulnerable plaque group (≥ 12). Variables with p < 0.05 in the univariate analysis were included in the multivariate model. Intra- and interobserver agreement for qualitative plaque morphologies determined by Ao-angioscopy was assessed using κ statistics. A significance level of 0.05 was used and 2-tailed tests were applied.
Results
This study recruited 37 patients with a mean age of 67 years, and 83.8% were men. We were able to observe and evaluate atherosclerotic changes from the aortic arch to the common iliac artery for all patients. No complications, such as acute aortic injury, stroke, acute renal failure, acute limb injury, blue toe syndrome, or abnormal changes in laboratory data occurred during aortic angioscopic examination or for 9 months after the procedure. Baseline demographics are reported in Table 1. A case of the patient with aortic vulnerable plaques identified by Ao-angioscopy is presented in Fig. 1.
Table 1. Baseline characteristics.
| All (N = 37) | VP in whole aorta | VP in whole aorta | p value | |
|---|---|---|---|---|
| 0–11 (N = 25) | ≥ 12 (N = 12) | |||
| Age | 67.3 ± 9.8 | 66.3 ± 10.9 | 69.4 ± 6.9 | 0.38 |
| Gender, male | 31 (83.8) | 22 (88.0) | 9 (75.0) | 0.33 |
| LV ejection fraction | 57.6 ± 15.6 | 55.3 ± 9.9 | 62.2 ± 13.7 | 0.09 |
| iabetes mellitus | 28 (75.7) | 10 (40.0) | 10 (83.3) | 0.01 |
| Hypertension | 22 (59.5) | 19 (76.0) | 9 (75.0) | 0.95 |
| Dyslipidemia | 27 (73.0) | 14 (56.0) | 8 (66.7) | 0.53 |
| Smoking history | 6 (16.2) | 18 (72.0) | 9 (75.0) | 0.85 |
| Hemodialysis | 6 (16.2) | 5 (20.0) | 1 (8.3) | 0.34 |
| OMI | 9 (24.3) | 8 (32.0) | 1 (8.3) | 0.093 |
| Past stroke | 3 (8.1) | 2 (8.0) | 1 (8.3) | 0.97 |
| PAD | 8 (21.6) | 2 (8.0) | 6 (50.0) | 0.005 |
| Medications | ||||
| Aspirin | 36 (97.3) | 25 (100.0) | 11 (91.7) | 0.13 |
| Dual antiplatelets | 35 (94.6) | 24 (96.0) | 11 (91.7) | 0.6 |
| Ca-Blocker | 14 (37.8) | 7 (28.0) | 7 (58.3) | 0.077 |
| ACE-I/ARB | 21 (56.8) | 14 (56.0) | 7 (58.3) | 0.89 |
| Diuretics | 7 (18.9) | 5 (20.0) | 2 (16.7) | 0.81 |
| Statin | 33 (89.2) | 22 (88.0) | 11 (91.7) | 0.73 |
| OHA | 16 (43.2) | 8 (32.0) | 8 (66.7) | 0.046 |
| Insulin | 8 (21.6) | 2 (8.0) | 6 (50.0) | 0.005 |
| Laboratory data | ||||
| Creatinine (mg/dl) | 0.94 (0.79–1.23) | 1.0 (0.8–1.3) | 0.8 (0.7–1.2) | 0.3 |
| eGFR (ml/min/1.73 m2) | 56.2 ± 29.1 | 53.7 ± 30.9 | 61.5 ± 25.2 | 0.45 |
| HbA1c (%) (JDS) | 6.4 ± 1.0 | 6.2 ± 0.9 | 6.8 ± 1.1 | 0.08 |
| HDL-C (mg/dl) | 44.5 ± 11.9 | 44.1 ± 12.7 | 45.3 ± 10.5 | 0.78 |
| LDL-C (mg/dl) | 78.6 ± 25.5 | 75.3 ± 24.6 | 85.4 ± 27.1 | 0.26 |
| Triglyceride (mg/dl) | 145.5 ± 90.2 | 142.0 ± 98.6 | 152.8 ± 73.0 | 0.74 |
| hs-CRP (mg/dl) | 0.12 (0.06–0.31) | 0.12 (0.09–0.31) | 0.12 (0.05–0.32) | 0.81 |
| BNP (pg/dl) | 71.2 (33.2–150.5) | 75.7 (24.2–166.0) | 49.3 (34.9–108.9) | 0.21 |
| Coronary angioscopy findings | ||||
| Yellow grade (2–3) | 18 (48.6) | 11 (44.0) | 7 (58.3) | 0.41 |
| Thrombi | 16 (43.2) | 11 (44.0) | 5 (41.7) | 0.89 |
Data are presented as n (%), mean ± SD, or median (IQR).
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BNP, brain natriuretic peptide; Ca, calcium; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol; LV, left ventricular; OHA, oral hypoglycemic agent; OMI, old myocardial infarction; PAD, peripheral artery disease; VP, vulnerable plaque.
Fig. 1.
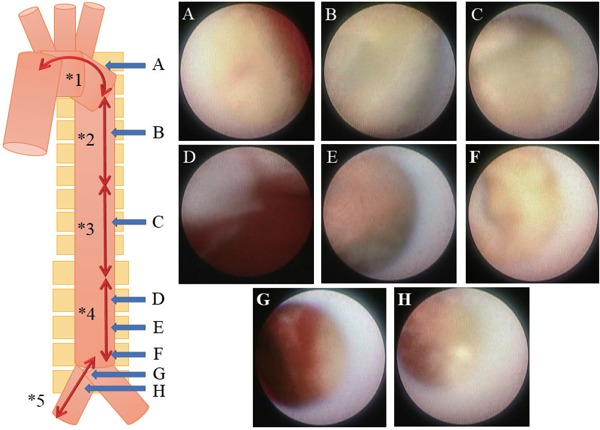
A representative case of aortic vulnerable plaques with Ao-angioscopy
A 75 year-old-female patient had effort angina pectoris, diabetes mellitus, and peripheral artery disease. She had been using rosuvastatin and insulin. Her low-density lipoprotein level was 109 mg/dL and HbA1c level was 7.0%. She was diagnosed with a chronic total occlusion lesion and intense yellow plaques identified by coronary angioscopy. We then performed aortic angioscopy immediately after percutaneous coronary intervention. We were able to observe several aortic plaques across the aorta.
Aortic angioscopic findings are shown below.
*1. aortic arch: *2. descending thoracic aorta: *3. suprarenal abdominal aorta: *4. infrarenal abdominal aorta: *5. common iliac artery
=(Maximum yellow grade) 3:2:2:3:2
=(Number of intense yellow plaque) 1:0:0:2:0
=(Number of ruptured plaque) 1:0:1:2:0
=(Number of thrombi) 1:0:0:2:2
There were 12 vulnerable plaques in whole aorta.
Prevalence and Distribution of Aortic Plaques with Aortic Angioscopy
Atherosclerotic changes of the aorta were identified in all patients by using angioscopy. The frequency of aortic plaques (yellow plaque, thrombus, or ruptured plaque) was the greatest at the IAA of the aorta (p < 0.001) (Fig. 2). The prevalence of intense yellow plaques and thrombi was the greatest at the IAA of the aorta, although there was no significant difference in the prevalence of ruptured plaques between the segments of the aorta (Fig. 3). The maximum yellow grade (Fig. 4A), the number of intense yellow plaques (Fig. 4B), ruptured plaques (Fig. 4C), thrombi (Fig. 4D), and total number of vulnerable plaques (Fig. 5) were the greatest at the IAA of the aorta (p < 0.001, < 0.001, 0.021, < 0.001, < 0.001, respectively)(Figs. 4 and 5).
Fig. 2.
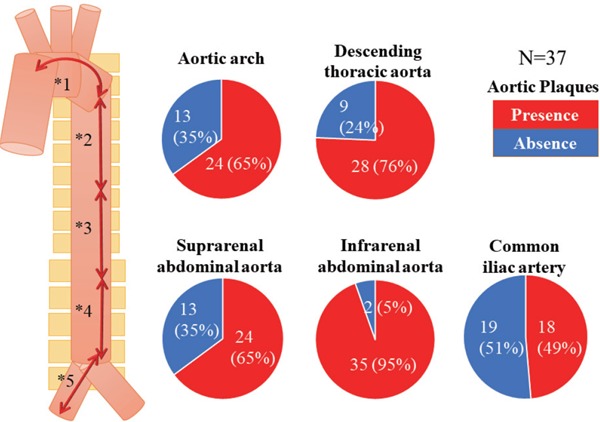
Distribution of all atherosclerotic plaques at each segment of the aorta
All atherosclerotic plaques were defined as yellow plaque, thrombus, or ruptured plaque.
Data are presented as n (%). The frequency of aortic plaques was the greatest at the IAA in the aorta (p < 0.001).
*1 (aortic arch); *2 (descending thoracic aorta); *3 (suprarenal abdominal aorta); *4 (infrarenal abdominal aorta); *5 (common iliac artery).
*1 vs. *4, p < 0.001; *2 vs. *4, p = 0.017; *3 vs. *4, p = 0.003; *4 vs. *5, p < 0.001.
Fig. 3.
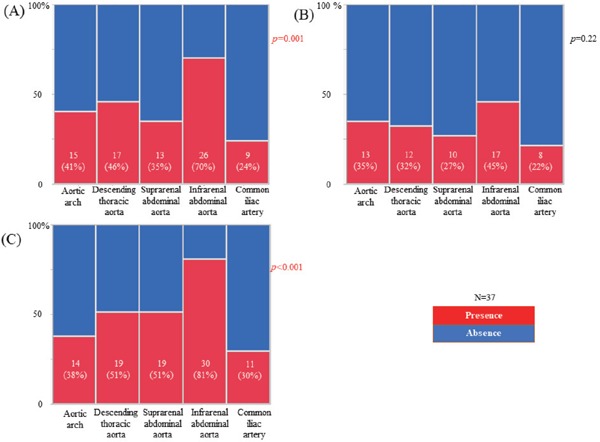
The prevalence of intense yellow plaques (A), ruptured plaques (B), and thrombi (C) at each segment of the aorta
Data are presented as number (%).
Fig. 4.
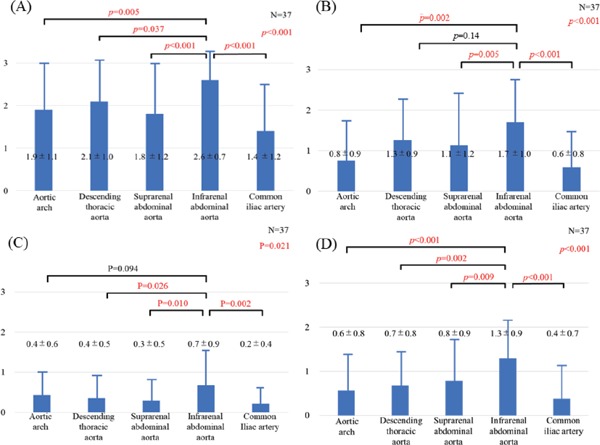
The maximum yellow grade (A), the number of intense yellow plaques (B), ruptured plaques (C), and thrombi (D) at each segment of the aorta
Data are presented as mean ± SD.
Fig. 5.
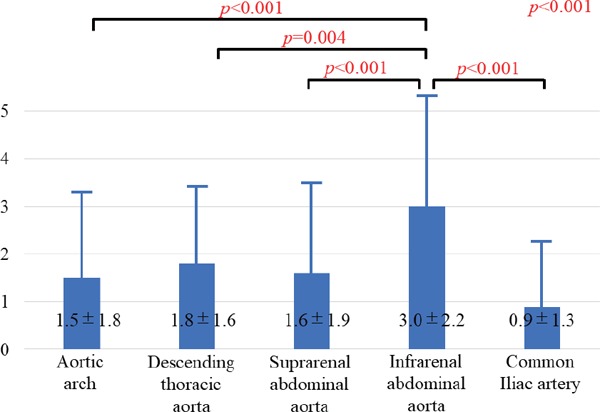
The number of vulnerable plaques at each segment of the aorta
Data are presented as mean ± SD.
Relationship between Patient Characteristics and Aortic Angioscopic Findings According to the Total Number of Vulnerable Plaques in the Whole Aorta
The frequency of DM and PAD was significantly higher in the high vulnerable plaque group. Use of OHA and insulin was higher in the high vulnerable plaque group. Use of statin or antihypertensive drugs did not differ significantly in the two groups. Laboratory data were not significantly different in the two groups. According to the coronary angioscopic findings, the presence of thrombi and yellow plaques was not significantly different in the two groups. Multivariate analysis indicated that the prevalence of DM and PAD was an independent predictor for high vulnerable plaque group (Tables 1 and 2).
Table 2. Factors associated with number of VP in whole aorta (≥ 12).
| OR | 95% CI | p-value | |
|---|---|---|---|
| Univariate logistic regression analysis | |||
| Age | 1.04 | 0.96–1.12 | 0.35 |
| Diabetes mellitus | 7.5 | 1.56–56.0 | 0.01 |
| Dyslipidemia | 1.57 | 0.38–7.18 | 0.53 |
| Hypertension | 0.95 | 0.19–5.29 | 0.95 |
| OMI | 0.19 | 0.01–1.27 | 0.093 |
| PAD | 11.5 | 2.08–93.9 | 0.005 |
| hs-CRP | 1.38 | 0.09–17.2 | 0.19 |
| Presence of YP in the coronary | 1.78 | 0.45–7.54 | 0.037 |
| Multivariate logistic regression analysis | |||
| Diabetes mellitus | 6.84 | 1.20–62.8 | 0.029 |
| PAD | 10.5 | 1.62–106.1 | 0.013 |
CI, confidence interval; hs-CRP, high-sensitivity C-reactive protein; OMI, old myocardial infarction; OR, odds ratio; PAD, peripheral artery disease; VP, vulnerable plaque; YP, yellow plaque.
Intra- and Interobserver Agreement
Intra- and interobserver agreement values were good regarding the identification of Ao-angioscopic findings (atheromatous plaque: intraobserver variability, κ = 0.95; interobserver variability, κ = 0.82).
Discussion
Aortic plaques were the most frequent and most severe at the IAA of the aorta. The total number of vulnerable plaques in the whole aorta was associated with the prevalence of DM and PAD, and the use of OHA and insulin.
To the best of our knowledge, this is the first study to evaluate the distribution of aortic plaques in each segment of the aorta in vivo, and the relationships between the severity of aortic plaques and atherosclerotic risk factors using Ao-angioscopy. Our first main finding was that atherosclerotic changes in the aorta could be detected in vivo, by using Ao-angioscopy. This new modality safely and effectively identified atherosclerotic changes of the aorta within the aortic arch and common iliac artery. Although atheromatous plaques of the aorta, such as plaque ruptures, can be detected using pathological and open surgical approaches, it has previously been difficult to detect aortic plaques in vivo9, 16). Historically, angioscopy for the coronary artery was developed to detect vulnerable plaques as intensive yellow plaques or thrombi, and to predict a cardiovascular event15, 17, 18). Our findings suggest that angioscopy could also evaluate plaque instability in the aorta.
Another finding was that aortic plaques identified using Ao-angioscopy were observed more frequently and were more severe at the IAA. These results were consistent with previous studies using conventional modalities or pathology. The prevalence and number of aortic plaques varies from segment to segment, and aortic plaques were much common and severe at the IAA in particular19, 20). However, many studies using conventional modalities have assessed wall thickness, calcification, or superficial irregularity as factors of aortic atherosclerosis21), but have not fully evaluated the quality of aortic plaque in vivo. In a report by DeBakey et al., atherosclerotic occlusive disease was most common in the IAA segment and iliac arteries22). Aortic aneurysms, as well as severe atheromatous lesions, have been reported to contain the most frequent number of plaques at the IAA19, 20). Atherosclerosis and aneurysm at the IAA tended to progress, because of the influence of hemodynamic mechanisms. The preferential growth of atherosclerotic plaques at the IAA has been attributed to variations in blood flow and wall shear stress23, 24).
We also found that DM and the use of OHA and insulin were more common in patients with severe atherosclerosis of the aorta. It is well documented that DM and the use of OHA and insulin are atherosclerotic risk factors25, 26). Aortic angioscopy showed that the frequency of DM in patients with aortic ruptured plaques was higher than in patients without ruptured plaques10). Insulin resistance disturbs vascular endothelial function and induces inflammatory cytokines, and hyperglycemia induces polyol accumulation, oxidative stress, formation of advanced glycation end products, and stimulation of activation pathways such as protein kinase C. These factors are implicated in the progression of atherosclerosis25, 26).
Another finding of our study was that PAD was more common in patients with severe atherosclerosis of the aorta. One previous study reported that the presence of an abdominal aortic aneurysm was detected using abdominal ultrasonography in 14% patients with PAD27). Calcification of the thoracic aorta is a risk factor for PAD28). PAD is an independent predictor of cardiovascular disease, reflecting systemic atherosclerosis29). The prevalence of PAD was sharply age-related, with patients in their 70s being > 10% more likely to have PAD compared to patients in their 60s30), whereas aortic atherosclerosis began in youth31). If PAD has already occurred, careful management not only of the PAD but also for the aortic disease may be necessary because of the possibility that the atherosclerotic lesion of the aorta has also progressed. In addition, one abdominal ultrasound study showed that lower extremity embolic arterial occlusion occurred due to vulnerable plaques of the abdominal aorta32). Aortic ruptured plaques detected using Aoangioscopy were commonly scattered, and their dimensions were smaller than previously recognized11). We speculate that one of the mechanisms behind PAD might be related to gradual progression of asymptomatic subclinical accumulation plaque embolism.
We were able to observe various types of atherosclerotic plaques using Ao-angioscopy, including intense yellow plaques, ruptured plaques, and thrombi. However, the clinical significance of aortic vulnerable plaques detected using Ao-angioscopy remains to be clearly understood. Severe atherosclerotic plaques contribute to the development of aortic aneurysms or penetrating atherosclerotic ulcers, due to weakening and thinning aortic wall2, 3). In the present study, we observed many intimal injuries of the aorta, but did not observe any acute aortic syndromes, such as ruptured aneurysm or dissection. In one angioscopic case report, aortic atherosclerotic changes with subintimal hemorrhage and peeled intima that were partly detected by Ao-angioscopy had progressed to a large cavity after 2 years33). We have not fully evaluated on how aortic plaques identified using Ao-angioscopy will progress in vivo or what clinical events may occur as a result of these aortic plaques. Aortic plaques observed with Ao-angioscopy, which is capable of detailed atherosclerotic assessment, might lead to cardiovascular events with time. Aortic plaques may be a source of aortogenic embolism. Komatsu et al. reported that atheromatous material, including cholesterol crystal, was observed in the sampled blood that included a scattered puff from aortic ruptured plaque detected by Ao-angioscopy. They suggested that the asymptomatic subclinical accumulation plaque embolism might cause gradual asymptomatic organ insufficiency, including the brain. Aortic ruptured plaques may be vulnerable and may relate to cardiovascular events.
Another important finding was that patients with the many vulnerable plaques in the whole aorta may be at high risk of future aortic events. Patients with the many vulnerable plaques had a high prevalence of DM and PAD, which were related to the progression of atherosclerosis including the aorta. Evaluation of vulnerable aortic plaques according to the total number of vulnerable plaques in the whole aorta may enable risk stratification for cardiovascular events.
Study Limitations
Several limitations of this study should be acknowledged. This was a small sample sized, single-centered, retrospective study. A larger scale study is needed to more accurately assess aortic plaques identified by Ao-angioscopy. Some selection bias may exist because we included patients who underwent PCI and angioscopy of both the coronary artery and the aorta, and excluded several types of coronary lesions, several clinical settings, and observations at the ascending aorta. Previous studies have shown that severe atherosclerosis was not observed at the ascending aorta11). Therefore, we suppose that exclusion of observations at the ascending aorta did not have a large impact on the result. Aortic atherosclerosis has been reported as a significant marker for coronary artery disease10, 16, 21), but our study did not show any relationships between aortic atherosclerosis and coronary angioscopic findings. One of the reasons may be that the aortic plaques identified using Ao-angioscopy were in a subclinical stage. Another possibility was the insufficient evaluation of the coronary artery with angioscopy. Ideally, we should have observed the three vessels of the coronary artery. In additionally, our data did not include aortic plaques in patients without coronary artery disease. Further studies are needed to assess the relationship between atherosclerosis of the aorta and the coronary artery. Our aortic observational method may not have been able to detect all plaques in the aorta and to observe the entire aorta. We tried to quantitatively evaluate all aortic plaques as accurately as possible by using our method. In order to minimize the influence of this limitation, we chose the approach site as the femoral artery in all the cases, and performed an observation method in which the catheter was rotated and lowered at a constant speed, as previously described14).
Conclusions
Aortic atherosclerosis identified by non-obstructive angioscopy was the most severe at the IAA, and it was associated with DM and PAD in patients with stable angina pectoris. The detailed evaluation of aortic atherosclerosis using Ao-angioscopy may elucidate the mechanisms behind critical aortic diseases, such as those caused by aortic atherosclerosis. In the clinical setting, non-obstructive angioscopy may help physicians identify patients at high risk for future aortic events.
Acknowledgments
None.
Conflict of Interest
The authors have no conflict of interest to declare.
References
- 1). Budoff MJ, Nasir K, Katz R, Takasu J, Carr JJ, Wong ND, Allison M, Lima JA, Detrano R, Blumenthal RS, Kronmal R. Thoracic aortic calcification and coronary heart disease events: the multi-ethnic study of Atherosclerosis (MESA). Atherosclerosis, 2011; 215: 196-202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Zarins CK, Xu C, Glagov S. Atherosclerotic enlargement of the human abdominal aorta. Atherosclerosis, 2001; 155: 157-164 [DOI] [PubMed] [Google Scholar]
- 3). Wada H, Sakata N, Tashiro T. Clinicopathological study on penetrating atherosclerotic ulcers and aortic dissection: distinct pattern of development of initial event. Heart Vessels, 2016; 31: 1855-1861 [DOI] [PubMed] [Google Scholar]
- 4). Kronzon I, Tunick PA. Aortic atherosclerotic disease and stroke. Circulation, 2006; 114: 63-75 [DOI] [PubMed] [Google Scholar]
- 5). Amarenco P, Cohen A, Tzourio C, Bertrand B, Hommel M, Besson G, Chauvel C, Touboul PJ, Bousser MG. Atherosclerotic disease of the aortic arch and the risk of ischemic stroke. N Engl J Med, 1994; 331: 1474-1479 [DOI] [PubMed] [Google Scholar]
- 6). Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, Templin B, White R, Zhang Z, Serruys PW. A prospective natural-history study of coronary atherosclerosis. N Engl J Med, 2011; 364: 226-235 [DOI] [PubMed] [Google Scholar]
- 7). Mintz GS, Guagliumi G. Intravascular imaging in coronary artery disease. Lancet, 2017; 390: 793-809 [DOI] [PubMed] [Google Scholar]
- 8). Ohtani T, Ueda Y, Mizote I, Oyabu J, Okada K, Hirayama A, Kodama K. Number of yellow plaques detected in a coronary artery is associated with future risk of acute coronary syndrome: detection of vulnerable patients by angioscopy. J Am Coll Cardiol, 2006; 47: 2194-2200 [DOI] [PubMed] [Google Scholar]
- 9). Komatsu S, Ohara T, Takahashi S, Takewa M, Minamiguchi H, Imai A, Kobayashi Y, Iwa N, Yutani C, Hirayama A, Kodama K. Early detection of vulnerable atherosclerotic plaque for risk reduction of acute aortic rupture and thromboemboli and atheroemboli using non-obstructive angioscopy. Circ J, 2015; 79: 742-750 [DOI] [PubMed] [Google Scholar]
- 10). Aono J, Ikeda S, Katsumata Y, Higashi H, Ohshima K, Ishibashi K, Matsuoka H, Watanabe K, Hamada M. Correlation between plaque vulnerability of aorta and coronary artery: an evaluation of plaque activity by direct visualization with angioscopy. Int J Cardiovasc Imaging, 2015; 31: 1107-1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Komatsu S, Yutani C, Ohara T, Takahashi S, Takewa M, Hirayama A, Kodama K. Angioscopic evaluation of spontaneously ruptured aortic plaques. J Am Coll Cardiol, 2018; 71: 2893-2902 [DOI] [PubMed] [Google Scholar]
- 12). Ueda Y, Asakura M, Hirayama A, Komamura K, Hori M, Kodama K. Intracoronary morphology of culprit lesions after reperfusion in acute myocardial infarction: serial angioscopic observations. J Am Coll Cardiol, 1996; 27: 606-610 [DOI] [PubMed] [Google Scholar]
- 13). Kimura S, Sugiyama T, Hishikari K, Nakagama S, Nakamura S, Misawa T, Mizusawa M, Hayasaka K, Yamakami Y, Sagawa Y, Kojima K, Ohtani H, Hikita H, Takahashi A. Intravascular Ultrasound and Angioscopy Assessment of Coronary Plaque Components in Chronic Totally Occluded Lesions. Circ J, 2018; 82: 2032-2040 [DOI] [PubMed] [Google Scholar]
- 14). Hiro T, Komatsu S, Fujii H, Takayama T, Ueda Y, Higuchi Y, Abe S, Kimura S, Kakuta T, Sato A, Matsuoka H, Kawakami H, Ikeda Y, Asakura M, Hayashi H, Yutani C, Saito S, Hirayama A, Kodama K. Consensus standards for acquisition, measurement, and reporting of non-obstructive aortic angioscopy studies: A report from the Working Group of Japan Vascular Imaging Research Organization for Standardization of Non-obstructive Aortic Angioscopy (Version 2017). Angioscopy, 2018; 4: 1-11 [Google Scholar]
- 15). Ueda Y, Matsuo K, Nishimoto Y, Sugihara R, Hirata A, Nemoto T, Okada M, Murakami A, Kashiwase K, Kodama K. In-stent yellow plaque at 1 year after implantation is associated with future event of very late stent failure: The DESNOTE study (Detect the Event of Very late Stent Failure From the Drug-Eluting Stent Not Well Covered by Neointima Determined by Angioscopy). JACC Cardiovasc Interv, 2015; 8: 814-821 [DOI] [PubMed] [Google Scholar]
- 16). Serfaty JM, Chaabane L, Tabib A, Chevallier JM, Briguet A, Douek PC. Atherosclerotic plaques: classification and characterization with T2-weighted high-spatial-resolution MR imaging-- an in vitro study. Radiology, 2001; 219: 403-410 [DOI] [PubMed] [Google Scholar]
- 17). Asakura M, Ueda Y, Yamaguchi O, Adachi T, Hirayama A, Hori M, Kodama K. Extensive development of vulnerable plaques as a pan-coronary process in patients with myocardial infarction: an angioscopic study. J Am Coll Cardiol, 2001; 37: 1284-1288 [DOI] [PubMed] [Google Scholar]
- 18). Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, Fayad Z, Stone PH, Waxman S, Raggi P, Madjid M, Zarrabi A, Burke A, Yuan C, Fitzgerald PJ, Siscovick DS, de Korte CL, Aikawa M, Juhani Airaksinen KE, Assmann G, Becker CR, Chesebro JH, Farb A, Galis ZS, Jackson C, Jang IK, Koenig W, Lodder RA, March K, Demirovic J, Navab M, Priori SG, Rekhter MD, Bahr R, Grundy SM, Mehran R, Colombo A, Boerwinkle E, Ballantyne C, Insull W, Jr., Schwartz RS, Vogel R, Serruys PW, Hansson GK, Faxon DP, Kaul S, Drexler H, Greenland P, Muller JE, Virmani R, Ridker PM, Zipes DP, Shah PK, Willerson JT. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation, 2003; 108: 1664-1672 [DOI] [PubMed] [Google Scholar]
- 19). Seo JS, Lee SY, Kim HD. Quantitative analysis of aortic atherosclerosis in Korean female: A necropsy study. J Korean Med Sci, 2007; 22: 536-545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Zarins CK, Xu C, Glagov S. Atherosclerotic enlargement of the human abdominal aorta. Atherosclerosis, 2001; 155: 157-164 [DOI] [PubMed] [Google Scholar]
- 21). Fayad ZA, Fuster V. Clinical imaging of the high-risk or vulnerable atherosclerotic plaque. Circ Res, 2001; 89: 305-316 [DOI] [PubMed] [Google Scholar]
- 22). DeBakey MM, Lawrie GM, Glaeser DH. Patterns of atherosclerosis and their surgical significance. Ann Surg, 1985; 201: 115-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Glagov S, Zarins C, Giddens DP, Ku DN. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Arch Pathol Lab Med, 1988; 112: 1018-1031 [PubMed] [Google Scholar]
- 24). Ruddy JM, Jones JA, Spinale FG, Ikonomidis JS. Regional heterogeneity within the aorta: relevance to aneurysm disease. J Thorac Cardiovasc Surg, 2008; 136: 1123-1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab, 2011; 14: 575-585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Howard G, O'Leary DH, Zaccaro D, Haffner S, Rewers M, Hamman R, Selby JV, Saad MF, Savage P, Bergman R. Insulin sensitivity and atherosclerosis. The Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Circulation, 1996; 93: 1809-1817 [DOI] [PubMed] [Google Scholar]
- 27). Galland RB, Simmons MJ, Torrie EP. Prevalence of abdominal aortic aneurysm in patients with occlusive peripheral vascular disease. Br J Surg, 1991; 78: 1259-1260 [DOI] [PubMed] [Google Scholar]
- 28). Churchill TW, Rasania SP, Rafeek H, Mulvey CK, Terembula K, Ferrari V, Jha S, Lilly SM, Eraso LH, Reilly MP, Qasim AN. Ascending and descending thoracic aorta calcification in type 2 diabetes mellitus. J Cardiovasc Comput Tomogr, 2015; 9: 373-381 [DOI] [PubMed] [Google Scholar]
- 29). Criqui MH, Aboyans V, Allison MA, Denenberg JO, Forbang N, McDermott MM, Wassel CL, Wong ND. Peripheral artery disease and aortic disease. Glob Heart, 2016; 11: 313-326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res, 2015; 116: 1509-1526 [DOI] [PubMed] [Google Scholar]
- 31). Strong JP, Malcom GT, McMahan CA, Tracy RE, Newman WP, 3rd, Herderick EE, Cornhill JF. Prevalence and extent of atherosclerosis in adolescents and young adults: implications for prevention from the Pathobiological Determinants of Atherosclerosis in Youth Study. JAMA, 1999; 281: 727-735 [DOI] [PubMed] [Google Scholar]
- 32). Spittell PC, Seward JB, Hallett JW., Jr. Mobile thrombi in the abdominal aorta in cases of lower extremity embolic arterial occlusion: value of extended transthoracic echocardiography. Am Heart J, 2000; 139: 241-244 [DOI] [PubMed] [Google Scholar]
- 33). Komatsu S, Takahashi S, Takewa M, Kodama K. Subintimal intraplaque haemorrhage prior to aortic plaque rupture: 2-year angioscopic follow-up. BMJ Case Rep, 2018. pii: bcr-2018-224801. 10.1136/bcr-2018-224801 [DOI] [PMC free article] [PubMed] [Google Scholar]


