Abstract
Aim: The primary percutaneous procedure resulted in a significant improvement in the prognosis of myocardial infarction. However, no-reflow phenomenon restrains this benefit of the process. There are studies suggesting that soluble suppression of tumorigenicity (sST2) can be valuable in the diagnosis and progression of heart failure and myocardial infarction. In this study, we aimed to investigate the effect of sST2 on no-reflow phenomenon in ST-elevated myocardial infarction (STEMI).
Method: This study included 379 patients (258 men; mean age, 60 ± 11 years) who underwent primary percutaneous treatment for STEMI. sST2 levels were measured from blood samples taken at admission. Patients were divided into two groups according to Thrombolysis in Myocardial Infarction(TIMI) flow grade: group 1 consists of TIMI 0,1,2, accepted as no-reflow, and group 2 consists of TIMI 3, accepted as reflow.
Results: No-reflow phenomenon occurred in 60 patients (15.8%). The sST2 level was higher in the no-reflow group (14.2 ± 4.6 vs. 11.3 ± 5.0, p = 0.003). Moreover, regression analysis indicated that diabetes mellitus, lower systolic blood pressure, multivessel vascular disease, high plaque burden, and grade 0 initial TIMI flow rate were other independent predictors of the no-reflow phenomenon in our study. Besides, when the patients were divided into high and low sST2 groups according to the cut-off value from the Receiver operating characteristics analysis, being in the high sST2 group was associated with 2.7 times increased odds for no-reflow than being in the low sST2 group.
Conclusion: sST2 is one of the independent predictors of the no-reflow phenomenon in STEMI patients undergoing primary percutaneous coronary intervention.
Keywords: Myocardial infarction, soluble ST2, No-reflow-phenomenon, Inflammation, In-hospital mortality
Introduction
Acute coronary syndrome (ACS) is considered to be the most important cause of death throughout the world, especially in western countries, despite technological improvements, new drugs, and an increasing level of awareness1). Percutaneous coronary intervention (PCI) provides normal blood flow in the responsible artery in patients with myocardial infarction, contributing significantly to the regression of symptoms and better prognosis2, 3). But no-reflow phenomenon reduces the positive effects of PCI.
No-reflow phenomenon is defined as the insufficiency of myocardial perfusion despite the mechanically responsible lesion being opened. The rate of no-reflow can reach 50% depending upon assessing methods especially in patients with ACS4). This phenomenon has been shown to be associated with poor prognoses, such as in-hospital and long-term mortality and advanced heart failure5, 6). The methods used in the treatment of no-reflow provide very limited benefits. Therefore, no-reflow is becoming more important to prevent it from occurring. For this reason, our priority should be to find out the causes of this phenomenon and the markers or clinical conditions that can predict no-reflow. Although some independent risk factors of the no-reflow phenomenon have been shown, new biomarkers are needed for the predictability of this complication.
The soluble suppression of tumorigenicity 2 (sST2), a member of the interleukin (IL)-1 family, is thought to play a role in cardiac remodeling and the inflammatory process7). sST2 levels are considered to increase with cardiovascular stress and cardiac fibrosis that ultimately creates a substrate for fatal arrhythmia8, 9). On a cellular basis, this molecule binds IL-33 to reduce its bioavailability. Thus, circulating sST2 eliminates the positive effects of IL-33 on myocardial fibrosis, hypertrophy, apoptosis, and myocardial function (MI)10). There are studies suggesting that sST2 is a predictor of mortality in patients with ACS. In these studies, independent of MI type, sST2 has been shown to be an independent predictor in both short-term and long-term follow-up11, 12). Furthermore, as stated in the guidelines, sST2 can be used in the diagnosis and follow-up of heart failure in addition to N-terminal prohormone of brain natriuretic peptide (NT-proBNP)13).
The relationship between high sST2 levels and major cardiovascular events are well-established in patients with MI14); however, as far as we know, the predictive value of sST2 on the no-reflow prediction in ST-elevation myocardial infarction (STEMI) patients has not been described to date. In this study, we aimed to investigate the no-reflow rate in our study population and the relationship of the no-reflow phenomenon with sST2 levels in patients undergoing primary PCI due to STEMI.
Methods
Study Population
In this single-center study, we included 379 patients who were admitted to a large-volume center between May 2017 and April 2018 with a diagnosis of STEMI and underwent primary PCI. We excluded patients who have missing or unavailable data or malignant and infectious diseases or other systemic inflammatory conditions or no indication of PCI. STEMI patients were defined as patients with typical chest discomfort or other ischemic symptoms, who develop new ST-segment elevations in two or more contiguous leads or new bundle branch blocks with ischemic repolarization patterns on the standard 12-lead electrocardiogram. All PCI procedures were performed by operators who execute more than 100 PCIs/year at a single center (> 1000 PCIs/year). Informed consent was taken from all patients, along with approval from the local ethical committee. The study follows the guidelines of the Helsinki Declaration.
Analysis of Patient Data
Patient demographics, medical charts, and laboratory parameters were recorded. Patients who had smoked within the previous month were regarded as smokers. Hypercholesterolemia was defined as having a low-density lipoprotein (LDL) level above 130 mg/dL or using antihyperlipidemic drugs. Diabetes mellitus (DM) was defined as having a fasting glucose level above 125 mg/dL or using antidiabetic drugs. Hypertension was defined as systolic blood pressure above 140 mmHg and diastolic blood pressure above 90 mmHg or having used antihypertensive drugs for longer than two weeks.
Five milliliters of peripheral venous blood was taken from all patients and placed in EDTA-coated vacutainer tubes at the time of hospital admission. Immediately after that, the blood samples were separated by centrifugation and eventually stored at −70°C. We reached sST2 levels by measuring with sandwich enzyme immunoassay using ELISA kits (Shanghai YL Biotech, Shanghai, China) by following the manufacturer's protocol. The coefficients of intra-assay and inter-assay variation of sST2 were 8%–10%. The sensitivity of both sST2 assays, intra- and inter-assay for plasma samples is 0.13 ng/L. Patients were divided into two groups according to Thrombolysis in Myocardial Infarction (TIMI) flow grade:15) group 1 (n = 60, mean age: 59.5 ± 11.8 years) consists TIMI 0,1,2, accepted as no-reflow, and group 2 (n = 319, mean age: 64.0 ± 11.6 years) consists of TIMI 3 as accepted reflow.
Coronary Intervention Procedure
All participants received a chewable acetylsalicylic acid (loading dosage 300 mg) and clopidogrel (600 mg loading dosage) or ticagrelor (180 mg loading dosage) before coronary angiography. In our study, the first choice for second antiplatelet therapy was ticagrelor. Clopidogrel was preferred as the second antiplatelet agent in patients with contraindicated ticagrelor use. When we looked at the second antiplatelet agent ratio, 83.3% of the patients had ticagrelor use, and 16.6% of the patients had clopidogrel. There was also no difference between the two groups in terms of ticagrelor use (reflow 82.9% vs. no-reflow 85.0%, p = 0.919). Heparin (100 IU/kg) was administered after the decision to perform a coronary intervention. After angioplasty, all patients were admitted to the coronary care unit where routine antithrombotic therapy was given as a daily dose of 100 mg of aspirin, 75 mg of clopidogrel or 90 mg ticagrelor, and subcutaneous administration of enoxaparin. Patients' angiographic data were reached by assessing catheter laboratory records. The artery that was assumed to be a non-infarct-related artery (IRA) was injected first. Blood flow in the IRA was calculated by using TIMI classification. Primary angioplasty was performed only for IRA occlusion (either total or partial). Multivessel disease was defined as more than 50% stenosis in one or more major epicardial vessels, or in major branches except for IRA. TIMI thrombus scale was used to determine the thrombus burden16). According to this scale, Grade 4 is definite thrombus with the largest dimension > 2 vessel diameters and grade 5 was total occlusion. Those which did not provide these features angiographically were defined as grade 0, 1, 2, 3. Finally, we classified patients as a high thrombus burden (grade 4 and 5) and low thrombus burden (grade 0, 1, 2, 3) in our study17).
Statistical Analysis
Statistical analysis was performed using the SPSS software version 21.0 for Windows (SPSS Inc., Chicago, Illinois, USA). Whether the values are normally distributed or not were determined by using visual and analytical methods. Descriptive analyses are presented as means and standard deviations for variables with normal distribution, as the median and interquartile range for non-normal distribution. The categorical variables are expressed as numbers and percentages. Study groups were compared using the unpaired Student's t-test for continuous variables that presented normal distribution and using the Mann–Whitney U test for continuous variables that did not present normal distribution. Categorical data were compared with a chi-square test. Receiver operating characteristics (ROC) curves were drawn to distinguish the no-reflow phenomenon. Youden's index was used to derive the best cut-offs of plasma concentrations of sST2 value. With the obtained value, patients were divided into two groups as high- and low-sST2 groups. Multivariate logistic regression backward stepwise that included variables with P < 0.1 on univariate analysis was carried out to identify independent predictors of the no-reflow phenomenon. A P value less than 0.05 was considered statistically significant.
Results
After excluding patients with exclusion criteria, a total of 379 patients were included. The prevalence of the no-reflow phenomenon was 16.5%. There was no statistical difference in terms of traditional risk factors except age and DM. Patients with no-reflow were older (64.0 ± 10.6 vs. 59.5 ± 11.8) and more had DM (40.0% vs 24.8%) compared with TIMI 3 patients (p = 0.006, p = 0.015, respectively). Medical treatment was compared before the occurrence of MI, and a lower rate of statin treatment was detected in the no-reflow group (25.0% vs. 38.6%, p = 0.046). In addition, patients with no-reflow had lower systolic blood pressure upon admission (128.8 ± 24.4 vs. 118.0 ± 22.5, p = 0.002). When two groups were compared in terms of laboratory values, there was only a difference in sST2 levels between the groups (no-reflow group = 14.2 ± 4.6 vs. reflow group = 11.3 ± 5.0, p = 0.003) (Table 1).
Table 1. Baseline Characteristics of Study Population, mean ± SD or n (%).
| Variable | Reflow | No-reflow | P |
|---|---|---|---|
| (n = 319) | (n = 60) | ||
| Age, years | 59.5 ± 11.8 | 64.0 ± 10.6 | 0.006 |
| Male, n (%) | 258 (80.9%) | 50 (83.3%) | 0.655 |
| BMI(kg/m2) | 26.2 ± 2.4 | 25.8 ± 3.0 | 0.396 |
| Smoking, n (%) | 160 (50.2%) | 25 (41.7%) | 0.227 |
| DM, n (%) | 79 (24.8%) | 24 (40.0%) | 0.015 |
| HT, n (%) | 108 (33.9%) | 23 (38.3%) | 0.503 |
| HL, n (%) | 90 (28.2%) | 22 (36.7%) | 0.188 |
| Family History, n (%) | 34 (10.7%) | 5 (8.3%) | 0.587 |
| Stroke, n (%) | 1 (0.3%) | 1 (1.7%) | 0.184 |
| MI history, % | 48 (15.0%) | 11 (18.3%) | 0.519 |
| eGFR | 91.6 ± 23.7 | 85.8 ± 24.4 | 0.205 |
| Total cholesterol (mg/dL) | 200.2 ± 93.3 | 173.6 ± 48.4 | 0.104 |
| LDL(mg/dL) | 124.8 ± 39.1 | 116.5 ± 39.8 | 0.272 |
| HDL (mg/dL) | 40.7 ± 14.1 | 40.6 ± 7.8 | 0.966 |
| Peak troponin (mg/dL) | 4.0 ± 3.4 | 4.9 ± 3.4 | 0.181 |
| Peak CK-MB (mg/dL) | 130.1 ± 114.2 | 147.7 ± 101.7 | 0.423 |
| WBC count (103/L) | 12.7 ± 8.5 | 13.3 ± 4.1 | 0.707 |
| Platelet (103/L) | 244.8 ± 71.4 | 245.5 ± 72.7 | 0.964 |
| Hemoglobin (g/dL) | 13.9 ± 5.2 | 12.8 ± 2.1 | 0.279 |
| SBP, mmHg | 128.8 ± 24.4 | 118.0 ± 22.5 | 0.002 |
| DBP, mmHg | 73.1 ± 13.3 | 69.8 ± 12.5 | 0.080 |
| Periprocedural Medications | |||
| Aspirin, n (%) | 79 (24.8%) | 18 (30.0%) | 0.394 |
| Beta Bloker, n (%) | 52 (16.3%) | 11 (18.3%) | 0.698 |
| RAS bloker, n (%) | 79 (24.8%) | 19 (31.7%) | 0.263 |
| Ca chanel bloker, n (%) | 66 (20.7%) | 13 (21.7%) | 0.864 |
| Oral antidiabetic, n (%) | 75 (23.5%) | 17 (28.3%) | 0.424 |
| Insulin, n (%) | 40 (12.5%) | 10 (16.7%) | 0.386 |
| Statin, n (%) | 123 (38.6%) | 15 (25.0%) | 0.046 |
| sST2 (ng/ml) | 11.3 ± 5.0 | 14.2 ± 4.6 | 0.003 |
Abbreviations; sST2, soluble ST2; DM, diabetes mellitus; HL, hyperlipidemia; HT, hypertension; PCI, percutaneous coronary intervention; MI, myocardial infarction; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; WBC; white blood cell; LVEF, left ventricular ejection fraction; eGFR, estimated glomerular filtration rate; CK, creatine kinase; RAS, Renin angiotensine system; SD, standard deviation
Remark: eGFR was not mentioned in the main text. Therefore, including this in the table is not clear. Please check this for all such parameters, if applicable and revise as necessary.
These two groups were compared according to the process characteristics (Table 2). There were no differences in terms of IRA, stent type, stent length, and diameter in the two groups. No-reflow was more common in patients with grade 0 initial TIMI flow rate (82.8% vs. 50.0%, p < 0.001). The high thrombus burden was more frequent in the no-reflow group (61.7% vs. 38.6%, p = 0.002). The presence of a multivessel lesion, Syntax, and Gensini score, which are the indicators of severity of coronary artery disease, was found to be statistically significant in the no-reflow group. Furthermore, an sST2 cut-off of 11.6 ng/ml had an AUC of 0.699 for distinguishing no-reflow with an 82.7% sensitivity, and 64.0% specificity showed in the ROC analysis (Fig. 1).
Table 2. Angiographic procedural charecteristics and drug use of the study population mean ± SD or n (%).
| Variable | Reflow | No-reflow | P |
|---|---|---|---|
| (n = 319) | (n = 60) | ||
| IRA, n (%) | 0.824 | ||
| LAD | 133 (41.7) | 27 (45.0) | |
| CX | 65 (17.2) | 9 (15.0) | |
| RCA | 112 (35.1) | 19 (31.7) | |
| Others | 19 (6.0) | 5 (8.3) | |
| Grade 0 initial TIMI flow rate, n (%) | 153 (50.0) | 24 (82.8) | < 0.001 |
| Pretimi, n (%) | 0.001 | ||
| 0 | 155 (48.6) | 44 (73.3) | |
| 1 | 4 (1.3) | 3 (5.0) | |
| 2 | 29 (9.1) | 2 (3.3) | |
| 3 | 131 (41.1) | 11 (18.3) | |
| TIMI thrombus grade, n (%) | 0.002 | ||
| High thrombus burden | 123 (38.6%) | 37 (61.7%) | |
| Low thrombus burden | 196 (61.4%) | 23 (38.3%) | |
| Gensini Score | 42.8 ± 21.1 | 51.6 ± 24.6 | 0.033 |
| Syntax score | 9.3 ± 5.5 | 12.9 ± 6.6 | 0.009 |
| Multi vessel, n (%) | 66 (20.7%) | 25 (41.7%) | 0.002 |
| PCI procedure, n (%) | 0.027 | ||
| Baloon and stenting | 191 (59.9%) | 45 (75.0%) | |
| Direct stenting | 128 (40.1%) | 15 (25.0%) | |
| Stent type, n (%) | 0.376 | ||
| BMS | 174 (54.5%) | 29 (48.3%) | |
| DES | 145 (45.5%) | 31 (51.7%) | |
| Stent Length, mm | 19.2 ± 5.5 | 19.1 ± 6.2 | 0.844 |
| Stent Daimeter, mm | 2.95 ± 0.31 | 3.01 ± 0.34 | 0.148 |
| > 1 Stent used, n (%) | 81 (25.4%) | 20 (33.3%) | 0.202 |
| In-hospital mortality | 10 (3.1%) | 7 (11.7%) | 0.003 |
Abbreviations: LAD, left anterior descending; CX, circumflex; RCA, right coronary artery; PCI, percutaneous coronory intervention; DES, drug eluting stent; SD, standard deviation
Fig. 1.
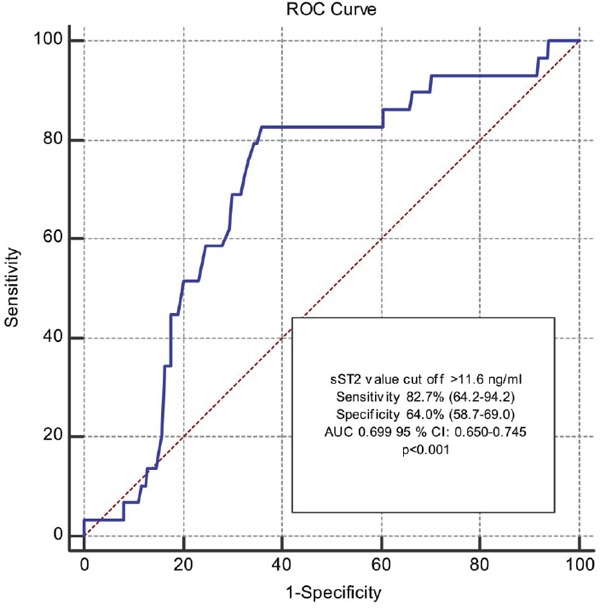
ROC curve showing the distinguishing ability of soluble ST2 level for the no-reflow phenomenon
AUC, area under the ROC curve; CI, confidence interval; ROC, receiver operating characteristics.
A total of 17 deaths (4.4%) occurred in-hospital follow-up. We found that 7 deaths occurred in the no-reflow group (11.7%) and 10 deaths occurred in the reflow group (3.1%) after examining the groups separately. The mortality rate was significantly higher in the no-reflow group as expected (P = 0.003) (Table 2). Furthermore, more in-hospital mortality occurred in the high-sST2 group than low-sST2 group (9.1% vs. 2.0%, p = 0.002).
In the logistic regression analysis; DM, high thrombus burden, grade 0 initial TIMI flow rate, the presence of multivessel disease, lower systolic blood pressure were independent risk factors of the no-reflow phenomenon. Besides, being in the high-sST2 group had 2.7 times increased odds for the no-reflow compared to the low-sST2 group independent of other risk factors (Table 3).
Table 3. Multivariate Logistic Regression Analysis for Potential Predictors of No-Reflow Phenomenon.
| Univariate analysis |
Multivariate analysis¶ |
|||
|---|---|---|---|---|
| OR (CI 95%) | p value | OR (CI 95%) | p value | |
| Demographic variables | ||||
| Age, years | 1.034 (1.010–1.060) | 0.006 | 1.016 (0.989–1.045) | 0.253 |
| Male, yes | 1.144 (0.448–2.922) | 0.779 | ----- | ----- |
| DM, yes | 2.025 (1.139–3.602) | 0.016 | 2.126 (1.096–4.125) | 0.026 |
| Statin use, yes | 0.531 (0.284–0.994) | 0.048 | 0.554 (0.276–1.111) | 0.096 |
| SBP, mmHg | 0.974 (0.958–0.990) | 0.002 | 0.982 (0.969–0.995) | 0.007 |
| Killip Class > 1 | 1.708 (0.725–4.343) | 0.208 | ----- | ----- |
| Labaratory data | ||||
| eGFR, (mL/min/1.73m2) | 0.990 (0.975.1.005) | 0.206 | ----- | ----- |
| WBC, count (103/L) | 1.007 (0.972–1.043) | 0.710 | ----- | ----- |
| Peak troponin, mg/dl | 1.053 (0.972–1.140) | 0.206 | ----- | ----- |
| Procedural features | ||||
| IRA* | 1.505 (0.709–3.235) | 0.283 | ----- | ----- |
| Total lesion, yes | 3.565 (1.885–6.743) | < 0.001 | 2.505 (1.249–5.023) | 0.010 |
| Multivessel disease, yes | 2.738 (1.533–4.892) | 0.001 | 2.288 (1.174–4.456) | 0.015 |
| High thrombus burden, yes | 2.563 (1.464–4.520) | 0.002 | 2.737 (1.464–5.118) | 0.002 |
| Predilatation, yes | 2.010 (1.075–3.759) | 0.029 | 1.287 (0.606–2.735) | 0.511 |
| Syntax score | 1.081 (1.033–1.131) | 0.001 | 0.979 (0.919–1.041) | 0.489 |
| High sST2‡ | 3.432 (1.925–6.119) | < 0.001 | 2.741 (1.433–5.244) | 0.002 |
IRA was divided into two groups as LAD and non LAD lesions.
High sST2 was formed according to the cut-off value 11.6 ng/ ml which is derived from ROC analysis
Nagelkerke R square of the full model was 29.8%.
Abbreviations: CI, confidence interval; DM, Diabetes mellitus; OR, odds ratio; SBP, systolic blood pressure; WBC; white blood cell; eGFR, estimated glomerular filtration rate; sST2, soluble ST2; IRA, infarct-related artery
Discussion
We can summarize the findings of our study as follows: (a) among STEMI patients who underwent primary PCI, 16.5% had no-reflow complications, (b) higher levels of sST2 patients had a significantly higher level of no-reflow compared with lower levels of sST2, (c) after adjustment for potential confounders, it was found that being in a high-sST2 group was one of the independent predictors of no-reflow (OR: 2.741 CI 95% 1.433–5.244, p = 0.002). Moreover, DM, grade 0 initial TIMI flow rate, the presence of multivessel disease, lower systolic blood pressure, and high thrombus burden were the other independent predictors of no-reflow.
Early mortality risk may vary considerably in STEMI patients undergoing PCI. One of the most important parameters used in risk classification is the success of the primary percutaneous procedure. Rapid restoration of the arterial flow in total lesions provides serious benefits for STEMI patients in the short- and long-term18). However, the inability to provide coronary flow prevents us from benefiting from the process19).
Since it is clearly demonstrated that the inflammatory conditions have a role in both onset and progression of atherosclerosis, studies were conducted to investigate the relationship between the inflammatory status and adverse events in patients with ACS in the next step. No-reflow phenomenon, which is one of the determinants of early mortality, especially, can be defined as incomplete reperfusion at the microvascular level despite the reopening of the IRA. This may be caused by tissue edema, the presence of microthrombi, neutrophil accumulation, and free radical formation, which are thought to be associated with inflammation following coronary perfusion20). The possible risk factors of no-reflow are considered to be associated with inflammatory activity after MI. Moreover, the complexity of the no-reflow mechanism, as mentioned before, makes the predictability of this phenomenon difficult.
Since the contractility and relaxation capacities of both the necrotic and penumbra parts of the left ventricle decrease after acute MI, the interventricular pressure causes extra wall tension in the healthy myocardium. Depending on this wall tension, cardiomyocytes release a number of molecules to increase MI. sST2 is one of these molecules21). Recently, especially after entering guidelines in heart failure, this cardiac biomarker has been used in clinical practice, but its clinical and pathophysiological features have not been clearly determined. A lot of studies have been carried out on the relationship of sST2 with both diagnosis and prognosis in heart failure. However, there is little and contradictory information about the role of sST2 in STEMI patients22, 23). In addition, a study examining the relationship between no-reflow and sST2 is not present in the literature.
Studies showed that sST2 levels were found to be significantly increased as a result of neutrophil infiltration during the exacerbation of diseases such as chronic obstructive pulmonary disease (COPD) and pneumonia24, 25). Since sST2 has been shown to play a role in the release of proinflammatory cytokines from macrophages, it is thought that these cytokines may cause inflammation and free radicals in the acute phase and cause no-reflow in STEMI patients. Given the correlation of no-reflow frequency with inflammatory activity, this assumption supports our hypothesis that no-reflow may be more common in individuals with high sST2 levels. Furthermore, studies have found a poor association with sST2 and other clinical features of MI which indicate that sST2 elevation reflects a separate mechanical pathway from other known biomarkers26). So, sST2 can provide us with new information about the course of MI.
The IL-33/sST2 pathway also plays a role in the induction of allergic inflammation. In particular, this pathway is thought to be one of the primary pathways responsible for allergic diseases such as asthma, atopic dermatitis, and autoimmune diseases such as rheumatoid arthritis27). In a study on smoke-induced COPD mice, the IL-33/sST2 pathway has been shown to induce IL-6 and IL-8 and cause systemic inflammation28). In addition, in another study, the increase in IL 6 via ST2 receptor in human arterial endothelial cells may have a role in the pathophysiology of idiopathic pulmonary arterial hypertension29). Moreover, the study30) that shows a relationship with IL-6 and a slow coronary flow phenomenon may explain the high ST2 levels leading to an increase in the no-reflow phenomenon. The reflection of the above-mentioned pathophysiological mechanisms to the clinical status has been shown in our study; that being in the high-sST2 group had 2.7 times increased odds for the no-reflow compared to the low-sST2 group independent of known risk factors such as age, DM, thrombus burden, and the presence of a total lesion before the intervention.
The findings of our study confirm the data in the studies investigating the role of sST2 in predicting the adverse outcome after STEMI. Shimpo et al. showed that high-sST2 levels were associated with increased major cardiovascular adverse events in the short-term follow-up after STEMI31). This study has also shown that sST2 is the predictor of mortality independent of parameters such as age, heart rate, blood pressure, and Killip class during the 30-day period after PCI. In parallel to this study, Sabatine and his colleagues found that sST2 levels above the median value predicted in-hospital mortality32). No-reflow rate in PCI-treated STEMI patients was 15.4% in our study. In addition, in-hospital mortality was higher in the high-sST2 group compared to the low-sST2 group similar to the above-mentioned studies (9.1% vs. 2.0%, p = 0.002).
In addition to sST2, other parameters that may affect no-reflow were also investigated. More no-reflow was observed in elderly individuals in parallel with other studies33). DM was found to be an independent predictor of no-reflow as shown in most of the studies (OR: 2.12, 95% CI 1.09–4.12, p = 0.026)34, 35). The burden of thrombus in the occluded vessel is generally high, which may increase the risk of no-reflow36). Particularly, as a possible mechanism, the removal of atherothrombus plates after stent placement and embolism of the thrombus fragments to the distal region leads to no-reflow, and consequently, the poor prognosis will become inevitable with decreasing myocardial perfusion and increasing infarct area37). In our study, the high thrombus burden was one of the independent predictors of no-reflow (OR: 2.73, 95% CI 1.46–5.11, p = 0.002).
There are some limitations in our study. First, this was a single-center study which may result in selection bias. Second, it seems difficult to compare our results with other studies on this subject due to differences in pre-analytical medical therapy, storage techniques, and ELISA kits. Besides, repeated measurements of sST2 were not calculated, so, changes which may affect the result could not be derived. We investigated the predictive value of the sST2 using the 11.6 ng/ml value acquired from the ROC analysis. Therefore, our results can be evaluated only for our cohort and needs validation in other large populations. But, this limitation is present in all studies with the particular population on the use of biomarkers. Finally, there were no data about the admission time of the patients in our study. Patient or system-induced delays in STEMI patients may also affect the no-reflow phenomenon38). In particular, the delayed presentation has been shown to be associated with edema in the capillary bed, swelling of the myocardial cells, and neutrophil accumulation39). Since the pathophysiological mechanisms in no-reflow in delayed patients may be triggered by sST2, it can be speculated that delayed patients may have high levels of sST2, although the admission time was not included in our study.
Conclusion
In summary, sST2 can help physicians predict the no-reflow phenomenon after primary PCI in STEMI patients. Moreover, patients with DM, more severe coronary artery disease, grade 0 initial TIMI flow rate, and lower systolic blood pressure constitute the risk for the no-reflow group. Our choice of treatment is very limited after the occurence of this complication, so it is important to recognize patients who may develop no-reflow after PCI. As STEMI patients with high levels of sST2 have lower successful procedure rates and consequently have adverse events in the hospital more frequently, it may be considered to use methods such as more intensive antithrombotic use or stent implantation without predilatation to reduce no-reflow in this group of patients. Further studies are needed to understand the effect of sST2 on the no-reflow pathogenesis and to identify protective strategies in high-risk patients.
Conflicts of Interest
The authors declare that there was no conflict of interest.
Funding
No funding was received for this research.
References
- 1). Gaziano JM: Global burden of cardiovascular disease. In Heart disease: A textbook of cardiovascular medicine 6th edition. Edited by: Braunwald E, Zipes DP, Libby P. Philadelphia: WB Saunders Company; 2001: 1-17 [Google Scholar]
- 2). The Lancet: 40 years of percutaneous coronary intervention: Where next? Lancet, 2017; 390: 715. [DOI] [PubMed] [Google Scholar]
- 3). Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol, 2011; 58: 2550-2583 [DOI] [PubMed] [Google Scholar]
- 4). Durante A, Camici PG. Novel insights into an old phenomenon: the no reflow. Int J Cardiol, 2015; 187: 273-280 [DOI] [PubMed] [Google Scholar]
- 5). Stone GW, Peterson MA, Lansky AJ, Dangas G, Mehran R, Leon MB. Impact of normalized myocardial perfusion after successful angioplasty in acute myocardial infarction. J Am Coll Cardiol, 2002; 39: 591e597. [DOI] [PubMed] [Google Scholar]
- 6). Resnic FS, Wainstein M, Lee MK, Behrendt D, Wainstein RV, Ohno-Machado L, Kirshenbaum JM, Rogers CD, Popma JJ, Piana R. No-reflow is an independent predictor of death and myocardial infarction after percutaneous coronary intervention. Am Heart J, 2003; 145: 42-46 [DOI] [PubMed] [Google Scholar]
- 7). Kohli P, Bonaca MP, Kakkar R, Kudinova AY, Scirica BM, Sabatine MS, Murphy SA, Braunwald E, Lee RT, Morrow DA. Role of ST2 in non-ST-elevation acute coronary syndrome in the MERLIN-TIMI 36 trial. Clin Chem, 2012; 58: 257-266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Pascual-Figal DA, Januzzi JL. The Biology of ST2: The International ST2 Consensus Panel. Am J Cardiol, 2015; 115: 3B±7B [DOI] [PubMed] [Google Scholar]
- 9). LepojaÈrvi ES, Piira O-P, PaÈaÈkkoÈ E, Lammentausta E, Risteli J, Miettinen JA, Perkiömäki JS, Huikuri HV, Junttila MJ. Serum PINP, PIIINP, galectin-3, and ST2 as surrogates of myocardial fibrosis and echocardiographic left venticular diastolic filling properties. Front Physiol, 2015; 6: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Weinberg EO, Shimpo M, De Keulenaer GW, MacGillivray C, Tominaga S, Solomon SD, Rouleau JL, Lee RT. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation, 2002; 106: 2961-2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Manzano-Fernández S, Mueller T, Pascual-Figal D, Truong QA, Januzzi JL. Usefulness of soluble concentrations of interleukin family member ST2 as predictor of mortality in patients with acutely decompensated heart failure relative to left ventricular ejection fraction. Am J Cardiol, 2011; 107: 259-267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Eggers KM, Armstrong PW, Califf RM, Simoons ML, Venge P, Wallentin L, James SK. ST2 and mortality in non-ST-segment elevation acute coronary syndrome. Am Heart J, 2010; 159: 788-794 [DOI] [PubMed] [Google Scholar]
- 13). Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol, 2013. 15; 62: e147-239 [DOI] [PubMed] [Google Scholar]
- 14). Jenkins WS, Roger VL, Jaffe AS, Weston SA, AbouEzzeddine OF, Jiang R, Manemann SM, Enriquez-Sarano M. Prognostic Value of Soluble ST2 After Myocardial Infarction: A Community Perspective. Am J Med, 2017; 130: 1112.e9-1112.e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Chesebro JH, Knatterud G, Roberts R, Borer J, Cohen LS, Dalen J, Dodge HT, Francis CK, Hillis D, Ludbrook P. Thrombolysis in Myocardial Infarction (TIMI) Trial. Phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation, 1987; 76: 142-154 [DOI] [PubMed] [Google Scholar]
- 16). Gibson CM, de Lemos JA, Murphy SA, Marble SJ, McCabe CH, Cannon CP, Antman EM, Braunwald E, TIMI Study Group Combination therapy with abciximab reduces angiographically evident thrombus in acute myocardial infarction: a TIMI 14 substudy. Circulation, 2001; 103: 2550-2554 [DOI] [PubMed] [Google Scholar]
- 17). Miranda-Guardiola F, Rossi A, Serra A, Garcia B, Rumoroso JR, Iñiguez A, Vaquerizo B, Triano JL, Sierra G, Bruguera J, Spanish AMIcath Registry Angiographic quantification of thrombus in ST-elevation acute myocardial infarction presenting with an occluded infarct-related artery and its relationship with results of percutaneous intervention. J Interv Cardiol, 2009; 22: 207-215 [DOI] [PubMed] [Google Scholar]
- 18). The GUSTO Angiographic Investigators The effect of tissue plasminogenactivator, streptokinase, or both on coronary-artery patency, ventricular function, and survival after acute myocardial infarction. N Engl J Med, 1993; 329: 1615e1622. [DOI] [PubMed] [Google Scholar]
- 19). Ndrepepa G, Tiroch K, Fusaro M, Keta D, Seyfarth M, Byrne RA, Pache J, Alger P, Mehilli J, Schömig A, Kastrati A. 5-year prognostic value of no-reflow phenomenon after percutaneous coronary intervention in patients with acute myocardial infarction. J Am Coll Cardiol, 2010; 55: 2383-2389 [DOI] [PubMed] [Google Scholar]
- 20). Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial no-reflow in humans. J Am Coll Cardiol, 2009; 54: 281-292 [DOI] [PubMed] [Google Scholar]
- 21). Weinberg E. O., Shimpo M., De Keulenaer G. W., Mac-Gillivray C, Tominaga S, Solomon SD, Rouleau JL, Lee RT. Expression and regulation of ST2, an interleukin 1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation, 2002; 106: 2961-2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Bayes-Genis A, Zhang Y, Ky B. ST2 and patient prognosis in chronic heart failure. Am J Cardiol, 2015; 115: 64B-69B [DOI] [PubMed] [Google Scholar]
- 23). Diez J. Serum soluble ST2 as a biochemical marker of acute heart failure: future areas of research. J Am Coll Cardiol, 2008; 52: 1466-1467 [DOI] [PubMed] [Google Scholar]
- 24). Watanabe M, Takizawa H, Tamura M, Nakajima A, Kurai D, Ishii H, Takata S, Nakamoto K, Sohara E, Honda K, Nakamura M, Inui T, Wada H, Goto H. Soluble ST2 as a prognostic marker in community-acquired pneumonia. J Inf Secur, 2015; 70: 474-482 [DOI] [PubMed] [Google Scholar]
- 25). Xia J, Zhao J, Shang J, Li M, Zeng Z, Wang J, Xu Y, Xie J. Increased IL-33 expression in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol, 2015; 308: 619-627 [DOI] [PubMed] [Google Scholar]
- 26). Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov, 2008; 7: 827-840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Takatori H, Makita S, Ito T, Matsuki A, Nakajima H. Regulatory Mechanisms of IL-33-ST2-Mediated Allergic Inflammation. Front Immunol, 2018; 4; 9: 2004. 10.3389/fimmu.2018.02004. eCollection 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Wu H, Yang S, Wu X, Zhao J, Zhao J, Ning Q, Xu Y, Xie J. Interleukin-33/ST2 signaling promotes production of interleukin-6 and interleukin-8 in systemic inflammation in cigarette smoke-induced chronic obstructive pulmonary disease mice. Biochem Biophys Res Commun, 2014; 450: 110-116 [DOI] [PubMed] [Google Scholar]
- 29). Shao D, Perros F, Caramori G, Meng C, Dormuller P, Chou PC, Church C, Papi A, Casolari P, Welsh D, Peacock A, Humbert M, Adcock IM, Wort SJ. Nuclear IL-33 regulates soluble ST2 receptor and IL-6 expression in primary human arterial endothelial cells and is decreased in idiopathic pulmonary arterial hypertension. Biochem Biophys Res Commun, 2014; 451: 8-14 [DOI] [PubMed] [Google Scholar]
- 30). Liu CL, Xue ZQ, Gao SP, Chen C, Chen XH, Pan M, Wang ZX. The Relationship between Interleukin-6 Promotor Polymorphisms and Slow Coronary Flow Phenomenon. Clin Lab, 2016; 62: 947-953 [DOI] [PubMed] [Google Scholar]
- 31). M Shimpo, Morrow D. A., Weinberg E. O., Sabatine MS, Murphy SA, Antman EM, Lee RT. Serum levels of the interleukin-1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation, 2004; 109-2186-2190 [DOI] [PubMed] [Google Scholar]
- 32). Sabatine MS, Morrow DA, Higgins LJ, MacGillivray C, Guo W, Bode C, Rifai N, Cannon CP, Gerszten RE, Lee RT. Complementary roles for biomarkers of biomechanical strain ST2 and N-terminal prohormone B-type natriuretic peptide in patients with ST-elevation myocardial infarction. Circulation, 2008; 117: 1936-1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Chan W., Stub D., Clark D.J., Ajani A.E., Andrianopoulos N., Brennan A.L., New G, Black A, Shaw JA, Reid CM, Dart AM, Duffy SJ, Melbourne Interventional Group Investigators Usefulness of transient and persistent no reflow to predict adverse clinical outcomes following percutaneous coronary intervention. Am J Cardiol, 2012; 109: 478-485 [DOI] [PubMed] [Google Scholar]
- 34). Mirbolouk F, Gholipour M, Salari A, Shakiba M, Kheyrkhah J, Nikseresht V, Sotoudeh N, Moghadam N, Mirbolouk MJ, Moayeri Far M. CHA2DS2-VASc Score Predict No-Reflow Phenomenon in Primary Percutaneous Coronary Intervention in Primary Percutaneous Coronary Intervention. J Cardiovasc Thorac Res, 2018; 10: 46-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Balta S, Celik T, Ozturk C, Kaya MG, Aparci M, Yildirim AO, Demir M, Kilic S, Aydin İ, Iyisoy A. The relation between monocyte to HDL ratio and no reflow phenomenon inthe patients with acute Stsegmentelevation myocardial infarction. Am J Emerg Med, 2016; 34: 1542-1547 [DOI] [PubMed] [Google Scholar]
- 36). Gibson C.M., Dotani M.I., Murphy S.A., Marble S.J., Dauterman K.W., Michaels A.D., Dodge JT, Jr, RESTORE Investigators Correlates of coronary blood flow before and after percutaneous coronary intervention and their relationship to angiographic and clinical outcomes in the RESTORE trial. Randomized Efficacy Study of Tirofiban for Outcomes and REstenosis. Am Heart J, 2012; 144: 130-135 [DOI] [PubMed] [Google Scholar]
- 37). Henriques J.P., Zijlstra F., Ottervanger J.P., de Boer M.J., van 't Hof A.W., Hoorntje J.C., Suryapranata H. Incidence and clinical significance of distal embolization during primary angioplasty for acute myocardial infarction. Eur Heart J, 2002; 23: 1112-1117 [DOI] [PubMed] [Google Scholar]
- 38). Harrison R.W., Aggarwal A., Ou F.S., Klein L.W., Rumsfeld J.S., Roe M.T., Wang TY, American College of Cardiology National Cardiovascular Data Registry Incidence and outcomes of no-reflow phenomenon during percutaneous coronary intervention among patients with acute myocardial infarction. Am J Cardiol, 2013; 111: 178-184 [DOI] [PubMed] [Google Scholar]
- 39). Schwartz B.G., Kloner R.A. Coronary no reflow. J Mol Cell Cardiol, 2012; 52: 873-882 [DOI] [PubMed] [Google Scholar]


