Abstract
Aim: Motivated by the paradoxical and differing results of the early atherosclerosis related indices – Cardio-Ankle Vascular Index (CAVI) reflecting arterial stiffness and Reactive Hyperemia Index (RHI) evaluating endothelium dependent flow-induced vasodilation – in obesity, we aimed to assess CAVI and RHI in obese adolescents and young adults in the context of differences in systemic vascular resistance (SVR).
Methods: We examined 29 obese (14f, 15.4 [12.3–18.5] y; BMI: 33.2 ± 4.4 kg.m−2) and 29 non-obese gender and age matched adolescents and young adults (BMI: 21.02 ± 2.3 kg.m−2). CAVI and RHI were measured using VaSera VS-1500 (Fukuda Denshi, Japan) and Endo-PAT 2000 (Itamar Medical, Israel), respectively. Hemodynamic measures were recorded using volume-clamp plethysmography (Finometer Pro, FMS, Netherlands) and impedance cardiography (CardioScreen 2000, Medis GmbH, Germany). SVR and sympathetic activity related indices – Velocity Index (VI) and Heather Index (HI), and LFSAP (spectral power in low frequency band of systolic blood pressure oscillations) were determined.
Results: In obese group, CAVI (4.59 ± 0.88 vs. 5.18 ± 0.63, p = 0.002) and its refined version CAVI0 (6.46 ± 1.39 vs. 7.33 ± 0.99, p = 0.002) were significantly lower. No significant difference in RHI was found. SVR and sympathetic activity indices were all significantly lower in the obese group than in the non-obese group. RHI correlated positively with SVR (r = 0.390, p = 0.044) in obese subjects.
Conclusion: Our results indicate that both indices used for the detection of early atherosclerotic changes are influenced by vascular tone. Vascular resistance could influence CAVI and RHI results impairing their interpretation.
Keywords: Adolescent obesity, Atherosclerosis, Cardio-ankle vascular index, Reactive hyperemia index, Systemic vascular resistance, Sympathetic activity
Introduction
Cardiovascular diseases are associated with a high mortality rate and form a major socio-economic problem. In many of these pathological states, the key role is played by the process of atherosclerosis (ATS). Since the incidence of risk factors for the development of ATS – including diabetes mellitus, hypertension, dyslipidemia, obesity – is increasing worldwide not only in adult population but also in children and adolescents1, 2), the detection of initial and still reversible stages of ATS process is highly relevant3).
The measurement of arterial stiffness is the most common non-invasive examination method for the detection of ATS related changes. Arterial stiffness is estimated by pulse wave velocity (PWV), where higher PWV corresponds to lower vessel distensibility and compliance and, therefore, to higher arterial stiffness4). The dependence of PWV on the current blood pressure value considered as a major disadvantage of this method was minimized by the introduction of the cardio-ankle vascular index – CAVI5). Applying this methodology, increased values of CAVI have been observed in patients with diseases associated with ATS (ischemic heart disease, stroke) as well as in patients at an increased risk of ATS development (hypertension, diabetes mellitus, dyslipidemia)6). With an increasing number of studies focused on CAVI, the influence of an individual's body mass index (BMI) on CAVI was evidenced. Paradoxically, most studies show a negative correlation between CAVI and BMI in children and adolescents7, 8) as well as in middle-aged healthy adults9–11) – i.e., lower values of CAVI associated with obesity.
Early ATS related changes involve impairment in vascular endothelial function as an important initial step in the atherosclerotic process12). The method of reactive hyperemia peripheral arterial tonometry (RH-PAT) enables automatic and noninvasive quantification – using a derived index RHI (reactive hyperemia index) – of flow-induced arterial dilation mediated by endothelial cells function. The results of studies designed to assess RHI in relation to obesity status in children and adolescents are still scarce and inconsistent, with results varying from no significant influence13) to significantly decreased values14).
Both indices quantifying changes potentially related to atherosclerotic process are also influenced by other factors, including sympathetic activity influencing vascular tone and age, potentially complicating the results interpretation15, 16).
We hypothesize that paradoxical results observed in previous studies assessing noninvasively early atherosclerotic changes related to obesity could be – at least partially – explained by differences in vascular tone resulting from the altered sympathetic activity in obese patients. Therefore, the aim of our study was to compare CAVI and RHI values in young obese subjects with a control group of normal weight subjects. The changes were interpreted in the context of alterations in vascular resistance as an effect of changed sympathetic activity to further elucidate the possible mechanisms in observed differences.
Methods
Subjects
A total of 58 Caucasian participants, aged 12–23 years, were enrolled in this study. They were divided into two groups based on their body mass index (BMI) and age according to the Cole's chart17). The obese group involved 29 participants (14 female, 15 male) aged 15.4 [12.3–18.5] (median [interquartile range]) years (range: 12.4–22.7 years). The control group consisted of 29 age- and gender-matched subjects (median age 15.8 [interquartile range: 12.7–18.9] years, range: 12.5–22.1 years). Detailed study groups characteristics are presented in Table 1.
Table 1. Characteristics of study population.
| VARIABLE | CONTROLS | OBESE | p value |
|---|---|---|---|
| Age (years) | 16.52 (2.6) | 16.44 (2.7) | 0.898 |
| Height (cm) | 170.08 (12.2) | 170.50 (8.7) | 0.881 |
| Weight (kg) | 61.34 (12.1) | 96.71 (15.1) | < 0.001 |
| BMI (kg/m2) | 21.02 (2.3) | 33.2 (4.4) | < 0.001 |
| Fat mass (%) | 18.67 (7.2) | 38.70 (7.3) | < 0.001 |
| Skeletal Muscle Mass (kg) | 27.81 (6.9) | 33.31 (7.0) | 0.004 |
| Waist circumference (cm) | 72.29 (7.0) | 99.07 (11.8) | < 0.001 |
| Hip circumference (cm) | 94.61 (7.7) | 117.55 (7.4) | < 0.001 |
| WHR (-) | 0.76 (0.05) | 0.84 (0.09) | < 0.001 |
| VFA (cm2) | 32.51 (20.0) | 130.0 (38.4) | < 0.001 |
| BSA (m2) | 1.709 (0.22) | 2.077 (0.19) | < 0.001 |
| office SBP (mmHg) | 115 (14) | 117 (19) | 0.232 |
| office DBP (mmHg) | 75.2 (10.3) | 80.6 (12.5) | 0.025 |
Values are expressed as mean (SD). BMI – Body Mass Index, WHR – Waist to Hip Ratio; VFA – Visceral Fat Area; BSA – Body Surface Area (Du Bois formula); office SBP, office DBP – systolic and diastolic blood pressure (means of the 2nd and 3rd sphygmomanometric office blood pressure measurements);
Italic = Mann-Whitney U test; non-italic = t-test (based on Shapiro-Wilk normality test)
All subjects were instructed not to use substances influencing autonomic nervous system activity or the cardiovascular system (caffeine, alcohol, energetic beverages) and were asked to refrain from smoking for 12 hours before examination. All measurements were performed in quiet thermo-neutral environment (22–25°C) in the morning hours (8 AM–11 AM).
We excluded subjects with any current infectious disease (including 3 weeks of post-infection reconvalescence period), cardiovascular disease including hypertension (diagnosed using 24-hours of ambulatory blood pressure monitoring following examination), diabetes mellitus, psychiatric disorders, and hypothyroidism. All female subjects were examined during the proliferative phase (5th–12th day) of their menstrual cycle.
All subjects or their legal representatives (in participants under 18 years of age) gave written informed consent prior to examination. The study was approved by the Ethics Committee of Jessenius Faculty of Medicine, Comenius University. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Procedures and Measurements
Clinical and Anthropometric Data
A detailed medical history was obtained from each participant and an experienced physician checked current health status to exclude subjects meeting exclusion criteria. Office blood pressure was measured three times during the initial interview in a sitting position using the auscultatory method.
Anthropometric measures were taken immediately after arrival (before light breakfast) using an InBody J10 device (InBody, South Korea). A body composition analyzer uses segmental multi-frequency bioelectrical impedance analysis method (MF-BIA) and provides a detailed analysis of body composition (height, weight, BMI, fat mass, skeletal muscle mass, percentage body fat, visceral fat area etc.). This method was validated against dual X-ray absorptiometry and was recommended as an acceptable surrogate method for the estimation of total body composition in research studies18). Waist and hips circumferences were measured using measuring tape and WHR (waist-to-hip ratio) was calculated. Body surface area (BSA) was calculated using Du Bois formula19).
CAVI Measurement
Arterial stiffness parameter CAVI (Cardio-Ankle Vascular Index) was measured using VaSera VS-1500 (Fukuda Denshi, Japan). A detailed description of the method implemented in this device can be find elsewhere6). Briefly, CAVI determination is based on the measurement of PWV and systolic and diastolic blood pressures. A procedure requires placement of the pressure cuffs on all four extremities (arms and ankles), the positioning of the phonocardiographic microphone over the sternal angle at the second intercostal space, and ECG lead. PWV is obtained by dividing the vascular length (estimated from the subject's height) by time of the pulse wave propagation from the aortic valve to the ankle. Systolic and diastolic blood pressures are measured oscilometrically using pressure cuffs. CAVI is automatically calculated as follows:
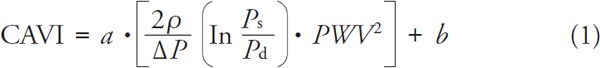 |
where: Ps–systolic blood pressure, Pd–diastolic blood pressure, ΔP = Ps–Pd, PWV–heart-to-ankle pulse wave velocity, ρ –blood density, a, b–constants.
Subjects were placed in a supine position for at least 10 minutes prior to CAVI measurement. All measurements and calculations were performed automatically. The mean of the right and left CAVI values was used for the analysis.
Recently, Spronck20) challenged the independence of CAVI of actual blood pressure and proposed improved parameter CAVI0, which does not show the residual BP dependence. CAVI0 was calculated from CAVI values (as reported by the VaSera device) by the following equation21):
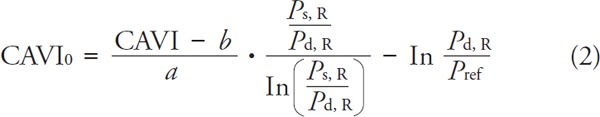 |
where: CAVI–values measured by VaSera device Ps, R–right brachial systolic blood pressure Pd, R–right brachial diastolic blood pressure, Pref–reference blood pressure (Pref = 100 mmHg), a, b–constants
RHI Measurement
Endothelial function was assessed by Reactive Hyperemia Peripheral Arterial Tonometry (RH-PAT) using Endo-PAT 2000 device (Itamar Medical, Israel). Using this method, it is possible to evaluate flow-induced vasodilation after a provocation of reactive hyperemia elicited by the rapid release of brachial artery occlusion lasting for 5 min. The reactive hyperemia index (RHI) was defined as the ratio of the postdeflation pulse amplitude to the baseline pulse amplitude22). Subjects were placed in a supine position for at least 5 minutes prior to examination and asked to minimize their movements during the examination.
Sympathetic Activity Assessment
Several measures reflecting different aspects of sympathetic cardiovascular control were measured noninvasively in a supine position in examined subjects. To derive cardiac inotropy related measures and peripheral (systemic) vascular resistance, impedance cardiography signal and reconstructed brachial arterial blood pressure from the finger arterial blood pressure were used. Cardiovascular parameters were recorded for 15 min after a 10 min rest period to achieve quasistationary condition. Subjects were asked to avoid movements or speaking during recording.
Impedance cardiography (ICG) enabling continuous beat-to-beat non-invasive monitoring of several indices characterizing myocardial performance and hemodynamics was performed using CardioScreen 2000 (Medis, Germany) device. This method calculates the changes in blood volume in the transthoracic region over time in terms of the changes in the transthoracic impedance and estimates the cardiovascular measures, including stroke volume (SV). In addition, several indices describing the cardiac ejection characteristics related to cardiac contractility (predominantly under sympathetic control)–Velocity Index (VI), Heather Index (HI)–were calculated from ICG recording. The continuous finger arterial blood pressure was measured simultaneously using the photoplethysmographic volume-clamp method (Finometer Pro, FMS, Netherlands) with the subsequent brachial arterial pressure reconstruction to estimate systemic mean blood pressure (MBP). Systemic vascular resistance (SVR) and systemic vascular resistance index (SVRI), as measures of overall vasoconstriction/vasodilation, were calculated as follows: SVR = 80*(median MBP/median CO); SVRI = 80*(median MBP/median CI); CO–cardiac output and CI–cardiac index (cardiac output divided by BSA) from ICG. We considered central venous pressure as negligible. Finally, we calculated power in low frequency band of spontaneous systolic blood pressure oscillations (LFSAP, power in 0.04–0.15 Hz) by spectral analysis as another sympathetic activity related index.
Statistical Analysis
The normality of the data distribution was assessed using the Shapiro-Wilk test. Variables are presented as mean ± SD or median [interquartile range]. To analyze the differences between obese and control groups, Student's t-test was used for the data with normal distribution and the Mann-Whitney U-test was used if the data were not normally distributed. The associations between parameters were analyzed using Pearson's (normal distribution) or Spearman (non-normal distribution) correlation coefficients. A p-value < 0.05 was considered as statistically significant. The statistical analysis was performed using SPSS software version 25 (IBM Corporation, New York, USA).
Results
Between Groups Comparison
Study groups characteristics are presented in Table 1. All anthropometric measures except body height were significantly higher in the obese group. Diastolic, but not systolic, office blood pressure was significantly higher in the obese group.
As expected, significantly lower CAVI values were found in obese group compared to controls (4.59 ± 0.88 vs. 5.18 ± 0.63, p = 0.005, Fig. 1 left panel). Similarly, CAVI0 was significantly lower in obese subjects (6.46 ± 1.39 vs. 7.33 ± 0.99, p = 0.011, Fig. 1 middle panel). Since CAVI and CAVI0 exhibit strong correlation in both groups (control: Pearson's r = 0.914, p < 0.001, obese: Spearman's ρ = 0.937, p < 0.001), only CAVI0 was used for the further analysis considering its methodological advantages. We found no significant difference in RHI between groups (1.387 ± 0.37 in controls and 1.453 ± 0.28 in obese, p = 0.441, Fig. 1 right panel).
Fig. 1.
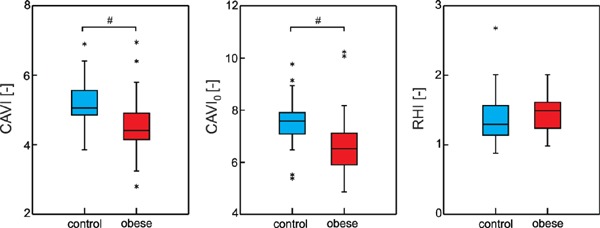
Box plots of CAVI – Cardio-Ankle Vascular Index; CAVI0 – refined Cardio-Ankle Vascular Index; RHI – Reactive Hyperemia Index; asterisks correspond to outliers; # denotes significant between-groups difference
Systemic vascular resistance was significantly lower in the obese group when expressed in absolute units (SVR: 843.89 ± 143.5 vs. 1094.29 ± 218.4 dyn·s·cm−5, p < 0.001) (Fig. 2). Other sympathetic activity related indices (Fig. 3)–measures related to cardiac contractility–VI and HI were also significantly lower in the obese group compared to control group (VI: 60.99 ± 17.5 for obese group vs. 88.77 ± 16.4 1/1000/s for control group, p < 0.001; HI: 15.83 ± 5.1 for obese group vs. 21.30 ± 6.6 Ω/s2 for control group, p = 0.001). Accordingly, spectral power in low frequency band of spontaneous systolic blood pressure oscillations LFSAP reflecting vascular sympathetic control was lower in the obese group (2.87 ± 1.7 vs. 4.78 ± 2.1 mmHg2 for obese and control groups, respectively, p < 0.001).
Fig. 2.
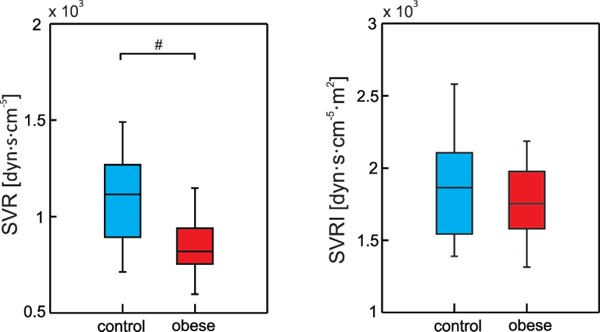
Box plots of SVR – Systemic Vascular Resistance; SVRI – Systemic Vascular Resistance Index; # denotes significant between-groups difference
Fig. 3.
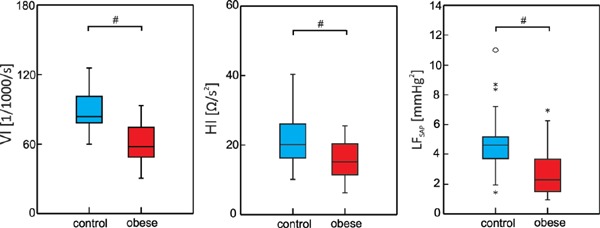
Box plots of VI – Velocity Index; HI – Heather Index; LFSAP – power in low frequency band of spontaneous systolic blood pressure oscillations; circles and asterisks correspond to outliers; # denotes significant between-groups difference
Correlations of CAVI0 and RHI with anthropometric and sympathetic activity related variables
Several anthropometric and hemodynamic measures were correlated with age in non-obese controls (results not shown). To reveal a relation of CAVI0 and RHI to anthropometric and hemodynamic variables in obese subjects, we performed a correlation analysis inside this group. To minimize the effect of age, the correlation analysis was adjusted for age as a potentially confounding variable.
No significant correlation between CAVI0 index and any anthropometric or sympathetic activity related variable was found in the obese group (Table 2). RHI correlated positively with systemic vascular resistance (RHI with SVR: Pearson's r = 0.390, p = 0.044, RHI with SVRI: Pearson's r = 0.456, p = 0.017) (Table 2).
Table 2. CAVI0 and RHI correlations with anthropometric and sympathetic activity related variables in obese group adjusted for age.
| CAVI0 vs. |
RHI vs. |
|||
|---|---|---|---|---|
| Correlation coefficient | p | Correlation coefficient | p | |
| Height (cm) | 0.368 | 0.070 | 0.355 | 0.064 |
| Weight (kg) | 0.115 | 0.584 | 0.077 | 0.698 |
| BMI (kg/m2) | −0.031 | 0.884 | −0.245 | 0.209 |
| Fat mass (%) | −0.197 | 0.345 | −0.279 | 0.150 |
| Skeletal Muscle Mass (kg) | 0.185 | 0.377 | 0.269 | 0.166 |
| WHR (-) | −0.170 | 0.417 | 0.080 | 0.685 |
| VFA (cm2) | −0.045 | 0.830 | −0.143 | 0.468 |
| BSA (m2) | 0.189 | 0.367 | 0.201 | 0.305 |
| SVR (dyn·s·cm−5) | 0.037 | 0.865 | 0.390 | 0.044 |
| SVRI (dyn·s·cm−5·m2) | 0.051 | 0.813 | 0.456 | 0.017 |
| VI (1/1000/s) | −0.050 | 0.817 | −0.290 | 0.143 |
| HI (Ω/s2) | −0.107 | 0.619 | −0.217 | 0.277 |
| LFSAP (mmHg2) | −0.029 | 0.892 | 0.151 | 0.442 |
BMI – Body Mass Index, WHR – Waist to Hip Ratio; VFA – Visceral Fat Area; BSA – Body Surface Area (Du Bois formula); SVR – Systemic Vascular Resistance, SVRI – Systemic Vascular Resistance Index, VI – Velocity Index, HI – Heather Index, LFSAP – power in low frequency band of spontaneous systolic blood pressure oscillations Italic = Spearman correlation; non-italic =Pearson correlation (based on Shapiro-Wilk normality test)
Discussion
The major findings of our study include: i) significantly lower values of arterial stiffness index CAVI in obese adolescents, together with no significant differences in RHI; ii) lower systemic vascular resistance in obesity associated with a decreased cardiovascular sympathetic activity; and iii) significant correlation of the endothelial function index RHI with systemic vascular resistance in the obese group.
Our study was focused on novel indices quantifying changes potentially related to atherosclerotic process. Two indices – CAVI, quantifying arterial stiffness and RHI, reflecting endothelial function – were measured in obese adolescents in relation to anthropometric, hemodynamic and sympathetic activity related indices with the aim to elucidate the factors possibly influencing their values in this group of participants associated with higher risk of atherosclerosis development in future. To exclude the confounding effect of pathological states potentially associated with obesity, including diabetes mellitus, dyslipidemia, hypertension, etc., our study group comprised otherwise healthy adolescents and young adults.
CAVI
In accordance with paradoxical results of previous studies, we found significantly lower values of CAVI and CAVI0 in obese adolescents. The association of CAVI with BMI and adiposity measures has already been described in previous studies. Nagayama9) and Tabara11) found the negative correlation between BMI and CAVI in metabolically healthy Japanese middle-aged adults. Similar results were found in the study of Gomez-Sanchez10), where the authors demonstrated the inverse relationship between CAVI and various adiposity measures (BMI, waist-to-height ratio, body fat percentage, body roundness) in a Caucasian population. All these studies were performed on an adult population. Studies on young subjects are rare and generally include a lower number of subjects. To the best of our knowledge, there are only two studies focused on CAVI in obese children and adolescents and both of them confirmed lower CAVI values in obese participants7, 8).
A negative association between BMI and CAVI seems discrepant since obesity is considered as one of the risk factors of atherosclerosis. Lower CAVI values in obese metabolically healthy people may be explained by alterations in hemodynamics associated with obesity. Increased BMI (adipose tissue + lean tissue) in obese subjects leads to an increase in blood volume, which in turn predisposes to an increase in cardiac output (CO). In normotensive obese patients, this high-output state is counterbalanced by decreased systemic vascular resistance23–25). Increased blood vessels diameter associated with decreased SVR results in a decreased PWV (based on Moens-Korteweg equation: 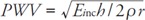 , where Einc–incremental Young's modulus, h – vessel wall thickness, r–vessel radius, ρ–blood density) and hence decreased CAVI values. Importantly, our results confirmed this concept through the simultaneous measurement of SVR and CAVI in one study sample–both lower SVR and CAVI were associated with obesity in young subjects.
, where Einc–incremental Young's modulus, h – vessel wall thickness, r–vessel radius, ρ–blood density) and hence decreased CAVI values. Importantly, our results confirmed this concept through the simultaneous measurement of SVR and CAVI in one study sample–both lower SVR and CAVI were associated with obesity in young subjects.
CAVI reflects the elastic properties of arterial walls from the aortic arch to the distal arteries of the lower extremities. It is a long arterial pathway comprised of large elastic arteries and smaller peripheral resistance arteries. The diameter of the latter is under the control of sympathetic part of autonomic nervous system (ANS). Thus, a shift in sympatho-vagal balance may have an effect on values of CAVI. Zwain16) and Maliha26) found a positive correlation between CAVI and the head-up tilt (HUT) angle and showed that increased sympathetic activity may increase CAVI values. The studies on CAVI in hypertensive individuals, where increased sympathetic activity results in increased SVR27), speak also in favor of the concept suggesting a significant influence of sympathetic activity on CAVI. Mešťaník8) found an increased CAVI in a group of obese hypertensive adolescents compared to obese normotensives. CAVI of obese hypertensives was not significantly different from the CAVI values of non-obese normotensive adolescents, suggesting increased vasoconstriction in obese hypertensive patients overriding decreased SVR in obesity.
Taken together, CAVI reportedly reflects not only the structural changes in the vessel wall (related to the ATS process), but also the functional stiffness – arterial vasomotor tone6, 28, 29). Our results confirmed this concept – a lower SVR in obese group was associated with a paradoxically decreased CAVI. Lower cardiovascular sympathetic activity indices based on cardiac contractility (VI and HI) as well as reflecting vascular sympathetic control (LFSAP) found in our group of obese adolescents indicate a decreased sympathetic activity as a potential mechanism of decreased SVR in this group.
In the obese patients subgroups where functional (e.g., increased sympathetic activity in hypertensives) and/or structural (e.g., accelerated atherosclerotic process in metabolic syndrome patients) changes override the effect of decreased SVR on CAVI, increased values of arterial stiffness were found. The involvement of different factors in CAVI makes its interpretation as an index of atherosclerosis less straightforward and the results in different studies can substantially differ; e.g., in patients with metabolic syndrome (MetS) where abdominal obesity is an important component, Gomez-Sanchez30) and Topouchian31) found a negative correlation between waist circumference and CAVI. In contrast, Liu32) observed significantly higher values of CAVI in the group with markedly increased waist circumference simultaneously with a negative correlation between BMI and CAVI. Moreover, the majority of MetS studies assert a positive correlation between the number of MetS components and CAVI with the strongest impact of elevated blood pressure and high fasting blood glucose level32, 33). The lack of correlation between CAVI and anthropometric or sympathetic activity related measures in obese patients in our study could be also explained by the individual heterogeneity of functional and structural changes in arterial tree.
RHI
Studies on obese adults demonstrated impaired vascular function assessed by RH-PAT method in obese group expressed by decreased RHI values34, 35) and a significant increase in RHI after lifestyle changes associated with weight loss36, 37). However, the results of studies on children and adolescents showed discrepant results. While several studies14, 38, 39) found significantly lower values of RHI in obese adolescents and an inverse relation of RHI to percentage of body fat40), other studies found no difference in endothelial function between obese and lean participants13, 41, 42).
In our study we found no difference in RHI between obese subjects and lean controls suggesting similar endothelial function in both groups. This finding could indicate no harmful effect of obesity in young age on endothelial function. Notably, being based on the arterial diameter increase associated with a restoration of blood flow through vessels, the results of RH-PAT method are also influenced by the level of initial vasoconstriction/vasodilation and thus the activity of sympathetic part of the ANS directed to vessels. Goswami43) demonstrated a significantly increased RHI in orthostasis (usually accompanied by vasoconstriction) compared to supine position in the same subjects. In our sample of obese adolescents, we found a positive correlation between RHI and SVR either expressed in absolute values (SVR) or considering the body size (SVRI). It indicates that in obese subjects with lower initial level of vasoconstriction (subjects with relative vasodilation), the RHI was lower. We suggest that starting from initial vasodilation the response to reperfusion could not be so large compared to the subjects/states where initially more prominent vasoconstriction occurred (vasodilation has its natural mechanical limits). On average, since the initial vasodilation was found in obese subjects (lower SVR), nonsignificant difference between groups indicates a well-preserved endothelial function in obese adolescents.
The mechanisms of the decreased vascular tone in obesity are currently not well known. The lack of information on this issue is determined by the difficulties associated with the noninvasive measurement of the vascular resistance. It is considered that changing the concentration of some biochemical substances (e.g., adipocytokines, inflammatory cytokines, etc.) could influence the vascular tone directly by their effects on vascular smooth muscles or indirectly through changed vasomotor centre activity. For example, it is considered that leptin as an important adipocytokine plays an important role in the initiation and progression of ATS44, 45). On the other hand, a recent study demonstrated potential vascular protective role of leptin in young healthy adults46). This paradoxical result could be at least partially attributed to the vasodilatory effect of the leptin demonstrated in rats and humans47). Nevertheless, a better understanding of the underlying mechanisms of vasodilation in obesity still require more detailed studies.
Clinical Implications
Our results indicate that both indices (RHI and CAVI) used for the detection of early atherosclerotic changes are also influenced by vascular tone. The level of vasoconstriction or vasodilation influences the capacity to perform further vasodilation after reperfusion during RH-PAT method protocol and limits the RHI measurement. Regarding CAVI method, vascular tone directly influences PWV – a crucial parameter in arterial stiffness quantification by CAVI. We stress that potential changes in vascular resistance associated with pathological and physiological conditions (e.g., arterial hypertension, obesity) could influence the detection of atherosclerotic changes employing RHI and CAVI examination. In studies focused on the assessment of CAVI and RHI, potential vascular resistance changes in relation to time (e.g., during treatment or disease development) and differing between groups (e.g., obese vs. lean subjects) should be considered.
Study Limitations
The lack of information on arterial/aortal diameter is an important limitation of our study. Based on Moens–Korteweg equation, PWV is indirectly related to arterial diameter. CAVI increases with an increase in PWV and thus an increased arterial diameter could result in a decreased CAVI. Taken together, one of the possible explanations of the decreased CAVI in the obese group could include an effect of the larger arterial diameter in this group.
Several previous studies investigated relations between aortic diameter or cross-sectional area and age, gender and basic anthropometric measures. It was shown that age and height and to a lower extent body weight or BMI are correlated with aortic diameter measured by ultrasonography or computed tomography in a wide age range from children to elderly adults48–52) The gender effect with the larger diameter in male subjects was also demonstrated49, 50). The selection of our groups of subjects (age and gender matching) was focused to avoid the effect of these potentially confounding factors. Since there were no significant between group differences in the body height, the observed differences in CAVI between obese and control groups in our study could not be attributed to the height effect. However, we cannot exclude the effect of body weight on the arterial diameter and hence CAVI for between groups differences. Several facts indicate that this effect is probably less important than the effect of the lower vascular tone in obesity. Firstly, the effect of body weight independent of body height (e.g., as included in the BMI) was found in morphological studies to be only minor compared to age or height effect50–52). Secondly, the correlation of CAVI0 index with weight in healthy controls in our study adjusted for age (ρ = −0.155, p = 0.430) or age and height together (ρ = −0.077, p = 0.702) was not significant. Thirdly, in previous studies the presence of obesity in adults resulted in an increase in arterial diameter ranging from 0 to 9 % depending on the measuring site53, 54). Deriving from the biophysical theory, it could theoretically lead to ≤ 9 % reduction in CAVI in obese group compared to controls. Since we found more than 15% difference in median CAVI0 values between groups, we assume that other factors – most importantly vascular resistance – contribute to the observed findings.
On the other hand, while RHI is calculated from the change in arterial diameter after occlusion (and not from the absolute value of arterial diameter), we assume that this index is less influenced by the body size effect.
Our study involved subjects in the period of adolescence, when pubertal development occurs. Considering the significant influence of pubertal stage on many physiological characteristics, including body composition and autonomic nervous system activity, another limitation of our study is that we lack the information on the stage of pubertal development (e.g., Tanner score).
In addition, the sample size was relatively small to generalize the results for the general population. Therefore, we could not exclude the existence of weaker associations and Type II statistical error (false negative conclusions from the statistical analysis). The observed differences and associations should be confirmed on a larger study sample.
Conclusion
We conclude that both CAVI and RHI are potentially influenced by vascular tone controlled by the sympathetic part of autonomic nervous system. Consequently, lower CAVI value observed in young obese subjects may not indicate the absence of atherosclerotic damage of the vessels. We suggest considering potential vascular resistance changes when interpreting CAVI and RHI data.
Acknowledgment
Research was supported by grants VEGA 1/0117/17, VEGA 1/0200/19, ITMS project “Center of Excellence for perinatological research II” no.26220120036 and ITMS project “BioMed Martin” no.26220220187
Conflict of Interest
All authors have no conflict of interest to declare.
References
- 1). Mendis S, Nordet P, Fernandez-Britto JE, Sternby N: Atherosclerosis in children and young adults: An overview of the World Health Organization and International Society and Federation of Cardiology study on Pathobiological Determinants of Atherosclerosis in Youth study (1985- 1995). Prev Control, 2005; 1: 3-15 [Google Scholar]
- 2). Umer A, Kelley GA, Cottrell LE, Giacobbi P, Innes KE, Lilly CL: Childhood obesity and adult cardiovascular disease risk factors: A systematic review with meta-analysis. BMC Public Health, 2017; 17:683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Nissen S E, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne ChM, et al. : Effect of Very High-Intensity Statin Therapy. J Am Med Assoc, 2006; 295:1556-1565 [DOI] [PubMed] [Google Scholar]
- 4). Pereira T, Correia C, Cardoso J: Novel methods for pulse wave velocity measurement. J Med Biol Eng, 2015; 35: 555-565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Shirai K, Utino J, Otsuka K, Takata M: A Novel Blood Pressure-independent Arterial Wall Stiffness Parameter; Cardio-Ankle Vascular Index (CAVI). J Atheroscler Thromb, 2006; 13: 101-107 [DOI] [PubMed] [Google Scholar]
- 6). Shirai K, Hiruta N, Song M, Kurosu T, Suzuki J, Tomaru T, et al. : Cardio-Ankle Vascular Index (CAVI) as a Novel Indicator of Arterial Stiffness: Theory, Evidence and Perspectives. J Atheroscler Thromb, 2011; 18: 924-938 [DOI] [PubMed] [Google Scholar]
- 7). Philip R, Alpert BS, Schwingshackl A, Huang X, Blakely D, Rovnaghi CR, et al. : Inverse Relationship between Cardio-Ankle Vascular Index and Body Mass Index in Healthy Children. J Pediatr, 2015; 167: 361-365 [DOI] [PubMed] [Google Scholar]
- 8). Mestanik M, Jurko A, Spronck B, Avolio AP, Butlin M, Jurko T, et al. : Improved assessment of arterial stiffness using corrected cardio-ankle vascular index (CAVI0) in overweight adolescents with white-coat and essential hypertension. Scand J Clin Lab Invest, 2017; 77: 665-672 [DOI] [PubMed] [Google Scholar]
- 9). Nagayama D, Imamura H, Sato Y, Yamaguchi T, Ban N, Kawana H, et al. : Inverse relationship of cardioankle vascular index with BMI in healthy Japanese subjects: A crosssectional study. Vasc Health Risk Manag, 2017; 13: 1-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Gomez-Sanchez L, Garcia-Ortiz L, Patino-Alonso MC, Recio-Rodriguez JI, Rigo F, Martí R, et al. : Adiposity measures and arterial stiffness in primary care: The MARK prospective observational study. BMJ Open, 2017; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Tabara Y, Setoh K, Kawaguchi T, Takahashi Y, Kosugi S, Nakayama T, et al. : Factors affecting longitudinal changes in cardio-ankle vascular index in a large general population: The Nagahama study. J Hypertens, 2018; 36: 1147-1153 [DOI] [PubMed] [Google Scholar]
- 12). Okumura K, Imamura A, Murakami R, Matsui H, Tokoaki M: Endothelial function and early atherosclerotic changes. Futur Cardiol, 2005; 1: 501-508 [DOI] [PubMed] [Google Scholar]
- 13). Mueller UM, Walther C, Adam J, Fikenzer K, Erbs S, Mende M, et al. : Endothelial Function in Children and Adolescents Is Mainly Influenced by Age, Sex and Physical Activity — An Analysis of Reactive Hyperemic Peripheral Artery Tonometry —. Circ J, 2017; 81: 717-725 [DOI] [PubMed] [Google Scholar]
- 14). Pareyn A, Allegaert K, Verhamme P, Vinckx J, Casteels K: Impaired endothelial function in adolescents with overweight or obesity measured by peripheral artery tonometry. Pediatr Diabetes, 2015; 16: 98-103 [DOI] [PubMed] [Google Scholar]
- 15). Sverrisdóttir YB, Jansson LM, Hägg U, Gan L-M: Muscle Sympathetic Nerve Activity Is Related to a Surrogate Marker of Endothelial Function in Healthy Individuals. PLoS One, 2010; 5: e9257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Zwain A, a MH, Al Esawi RW, Al-Dejeli A a. B: Cardiac index (CI) versus cardio ankle vascular index (CAVI) at different degrees of head-up tilt (HUT) in healthy subjects. Open J Mol Integr Physiol, 2013; 3: 71-79 [Google Scholar]
- 17). Cole TJ, Bellizzi MC, Flegal KM, Dietz WH: Establishing a standard definition for child overweight and obesity worldwide: international survey. Bmj, 2000; 320: 1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Karelis AD, Chamberland G, Aubertin-Leheudre M, Duval C: Validation of a portable bioelectrical impedance analyzer for the assessment of body composition. Appl Physiol Nutr Metab, 2013; 38: 27-32 [DOI] [PubMed] [Google Scholar]
- 19). Du Bois EF: Clinical calorimetry: Tenth paper a formula to estimate the approximate surface area if height and weight be known. Arch Intern Med, 1916; XVII(6_2): 863-871 [Google Scholar]
- 20). Spronck B, Avolio AP, Tan I, Butlin M, Reesink KD, Delhaas T: Arterial stiffness index beta and cardio-ankle vascular index inherently depend on blood pressure but can be readily corrected. J Hypertens, 2017; 35: 98-104 [DOI] [PubMed] [Google Scholar]
- 21). Spronck Bart, Mestanik M, Tonhajzerova I, Jurko A, Jurko T, Avolio AP, et al. : Direct means of obtaining CAVI0- A corrected cardio-ankle vascular stiffness index (CAVI) - From conventional CAVI measurements or their underlying variables. Physiol Meas, 2017; 38: N128-N137 [DOI] [PubMed] [Google Scholar]
- 22). Kuvin JT, Patel AR, Sliney K, Pandian NG, Sheffy J, Schnall RP, et al. : Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J, 2003; 146: 168-174 [DOI] [PubMed] [Google Scholar]
- 23). Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer F, et al. : Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on obesity and heart disease from the Obesity Committee of the Council on Nutrition, Physical. Circulation, 2006; 113: 898-918 [DOI] [PubMed] [Google Scholar]
- 24). Alpert MA, Lavie CJ, Agrawal H, Kumar A, Kumar SA: Cardiac effects of obesity: Pathophysiologic, clinical, and prognostic consequences-A review. J Cardiopulm Rehabil Prev, 2016; 36: 1-11 [DOI] [PubMed] [Google Scholar]
- 25). Santos C, Marques da Silva P: Hemodynamic patterns in obesity associated hypertension. BMC Obes, 2018; 5: 1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Maliha G, Townsend RR: A study of the VaSera arterial stiffness device in US patients. J Clin Hypertens, 2017; 19: 661-668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Grassi G: Sympathetic overdrive and cardiovascular risk in the metabolic syndrome. Hypertens Res, 2006; 29: 839-847 [DOI] [PubMed] [Google Scholar]
- 28). Takahashi M, Shiba T, Hirano K, Hitsumoto T, Shirai K: Acute Decrease of Cardio-Ankle Vascular Index with the Administration of Beraprost Sodium. J Atheroscler Thromb, 2012; 19: 479-484 [DOI] [PubMed] [Google Scholar]
- 29). Shimizu K, Yamamoto T, Takahashi M, Sato S, Noike H, Shirai K: Effect of nitroglycerin administration on cardio-ankle vascular index. Vasc Health Risk Manag, 2016; 12: 313-318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Gomez-Sanchez L, Garcia-Ortiz L, Patino-Alonso MC, Recio-Rodriguez JI, Fernando R, Marti R, et al. : Association of metabolic syndrome and its components with arterial stiffness in Caucasian subjects of the MARK study: A cross-sectional trial. Cardiovasc Diabetol, 2016; 15: 148: 1-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Topouchian J, Labat C, Gautier S, Bäck M, Achimastos A, Blacher J, et al. : Effects of metabolic syndrome on arterial function in different age groups: The Advanced Approach to Arterial Stiffness study. J Hypertens, 2018; 36: 824-833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Liu H, Zhang X, Feng X, Li J, Hu M, Yambe T: Effects of Metabolic Syndrome on Cardio-Ankle Vascular Index in Middle-Aged and Elderly Chinese. Metab Syndr Relat Disord, 2011; 9: 105-110 [DOI] [PubMed] [Google Scholar]
- 33). Nam S, Kang S, Lee Y, Song S, Rho J: Association of Metabolic Syndrome with the Cardioankle Vascular Index in Asymptomatic Korean Population. J Diabetes Res, 2015; 2015: 1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34). Gupta AK, Ravussin E, Johannsen DL, Stull AJ, Cefalu WT, Johnson WD: Endothelial Dysfunction: An Early Cardiovascular Risk Marker in Asymptomatic Obese Individuals with Prediabetes. Br J Med Med Res, 2012; 2: 413-423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Merino J, Megias-Rangil I, Ferré R, Plana N, Girona J, Rabasa A, et al. : Body weight loss by very-low-calorie diet program improves small artery reactive hyperemia in severely obese patients. Obes Surg, 2013; 23: 17-23 [DOI] [PubMed] [Google Scholar]
- 36). Ferré R, Plana N, Merino J, Aragonès G, Girona J, Heras M, et al. : Effects of therapeutic lifestyle changes on peripheral artery tonometry in patients with abdominal obesity. Nutr Metab Cardiovasc Dis, 2012; 22: 95-102 [DOI] [PubMed] [Google Scholar]
- 37). Kurose S, Tsutsumi H, Yamanaka Y, Shinno H, Miyauchi T, Tamanoi A, et al. : Improvement in endothelial function by lifestyle modification focused on exercise training is associated with insulin resistance in obese patients. Obes Res Clin Pract, 2014; 8: e106-e114 [DOI] [PubMed] [Google Scholar]
- 38). Agarwal C, Cohen HW, Muzumdar RH, Heptulla RA, Renukuntla VS, Crandall J: Obesity, hyperglycemia and endothelial function in inner city Bronx adolescents: a cross-sectional study. Int J Pediatr Endocrinol, 2013; 2013: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39). Mahmud FH, Hill DJ, Cuerden MS, Clarson CL: Impaired Vascular Function in Obese Adolescents with Insulin Resistance. J Pediatr, 2009; 155: 678-682 [DOI] [PubMed] [Google Scholar]
- 40). Tomsa A, Klinepeter Bartz S, Krishnamurthy R, Krishnamurthy R, Bacha F: Endothelial Function in Youth: A Biomarker Modulated by Adiposity-Related Insulin Resistance. J Pediatr, 2016; 178: 171-177 [DOI] [PubMed] [Google Scholar]
- 41). Tryggestad JB, Thompson DM, Copeland KC, Short KR: Obese children have higher arterial elasticity without a difference in endothelial function: The role of body composition. Obesity, 2012; 20: 165-171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42). Singh R, Verma A, Aljabari S, Vasylyeva TL: Urinary biomarkers as indicator of chronic inflammation and endothelial dysfunction in obese adolescents. BMC Obes, 2017; 4: 2-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43). Goswami N, Gorur P, Pilsl U, Anyaehie B, Green DA, Bondarenko AI, et al. : Effect of Orthostasis on Endothelial Function: A Gender Comparative Study. PLoS One, 2013; 8: 1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44). Dubey L, Hesong Z.: Role of leptin in atherogenesis. Exp Cardiol, 2006; 11: 269-275 [PMC free article] [PubMed] [Google Scholar]
- 45). Lu C-W, Lee C-J, Hou J-S, Wu D, Hsu B.: Positive correlation of serum leptin levels and peripheral arterial stiffness in patients with type 2 diabetes. Tzu Chi Med J, 2018; 30: 10-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46). Ahiante BO, Smith W, Lammertyn L, Schutte A.: Leptin and the vasculature in young adults: The African - PREDICT study. Eur J Clin Invest, 2019; 49: 1-10 [DOI] [PubMed] [Google Scholar]
- 47). Nakagawa K, Higashi Y, Sasaki S, Oshima T, Matsuura H, Chayama K.: Leptin Causes Vasodilation in Humans. Hypertens Res, 2002; 25: 161-165 [DOI] [PubMed] [Google Scholar]
- 48). Gautier M, Detaint D, Fermanian C, et al. : Nomograms for Aortic Root Diameters in Children Using Two-Dimensional Echocardiography. Am J Cardiol, 2010; 105: 888-894 [DOI] [PubMed] [Google Scholar]
- 49). Pearce WH, Slaughter MS, LeMaire S, et al. : Aortic diameter as a function of age, gender, and body surface area. Surgery, 1993; 114: 691-697 [PubMed] [Google Scholar]
- 50). Hannuksela M, Lundqvist S, Carlberg BO.: Thoracic aorta - dilated or not? Scand Cardiovasc J, 2006; 40: 175-178 [DOI] [PubMed] [Google Scholar]
- 51). Roman MJ, Devereux RB, Kramer-Fox R, O'Loughlin J.: Two-Dimensional Echocardiographic Aortic Root Dimensions in Normal Children and Adults. Am J Cardiol, 1989: s507-512 [DOI] [PubMed] [Google Scholar]
- 52). Liddington MI, Heather BP.: The Relationship Between Aortic Diameter and Body Habitus. Eur J Vasc Surg, 1992; 6: 89-92 [DOI] [PubMed] [Google Scholar]
- 53). Wildman RP, Mehta V, Thompson T, Sarah B, Sutton-Tyrrell K.: Obesity Is Associated With Larger Arterial Diameters in Caucasian and African-American Young Adults. Diabetes Care, 2004; 27: 2-4 [DOI] [PubMed] [Google Scholar]
- 54). Kappus RM, Fahs CA, Smith D, et al. : Obesity and Overweight Associated With Increased Carotid Diameter and Decreased Arterial Function in Young Otherwise Healthy Men. Am J Hypertens, 2014; 27: 628-634 [DOI] [PMC free article] [PubMed] [Google Scholar]


