Supplemental Digital Content is available in the text.
Background:
Hyperbaric oxygen therapy (HBOT) can improve wound healing and has been found to have positive preconditioning effects in animal models. Among esthetic surgical procedures, abdominoplasty poses the highest rate of postoperative complications. The aim of this study was to evaluate the effect of preoperative HBOT as a preconditioning treatment for expected postsurgical complications.
Methods:
We conducted a retrospective cohort study among patients who underwent abdominoplasty at our institute and private practice between January 2012 and November 2017. Patients who received preoperative HBOT were compared with patients who did not receive HBOT. Surgical complication data and demographic, preoperative and postoperative data from patient records were collected.
Results:
The study included 356 patients. Of them, 83 underwent HBOT preoperatively. Using preoperative HBOT, postoperative complications were significantly reduced from 32.6% (89 patients) to 8.4% (7 patients), P <0.001. Moreover, 17 (6.2%) patients in the comparison group and none in the HBOT group experienced necrosis (P = 0.016). In the multivariate analysis, preoperative HBOT was an independent protective factor against postoperative complications (odds ratio, 0.188; 95% CI, 0.082–0.432; P < 0.001). After propensity score matching, the study results remained the same.
Conclusions:
Preoperative HBOT can reduce postoperative complication rate in abdominoplasty patients. Further prospective studies are necessary to validate the findings and characterize patients who benefit the most from this treatment.
INTRODUCTION
Abdominoplasty is a common procedure to repair a protruding abdomen, loose, or lax abdominal skin, by removing the excess skin and fat from the abdomen and waist area. In addition, the procedure comprises of the tightening of abdominal wall muscles, especially when esthetic improvement attained by exercise is limited.1Among esthetic surgical procedures, abdominoplasty poses the highest rate of postoperative complications, accounting for a staggering 38%. The high complication rate of abdominoplasty, due to the nature of the surgery, is attributable to blood supply disruption at the edges of the abdominal flap, resulting in ischemia, necrosis, infection, wound dehiscence, seromas, and excessive scarring.2–4 Although patient factors that adversely affect blood supply are diabetes, smoking, and old abdominal scars, surgical factors include concomitant liposuction, excessive skin separation, wound undermining, and excessive tension on the final skin closure, placing patients at the highest risk for postoperative complications.1–3
Israel’s health system is governed by the National Health Insurance Law, under which all Israeli residents are entitled to healthcare, with monthly premiums paid to the National Insurance Institute. Citizens select from 4 Health Maintenance Organizations, each providing an identical basket of services including, but not limited to, hospital, primary, and specialty care.5 Select specialty services, such as hyperbaric oxygen therapy (HBOT), are currently included in basket services.
HBOT involves the inhalation of 100% oxygen at a pressure of >1 atmospheres absolute to enhance the amount of oxygen dissolved in body tissues. During HBOT, arterial O2 tension typically escalates from the normal non-HBOT range of 75–100 mm Hg to tensions of >1,400 mm Hg and O2 tension levels in the tissues rise as high as 200–400 mm Hg.6 In clinical practice, nonhealing wounds and compromised grafts and flaps are usually treated with HBOT. HBOT has been found to improve the underlying hypoxia,7 induce both angiogenesis and vasculogenesis and exhibits anti-inflammatory effects, and promote collagen synthesis for wound healing.8 Furthermore, the fluctuation in circulating oxygen during HBOT from very high levels back to normal is interpreted by the body as relative hypoxia: the “hyperoxic-hypoxic paradox.” In response to relative hypoxia, hypoxia-inducible factor and its downregulated gene cascade are activated during but in the hyperoxidized environment. As preconditioning, HBOT can be utilized as prevention strategy to activate protective mechanisms, which could reduce the risk of morphological and functional sequelae. Some animal studies have reported that preconditioning treatment with HBOT can induce an independent protective effect on skin flaps and grafts, preventing ischemia–reperfusion injury and improving surgical wound healing.9–14 In addition, previous randomized controlled trials have reported that patients who underwent on-pump coronary artery bypass grafting having underwent HBOT as a preconditioning treatment encountered fewer postoperative complications and surgical site infections.15 To the best of our knowledge, the effect of preoperative HBOT on abdominal surgical wound healing and postoperative complications in humans remains only partially elucidated.
The aim of this study was to evaluate the effect of preoperative HBOT as a preconditioning treatment on the expected postabdominoplasty complication rate.
METHODS
Study Design
A historical cohort study of all female patients who underwent abdominoplasty between January 2012 and November 2017 at the Department of Plastic and Reconstructive Surgery, at Assaf Harofeh Medical Center, Israel, and all patients who underwent the procedure by Dr. Tali Friedman at Ramat Aviv Medical Center, a private hospital located in Tel Aviv, Israel. Throughout the study period, every patient who underwent abdominoplasty had been offered a referral to HBOT. Every surgery was performed using the same techniques. The study included only female patients over 18 years of age. No informed consent was required as this was a retrospective study. The study was approved by the Assaf Harofeh Medical Center Ethics Review Board.
The study included 2 groups of patients: a study group of patients who underwent preoperative HBOT and a comparison group of patients who did not undergo preoperative HBOT.
Patients were referred for preoperative HBOT by the surgeon after a comprehensive explanation about the benefits and risks of undergoing HBOT. HBOT was conducted at the Sagol Center for Hyperbaric Medicine and Research (Assaf Harofeh Medical Center). The treatment consisted of 1–3 daily sessions of 90 minutes of 100% oxygen at 2 atmospheres absolute with 5-minute air breaks at 20-minute intervals. As preoperative HBOT is still in the pilot stages, the number of treatment sessions has not yet been characterized and was at the discretion of the referring physician. The final HBOT session was conducted the day before surgery.
Data were collected from patients’ medical records for preoperative medical history and postoperative records for the 6-month period following surgery. Primary outcomes were postoperative complications, including skin necrosis, infection, seroma, hematoma, wound dehiscence, or hypertrophic scarring.
Statistical Analysis
Categorical variables were described as frequency and percentage. Continuous variables were evaluated for normal distribution using histogram and Q–Q plot and described as median and interquartile range, or mean and SD. Categorical variables were compared between patients treated before surgery and those not treated using a chi-squared test or Fisher’s exact test, and continuous variables were compared between the 2 groups using independent samples t test or Mann-Whitney U test. Multivariate logistic regression was used to study the association between preoperative treatment and complication while controlling for confounders. We used the forward method to select variables for inclusion in the multivariate analysis. To control for differences in the baseline characteristics, patients in the pretreatment group were matched with patients in the comparison group using a propensity score. The propensity score was calculated as the probability of patients being in the pretreatment group. Logistic regression was used to calculate the propensity score. Age, smoking, regular sporting activity, bariatric surgery, maximal body mass index (BMI, from patient charts), current BMI (on day of surgery), weight stability in months, diabetes mellitus, thyroid disease, hypertension, and mental disorder were included in the logistic regression. An absolute difference up to 5% in the propensity score was considered acceptable for matching. After matching, patients were compared using paired-samples t test, Wilcoxon test, and McNemar’s test. When McNemar’s test was not applicable, we used Fisher’s exact test. Conditional logistic regression was used to describe the odds ratio (OR) after matching. All statistical tests were 2 sided. P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS (IBM SPSS Statistics for Windows, version 22, 2013; IBM Corp., Armonk, NY).
RESULTS
The study included 356 patients. Preconditioning with HBOT had been conducted in 83 patients (HBOT group), and was not conducted in 273 patients (comparison group). From the HBOT group, 53 patients had undergone 1 HBOT session, 14 patients had undergone 2 HBOT sessions, and 16 patients had undergone 3 HBOT sessions. Table 1 summarizes the patients’ baseline characteristics.
Table 1.
Patient Characteristics
| Patient Characteristics | (n = 356) |
|---|---|
| Age (years), mean (SD) | 42.2 (10.23) |
| Smoking, n (%) | 81 (22.8) |
| Regular sporting activity, n (%) | 114 (32) |
| Bariatric surgery n (%) | 196 (55.1) |
| Abdominoplasty, n (%) | 212 (59.6) |
| Lower body lift, n (%) | 144 (40.4) |
| Liposuction, n (%) | 247 (69.4) |
| BMI (kg/m2), mean (SD) | |
| Maximal | 38.88 (8.99) |
| Minimal | 25.46 (3.99) |
| Current | 26.45 (3.99) |
| Maximal weight loss (kg), median (IQR) | 37 (24–48) |
| Current weight (kg), mean (SD) | 70.76 (11.43) |
| Current weight loss (kg), median (IQR) | 35 (20–45) |
| HBOT before surgery, n (%) | 83 (23.3) |
| Comorbidity, n (%) | |
| Hypertension | 29 (8.1) |
| Cardiovascular | 9 (2.5) |
| Thyroid disease | 39 (11) |
| Asthma | 14 (3.9) |
| Diabetes mellitus | 25 (7) |
| Oncology | 11 (3.1) |
| Mental disorder | 19 (5.3) |
IQR, interquartile range.
Patients in the HBOT group were older than those in the comparison group, and less likely to have underwent bariatric surgery and lower body lift procedures. In contrast, patients in the comparison group were less likely to have underwent abdominoplasty procedures and liposuction. In addition, maximal BMI and weight were lower in the HBOT group than in the comparison group. In all other studied parameters, there was no significant difference between the 2 groups. Table 2 presents the comparison of patients’ characteristics between the treatment and comparison groups.
Table 2.
Comparison of Patient Characteristics between the Treatment and Control Groups
| HBOT before Surgery | |||
|---|---|---|---|
| No | Yes | ||
| Patient Characteristics | (n = 273) | (n = 83) | P |
| Age (years), mean (SD) | 41.49 (10.56) | 44.56 (8.71) | 0.008 |
| Smoking, n (%) | 56 (20.5) | 25 (30.1) | 0.068 |
| Regular sporting activity, n (%) | 86 (31.5) | 28 (33.7) | 0.703 |
| Bariatric surgery, n (%) | 177 (64.8) | 19 (22.9) | <0.001 |
| Abdominoplasty, n (%) | 152 (55.7) | 60 (72.3) | 0.007 |
| Lower body lift, n (%) | 121 (44.3) | 23 (27.7) | 0.007 |
| Liposuction, n (%) | 174 (63.7) | 73 (88.0) | <0.001 |
| BMI (kg/m2), mean (SD) | |||
| Maximal | 41.13 (8.2) | 31.46 (7.03) | <0.001 |
| Minimal | 25.81 (3.86) | 24.32 (4.21) | 0.003 |
| Current | 26.77 (3.96) | 25.40 (3.89) | 0.006 |
| Maximal weight loss (kg), median (IQR) | 40.00 (30.5–50.5) | 16 (2–35) | <0.001 |
| Current weight (kg), mean (SD) | 71.48 (11.29) | 68.37 (11.64) | 0.03 |
| Current weight loss (kg), median (IQR) | 39 (28–48) | 11 (0.0–30) | <0.001 |
| Comorbidity, n (%) | |||
| Hypertension | 28 (10.3) | 1 (1.2) | 0.008 |
| Cardiovascular | 8 (2.9) | 1 (1.2) | 0.691 |
| Thyroid disease | 31 (11.4) | 8 (9.6) | 0.661 |
| Asthma | 11 (4) | 3 (3.6) | >0.999 |
| Diabetes mellitus | 18 (6.6) | 7 (8.4) | 0.566 |
| Oncology | 8 (2.9) | 3 (3.6) | 0.723 |
| Mental disorder | 14 (5.1) | 5 (6) | 0.781 |
IQR, interquartile range.
Overall, 96 patients (27%) experienced complications. The most frequent complication was infection (n = 33), followed by seroma (n = 29), hypertrophic scar (n = 20), necrosis (n = 17), dehiscence wound breakdown (n = 13), hematoma (n = 7), pulmonary embolism/deep vein thrombosis (n = 4), and lymphedema (n = 3). Of the 83 patients in the HBOT group, only 7 patients (8.4%) experienced complications as opposed to 89 patients (32.6%) in the comparison group (OR, 0.182; 95% CI, 0.08–0.413; P < 0.001). Necrosis, infection, and hypertrophic scars were only present in the comparison group and not in the HBOT group (P = 0.016, P = 0.001, and P = 0.006, respectively). The multivariate analysis identified preoperative HBOT as an independent protective predictor against complications (OR, 0.188; 95% CI, 0.08–0.432; P <0.001). Comparison of the complications between the 2 groups is presented in Table 3 and Figure 1.
Table 3.
Comparison of the Incidence of Specific Complications between the Treatment and Comparison Groups
| HBOT before Surgery | |||
|---|---|---|---|
| No (n = 273) (%) | Yes (n = 83) (%) | P | |
| Any complication | 89 (32.6) | 7 (8.4) | <0.001 |
| Seroma | 25 (9.2) | 4 (4.8) | 0.206 |
| Necrosis | 17 (6.2) | 0 (0.0) | 0.016 |
| Dehiscence wound breakdown | 12 (4.4) | 1 (1.2) | 0.314 |
| Infection | 33 (12.1) | 0 (0) | 0.001 |
| PE/DVT | 3 (1.1) | 1 (1.2) | >0.999 |
| Hypertrophic scar | 20 (7.3) | 0 (0.0) | 0.006 |
| Lymphedema | 3 (1.1) | 0 (0.0) | >0.999 |
| Hematoma | 6 (2.2) | 1 (1.2) | >0.999 |
DVT, deep vein thrombosis; PE, pulmonary embolism.
Fig. 1.
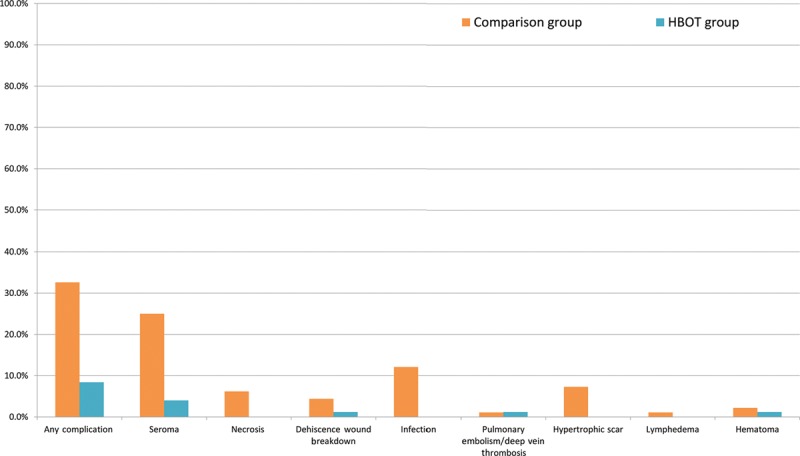
Comparison of patient characteristics between the treatment and comparison groups.
To control for differences in baseline characteristics between the groups, we performed a propensity score matching and 63 patients were matched. Comparison of baseline characteristics between the 2 matched groups is presented in table (Supplemental Digital Content 1, which displays preoperative characteristics of the matched groups. http://links.lww.com/PRSGO/B192).
None of the characteristics were significantly different between the groups. After matching, 4 (6.3%) patients in the treatment group experienced postoperative complications compared with 25 (39.7%) patients in the comparison group (OR, 0.16; 95% CI, 0.056–0.46; P = 0.001). Necrosis, infection, and hypertrophic scar remained statistically different between the groups. Table 4 presents incidence comparisons of specific complications between the matched groups.
Table 4.
Patient-specific Complications in the Matched Groups
| HBOT before Surgery | |||
|---|---|---|---|
| No (n = 63) (%) | Yes (n = 63) (%) | P | |
| Any complication | 25 (39.7) | 4 (6.3) | <0.001 |
| Seroma | 7 (11.1) | 3(4.8) | 0.344 |
| Necrosis | 6 (9.5) | 0 (0.0) | 0.028 |
| Dehiscence wound breakdown | 1 (1.6) | 0 (0.0) | >0.999 |
| Infection | 7 (11.1) | 0 (0.0) | 0.013 |
| PE/DVT | 1 (1.6) | 1 (1.6) | >0.999 |
| Hypertrophic scar | 6 (9.5) | 0 (0.0) | 0.028 |
| Lymphedema | 1 (1.6) | 0 (0.0) | >0.999 |
| Hematoma | 2 (3.2) | 0 (0.0) | 0.496 |
DVT, deep vein thrombosis; PE, pulmonary embolism.
DISCUSSION
Typically, preconditioning treatment is defined by the response to a stimulus that extends beyond its presence in the system, whereas effective response is expected to promote a protective effect. Several studies have demonstrated the efficacy of HBOT preconditioning in expected ischemia–reperfusion injury to the skin, spinal cord, brain, liver, kidney, and heart in various animal models.9–13,16–19 In addition, some clinical studies in humans reported that HBOT preconditioning in patients with coronary artery disease before coronary artery graft bypass demonstrated decreased myocardial injury, decreased duration of postoperative stay in the cardiac intensive care unit, decreased blood loss, fewer postoperative complications, and lesser financial burden.14,19 The results of this study support the use of HBOT as an effective preconditioning treatment before abdominoplasty. Case examples can be seen in Figures 2 and 3.
Fig. 2.
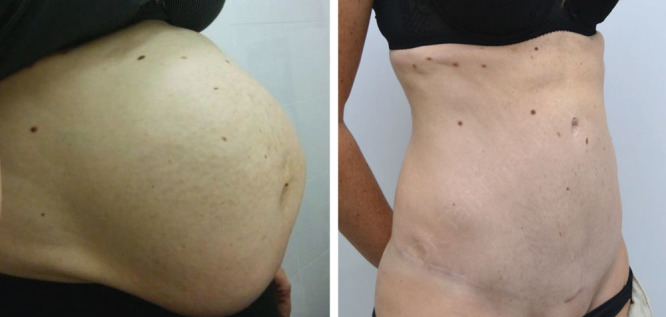
A 34-year-old patient, status postmultiple cesarean sections including 2 pairs of twins. She had severe diastasis recti and expected to have a wide undermining as part of her abdominoplasty. She was an active smoker. She underwent 1 HBOT session the morning of abdominoplasty and had an uneventful surgery.
Fig. 3.
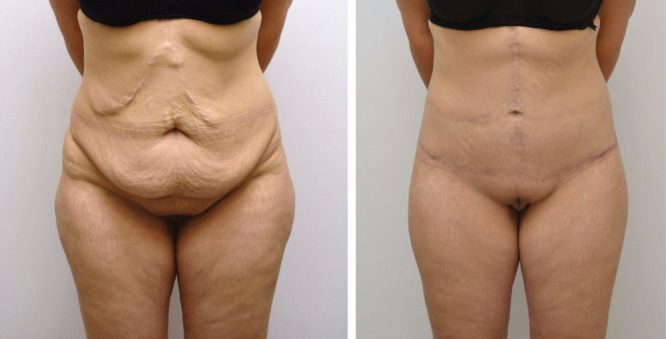
This is a 38-year-old patient, who had a few abdominal surgeries due to a motor vehicle accident. She was an active smoker who did not stop smoking before surgery. She underwent 1 HBOT session the morning of fleur-de-lis lower body lift and had an uneventful surgery and recovery.
The major beneficial effects of HBOT could be attributed to the increased amount of free oxygen molecules dissolved in the blood stream, which are then transferred to the tissues, inducing various processes:
Hyperoxic-hypoxic paradox and hypoxia-inducible factor cascade (as discussed above).
Oxidative stress tolerance: HBOT preconditioning results in an elevation in the partial pressure of oxygen along with a transient increase in the levels of reactive oxygen species, triggering an upsurge in the expression of cell-protective proteins, which in turn enhances the cellular tolerance against harmful stimuli. In addition, endothelial cells exposed to high oxygen pressure exhibit an elevation in antioxidant production.20 In particular, several antioxidant proteins and free-radical scavengers (such as glutathione, Heme Oxygenase-1 (HO-1), catalase, and superoxide dismutase) are upregulated following HBOT,21,22 facilitating the reduction of the oxidative stress in cells following reperfusion injury. Furthermore, the levels of pro-oxidant enzymes (such as inducible nitric oxide synthase and gp91-phox) are considerably decreased following HBOT.19
Anti-inflammatory mediators: HBOT treatment inhibits neutrophil adherence molecules (such as β2-integrins, intercellular adhesion molecule [ICAM]-1,23 integrin beta chain-2 [CD18], and others),24 thereby temporarily inhibiting the ability of circulating neutrophils to adhere to target tissues and subsequently reducing postsurgical inflammation. Inflammation is also reduced by the inhibition of proinflammatory cytokine production (such as IL-1β, IL-6, TNF-α, and prostaglandin-E2) by monocyte-macrophages.19,24,25 The immune system is not compromised by HBOT.26
Increased microcirculatory perfusion and neovascularization: Previous animal studies have reported that HBOT preconditioning increases perfusion in compromised flaps compared with nonpreconditioned animals.7,12 A histological investigation revealed that an increased density of microvessels was accountable for increased perfusion.11 HBOT is also known to promote neovascularization by increasing angiogenic molecules such as stromal cell–derived factor-1 and CXC chemokine receptor 4.11
Decreased apoptosis: HBOT increases antiapoptotic molecules (such as BCL-2) while reducing proapoptotic molecules (such as pASK-1 and Bax). In addition, the antiapoptotic effects markedly decrease the ischemia–reperfusion injury in skin flaps.10
Although HBOT has been used for several years for various indications, this study is the first to report its utility as a preoperative treatment to reduce postoperative complications of major plastic surgery. Thus, this study determined the efficacy of preoperative HBOT, conducted before abdominoplasty, on postoperative complications.
The benefit of HBOT in our study is highlighted by the fact that patients in the HBOT group tended to have several risk factors for poor results and complications, that is, older age, smoking, and a previous abdominoplasty or liposuction. In addition, the benefit was retained when propensity score matching was performed to create matched pairs between the control and the HBOT groups.
Based on the beneficial effects of preoperative HBOT in our study, it could be argued that the use of preoperative HBOT preconditioning could potentially benefit patients undergoing several other surgical procedures. Although apparent costs are involved with HBOT preconditioning, including patient time investment and financial costs associated with the treatment, these might be easily offset by the potential benefits including a shorter hospital stay, lower overall costs, decreased morbidity related to complications, increased patient satisfaction with surgical results, and fewer physician visits.
HBOT is considered a very safe treatment with minimal, if any, adverse effects.26 Primary side effects might include middle ear or sinus barotraumas, which are usually mild and temporary and completely resolved after several days. In this study, no side effects of HBOT were observed. Although preoperative HBOT is not yet a prominent and frequently used technique, and there are several established methods commonly used to promote postoperative wound healing and minimize complications, HBOT preconditioning could potentially be of great benefit to both patients and physicians.
LIMITATIONS
This study has several limitations, most of which are associated with the fact that data were retrospectively collected. To eliminate the risk of selection bias, all eligible patients who underwent abdominoplasty at the medical centers were included in the study, without any selection. As there was patient self-selection involved, it is possible smokers may have been more willing to undergo HBOT. Due to the different baseline characteristics of patients referred to receive HBOT, propensity score matching was performed, and the beneficial effects of HBOT were statistically significant even after matching.
Further studies in multiple clinical centers with larger, more heterogeneous patient populations are needed to evaluate and corroborate the findings presented here. Future studies will enable us to define which patients will benefit the most from preoperative HBOT and the optimal number of sessions needed before abdominoplasty based on patient risk factors. Additional studies may also explore the cost–benefit trade-offs for this procedure in global health systems with different cost structures.
CONCLUSIONS
Preoperative HBOT can decrease the rate of abdominoplasty-related postoperative complications. The treatment is safe and well-tolerated and can be easily conducted for most patients. Nevertheless, further prospective studies are warranted to validate the findings and characterize patients who benefit the most from this treatment.
Supplementary Material
Footnotes
Published online 24 October 2019.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
Drs. Friedman and Menashe contributed equally to this work.
REFERENCES
- 1.Winocour J, Gupta V, Ramirez JR, et al. Abdominoplasty: risk factors, complication rates, and safety of combined procedures. Plast Reconstr Surg. 2015;136:597e–606e. [DOI] [PubMed] [Google Scholar]
- 2.Neaman KC, Hansen JE. Analysis of complications from abdominoplasty: a review of 206 cases at a university hospital. Ann Plast Surg. 2007;58:292–298. [DOI] [PubMed] [Google Scholar]
- 3.Neaman KC, Armstrong SD, Baca ME, et al. Outcomes of traditional cosmetic abdominoplasty in a community setting: a retrospective analysis of 1008 patients. Plast Reconstr Surg. 2013;131:403e–410e. [DOI] [PubMed] [Google Scholar]
- 4.Staalesen T, Elander A, Strandell A, et al. A systematic review of outcomes of abdominoplasty. J Plast Surg Hand Surg. 2012;46:139–144. [DOI] [PubMed] [Google Scholar]
- 5.Rosen B. Mossialos E, Wenzl M, Osborn R, Sarnak D. The Israeli Health Care System. In: International Profiles of Health Care Systems. 2016:New York, NY: Commonwealth Fund; 87–95. [Google Scholar]
- 6.Fosen KM, Thom SR. Hyperbaric oxygen, vasculogenic stem cells, and wound healing. Antioxid Redox Signal. 2014;21:1634–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Francis A, Baynosa RC. Hyperbaric oxygen therapy for the compromised graft or flap. Adv Wound Care (New Rochelle). 2017;6:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang N, Hai Y, Liang F, et al. Preconditioned hyperbaric oxygenation protects skin flap grafts in rats against ischemia/reperfusion injury. Mol Med Rep. 2014;9:2124–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poyrazoglu Y, Topal T, Yuksel R, et al. Effects of hyperbaric oxygen and preconditioning on wound healing in colonic anastomoses. J Invest Surg. 2015;28:188–195. [DOI] [PubMed] [Google Scholar]
- 10.Xiao YD, Liu YQ, Li JL, et al. Hyperbaric oxygen preconditioning inhibits skin flap apoptosis in a rat ischemia-reperfusion model. J Surg Res. 2015;199:732–739. [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Yang J, Li Z, et al. Hyperbaric oxygen preconditioning promotes neovascularization of transplanted skin flaps in rats. Int J Clin Exp Pathol. 2014;7:4734–4744. [PMC free article] [PubMed] [Google Scholar]
- 12.Qi Z, Gao CJ, Wang YB, et al. Effects of hyperbaric oxygen preconditioning on ischemia-reperfusion inflammation and skin flap survival. Chin Med J (Engl). 2013;126:3904–3909. [PubMed] [Google Scholar]
- 13.Koca K, Yurttas Y, Bilgic S, et al. Effect of preconditioned hyperbaric oxygen and ozone on ischemia-reperfusion induced tourniquet in skeletal bone of rats. J Surg Res. 2010;164:e83–e89. [DOI] [PubMed] [Google Scholar]
- 14.Yu SY, Chiu JH, Yang SD, et al. Preconditioned hyperbaric oxygenation protects the liver against ischemia-reperfusion injury in rats. J Surg Res. 2005;128:28–36. [DOI] [PubMed] [Google Scholar]
- 15.Yogaratnam JZ, Laden G, Guvendik L, et al. Hyperbaric oxygen preconditioning improves myocardial function, reduces length of intensive care stay, and limits complications post coronary artery bypass graft surgery. Cardiovasc Revasc Med. 2010;11:8–19. [DOI] [PubMed] [Google Scholar]
- 16.Gao ZX, Rao J, Li YH. Hyperbaric oxygen preconditioning improves postoperative cognitive dysfunction by reducing oxidant stress and inflammation. Neural Regen Res. 2017;12:329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Liu W, Ding S, et al. Hyperbaric oxygen preconditioning induces tolerance against brain ischemia-reperfusion injury by upregulation of antioxidant enzymes in rats. Brain Res. 2008;1210:223–229. [DOI] [PubMed] [Google Scholar]
- 18.He X, Xu X, Fan M, et al. Preconditioning with hyperbaric oxygen induces tolerance against renal ischemia-reperfusion injury via increased expression of heme oxygenase-1. J Surg Res. 2011;170:e271–e277. [DOI] [PubMed] [Google Scholar]
- 19.Huang G, Xu J, Xu L, et al. Hyperbaric oxygen preconditioning induces tolerance against oxidative injury and oxygen-glucose deprivation by up-regulating heat shock protein 32 in rat spinal neurons. Plos One. 2014;9:e85967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Dong H, Chen M, et al. Preconditioning with repeated hyperbaric oxygen induces myocardial and cerebral protection in patients undergoing coronary artery bypass graft surgery: a prospective, randomized, controlled clinical trial. J Cardiothorac Vasc Anesth. 2011;25:908–916. [DOI] [PubMed] [Google Scholar]
- 21.Li Q, Li J, Zhang L, et al. Preconditioning with hyperbaric oxygen induces tolerance against oxidative injury via increased expression of heme oxygenase-1 in primary cultured spinal cord neurons. Life Sci. 2007;80:1087–1093. [DOI] [PubMed] [Google Scholar]
- 22.Thom SR. Oxidative stress is fundamental to hyperbaric oxygen therapy. J Appl Physiol (1985). 2009;106:988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buras JA, Stahl GL, Svoboda KK, et al. Hyperbaric oxygen downregulates ICAM-1 expression induced by hypoxia and hypoglycemia: the role of NOS. Am J Physiol Cell Physiol. 2000;278:C292–C302. [DOI] [PubMed] [Google Scholar]
- 24.Benson RM, Minter LM, Osborne BA, et al. Hyperbaric oxygen inhibits stimulus-induced proinflammatory cytokine synthesis by human blood-derived monocyte-macrophages. Clin Exp Immunol. 2003;134:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niu KC, Huang WT, Lin MT, et al. Hyperbaric oxygen causes both antiinflammation and antipyresis in rabbits. Eur J Pharmacol. 2009;606:240–245. [DOI] [PubMed] [Google Scholar]
- 26.hadanny A, Meir O, Bechor Y, et al. The safety of hyperbaric oxygen treatment–retrospective analysis in 2,334 patients. Undersea Hyperb Med. 2016;43:113–122. [PubMed] [Google Scholar]


