Introduction:
Comparative data on clinical outcomes and cost of deep inferior epigastric perforator (DIEP) and implant-based reconstruction (IBR) are limited. We conducted a Preferred Reporting Items for Systematic Review and Meta-analysis-compliant systematic review and meta-analysis to compare clinical, patient-reported outcomes (PROs) and cost.
Methods:
The protocol was published a priori on PROSPERO (CRD42017072557). EMBASE, MEDLINE, Google Scholar, Cochrane Controlled Register of Trials, Science Citation Index, and ClinicalTrials.gov were searched from January 1994 to August 2018. Two independent reviewers evaluated the articles for inclusion. Study quality was assessed using Grading of Recommendations Assessment, Development, and Evaluation, and risk of bias (RoB) was assessed using Cochrane’s RoB in Nonrandomized Studies of Interventions tool.
Results:
Out of 6,381 articles screened, 16 were included [unilateral 782 DIEPs, 376 implants; mean age 49 years, follow-up (months): DIEP 29.9; IBR 35.5]. Mean flap loss and fat necrosis rates were 3.97% (SD 4.90) and 9.67% (SD 17.0), respectively. There was no difference in mean length of stay {standard mean difference 0.63 [confidence interval (CI) −9.17 to 10.43]; P =0.90}. The number of reoperations for complications was significantly lower in DIEP versus IBR [SMD −0.29 (CI −0.48 to −0.09); P < 0.01]. There were no randomized controlled trials. Study quality was low with high RoB. One study reported $11,941/Quality-adjusted Life Year incremental cost-effectiveness ratio for DIEP, with higher breast Quality-adjusted Life Year (DIEP 19.5; IBR 17.7) using Breast Questionnaire; 3 studies evaluated cost, favoring DIEP. Two comparative studies evaluating PROs favored DIEP.
Conclusions:
DIEP reconstruction maybe more cost-effective and yield superior PROs. However, poor-quality, bias-ridden studies limit the findings. Adequate reporting of core outcome measures is required to minimize reporting bias and facilitate evidence synthesis. Prospective, multicenter, cohort studies using robust patient-reported outcome measures (PROMs) tools, evaluating cost-effectiveness and contributing to national/international registries, will facilitate national-level policy and shared decision-making.
INTRODUCTION
Breast cancer is the most common malignancy and the principal cause of cancer-related mortality in women.1,2 Breast-conserving surgery or mastectomy is normally offered as management strategies.3 However, mastectomy has been associated with a profoundly negative impact on a woman’s physical, psychological, and sexual well-being.4 Assessment of quality of life (QoL) and patient-reported outcomes (PROs) is thus especially pertinent in breast reconstruction (BRR) surgery, and morbidity and mortality are necessary but not sufficient for adequate outcome assessment.5–17 The reconstruction must satisfy the patient with regard to physical, psychological, and sexual well-being. The exponential rise in QoL and PRO research highlights their importance.12,18 Development and validation of psychometrically robust, validated disease-specific PRO tools such as the Breast Questionnaire (BREAST-Q) and the European Organization for Research and Treatment of Cancer (EORTC) Breast Cancer-specific Quality of Life Questionnaire-23 further exemplify this. Their development and validation have been described previously.9,18–21
Patient demands for BRR have significantly increased over the last 2 decades with the doubling of postmastectomy BRR rates from 13% to 26% between 1998 and 2007.22 This is not only due to advances in oncological management but also due to the clearly demonstrable functional and psychological benefits.23–26 Two of the commonest reconstructive modalities include autologous reconstruction using the deep inferior epigastric perforator (DIEP) flap and implant-based reconstruction (IBR).27 The treatment choice is determined by patient factors (individual preference, body image) and surgeon factors (resource availability and experience).28 Nevertheless, many plastic surgery units worldwide regard autologous reconstruction, compared with IBR, as the superior modality, replacing “like with like.”22 There is emerging evidence that autologous abdominal-based flap BRR may yield superior clinical and PROs.15,27,29–31
IBR is associated with complications, including implant rupture, infection, migration, exposure/extrusion, patient dissatisfaction with edge visibility/implant animation and reduced/absent sensation at the nipple.32 Capsular contracture can culminate in pain, increased palpability, asymmetry, and implant removal requirement.33 Allergan’s 10-year cumulative risk study found that 24.6% of patients who underwent IBR developed capsular contracture.34 Conversely, DIEP flap is widely considered the “gold standard” for postmastectomy BRR. It has largely superseded the traditional transverse rectus abdominus myocutaneous (TRAM) flap, by preserving the rectus abdominis muscle continuity and integrity, limiting donor site complications such as abdominal bulge/hernia.35
From an economic standpoint, some protagonists have argued that DIEP reconstruction is more cost-effective, yielding fewer overall complications and superior PROs, compared with IBR.15,27,30 Although some European and North American centers have published cost-analyses on DIEP and IBR, the data are sparse with relative scarcity of data from public and free universal health care system settings.
We systematically evaluated the quality of evidence and analyzed cost, clinical outcomes, and PROs of unilateral DIEP versus IBR in context of breast malignancy. The aim was to help evaluate which technique is superior in terms of clinical outcomes, PROs, and cost and thus inform worldwide clinical practice and facilitate informed consent and patient–clinician-shared decision-making.
METHODS
Our protocol was registered and published a priori on the National Institute of Health Research Prospective Register of Systematic Reviews PROSPERO (CRD42017072557) and Systematic Reviews peer-reviewed journal.35-37 In the section below, we have detailed the search strategy used, the identification and selection of studies, and the design with inclusion/exclusion criteria. We have subsequently described the risk of bias (RoB) and quality assessment, outcomes, data extraction, collection and management, and the statistical methods utilized.
Search Strategies
We conducted a comprehensive search of the MEDLINE (OVID SP), EMBASE (OVID SP), Google Scholar, Cochrane Controlled Register of Trials, Science Citation Index databases, and ClinicalTrials.gov from January 1994 up to August 2018 to identify studies relevant to the review. A combination of Medical Subject Headings terms, free text, and Boolean logical operators were used to construct the search strategy, in consultation with a literature search expert. Explode function was utilized to capture narrower terms. No language restrictions were applied. The reference list of all included articles was also screened for relevance. A sample search strategy, for EMBASE (OVID SP), is shown below; a similar search strategy was adapted for the other databases:
exp Breast Neoplasms/OR ((breast adj6 cancer*) or (breast adj6 neoplasm*) or (breast adj6 carcinoma*) or (breast adj6 tumour*) or (breast adj6 tumor*) or (breast* adj4 reconstruct*))
exp deep inferior epigastric perforator flap/ OR DIEP flap* OR DIEAP flap* OR ((Deep and inferior and epigastric and perforator) adj2 flap*) OR Deep and inferior and epigastric and perforator and flap*)
exp breast implant/ OR breast adj3 implant* OR exp silicone prosthesis/147 – [(1) AND (2)] OR [(1) AND (3)]; publication date: January 1994 to August 2018
Identification and Selection of Studies
Studies were extracted following database searching and were populated into an Endnote X8 library (Clarivate Analytics, USA). Using prespecified screening criteria, the screening was carried out in 2 stages, by 2 independent reviewers.
Stage 1: Title and abstract screening carried out by 2 researchers independently (MP, MG). Any discrepancies were resolved by consensus. If any doubts remained, the article proceeded to full-text review.
Stage 2: The full texts of the studies included in stage 1 were downloaded and screened for eligibility by 2 researchers independently (MP, MG). Discrepancies were resolved by consensus. If this was not possible, the senior author (AM) was consulted for the final determination for inclusion/exclusion of the article.
Study Design
All primary human studies evaluating clinical outcomes, PROs, or cost for unilateral DIEP flap BRR or IBR in context of breast malignancy were included. The intervention included unilateral DIEP BRR, and the comparator was IBR. The inclusion and exclusion criteria are highlighted below.
Inclusion Criteria
Studies involving adult patients aged ≥18 years old
Studies involving unilateral autologous DIEP flap BRR or IBR in context of breast malignancy
Clinical studies [randomized controlled trials (RCTs), prospective and retrospective cohort studies and case series with 10 or more patients]
Exclusion Criteria
Duplicates, case reports, conference abstracts, simulation studies, review articles, clinical studies in nonhuman subjects, patients with segmental or partial mastectomy, technical operative repair descriptions with no outcome measures, BRR unrelated to cancer, and autologous flap techniques other than DIEP were excluded. Studies of patients receiving adjuvant postmastectomy radiotherapy (PMRT) were also excluded, as adjuvant PMRT is associated with serious adverse events and reduced QoL in IBR, although the evidence is more equivocal for autologous reconstruction, and thus would introduce bias and preclude outcome analysis when comparing IBR and DIEP. Our group is currently conducting a separate systematic review and meta-analysis to investigate outcomes for immediate versus delayed autologous reconstruction in context of PMRT (PROSPERO CRD42017077945).38
RoB and Quality Assessment
For nonrandomized comparative studies, the RoB in Nonrandomized Studies of Interventions (ROBINS-I) by the Cochrane collaboration was used.39 ROBINS-I covers 7 domains from which bias may be introduced, with “signaling questions” facilitating judgments about RoB. These domains include: (1) bias due to confounding; (2) bias in the selection of participants into the study; (3) bias in the classification of interventions; (4) bias due to deviations from intended interventions; (5) bias due to missing data; (6) bias in the measurement of outcomes; and (7) bias in the selection of the reported result. The judgments within each domain were carried forward for an overall RoB judgment across bias domains.39 To assess individual study methodological quality, the Grading of Recommendations, Assessment, Development and Evaluation approach40 was utilized.
Outcomes
The primary outcomes were as follows:
Clinical (complications: fat necrosis, partial/total flap loss, infection, number of reoperation procedures for complications and implant-specific complications, including capsular contracture, implant rupture, displacement, deflation and scarring), with grades of complications where reported
PRO measures (generic and disease-specific PROMs tools, eg, BREAST-Q and EORTC-QLQ-BR23)
Cost-analyses
Data Extraction, Collection, and Management
A standardized extraction form was used to extract data from the full-text articles by 2 independent authors (MP, MG). Any discrepancy was resolved by consensus or with referral to the senior author (AM). The following data were extracted:
first author; year of publication; study design; participant demographics (sex, age, BMI and comorbidity, where reported); study setting; length of follow-up;
primary outcomes, as above.
Statistical Methods
Using Review Manager 5.3,41 provided by the Cochrane Collaboration, an assessment of heterogeneity was performed. The Higgins and Thompson’s I2 statistic was used to quantify statistical heterogeneity.42 The DerSimonian and Laird random-effects model, which is well established for evaluating heterogeneous cohorts, was employed.43 Odds ratios (ORs) with 95% confidence interval (CI) were used to determine dichotomous outcomes (complications). Continuous outcomes were evaluated by standardized mean differences with 95% CI.
RESULTS
A total of 6,381 records were identified. Out of those, 16 fulfilled the inclusion criteria and were considered for quantitative synthesis.15,27,30,31,44–55 The Preferred Reporting Items for Systematic Review and Meta-analysis diagram (Fig. 1) depicts how studies were included and the reasons for exclusion. The 16 studies included 782 unilateral DIEPs and 376 implants; mean age 49 years, mean follow-up (months): DIEP 29.9; IBR 35.5. There were 6 prospective cohort studies,27,31,44,47,50,52 8 retrospective cohort studies,15,30,45,48,51,53–55 2 case series,46,49 and no RCTs. There was 1 multicenter study15; the remaining 15 were single-center studies. The overall quality of the studies using the Grading of Recommendations, Assessment, Development and Evaluation criteria was low, with serious RoB using the ROBINS-I tool. Tables 1 and 2 summarize the baseline characteristics and results (clinical, PROs, and cost).
Fig. 1.
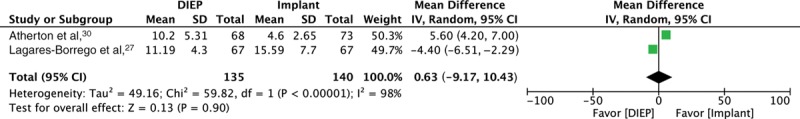
Forest plot for 2 comparative studies, evaluating mean length of stay (days).
Table 1.
Studies Evaluating DIEP or IBR Reconstruction; Comparative Studies’ Data Presented as DIEP versus IBR
| Reference Study, Location, Design | GRADE | ROBINS-I | No. Pts (DIEP) |
No. Pts (IBR) | Mean F/u (mo) with SD/Range Where Reported | No. Overall Comps. | Fat Necrosis | Venous Congestion | Arterial Thrombosis | Flap Loss | Infection | Hematoma /Seroma | Mean LOS (days) with SD/Range Where Reported | Mean Number of Times Return to Theatre for Correction of Complications ± SD | Other Implant Comps.* | Cost-Analysis | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Atherton et al,30 UK, Cohort† | Low | Serious | 68 | 73 | 36 | NA | NA | NA | NA | NA | NA | NA | 10.20 ± 5.31 cw 4.60 ± 2.65‡ | 0.8 ± 6.80 cw 1.5 ± 3.24§ | NA | £10,910 cw £8,034 (cost at 3 y)¶ |
||||||||||||||||
| Cheng et al,44 Taiwan, one-arm clinical trial‖ | Moderate | Moderate | 30 | NA | NA | 1 | 0 | NA | NA | 0 | 0 | 1 | 8.40 | 0.03 | NA | £2,951 | ||||||||||||||||
| Kroll et al,45 USA, Cohort† | Low | Serious | 21 | NA | NA | NA | NA | NA | NA | NA | NA | NA | 6.29 | NA | NA | $18,941 | ||||||||||||||||
| Lagares-Borrego et al,27 Spain, Cohort‖ | Moderate | Moderate | 67 | 67 | 45.31 ±15.65 cw 80.38 ±11.60 |
22 cw 26 (NS) |
1 cw 0¶ | 1 cw 1¶ | 0 cw 0¶ | 2 cw 1¶ | 3 cw 5¶ | 2 cw 4¶ | 11.19 ± 4.3 [7–32] cw 15.59 ± 7.7 [6–51]‡ | 0.10±0.34 [0–1] cw 0.38±0.75§ [0–3] |
15 | €18,857.77 cw €20,502.08 (NS) | ||||||||||||||||
| Matros et al,15 USA, Cohort† | Moderate | Moderate | 103 | 172 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | $75,184 cw $53,571¶ ICER: $11,941 |
||||||||||||||||
| McGeorge et al,46 UK, Case series† | Low | Serious | NA | 13 | 6 | 2 | NA | NA | NA | NA | NA | 1 | NA | NA | 1 | NA | ||||||||||||||||
| Moradi et al,50 UK, Cohort‖ | Moderate | Moderate | 27 | NA | NA | NA | 1 | NA | NA | NA | NA | 1 | 9.1 | NA | NA | NA | ||||||||||||||||
| Nahabedian et al,47 USA, Cohort‖ | Moderate | Moderate | 66 | NA | NA | NA | 6 | 1 | NA | 1 | NA | NA | NA | NA | NA | NA | ||||||||||||||||
| Niddam et al,48 France, Cohort† | Low | Serious | 50 | NA | Median 18.3 [6–34] |
NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||||||||||||||||
| Paget et al,49 UK, Case series‖ | Low | Serious | 10 | NA | NA | NA | 0 | 0 | 0 | 0 | 0 | 0 | 5.7 [5–7] | NA | NA | £7,628 ± £754 [£6,324.06–£8,332.68] | ||||||||||||||||
| Paik et al, 51 South Korea, Cohort† | Moderate | Moderate | 217 | NA | NA | 51 | 8 | NA | NA | 0 | NA | 17 | NA | 0.17 | NA | NA | ||||||||||||||||
| Schaverian et al,55 UK, Cohort† | Low | Serious | 26 | NA | 14 [6–20] |
6 | NA | NA | NA | 2 | 0 | NA | 7.4 ± 3.7 | 0.1 | NA | NA | ||||||||||||||||
| Scheer et al,54 Canada, Cohort† | Low | Serious | 52 | NA | NA | 45 | 24 | 3 | 1 | 3 | 2 | 6 | NA | NA | NA | NA | ||||||||||||||||
| Tan et al, 53 Singapore, Cohort† | Low | Serious | 16 | NA | 24 | 5 | 4 | NA | NA | NA | NA | 1 | 7.56 [5–10] | 0.06 | NA | $8,864.67 | ||||||||||||||||
| Tønseth et al,31 Norway, Cohort‖ | Moderate | Moderate | 29 | 21 | 30±12 cw 33.6±12 | 9 cw 0 | NA | NA | NA | 4 | NA | NA | NA | 0.26 cw NA | NA | NA | ||||||||||||||||
| Wang et al,52 Taiwan, Cohort‖ | Moderate | Moderate | NA | 30 | 21.5 [6–40] | 4 | NA | NA | NA | NA | 1 | NA | 4.0±NA | 0.13 | 1** | NA | ||||||||||||||||
[], brackets for range. GRADE, tool for grading the quality of evidence; ICER, the additional cost of obtaining 1 year of perfect breast-related health for DIEP cw IBR; ROBINS-I, tool for assessing the risk of bias.
*Capsular contracture, scarring, implant deflation/rupture/displacement.
†Retrospective.
‡Statistically significant (P < 0.01).
§Statistically significant (P < 0.05).
¶Statistical significance not reported.
‖Prospective.
**Grade IV contracture (Baker’s classification); ± SD.
F/u, follow up; Comps, complications; cw, compared with; GRADE, Grading of Recommendations, Assessment, Development and Evaluation; NA, not applicable/available; NS, no significance; pts, patients; UL, unilateral.
Table 2.
Comparative Studies Evaluating PROs for DIEP versus IBR Reconstruction
| Reference Study, Location, Design | No. Pts (DIEP) | No. Pts (IBR) | Mean F/u (mo) with SD Where Reported | PROs | ||||
|---|---|---|---|---|---|---|---|---|
| Matros et al,15 USA, cohort* | 103 | 172 | NA | BREAST-Q scores consistently higher for DIEP, 1–8 y postoperatively† Breast QALY: 19.5 cw 17.7† |
||||
| Tønseth et al,31 Norway, Cohort‡ | 29 | 21 | 30±12 cw 33.6±12 | SF-36 scores: Physical functioning 85.0 cw 89.0 (NS); role physical 77.5 cw 78.7 (NS); bodily pain 72.9 cw 74.6 (NS); general health 78.0 cw 80.4 (NS); vitality 60.0 cw 63.8 (NS); social functioning 87.3 cw 90.0 (NS); role emotional 75.6 cw 69.8 (NS); mental health 79.6 cw 77.2 (NS) Study-specific questionnaire scores: Satisfied with appearance of breast: Yes: 24 cw 5; neither yes/no: 3 cw 8; no: 2 cw 8§ (P < 0.0005) Social relationship: Improved: 5 cw 0; unchanged: 24 cw 20; worse: o cw 1¶ (P = 0.02) Sad about body image: Yes: 3 cw 5; neither yes/no: 1 cw 6; no: 25 cw 10¶ (P = 0.01) Study-specific questions concerning self image (NS), social and intimate relationship (NS), general health (NS), and general satisfaction (NS) Visual Analog Scale: Breast shape: 7.9±2.2 cw 5.1±2.5§ (P < 0.0005) Breast symmetry: 7.6±2.1 cw 6.0±2.9¶ (P = 0.023) Breast volume: 7.7±2.1 cw 5.4±2.7§ (P = 0.006) Breast position: 8.8±1.3 cw 6.8±2.6§ (P = 0.003) Breast consistency: 5.6±2.9 cw 3.8±3.0§ (P = 0.008) |
||||
*Retrospective.
†Statistical significance not reported.
‡Prospective.
§Statistically significant (P < 0.01).
¶Statistically significant (P < 0.05).
Cw, compared with (assessing the RoB); QALY, Quality-Adjusted Life Year; NA, not available; NS, no significance.
Clinical Outcomes
Two studies provided comparative data on mean length of stay (days),27,30 with no difference between DIEP and IBR [SMD 0.63 (CI −9.17 to 10.43); P = 0.90] and significant statistical heterogeneity (I2 = 98%) (Fig. 2). Moreover, combining data from single-arm studies (7 studies),44,45,49,50,52,53,55 further revealed no difference in mean length of stay in days [DIEP (8.32; SD 2.05) versus IBR (9.80; SD 8.20), P = 0.89]. Two studies provided comparative data on the mean number of reoperations for complications,27,30 with a statistically significant lower number for DIEP versus IBR [SMD −0.29 (CI −0.48 to −0.09), P < 0.01] with I2 = 0%. The combined data from single-arm studies (7 studies)44,51–53,55 showed lower mean number of revision procedures for DIEP (0.22; SD 0.27) versus IBR (0.50; SD 0.68), but without statistical significance (P = 0.65). There was no statistically significant difference in mean infection rates between DIEP (5 studies)27,44,49,54,55 [1.67% (SD 2.29)] and IBR (2 studies)27,52 [5.40% (SD 2.92)], P = 0.38. Three studies27,46,52 reported mean implant-specific complication rates of 11.1% (SD 9.98); 1 classified capsular contracture grades as per the Baker’s classification, with 1/30 (3.33%) grade IV contracture.52 Out of all 6 IBR studies, 2 studies did not specify whether direct to implant (DTI) or expander–prosthesis (EP) reconstruction was employed.15,30 One reported DTI,52 and 3 reported EP reconstruction.27,31,46 Mean flap loss and fat necrosis rates, reported by 9 studies, were 3.97% (SD 4.90) and 9.67% (SD 17.0), respectively.27,31,44,47,49,51,53–55 No studies were reported as per the Clavien–Dindo classification (CDC).56 Other than capsular contracture being graded as per the Baker’s classification by 1 study,52 none of the other complications were graded.
Fig. 2.
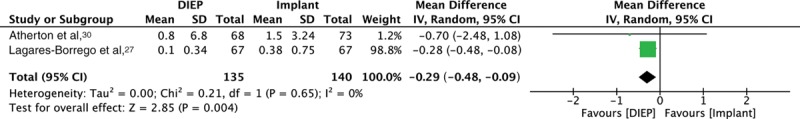
Forest plot for 2 comparative studies, evaluating mean number of reoperations for complications.
QoL
Two comparative studies evaluated QoL.15,31 Tønseth et al31 evaluated 29 patients with DIEP BRR and 21 patients with IBR. They utilized a generic PRO tool, Short-Form 36 (SF-36), which showed no difference in QoL, a nonvalidated study-specific questionnaire that showed higher breast satisfaction (P < 0.001), improved social relationship (P = 0.02) and body image satisfaction (P = 0.01) for DIEP, and a nonvalidated Visual Analog Scale, with superior cosmetic outcome with DIEP (Table 2). Matros et al15 prospectively evaluated 103 patients with DIEP BRR and 172 patients with IBR and utilized the BREAST-Q. BREAST-Q scores were consistently higher for DIEP compared with implants in postoperative years 1–8, with a higher breast Quality-adjusted Life Year for DIEP (19.5) versus IBR (17.7).
Cost
Three comparative studies evaluated cost.15,27,30 Matros et al (USA) calculated an incremental cost-effectiveness ratio (ICER) of $11,491 for DIEP, ie, the additional cost of DIEP BRR to obtain 1 year of perfect breast-related QoL compared with IBR. Lagares-Borrego et al, 2015 (Spain) reported no difference in overall cost between DIEP BRR (€18,857.77) versus IBR (€20,502.08); P = 0.89. However, when considering surgical complications, cost of DIEP (€2,859.90) was significantly lower than IBR (€5,837.9), P < 0.001. Cost of DIEP was also lower owing to length of hospital stay (P < 0.001), consultations (P < 0.001), and materials and tests used (P < 0.001), but higher owing to duration of procedure (P < 0.001). Atherton et al estimated cost at 3 years: DIEP £10,910 versus IBR £8,034. No statistics were performed; however, the authors reported that the cost “difference is small and patient will still require more revisions (with IBR), and if followed up enough will lose this small financial benefit”; the cost difference maybe “justified by the increased patient satisfaction and cosmetic outcome (with DIEP).”
DISCUSSION
To our knowledge, this is the first systematic review and meta-analysis in available literature to evaluate clinical outcomes, PROs, and cost of DIEP versus IBR. Overall study quality is low with serious RoB, weakly supporting DIEP as a more cost-effective strategy that confers higher QoL compared with IBR. Factors limiting the quality of evidence include study designs, absence/heterogeneous reporting of clinical outcomes, study exclusion due to combined reporting of different flaps, and no breakdown of outcomes between unilateral and bilateral reconstruction. Majority of the studies have small sample sizes, were conducted in a retrospective manner with potentially biased patient recall after variable and delayed lengths of time post surgery, and failed to achieve adequate follow-up periods. Complications such as capsular contracture may occur well beyond this time frame. Fifteen or sixteen studies were single-center studies, negatively impacting generalizability.
Our systematic review demonstrates the inconsistency and heterogeneity in clinical outcome reporting, which presents a limitation. Only 8/14 (57.1%) studies evaluating DIEP reported flap loss rates. Likewise, only 3/6 (50.0%) studies evaluating IBR reported implant-specific complications, including capsular contracture. Only 1 of these studies classified capsular contracture according to the Baker’s classification.57 Because classification/grades help inform management strategies, inaccurate classification, and grading of these complications, risks biased comparisons of clinical outcomes between studies, rendering it difficult to interpret the study findings. This corroborates the results from the systematic review by Potter et al,58 on reporting quality of BRR clinical outcomes, that identified poor reporting quality and need for a core outcome set to facilitate outcome assessment in effectiveness studies. Furthermore, no studies reported outcomes using the validated CDC.56 Moreover, no studies reported grade of fat necrosis.
Standardization of outcome reporting, with uptake of validated tools such as CDC and incorporation into journal submission guidelines by editors, may promote higher quality, standardized reporting and facilitate homogeneity and meta-analysis.
Out of 6 IBR studies, 3 reported implant-specific complications; 2 out of 6 studies did not categorize type of IBR and reported as “implant reconstruction”. Three out of 6 studies reported EP reconstruction, and 1 reported DTI. Due to the scarcity of IBR data, further subgroup analysis was not possible. Future studies should clearly specify the type of reconstruction – DTI/EP; subpectoral or prepectoral and whether acellular dermal matrix was utilized. Adequate reporting as part of a core outcome set will facilitate inter-study comparisons and meta-analyses.
The Mastectomy Reconstruction Outcomes Consortium (MROC) is a large, multicenter prospective cohort study involving 9 academic and 2 private practices in the United States and Canada with high volumes of BRR.59 This did not meet our systematic review’s inclusion criteria, due to combined data reporting of unilateral and bilateral reconstructions, as well as reporting of clinical outcomes with combined results from a range of autologous reconstruction techniques, including DIEP, TRAM, free TRAM, and latissimus dorsi (LD) and superficial inferior epigastric perforator flaps. Nevertheless, it is pertinent to discuss the results from this cohort.
Bennett et al8 prospectively evaluated 2,343 patients undergoing postmastectomy autologous reconstruction (706), using DIEP, pedicled TRAM, free TRAM, superficial inferior epigastric perforator, latissimus dorsi or IBR (1,637), with comparison of 2-year complication rates. The authors found that DIEP had lower failure rates compared with IBR (1.3% versus 7.1%, P < 0.001) and lower odds of developing infection (OR 0.45; CI: 0.25–0.29; P = 0.006). This corroborates with the findings from our systematic review with lower rates of infection and revision procedures in DIEP compared with IBR. However, Bennett et al8 reported higher odds of developing any complication with DIEP (OR 1.97; CI 1.41–2.76; P < 0.001), including reoperative complications (OR 2.76; CI 1.87–4.07; P < 0.001). This in part could be explained by outcomes following adjuvant radiotherapy. Although the detrimental effect of PMRT on IBR is well established, the effect on autologous reconstruction is more equivocal. This is being evaluated in a separate systematic review and meta-analysis by our group (PROSPERO CRD42017077945).38 Moreover, confounders such as the level-of-care provider expertise, nonstandardized operative technique, differences in centers’ volume, and learning curves may further bias the results and their interpretability. Indeed, another MROC study evaluating hospital variations in clinical complications and PROs at 2 years post autologous BRR or IBR demonstrated that complications varied widely between hospitals.10 It also highlighted the limitations of extrapolating single-institution level data and the challenges of evaluating hospital-based outcomes in BRR patients.
In our systematic review, out of 16 studies, only 2 comparative studies (12.5%)15,31 reported PROs. A major paradigm shift is needed to incorporate PROs in all studies evaluating BRR, as also supported by the recent publication of the “Gap analysis” in BRR.12 Evaluating clinical outcomes without PROs is a major drawback in evaluating outcomes in BRR, as the reconstruction must satisfy the patient with regard to physical, psychological, and sexual well-being.59 Disregard of these domains renders outcome assessment incomplete and suboptimal.
Two comparative studies that evaluated PROs in our review favored DIEP reconstruction. Matros et al15 utilized a robust, validated, disease-specific questionnaire, BREAST-Q. BREAST-Q scores were reported as consistently higher for DIEP compared with IBR in postoperative years 1–8, with a higher breast Quality-adjusted Life Year for DIEP. Conversely, Tønseth et al31 used generic PRO tools, SF-36, which revealed no difference in QoL between DIEP and IBR, and Visual Analog Scale, with superior cosmetic outcome with DIEP. The study also used a nonvalidated study-specific questionnaire that demonstrated higher breast satisfaction, improved social relationship, and body image satisfaction for DIEP.
The results from our review corroborate results from Santosa et al29 who evaluated PROs for 2,013 patients (523 autologous reconstructions; 1,490 IBR) from the MROC cohort, pre and 2 years post BRR, using the BREAST-Q. The 4 domains evaluated were as follows: satisfaction with breasts, psychosocial well-being, physical well-being, and sexual well-being. At 2 years, patients who underwent autologous reconstruction had higher breast satisfaction, higher psychosocial well-being, and sexual well-being than did those who underwent IBR.29 Lack of a significant difference in QoL between DIEP and IBR reported by Tønseth et al in our review may be due to the small sample size in the study (n = 50) and use of a nonspecific, generic QoL tool, SF-36, which may not be sensitive enough to measure changes as a result of BRR intervention or to capture all aspects of outcome specific to breast surgery.18 Moreover, as purported by our group, QoL domains should be defined a priori, facilitating estimations of potential effect size.17
Three comparative studies evaluated cost, all favoring DIEP.15,27,30 Two studies were conducted in a universal health care system (UK and Spain)27,30 and 1 was conducted in a health insurance-based model (USA),15 making direct comparisons on cost difficult. This is exacerbated by only 1 study performing robust cost-effectiveness analysis, calculating an ICER of $11,491 for DIEP.15 An ICER is the additional cost for DIEP to obtain 1 year of perfect breast-related QoL compared with IBR; a threshold of $50,000–$100,000 for a year in perfect overall health has been deemed as acceptable for the adoption of new technologies or techniques in developed countries.60 Heterogeneity in cost-evaluation methods and reporting prevented the calculation of an overall cost-effectiveness summary measure in our systematic review.
CONCLUSIONS
Limitations in study design and outcome reporting preclude firm consensus on best recommendations for postmastectomy BRR. However, the evidence supports a weak recommendation for DIEP reconstruction being more cost-effective and yielding higher QoL compared with IBR. There is a pressing need for level I and II data, in the form of RCTs and prospective, multicenter, longitudinal cohort studies, with long-term follow-up. These must incorporate validated, disease-specific PRO tools such as BREAST-Q. Evaluation of a priori core outcome set and cost-effectiveness is required for national guidelines, optimizing informed consent and facilitating clinician–patient-shared decision-making.
Footnotes
Published online 25 October 2019.
Presented at the World Society of Reconstructive Microsurgery Meeting, 2019, Bologna, Italy (podium presentation).
Disclosure: AK is a Kellogg Scholar at the University of Oxford and receives funding equating to the scholarship amount. ALP is the codeveloper of the Breast Questionnaire and receives royalties when the Breast Questionnaire is used in industry-sponsored clinical trials. None Neither of the other authors has any financial disclosures.
REFERENCES
- 1.Ginsburg O, Bray F, Coleman MP, et al. The global burden of women’s cancers: a grand challenge in global health. Lancet. 2017;389:847–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winters S, Martin C, Murphy D, et al. Breast cancer epidemiology, prevention, and screening. Prog Mol Biol Transl Sci. 2017;151:1–32. [DOI] [PubMed] [Google Scholar]
- 3.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. [DOI] [PubMed] [Google Scholar]
- 4.Helms RL, O’Hea EL, Corso M. Body image issues in women with breast cancer. Psychol Health Med. 2008;13:313–325. [DOI] [PubMed] [Google Scholar]
- 5.Klassen AF, Pusic AL, Scott A, et al. Satisfaction and quality of life in women who undergo breast surgery: a qualitative study. BMC Womens Health. 2009;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atisha DM, Tessiatore KM, Rushing CN, et al. A national snapshot of patient-reported outcomes comparing types of abdominal flaps for breast reconstruction. Plast Reconstr Surg. 2019;143:667–677. [DOI] [PubMed] [Google Scholar]
- 7.Atisha D, Alderman AK, Lowery JC, et al. Prospective analysis of long-term psychosocial outcomes in breast reconstruction: two-year postoperative results from the Michigan breast reconstruction outcomes study. Ann Surg. 2008;247:1019–1028. [DOI] [PubMed] [Google Scholar]
- 8.Bennett KG, Qi J, Kim HM, et al. Comparison of 2-year complication rates among common techniques for postmastectomy breast reconstruction. JAMA Surg. 2018;153:901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winters ZE, Balta V, Thomson HJ, et al. ; European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Group. Phase III development of the European organization for research and treatment of cancer quality of life questionnaire module for women undergoing breast reconstruction. Br J Surg. 2014;101:371–382. [DOI] [PubMed] [Google Scholar]
- 10.Berlin NL, Tandon VJ, Qi J, et al. Hospital variations in clinical complications and patient-reported outcomes at 2 years after immediate breast reconstruction. Ann Surg. 2019;269:959–965. [DOI] [PubMed] [Google Scholar]
- 11.Cohen WA, Mundy LR, Ballard TN, et al. The BREAST-Q in surgical research: a review of the literature 2009-2015. J Plast Reconstr Aesthet Surg. 2016;69:149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cutress RI, McIntosh SA, Potter S, et al. ; Association of Breast Surgery Surgical Gap Analysis Working Group. Opportunities and priorities for breast surgical research. Lancet Oncol. 2018;19:e521–e533. [DOI] [PubMed] [Google Scholar]
- 13.Erdmann-Sager J, Wilkins EG, Pusic AL, et al. Complications and patient-reported outcomes after abdominally based breast reconstruction: results of the mastectomy reconstruction outcomes consortium study. Plast Reconstr Surg. 2018;141:271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howard MA, Sisco M, Yao K, et al. Patient satisfaction with nipple-sparing mastectomy: a prospective study of patient reported outcomes using the BREAST-Q. J Surg Oncol. 2016;114:416–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matros E, Albornoz CR, Razdan SN, et al. Cost-effectiveness analysis of implants versus autologous perforator flaps using the BREAST-Q. Plast Reconstr Surg. 2015;135:937–946. [DOI] [PubMed] [Google Scholar]
- 16.Macadam SA, Zhong T, Weichman K, et al. Quality of life and patient-reported outcomes in breast cancer survivors: a multicenter comparison of four abdominally based autologous reconstruction methods. Plast Reconstr Surg. 2016;137:758–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winters ZE, Khajuria A. Quality of life after breast reconstruction-the BRIOS study. Lancet Oncol. 2018;19:e579. [DOI] [PubMed] [Google Scholar]
- 18.Pusic AL, Klassen AF, Scott AM, et al. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009;124:345–353. [DOI] [PubMed] [Google Scholar]
- 19.Cano SJ, Klassen AF, Scott AM, et al. The BREAST-Q: further validation in independent clinical samples. Plast Reconstr Surg. 2012;129:293–302. [DOI] [PubMed] [Google Scholar]
- 20.Winters ZE, Afzal M, Rutherford C, et al. ; European Organisation for Research and Treatment of Cancer Quality of Life Group. International validation of the European organisation for research and treatment of cancer QLQ-BRECON23 quality-of-life questionnaire for women undergoing breast reconstruction. Br J Surg. 2018;105:209–222. [DOI] [PubMed] [Google Scholar]
- 21.Thomson HJ, Winters ZE, Brandberg Y, et al. The early development phases of a European Organisation for Research and Treatment of Cancer (EORTC) module to assess patient reported outcomes (pros) in women undergoing breast reconstruction. Eur J Cancer. 2013;49:1018–1026. [DOI] [PubMed] [Google Scholar]
- 22.Sisco M, Du H, Warner JP, et al. Have we expanded the equitable delivery of postmastectomy breast reconstruction in the new millennium? Evidence from the national cancer data base. J Am Coll Surg. 2012;215:658–666; discussion 666. [DOI] [PubMed] [Google Scholar]
- 23.Eltahir Y, Werners LL, Dreise MM, et al. Quality-of-life outcomes between mastectomy alone and breast reconstruction: comparison of patient-reported BREAST-Q and other health-related quality-of-life measures. Plast Reconstr Surg. 2013;132:201e–209e. [DOI] [PubMed] [Google Scholar]
- 24.Guyomard V, Leinster S, Wilkinson M. Systematic review of studies of patients’ satisfaction with breast reconstruction after mastectomy. Breast. 2007;16:547–567. [DOI] [PubMed] [Google Scholar]
- 25.Dean C, Chetty U, Forrest AP. Effects of immediate breast reconstruction on psychosocial morbidity after mastectomy. Lancet. 1983;1:459–462. [DOI] [PubMed] [Google Scholar]
- 26.Rowland JH, Holland JC, Chaglassian T, et al. Psychological response to breast reconstruction. Expectations for and impact on postmastectomy functioning. Psychosomatics. 1993;34:241–250. [DOI] [PubMed] [Google Scholar]
- 27.Lagares-Borrego A, Gacto-Sanchez P, Infante-Cossio P, et al. A comparison of long-term cost and clinical outcomes between the two-stage sequence expander/prosthesis and autologous deep inferior epigastric flap methods for breast reconstruction in a public hospital. J Plast Reconstr Aesthet Surg. 2016;69:196–205. [DOI] [PubMed] [Google Scholar]
- 28.Gu J, Groot G, Boden C, et al. Review of factors influencing women’s choice of mastectomy versus breast conserving therapy in early stage breast cancer: a systematic review. Clin Breast Cancer. 2018;18:e539–e554. [DOI] [PubMed] [Google Scholar]
- 29.Santosa KB, Qi J, Kim HM, et al. Long-term patient-reported outcomes in postmastectomy breast reconstruction. JAMA Surg. 2018;153:891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atherton DD, Hills AJ, Moradi P, et al. The economic viability of breast reconstruction in the UK: comparison of a single surgeon’s experience of implant; LD; TRAM and DIEP based reconstructions in 274 patients. J Plast Reconstr Aesthet Surg. 2011;64:710–715. [DOI] [PubMed] [Google Scholar]
- 31.Tønseth KA, Hokland BM, Tindholdt TT, et al. Quality of life, patient satisfaction and cosmetic outcome after breast reconstruction using DIEP flap or expandable breast implant. J Plast Reconstr Aesthet Surg. 2008;61:1188–1194. [DOI] [PubMed] [Google Scholar]
- 32.Agha RA, Fowler AJ, Herlin C, et al. Use of autologous fat grafting for breast reconstruction: a systematic review with meta-analysis of oncological outcomes. J Plast Reconstr Aesthet Surg. 2015;68:143–161. [DOI] [PubMed] [Google Scholar]
- 33.Steiert AE, Boyce M, Sorg H. Capsular contracture by silicone breast implants: possible causes, biocompatibility, and prophylactic strategies. Med Devices (Auckl). 2013;6:211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spear SL, Murphy DK; Allergan Silicone Breast Implant U.S. Core Clinical Study Group. Natrelle round silicone breast implants: core study results at 10 years. Plast Reconstr Surg. 2014;133:1354–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bajaj AK, Chevray PM, Chang DW. Comparison of donor-site complications and functional outcomes in free muscle-sparing TRAM flap and free DIEP flap breast reconstruction. Plast Reconstr Surg. 2006;117:737–746; discussion 747. [DOI] [PubMed] [Google Scholar]
- 36.Khajuria A, Smith O, Mosahebi A. A systematic review and meta-analysis of the clinical outcomes and cost of using autologous deep inferior epigastric perforator (DIEP) flap versus implants for breast reconstruction. http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017072557. Accessed June 2, 2019. [DOI] [PMC free article] [PubMed]
- 37.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khajuria A, Winters Z, Mosahebi A. A systematic review and meta-analysis of clinical and patient-reported outcomes (PROs) of immediate versus delayed autologous abdominal-based flap breast reconstruction in the context of post-mastectomy radiotherapy [PROSPERO CRD42017077945]. http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017077945.
- 39.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atkins D, Best D, Briss PA, et al. ; GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collaboration TC. Review Manager (RevMan) Version 5.2. 2012. Copenhagen, Denmark: The Nordic Cochrane Centre; https://community.cochrane.org/help/tools-and-software/revman-5. [Google Scholar]
- 42.Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45(Pt A):139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng MH, Lin JY, Ulusal BG, et al. Comparisons of resource costs and success rates between immediate and delayed breast reconstruction using DIEP or SIEA flaps under a well-controlled clinical trial. Plast Reconstr Surg. 2006;117:2139–2142; discussion 2143. [DOI] [PubMed] [Google Scholar]
- 45.Kroll SS, Reece GP, Miller MJ, et al. Comparison of cost for DIEP and free TRAM flap breast reconstructions. Plast Reconstr Surg. 2001;107:1413–1416; discussion 1417. [DOI] [PubMed] [Google Scholar]
- 46.McGeorge DD, Mahdi S, Tsekouras A. Breast reconstruction with anatomical expanders and implants: our early experience. Br J Plast Surg. 1996;49:352–357. [DOI] [PubMed] [Google Scholar]
- 47.Nahabedian MY, Tsangaris T, Momen B. Breast reconstruction with the DIEP flap or the muscle-sparing (MS-2) free TRAM flap: is there a difference? Plast Reconstr Surg. 2005;115:436–444; discussion 445–446. [DOI] [PubMed] [Google Scholar]
- 48.Niddam J, Bosc R, Lange F, et al. DIEP flap for breast reconstruction: retrospective evaluation of patient satisfaction on abdominal results. J Plast Reconstr Aesthet Surg. 2014;67:789–796. [DOI] [PubMed] [Google Scholar]
- 49.Paget JT, Young KC, Wilson SM. Accurately costing unilateral delayed DIEP flap breast reconstruction. J Plast Reconstr Aesthet Surg. 2013;66:926–930. [DOI] [PubMed] [Google Scholar]
- 50.Moradi P, Durrant C, Glass GE, et al. SIEA flap leads to an increase in abdominal seroma rates compared to DIEP flap for breast reconstruction. Eur J Plast Surg. 2010;34:87–91. [Google Scholar]
- 51.Paik JM, Lee KT, Jeon BJ, et al. Donor site morbidity following DIEP flap for breast reconstruction in Asian patients: is it different? Microsurgery. 2015;35:596–602. [DOI] [PubMed] [Google Scholar]
- 52.Wang HY, Ali RS, Chen SC, et al. One-stage immediate breast reconstruction with implant following skin-sparing mastectomy in Asian patients. Ann Plast Surg. 2008;60:362–366. [DOI] [PubMed] [Google Scholar]
- 53.Tan S, Lim J, Yek J, et al. The deep inferior epigastric perforator and pedicled transverse rectus abdominis myocutaneous flap in breast reconstruction: a comparative study. Arch Plast Surg. 2013;40:187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scheer AS, Novak CB, Neligan PC, et al. Complications associated with breast reconstruction using a perforator flap compared with a free TRAM flap. Ann Plast Surg. 2006;56:355–358. [DOI] [PubMed] [Google Scholar]
- 55.Schaverien MV, Perks AG, McCulley SJ. Comparison of outcomes and donor-site morbidity in unilateral free TRAM versus DIEP flap breast reconstruction. J Plast Reconstr Aesthet Surg. 2007;60:1219–1224. [DOI] [PubMed] [Google Scholar]
- 56.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spear SL, Baker JL., Jr. Classification of capsular contracture after prosthetic breast reconstruction. Plast Reconstr Surg. 1995;96:1119–1123; discussion 1124. [PubMed] [Google Scholar]
- 58.Potter S, Brigic A, Whiting PF, et al. Reporting clinical outcomes of breast reconstruction: a systematic review. J Natl Cancer Inst. 2011;103:31–46. [DOI] [PubMed] [Google Scholar]
- 59.Pusic AL, Matros E, Fine N, et al. Patient-reported outcomes 1 year after immediate breast reconstruction: results of the mastectomy reconstruction outcomes consortium study. J Clin Oncol. 2017;35:2499–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laupacis A, Feeny D, Detsky AS, et al. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ. 1992;146:473–481. [PMC free article] [PubMed] [Google Scholar]


