Background:
Volume replacement oncoplastic breast techniques have become one of the standard lines in the treatment of early breast cancer. They have better cosmetic outcome and patient satisfaction. Latissimus dorsi (LD) flap is one of the most commonly used flaps for these techniques. Although it shows satisfactory surgical outcomes, postoperative shoulder dysfunction is an obvious drawback. The aim of this study was to compare LD flap with thoracodorsal artery perforator (TDAP) flap after breast-conserving surgery regarding surgical outcomes, patient satisfaction, and impact on shoulder function.
Methods:
The study included 42 adult female patients with early breast cancer who were eligible for conservative breast surgery and immediate breast reconstruction. Patients were divided into 2 equal groups: group A where patients underwent immediate reconstruction using LD flap and group B where patients underwent reconstruction using TDAP flap. Follow-up was designed for 12 months for early outcome, patient satisfaction, and shoulder functions.
Results:
The mean age of the included patients in group A and group B was 40.95 ± 5.06 and 40.33± 5.25 years, respectively. There was no significant difference in flap dimensions, postoperative complications, or cosmetic outcome in both groups. However, significantly less shoulder dysfunction was documented in cases of TDAP compared to LD flap at 3, 6, and 12 months postoperatively.
Conclusions:
TDAP flap is as reliable a technique as LD flap regarding the feasibility, postoperative complications, and the cosmetic outcome with significantly better functional outcome of the shoulder.
INTRODUCTION
Multiple meta-analyses and randomized trials have documented the use of conservative surgery and radiation therapy for the treatment of early-stage breast cancer.1 This conception in breast surgery has developed to attain both minimal surgical intervention and more satisfactory aesthetic results.2 It is important for surgeons performing breast surgery to have a basic understanding of which patients are candidates for breast reconstruction and the reconstructive options.3 Excision of more than 20% of the breast volume will increase the risk of worse cosmetic outcome.4,5 Oncoplastic breast surgery has emerged with the concept of combining tumor excision with clear safety margin followed by breast reconstruction. These procedures include either volume displacement or volume replacement techniques with a clear shift toward immediate reconstruction for a better psychological outcome.6,7 The aim of breast reconstruction using different oncoplastic techniques is not just creating a mound on the chest wall but achieving symmetry with the contralateral native breast as well.3
Conservative breast surgery with immediate partial reconstruction using the latissimus dorsi (LD) flap has been widely applied as a part of oncoplastic breast surgery. Several studies have documented acceptable surgical outcomes in terms of cosmoses and oncological safety.8 It is suitable for patients with small-to-medium-sized breasts and those refusing contralateral surgery. Transposition of the LD flap will not interfere with the subsequent mammogram, because the fatty tissue and the muscle are radiolucent.9–11 However, functional impairment has been observed in clinical practice following the use of LD flap. This observation has been documented by a lot of studies12–14 in the form of early postoperative arm and shoulder disability that may interfere with usual daily activities.
The thoracodorsal artery perforator (TDAP) flap is a fasciocutaneous flap that can be an alternative solution. It can offer the theoretical advantage of sparing the LD muscle and thus reducing the donor site morbidity.15 It is based on a musculocutaneous perforator or perforators from the thoracodorsal artery. The TDAP flap is well suited for head, neck, and extremity defects. A considerable size of the flap can be harvested on a single perforator, with the advantage of both the avoidance of postoperative partial or complete loss of the flap and primary closure of the donor site.16
The aim of the present study was to compare the TDAP flap with the LD flap in partial breast reconstruction regarding feasibility, cosmoses, postoperative complications, and early musculoskeletal functional outcome.
PATIENTS AND METHODS
The study was conducted at Surgery Departments of the University Main Hospitals in Benha, Menoufia, and Tanta Faculty of Medicine throughout the period from August 2016 to January 2019. Approval to conduct the research was obtained from the institutes’ ethical and research committees. The study included 42 adult female patients diagnosed with early breast cancer (T1, T2) and ductal carcinoma in situ, who were eligible and motivated for conservative breast therapy and immediate breast reconstruction. Exclusion criteria included patients with locally advanced disease, inflammatory breast carcinoma or metastatic disease, those who had a contraindication for radiotherapy or with collagen diseases such as scleroderma, or those with the score of >3 on American Society of Anesthesiologists scale. Written informed consent was obtained from all included patients. Preoperative assessment included full history taking, complete general and local assessment, bilateral mammography, and tissue biopsy. Also, full preoperative laboratory and metastatic work up were done. Included patients were randomized by computer-generated random allocation software, into 2 equal study groups: A and B. Patients in both groups were subjected to conservative breast surgery. Group A included patients for whom immediate reconstruction was performed using LD flap, whereas TDAP flap was used in group B.
For LD flap, preoperative flap design was performed with the patient in sitting position by marking a transverse elliptical skin paddle (Fig. 1). Size of the skin ellipse was adjusted in accordance to the estimated breast defect after conservative breast surgery and to enable primary closure of the donor site. The deep dissection was performed to the thoracodorsal fascia till the separation of the LD muscle from the serratus anterior, paraspinous, and trapezius muscles. The LD muscle was then separated from the humorous after identification of the thoracodorsal artery. A subcutaneous tunnel was created for the transfer of the flap into the breast defect. Finally, closure of the donor site by direct suturing was applied.
Fig. 1.
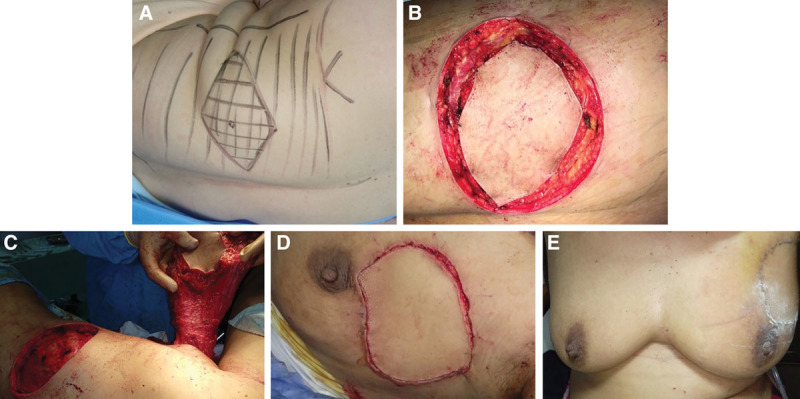
LD flap. A, Marking of the flap. B, Incision and dissection. C, Full mobilization and tunnel formation. D, Insetting. E, Final aesthetic outcome.
For TDAP flap, preoperative marking of the site of the TDAP was performed using handheld Doppler (Fig. 2). Two anatomical landmarks were determined. The first point would be to the center of the flap and was located 8 cm below the posterior axillary fold and 2 cm behind the lateral border of the LD muscle. This point is corresponding to the site of emergence of the proximal skin perforator from the descending branch of the thoracodorsal artery and its exit off the LD muscle to pass into the SC tissue. The second point was located 3–6 cm below the inferior scapular tip and 1–4 cm medial to the lateral free margin of the LD muscle. This point is corresponding to the site of thoracodorsal artery bifurcation. After the evaluation of volume deficit and location, the TDAP flap was marked in standing position with the arms at sides and hands on waist. It was designed to exceed the lateral edge of the LD muscle and to contain the point of the previously localized artery within its center. As for LD flap, the width of the TDAP flap was designed with the possibility of direct closure of the donor site. Dissection was beveled outward to include the maximum fat, beginning from the anterior side along the supra-fascial plane till pulsation of the perforator was felt and easily observed. When the anterior border of the muscle was reached, a tunnel was created under the lateral breast mound and lateral thoracic wall for passage for the flap in the setting. The vascular pedicle was dissected until enough length was achieved to allow insetting of the flap in the breast defect without tension; then, the donor area was closed directly in 2 layers. Comparison between the 2 groups as regards flap size, operative time, and immediate postoperative complications was performed.
Fig. 2.
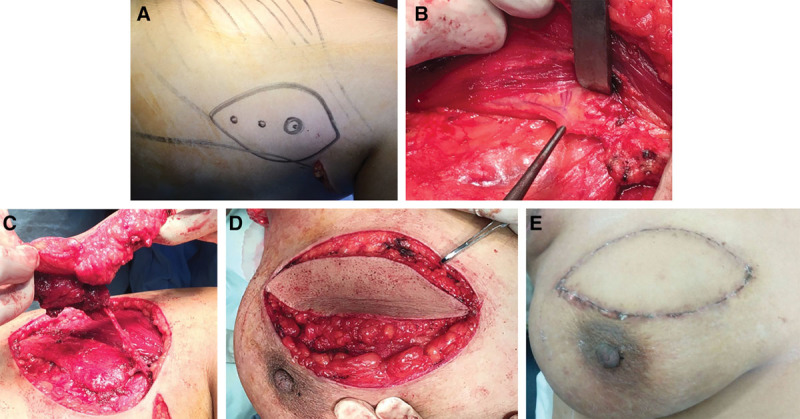
TDAP flap. A, Marking of the flap. B, Identification of thoracodorsal artery. C, Full mobilization on the vascular pedicle. D, Insetting. E, Final aesthetic outcome.
Immediate postoperative management included prophylactic anticoagulation in the form of an intravenous infusion of heparin together with prophylactic antibiotic therapy.
Immediate postoperative close monitoring of the viability of the flaps with documentation of any partial or total flap loss was performed.
Postoperative adjuvant therapy was planned for all our patients, and the delivery time was determined to start from 4 to 6 weeks postoperatively to achieve maximum effect. Follow-up for early postoperative complications, patient satisfaction, and range of shoulder movement was done for at least 12 months.
The aesthetic outcome and patient satisfaction were evaluated by patient questionnaire regarding the symmetry of both breasts, the shape of the scar, keloid, and finally the nipple areola complex. This was achieved through a 5-point score (1 = bad, 2 = poor, 3 = fair, 4 = good, and 5 = excellent). The functional outcome of the shoulder was evaluated through Shoulder Pain And Disability Index (SPADI).17 It was assessed with 8 questions designed to measure the degree of difficulty an individual has with various activities of daily living that require upper-extremity use. To answer the questions, patients were asked to place a mark on a 10-cm visual analog scale for each question. Verbal anchors for the pain dimension are “no pain at all” and “worst pain imaginable,” and those for the functional activities are “no difficulty” and “so difficult it required help.” The scores from both dimensions are averaged to derive a total score. Total disability score is as follows: patient score/80 × 100 = ___%. The original SPADI does not provide specific cutoff points to separate the results into limited, medium, high, or extreme disability. It is considered that the higher the score in each scale, the higher the impairment to the shoulder function. The minimal detectable changes at 90% confidence interval is 13% for the functional disability score. This functional outcome was performed at 3 months postoperatively and repeated at 6 and 12 months. Comparison between the 2 groups was performed.
Statistical analysis was performed using Student’s t test for quantitative parameters that were described using range (minimum and maximum), mean, and SD. Chi-square test was used for qualitative parameters that were described as frequency with percent. SPSS-20 (Statistical Package for Social Sciences version 21) was used. Probability values of <0.05 were considered significant.
RESULTS
The mean age of patients in group A was 40.95±5.06 years whereas it was 40.33±5.25 years in group B (P = 0.699). There was no significant difference between the 2 groups as regards sociodemographic data or comorbidities as shown in Table 1, nor tumor clinical and pathological data as shown in Table 2.The mean operative time was 154.3 ± 11.54 and 155.7 ± 9.26 minutes in group A and group B, respectively (P = 0.661). Table 3 shows no statistically significant difference between the 2 groups regarding postoperative results as hematoma or seroma formation, wound infection or dehiscence. There was no total flap loss in any of the patients in either group. However, partial flap loss has been encountered in 1 patient (4.8%) in group A and in 2 patients (9.5%) in group B (P = 0.99).
Table 1.
Sociodemographic Data and Comorbidities
| Group A (n = 21) | Group B (n = 21) | P | |||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Marital status | |||||
| Single | 2 | 9.5 | 2 | 9.5 | MCP = 1.000 |
| Married | 16 | 76.2 | 16 | 76.2 | |
| Divorce | 3 | 14.3 | 3 | 14.3 | |
| Age, y | |||||
| Minimum–maximum | 31.0–50.0 | 32.0–51.0 | 0.699 | ||
| Mean ± SD | 40.95 ± 5.06 | 40.33 ± 5.25 | |||
| Median | 41.0 | 40.0 | |||
| Parity | |||||
| M | 16 | 76.2 | 17 | 81.0 | FEP = 1.000 |
| N | 5 | 23.8 | 4 | 19.0 | |
| Comorbidities | |||||
| DM | 3 | 14.3 | 4 | 19 | 0.866 |
| IHD | 1 | 4.8 | 1 | 4.8 | 1.000 |
| HTN | 2 | 9.5 | 3 | 14.3 | 0.927 |
DM, diabetes mellitus; FE, Fisher exact; HTN, hypertension; IHD, ischemic heart disease; MC, Monte Carlo; M, multiparity; N, nulliparity.
Table 2.
Clinical and Pathological Tumor Data
| Group A (n = 21) | Group B (n = 21 | P | |||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Quadrant | 1.000 | ||||
| UOQ | 10 | 47.6 | 9 | 42.9 | |
| UIQ | 4 | 19.0 | 5 | 23.8 | |
| LIQ | 3 | 14.4 | 2 | 9.5 | |
| LOQ | 4 | 19.0 | 5 | 23.8 | |
| Side | 1.000 | ||||
| RT | 13 | 61.9 | 12 | 57.1 | |
| LT | 8 | 38.1 | 9 | 42.9 | |
| Size | 1.000 | ||||
| T1 | 7 | 33.3 | 6 | 28.6 | |
| T2 | 14 | 66.7 | 15 | 71.4 | |
| Safety margin, mm | 0.866 | ||||
| Minimum–maximum | 11.0–25.0 | 12.0–23.0 | |||
| Mean ± SD | 17.52 ± 4.06 | 17.33 ± 3.17 | |||
| LN status | 0.726 | ||||
| Negative | 13 | 61.9 | 11 | 52.4 | |
| +1 | 3 | 14.3 | 2 | 9.5 | |
| +2 | 1 | 4.8 | 4 | 19.0 | |
| +3 | 3 | 14.3 | 2 | 9.5 | |
| +4 | 1 | 4.8 | 2 | 9.5 | |
LIQ, lower inner quadrant; LOQ, lower outer quadrant; UIQ, upper inner quadrant; UOQ, upper outer quadrant; RT, right; LT, left; LN, lymph node.
Table 3.
Postoperative Results
| Group A (n = 21) | Group B (n = 21) | FEP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | |||||||
| Hematoma | 4 | 19.0 | 1 | 4.8 | 0.343 | |||||
| Seroma | 3 | 14.3 | 1 | 4.8 | 0.606 | |||||
| Infection | 2 | 9.5 | 1 | 4.8 | 1.000 | |||||
| Wound dehiscence | 1 | 4.8 | 1 | 4.8 | 1.000 | |||||
| Partial flap loss | 1 | 4.8 | 2 | 9.5 | 1.000 | |||||
FE, Fisher exact.
Postoperative hospital stay was calculated from the day of the operation to the day of discharge. It was 7.0 ± 1.22 days for group A and 6.71 ± 0.96 days for group B (P = 0.404). There was no significant difference comparing both groups as regards patient satisfaction for the cosmetic outcome as shown in Table 4 (P = 0.927). Patients showed satisfactory results including “excellent” and “good” outcomes in 80.9% and 76.2% for group A and B, respectively.
Table 4.
Patient Satisfaction
| Patient Satisfaction Score | Group A (n = 21) | Group B (n = 21) | MCP | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Excellent | 5 | 23.8 | 6 | 28.6 | 0.927 |
| Good | 12 | 57.1 | 10 | 47.6 | |
| Fair | 2 | 9.5 | 3 | 14.3 | |
| Poor | 2 | 9.5 | 2 | 9.5 | |
| Bad | 0 | 0.0 | 0 | 0.0 | |
MC, Monte Carlo.
Using SPADI, patients in each individual group showed less shoulder function disability and significant improvement with time when compared at 3-, 6-, and 12-month intervals (P < 0.001). Comparing both groups together, patients in groups B had a significantly less shoulder disability compared with group A at the same intervals (P < 0.001) (Tables 5 and 6).
Table 5.
Comparison between the Different Studied Periods According to SPADI
| Shoulder Functional Disability | 3 mo | 6 mo | 12 mo | P |
|---|---|---|---|---|
| Group A (n = 21) | ||||
| Minimum–maximum | 15.0–58.0 | 9.0–28.0 | 2.0–16.0 | <0.001* |
| Mean ± SD | 28.10 ± 9.78 | 17.43 ± 5.66 | 5.62 ± 3.09 | |
| Significant between periods | P1 = 0.002*, P2 <0.001*, P3 = 0.001* | |||
| Group B (n = 21) | ||||
| Minimum–maximum | 9.0–27.0 | 4.0–14.0 | 0.0–5.0 | <0.001* |
| Mean ± SD | 17.24 ± 5.36 | 7.57 ± 3.09 | 3.05 ± 1.47 | |
| Significant between periods | P1 = 0.001*, P2 < 0.001*, P3 = 0.001* | |||
Table 6.
Comparing Both Groups Regarding Shoulder Functional Disability
| Shoulder Functional Disability | Group A (n = 21) | Group B (n = 21) | P |
|---|---|---|---|
| 3 mo | |||
| Minimum–maximum | 15.0–58.0 | 9.0–27.0 | <0.001* |
| Mean ± SD | 28.10 ± 9.78 | 17.24 ± 5.36 | |
| 6 mo | |||
| Minimum–maximum | 9.0–28.0 | 4.0–14.0 | <0.001* |
| Mean ± SD | 17.43 ± 5.66 | 7.57 ± 3.09 | |
| 12 mo | |||
| Minimum–maximum | 2.0–16.0 | 0.0–5.0 | <0.001* |
| Mean ± SD | 5.62 ± 3.09 | 3.05 ± 1.47 |
DISCUSSION
Oncoplastic breast surgery in the treatment of breast cancer is an intermediate option between conventional breast conservative surgery and mastectomy.9 The optimal oncological outcome of conservative breast surgery entails complete excision of malignant tumor with negative resection margins, as involved margins are highly associated with local recurrence.18,19 However, wide resection can compromise the cosmetic outcome and result in breast deformity or bilateral asymmetry. Oncoplastic breast surgery using volume replacement techniques has the advantage of achieving both wide resection and acceptable cosmetic outcome by partial breast reconstruction.20 As oncoplastic procedure was scheduled for all cases in the current study, a wide safety margin of resection was achieved and there were no involved surgical margins with excised tumors in both groups.
Donor site complications such as hematoma and seroma formation are very common after harvesting flaps for breast reconstruction.21 Harvesting of the LD muscle is expected to have more donor site morbidity than flaps with muscle preservation.22,23 This has been demonstrated by Sowa et al24 who documented that less seroma has developed at the donor site of muscle-sparing LD flap compared with conventional LD flap, with no statistically significant difference. This matches with the results of the current study that showed more incidence of hematoma and seroma formation in group A than group B, although the difference did not achieve statistical significance. In many studies, the incidence of seroma following LD flap ranged from 40% up to 76% in some obese patients.25–27 However, in the current study, the incidence of seroma in either group was much less. This can be explained by the smaller size of the designed flap required for partial breast reconstruction compared with wider flaps used in those studies for total breast reconstruction. It is quite logical to have the incidence of seroma to be directly related to the size of the dead space left after harvesting the flap.
LD flap is a highly reliable flap with minimal ischemic complications due to the sufficient vascular supply of the thoracodorsal artery.28 Even in patients with diabetes or tobacco use, there is a minimal risk of flap necrosis. Significant flap necrosis is usually secondary to vascular pedicle injury during the operative dissection or pedicle thrombosis from twisting of the flap on its pedicle.21 Some studies have reported no flap necrosis at all among the included cases as in the study performed by Lee et al.29 Hokin and Silfverskiold30 reported 7% rate of partial flap necrosis. The same has been documented regarding TDAP flap. Adler et al15 reported no flap necrosis among the 18 cases of TDAP flap, whereas Angrigiani et al31 demonstrated 4.44% total flap loss and 8.88% partial flap loss among the included cases. In the current study, there was no total flap loss in either group; however, partial flap loss occurred in 4.8% and 9.5% in group A and group B, respectively, with no significant difference between the 2 groups.
There is a general agreement that defects following breast-conserving surgery can be managed with primary closure; however, the cosmetic outcome may be unpredictable and frequently patients are unsatisfied.32–34 Breast-conserving surgery may lead to varying amounts of volume deficit depending on the dimensions of the resected tissue,31 with the consequence that approximately 10%–30% of these patients are not satisfied with the aesthetic outcome.35 In the current study, the final cosmetic outcome in both groups was assessed depending on the symmetry, wound scar, and nipple and areola. Satisfied patients for their aesthetic outcome accounted for 80.9% for LD flap and 76.2% for TDAP flap, and none of the patients evaluated their outcome to be bad. These are similar to results reported by other studies using either LD flap8,29,36 or TDAP flap,29,31,37 which showed a high percentage of patient satisfaction with either technique. Adler et al15 explained that the aesthetic result might be somewhat superior with the TDAP flap because of better preservation of the posterior axillary fold. In the current study, there was no significant difference in the patient’s satisfaction comparing the 2 groups.
Although LD flap has demonstrated positive aesthetic outcomes in breast reconstruction with apparent minimal reported complications, postoperative shoulder dysfunction has been overlooked.38 Dejode et al39 in their study demonstrated that LD muscle transfer has sequelae on the ipsilateral shoulder range of movement. However, the exact functional impairment was still a subject of debate. Garusi et al40 combined DASH score and objective evaluation of shoulder functions after harvesting LD flap in breast reconstruction and recorded a percentage of recovery. They demonstrated minimal disability in general and up to 80% recovery within 1 year, especially with sports practice.
Blackburn et al38 have documented that breast reconstruction using the LD had an impact on the shoulder function and on some daily life activities, with a significant negative impact not only on the patients themselves but their families as well.38 This significant functional impairment that has been observed in clinical practice was the motivation to explore this area and even to find an alternative to muscle harvesting.12
The current study revealed significantly better shoulder functional outcome in cases of TDAP flap compared with LD flap using SPADI. The study also demonstrated interperiodic significant difference within each group separately, during the follow-up at 3, 6, and 12 months, indicating the improvement of the disability in functions of the shoulder in both groups. Despite this individual group improvement, still, the function after TDAP flap was significantly better.
CONCLUSIONS
Our study considers the TDAP flap as reliable a technique as LD flap regarding the feasibility, postoperative complications, cosmetic outcome, and finally early functional outcome, which is significantly better than that of LD flap. There is still a grey area that should be studied further, and we are still in need of algorithm for choosing the appropriate oncoplastic technique depending on the previous parameters.
Footnotes
Published online 29 October 2019.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Tailby E, Boyages Am J. Conservation surgery and radiation therapy in early breast cancer - an update. Aust Fam Physician. 2017;46:214–219. [PubMed] [Google Scholar]
- 2.Xing L, He Q, Wang YY, et al. Advances in the surgical treatment of breast cancer. Chin Clin Oncol. 2016;5:34. [DOI] [PubMed] [Google Scholar]
- 3.Townsend CM, Beauchamp RD, Evers BM, et al. Sabiston Textbook of Surgery: The Biological Basis of Modern Surgical Practice. 201720th ed Philadelphia: Elsevier. [Google Scholar]
- 4.Chan SW, Cheung PS, Chueng PS, et al. Cosmetic outcome and percentage of breast volume excision in oncoplastic breast conserving surgery. World J Surg. 2010;34:1447–1452. [DOI] [PubMed] [Google Scholar]
- 5.Dahlbäck C, Manjer J, Rehn M, et al. Determinants for patient satisfaction regarding aesthetic outcome and skin sensitivity after breast-conserving surgery. World J Surg Oncol. 2016;14:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bulstrode NW, Shrotria S. Prediction of cosmetic outcome following conservative breast surgery using breast volume measurements. Breast. 2001;10:124–126. [DOI] [PubMed] [Google Scholar]
- 7.Cochrane RA, Valasiadou P, Wilson AR, et al. Cosmesis and satisfaction after breast-conserving surgery correlates with the percentage of breast volume excised. Br J Surg. 2003;90:1505–1509. [DOI] [PubMed] [Google Scholar]
- 8.Kim KD, Kim Z, Kuk JC, et al. Long-term results of oncoplastic breast surgery with latissimus dorsi flap reconstruction: a pilot study of the objective cosmetic results and patient reported outcome. Ann Surg Treat Res. 2016;90:117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maxwell GP. Iginio Tansini and the origin of the latissimus dorsi musculocutaneous flap. Plast Reconstr Surg. 1980;65:686–692. [DOI] [PubMed] [Google Scholar]
- 10.Monticciolo DL, Ross D, Bostwick J, III, et al. Autologous breast reconstruction with endoscopic latissimus dorsi musculosubcutaneous flaps in patients choosing breast-conserving therapy: mammographic appearance. AJR Am J Roentgenol. 1996;167:385–389. [DOI] [PubMed] [Google Scholar]
- 11.Rainsbury RM, Paramanathan N. Recent progress with breast-conserving volume replacement using latissimus dorsi miniflaps in UK patients. Breast Cancer. 1998;5:139–147. [DOI] [PubMed] [Google Scholar]
- 12.Koh CE, Morrison WA. Functional impairment after latissimus dorsi flap. ANZ J Surg. 2009;79:42–47. [DOI] [PubMed] [Google Scholar]
- 13.Lee KT, Mun GH. A systematic review of functional donor-site morbidity after latissimus dorsi muscle transfer. Plast Reconstr Surg. 2014;134:303–314. [DOI] [PubMed] [Google Scholar]
- 14.Blackburn NE, Mc Veigh JG, Mc Caughan E, et al. The musculoskeletal consequences of breast reconstruction using the latissimus dorsi muscle for women following mastectomy for breast cancer: a critical review. Eur J Cancer Care (Engl). 2018;27:e12664. [DOI] [PubMed] [Google Scholar]
- 15.Adler N, Seitz IA, Song DH. Pedicled thoracodorsal artery perforator flap in breast reconstruction: clinical experience. Eplasty. 2009;9:e24. [PMC free article] [PubMed] [Google Scholar]
- 16.Jain L, Kumta SM, Purohit SK, et al. Thoracodorsal artery perforator flap: indeed a versatile flap. Indian J Plast Surg. 2015;48:153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roach KE, Budiman-Mak E, Songsiridej N, et al. Development of a shoulder pain and disability index. Arthritis Care Res. 1991;4:143–149. [PubMed] [Google Scholar]
- 18.Campbell EJ, Romics L. Oncological safety and cosmetic outcomes in oncoplastic breast conservation surgery, a review of the best level of evidence literature. Breast Cancer (Dove Med Press). 2017;9:521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White J, Achuthan R, Turton P, et al. Breast conservation surgery: state of the art. Int J Breast Cancer. 2011;2011:107981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Down SK, Jha PK, Burger A, et al. Oncological advantages of oncoplastic breast-conserving surgery in treatment of early breast cancer. Breast J. 2013;19:56–63. [DOI] [PubMed] [Google Scholar]
- 21.Sood R, Easow JM, Konopka G, et al. Latissimus dorsi flap in breast reconstruction: recent innovations in the workhorse flap. Cancer Control. 2018;25:1073274817744638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Randolph LC, Barone J, Angelats J, et al. Prediction of postoperative seroma after latissimus dorsi breast reconstruction. Plast Reconstr Surg. 2005;116:1287–1290. [DOI] [PubMed] [Google Scholar]
- 23.Tomita K, Yano K, Masuoka T, et al. Postoperative seroma formation in breast reconstruction with latissimus dorsi flaps: a retrospective study of 174 consecutive cases. Ann Plast Surg. 2007;59:149–151. [DOI] [PubMed] [Google Scholar]
- 24.Sowa Y, Numajiri T, Nakatsukasa K, et al. Comparison of morbidity-related seroma formation following conventional latissimus dorsi flap versus muscle-sparing latissimus dorsi flap breast reconstruction. Ann Surg Treat Res. 2017;93:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdalla HM, Shalaan MA, Fouad FA, et al. Immediate breast reconstruction with expander assisted latissimus dorsi flap after skin sparing mastectomy. J Egypt Natl Canc Inst. 2006;18:134–140. [PubMed] [Google Scholar]
- 26.Yan WH, Mang JB, Ren LL, et al. Natural history of seroma following the immediate latissimus dorsi flap method of breast reconstruction. Chin Med J (Engl). 2018;131:1674–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin IS, Lee DW, Lew DH. Efficacy of quilting sutures and fibrin sealant together for prevention of seroma in extended latissimus dorsi flap donor sites. Arch Plast Surg. 2012;39:509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burgic M, Bruant Rodier C, Wilk A, et al. Complications following autologous latissimus flap breast reconstruction. Bosn J Basic Med Sci. 2010;10:65–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JW, Kim MC, Park HY, et al. Oncoplastic volume replacement techniques according to the excised volume and tumor location in small- to moderate-sized breasts. Gland Surg. 2014;3:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hokin JA, Silfverskiold KL. Breast reconstruction without an implant: results and complications using an extended latissimus dorsi flap. Plast Reconstr Surg. 1987;79:58–66. [PubMed] [Google Scholar]
- 31.Angrigiani C, Rancati A, Escudero E, et al. Extended thoracodorsal artery perforator flap for breast reconstruction. Gland Surg. 2015;4:519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clough KB, Thomas SS, Fitoussi AD, et al. Reconstruction after conservative treatment for breast cancer: cosmetic sequelae classification revisited. Plast Reconstr Surg. 2004;114:1743–1753. [DOI] [PubMed] [Google Scholar]
- 33.Hamdi M, Wolfli J, Van Landuyt K. Partial mastectomy reconstruction. Clin Plast Surg. 2007;34:51–62; abstract vi. [DOI] [PubMed] [Google Scholar]
- 34.Munhoz AM, Aldrighi CM, Ferreira MC. Paradigms in oncoplastic breast surgery: a careful assessment of the oncological need and esthetic objective. Breast J. 2007;13:326–327. [DOI] [PubMed] [Google Scholar]
- 35.Munhoz AM, Montag E, Gemperli R. Oncoplastic breast surgery: indications, techniques and perspectives. Gland Surg. 2013;2:143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Marakby HH, Kotb MH. Oncoplastic volume replacement with latissimus dorsi myocutaneous flap in patients with large ptotic breasts. Is it feasible? J Egypt Natl Canc Inst. 2011;23:163–169. [DOI] [PubMed] [Google Scholar]
- 37.Kim JB, Kim DK, Lee JW, et al. The usefulness of pedicled perforator flap in partial breast reconstruction after breast conserving surgery in Korean women. Arch Plast Surg. 2018;45:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blackburn NE, Mc Veigh JG, Mc Caughan EM, et al. The musculoskeletal consequences of latissimus dorsi breast reconstruction in women following mastectomy for breast cancer. Plos One. 2018;13:e0202859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dejode M, Bordes V, Jaffré I, et al. [Oncologic, functional, and aesthetics results; evaluation of the quality of life after latissimus dorsi flap breast reconstruction. About a retrospective series of 450 patients [in French]]. Ann Chir Plast Esthet. 2011;56:207–215. [DOI] [PubMed] [Google Scholar]
- 40.Garusi C, Manconi A, Lanni G, et al. Shoulder function after breast reconstruction with the latissimus dorsi flap: a prospective cohort study - combining DASH score and objective evaluation. Breast. 2016;27:78–86. [DOI] [PubMed] [Google Scholar]


