Abstract
Ocean acidification (OA), from seawater uptake of anthropogenic CO2, has a suite of negative effects on the ability of marine invertebrates to produce and maintain their skeletons. Increased organism pCO2 causes hypercapnia, an energetically costly physiological stress. OA alters seawater carbonate chemistry, limiting the carbonate available to form the calcium carbonate (CaCO3) minerals used to build skeletons. The reduced saturation state of CaCO3 also causes corrosion of CaCO3 structures. Global change is also accelerating coastal acidification driven by land-run off (e.g. acid soil leachates, tannic acid). Building and maintaining marine biomaterials in the face of changing climate will depend on the balance between calcification and dissolution. Overall, in response to environmental acidification, many calcifiers produce less biomineral and so have smaller body size. Studies of skeleton development in echinoderms and molluscs across life stages show the stunting effect of OA. For corals, linear extension may be maintained, but at the expense of less dense biomineral. Conventional metrics used to quantify growth and calcification need to be augmented by characterisation of the changes to biomineral structure and mechanical integrity caused by environmental acidification. Scanning electron microscopy and microcomputed tomography of corals, tube worms and sea urchins exposed to experimental (laboratory) and natural (vents, coastal run off) acidification show a less dense biomineral with greater porosity and a larger void space. For bivalves, CaCO3 crystal deposition is more chaotic in response to both ocean and coastal acidification. Biomechanics tests reveal that these changes result in weaker, more fragile skeletons, compromising their vital protective roles. Vulnerabilities differ among taxa and depend on acidification level. Climate warming has the potential to ameliorate some of the negative effects of acidification but may also make matters worse. The integrative morphology-ecomechanics approach is key to understanding how marine biominerals will perform in the face of changing climate.
Keywords: Climate change, marine biominerals, corals, molluscs, sea urchins, serpulid worms
Introduction
Ocean acidification (OA) resulting from increased ocean uptake of CO2, driven by the increase in anthropogenic greenhouse gas emissions, is unprecedented on geological time scales (Zeebe et al., 2016). This uptake is causing major changes to ocean chemistry with a suite of negative effects on the ability of marine invertebrates to produce and maintain their skeletons. Firstly, increased organism pCO2 causes hypercapnia, a stress that can affect many physiological processes. Hypercapnia can be energetically costly reducing the resources that animals need to calcify because maintenance of essential metabolic processes (e.g. acid-base homeostasis) takes priority over diverting energy to growth and calcification (Stumpp et al., 2012; Pan et al., 2015). The production and maintenance of calcium carbonate (CaCO3) structures has been shown to be modulated by energy acquisition (Melzner et al., 2011). Secondly, OA reduces the saturation state of CaCO3 minerals, limiting the carbonate available for calcification (IPCC, 2014). These minerals are the building blocks of biocalcification and include aragonite and calcite. Thirdly, the direct corrosive effect of OA can cause pitting and erosion of CaCO3 structures, as reported for calcifiers that are resident in low pH CO2 seep environments (Harvey et al., 2018). Calcite is less susceptible to dissolution at lower pH values than aragonite, unless it contains high levels of magnesium (Ries et al., 2009; Chan et al., 2012). However, calcite is more brittle compared to aragonite, making it mechanically weaker (Fitzer et al., 2015a; Meng et al., 2018).
Due to sea-level rise and changing weather patterns, global change is exacerbating coastal acidification in areas where freshwater runoff results in reduced pH due to leachate from acid sulphate soils and humic acids and tannic acids from groundwater (Amaral et al., 2011, 2012; Duarte et al., 2013; Jiang et al., 2017; Fitzer et al., 2018). Environmental acidification in a changing ocean is being caused by both ocean and coastal acidification. These two forms of acidification (CO2 and land run off) differ in chemistry mechanisms (Fig. 1). Environmental acidification also occurs at CO2 seeps (Foo et al. 2018; González-Delago and Hernández, 2018).
Figure 1.
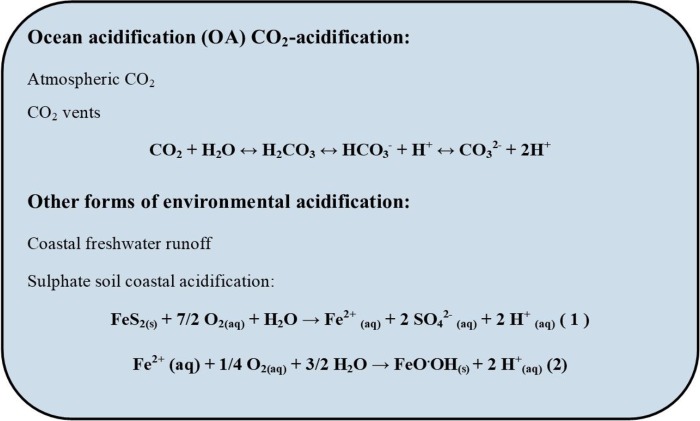
Equations for the mechanisms of acidification from CO2-OA and other forms of environmental acidification for example from acid sulphate soil leachates. Modified from Fitzer et al. (2018).
Marine invertebrate skeletons are complex and ancient structures (Wilkinson, 1979) produced through the superb biocontrol of mineral production, resulting in a great diversity of taxon-specific structures (Wilbur, 1972; Cavey and Markel, 1994; Von Euw et al., 2017). For a broad range of marine invertebrates (e.g. corals, molluscs, echinoderms) experimental acidification in laboratory studies and environmental acidification at natural CO2 seeps and in low pH coastal waters (Amaral et al., 2011, 2012; Duarte et al., 2013; Jiang et al., 2017; Fitzer et al., 2018) show the negative effect of acidification on biomineralisation. Faced with these challenges, building and maintaining calcified structures by marine invertebrates will depend on the balance between calcification and dissolution.
Calcified structures are expensive to produce (Comeau et al., 2017) and, in response to the physiological challenges presented by OA, many calcifiers produce smaller skeletons and so have smaller body sizes. This is seen in the stunting effect of OA at the larval and adult stage of molluscs and echinoderms (Parker et al., 2012, 2013; Byrne et al., 2013; Garilli et al., 2015; Dworjanyn and Byrne, 2018). Vulnerabilities with respect to the amount of biomineral produced, its mechanical properties and propensity for dissolution or etching in OA conditions differ greatly among taxa, even within the same phylum that have similar calcification mechanisms (Table 1). Most laboratory studies have involved translocation of animals that that have calcified (i.e. ‘grown up’) in control conditions to OA conditions for short- or long-term exposure, an approach that shows the effects of OA on established skeleton and the ability to make new skeleton. For insight into the inherent differences of the production of biomineral in normal ambient and acidification conditions, many studies have reared larvae and juveniles under OA (Parker et al., 2012, 2013; Byrne et al., 2013; Dworjanyn and Byrne, 2018) or have availed of naturally low pH habitats (e.g. CO2 seeps or low pH coastal waters) where biomineralisation has occurred through life (Crook et al., 2013; Garilli et al., 2015; Fitzer et al., 2018; Foo et al., 2018; González-Delago and Hernández, 2018; Migliaccio et al., 2019).
Table 1.
Studies that have investigated the impacts of environmental acidification on the microstrucure and/or mechanics of marine invertebrate skeletons and methods used. pH levels are listed as published on the total (pHT) or NBS (pHNBS) scales or no scale (pH*) if not indicated
| Phylum/ species |
Time | pH or Ω levels/location | Morph methods |
Morph results | Biomechanics methods | Biomechanics results | References |
|---|---|---|---|---|---|---|---|
| Cnidaria | |||||||
|
Balanophyllia
europaea |
Resident | pHT 7.7, 7.9, 8.1; Vent |
μCT, SEM | pHT 7.7, 21-31% ↑ in porosity & 7% ↓ in bulk density | Nanoindentation | pHT 7.7, hardness - no effect of pH; ↓ in stiffness |
Fantazzini et al., (2015) |
| Favia fragum | 8 d to juvenile** | Ω Ar 0.22-3.71; Lab | SEM | Ω Ar ≤ 1; change in crystal size, shape & orientation, thin septa | - | - | Cohen et al., (2009) |
| Porites astreoides | Resident | Ω Ar < 1-3.71; Coast | μCT | Ar < 2; ↓ density | - | - | Crook et al., (2013) |
| Stylophora pistillata | 12 mo | pHT 7.2, 7.4, 7.8, 8.0; Lab |
μCT | pHT 7.2-7.4, ↑ in porosity & ↓ in bulk density, thinner skeleton, no change in crystallography |
- | - | Tambutté et al., (2015) |
| Stylophora pistillata | 14 mo | pH NBS 7.3, 7.6, 8.2; Lab |
SEM, EBSD | pH NBS 7.3, 7.6, crystallographic changes, shorter less round fibre bundles |
- | - | Coronado et al., (2019) |
| Brachiopoda | |||||||
| Magellania venosa | 335 d | pH* 7.35-8.15 Lab, ASW |
SEM | pH* 7.35-7.6, ↑ pore size; ↑ thickness primary layer; ↓ thickness secondary layer, more organic rich shell | - | - | Ye et al., (2018) |
| Annelida | |||||||
| Hydroides elegans | 9-18 d** | pHNBS 7.4, 7.6, 7.8,7.9, 8.0; Lab |
μCT, SEM | pHNBS 7.4-7.8, ↑ porosity, irregular layers, surface pitting & erosion, thinner; change in mineral layers & crystallography | Nanoindentation, crushing test | pHNBS 7.4-7.8, ↓ hardness, elasticity & 62% ↓ in crushing force | Chan et al., (2012); Li et al., (2014; 2016) |
| Mollusca | |||||||
| Gastropoda | |||||||
| Austrocochlea constricta | 4 mo | pHNBS 7.8, 8.1; Lab |
- | - | Vickers hardness, Elastic modulus | pHNBS 7.8, ↑ shell hardness, ↑ stiffness | Leung et al., (2017) |
| Austrocochlea odontis | 4 mo | pHNBS 7.86, 8.0; Lab |
- | - | Vickers hardness, Elastic modulus | No effect of pH | Leung et al., (2017) |
| Austrocochlea porcata | 95 d | pHNBS 7.7, 7.9, 8.1; Lab |
- | No effect of pH | Crushing test | pHNBS 7.7, ↓ shell strength by 28% | Coleman et al., (2014) |
| Bulla quoyii | 4 mo | pHNBS 7.9, 8.1; Lab |
- | - | Vickers hardness, Elastic modulus | No effect of pH | Leung et al., (2017) |
| Charonia lampas | Resident | pHT 7.8, 8.1; Vent |
μCT | pHT 7.8, Two-fold ↓ in density and thickness | - | - | Harvey et al., (2018) |
| Columbella rustica | 3 mo | pHNBS 7.6, 8.1, Lab | μCT | pHNBS 7.6, 0.8-8% ↓ in density depending on shell region | - | - | Chatzinikolaou et al., (2017) |
| Littorina littorea | 5 mo (small and large) |
pHNBS 7.8, 8.0; Lab | - | - | Crushing test | pHNBS 7.8, Large snails, ↓ in crushing force, Small snails, no effect of pH | Landes and Zimmer, (2012) |
| Nassarius nitidus | 3 mo | pHNBS 7.6, 8.1, Lab | μCT | pHNBS 7.6, 38-51% ↓ in density depending on shell region | - | - | Chatzinikolaou et al., (2017) |
| Nerita atramentosa | 4 mo | pHNBS 7.8, 8.1; Lab |
- | - | Vickers hardness, Elastic modulus | No effect of pH | Leung et al., (2017) |
| Nucella lapillus | 14 mo (juveniles) |
pHT 7.8, 7.9, 8.0; Lab |
μCT, SEM |
pHT 7.8, 20-30% ↓ in density; surface erosion, lack of layering in shell, younger shells tinner, older shells thicker | - | - | Queirós et al., (2015); Rühl et al., (2017) |
| Nucella ostrina | 6 mo. | pHT ~ 7.6, 8.1; Lab |
- | - | Crushing test | 10% reduction in strength | Barclay et al., (2019) |
| Phasianella australis | 4 mo | pHNBS 7.9, 8.1; Lab |
- | - | Vickers hardness, Elastic modulus | No effect of pH | Leung et al., (2017) |
| Phorcus sauciatus | Resident | pHNBS 7.52-8.2 Vent |
- | Vent site – corrosion and cracks in shell | Crushing test | Weaker shells | Viotti et al., (2019) |
| Subninella undulata | 65 d | pHNBS 7.7, 7.9, 8.1; Lab |
- | pHNBS 7.7, ↓ shell thickness | Crushing test | No effect of pH | Coleman et al., (2014) |
| Tegula funebralis | 6 mo. | pHT 7.4-8.1; Lab |
- | - | Crushing test | 50% reduction in strength | Barclay et al., (2019) |
| Thalotia conica | 4 mo | pHNBS 7.9, 8.1; Lab |
- | - | Vickers hardness, Elastic modulus | No effect of pH | Leung et al., (2017) |
| Turbo undulatus | 4 mo | pHNBS 7.86, 8.0; Lab |
- | - | Vickers hardness, Elastic modulus | No effect of pH | Leung et al., (2017) |
| Bivalvia | |||||||
| Adamussium colbecki | 1 mo | pHT 7.6, 8.1; Lab |
SEM | No effect on crystals | Nanoindentation | No effect of pH | Dell’Acqua et al., (2019) |
| Arctica islandica | 3 mo | pHNBS7.7, 7.9, 8.0 | SEM | No effect on shape or size of crystals | - | - | Stemmer et al., (2013) |
| Cerastoderma edule | 2 mo | pHNBS, 6.4 6.7, 7.0, 7.4, 7.8, Lab |
SEM | pHNBS 6.4, 6.7 ↓ net calcification, no change in dissolution | Nanoindentation | No effect of pH | Milano et al., (2016) |
| Crassostrea virginica | 20 wk (juveniles) 2 wk (adults) |
pHNBS 7.5, 8.2, Lab |
FTIR SEM |
pHNBS 7.5 40% ↓ in shell mass in juveniles | Microindentation, fracture toughness | pHNBS 7.5 ↓ calcite hardness and fracture toughness in juveniles | Beniash et al., (2010) |
| Crassostrea virginica | 11 wk | pHNBS 7.97/8.1 8.11/8.36 Lab |
SEM | No difference in shell body mass | Microindentation, fracture toughness | pHNBS 7.9/8.1 ↓ hardness and fracture resistance at salinity | Dickinson et al., (2012) |
| Magallana angulata | 35 d early juveniles | pHNBS 7.8, 8.1, Lab | SEM, EBSD μCT, |
pHNBS 7.8 ↑ porosity of microstructure, ↓ density | Nanoindentation | pHNBS 7.8 ↓ hardness and stiffness | Meng et al., (2018) |
| Magallana gigas | 6 wk | pHNBS, 7.7, 8.1, Lab | - | - | Crushing test | pHNBS 7.7 ↓ crushing force | Wright et al., (2018) |
| Mytilus californianus | 8 d larva** | pHNBS 7.8, 8.0, 8.1, Lab | SEM | pHNBS 7.8 15% thinner | Crushing test | pHNBS 7.8 15-20% weaker pHNBS 8.0 13-15% weaker | Gaylord et al., (2011) |
| Mytilus edulis | 6 mo | pHNBS7.2, 7.3, 7.4, 7.5, 7.7, 8.1, 8.2, Lab | SEM, EBSD | pHNBS 7.2, 7.3, 7.4, 7.5. 7.7 disorganised crystals & altered layer structure | - | - | Fitzer et al., (2014b) |
| Mytilus edulis | 6 mo (juveniles) |
pHNBS7.2, 7.3, 7.4, 7.5, 7.7, 8.1, 8.2, Lab | SEM, EBSD | pHNBS7.2, 7.3, 7.4, 7.5, thinner calcite layer and altered layer structure and crystallography | - | - | Fitzer et al., (2014a) |
| Mytilus edulis | 6 mo | pHT 7.65, 8.0; Lab | - | - | Crushing test | pHT 7.65 ↓ flex before failure; Strength not affected | Mackenzie et al. (2014) |
| Mytilus edulis | 6 mo | pHNBS7.2, 7.3, 7.4, 7.5, 7.7, 8.1, 8.2, Lab | - | - | Microindentation fracture toughness, nanoindentation | pHNBS7.4, 7.5,7.7 ↑ calcite hardness and ↓ fracture toughness | Fitzer et al., (2014a) |
| Mytilus edulis | 2 mo | pHNBS 7.8, 8.1, Lab | SEM | Disordered crystals | Crushing test | pHNBS 7.8, ↓ crushing strength 22-24% | Li et al., (2015) |
| Mytilus edulis | 6 mo | pHNBS 7.4, 8.1, Lab | SEM-EBSD, XPEEM | pHNBS 7.2, altered crystallography structure and more ACC. | - | - | Fitzer et al., (2016) |
| Mytilus edulis | 9 months | pHNBS 7.2, 7.3, 7.4, 7.5, 7.7, 8.1, 8.2, 8.2, 8.1, Lab |
Shell shape analysis, shell thickness index (STI) | pHNBS 7.2, 7.3, 7.4, 7.5, 7.7, ↓ shell thickness and rounder flatter shells. | - | - | Fitzer et al., (2015b) |
| Mytilus edulis | 7 weeks | pHNBS7.2, 7.4, 7.7, 8.0, Lab |
SEM | pHNBS7.2, corrosion of internal aragonite layers | - | - | Melzner et al., (2011) |
| Mytilus edulis | 6 mo | pHT 7.65, 8.0; Lab | - | - | Crushing test | pHT 7.65 ↓ flex before failure; Strength not affected | Mackenzie et al. (2014) |
| Mytilus galloprovincialis | 68 d | pHT 7.25, 8.07; Vent transplant | SEM, EBSD | pHT 7.25, thinner shell, disturbed less ordered structure | - | - | Hahn et al., (2012) |
| Mytilus galloprovincialis | 21 d - 5 mo | pHT 6.8, 7.2, 7.8, Vent transplant to lab | SEM | Calcification continued in vents; Isolated shells, dissolution at low pH |
- | - | Rodolfo-Metalpa et al., (2011) |
| Pinctada fucata | 28 d | pHNBS7.6, 7.8, 8.1 |
SEM | Shell dissolution | Crushing test | pHNBS 7.6, 25.9% and 26.8% weaker shells | Welladsen et al., (2011) |
| Saccostrea glomerata | 2 yr | pHNBS 7.7, 8.1 Coast*** |
SEM-EBSD | pH 7.7, disordered crystallographic structure | - | - | Fitzer et al., (2018, Fitzer et al., 2019b) |
| Saccostrea glomerata | N/A | pHNBS 6.6, 6.8, 6.9, 7.8, 7.9 Coast*** |
- | - | Crushing test | pH pHNBS 6.6, 6.8, 6.9, Weaker shells | Amaral et al., (2012); Fitzer et al., (2019b) |
| Cephalopoda | |||||||
| Argonauta nodosa | N/A | pHT 7.35,7.55,7.8,8.0, Lab | SEM, EBSD | Shell only pHT 7.35-7.8, dissolution and etching, altered crystallography and structure |
- | - | Wolfe et al., (2012, 2013a) |
| Echinodermata | |||||||
| Diadema africanum | 100 d, juveniles | pHT 7.6, 8.0, Lab | SEM | pHT 7.6, Test plates thinner, spines, dissolution/etching | Crushing test (whole urchin dried) | pHT 7.6, ↓ crushing force | Rodríguez et al., (2017) |
| Echinometra sp. | 343 d | pHNBS 7.7, 8.1, Lab | SEM | Test plates and sines, no dissolution | - | - | Hazan et al., (2014) |
| Echinometra mathei | 13 mo | pHT 7.65, 8.1, Lab | - | - | Ambital and apical plate fracture force and elasticity | No effect of pH | Moulin et al., (2015) |
| Heliocidaris erythrogramma | 2 wk, early juveniles | pHNBS7.4, 7.6, 7.8, 8.1, Lab | SEM | pHNBS 7.4, Spines, ↑ pore size and dissolution | - | - | Wolfe et al., (2013b) |
| Heliocidaris erythrogramma | 9 mo | pHNBS 7.6, 8.1, Lab | SEM | pHNBS 7.6, ↑ pore size apical but not ambital plates | Nanoindentation, elasticity | pHNBS 7.6, ↓ hardness and elasticity | Johnson and Byrne (unpublished data) |
| Lytechinus variegatus | 3 mo, early juveniles | pHNBS 7.8, 8.0, 8.1, Lab | SEM | pHNBS 7.4, Spines, dissolution and malformation | - | - | Albright et al., (2012) |
| Lytechinus variegatus | 59 d | pH* 7.47, 7.7, 7.9, Lab | SEM | pH* 7.47, Spines, reduced barbs | Snap test | pH* 7.47, ↓ snap force | Emerson et al., (2017) |
| Paracentrotus lividus | 1 mo (juveniles) |
pHT 7.7, 7.8, 8.0, 8.1, Lab | SEM | pHT 7.7, larger pore size in tooth, no change test plate thickness; no spines dissolution/etching | Crushing test (whole urchin dried) Fracture force plate |
pHT 7.7, ↓ crushing force; No effect of pH on test plate |
Asnaghi et al., (2013), 2014, (2019) |
| Paracentrotus lividus | 12 mo | pHNBS 7.8, 7.9, 8.0, 8.1, Lab | - | - | Crushing tests, ambital and apical plate fracture force, nanoindentation, elasticity | No effect of pH | Collard et al., (2016) |
| Paracentrotus lividus | Resident | pHNBS 7.8, 8.2, Vent | - | - | Crushing force, ambital and apical plate fracture force, nanoindentation, elasticity | No effect of pH | Collard et al., (2016) |
| Paracentrotus lividus | 100 d, juveniles | pHT 7.6, 8.0, Lab | SEM | pHT 7.6, Test plates thinner, Spines, no dissolution/etching | Crushing test (whole urchin dried) |
pHT 7.6, ↓ crushing force | Rodríguez et al., (2017) |
| Tripneustes gratilla | 146 d juvenile to adult | pHNBS 7.6, 7.8, 8.1 Lab | SEM | pHNBS 7.6, thinner test, spines no dissolution/etching | Crushing test (live whole urchin) |
pHNBS 7.6, ↓ crushing force, rupture at sutures not skeleton | Byrne et al., (2014) |
| Tripneustes ventricosus | 5 wk | pHT 7.4, 7.7, 8.1, Lab | SEM | pHT 7.4, 7.7, spine etching Test plates, no dissolution/etching |
Fracture force spines, two-point bending, elasticity | pHT 7.4, 7.7, spine more brittle, ↓ fracture force, 35% and 16%, no effect of pH on elasticity | Dery et al., (2017) |
| Strongylocentrotus droebachiensis | 45 d | pHNBS 7.2, 7.7, 8.0, Lab | SEM | pHNBS ~ 7.2, test plates pitted; spine dissolution | Fracture force spines | pHNBS ~ 7.2, spines, ↓ fracture force; No effect of pH on test plates | Holtmann et al., (2013) |
| Crustaceans | |||||||
| Amphibalanus amphitrite | 86 d-20 wk | pHNBS 7.4, 8.2; Lab | - | - | Penetrometry, breaking test | pHNBS 7.4, ↓ force to penetrate shells | McDonald et al., (2009) |
| Amphibalanus improvisus | 86d-20 wk | pHT 7.3-7.9 Lab | SEM | Low pH increasing corrosion | Crushing test | No effect of pH | Panch et al., (2013, 2014) |
Morphology methods: EBSD, electron back scatter defractometry; FTIR, Fourier-transform infrared spectroscopy; μCT, micro-computed tomography; SEM, scanning electron microscopy; XPEEM, photo emission electron microscopy. Biomechanics methods: crushing tests (force of rupture); nanoindentation (hardness; Young’s modulus of elasticity—a measure of stiffness). ACC, amorphous calcium carbonate; ASW, artificial seawater; Coast, ***coastal runoff pH gradient; Lab, laboratory; Morph, morphology; Ω, saturation state calcium carbonate minerals; Time, exposure duration; Vent, CO2 seep pH gradient; −, not investigated; **, larva to juvenile studies, all others are of adults; ***, includes pH flux
The impact of OA on calcification depends on level of pCO2, with near future projections (e.g. pHT 7.8) having milder impacts than far future ones (e.g. pHT 7.6 and lower) and also depends on the duration of exposure (see Table 1). For instance, many calcifiers can reside at a mean pHT 7.7–7.9 at low pH vent sites zones through their life, but are absent at zones with a mean pH ≤ 7.6 (Calosi et al., 2013; Kroeker et al., 2013; Foo et al., 2018). The impact of environmental acidification also varies among regions and habitats with some species or populations appearing to be adapted or phenotypically adjusted to environmental acidification. This is seen in the resilience of sea urchins living in low pH upwelling zones and vent sites (Calosi et al., 2013; Hofmann et al. 2014; Migliaccio et al., 2019). Some molluscs can alter the amount of the form of CaCO3 produced (e.g. aragonite or calcite) to a more favourable form making them more resilient to acidification (Fitzer et al., 2014a, 2016; Langer et al., 2014; Rühl et al., 2017).
In calcifying marine invertebrates, environmental carbon for calcification such as dissolved inorganic carbon (DIC) can be sourced in the form of CO32− or hydrogen carbonate (HCO3−) from ambient seawater. Under CO2-driven acidification, this may impair shell growth as pH and CaCO3 saturation are lowered resulting in reduced carbonate available for biomineralisation (Doney et al., 2009). Therefore, under OA, where CO32− becomes less available from seawater limited shell growth and increased abnormalities become a coping mechanism for continued biomineralisation (Wittmann and Pörtner, 2013). However, respiratory CO2 can also be a source of carbon for calcification where it is used to form HCO3− through hydrolysis, a process catalysed by carbonic anhydrase (Wilbur, 1972; Roleda et al., 2012), a highly conserved enzyme that functions in CO2 regulation. Therefore, as DIC sources required for calcification vary between calcifying species, it might also be expected that shell growth responses to OA may be species specific. This is an important consideration in understanding the vulnerability of species and their calcification response in ocean and coastal acidification conditions.
The production and maintenance of CaCO3 structures are vital to the success and survival of a vast diversity of marine species as they play essential roles in body support and protection. Corals and oysters provide the structural foundation of reef ecosystems that many species depend on for habitat. Marine calcifiers also provide vital ecosystem services to humanity as species for fisheries and aquaculture and in shoreline protection and are vital to the livelihoods of millions globally (Cooley et al., 2009; 2011; Gattuso et al., 2015). There has been a wealth of studies on the impacts of OA on marine invertebrates with respect to development, physiology and ecology, as detailed in a number of reviews and meta-analyses (Andersson and Gledhill, 2013; Kroeker et al., 2013; Gazeau et al., 2013; Byrne et al., 2013; Wittmann and Pörtner, 2013; Dubois, 2014; Prezeslawski et al., 2015; Foo et al., 2018). The mineralogy of marine skeletons and their vulnerability to OA has also been reviewed (Ries, 2010; Smith et al., 2013, 2016).
Despite concerns for the prospects for marine calcifiers in a changing ocean, the impacts of environmental acidification on the structure of the biomineral itself remains largely underexplored. We need to understand how acidification alters biomineral production and its microstructure and mechanical integrity to address uncertainties on the biological consequences of climate change. This is addressed here in a review of recent research, where advanced microscopy is used to visualize the internal microstructure and crystallography of biomineral in calcifiers that have been exposed to experimental (laboratory) or natural (vents, coastal run off) acidification (Table 1). Changes in microstructure have been revealed by the application of scanning electron microscopy (SEM) to view surface changes and microcomputed tomography (μCT) to generate three-dimensional reconstructions of entire shells and skeletons (Fitzer et al., 2019a).
Several studies have investigated the biomechanical changes to marine skeletons exposed to environmental acidification (Table 1). These studies have largely used crushing tests and nanoindentation. Nanoindentation provides a high-resolution assessment of material hardness and is especially useful on smaller, thinner shell samples using nanoscale indents (depth of 10–100’s nm). In addition, nanoindentation can be used to determine the elastic modulus (Young’s modulus—E) of the surface of the shell (Fitzer et al., 2015a; Milano et al., 2016; Meng et al., 2018). Details of the advanced microscopy and materials science techniques that have been used to investigate marine skeletons in an OA context are reviewed in Fitzer et al. (2019a).
The list of studies (Table 1) provides an overview of the impacts of environmental acidification on skeletal structure and biomechanics and the pH levels and scales (e.g. pHT vs pHNBS) used are important to note. In the following text, we review research on the major marine calcifying taxa (tube worms, corals, molluscs, echinoderms) with a focus on those most studied, bivalves and sea urchins. Only a few studies have investigated the impact of habitat acidification and warming, and the interaction of these factors, on the structure and integrity of marine biomineral, and we highlight a few of these.
Corals
Corals produce an aragonite skeleton that is covered by the tissues of the coral polyps (Von Euw et al., 2017). Several studies have investigated the impact of OA on the microstructure of coral skeletons using SEM or μCT and one study has incorporated mechanical tests (Table 1). The number of studies appears surprisingly limited considering the ecological importance of reef-building corals. For this important taxon more research is needed on the impact of projected near-future acidification on skeletal structure and mechanical integrity and in context with habitat warming.
The temperate solitary cup coral Balanophyllia europaea living at a low pH vent site had a more porous and thinner skeleton than those living at nearby control sites (Fantazzini et al., 2015). Skeletal pore size increased and bulk density decreased, with decreasing pH (8.1, 7.9, 7.7). However, growth, with respect to size, was maintained by these corals along the vent pH gradient. Nanoindentation revealed that the skeleton of B. europaea living at low pH (pH 7.7) had a lower hardness and stiffness compared with corals residing at pH 8.1. While this species is able to maintain growth at reduced pH, the deposition of less biomineral is likely to increase susceptibility to physical damage (Fantazzini et al., 2015).
For colonies of the tropical coral, Stylophora pistilla, exposed to pH 7.2 for 12 months, SEM and μCT revealed that the biomineral produced had greater porosity and lower bulk density compared with conspecifics maintained at ambient pH (Table 1). Note the caveat that the low pH treatments used are well beyond OA scenarios (IPCC, 2014) (as the case for many of the studies listed in Table 1). Tambutté et al., (2015) suggest that these extreme treatments are useful to help identify trends. While the growth of Stylophora pistilla, as linear extension did not change in pH 7.2, the density of the biomineral did, potentially as a trade-off strategy under competition to maintain access to light and space with the compromise of a weaker skeleton (Tambutté et al., 2015). This change in the skeleton also reflects the decline in S. pistilla colonies at low pH sites in nature (Tambutté et al., 2015). A recent study of these species maintained at low pH for 14 months showed a change in skeletal chrystallography (Coronado et al., 2019).
Colonies of Porites astreoides living in a naturally low pH environment from coastal run off also had a lower density skeleton (Crook et al., 2013) and juvenile Favia fragum exposed to CO2 driven under saturation conditions (pH not provided) for 8 days had a thinner skeleton compared with controls (Cohen et al., 2009).
In these coral studies skeletal dissolution was not noted. It appears that the thin veneer of polyp/corallite tissue protects the underlying skeleton from dissolution. Importantly, the most universal measure of coral health and biomineralisation rate (linear extension) is unlikely to be a reliable metric of coral health and growth in the face of OA, with potential production of more fragile phenotypes (Tambutté et al., 2015).
Tube worms
Serpulid polychaetes are a major group of marine worms that produce a calcareous tube that varies in composition from being entirely aragonitic, to entirely high-Mg calcite to mixtures of these two (Smith et al., 2013). The serpulid, Hydroides elegans, is one of the most important species in tropical biofouling communities and the microstructure and mechanics of its calcareous tube has been investigated in the OA context (Chan et al., 2012; Li et al., 2014, 2016).
In studies where H. elegans was reared in experimental OA conditions across larval development through to tube formation, SEM and μCT revealed that at low pH (7.4–7.8), the tube had increased porosity, decreased thickness and had surface pitting and erosion. There was also a change in the mineral layers and crystallography. These changes reduced the hardness of the tube. The force needed to crush the tube decreased by 62%. The composition of aragonite in the tube is also influenced by OA with H. elegans depositing more calcite in the tube at low pH. This species seems to be able to adjust the mineralogy of its tube as a potential adaptation to the changes in water chemistry driven by OA, but this compromises the strength and elasticity of the tube (Li et al., 2014, 2016). In comparison, the serpulid, Spirobranchus triqueter was similarly fragile when reared under moderate (pH 7.7) and severe (pH 7.4) reductions in pH. Tube fracture toughness was not linearly related to the reduced pH, but was related to changes in porosity as found for H. elegans. Larger pores in S. triqueter resulted in thinner calcareous layers in the tubes and therefore reduced fracture toughness (Díaz-Castañeda et al., 2019).
In experiments where ocean warming was also considered, increased temperature (+6°C) had the opposite effect, increasing skeletal hardness and elasticity (Chan et al. 2012). Although the temperature increase used was more extreme than predicted under future climate scenarios, the outcome for this important polychaete indicated that in a future ocean biomineral production may be more dictated by climate warming than acidification.
Gastropods
Depending on the species and life stage, gastropods produce aragonitic or calcitic shells or a combination of these (Marin et al., 2008; Rühl et al., 2017). The impact of OA on shell microstructure of several species, maintained in experimental OA for various lengths of time has been investigated (Table 1). For the ecologically important species, Nucella lapillus and Nassarius nitidus, the reduction in shell density determined using μCT was marked (20–50%), while that for Columbella rustica was much less (0.8–8%) (Queirós et al., 2015; Chatzinikolaou et al., 2017). This shows differences in vulnerability of shells to OA between closely related gastropod species. For the two species greatly affected, the extent of biomineral reduction varied across locations in the shell which would make weaker regions a target for durophagous predators such as crabs. In a study of the mechanical integrity of the shells of Littorina littorea maintained in pH 7.8 for 5 months, the shells of larger snails were more vulnerable to the crushing action of a crab predator than those of smaller snails (Landes and Zimmer, 2012). In a study of 7 gastropod species, the mechanical properties of the shells of six of these were not affected by acidification (pH 7.8-7.9) (Leung et al., 2017). For Austrochochlea constricta, shell hardness and stiffness increased. This species also produced a shell with higher calcite to aragonite and magnesium to calcium ratios under acidification conditions (Leung et al., 2017). In this study elevated temperature (+ 2.5-4.0 °C) was the more important factor on shell mechanics. For juvenile N. lapillus, grown under OA conditions, a 2°C warming appeared to counter the negative effect of OA, but as this differed between small and large snails, the trend was difficult to interpret (Rühl et al., 2017).
Gastropods resident at vents also have thinner, less dense shells as seen in μCT reconstructions (Garilli et al. 2015; Harvey et al. 2018). The shell surface of Charonia lampas exhibited the corrosive effects of low pH water even to the extent that the soft tissue was exposed (Harvey et al. 2018). Overall, gastropods living at CO2 vent sites tend to have a smaller adult body size, similar to the Lilliput effect seen in the fossil record for mollusc species that survived past low pH driven extinction events (Garilli et al., 2015). It is suggested that smaller body size is a physiological adaptation to environmental acidification in order to maintain calcification as well as to have the energy to repair shell dissolution (Garilli et al., 2015). For herbivorous gastropods living at vent sites, the enhanced algal food levels due to high CO2 can buffer, to a variable extent, the negative effect of OA on calcification (Doubleday et al., 2019).
Bivalves
Bivalve skeletons are comprised of two shells joined at a hinge and vary in their composition of calcite and aragonite (Marin et al., 2008; Wehrmeister et al., 2011). There has been extensive research on the impacts of OA on these animals as they form the basis of significant aquaculture productivity. They are also ecologically important as being major prey for many species and their filter feeding activity strongly influences water clarity and quality.
Many bivalve studies report reduced shell growth, reduced shell thickness and mechanically weaker shells under OA conditions (Gazeau et al., 2007; Ries et al. 2009; Beniash et al., 2010; Dickinson et al., 2012; Fitzer et al., 2015a, b, 2018). Reduced shell growth and thinning of shells under experimental CO2 acidification have been observed using SEM at both the larval (Gazeau et al., 2010; Parker et al., 2012; Fitzer et al., 2014a) and adult stages (Gazeau et al., 2013; Fitzer et al., 2014b). However, no effect on shape or size of crystals was observed in the clam Arctica islandica or the scallop Adamussium colbecki under elevated CO2 (Stemmer et al., 2013, Dell’ Acqua et al., 2019). The abnormalities and changes to shell growth can affect bivalves at the microstructure level of the forming phases of aragonite and calcite (Wehrmeister et al., 2011), and can also impact the amorphous calcium carbonate (ACC) that is an important precursor of crystalline carbonate minerals (Addadi et al. 2003).
Microstructural observations of bivalves exposed to acidification conditions have been determined using a range of microscopy techniques including SEM coupled with electron backscatter diffraction (EBSD) (Melzner et al., 2011; Fitzer et al., 2014a, b), Fourier-transform infrared (FTIR) spectroscopy (Beniash et al., 2010) and X-ray photo emission electron microscopy (XPEEM) (Fitzer et al., 2016) to characterize crystallography and the form of calcium carbonate. μCT has been used to examine skeletal density (Meng et al., 2018).
In the mussel Mytilus edulis, which contains an outer calcite and an inner aragonite layer (Fig. 2), experimental OA resulted in a thinning of the shell (Fitzer et al., 2015b), with disordered crystallography as revealed by SEM-EBSD as well as a corroded aragonite layer (Melzner et al., 2011; Fitzer et al., 2014a), in favour of a disordered calcite layer (Fitzer et al., 2014a, b). Examination of the shells of M. edulis exposed to CO2 acidification with XPEEM revealed that more ACC was produced (Fitzer et al., 2016). This was suggested to aid repair to the disordered calcite layer in the shell (Fitzer et al., 2016).
Figure 2.
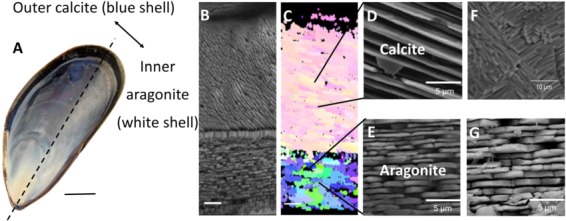
A schematic representation of the use of SEM-EBSD as an analytical tool to assess the effects of acidification on shell microstructure. (A) M. edulis has been a focal study species to understand the impacts of OA on marine biomineral. (B) Section imaged using SEM is a cross section through a shell grown under OA (pHNBS 7.5). (C) The same section imaged using EBSD and analysed for crystallographic orientation displayed as a crystallographic orientation map. (D) Calcite and (E) aragonite crystals at higher magnification from the same cross section of the M. edulis shell. (F) Disordered microstructure of the calcite layer and (G) dissolution of the aragonite tablets (edges more rounded compared to panel E and tablets are less tightly packed) of the shells grown in OA. Images adapted from Fitzer et al. (2014a).
While M. edulis has been a focal case study species (Table 1), many bivalves are similarly affected by OA with commonly observed thinning of the shells and disorder in the crystallography of the calcite layers (Figs 2 and 3). An SEM-EBSD investigation of Mytilus galloprovincialis transplanted to a CO2 vent (~pH = 7.2–7.8) revealed that they grew a thinner shell with disordered crystallography (Rodolfo-Metalpa et al., 2011; Hahn et al., 2012). This was also the case the shells of the oysters Magallana angulata (pH 7.2, 7.5) and M. hongkongensis (pH 7.3) grown in laboratory (CO2 dosing) (Meng et al., 2018, 2019), and Saccostrea glomerata farmed in coastal acidified environments (pH 7.6–7.8) (Fitzer et al., 2018, 2019b). The disordered crystallography in S. glomerata wild type oysters grown in coastal acidified environments was as a result of altered biomineralisation pathways shown by changing shell carbon isotopes (Fitzer et al., 2019b). Oysters such as the pearl oyster Pinctada fucata that have an internal layer of aragonite, dissolution occurred internally under OA conditions (Welladson et al., 2011).
Figure 3.
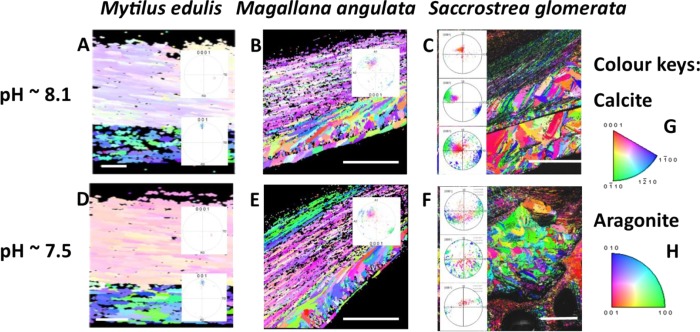
Crystallographic orientation maps with accompanying pole figures for the calcite and aragonite shell of M. edulis (A, D), and the calcite shells of Magallana angulata (B, E) and Saccostrea glomerata (C, F) grown at pH 8.1NBS and pHNBS 7.5 under CO2 acidfication and sulphate soil acidification. The figures highlight the similarly altered crystallographic orientation of the mussel and oyster shells with increased disorder at pH 7.5. This is highlighted by the increased range of crystallographic orientation shown by the increased variation of colours. The colours here represent a change in the angle of crystallographic orientation as per the calcite (0001) (G) and aragonite (001) (H) colour keys. Scale bars represent 5 μm for M. edulis, 45 μm for M. angulata and 200 μm for S. glomerata. Adapted from Fitzer et al. (2014a, 2018) and Meng et al. (2018).
The impacts of OA on the biomechanics of bivalve shells have been studied using a variety of techniques, in particular microindentation of the shell surface to calculate hardness. The resultant cracks propagating from the indents can also be applied to calculate fracture toughness (Mackenzie et al., 2014; Fitzer et al., 2015a). The changes to the shell microstructure in M. edulis grown in OA conditions resulted in a mechanically weaker shell as indicated by nanoindentation. The calcite produced was harder and more brittle. Microindentation indicated reduced fracture toughness (Fitzer et al., 2015a) and reduced bend/flex before failure (Mackenzie et al., 2014).
While OA (pH 7.5) as a single stressor reduced shell hardness and stiffness in M. edulis, when this level of acidification is used in combination with moderate warming (+2°C), these negative effects were reduced (Fitzer et al., 2015b). Temperature alone did not alter shell size of thickness; however, a combination of +2°C warming with acidification (pH 7.5) reduced the aragonite layer thickness in M. edulis (Fitzer et al., 2015a). A +4°C warming reduced the maximum crushing load of mussel shells in both ambient and reduced pH (pH 7.6) (Mackenzie et al., 2014).
The mechanical properties of bivalve shells that have different forms of CaCO3 differ in response to OA. In the shell of M. edulis, which contains both calcite and aragonite, the calcite becomes harder or more brittle when exposed to OA. For the calcitic shell of the oyster Crassostrea virginica grown under experimental OA, microindentation revealed that shell hardness is reduced along with a resultant reduced fracture toughness (Beniash et al., 2010; Dickinson et al., 2012). Similarly, the shells of juvenile Magallana angulata grown under experimental OA (pH 7.2, 7.5) had reduced hardness, as a result of increased porosity as visualized by μCT (Meng et al., 2018). In the more resistant M. hongkongensis, juvenile shells were similarly affected, but at a much lower pH (pH 7.3) (Meng et al., 2019). For this species, shell hardness and crystallography remained unchanged at pH 7.6 (Meng et al., 2019). Oysters such as Pinctada fucata that have an internal layer of aragonite had a weaker shell when grown under experimental OA conditions (Welladsen et al 2011) than oysters that have a calcitic internal layer. The shells of S. glomerata growing in estuarine habitats with sulphate soil acidification (pH 6.6, 6.8, 6.9) were also significantly weaker in crushing tests (Amaral et al., 2012). Although these pH levels are much lower than predicted OA, this level of acidification occurs in acid sulphate regions during increased rainfall (Amaral et al., 2012). Reduced growth of S. glomerata during such extreme events may be offset by positive growth in dry periods (Amaral et al., 2012).
Overall, OA affects the microstructure of bivalve shells through reduced shell growth with thinner, more porous shells that have reduced fracture toughness. Trends appear similar in mussels and oysters in terms of microstructural responses to OA regardless of whether laboratory CO2 acidfication or environmental acidification from CO2 or coastal run off are the source of acidification (Table 1).
Echinoderms
Echinoderms produce a unique endoskeleton laid down as a three-dimensional mesh-like calcite lattice (Fig. 4), with cells and connective tissue living in the void space (Cavey and Markel, 1994). The impacts of OA on the production and maintenance of the skeleton are extensively investigated for echinoids (sea urchins) across their planktonic and benthic life phases (Byrne et al. 2013; Dubois, 2014). These animals are ecologically and economically important grazers across world oceans (Lawrence, 2013). The sea urchin skeleton is composed of Mg-calcite, ~3–16 wt% MgCO3, where Mg2+ is substituted for Ca2+ during calcification (Chave, 1954; Smith et al., 2016). As a result of this chemical composition, the echinoderm skeleton is one of the most soluble forms of CaCO3, and so is vulnerable to dissolution in OA conditions (Andersson et al., 2008; McClintock et al., 2011; Dubois, 2014).
Figure 4.
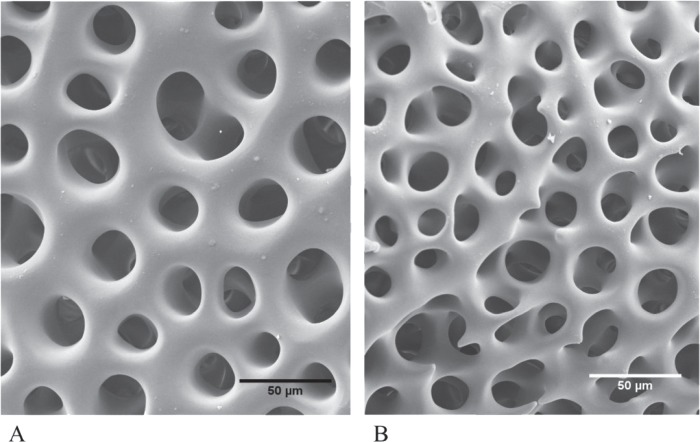
SEM of the surface of the apical test plates of the adult sea urchin, Heliocidaris erythrogramma maintained in control (pHNBS 8.1) (A) and decreased pH (pHNBS 7.6) (B) for 9 months. The skeleton formed in the OA treatment has thinner calcite. Images courtesy of Ms R. Johnson.
Despite the echinoderm skeleton being a form that is vulnerable to dissolution, the epithelium largely protects the living skeleton from direct exposure to low pH conditions (Dubois, 2014). The exceptions are the tips of the spines, potentially due to their thin epithelial cover. Overall, the established sea urchin skeleton does not appear to be vulnerable to direct dissolution under OA conditions (Table 1). Surface pitting of the test has only been reported at very low pH (Holtmann et al., 2013). Interesting, the cidaroids, a group of sea urchins with many species living in deep water below the isocline, produce spines that do not have an epithelial cover, but have a calcitic cortical layer that makes them resistant to dissolution in OA conditions, as shown for 15 species, including in tests where species were maintained in pH 7.2–7.4 for 3–5 weeks (Dery et al., 2014, 2017, 2018).
With respect to production of biomaterial, calcification in sea urchins is constrained by OA. This is shown by the comparatively smaller skeletons of larvae and juveniles (Figs 5 and 6) reared in OA conditions through development and the smaller tests of adult sea urchins (Fig. 7) grown in long-term OA experiments (Byrne et al., 2013, 2014; Dubois, 2014; Dworjanyn and Byrne, 2018). It appears that sea urchins have a reduced ability to deposit biomineral under OA conditions (Figs 4 and 5). This may be due to energetic constraints associated with the higher metabolic costs of life at low pH and the priority to maintain acid-base balance (Stumpp et al., 2012; Carey et al., 2016). The impacts of OA on the skeleton may be mitigated by near future warming. In Tripneustes gratilla, a +3°C warming mitigated the negative effects of low pH (pH 7.6, 7.8) on test growth (Fig. 7). Further warming made matters worse (Byrne et al., 2014; Dworjanyn and Byrne, 2018).
Figure 5.
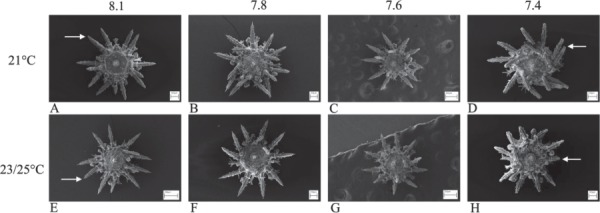
SEM of juvenile Heliocidaris erythrogramma reared in four pH and three temperature levels in all combinations for 14 days. Urchins had shorter spines and smaller tests at pHNBS 7.4 (see Wolfe et al. 2013b). At control pH (A, E) the arrows point to the terminal spike which is the calcite growing region of the spines compared with the flat-ended spines of juveniles reared in pHNBS 7.4 indicating retarded or no calcification. Images courtesy of Dr K Wolfe.
Figure 6.
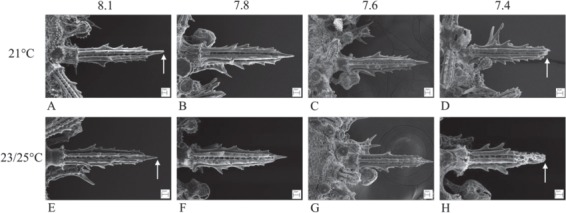
SEM of the spines of juvenile Heliocidaris erythrogramma reared in four pH and three temperature levels in all combinations for 14 days. The arrows point to the pointed end of the spines in control pHNBS 8.1 (A, E) at the calcite growing region. At pHNBS 7.4 and at warmer temperature the spines were shorter, more porous, had blunt ends (D) and were eroded (H) (see Wolfe et al. 2013b). Images courtesy of Dr K Wolfe.
Figure 7.
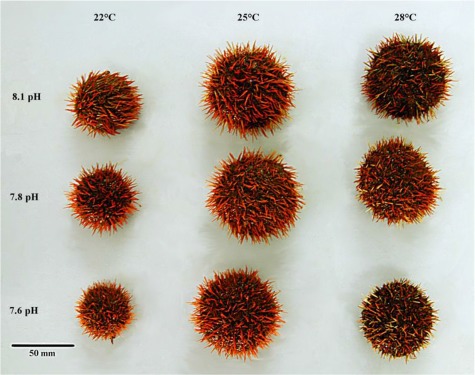
Tripneustes gratilla reared in three pH and three temperature levels in all combinations from the early juvenile (5.0 mm test diameter) for 146 days. A +3°C warming mitigated the negative effects of low pHNBS (pH 7.6, 7.8), but further warming was deleterious. From Dworjanyn and Byrne (2018).
In addition to the smaller amount of skeleton produced under OA conditions, skeletal microstructure may also be altered. Several SEM and/or μCT investigations have compared the pore size, the calcitic trabecular connections and the void space of the skeleton of sea urchins exposed to OA with these traits in the skeleton formed under ambient pH (Table 1). Larger pore size is reported for the spines of juvenile Heliocidaris erythrogramma (Fig. 6), but only at very low pH (pH 7.4) (Wolfe et al., 2013b) and for the teeth of Paracentrotus lividus where the tips were regenerated under OA (pH 7.7) (Asnaghi et al., 2013).
For H. erythrogramma maintained on OA conditions for 9 months, the mid body ambital test plates produced prior to exposure to OA exhibited no change in skeletal structure. In contrast, the younger apical plates from the active growth region of the test, that had likely grown over the 9-month exposure at low pH had thinner trabeculae and a greater void space compared to those from control urchins as visualized with SEM (Fig. 4A and B) and μCT (Johnson, 2019).
The μCT study of Strongylocentrotus fragilis that had likely grown from the juvenile stage in chronic low pH (pH 7.69–7.57) and oxygen saturation on the Californian continental shelf, showed that that skeletal porosity and pore size is higher than that for conspecifics living at higher pH. For this species, skeletal porosity increased with decreasing pH and oxygen saturation, but it is not known how hypoxia and low pH interact with respect to the differences in the skeleton (Sato et al., 2018).
The impact of OA on the mechanical properties of the test plates has been documented by nanoindentation and in crushing and bending tests (Table 1). In P. lividus maintained in OA (pH 7.8, 7.9) for 12 months, there was no effect of pH on the hardness or breaking force of the test plates (Collard et al., 2016), as also the case for Echinometra mathaei (Moulin et al., 2014). Exposure to low pH reduced the hardness of the apical plates of H. erythrogramma that had likely been produced in OA conditions, but the previously established ambital plates were not affected (Johnson and Byrne, unpublished data). There was also no change in the mechanical properties of the test of P. lividus resident at a vent site (~pH 7.7) compared with conspecifics from ambient conditions.
In studies where the whole test was crushed as might occur in an attack by a predatory fish (Guidetti and Mori, 2005), the force required to crush the dried tests of juvenile P. lividus and Diadema africanum maintained in OA was lower compared to juveniles from control treatments (Asnaghi et al., 2013; Rodríguez et al. 2017). However, as dried tests were used, the ecological relevance of these results is not clear. The force required to crush the test was lower in live Tripneustes gratilla reared in OA (pH 7.6) from juvenile to adult in a test designed to mimic the ram force of a predatory fish, but the break occurred at the suture lines, not in the skeleton itself (Byrne et al., 2014). These tests need to be repeated across species with live urchins being most relevant test subjects, to incorporate the elasticity of the ligaments that bind the test plates (Ellers et al., 1998), and with application of forces modelled with respect to the jaw crushing or ramming action of predators, although these forces are poorly characterized.
With respect to the spines of sea urchins maintained at pH 7.4 and 7.7, the thinner, more brittle spines formed in these conditions by Tripneustes ventricosus and Eucidaris tribuloides are weaker in bending tests, but not the spines of Prionocidaris baculosa (Dery et al., 2017). While the spines are the most vulnerable skeletal element in sea urchins to OA, these structures are designed to break in a predatory attack, and regenerate quickly (Dery et al., 2017). But this entails an energetic cost (Haga et al., 2016)
Overall, OA affects the material properties of the sea urchin skeleton through changes in growth rate with less biomineral produced. While it appears that there is weakening of the sea urchin skeleton in response to OA, thereby compromising its protective roles, more studies are needed where characterisation of the impacts of OA on biomineral microstructure is investigated in tandem with biomechanical tests and in skeletal elements that have been completely produced at low pH. Finally, the 3D void space of the sea urchin endoskeleton is important for the resident cells and tissues. Any change in the stereotypic arrangement of the skeleton is likely to have impacts for organism function beyond the skeleton itself.
Crustaceans
The crustacean exoskeleton is made of chitin and calcite and is comparatively tolerant of acidification due to the lower degree of calcification (Whiteley, 2011), the exception being adult barnacles, with several studies on these animals (Table 1). For Amphibalanus amphitrite, there were no effects of low pH (to pH 7.4) through the larval stage, but the adult shell was weaker (McDonald et al., 2009). Corrosion of the shell of A. improvises was evident at low pH (Pansch et al., 2014). Increased temperature (+4°C) resulted in an increase in shell strength of this species, but there was no effect of pH (Pansch et al. 2013, 2014). For Balanus improvises, low pH (stable pH 7.7, fluctuating pH 7.5–7.9) reduced the strength of the shell (Eriander et al., 2016).
Discussion
The morphological and biomechanical properties of marine invertebrate skeletons are integral to animal function and individual fitness which in turn affects population dynamics and community structure (Kroeker et al., 2014). In a climate change world, it is important to understand how calcification systems, and the biomineral that is produced, respond to environmental acidification. The combination of detailed morphology using advanced imaging techniques and biomechanics, is emerging as a leading-edge approach to quantify the vulnerability of marine skeletons. This approach is also key to detecting the potential for phenotypic adjustment in biomineralisation as a means to produce and maintain biomineral in the face of environmental acidification.
Although the field of marine climate change has focused on anthropogenic CO2-driven acidification, many important calcifying species live in shallow coastal habitats that are vulnerable to acidification from increasing run off due to pressures from sea level rise and precipitation (Amaral et al., 2011; Fitzer et al., 2018). The chemistry of ‘ocean’ and ‘coastal’ acidification, and how they impact the carbonate system, the basis for calcification, are fundamentally different (Fig. 1). For bivalves, despite these differences, the resulting morphology and biomechanics of the biomineral produced under the two forms of acidification are similar (Beniash et al., 2010; Rodolfo-Metalpa et al., 2011; Dickinson et al., 2012; Hahn et al., 2012; Fitzer et al., 2018; Meng et al., 2018). Coastal waters have a more variable chemistry than the ocean and understanding how this chemistry interacts with CO2-driven acidification, remains a significant challenge. This is especially important for socioeconomically important mollusc resources that are typically fished and cultured in coastal bays and estuaries.
In response to environmental acidification, the biomineral produced by marine invertebrates across many taxa, is more porous and less dense. Increased skeletal porosity in diverse species has been revealed by SEM and/or high-resolution 3D reconstructions generated by μCT. Indeed, some skeletal malformations are only evident through application of these methods (Fitzer et al., 2014a, b, 2015a, b, 2018; Rühl et al., 2017). There may be a balance between the absolute amount of mineral that can be physiologically deposited in the skeleton in order to maintain its key physiological functions and the energy that can be devoted to this function under OA. For molluscs and echinoderms, constraints in biomineral production result in smaller body size, similar to the Lilliput effect seen in the fossil record when smaller mollusc species survived global warming extinction events (Garilli et al., 2015). In contrast for corals, growth, as measured by linear extension of the skeleton, the standard metric of coral health appears to be maintained under CO2-driven acidification, but the skeleton produced has a lower density and is thus more vulnerable to physical damage (Tambutté et al., 2015). OA also impacts biomineral directly through dissolution and this is most commonly noted for molluscs that lack an outer protective cover of conchiolin (Ries et al., 2009; Rodolfo-Metalpa et al., 2011; Harvey et al., 2018). Echinoderm skeletons are protected from corrosion by their epithelial cover, and in corals, the polyp tissue protects the skeleton.
Within each of the groups considered here, environmental acidification impacts skeletal microstructure and biomechanics in different ways, reflecting the diverse calcification biology of the species investigated. In bivalves, where skeletal CaCO3 varies in composition of calcite and aragonite, the responses to CO2-driven acidification are often similar within the same family. For example, the mytellids (Mytilus sp) have harder, brittle shells prone to fracture under OA, compared with the Ostreidae (Crassostrea sp.) where the shells have reduced hardness. Despite these different responses, the outcome with regard to the mechanical integrity of the shell of mussels and oysters is similar with reduced fracture toughness of shells formed in OA conditions.
Volcanic CO2 vent systems have been used as natural laboratories to investigate the impacts of near and far future OA (Foo et al., 2018; González-Delago and Hernández, 2018). The CO2 gradient at these sites provides a pH range from very low ~pH 6 to ambient levels in nearby habitats not affected by CO2 venting. Investigation of marine calcifiers resident along these CO2 gradients where all or most of their skeleton has formed under low pH, has been an important means to understand how acidification impacts calcification. Overall, the results of laboratory and vent studies, at similar pH/acidification levels, are similar with respect to the response of marine invertebrate calcification. For herbivorous species resident at vents, higher levels of algal resources promoted by the CO2 helps to ameliorate the energetic stress caused by acidification (Uthicke et al., 2016).
Biomechanical tests of the biomineral produced under experimental and natural acidification indicate that the shells and skeletons of many species are weaker than those produced in ambient conditions. This has been largely shown for molluscs with some studies of tube worms and corals, although the shells of many gastropod species are not affected by acidification (Leung et al., 2017). For echinoderms, biomechanics tests indicated that the spines are most affected by acidification (Collard et al., 2016; Dery et al., 2017) and that the newly formed apical plates were weaker (Johnson and Byrne, unpublished data). Overall, the shift to producing weaker skeletons with increased porosity decreased density under acidification conditions in many species will compromise the function of these structures in protection against physical stress and defence against predators.
It is key to consider the fitness consequences of observed change. Change to animal size, strength or defence will have effects to marine communities beyond the fitness of the individual. Changes to predator–prey interactions may change predation rates of vulnerable species and thereby alter population dynamics and community structure in the future (Kroeker et al., 2014). Weaker shells change predator–prey dynamics as durophagous predators such as crabs optimize their foraging to preferentially target weaker-shelled individuals or weaker regions of the shell of their molluscan prey (Kroeker et al., 2014). Predatory mollusc and boring epibionts also target the weaker, less dense areas of shells to drill and bore through the biomineral. Many molluscs also exhibit an inducible defence behaviour producing stronger, more calcified shells in response to the presence of a predator or in response to hydrodynamic conditions and the latter also applies to sea urchins (Bibby et al., 2007; Collard et al., 2016). For the gastropod Littorina obtusata, the inducible defence response expressed in the presence of a crab predator was inhibited at low pH, albeit at a rather extreme level (pH 6.6, ~ 14 000 μatm pCO2) (Bibby et al., 2007). The thinner shells of the oyster Ostrea lurida reared from the larval stage in OA appears to have made them more vulnerable to predatory gastropods (Sanford et al., 2014). In a study of M. edulis and the drilling gastropod Nucella lapillus maintained in the same acidification conditions, the thinner mussel shells produced in OA conditions made them more vulnerable to predation by the gastropod (Sadler et al., 2018). The outcomes for marine calcifiers in the face of OA will also be influenced by other environmental factors such as food levels. Mussels and barnacles are able to maintain a higher-level calcification in the presence of high food levels (Melzner et al., 2011; Pansch et al., 2014). Sea urchins with a higher level of calcified algae in their diet produce stronger skeletons (Asanaghi et al., 2013). Species adapted to fluctuations in intertidal habitats are also more tolerant of acidification (Wolfe et al., 2013b; Leung et al., 2017).
For several marine invertebrate groups across a variety of biomineralized structures, the lack of studies of the microstructure and mechanics stands out as gaps to address. In particular, the response of the crustacean chitin-calcite exoskeleton to acidification is poorly studied. The impact of acidification on jellyfish calcium sulfate (basanite) statoliths (Mooney and Kingsford, 2016; Sötje et al., 2017) is not known. Understanding the effects of acidification on the organic matrix of marine skeletons is also an important gap in knowledge, especially as shell matrix proteins are essential for crystal nucleation and other key aspects of skeletogenesis (Marin et al., 2008).
Finally, while the integrated morphology-mechanics approach has great potential to characterize the impacts of environmental acidification on marine invertebrate skeletons and advance our understanding of the biological consequences of climate change, this approach has only been applied to a relatively small number of species (Table 1). There is great interest in how calcification and the biomineral produced will be altered in a changing ocean and it is clear that application of morphology and ecomechanics, in context with use of realistic pH levels, will be particularly informative.
Acknowledgments
We thank colleagues that provided images, Dr K. Wolfe (University of Queensland) and Roberta Johnson (University of Barcelona). Hamish Campbell helped in preparing the manuscript.
Funding
Supported by an ARC Discovery Grant (DP150102771 to M.B.) and a NERC Independent Research Fellowship (NE/N01409X/1 to S.F.).
References
- Addadi L, Raz S, Weiner S (2003) Taking advantage of disorder: amorphous calcium carbonate and its roles in biomineralization. Adv Mater 15: 959–970. [Google Scholar]
- Albright R, Bland C, Cillette P, Serafy JE, Kangdon C, Capo T (2012) Juvenile growth of the tropical sea urchin Lytechinus variegatus exposed to near-future ocean acidification scenarios. J Exp Mar Biol Ecol 426: 12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral V, Cabral H, Bishop MJ (2011) Resistance among wild invertebrate populations to recurrent estuarine acidification. Estuar Coast Shelf Sci 9: 460–467. [Google Scholar]
- Amaral V, Cabral H, Bishop MJ (2012) Effects of estuarine acidification on predator-prey interactions. Mar Ecol Prog Ser 445: 117–127. [Google Scholar]
- Andersson AJ, Mackenzie FT, Bates NR (2008) Life on the margin: implications of ocean acidification on mg-calcite, high latitude and cold-water marine calcifiers. Mar Ecol Prog Ser 373: 265–273. [Google Scholar]
- Andersson AJ, Gledhill D (2013) Ocean acidification and coral reefs: effects on breakdown, dissolution, and net ecosystem calcification. Ann Rev Mar Sci 5: 321–348. [DOI] [PubMed] [Google Scholar]
- Asnaghi V, Chiantore M, Mangialajo L, Gazeau F, Francour P, Alliouane S, Gattuso JP (2013) Cascading effects of ocean acidification in a rocky subtidal community. PLoS One 8: e6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asnaghi V, Magialajo L, Gattuso JP, Francour P, Privitera D, Chiantore M (2014) Effects of ocean acidification and diet on thickness and carbonate elemental composition of the test of juvenile sea urchins. Mar Environ Res 93: 78–84. [DOI] [PubMed] [Google Scholar]
- Asnaghi V, Collard M, Mangialajo L, Gattuso JP, Dubois P (2019) Bottom-up effects on biomechanical properties of the skeletal plates of the sea urchin Paracentrotus lividus (Lamarck, 1816) in an acidified ocean. Mar Environ Res 144: 56–61. [DOI] [PubMed] [Google Scholar]
- Barclay KM, Gaylord B, Jellison B, Shukla P, Sanford E, Leighton LR (2019) Variation in the effects of ocean acidification on shell growth and strength of two intertidal gastropods. Mar Ecol Prog Ser 626: 109–121. [Google Scholar]
- Beniash E, Ivanina A, Lieb NS, Kurochkin I, Sokolova IM (2010) Elevated level of carbon dioxide affects metabolism and shell formation in oysters Crassostrea virginica. Mar Ecol Prog Ser 419: 95–108. [Google Scholar]
- Bibby R, Cleall-Harding P, Rundle S, Widdicombe S, Spicer J (2007) Ocean acidification disrupts induced defences in the intertidal gastropod Littorina littorea. Biol Lett 3: 699–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne M, Lamare M, Winter D, Dworjanyn SA, Uthicke S (2013) The stunting effect of a high CO2 ocean on calcification and development in sea urchin larvae, a synthesis from the tropics to the poles. Philos Trans R Soc Lond B Biol Sci 368: 20120439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne M, Smith AM, West S, Collard M, Dubois P, Graba-landry A, Dworjanyn SA (2014) Warming influences Mg2+ content, while warming and acidification influence calcification and test strength of a sea urchin. Environ Sci Technol 48: 12620–12627. [DOI] [PubMed] [Google Scholar]
- Calosi P, Rastrick SPS, Graziano M, Thomas SC, Baggini C, Carter HA, Hall-Spencer JM, Milazzo M, Spicer JI (2013) Distribution of sea urchins living near shallow water CO2 vents is dependent upon species acid-base and ion-regulatory abilities. Mar Pollut Bull 73: 470–484. [DOI] [PubMed] [Google Scholar]
- Carey N, Harianto J, Byrne M (2016) Urchins in a high CO2 world: partitioned effects of body-size, ocean warming and acidification on metabolic rate. J Exp Biol 219: 1178–1186. [DOI] [PubMed] [Google Scholar]
- Cavey MJ, Markel K (1994) Class echinoidea In Harrison F, Chia FS (eds) Microanatomy of the Invertebrates. Vol 14: Echinodermata Alan R. Liss, New York, NY, p 345–400. [Google Scholar]
- Chan VBS, Li C, Lane AC, Wang Y, Lu X, Shih K, Zhang T, Thiyagarajan V (2012) CO2-driven ocean acidification alters and weakens integrity of the calcareous tubes produced by the serpulid tubeworm, Hydroides elegans. PLoS One 7: e42718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzinikolaou E, Grigoriou P, Keklikoglou K, Faulwetter S, Papageorgiou N (2017) The combined effects of reduced pH and elevated temperature on the shell density of two gastropod species measured using micro-CT imaging. ICES J Mar Sci 74: 1135–1149. [Google Scholar]
- Chave KE. (1954) Aspects of the biogeochemistry of magnesium 1. Calcareous marine organisms. J Geol 62: 266–283. [Google Scholar]
- Cohen AL, McCorkle DC, de Putron S, Gaetani GA, Rose KA (2009) Morphological and compositional changes in the skeletons of new coral recruits reared in acidified water: insights into the biomineralization response to ocen acidification. Geochem Geophys Geosyst 10: 7. [Google Scholar]
- Coleman D, Byrne M, Davis A (2014) Molluscs on acid gastropod shell repair and integrity in acidifying oceans. Mar Ecol Prog Ser 509: 203–211. [Google Scholar]
- Collard M, Rastrick SPS, Calosi P, Demolder Y, Dille J, Findlay HS, Hall-Spencer JM, Milazzo M, Moulin L, Widdicombe S, Dehairs F (2016) The impact of ocean acidification and warming on the skeletal mechanical properties of the sea urchin Paracentrotus lividus from laboratory and field observations. ICES J Mar Sci 73: 727–738. [Google Scholar]
- Comeau S, Tambutté E, Carpenter RC, Edmunds PJ, Evensen NR, Allemand D, Ferrier-Pages C, Tambutté S, Venn AA (2017) Coral calcifying fluid pH is modulated by seawater carbonate chemistry not solely seawater pH. Proc Roy Soc B 284: 20161669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley SR, Doney SC (2009) Anticipating ocean acidification’s economic consequences for commercial fisheries. Environ Res Lett 4: 024007. [Google Scholar]
- Cooley SR, Lucey N, Kite-Powell H, Doney SC (2011) Nutrition and income from molluscs today imply vulnerability to ocean acidification tomorrow. Fish Fish 13: 182–215. [Google Scholar]
- Coronado I, Fine M, Bosellini FR, Stolarski J (2013) Impact of ocean acidification on crystallographic vital effect of the coral skeleton. Nature communications 10: 2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook ED, Cohen AL, Rebolledo-Vieyra M, Hernandez L, Paytam A (2013) Reduced calcification and lack of acclimatization by coral colonies growing in areas of persistant natural acidification. Proc Natl Acad Sci U S A 110: 11044–11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Acqua O, Trębala M, Chiantore M, Hannula SP (2019) Robustness of Adamussium colbecki shell to ocean acidification in a short-term exposure.. Mar Environ Res 149: 90–99. [DOI] [PubMed] [Google Scholar]
- Dery A, Guibourt V, Catarino AI, Compère P, Dubois P (2014) Properties, morphogenesis and effect of acidification on spines of the cidaroid sea urchin Phyllacanthus imperialis. Invertebr Biol 133: 188–199. [Google Scholar]
- Dery A, Collard M, Dubois P (2017) Ocean acidification reduced spine mechanical strength on euechinoid but not cidaroid sea urchins. Environ Sci Technol 51: 3640–3648. [DOI] [PubMed] [Google Scholar]
- Dery A, Tran PD, Compère P, Dubois P (2018) Cidaroid spines facing ocean acidification. Mar Environ Res 133: 9–18. [DOI] [PubMed] [Google Scholar]
- Díaz-Castañeda V, Cox TE, Gazeau F, Fitzer S, Delille J, Alliouane S, Gattuso J-P (2019) Ocean acidification affects calcareous tube growth in adult stage and reared offspring of serpulid polychaetes. J Exp Biol 222: jeb194543. [DOI] [PubMed] [Google Scholar]
- Dickinson GH, Ivanina AV, Matoo OB, Pörtner HO, Lannig G, Bocl C, Beniash E, Sokolova IM (2012) Interactive effects of salinity and elevated CO2 levels on juvenile eastern oysters, Crassostrea virginica. J Exp Biol 215: 29–43. [DOI] [PubMed] [Google Scholar]
- Doney SC, Fabry VJ, Feely RA, Kleypas JA (2009) Ocean acidification: the other CO2 problem. Ann Rev Mar Sci 1: 169–192. [DOI] [PubMed] [Google Scholar]
- Doubleday ZA, Nagelkerken I, Coutts MD, Goldenberg SU, Connell SD (2019) A triple trophic boost: how carbon emissions indirectly change a marine food chain. Glob Chang Biol 25: 978–984. [DOI] [PubMed] [Google Scholar]
- Duarte CM, Hendriks IE, Moore TS, Olsen YS, Steckbauer A, Ramajo L, McCulloch M (2013) Is ocean acidification an open-ocean syndrome? Understanding anthropogenic impacts on seawater pH. Estuaries Coast 36: 221–236. [Google Scholar]
- Dubois P. (2014) The skeleton of postmetamorphic echinoderms in a changing world. Biol Bull 226: 223–236. [DOI] [PubMed] [Google Scholar]
- Dworjanyn SA, Byrne M (2018) Impacts of ocean acidification on sea urchin growth across the juvenile to mature adult life-stage transition is mitigated by warming. Proc Roy Soc B 285: 20172684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellers O, Johnson AS, Moberg PE (1998) Structural strengthening of urchin skeletons by collagenous sutural ligaments. Biol Bull 195: 136–144. [DOI] [PubMed] [Google Scholar]
- Emerson CE, Reinardy HC, Bates NR, Bodnar AG (2017) Ocean acidification impacts spine integrity but not regenerative capacity of spines and tube feet in adult sea urchins. R Soc Open Sci 4: 170140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriander L, Wrange A-L, Havenhand JN (2016) Simulated diurnal pH fluctuations radically increases variance in—but not the mean of—growth in the barnacle Balanus improvisus. ICES J Mar Sci 73: 596–603. [Google Scholar]
- Fantazzini P, Mengoli S, Pasquini L, Bortolotti V, Brizi L, Mariani M, Di Giosia M, Fermani S, Capaccioni B, Caroselli E, Prada F (2015) Gains and losses of coral skeletal porosity changes with ocean acidification acclimation. Nat Comm 6: 7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzer SC, Cusack M, Phoenix VR, Kamenos NA (2014a) Ocean acidification reduces the crystallographic control in juvenile mussel shells. J Struct Biol 188: 39–45. [DOI] [PubMed] [Google Scholar]
- Fitzer SC, Phoenix VR, Cusack M, Kamenos NA (2014b) Ocean acidification impacts mussel control on biomineralisation. Sci Rep 4: 6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzer SC, Zhu W, Tanner KE, Kamenos NA, Phoenix VR, Cusack M (2015a) Ocean acidification alters the material properties of Mytilus edulis shells. J R Soc Interface 12: 20141227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzer SC, Vittert L, Bowman A, Kamenos NA, Phoenix VR, Cusack M (2015b) Ocean acidification and temperature increase impact mussel shell shape and thickness: problematic for protection? Ecol Evol 5: 4875–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzer S, Chung CP, Maccherozzi F, Dhesi SS, Kamenos NA, Phoenix VR, Cusack M (2016) Biomineral shell formation under ocean acidification: a shift from order to chaos. Sci Rep 6: 21076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzer SC, Torres Gabarda S, Daly L, Hughes B, Dove M, O’Connor W, Potts J, Scanes P, Byrne M (2018) Coastal acidification impacts on shell mineral structure of bivalve mollusks. Ecol Evol 8: 8973–8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzer SC, Chan VBS, Meng Y, Rajan LC, Suzuki M, Not C, Toyofuko T, Falkenberg L, Byrne M, Harvey BP (2019a) Established and emerging techniques for characterising the formation, structure and performance of calcified structures under ocean acidification In Oceanography and Marine Biology: An Annual Review, 57 CRC Press, Boca Raton: pp 89–126. [Google Scholar]
- Fitzer S, McGill RAR, Gabarda ST, Hughes B, Dove M, O’Connor, Byrne (2019b) Selectively bred oysters can alter their biomineralization pathways, promoting resilience to environmental acidification. Global Change Biology. doi: 10.1111/gcb.14818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo SA, Byrne M, Ricevuto E, Gambi MC (2018) The carbon dioxide vents of Ischia, Italy, a natural laboratory to assess impacts of ocean acidification on marine ecosystems: an overview of research and comparisons with other vent systems In Oceanography and Marine Biology: An Annual Review, 56 CRC Press, Boca Raton: pp 237–310. [Google Scholar]
- Garilli V, Rodolfo-Metalpa R, Scuderi D, Brusca L, Parrinello D, Rastrick SPS, Foggo A, Twitchett RJ, Hall-Spencer JM, Milazzo M (2015) Physiological advantages of dwarfing in surviving extinctions in high-CO2 oceans. Nat Clim Chang 5: 678–682. [Google Scholar]
- Gattuso JP Magnan A, Billé R, Cheung WW, Howes EL, Joos F, Allemand D, Bopp L, Cooley SR, Eakin CM, Hoegh-Guldberg O (2015) Oceanography. Contrasting futures for ocean and society from different anthropogenic CO₂ emissions scenarios. Science 349: aac4722. [DOI] [PubMed] [Google Scholar]
- Gaylord B, Hill TM, Sanford E, Lenz EA, Jacobs LA, Sato KN, Hettinger A (2011) Functional impacts of ocean acidification in an ecologically critical foundation species. J Exp Biol 214: 2586–2594. [DOI] [PubMed] [Google Scholar]
- Gazeau F, Quiblier C, Jansen JM, Gattuso JP, Middelburg JJ, Heip CHR (2007) Impact of elevated CO2 on shellfish calcification. Geophys Res Lett 34: L07603. [Google Scholar]
- Gazeau F, Gattuso JP, Dawber C, Pronker AE, Peene F, Peene J, Middelburg JJ (2010) Effect of ocean acidification on the early life stages of the blue mussel Mytilus edulis. Biogeosciences 7: 2051–2060. [Google Scholar]
- Gazeau F, Parker LM, Comeau S, Gattuso JP, O’Connor WA, Martin S, Ross PM (2013) Impacts of ocean acidification on marine shelled molluscs. Mar Biol 160: 2207–2245. [Google Scholar]
- González-Delgado S, Hernández JC (2018) The importance of natural acidified systems in the study of ocean acidification: what have we learned? Adv Mar Biol 80: 57–99. [DOI] [PubMed] [Google Scholar]
- Guidetti P, Mori M (2005) Morpho-functional defences of Mediterranean sea urchins, Paracentrotus lividus and Arbacia lixula, against fish predators. Mar Biol 147: 797–802. [Google Scholar]
- Haag N, Russell MP, Hernandez JC (2016) Effects of spine damage and microhabitat on resource allocation of the purple sea urchin Strongylocentrotus purpuratus (Stimpson 1857). J Exp Mar Biol Ecol 482: 106–117. [Google Scholar]
- Hahn S, Rodolfo-Metalpa R, Griesshaber E, Schmahl WW, Buhl D, Hall-Spencer JM, Baggini C, Fehr KT, Immenhauser A (2012) Marine bivalve shell geochemistry and ultrastructure from modern low pH environments: environmental effect versus experimental bias. Biogeosciences 9: 1897–1914. [Google Scholar]
- Harvey BP, Agostini S, Wada S, Inaba K, Hall-Spencer JM (2018) Dissolution: the Achilles’ heel of the triton shell in an acidifying ocean. Front Mar Sci 5: 371. [Google Scholar]
- Hazan Y, Wangensteen OS, Fine M (2014) Tough as a rock-boring urchin: adult Echinometra sp. EE from the Red Sea show high resistance to ocean acidi cation over long-term exposures. Mar Biol 161: 2531–2545. [Google Scholar]
- Hofmann GE, Evans TG, Kelly MW, Padilla-Gamiño JL, Blanchette CA, Washburn L, Chan F, McManus MA, Menge BA, Gaylord B, Hill TM (2014) Exploring local adaptation and the ocean acidification seascape—studies in the California current large marine ecosystem. Biogeosciences 11: 1053–1064. [Google Scholar]
- Holtmann WC, Stumpp M, Gutowska MA, Syré S, Himmerskus N, Melzner F, Bleich M (2013) Maintenance of coelomic fluid pH in sea urchins exposed to elevated CO2: the role of body cavity epithelia and stereom dissolution. Mar Biol 160: 2631–2645. [Google Scholar]
- IPCC (2014) Climate change 2014: synthesis report. contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. (eds Core Writing Team, Pachauri RK, Meyer LA) 151 pp. IPCC, Geneva, Switzerland.
- Jiang T, Kaal J, Liang J, Zhang Y, Wei S, Wang D, Green NW (2017) Composition of dissolved organic matter (DOM) from periodically submerged soils in the three gorges reservoir areas as determined by elemental and optical analysis, infrared spectroscopy, pyrolysis-GC–MS and thermally assisted hydrolysis and methylation. Sci Total Environ 603–604: 461–471. [DOI] [PubMed] [Google Scholar]
- Johnson R, Harianto J, Thomson M, Byrne M (2019) The effects of long-term exposure to low pH on the skeletal microstructure of the sea urchin Heliocidaris erythrogramma. J Exp Mar Biol Ecol.
- Kroeker KJ, Kordas RL, Crim R, Hendriks IE, Ramajo J, Singh GS, Duarte CM, Gatusso JP (2013) Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob Chang Biol 19: 1884–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeker KJ, Sanford E, Jellison BM, Gaylord B (2014) Predicting the effects skeleton of ocean acidification on predator-prey interactions: a conceptual framework based on coastal molluscs. Biol Bull 226: 211–222. [DOI] [PubMed] [Google Scholar]
- Landes A, Zimmer M (2012) Acidification and warming affect both a calcifying predator and prey, but not their interaction. Mar Ecol Prog Ser 450: 1–10. [Google Scholar]
- Langer G, Nehrke G, Baggini C, Rodolfo-Metalpa R, Hall-Spencer JM, Bijma J (2014) Limpets counteract ocean acidification induced shell corrosion by thickening of aragonitic shell layers. Biogeosciences 11: 7363–7368. [Google Scholar]
- Lawrence J. (2013) Edible Sea Urchins: Biology and Ecology. Academic Press, San Diego, California. [Google Scholar]
- Leung JY, Connell SD, Nagelkerken I, Russell BD (2017) Impacts of near-future ocean acidification and warming on the shell mechanical and geochemical properties of gastropods from intertidal to subtidal zones. Environ Sci Technol 51: 12097–12103. [DOI] [PubMed] [Google Scholar]
- Li C, Chan VBS, He C, Meng Y, Yao H, Shih K, Thiyagarajan V (2014) Weakening mechanisms of the serpulid tube in a high-CO2 world. Environ Sci Technol 48: 14158–14167. [DOI] [PubMed] [Google Scholar]
- Li C, Meng Y, He C, Chan VBS, Yao H, Thiyagarajan V (2016) Mechanical robustness of the calcareous tubeworm Hydroides elegans: warming mitigates the adverse effects of ocean acidification. Biofouling 32: 191–204. [DOI] [PubMed] [Google Scholar]
- Li S, Liu C, Huang J, Liu Y, Zheng G, Xie L, Rongqing Zhang R (2015) Interactive effects of seawater acidification and elevated temperature on biomineralization and amino acid metabolism in the mussel Mytilus edulis. J Exp Biol 218: 3623–3631. [DOI] [PubMed] [Google Scholar]
- Mackenzie CL, Ormondroyd GA, Curling SF, Ball RJ, Whiteley NM, Malham SK (2014) Ocean warming, more than acidification, reduces shell strength in a commercial shellfish species during food limitation. PLoS One 9: e86764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin F, Luquet G, Marie B, Medakovic M (2008) Molluscan shell proteins: primary structure, origin, and evolution. Curr Top Dev Biol 80: 209–276. [DOI] [PubMed] [Google Scholar]
- McClintock JB, Amsler MO, Angus RA, Challener RC, Schram JB, Amsler CD, Mah CL, Cuce J, Baker BJ (2011) The Mg-calcite composition of Antarctic echinoderms: important implications for predicting the impacts of ocean acidification. J Geo 119: 457–466. [Google Scholar]
- McDonald MR, McClintock JB, Amsler CD, Rittschof D, Angus RA, Orihuela B, Lutostanki K (2009) Effects of ocean acidification over the life history of the barnacle Amphibalanus amphitrite. Mar Ecol Prog Ser 385: 179–187. [Google Scholar]
- Melzner F, Stange P, Trubenbach K, Thomsen J, Casties I, Panknin U, Gorb SN, Gutowska MA (2011) Food supply and seawater pCO2 impact calcification and internal shell dissolution in the blue mussel Mytilus edulis. PLoS One 6: e24223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Guo Z, Fitzer SC, Upadhyay A, Chan VBS, Li C, Cusack M, Yao H, Yeung KWK, Thiyagarajan V (2018) Ocean acidification reduces hardness and stiffness of the Portuguese oyster shell with impaired microstructure: a hierarchical analysis. Biogeosciences 15: 6833–6846. [Google Scholar]
- Meng Y, Guo Z, Yao H, Yeung KWK, Thiyagarajan V (2019) Calcium carbonate unit realignment under acidification: a potential compensatory mechanism in an edible estuarine oyster. Mar Pollut Bull 139: 141–149. [DOI] [PubMed] [Google Scholar]
- Migliaccio O, Pinsino A, Maffioli E, Smith AM, Agnisola C, Matranga V, Nonnis S, Tedeschi G, Byrne M, Gambi MC, Palumbo A (2019) Living in future ocean acidification, physiological adaptive responses of the immune systems of sea urchins resident at a CO2 vent system. Sci Tot Environ 672: 938–950. [DOI] [PubMed] [Google Scholar]
- Milano S, Schöne BR, Wang S, Müller WE (2016) Impact of high pCO2 on shell structure of the bivalve Cerastoderma edule. Mar Environ Res 119: 144–155. [DOI] [PubMed] [Google Scholar]
- Mooney CJ, Kingsford MJ (2016) Statolith morphometrics can discriminate among cubozoan jellyfishes. PloS One 11: e0155719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulin L, Grosjean P, Leblud J, Batigny A, Dubois P (2014) Impact of elevated pCO2 on acid-base regulation of the sea urchin Echinometra mathaei and its relation to resistance to ocean acidification: a study in mesocosms. J Exp Mar Biol Ecol 457: 97–104. [Google Scholar]
- Pan TCF, Applebaum SL, Manahan DT (2015) Experimental ocean acidification alters the allocation of metabolic energy. Proc Natl Acad Sci U S A 112: 4696–4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pansch C, Nasrolahi A, Appelhans Y, Whal M (2013) Tolerance of juvenile barnacles (Amphibalanus improvisus) to warming and elevated pCO2. Mar Biol 160: 2023–2035. [Google Scholar]
- Pansch C, Schaub I, Havenhand J, Wahl M (2014) Habitat traits and food availability determine the response of marine invertebrates to ocean acidification. Glob Chang Biol 20: 765–777. [DOI] [PubMed] [Google Scholar]
- Parker L, Ross P, O’Connor W, Borysko L, Raftos D, Pörtner H (2012) Adult exposure influences offspring response to ocean acidification in oysters. Glob Chang Biol 18: 82–92. [Google Scholar]
- Parker L, Ross P, O’Connor W, Pörtner H, Scanes E, Wright J (2013) Predicting the response of molluscs to the impact of ocean acidification. Biology 2: 651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przeslawski R, Byrne M, Mellin C (2015) A review and meta-analysis of the effects of multiple abiotic stressors on marine embryos and larvae. Glob Chang Biol 21: 2122–2140. [DOI] [PubMed] [Google Scholar]
- Queirós AM, Fernandes JA, Faulwetter S, Nunes J, Rastrick SP, Mieszkowska N, Artioli Y, Yool A, Calosi P, Arvanitidis C, Findlay HS (2015) Scaling up experimental ocean acidification and warming research: from individuals to the ecosystem. Glob Chang Biol 21: 130–143. [DOI] [PubMed] [Google Scholar]
- Ries JB, Cohen AL, McCorkle DC (2009) Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 37: 1131–1134. [Google Scholar]
- Ries JB. (2010) Review: geological and experimental evidence for secular variation in seawater Mg/Ca (calcite- aragonite seas) and its effects on marine biological calcification. Biogeosciences 7: 2795–2849. [Google Scholar]
- Rodolfo-Metalpa R, Houlbrèque F, Tambutté É, Boisson F, Baggini C, Patti FP, Jeffree R, Fine M, Foggo A, Gattuso JP, Hall-Spencer JM (2011) Coral and mollusc resistance to ocean acidification adversely affected by warming. Nat Clim Change 1: 308–312. [Google Scholar]
- Rodríguez A, Hernández JC, Brito A, Clemente S (2017) Effects of ocean acidification on juveniles sea urchins: predator-prey interactions. J Exp Mar Biol Ecol 493: 31–40. [Google Scholar]
- Roleda MY, Boyd PW, Hurd CL (2012) Before ocean acidification: calcifier chemistry lessons. J Phycol 48: 840–843. [DOI] [PubMed] [Google Scholar]
- Rühl S, Calosi P, Faulwetter S, Keklikoglou K, Widdicombe S, Queirós AM (2017) Long-term exposure to elevated pCO2 more than warming modifies early-life shell growth in a temperate gastropod. ICES J Mar Sci 74: 1113–1124. [Google Scholar]
- Sadler DE, Lemasson AJ, Knights AM (2018) The effects of elevated CO2 on shell properties and susceptibility to predation in mussels Mytilus edulis. Mar Environ Res 139: 162–168. [DOI] [PubMed] [Google Scholar]
- Sanford E, Gaylord B, Hettinger A, Lenz EA, Meyer K, Hill TM (2014) Ocean acidification increases the vulnerability of native oysters to predation by invasive snails. Proc R Soc B 281: 20132681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato KN, Andersson AJ, Day JMD, Taylor JRA, Frank MB, Jung J-Y, McKittrick J, Levin LA (2018) Response of sea urchin fitness traits to environmental gradients across the Southern California oxygen minimum zone. Front Mar Sci 5: 258. [Google Scholar]
- Smith AM, Riedl MA, Winter DJ (2013) Temperate reefs in a changing ocean: skeletal carbonate mineralogy of serpulids. Mar Biol 160: 2281–2294. [Google Scholar]
- Smith AM, Clark DE, Lamare MD, Winter DJ, Byrne M (2016) Risk and resilience: variations in magnesium in echinoid skeletal calcite. Mar Ecol Prog Ser 561: 1–16. [Google Scholar]
- Sötje I, Dishon T, Hoffmann F, Holst S (2017) New methods of morphometric analyses on scyphozoan jellyfish statoliths including the first direct evidence for statolith growth using calcein as a fluorescent marker. Microsc Microanal 23: 553–568. [DOI] [PubMed] [Google Scholar]
- Stemmer K, Nehrke G, Brey T (2013) Elevated CO2 levels do not affect the shell structure of the bivalve Arctica islandica from the Western Baltic. PLoS One 8: e70106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpp M, Trubenbach K, Brennecke D, Hu MY, Melzner F (2012) Resource allocation and extracellular acid-base status in the sea urchin Strongylocentrotus droebachiensis. Aquat Toxicol 110–111: 194–207. [DOI] [PubMed] [Google Scholar]
- Tambutté E, Venn AA, Holcomb M, Segonds N, Techer N, Zoccola D, Allemand D, Tambutté S (2015) Morphological plasticity of the coral skeleton under CO2-driven seawater acidification. Nat Commun 6: 7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uthicke S, Ebert T, Liddy M, Johansson C, Fabricius KE, Lamare M (2016) Echinometra sea urchins acclimatized to elevated pCO2 at volcanic vents outperform those under present-day pCO2 conditions. Glob Chang Biol 22: 2451–2461. [DOI] [PubMed] [Google Scholar]
- Viotti S, Sangil C, Hernández CA, Hernández JC (2019) Effects of long-term exposure to reduced pH conditions on the shell and survival of an intertidal gastropod. DOI: 10.1016/j.marenvres.2019.104789 [DOI] [PubMed] [Google Scholar]
- Von Euw S, Zhang Q, Manichev V, Murali N, Gross J, Feldman LC (2017) Biological control of aragonite formation in stony corals. Science 356: 933–938. [DOI] [PubMed] [Google Scholar]
- Wehrmeister U, Jacob DE, Soldati AL, Loges N, Häger T, Hofmeister W (2011) Amorphous, nanocrystalline and crystalline calcium carbonates in biological materials. J Raman Spectrosc 42: 926–935. [Google Scholar]
- Welladson HM, Southgate PC, Heimann K (2011) The effects of exposure to near-future levels of ocean acidification on shell characteristics of Pinctada fucata (Bivalvia: Pteriidae). Molluscan Res 30: 125–130. [Google Scholar]
- Whiteley NM. (2011) Physiological and ecological responses of crustaceans to ocean acidification. Mar Ecol Prog Ser 430: 257–271. [Google Scholar]
- Wilbur KM. (1972) Shell formation in molluscs In Flourkin and Scheer, Chem Zool 103-145 Academic Press, New York. [Google Scholar]
- Wilkinson BH. (1979) Biomineralization, paleoceanography, and the evolution of calcareous marine organisms. Geology 7: 524–527. [Google Scholar]
- Wittmann AC, Pörtner HO (2013) Sensitivities of extant animal taxa to ocean acidification. Nat Clim Chang 3: 995–1001. [Google Scholar]
- Wolfe K, Smith AM, Trimby P, Byrne M (2012) Vulnerability of the paper nautilus (Argonauta nodosa) to a climate change ocean, potential for extinction by dissolution. Biol Bull 223: 236–244. [DOI] [PubMed] [Google Scholar]
- Wolfe K, Smith AM, Trimby P, Byrne M (2013a) Microstructure of the paper nautilus (Argonauta nodosa) shell and the novel application of electron backscatter diffraction (EBSD) to address effects of ocean acidification. Mar Biol 160: 2271–2278. [Google Scholar]
- Wolfe K, Dworjanyn S, Byrne M (2013b) Effects of ocean warming and acidification on survival, growth and skeletal development in the early benthic juvenile sea urchin (Heliocidaris erythrogramma). Glob Chang Biol 19: 2698–2707. [DOI] [PubMed] [Google Scholar]
- Wright J, Parker L, O’Connor W, Ross P (2018) Ocean acidification affects both the predator and prey to alter interactions between the oyster Crassostrea gigas (Thunberg, 1793) and the whelk Tenguella marginalba (Blainville, 1832). Mar Biol 165: 3. [Google Scholar]
- Ye F, Jurikova H, Angiolini L, Brand U, Crippa G, Henkel D, Smajgl D (2018) Variation in brachiopod microstructure and isotope geochemistry under low pH–ocean acidification–conditions. Biogeosciences 16: 617–642. [Google Scholar]
- Zeebe RE, Rigwell A, Zachos J (2016) Anthropogenic carbon release rate unprecedented during the past 66 million years. Nat Geosci 9: 325–329. [Google Scholar]


