Abstract
Aim: To evaluate the prognostic value of plasma big endothelin-1 level in the context of three-vessel disease (TVD) with heavy atherosclerotic burden.
Methods: A total of 6,150 patients with TVD and available big endothelin-1 data were included in the study. Participants were divided into two groups according to the optimal cutoff value of big endothelin-1 for mortality prediction. The primary endpoint was all-cause death. C-index, net reclassification improvement (NRI), and integrated discrimination improvement (IDI) were calculated to evaluate the added prognostic value of plasma big endothelin-1 level beyond the SYNTAX score II.
Results: On the basis of the optimal cutoff value of 0.79 pmol/L, 1,984 patients were assigned to the high big endothelin-1 group. During a median follow-up of 6.8 years, 818 patients experienced all-cause death. Plasma big endothelin-1 level was significantly higher in patients who died than in patients who survived. Multivariable analysis found that high big endothelin-1 level was independently associated with an increased risk of mortality (hazard ratio: 1.36, 95% confidence interval: 1.18–1.57, P < 0.001). The association of big endothelin-1 with all-cause death was relatively consistent across subgroups with no significant interactions. The predictive ability of the SYNTAX score II was significantly enhanced by addition of plasma big endothelin-1 level (C-index: 0.723 vs. 0.715, P = 0.029; NRI: 0.304, P < 0.001; IDI: 0.009, P < 0.001).
Conclusions: Plasma big endothelin-1 level is an independent predictor of long-term mortality in patients with TVD. It can improve the discrimination and reclassification of the SYNTAX score II for mortality prediction.
Keywords: Three-vessel coronary artery disease, Big endothelin-1, Prognosis
Introduction
Three-vessel disease (TVD), characterized by significant stenosis in all three major coronary arteries, is a severe form of coronary artery disease (CAD) that confers a substantial risk of mortality1). Risk stratification is important to identify high-risk patients with TVD who require intensive treatment and close follow-up. With this aim, the SYNTAX score (SS) and SYNTAX score II (SSII) have been established for risk assessment in patients with TVD2, 3). However, accurate risk stratification has always been challenging. Identification of additional biomarkers with prognostic significance may be able to improve the predictability of the established models.
Endothelin-1 (ET-1), a 21-amino acid peptide, is the most powerful constrictor of human vessels discovered to date4). It is primarily produced and released by vascular endothelial cells and can cause endothelial dysfunction and inflammation, which may contribute to atherosclerotic plaque formation5). However, clinical use of ET-1 as a biomarker is limited because of its instability in plasma6). As a precursor of ET-1, big ET-1 is relatively stable and can be used as an alternative approach for indirect estimation of ET-1 release6). Big ET-1 level was shown to be correlated with disease severity and clinical outcome in patients with acute myocardial infarction (MI) and stable CAD7–10). However, its clinical implications have not been evaluated in the setting of TVD with advanced coronary atheroma burden.
Aim
The present study aimed to assess the prognostic value of plasma big ET-1 level in patients with TVD.
Methods
Study Design and Participants
Data were obtained from a large prospective cohort study in which a total of 8,943 patients with TVD were consecutively enrolled from April 2004 to February 2011 at Fuwai Hospital, Chinese Academy of Medical Sciences (Beijing, China). Eligible patients were those who had TVD, defined as angiographically confirmed stenosis of ≥ 50% in all three main epicardial coronary arteries (left anterior descending, left circumflex, and right coronary arteries) with or without involvement of the left main artery, and were willing to undergo follow-up. There were no prespecified exclusion criteria. No treatment intervention was dictated by the protocol for the observational study. Patients received medical therapy (MT) alone, percutaneous coronary intervention (PCI), or coronary artery bypass grafting (CABG) according to contemporary practice guidelines and their preferences11, 12). After enrollment, the patients were followed up in accordance with the study protocol. Baseline and procedural data for all participants were collected into a database by independent clinical research coordinators.
The study complied with the principles of the Declaration of Helsinki and was approved by the Review Board of Fuwai Hospital. Written informed consent was obtained from all participants.
Definitions
The concentrations of plasma big ET-1 were measured in fasting venous blood samples after admission for coronary angiography, using a highly sensitive and specific commercial sandwich enzyme immunoassay (BI-20082H; Biomedica, Wien, Austria). The SS was calculated using an online calculator (http://www.syntaxscore.com) by a dedicated research group blinded to the clinical data. Calculation of the SSII was based on two anatomical variables and six clinical variables3). Creatinine clearance was calculated by the Cockcroft and Gault formula.
Outcomes
Outcome data were obtained by telephone interview, follow-up letter or clinic visit. All events were carefully checked and verified by an independent group of clinical physicians. Investigator training, blinded questionnaire filling, and telephone recording were performed to achieve high-quality results. The primary endpoint was all-cause death. Secondary endpoints included cardiac death, major adverse cardiac and cerebrovascular events (MACCE), a composite of all-cause death, MI, stroke, or unplanned revascularization, and the individual components of the composite. All deaths were considered cardiac unless an unequivocal non-cardiac cause could be established.
Statistical Analysis
A receiver operating characteristic (ROC) curve analysis was performed to determine the optimal cut-off value of plasma big ET-1 for mortality prediction (Supplementary Fig. 1). Participants were divided into high and low big ET-1 groups according to this cutoff value.
Supplementary Fig. 1.
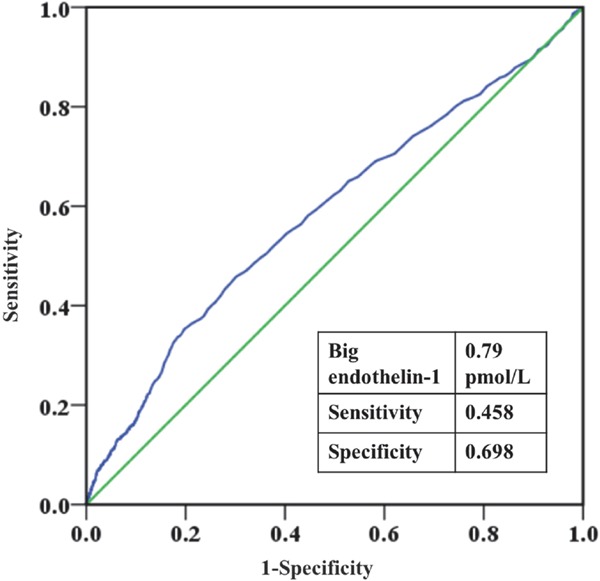
Receiver operating characteristic (ROC) curve of big endothelin-1 for mortality prediction.
Summary statistics were presented as frequency and percentage for categorical variables and mean ± standard deviation or median and interquartile range for continuous variables. An independent-sample Student's t-test or the Mann–Whitney U-test was performed for comparisons of continuous variables, and the Pearson chi-square test or Fisher's exact test was performed for comparison of categorical variables.
Survival curves were constructed by the Kaplan–Meier method and compared by the log-rank test. Univariable and multivariable Cox proportional hazards regressions were performed to calculate the hazard ratio (HR) and 95% confidence interval (CI) and evaluate the associations between big ET-1 level (as a categorical or continuous variable normalized by log10 transformation) and clinical outcomes. Covariates included in the multivariable model were age, sex, body mass index, hypertension, diabetes, previous MI, previous stroke, clinical presentation (stable angina pectoris [SAP] or acute coronary syndrome [ACS]), left main coronary artery involvement, left ventricular ejection fraction (LVEF), creatinine clearance, SS (≤ 22, 23–32, or ≥ 33), procedure (MT, PCI, or CABG), and aspirin. Patients who were lost to follow-up were censored at the last available contact.
Exploratory subgroup analyses of the primary outcome were performed according to age (< 65 or ≥ 65 years), sex (male or female), diabetes (yes or no), presentation (SAP or ACS), left main involvement (yes or no), LVEF (< 40% or ≥ 40%), SS (0–22, 23–32, or ≥ 33), and procedure (MT, PCI, or CABG). Interactions between plasma big ET-1 level (high or low) and these covariates were tested to interpret potential subgroup differences. The above-described multivariable Cox proportional hazards models were used for the interaction and subgroup analyses.
To assess the added prognostic value of big ET-1 for mortality prediction beyond the SSII, the C-index, continuous net reclassification improvement (NRI), and integrated discrimination improvement (IDI) were calculated using R software version 3.4.3 (R Core Team, Vienna, Austria).
Two-sided P-values of < 0.05 were considered statistically significant. Analyses were performed using SPSS software version 22.0 (IBM, Armonk, NY, USA) unless otherwise stated.
Results
A total of 6,150 patients with available big ET-1 data were included in the present analysis. On the basis of the optimal cutoff value of 0.79 pmol/L, the patients were divided into low and high big ET-1 groups (Fig. 1). At baseline, patients in the high big ET-1 group were older and had higher troponin I, N-terminal pro-B-type natriuretic peptide, and high-sensitivity C-reactive protein, but lower LVEF and creatinine clearance (Table 1). Higher rates of female sex, hypertension, diabetes, previous MI, previous stroke, chronic kidney disease, and high SS were observed in the high big ET-1 group. Patients in the high big ET-1 group were more likely to receive MT alone rather than CABG and to take aspirin.
Fig. 1.
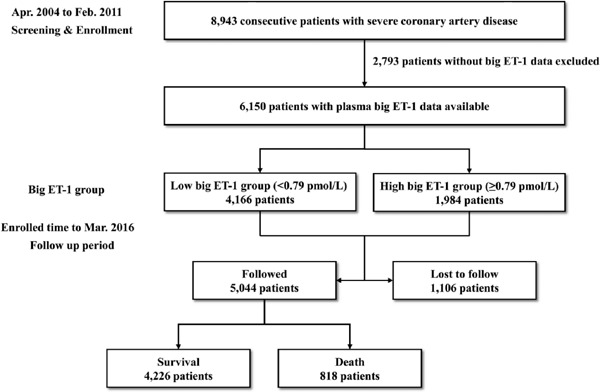
Flowchart of subject selection.
Table 1. Baseline characteristics of the study population grouped by the optimal cutoff value of big endothelin-1.
| Overall | Big ET-1 < 0.79 pmol/L | Big ET-1 ≥ 0.79 pmol/L | P-value | |
|---|---|---|---|---|
| (n = 6150) | (n = 4166) | (n = 1984) | ||
| Demographics | ||||
| Age, year | 60.9 ± 10.0 | 60.5 ± 9.9 | 61.8 ± 10.2 | < 0.001 |
| Male | 4924 (80.1) | 3367 (80.8) | 1557 (78.5) | 0.032 |
| BMI, kg/m2 | 25.9 ± 3.1 | 25.8 ± 3.0 | 26.0 ± 3.1 | 0.192 |
| Medical history and risk factor | ||||
| Hypertension | 4176 (67.9) | 2785 (66.9) | 1391 (70.1) | 0.010 |
| Diabetes | 2189 (35.6) | 1428 (34.3) | 761 (38.4) | 0.002 |
| Hyperlipidemia | 3691 (60.0) | 2529 (60.7) | 1162 (58.6) | 0.110 |
| Previous MI | 2204 (35.8) | 1454 (34.9) | 750 (37.8) | 0.027 |
| Previous stroke | 646 (10.5) | 406 (9.7) | 240 (12.1) | 0.005 |
| COPD | 76 (1.2) | 44 (1.1) | 32 (1.6) | 0.065 |
| PAD | 575 (9.3) | 376 (9.0) | 199 (10.0) | 0.206 |
| CKD | 57 (0.9) | 24 (0.6) | 33 (1.7) | < 0.001 |
| Smoker | 3392 (55.2) | 2300 (55.2) | 1092 (55.0) | 0.901 |
| Clinical characteristic | ||||
| SAP | 2406 (39.1) | 1662 (39.9) | 744 (37.5) | 0.072 |
| ACS | 3744 (60.9) | 2504 (60.1) | 1240 (62.5) | 0.072 |
| Left main involvement | 1435 (23.3) | 952 (22.9) | 483 (24.3) | 0.196 |
| LVEF, % | 58.3 ± 9.5 | 58.9 ± 8.9 | 57.2 ± 10.4 | < 0.001 |
| Big ET-1, pmol/L | 0.65 (0.50–0.86) | 0.56 (0.45–0.66) | 1.05 (0.87–2.14) | < 0.001 |
| Troponin I, ng/mL | 0.03 (0.01–0.09) | 0.02 (0.01–0.07) | 0.03 (0.02–0.14) | < 0.001 |
| NT-proBNP, pmol/L | 632.0 (445.8–972.8) | 596.1 (424.2–880.4) | 715.4 (497.5–1241.4) | < 0.001 |
| hsCRP, mg/L | 2.05 (1.00–5.45) | 1.93 (0.96–4.70) | 2.39 (1.08–6.93) | < 0.001 |
| Creatinine clearance, mL/min | 86.7 ± 27.5 | 87.9 ± 27.0 | 84.1 ± 28.5 | < 0.001 |
| Procedural characteristic | ||||
| SYNTAX score | ||||
| ≤ 22 | 2255 (36.7) | 1570 (38.7) | 685 (35.8) | 0.027 |
| 23–32 | 2271 (36.9) | 1534 (37.8) | 737 (38.5) | 0.642 |
| ≥ 33 | 1444 (23.5) | 950 (23.4) | 494 (25.8) | 0.048 |
| Treatment | ||||
| MT | 1690 (27.5) | 1073 (25.8) | 617 (31.1) | < 0.001 |
| PCI | 2576 (41.9) | 1757 (42.2) | 819 (41.3) | 0.506 |
| CABG | 1884 (30.6) | 1336 (32.1) | 548 (27.6) | < 0.001 |
| Medication at discharge | ||||
| Aspirin | 5908 (96.1) | 4036 (96.9) | 1872 (94.4) | < 0.001 |
| Clopidogrel | 3328 (54.1) | 2284 (54.8) | 1044 (52.6) | 0.105 |
| ACEI | 2215 (36.0) | 1495 (35.9) | 720 (36.3) | 0.757 |
| ARB | 1013 (16.5) | 671 (16.1) | 342 (17.2) | 0.264 |
| Beta-blocker | 5457 (88.7) | 3694 (88.7) | 1763 (88.9) | 0.825 |
| CCB | 2097 (34.1) | 1409 (33.8) | 688 (34.7) | 0.508 |
| Statin | 4149 (67.5) | 2799 (67.2) | 1350 (68.0) | 0.502 |
Values are presented as mean ± standard deviation, median (interquartile range), or number (%).
ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers; BMI, body mass index; CCB, calcium channel blocker; COPD, chronic obstructive pulmonary disease; hsCRP, high-sensitivity C-reactive protein; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PAD, peripheral artery disease.
The median follow-up was 6.8 (5.7–8.1) years, with a response rate of 82.0% (Fig. 1). The baseline characteristics of the patients followed up and lost to follow-up are listed in Supplementary Table 1. All patients included in the analysis completed at least one follow-up. During the follow-up period, 818 (13.3%) patients experienced all-cause death. The median level of plasma big ET-1 was significantly higher in the patients who died (0.74 [0.54–1.08] pmol/L) than in those who survived (0.64 [0.50–0.84] pmol/L, P < 0.001). Significantly more all-cause death, cardiac death, MACCE, and stroke occurred in the high big ET-1 group compared with the low big ET-1 group (all P % 0.05, Table 2).
Supplementary Table 1. Baseline characteristics of patients followed up and lost to follow up.
| Patients followed up | Patients lost to follow up | P-value | ||
|---|---|---|---|---|
| (n = 5,044) | (n = 1,106) | |||
| Demographics | ||||
| Age, year | 60.7 ± 9.9 | 62.0 ± 10.3 | < 0.001 | |
| Male | 4048 (80.3) | 876 (79.2) | 0.429 | |
| BMI, kg/m2 | 25.9 ± 3.0 | 25.6 ± 3.3 | 0.003 | |
| Medical history and risk factor | ||||
| Hypertension | 3434 (68.1) | 742 (67.1) | 0.522 | |
| Diabetes | 1753 (34.8) | 436 (39.4) | 0.003 | |
| Hyperlipidemia | 3110 (61.7) | 581 (52.5) | < 0.001 | |
| Previous MI | 1748 (34.7) | 456 (41.2) | < 0.001 | |
| Previous stroke | 505 (10.0) | 141 (12.7) | 0.007 | |
| COPD | 62 (1.2) | 14 (1.3) | 0.920 | |
| PAD | 456 (9.0) | 119 (10.8) | 0.075 | |
| CKD | 31 (0.6) | 26 (2.4) | < 0.001 | |
| Smoker | 2788 (55.3) | 604 (54.6) | 0.688 | |
| Clinical characteristic | ||||
| SAP | 1988 (39.4) | 418 (37.8) | 0.318 | |
| ACS | 3056 (60.6) | 688 (62.2) | 0.318 | |
| Left main involvement | 1131 (22.4) | 304 (27.5) | < 0.001 | |
| LVEF, % | 58.7 ± 9.2 | 56.7 ± 10.3 | < 0.001 | |
| Big ET-1, pmol/L | 0.65 (0.50–0.85) | 0.68 (0.52–1.01) | < 0.001 | |
| Troponin I, ng/mL | 0.03 (0.01–0.08) | 0.03 (0.02–0.13) | < 0.001 | |
| NT-proBNP, pmol/L | 622.2 (443.2–939.4) | 690.9 (462.0–1228.1) | < 0.001 | |
| hsCRP, mg/L | 2.01 (0.98–5.27) | 2.29 (1.05–6.19) | 0.015 | |
| Creatinine clearance, mL/min | 87.5 ± 27.5 | 83.0 ± 27.4 | < 0.001 | |
| Procedural characteristic | ||||
| SYNTAX score | ||||
| ≤ 22 | 1905 (37.8) | 350 (31.6) | < 0.001 | |
| 23–32 | 1841 (36.5) | 430 (38.9) | 0.137 | |
| ≥ 33 | 1148 (22.8) | 296 (26.8) | 0.004 | |
| Treatment | ||||
| MT | 1295 (25.7) | 395 (35.7) | < 0.001 | |
| PCI | 2168 (43.0) | 408 (36.9) | < 0.001 | |
| CABG | 1581 (31.3) | 303 (27.4) | 0.010 | |
| Medication at discharge | ||||
| Aspirin | 4871 (96.6) | 1037 (93.8) | < 0.001 | |
| Clopidogrel | 2758 (54.7) | 570 (51.5) | 0.058 | |
| ACEI | 1801 (35.7) | 414 (37.4) | 0.279 | |
| ARB | 816 (16.2) | 197 (17.8) | 0.185 | |
| Beta-blocker | 4469 (88.6) | 988 (89.3) | 0.486 | |
| CCB | 1681 (33.3) | 416 (37.6) | 0.006 | |
| Statin | 3388 (67.2) | 761 (68.8) | 0.292 | |
Values are presented as mean ± standard deviation, median (interquartile) or number (%).
ACEI, angiotensin-converting enzyme inhibitors; ACS, acute coronary syndrome; ARB, angiotensin II receptor blockers; BMI, body mass index; CABG, coronary artery bypass grafting; CCB, calcium channel blocker; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ET-1, endothelin-1; hsCRP, high sensitivity C reactive protein; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MT, medical therapy; NT-proBNP, N-terminal pro-B-Type natriuretic peptide; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; SAP, stable angina pectoris.
Table 2. Risks of primary and secondary outcomes.
| Outcome | No. of Patients with event (%) | Crude HR (95% CI) | Crude P-value | Adjusted HR (95% CI) | Adjusted P-value |
|---|---|---|---|---|---|
| All-cause death | |||||
| Low big ET-1 | 443 (10.6) | Reference | Reference | ||
| High big ET-1 | 375 (18.9) | 1.69 (1.47–1.94) | < 0.001 | 1.38 (1.19–1.59) | < 0.001 |
| log(big ET-1) | – | 1.93 (1.55–2.40) | < 0.001 | 1.43 (1.13–1.80) | 0.002 |
| Cardiac death | |||||
| Low big ET-1 | 231 (5.5) | Reference | Reference | ||
| High big ET-1 | 204 (10.3) | 1.75 (1.44–2.11) | < 0.001 | 1.37 (1.13–1.67) | 0.002 |
| log(big ET-1) | – | 2.11 (1.57–2.85) | < 0.001 | 1.53 (1.11–2.10) | 0.009 |
| MACCE | |||||
| Low big ET-1 | 1067 (25.6) | Reference | Reference | ||
| High big ET-1 | 695 (35.0) | 1.32 (1.20–1.45) | < 0.001 | 1.20 (1.09–1.32) | < 0.001 |
| log(big ET-1) | – | 1.38 (1.18–1.62) | < 0.001 | 1.20 (1.02–1.42) | 0.027 |
| Myocardial infarction | |||||
| Low big ET-1 | 180 (4.3) | Reference | Reference | ||
| High big ET-1 | 105 (5.3) | 1.13 (0.88–1.44) | 0.335 | 1.12 (0.88–1.44) | 0.358 |
| log(big ET-1) | – | 1.01 (0.69–1.48) | 0.969 | 1.02 (0.69–1.50) | 0.931 |
| Stroke | |||||
| Low big ET-1 | 266 (6.4) | Reference | Reference | ||
| High big ET-1 | 155 (7.8) | 1.17 (0.96–1.43) | 0.116 | 1.12 (0.92–1.38) | 0.265 |
| log(big ET-1) | – | 1.13 (0.81–1.57) | 0.469 | 1.06 (0.75–1.48) | 0.746 |
| Unplanned revascularization | |||||
| Low big ET-1 | 321 (7.7) | Reference | Reference | ||
| High big ET-1 | 167 (8.4) | 1.11 (0.92–1.34) | 0.264 | 1.11 (0.92–1.35) | 0.277 |
| log(big ET-1) | – | 1.08 (0.77–1.49) | 0.663 | 1.12 (0.81–1.56) | 0.494 |
Univariable analysis showed that high big ET-1 level was associated with higher risks of all-cause death, cardiac death, and MACCE, but not MI, stroke, or unplanned revascularization (Table 2). After adjustment for covariates, high big ET-1 level remained an independent risk factor for all-cause death (HR: 1.36, 95% CI: 1.18–1.57, P < 0.001), cardiac death (HR: 1.36, 95% CI: 1.12 –1.66, P = 0.002), and MACCE (HR: 1.19, 95% CI: 1.08–1.31, P = 0.001). Fig. 2 shows the Kaplan–Meier estimates for the primary and secondary endpoints (log-rank P < 0.001 for all-cause death, cardiac death, and MACCE).
Fig. 2.
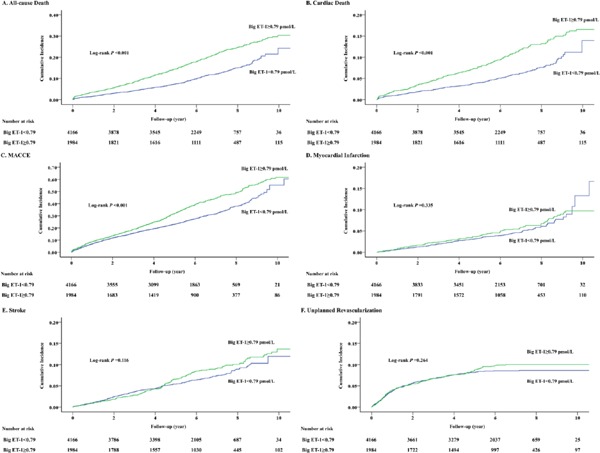
Cumulative incidence curves for primary and secondary endpoints.
(A–F) Cumulative incidences of all-cause death (A), cardiac death (B), MACCE (C), myocardial infarction (D), stroke (E), and unplanned revascularization (F).
The relationship of big ET-1 level (high or low) with all-cause death was relatively consistent across the subgroups of age, sex, diabetes, presentation, left main disease, LVEF, SS, and procedure (Fig. 3). There were no significant interactions between big ET-1 levels and these covariates (interaction P > 0.1 for all subgroups).
Fig. 3.
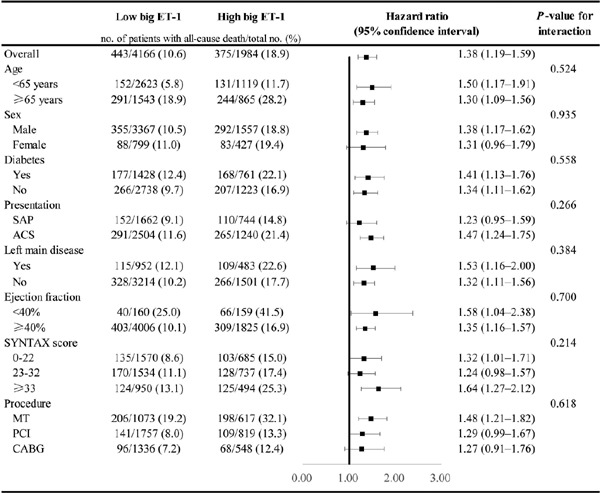
Subgroup analyses for the primary endpoint.
HRs and 95% CIs were calculated by reference to the low big ET-1 group. The interaction between big ET-1 level and each covariate was tested by a multivariable Cox proportional hazards regression model.
When big ET-1 level (high or low) was combined with the SSII for mortality prediction, there were significant improvements in C-index (0.723 [0.704–0.742] vs. 0.715 [0.697–0.734], P = 0.029), NRI (0.304 [0.229–0.378], P < 0.001), and IDI (0.009 [0.006–0.012], P < 0.001) compared with the SSII alone (Table 3).
Table 3. Additional prognostic information provided by big endothelin-1 level beyond SSII.
| C-index (95% CI) | P-value | NRI (95% CI) | P-value | IDI (95% CI) | P-value | |
|---|---|---|---|---|---|---|
| SSII | 0.715 (0.697–0.734) | Reference | – | Reference | – | Reference |
| SSII + big ET-1 | 0.723 (0.704–0.742) | 0.029 | 0.304 (0.229–0.378) | < 0.001 | 0.009 (0.006–0.012) | < 0.001 |
Discussion
The study shows that (i) high plasma big ET-1 level is an independent risk factor for all-cause death, cardiac death, and MACCE and is relatively consistent across subgroups and (ii) plasma big ET-1 level improves predictability of the SSII for long-term mortality in patients with TVD.
Plasma big ET-1 level has been identified as a novel marker of disease severity and clinical outcome in the context of CAD. Big ET-1 level was a predictor of severity in stable CAD, as reflected by the Gensini score9). Moreover, two cohort studies found that high big ET-1 level was associated with increased risks of adverse outcomes in patients with stable CAD8, 10). In another cohort study of 983 patients with acute MI, big ET-1 level was an independent predictor of death or heart failure, with an area under the ROC curve of 0.767). These studies demonstrated that higher level of big ET-1 was associated with higher risks of adverse events in stable CAD or ACS, in accordance with the present study performed in SAP and ACS patients. The present study further extended the association to the setting of TVD with heavy atherosclerotic burden.
Revascularization (PCI or CABG) has been considered to improve prognosis in patients with multivessel disease compared with MT alone13). It remains unknown whether there is an interaction between treatment (MT, PCI, or CABG) and big ET-1 level with outcomes. In our study, the association between big ET-1 level and mortality was relatively consistent across patients receiving MT, PCI, and CABG, with no significant interaction between big ET-1 level and treatment. Thus, high big ET-1 level remained an independent risk marker for mortality regardless of whether or not revascularization was performed.
ET-1 contributes to the poor prognosis in TVD patients through several mechanisms. First, as a vasoconstrictor, ET-1 accounts for nearly all the resting tone in atherosclerotic coronary arteries14). High ET-1 level can lead to vasoconstriction and decreased coronary blood flow, which may induce or aggravate myocardial ischemia. Second, ET-1 can decrease nitric oxide (NO) production and increase NO degradation, leading to an imbalance between NO and ET-1 and subsequent endothelial dysfunction in the coronary circulation15). Third, ET-1 can promote increases in oxidative stress and inflammatory cell infiltration, which contribute to atherosclerotic plaque formation, progression, and rupture16). These effects can be even more significant and more likely to lead to adverse events in the setting of TVD with heavy atherosclerotic burden in all three major vessels. Compared with traditional biomarkers representing myocardial injury (troponin I), cardiac stress (N-terminal pro-B-type natriuretic peptide), and inflammation (high-sensitivity C-reactive protein), big ET-1 can reflect endothelial function that is important for the development of atherosclerosis4).
TVD is present in 20%–30% of patients with obstructive CAD17, 18). As a severe type of CAD, it confers an almost two-fold higher risk of mortality compared with single-vessel disease1). Calculation of the SS is recommended for assessment of the long-term mortality risk in TVD patients19) but showed only modest predictability in previous studies20, 21). Despite the generally better performance of the SSII compared with the SS, some studies found only a moderate discrimination ability of the SSII for long-term mortality prediction in patients with multivessel disease22, 23). Biomarkers have been shown to provide additional prognostic information beyond clinical characteristics in CAD24). Addition of biomarkers to the SSII may enhance its predictability, because it is mainly based on clinical characteristics. Our study demonstrated significant improvements in the C-index, NRI, and IDI after incorporation of big ET-1 level into the SSII, indicating better predictive performance compared with the SSII alone. These findings are of great importance because better risk stratification can be achieved for guidance of treatment. Furthermore, similar to the traditional biomarkers, measurement of plasma big ET-1 is simple and economic using a commercial immunoassay. Although more evidence is needed regarding the prognostic value of big ET-1 level, our study has shown the feasibility of its addition to the established model to improve predictability.
There are some limitations that should be noticed in this study. First, this was an observational study that may suffer from potential selection and measurement biases. Second, all participants in the study were enrolled from a single specialized center for cardiovascular disease, which may limit the reliability and generalizability of our findings. Third, angiographic criteria were used as the main indications for revascularization, and physiological tests were only performed when a treatment decision could not be made using the angiographic findings alone. As a result, most of the participants in our study did not undergo preprocedural/intraprocedural ischemia evaluations. Fourth, the present analyses were based on a single plasma big ET-1 measurement, and thus, potential fluctuations in its levels remain uncountable.
Conclusions
In patients with TVD, plasma big ET-1 level was significantly higher in patients who died during follow-up than those who survived. Higher big ET-1 level was independently associated with increased risks of all-cause death, cardiac death, and MACCE. Big ET-1 level improves the discrimination and reclassification of the SSII to predict long-term mortality.
Acknowledgments
This work was supported by the CAMS Innovation Fund for Medical Sciences (CAMS-I2M, 2016-I2M-1-002), the National High Technology Research and Development Program of China (2015AA020407), and the National Natural Science Foundation of China (81470380). We thank all staff members for data collection, data entry, and monitoring as part of this study. We also thank Dr. Alison Sherwin from Liwen Bianji, Edanz Group China for editing the English text of this study.
Conflict of Interest
Dr. Lei Song reports grants from the CAMS Innovation Fund for Medical Sciences (CAMS-I2M, 2016- I2M-1-002), National High Technology Research and Development Program of China (2015AA020407), and National Natural Science Foundation of China (81470380). The other authors have nothing to disclose.
References
- 1). Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah M, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng V, Chinnaiyan K, Chow BJ, Delago A, Hadamitzky M, Hausleiter J, Kaufmann P, Maffei E, Raff G, Shaw LJ, Villines T, Berman DS, Investigators C : Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol, 2011; 58: 849-860 [DOI] [PubMed] [Google Scholar]
- 2). Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Stahle E, Feldman TE, van den Brand M, Bass EJ, Van Dyck N, Leadley K, Dawkins KD, Mohr FW, Investigators S : Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med, 2009; 360: 961-972 [DOI] [PubMed] [Google Scholar]
- 3). Farooq V, van Klaveren D, Steyerberg EW, Meliga E, Vergouwe Y, Chieffo A, Kappetein AP, Colombo A, Holmes DR, Jr., Mack M, Feldman T, Morice MC, Stahle E, Onuma Y, Morel MA, Garcia-Garcia HM, van Es GA, Dawkins KD, Mohr FW, Serruys PW: Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: development and validation of SYNTAX score II. Lancet, 2013; 381: 639-650 [DOI] [PubMed] [Google Scholar]
- 4). Davenport AP, Hyndman KA, Dhaun N, Southan C, Kohan DE, Pollock JS, Pollock DM, Webb DJ, Maguire JJ: Endothelin. Pharmacol Rev, 2016; 68: 357-418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Kolettis TM, Barton M, Langleben D, Matsumura Y: Endothelin in coronary artery disease and myocardial infarction. Cardiol Rev, 2013; 21: 249-256 [DOI] [PubMed] [Google Scholar]
- 6). Papassotiriou J, Morgenthaler NG, Struck J, Alonso C, Bergmann A: Immunoluminometric assay for measurement of the C-terminal endothelin-1 precursor fragment in human plasma. Clin Chem, 2006; 52: 1144-1151 [DOI] [PubMed] [Google Scholar]
- 7). Khan SQ, Dhillon O, Struck J, Quinn P, Morgenthaler NG, Squire IB, Davies JE, Bergmann A, Ng LL: C-terminal pro-endothelin-1 offers additional prognostic information in patients after acute myocardial infarction: Leicester Acute Myocardial Infarction Peptide (LAMP) Study. Am Heart J, 2007; 154: 736-742 [DOI] [PubMed] [Google Scholar]
- 8). Sabatine MS, Morrow DA, de Lemos JA, Omland T, Sloan S, Jarolim P, Solomon SD, Pfeffer MA, Braunwald E: Evaluation of multiple biomarkers of cardiovascular stress for risk prediction and guiding medical therapy in patients with stable coronary disease. Circulation, 2012; 125: 233-240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Chen J, Chen MH, Guo YL, Zhu CG, Xu RX, Dong Q, Li JJ: Plasma big endothelin-1 level and the severity of new-onset stable coronary artery disease. J Atheroscler Thromb, 2015; 22: 126-135 [DOI] [PubMed] [Google Scholar]
- 10). Zhou BY, Guo YL, Wu NQ, Zhu CG, Gao Y, Qing P, Li XL, Wang Y, Dong Q, Liu G, Xu RX, Cui CJ, Sun J, Li JJ: Plasma big endothelin-1 levels at admission and future cardiovascular outcomes: A cohort study in patients with stable coronary artery disease. Int J Cardiol, 2017; 230: 76-79 [DOI] [PubMed] [Google Scholar]
- 11). Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH, American College of Cardiology F, American Heart Association Task Force on Practice G, Society for Cardiovascular A and Interventions : 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol, 2011; 58: e44-122 [DOI] [PubMed] [Google Scholar]
- 12). Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, Cigarroa JE, Disesa VJ, Hiratzka LF, Hutter AM, Jr., Jessen ME, Keeley EC, Lahey SJ, Lange RA, London MJ, Mack MJ, Patel MR, Puskas JD, Sabik JF, Selnes O, Shahian DM, Trost JC, Winniford MD, American College of Cardiology F, American Heart Association Task Force on Practice G, American Association for Thoracic S, Society of Cardiovascular A and Society of Thoracic S : 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol, 2011; 58: e123-210 [DOI] [PubMed] [Google Scholar]
- 13). Hueb W, Lopes N, Gersh BJ, Soares PR, Ribeiro EE, Pereira AC, Favarato D, Rocha AS, Hueb AC, Ramires JA: Ten-year follow-up survival of the Medicine, Angioplasty, or Surgery Study (MASS II): a randomized controlled clinical trial of 3 therapeutic strategies for multivessel coronary artery disease. Circulation, 2010; 122: 949-957 [DOI] [PubMed] [Google Scholar]
- 14). Kinlay S, Behrendt D, Wainstein M, Beltrame J, Fang JC, Creager MA, Selwyn AP, Ganz P: Role of endothelin-1 in the active constriction of human atherosclerotic coronary arteries. Circulation, 2001; 104: 1114-1118 [DOI] [PubMed] [Google Scholar]
- 15). Iglarz M, Clozel M: Mechanisms of ET-1-induced endothelial dysfunction. J Cardiovasc Pharmacol, 2007; 50: 621-628 [DOI] [PubMed] [Google Scholar]
- 16). Li MW, Mian MO, Barhoumi T, Rehman A, Mann K, Paradis P, Schiffrin EL: Endothelin-1 overexpression exacerbates atherosclerosis and induces aortic aneurysms in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol, 2013; 33: 2306-2315 [DOI] [PubMed] [Google Scholar]
- 17). Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG, Douglas PS: Low diagnostic yield of elective coronary angiography. N Engl J Med, 2010; 362: 886-895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Bradley SM, Spertus JA, Kennedy KF, Nallamothu BK, Chan PS, Patel MR, Bryson CL, Malenka DJ, Rumsfeld JS: Patient selection for diagnostic coronary angiography and hospital-level percutaneous coronary intervention appropriateness: insights from the National Cardiovascular Data Registry. JAMA Intern Med, 2014; 174: 1630-1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Juni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO, Group ESCSD : 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J, 2018; [DOI] [PubMed] [Google Scholar]
- 20). Girasis C, Garg S, Raber L, Sarno G, Morel MA, Garcia-Garcia HM, Luscher TF, Serruys PW, Windecker S : SYNTAX score and Clinical SYNTAX score as predictors of very long-term clinical outcomes in patients undergoing percutaneous coronary interventions: a substudy of SIRolimus-eluting stent compared with pacliTAXel-eluting stent for coronary revascularization (SIRTAX) trial. Eur Heart J, 2011; 32: 3115-3127 [DOI] [PubMed] [Google Scholar]
- 21). Zhang YJ, Iqbal J, Campos CM, Klaveren DV, Bourantas CV, Dawkins KD, Banning AP, Escaned J, de Vries T, Morel MA, Farooq V, Onuma Y, Garcia-Garcia HM, Stone GW, Steyerberg EW, Mohr FW, Serruys PW: Prognostic value of site SYNTAX score and rationale for combining anatomic and clinical factors in decision making: insights from the SYNTAX trial. J Am Coll Cardiol, 2014; 64: 423-432 [DOI] [PubMed] [Google Scholar]
- 22). Sotomi Y, Cavalcante R, van Klaveren D, Ahn JM, Lee CW, de Winter RJ, Wykrzykowska JJ, Onuma Y, Steyerberg EW, Park SJ, Serruys PW: Individual Long-Term Mortality Prediction Following Either Coronary Stenting or Bypass Surgery in Patients With Multivessel and/or Unprotected Left Main Disease: An External Validation of the SYNTAX Score II Model in the 1,480 Patients of the BEST and PRECOMBAT Randomized Controlled Trials. JACC Cardiovasc Interv, 2016; 9: 1564-1572 [DOI] [PubMed] [Google Scholar]
- 23). Cavalcante R, Sotomi Y, Mancone M, Whan Lee C, Ahn JM, Onuma Y, Lemos PA, van Geuns RJ, Park SJ, Serruys PW: Impact of the SYNTAX scores I and II in patients with diabetes and multivessel coronary disease: a pooled analysis of patient level data from the SYNTAX, PRECOMBAT, and BEST trials. Eur Heart J, 2017; 38: 1969-1977 [DOI] [PubMed] [Google Scholar]
- 24). Lindholm D, James SK, Bertilsson M, Becker RC, Cannon CP, Giannitsis E, Harrington RA, Himmelmann A, Kontny F, Siegbahn A, Steg PG, Storey RF, Velders MA, Weaver WD, Wallentin L, Investigators P : Biomarkers and Coronary Lesions Predict Outcomes after Revascularization in Non-ST-Elevation Acute Coronary Syndrome. Clin Chem, 2017; 63: 573-584 [DOI] [PubMed] [Google Scholar]


