Abstract
Background:
Gestational diabetes mellitus (GDM) is a common complication during pregnancy. Obesity and overweight are closely related to metabolic diseases and diabetes. However, the role of adipose tissue in the pathogenesis of GDM remains to be studied. The aim of this study was to investigate the correlation of vitamin D (VD) levels, VD receptor (VDR), and peroxisome proliferator-activated receptor γ (PPARγ) expression with GDM in overweight or obese women.
Methods:
One hundred and forty pregnant women with full-term single-birth cesarean-section were selected as the study subjects and grouped (70 GDM women, including 35 non-overweight/non-obese women [group G1] and 35 women with overweight or obesity [group G2]; 70 pregnant women with normal glucose tolerance, including 35 non-overweight/non-obese women [group N1] and 35 overweight/obese women [group N2]). The levels of serum VD, blood biochemistry, and adiponectin were compared in these women. Subcutaneous adipose tissue was isolated from the abdominal wall incision. VDR and PPARγ messenger RNA (mRNA) transcript levels in these adipose tissues were quantified by real-time polymerase chain reaction. The differences between the levels of PPARγ protein and phosphorylated PPARγ Ser273 were detected by Western blotting.
Results:
The serum VD level of GDM women was lower in comparison to that of women with normal glucose tolerance (G1 vs. N1: 20.62 ± 7.87 ng/mL vs. 25.85 ± 7.29 ng/mL, G2 vs. N2: 17.06 ± 6.74 ng/mL vs. 21.62 ± 7.18 ng/mL, P < 0.05), and the lowest in overweight/obese GDM women. VDR and PPARγ mRNA expression was higher in the adipose tissues of GDM women in comparison to that of women with normal glucose tolerance (VDR mRNA: G1 vs. N1: 210.00 [90.58–311.46] vs. 89.34 [63.74–159.92], G2 vs. N2: 298.67 [170.84–451.25] vs. 198.28 [119.46–261.23], PPARγ mRNA: G1 vs. N1: 100.72 [88.61–123.87] vs. 87.52 [66.37–100.04], G2 vs. N2: 117.33 [100.08–149.00] vs. 89.90 [76.95–109.09], P < 0.05), and their expression was the highest in GDM + overweight/obese women. VDR mRNA levels positively correlated with the pre-pregnancy body mass index (BMI), pre-delivery BMI, fasting blood glucose (FBG), homeostasis model assessment of insulin resistance (HOMA-IR), and PPARγ mRNA while it negatively correlated with the VD and the adiponectin levels (r = 0.395, 0.336, 0.240, 0.190, 0.235, –0.350, –0.294, respectively, P < 0.05). The degree of PPARγ Ser273 phosphorylation increased in obese and GDM pregnant women. PPARγ mRNA levels positively correlated with pre-pregnancy BMI, pre-delivery BMI, FBG, HOMA-IR, serum total cholesterol, triglyceride, free fatty acid, and VDR mRNA, while it negatively correlated with the VD and adiponectin levels (r = 0.276, 0.199, 0.210, 0.230, 0.182, 0.214, 0.270, 0.235, –0.232, –0.199, respectively, P < 0.05).
Conclusions:
Both GDM and overweight/obese women had decreased serum VD levels and up-regulated VDR and PPARγ mRNA expression in adipose tissue, which was further higher in the overweight or obese women with GDM. VD may regulate the formation and differentiation of adipocytes through the VDR and PPARγ pathways and participate in the occurrence of GDM.
Keywords: Gestational diabetes mellitus, Overweight, Obesity, Serum vitamin D, Vitamin D receptor, Peroxisome proliferator-activated receptor gamma
Introduction
Gestational diabetes mellitus (GDM) is associated with normal glucose metabolism before pregnancy while diabetes-associated symptoms are exhibited during pregnancy. The incidence of GDM in China is on the rise, and has reached 17.5% according to the latest statistics.[1] GDM is a disease caused by multiple factors which seriously threatens maternal and child safety. Women with GDM are at an increased risk of developing metabolic syndrome, type 2 diabetes mellitus (T2DM), and cardiovascular disease.[2] Women with GDM were more likely to undergo cesarean deliveries, and their newborns had a higher birth weight.[3] GDM is associated with increased risks of depression,[4] significantly and independently associated with childhood impaired glucose tolerance.[5] Infants born to mothers with GDM also have a higher risk of developing T2DM in their teens or early adulthood.[6] Insulin resistance (IR) and decreased islet β cell secretion are considered to be important links in the pathogenesis of GDM.[7] Most studies have shown that low vitamin D (VD) level is also associated with GDM,[8] the risk of GDM increases with the increase of pre-pregnancy body mass index (BMI).[9] A meta-analysis of obesity and GDM relationship in pregnant women showed that the unadjusted odds ratio (OR) of GDM occurrence in women with overweight, obesity, and severe obesity, when compared with normal-weight pregnant women, were 2.14 (95% confidence interval [CI]: 1.82–2.53), 3.56 (95% CI: 3.05–4.21), and 8.56 (95% CI: 5.07–16.04), respectively.[10] Overweight or obesity refers to a condition of excess amount of adipose in the body and is caused by various reasons. The adipose tissue is an active endocrine organ that secretes various kinds of proteins and peptides,[11] including the adipocytokines such as leptin, adiponectin, and tumor-associated cytokines that are involved in insulin signal transduction, glycolipid metabolism, etc. Recent research supports that the adipose tissue is the origin of IR,[12] and VD interacts with adipose tissue and affects each other. A variety of enzymes that can synthesize 25(OH)D, 1,25-(OH)2D3 are expressed in the adipose tissue. Large amounts of adipocytes in obese individuals can inhibit the expression of 25 hydroxylase (CYP2J2) and 1α-hydroxylase (the expression of CYP27B1), thus leading to metabolic impairment of VD.[13] Fat can also be used as a reservoir of VD and limits its biological activity.[14] However, 1,25-(OH)2D3 affects adipocytes, and adipokine formation and cytokine secretion through the VD receptor (VDR) of adipocytes.[15] Peroxisome proliferator-activated receptor γ (PPARγ) is an adipocyte-specific nuclear transcription factor belonging to the nuclear receptor family. It is abundant in the adipose tissue and is a key factor in controlling the production of adipocytes and fatty acid metabolism. It is closely associated to the metabolic syndrome. PPARγ is necessary for the lipid formation both in vivo and in vitro.[16]
In the low vitamin status, obesity is closely related to GDM. Whether the alteration of serum VD in women with GDM is caused by the VDR and PPARγ in the adipose tissues is not clear. Here, we studied the changes in serum VD, VDR levels, and PPARγ expression in the subcutaneous adipose tissue of overweight or obese women with GDM, aiming to investigate if there is a correlation between VDR levels and PPARγ expression in the subcutaneous adipose tissue of overweight/obese and diabetic pregnant women and their effects on GDM.
Methods
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Nanjing Medical University. Written informed consent was obtained from all participants.
General information
Pregnant women admitted to the Changzhou Woman and Children Health-Care Hospital Affiliated to Nanjing Medical University from January 2015 to April 2017 for full-term delivery were selected as the subjects. Oral glucose tolerance test (75 g glucose) was performed at 24 to 28 weeks of gestation as it is a diagnostic criterion for GDM recognized by The International Association of Diabetes and Pregnancy Study Groups. Normal fasting blood glucose (FBG) and blood glucose levels at 1 and 2 h after glucose administration are less than 5.1, 10.0, and 8.5 mmol/L, respectively. Any individual with blood glucose levels higher than the reference range for any one of the above time points can be diagnosed as having GDM.[17] Seventy full-term single-birth women diagnosed with GDM, including 35 women with normal BMI (18.5–24.9 kg/m2) (group G1) and 35 women with overweight or obesity (BMI ≥25.0 kg/m2) (group G2). Another 70 normal glucose tolerance pregnant women with cesarean section due to malposition or scar uterus were selected as group non-GDM, among whom 35 had normal BMI (group N1) and 35 were women with overweight or obesity (group N2). Exclusion criteria were patients with a history of diabetes or hypertension before pregnancy or other pregnancy-related complications.
Sample conditions and data collection
The height and weight of each pregnant woman were measured and BMI was calculated by the assigned nurse after hospital admission. A face-to-face questionnaire was also performed. The questionnaire included questions related to the general health conditions during pregnancy, weight before pregnancy, and weight during childbirth. Specialists were assigned for the inquiry of VD and calcium tablets taken during the pregnancy and gestational weeks. The dosage of taken tablets was then investigated and converted to VD3 content (U) and calcium content (mg) according to the instructions. The product of supplemented oral VD (U) multiplied by the supplement gestational weeks was used to represent the oral supplemental dose of VD during pregnancy. The product of supplemental calcium content (mg) during pregnancy multiplied by the supplement gestational weeks was used to represent the supplemental dose of calcium during pregnancy. If no ectogenic oral VD or calcium were administered at 4 weeks before delivery, it was considered as no recent intake of VD and calcium. The daily sun exposure of each pregnant woman was also asked in detail, based on which the pregnant women were divided into four different grades: women with <0.5 h of sun exposure per week was considered as 0, ≥0.5 to <1 h of sun exposure per week was considered as 1; ≥1 to <2 h of sun exposure per week was considered as 2, and ≥2 h of sun exposure per week was considered as 3.
Detection of VD, biochemical indicators, and adiponectin
Four milliliters of fasting elbow venous blood anti-coagulated by ethylenediaminetetraacetic acid was sampled pre-operatively, followed by centrifugation at 1500 r/min for isolating the plasma and cryopreservation at –70°C. FBG levels were determined by the glucose oxidase method. Serum total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were determined by one automatic biochemical analyzer. Fasting insulin (FINS) was measured by the electrochemiluminescence method and free fatty acid (FFA) levels were measured by the enzyme colorimetry method (Beckman reagent, Shenzhen, China). The homeostasis model assessment of insulin resistance (HOMA-IR) = FBG × FINS/22.5. The serum VD level was detected by the electrochemiluminescence method (Roche Diagnostics GmbH, Mannheim, Germany), which was the sum of 25-OH-D2 and 25-OH-D3; according to the kit standards <26 ng/mL indicated VD deficiency. Adiponectin levels were detected by the enzyme-linked immunosorbent assay (R&D System, Minneapolis, MN, USA).
Collection of adipose tissue specimen
After having obtained the informed consent, we sampled two pieces of subcutaneous fat tissue at the abdominal wall during cesarean-section, with the size as approximately 1.0 cm × 1.0 cm × 1.0 cm; after saline rinsing, one piece of the sample was fixed in 10% formaldehyde, followed by paraffin embedding within 24 h. The other piece of the sample was suspended in the RNA later solution and stored at –20°C for 24 h and then cryopreserved at –70°C for later use. Immunohistochemistry was used to observe the location of VDR and PPARγ in the adipose tissue of the four groups, as well as to semi-quantitatively detect the expression level. The transcription level of VDR messenger RNA (mRNA) and PPARγ mRNA in each group was detected by quantitative real-time polymerase chain reaction (PCR). Western blotting was used to detect the expression of PPARγ protein and PPARγ Ser273 phosphorylation.
Immunohistochemistry
The paraffin-embedded specimens were serially sliced at 4 μm, de-waxed until water was removed, re-suspended in citrate saline bath for 20 min, incubated for 10 min, closed in freshly prepared 3% H2O2 for 5 min, washed with phosphate buffer saline (PBS), and then incubated with 1:100 mouse anti-human VDR (PPARγ) (Thermo Fischer Scientific, former Savant, MA, USA) overnight at 4°C. After PBS washing, the horseradish peroxidase (HRP)-labeled goat anti-mouse immunoglobulin G (IgG) (1:100, Thermo Scientific) was added for 30-min incubation at room temperature, followed by PBS washing, diaminobenzidine coloration, and hematoxylin counterstaining. The nuclei of positively stained cells showed brownish-yellow particles, and the negative control replaced the primary antibody with PBS. The cells were counted by the double-blind method with the microscopic brownish-yellow particles as the positive result. According to the percentage of positively stained cells, <5% was scored as 0 point, 5% to 25% was scored as 1 point, 26% to 50% was scored as 2 points, 51% to 75% was scored as 3 points, and >75% was scored as 4 points. The score was combined with the staining intensity: no coloration was scored as 0, weakly positive was scored as 1 point, positive was scored as 2 points, and strongly positive was scored as 3 points. The product of the positive rate score and the intensity score was finally calculated as the final score of the related group.
Quantitative real-time polymerase chain reaction
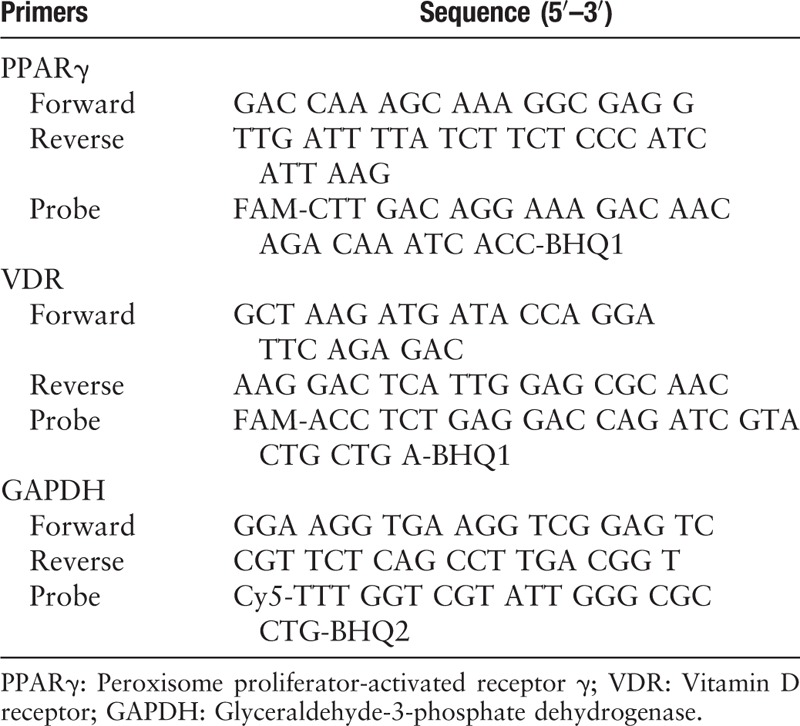
The total RNA of adipose tissue was extracted using the Trizol Reagent (InvivoGen, San Diego, CA, USA). After identifying the RNA integrity by electrophoresis, complementary DNA (cDNA) was synthesized by reverse transcription of 2 μg of total RNA. The primer reaction system: 10× PCR 2.5 μL, MgCl2 (25 mmol/L) 2.5 μL, deoxy-ribonucleoside triphosphate (10 mmol/L), 0.5 μL, Taq polymerase (5 U/μL) 0.25 μL, VDR (PPARγ) forward primer (F) (100 μmol/L) 0.04 μL, VDR (PPARγ) reverse primer (R) (100 μmol/L) 0.04 μL, VDR (PPARγ) probe (P) (100 μmol/L) 0.04 μL; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (F) (100 μmol/L) 0.04 μL, GAPDH (R) (100 μmol/L) 0.04 μL, GAPDH (P) (100 μmol/L) 0.04 μL, sterilized double distilled water supplement 17.01 μL, cDNA 2 μL, a total reaction volume of 25 μL. The amplification conditions of the PCR instrument (Roche 480II) were: pre-denaturation at 95°C for 3 min; 95°C for 5 s, 60°C for 15 s (temperature conversion rate is 20°C/s), and amplification rounds of 40 cycles at 40°C for 1 min per cycle. The fluorescence signals were acquired during the period of 60°C extension. The results were expressed as the ratio of the relative expression of the gene of interest to the expression of the internal reference gene GAPDH. Primer probe sequences in reaction system of real-time PCR are shown in Table 1.
Table 1.
The primer probe sequences in reaction system of real-time polymerase chain reaction.
Western blotting
Protein was extracted at low temperature, quantified by Bradford method, then transferred to nitrocellulose filter (NC) membrane by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and washed with Tris buffered saline Tween 20 (TBST) for 5 min ×3 times. NC film was placed in a dish covered by sealing liquid (5% skimmed milk powder) and shaken for 2 h, then TBST was used to wash the membrane for 5 min ×3 times. The membrane was incubated overnight in a shaking bed at 4°C in a dish containing 1:10,000 rabbit anti-GAPDH (Jiangsu Kaiji Biotechnology Co., Ltd., China), 1:200 mouse anti-PPARγ (Santa Cruz Biotechnology, Dallas, TX, USA), 1:200 rabbit anti-p-PPARγ (Beijing Boosen Biotechnology Co., Ltd., China). The next day, it was taken out and shaken at room temperature for 30 min. The primary antibody was absorbed and washed with TBST for 10 min ×3 times. The secondary antibody was diluted with the diluent of the second antibody (sheep anti-mouse IgG-HRP, sheep anti-rabbit IgG-HRP; Jiangsu Kaiji Biotechnology Co., Ltd.) and the shaking reaction at room temperature was 2 h. After the secondary antibody reaction, the secondary antibody was recovered. Thereafter, TBST was used to wash the film for 5 min ×3 times. The liquid A and B in the electrochemiluminescence kit were mixed in equal volume of 1:1 to form a working liquid for reserve. NC film was removed from TBST, and the relative content of the target protein was calculated with GADPH as internal parameter, by using G: BOX chemiXR5 (Syngene company, UK) to image the blot.
Observation indexes
The difference of VD level, biochemical indexes, and VDR and PPARγ expression between groups N1 and N2, groups G1 and G2 were compared and the difference between pregnant women with or without overweight/obesity were explored. Different study groups were weight matched, and the difference of VD level, biochemical indexes, VDR and PPARγ expression between group N1 and G1, as well as between group N2 and G2, were compared to explore the difference between diabetic and non-diabetic pregnant women with different BMI. Western blotting was used to detect the PPARγ protein and phosphorylation of PPARγ Ser273 in adipose tissue of the four groups. The correlation of serum VD and VDR and PPARγ with overweight/obesity in women with GDM were explored.
Statistical analysis
All the data were entered into Excel and analyzed using SPSS19.0 (SPSS Inc., Chicago, IL, USA). The data with normal distribution (age, gravidity, parity, gestational age, pre-pregnancy BMI, pre-delivery BMI, neonatal birth weight, VD, TC, LDL-C, adiponectin, VDR, PPARγ, and p-PPARγ) were expressed as mean ± standard deviation, and the median (M) and interquartile range (P25–P75) were used to describe the data with non-normal distribution (VD supplement, calcium supplement, FBG, FINS, HOMA-IR, TG, HDL-C, FFA, VDR mRNA, and PPARγ mRNA). The difference between women with GDM and women with group non-GDM was compared statistically by the t test or the Wilcoxon rank-sum test. The count data was expressed by n (%). The comparison between groups was performed by the Chi-square test, the spearman rank correlation analysis was used to analyze the correlation between indicators. Binary logistic regression was used to evaluate the relative risk (OR) and 95% CI. The test level was α = 0.05, and P < 0.05 was considered as statistical significant.
Results
Comparison of general conditions
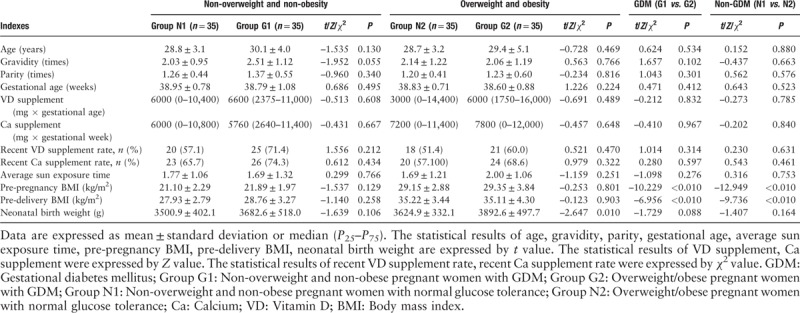
The comparison of the age, gravidity and parity, gestational age, VD supplement, calcium supplement, recent VD supplement rate, calcium supplement rate, average sun exposure time, and neonatal birth weight showed no statistical difference between the group G1 and group G2, the same results were found between group N1 and group N2 (P > 0.05). The pre-pregnancy BMI of group N2 was higher than that of group N1, and the pre-pregnancy BMI of group G2 was higher than that of group G1, whereas the pre-pregnancy BMI of the overweight/obese sub-group were higher than the non-overweight/non-obese sub-group (P < 0.05), there was no significant difference in the BMI between the two sub-groups of the non-overweight/non-obese group. While compared in pre-delivery BMI, the same results were found. The neonatal birth weight in the group G2 was significantly higher than that in the group N2 (3892.6 ± 497.7 g vs. 3624.9 ± 332.1 g, P < 0.05). There were no significant differences in other indexes of sub-groups of the overweight/obese group (P > 0.05) [Table 2].
Table 2.
Clinical characteristics of pregnant women with full-term single-birth cesarean-section (n = 140).
Detection of biochemical indexes during pregnancy
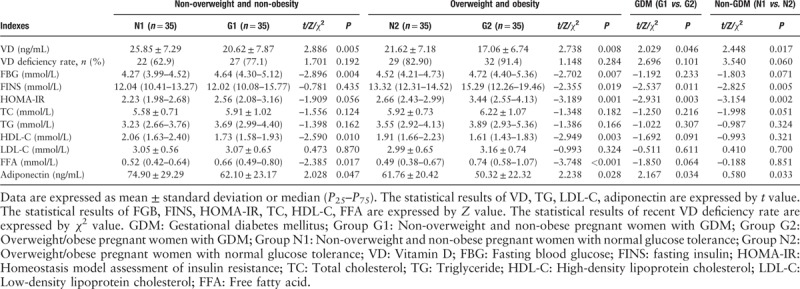
The levels of serum VD, HDL-C, and adiponectin decreased, while the FBG and FFA levels increased in group G1 when compared to the group N1 (P < 0.05). The levels of serum VD, HDL-C, and adiponectin decreased, while the FBG, FINS, HOMA-IR, and FFA levels increased in group G2 when compared to the group N2 (P < 0.05). The levels of serum VD and adiponectin decreased, while the FINS and HOMA-IR increased in group G2 when compared to the group G1 (P < 0.05). The levels of serum VD and adiponectin decreased while the FINS and HOMA-IR levels increased in group N2 when compared to the group N1 (P < 0.05).
In this study, 110 pregnant women with VD deficiency in the third trimester showed a VD deficiency rate of 78.6%, but there was no significant difference between overweight/obese group and non-overweight/non-obese group, or between group GDM and group non-GDM; the data in group G2 were significantly higher than that in the group N1 (32/35 vs. 22/35, χ2 = 8.102, P = 0.004), suggesting that GDM women with overweight/obesity showed more obvious VD deficiency than non-overweight/non-obesity pregnant women with normal glucose tolerance [Table 3].
Table 3.
Biochemical variables in pregnant women with full-term single-birth cesarean-section (n = 140).
Immunohistochemistry
VDR and PPARγ are the brown-yellow particles in the nuclei of adipocytes in each group [Figure 1]. VDR expression in group GDM increased significantly, and its expression in the group G1 was higher than its expression in the group N1. Similarly, its expression in the group G2 was higher than its expression in the group N2 (P < 0.05). PPARγ expression in group GDM was higher than that in group non-GDM, and its expression in the group G1 was higher than that in the group N1, and its expression in the group G2 was higher than that in the group N2 (P < 0.05).
Figure 1.
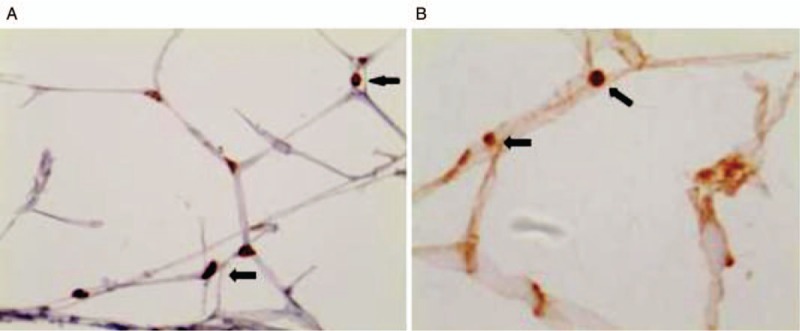
Immunohistochemistry was used to observe the location of VDR and PPARγ in adipose tissue (Streptomyces antibiotic protein-peroxidase ligation staining, original magnification ×400). Brown-yellow granules (arrows) were seen in the adipose tissue nucleus, indicating the location of VDR (A) and PPARγ (B) in the adipose tissue nucleus. PPARγ: Peroxisome proliferator-activated receptor γ; VDR: Vitamin D receptor.
Quantitative real-time polymerase chain reaction
The transcript levels of VDR mRNA in the group GDM was higher than that in the group non-GDM (the level of VDR mRNA in the group G1 was higher than that in the group N1, while its expression in the group G2 was higher than that in the group N2, P < 0.05). The VDR mRNA level of pregnant women with overweight/obesity was higher than that of non-overweight/non-obese pregnant women (the level of VDR mRNA in group N2 was higher than that in group N1, which its expression in group G2 was higher than that in group G1, P < 0.05). And the GDM pregnant women with overweight/obesity showed the statistically highest level of VDR mRNA.
Quantitative PCR analysis of adipose tissue showed that the expression level of PPARγ mRNA in group GDM was higher than that in group non-GDM (group G1 vs. group N1, group G2 vs. group N2, P < 0.05). The expression level of PPARγ mRNA in the group G2 was higher than that in the group G1 (P < 0.05). The GDM pregnant women with obesity showed the statistically highest level of PPARγ mRNA [Table 4 and Figure 2].
Table 4.
Immunohistochemical semi-quantitative expression intensity and qPCR expression intensity of VDR and PPARγ in the four groups.
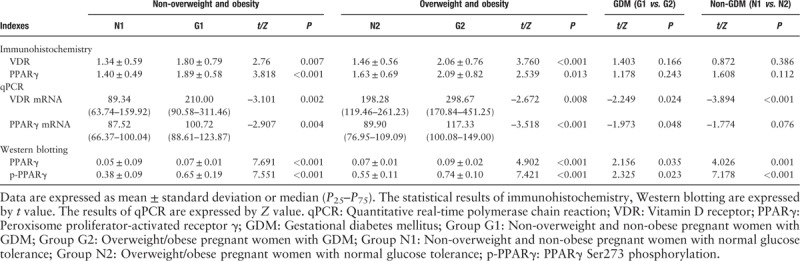
Figure 2.
Adipose tissue VDR/GAPDH and PPARγ/GAPDH expression in the four groups. ∗P < 0.05, compared with group N1; †P < 0.05, compared with group N2; ‡P < 0.05, compared with group G1. GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; GDM: Gestational diabetes mellitus; Group G1: Non-overweight and non-obesity pregnant women with GDM; Group G2: Overweight/obesity pregnant women with GDM; Group N1: Non-overweight and non-obesity pregnant women with normal glucose tolerance; Group N2: Overweight/obesity pregnant women with normal glucose tolerance; PPARγ: Peroxisome proliferator-activated receptor γ; VDR: Vitamin D receptor.
Western blotting
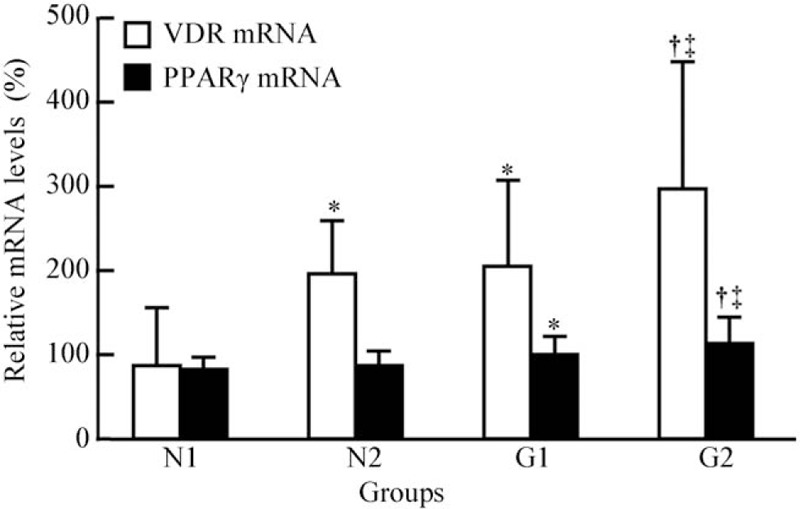
PPARγ levels in group GDM was higher than that in group non-GDM (group G1 vs. group N1, group G2 vs. group N2, P < 0.05). The expression levels of PPARγ in pregnant women with overweight/obesity was higher than that in the non-overweight/non-obese pregnant women (group N2 vs. group N1, group G2 vs. group G1, P < 0.05). The same changes were observed in the levels of Ser273 phosphorylated PPARγ [Table 4 and Figure 3].
Figure 3.
Western blotting of PPARγ/p-PPARγ in the four groups. GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; GDM: Gestational diabetes mellitus; Group G1: Non-overweight and non-obesity pregnant women with GDM; Group G2: Overweight/obesity pregnant women with GDM; Group N1: Non-overweight and non-obesity pregnant women with normal glucose tolerance; Group N2: Overweight/obesity pregnant women with normal glucose tolerance; PPARγ: Peroxisome proliferator-activated receptor γ; p-PPARγ: PPARγ Ser273 phosphorylation.
Correlation test
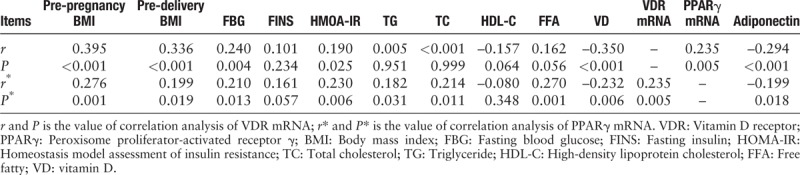
VDR mRNA levels positively correlated with the pre-pregnancy BMI, pre-delivery BMI, FBG, HOMA-IR, and PPARγ mRNA while negatively correlated with the VD and adiponectin levels (r = 0.395, 0.336, 0.240, 0.190, 0.235, –0.350, –0.294, P < 0.05) [Table 5], the PPARγ mRNA levels positively correlated with the pre-pregnancy BMI, pre-delivery BMI, FBG, HOMA-IR, TC, TG, FFA, and VDR mRNA while it negatively correlated with the VD and adiponectin levels (r = 0.276, 0.199, 0.210, 0.230, 0.182, 0.214, 0.270, 0.235, –0.232, –0.199, P < 0.05).
Table 5.
Spearman correlations between expression of VDR and PPARγ mRNA with BMI and biochemical parameters in all the pregnant women.
Logistic regression analysis
In the overweight/obese women, univariate logistic regression analysis showed that VD, adiponectin, VDR mRNA, PPAR mRNA, HDL-C, FFA, FINS, HOMA-IR levels correlated with GDM (P < 0.05). Multivariate logistic regression analysis showed that HDL-C (OR = 0.065, 95% CI: 0.007–0.592) and FFA (OR = 39.853, 95% CI: 2.363–672.154) correlated with GDM.
In the non-overweight/non-obese women, univariate logistic regression analysis showed that VD, VDR mRNA, PPAR mRNA, HDL-C, FFA, and HOMA-IR levels correlated to GDM, P < 0.05. Multivariate logistic regression analysis showed that VDR mRNA (OR = 1.007, 95% CI: 1.000–1.013), PPAR mRNA (OR = 1.029, 95% CI: 1.003–1.057), HOMA-IR (OR = 2.568, 95% CI: 1.004–6.564) levels correlated to GDM.
Discussion
We revealed that VD deficiency exists in pregnant women in their third trimester, and under the same conditions, such as the age of pregnancy, gravidity and parity, gestational weeks, calcium and VD supplement, and sun exposure, the VD level in GDM patients with overweight/obesity decreased significantly, but the VDR and PPARγ mRNA levels in the adipose tissue were up-regulated. Studies have shown that higher the BMI of obese people, lower the concentration of VD.[18] High BMI[19] and low vitamin status were closely related to GDM. Active VD bind to the VDR, which is a ligand-dependent nuclear transcription factor and has roles in regulating the metabolism of calcium and phosphorus, cell proliferation and differentiation, and immune function together with 1,25(OH)2D3.[20] Knabl et al[21] has reported that the maternal VD level in GDM patients is lower, and low-dose exogenous VD can up-regulate VDR in the trophoblasts. Specific transfer of VDR to VDR-knockout mice can promote the growth of adipose tissue and induce fat accumulation in the body,[22] thus leading to obesity. BMI and HOMA-IR were independent positive predictors of subcutaneous fat VDR gene expression.[23] This study is consistent with previous reports.
PPARγ is a member of the nuclear receptor superfamily. The binding of 1,25-(OH)2D3 to VDR and inhibition of adipogenesis are closely related to the activity of PPARγ. The 1,25-(OH)2D3 regulates lipogenesis mainly by reducing the formation of PPARγ ligand in early stages of adipocyte differentiation,[24] decreasing the transcriptional activity of PPARγ,[25] or directly regulating its upstream factors.[26] Studies have shown that the expression level of PPARγ gene positively correlated with the size of adipocyte volume and its differentiation degree,[27] and that the excessive activation of PPARγ is involved in the occurrence of obesity. Phosphorylation is the most common post-transcriptional modification of PPARγ. CDK5-mediated phosphorylation of PPARγ Ser273 in adipose tissue is considered to be associated with obesity. CDK5-mediated phosphorylation of PPARγ may be involved in the pathogenesis of insulin-resistance, and present an opportunity for development of an improved generation of anti-diabetic drugs through PPARγ.[28] The PPARγ ligands include polyunsaturated fatty acids, thiazolidinedione (TZD), etc, of which TZD has been used as an insulin sensitizer for the treatment of T2DM. Belenchia et al[29] studied the filial generation in pregnant mice with VD deficiency during perinatal period and their studies suggested that VD deficiency can directly affect the development of adipose tissue in the non-obese offspring, and the VD deficient progeny has stronger fat regulation genes that could regulate the expression of PPARγ and VDR. Studies by Nobre et al[30] suggested that CCAAT/enhancer binding protein beta (C/EBPβ) and PPARγ are highly expressed in the adipose tissue of obese animals. The cyp27b1-1 hydroxylase and VDR expression is decreased in prosome adipocyte 3T3L1 incubated with 1,25(OH)2D. C/EBPβ and PPARγ are decreased. The level of PPARγ in the plasma of GDM pregnant women is significantly higher than that of other groups. With the increase of PPARγ concentration, the cytoplasmic lipid uptake increases suggesting that PPARγ may participate in the regulation of lipid transport among the maternal-fetal interface cells and might have a role in the lipid dysmetabolism in GDM patients.[31]
In this study, the expression of FINS and HOMA-IR increased in patients with diabetes and overweight/obesity, whereas that of HDL-C and adiponectin decreased; the FFA levels in patients with GDM increased. In the non-overweight/obese women, VDR mRNA, PPAR mRNA, and HOMA-IR were related to GDM, while in the women with overweight/obesity, HDL-C and FFA levels were related to GDM. VDR/PPARγ expression correlated to the glucose levels and lipid metabolism. Herrera and Desoye[32] has shown that lipid metabolism is abnormal in diabetic patients, IR exists in the adipose tissue of obese and diabetic pregnant women, and adipose tissue plays an important role in the pathogenesis of diabetes.[12] VDR levels positively correlate with IR,[33] Pregnant women with high pre-pregnancy BMI or GDM have impaired FFA transport at the mother-fetal interface,[34]GDM and FFA levels also correlate with IR,[35] adiponectin is an adipokine and an endogenous insulin sensitizer that reduces the circulating level of insulin in patients obesity and diabetes. Mousa et al[36] showed that the baseline concentration of 25(OH)D negatively correlated with TC /TG and positively correlated with adiponectin in 102 high-risk women with overweight or obesity. Adiponectin can up-regulate the PPARγ expression through by regulating the insulin content and insulin secretion, and decreased levels of adiponectin in the circulation of obese individuals may be directly associated with the β-cell dysfunction in T2DM.[37]
PPARγ and VDR are the members of transcription factor and nuclear receptor superfamily, which regulates the signaling cascade by interacting with other nuclear receptors and transcription factors. Transcription factors, VDR, and PPARγ regulate the gene transcription by acting as VD's reactive elements or peroxisome proliferator response elements in the promoter of the target genes.[38,39] VDR and PPARγ also interact with nuclear receptors, and the corresponding heterodimers formed by their binding with the retinol X receptor regulate the activation of the target genes.[38,39] In this study, we found that the serum VD level is lower in patients with GDM and obese women, the transcription level of VDR and PPARγ in the adipose tissue increased, which may be caused by the negative feedback initiated by low VD. Increased VDR can result in lipopexia, increase IR, and lipid metabolism disorder; FFA, as a ligand of PPARγ, increases significantly, which can increase the transcriptional activity of PPARγ, thus further leading to lipopexia and obesity. Obese patients then suffer from lipid dysmetabolism, significant adiponectin decrease, β-cell dysfunction, and insulin sensitivity decrease, which further leads to diabetes and thus forms a vicious circle.
Therefore, our studies suggest a possibility that obese women with VD deficiency are at high risk of GDM. VD may regulate the formation and differentiation of fat cells through the nuclear receptor VDR and PPARγ pathways and participate in the occurrence of GDM. Adipokines play certain roles in the pathogenesis of GDM.
This study explored the correlation between VD and VDR levels, and PPARγ expression, in subcutaneous adipose tissue of overweight/obese diabetic pregnant women. However, the study had small sample size and did not investigate the molecular mechanisms of the VDR and PPARγ pathway. So the specific mechanism of how adipose tissue and adipokines contributes to the pathogenesis of GDM remains to be further studied. The roles of VD supplement in clinical work and weight control during pregnancy in reducing the incidence of GDM remains to be further studied.
Funding
This study was supported by the grants from the Applied Basic Research Project of Changzhou Science and Technology Bureau (No. CJ20159055), and Major Research and Development Special Projects of Provincial Science and Technology Department (No. BE2017650).
Conflicts of interest
None.
Footnotes
How to cite this article: Wang HY, She GT, Sun LZ, Lu H, Wang YP, Miao J, Liu KZ, Sun CF, Ju HH. Correlation of serum vitamin D, adipose tissue vitamin D receptor, and peroxisome proliferator-activated receptor γ in women with gestational diabetes mellitus. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000480
References
- 1.Xu T, Dainelli L, Yu K, Ma L, Silva Zolezzi I, Detzel P, et al. The short-term health and economic burden of gestational diabetes mellitus in China: a modelling study. BMJ Open 2017; 7:e018893.doi: 10.1136/bmjopen-2017-018893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poola-Kella S, Steinman RA, Mesmar B, Malek R. Gestational diabetes mellitus: post-partum risk and follow up. Rev Recent Clin Trials 2018; 13:5–14.. doi: 10.2174/1574887112666170911124806. [DOI] [PubMed] [Google Scholar]
- 3.Chiou YL, Hung CH, Liao HY. The impact of prepregnancy body mass index and gestational weight gain on perinatal outcomes for women with gestational diabetes mellitus. Worldviews Evid Based Nurs 2018; 5:313–322.. doi: 10.1111/wvn.12305. [DOI] [PubMed] [Google Scholar]
- 4.Pace R, Rahme E, Da Costa D, Dasgupta K. Association between gestational diabetes mellitus and depression in parents: a retrospective cohort study. Clin Epidemiol 2018; 10:1827–1838.. doi: 10.2147/CLEP.S184319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowe WL, Jr, Scholtens DM, Kuang A, Linder B, Lawrence JM, Lebenthal Y, et al. Hyperglycemia and adverse pregnancy outcome follow-up study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care 2019; 42:372–380.. doi: 10.2337/dc18-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Law KP, Zhang H. The pathogenesis and pathophysiology of gestational diabetes mellitus: deductions from a three-part longitudinal metabolomics study in China. Clin Chim Acta 2017; 468:60–70.. doi: 10.1016/j.cca.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Hou W, Meng X, Zhao W, Pan J, Tang J, et al. Heterogeneity of insulin resistance and beta cell dysfunction in gestational diabetes mellitus: a prospective cohort study of perinatal outcomes. J Transl Med 2018; 16:289.doi: 10.1186/s12967-018-1666-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ede G, Keskin U, Cemal Yenen M, Samur G. Lower vitamin D levels during the second trimester are associated with developing gestational diabetes mellitus: an observational cross-sectional study. Gynecol Endocrinol 2019; 35:525–528.. doi: 10.1080/09513590.2018.1548593. [DOI] [PubMed] [Google Scholar]
- 9.Najafi F, Hasani J, Izadi N, Hashemi-Nazari SS, Namvar Z, Mohammadi S, et al. The effect of prepregnancy body mass index on the risk of gestational diabetes mellitus: a systematic review and dose-response meta-analysis. Obes Rev 2019; 20:472–486.. doi: 10.1111/obr.12803. [DOI] [PubMed] [Google Scholar]
- 10.Chu SY, Callaghan WM, Kim SY, Schmid CH, Lau J, England LJ, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care 2007; 30:2070–2076.. doi: 10.2337/dc06-2559a. [DOI] [PubMed] [Google Scholar]
- 11.Borges MD, Franca EL, Fujimori M, Silva SMC, de Marchi PGF, Deluque AL, et al. Relationship between proinflammatory cytokines/chemokines and adipokines in serum of young adults with obesity. Endocr Metab Immune Disord Drug Targets 2018; 18:260–267.. doi: 10.2174/1871530318666180131094733. [DOI] [PubMed] [Google Scholar]
- 12.Lappas M. Effect of pre-existing maternal obesity, gestational diabetes and adipokines on the expression of genes involved in lipid metabolism in adipose tissue. Metabolism 2014; 63:250–262.. doi: 10.1016/j.metabol.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 2000; 72:690–693.. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 14.Carrelli A, Bucovsky M, Horst R, Cremers S, Zhang C, Bessler M, et al. Vitamin D storage in adipose tissue of obese and normal weight women. J Bone Miner Res 2017; 32:237–242.. doi: 10.1002/jbmr.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbas MA. Physiological functions of vitamin D in adipose tissue. J Steroid Biochem Mol Biol 2017; 165:369–381.. doi: 10.1016/j.jsbmb.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Rosen ED, Spiegelman BM. PPARgamma: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem 2001; 276:37731–37734.. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- 17.Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, et al. International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010; 33:676–682.. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vimaleswaran KS, Berry DJ, Lu C, Tikkanen E, Pilz S, Hiraki LT, et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. Plos Med 2013; 10:e1001383.doi: 10.1371/journal.pmed.1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashemi-Nazari SS, Najafi F, Rahimi MA, Izadi N, Heydarpour F, Forooghirad H. Estimation of gestational diabetes mellitus and dose-response association of BMI with the occurrence of diabetes mellitus in pregnant women of the west of Iran. Health Care Women Int 2018; 15:1–10.. doi: 10.1080/07399332.2018.1521812 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 20.Danescu LG, Levy S, Levy J. Vitamin D and diabetes mellitus. Endocrine 2009; 35:11–17.. doi: 10.1007/s12020-008-9115-5. [DOI] [PubMed] [Google Scholar]
- 21.Knabl J, Hüttenbrenner R, Hutter S, Günthner-Biller M, Riedel C, Hiden U, et al. Gestational diabetes mellitus upregulates vitamin D receptor in extravillous trophoblasts and fetoplacental endothelial cells. Reprod Sci 2015; 22:358–366.. doi: 10.1177/1933719114542020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narvaez CJ, Simmons KM, Brunton J, Salinero A, Chittur SV, Welsh JE. Induction of STEAP4 correlates with 1.25-dihydruxyvitamin D3 stimulation of adipogenesis in mesenchymal progenitor cells derived from human adipose tissue. J Cell Physiol 2013; 228:2024–2036.. doi: 10.1002/jcp.24371. [DOI] [PubMed] [Google Scholar]
- 23.Yuzbashian E, Asghari G, Hedayati M, Zarkesh M, Mirmiran P, Khalaj A. Determinants of vitamin D receptor gene expression in visceral and subcutaneous adipose tissue in non-obese, obese, and morbidly obese subjects. J Steroid Biochem Mol Biol 2019; 187:82–87.. doi: 10.1016/j.jsbmb.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Blumberg JM, Tzameli I, Astapova I, Lam FS, Flier JS, Hollenberg AN. Complex role of the vitamin D receptor and its ligand in adipogenesis in 3T3-L1 cells. J Biol Chem 2006; 281:11205–11213.. doi: 10.1074/jbc.M510343200. [DOI] [PubMed] [Google Scholar]
- 25.Kong J, Li YC. Molecular mechanism of 1,25-dihydroxyvitamin D3 inhibition of adipogenesis in 3T3-L1 cells. AM J Physiol Endocrinol Metab 2006; 290:E916–E924.. doi: 10.1152/ajpendo.00410.2005. [DOI] [PubMed] [Google Scholar]
- 26.Ji S, Doumit ME, Hill RA. Regulation of adipogenesis and key adipogenic gene expression by 1, 25-dihydroxyvitamin D in 3T3-L1 cells. PLoS One 2015; 10:e0126142.doi: 10.1371/journal.pone.0126142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee EK, Lee MJ, Abdelmohsen K, Kim W, Kim MM, Srikantan S, et al. miR-130 suppresses adipogenesis by inhibiting peroxisome proliferator-activated receptor gamma expression. Mol Cell Biol 2011; 31:626–638.. doi: 10.1128/MCB.00894-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi JH, Banks AS, Kamenecka TM, Busby SA, Chalmers MJ, Kumar N, et al. Antidiabetic actions of a non-agonist PPARγ ligand blocking Cdk5-mediated phosphorylation. Nature 2011; 4:477–481.. doi: 10.1038/nature10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belenchia AM, Jones KL, Will M, Beversdorf DQ, Vieira-Potter V, Rosenfeld CS, et al. Maternal vitamin D deficiency during pregnancy affects expression of adipogenic-regulating genes peroxisome proliferator-activated receptor gamma (PPARγ) and vitamin D receptor (VDR) in lean male mice off spring. Eur J Nutr 2018; 57:723–730.. doi: 10.1007/s00394-016-1359-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nobre JL, Lisboa PC, Carvalho JC, Martins MR, Vargas S, Barja-Fidalgo C, et al. Leptin blocks the inhibitory effect of vitamin D on adipogenesis and cell proliferation in 3T3-L1 adipocytes. Gen Comp Endocrinol 2018; 266:1–8.. doi: 10.1016/j.ygcen.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 31.Cheng Y, Liu S, Lin R, Wang J, Peng T, Zhang Q, et al. Plasma and amniotic fluid PPARγ is involved in the lipid metabolism of maternal-fetal interface cells. J Matern Fetal Neonatal Med 2018; 31:2656–2664.. doi: 10.1080/14767058.2017.1350641. [DOI] [PubMed] [Google Scholar]
- 32.Herrera E, Desoye G. Maternal and fetal lipid metabolism under normal and gestational diabetic conditions. Horm Mol Biol Clin Investigation 2016; 26:109–127.. doi: 10.1515/hmbci-2015-0025. [DOI] [PubMed] [Google Scholar]
- 33.Pramono A, Jocken JWE, Essers YPG, Goossens GH, Blaak EE. Vitamin D and tissue-specific insulin sensitivity in humans with overweight/obesity. J Clin Endocrinol Metab 2019; 104:49–56.. doi: 10.1210/jc.2018-00995. [DOI] [PubMed] [Google Scholar]
- 34.Segura MT, Demmelmair H, Krauss-Etschmann S, Nathan P, Dehmel S, Padilla MC, et al. Maternal BMI and gestational diabetes alter placental lipid transporters and fatty acid composition. Placenta 2017; 57:144–151.. doi: 10.1016/j.placenta.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Catalano PM, Nizielski SE, Shao J, Preston L, Qiao L, Friedman JE. Downregulated IRS-1 and PPARgamma in obese women with gestational diabetes: relationship to FFA during pregnancy. Am J Physiol Endocrinol Metab 2002; 282:E522–E533.. doi: 10.1152/ajpendo.00124.2001. [DOI] [PubMed] [Google Scholar]
- 36.Mousa A, Abell SK, Shorakae S. Relationship between vitamin D and gestational diabetes in overweight or obese pregnant women may be mediated by adiponectin. Mol Nut Food Res 2017; 61: Epub 2017 Aug 23. doi: 10.1002/mnfr.201700488. [DOI] [PubMed] [Google Scholar]
- 37.Rao JR, Keating DJ, Chen C, Parkington HC. Adiponectin increases insulin content and cell proliferation in MIN6 cells via PPARγ-dependent and PPARγ-independent mechanisms. Diabetes Obes Metab 2012; 14:983–989.. doi: 10.1111/j.1463-1326.2012.01626.x. [DOI] [PubMed] [Google Scholar]
- 38.Nettles KW. Insights into PPAR gamma from structures with endogenous and covalently bound ligands. Nat Struct Mol Biol 2008; 15:893–895.. doi: 10.1038/nsmb0908-893. [DOI] [PubMed] [Google Scholar]
- 39.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer 2007; 7:684–700.. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]


