Summary:
Most commercially available breast implants feature some degree of elastomer surface modifications to increase surface roughness, in part because several clinical series have demonstrated positive outcomes from texturizing. However, the literature shows that textured implants support higher rates of bacterial growth, and there is a clear association between increased bacterial contamination and host response in vivo, such as capsular contracture. Furthermore, the infectious theory related to bacterial contamination has recently been described as a potential cause in the etiology of anaplastic large-cell lymphoma. Recent research has focused on the physiology of breast implant surfaces advances and how they interact with the body, creating new surface technologies which have the potential to affect all aspects of breast surgery. Understanding how surface properties affect inflammatory cell response will be essential in designing implants that can provide an esthetic solution while also minimizing long-term clinical complications. This special topic highlights the current knowledge on silicone implant surfaces, as well as innovations that have shaped and will continue to change the silicone breast implant industry in the future. It also provides an overview of the principal surfaces that exist and may find clinical applications in esthetic and reconstructive breast surgery. As additional advances emerge, objective tools will be required to evaluate the different surfaces available on the market, along with the long-term efficacy of new technologies.
INTRODUCTION
Silicone gel breast implants are the most commonly used devices for esthetic and reconstructive breast surgery.1,2 Silicone gel implants have evolved over the years, and that includes technical alterations and improvements to enhance surgical outcomes.3,4 The composition of the silicone gel as well as the degree of texturing of the outer elastomer has been modified by all manufacturers such that a wide array of implant types and surfaces can be selected to best suit each patient and individual breast anatomy.4
Despite improvements in design and technology, commercially available breast implants are not without long-term complications, and the different surface types differ in the way they interact with the soft tissue. Capsular contracture (CC) continues to be the most significant complication, occurring in 14.8%–20.5% of cases.5 Textured implant shells were introduced in the 1970s to minimize this phenomenon, on the assumption that an irregular surface would avoid the parallel alignment of collagen fibers causing the centripetal forces responsible for CC.6 However, the controversy surrounding textured surface implants and whether they reduce the incidence of CC remains.7–12 Recent retrospective long-term studies have demonstrated minimal difference between textured and smooth implants with regard to the occurrence of CC.10–12
On the other hand, the literature shows that textured implants have the potential to support higher rates of bacterial growth, and there is a clear association between greater bacterial contamination and host response in vivo.7,13,14 Textured implants have demonstrated a 3 times higher infection rate over comparable smooth implants in a prospective series.15 Studies have associated biofilms with breast implant complications including CC16,17 and double-capsule formation.18 More recently, the infectious theory related to bacterial contamination has been implicated as a potential etiology for breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL).7,8,13,14
Bacterial adhesion to different abiotic surfaces and biofilm development are proposed mechanisms, regulated by the interaction between the secretion of bacteria extracellular materials and appendages, and surface charge, topography, and surface energy.19 According to some authors, bacteria rarely attach directly to an abiotic surface. Rather, cytokines and matrix proteins produced by immunological cells impacted further by electrostatic charge, pH, and temperature interact with both the foreign body and themselves to promote bacterial adhesion.20
Over recent decades, the engineering of silicone breast implants has progressed to achieve an ideal aesthetic appearance while reducing unsatisfactory results.21–34 This report examines different texture morphology to evaluate the main objective characteristics and potential influences on long-term outcomes. An overview of recent technological developments in breast implant surfaces and the potential impact of these surfaces on surgical outcomes will be reviewed.
Objective Methodology for Assessing Implant Surfaces
Solid surfaces have complex structures depending on the nature of their composition and the surface preparation method. Surface properties are critical in the interaction of breast implants since they affect the area of contact, friction, wear, and wettability7,13,35–37 and some of these properties stimulate soft tissue growth.22,27,35
Profilometry is often used for surface analysis, with a probe tracing along a straight line on a flat surface.35 When a contact profilometer is used, a diamond stylus is moved vertically in contact with a surface and then moved laterally for a determined distance and specified contact force.38,39 This method can assess small vertical aspects ranging from 10 nm to 1 mm. Noncontact profilometers are also used, based on different calculation systems such as laser triangulation, confocal microscopy, and digital holography;38 even so, most global surface standards are written for contact profilometers, including the International Organization for Standardization (ISO) norms.40 Profilometric analysis can define surfaces according to random deviation from the nominal surface that forms the 3D surface topography,38,41 with the main parameters defining roughness (nano- and micro-roughness), waviness (macro-roughness), lay, and flaws.35,38
Micro-computed tomography and confocal laser scanning microscopy (CLSM) are also generally used to measure surface area (SA) and roughness as well as various material properties.7 Micro-computed tomography is essentially 3D X-ray imaging on a very small scale, with massively increased resolution. In this last device, surface specimens (1 cm2) are scanned in a MCTS system every 0.25 degrees over a total rotation of 180 degrees, and saved as 16-bit images in a file format, and the projections are reconstructed using special software.7,42
Wear and Friction
Tribology is defined as the science and technology of interacting surfaces in relative motion, with wear and friction being the main parameters utilized.35 Tribology is of practical importance, because biocompatability of a device in part depends on friction and wear values. Friction is defined as the resistance to motion and is measured by static friction (the force that must be overcome to start moving the object), and dynamic friction (the force needed to keep a surface in motion at a constant velocity) (Fig. 1). Wear is defined as progressive loss of substance from the operating surface of a body occurring as a result of relative motion at the surface.43
Fig. 1.
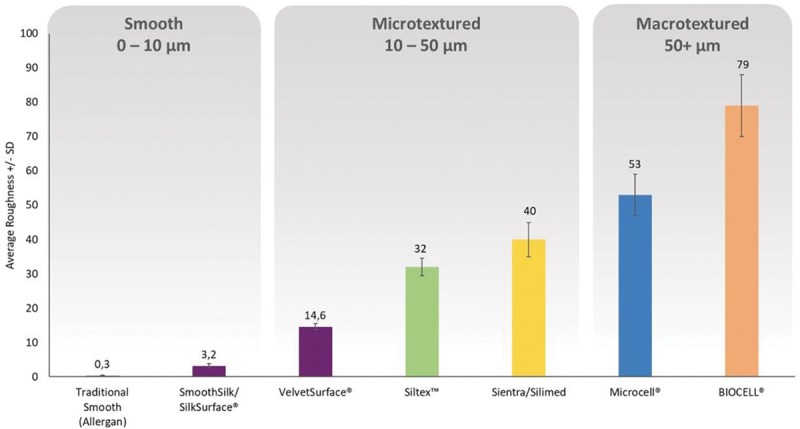
Implant surface interaction with soft tissue. Friction is defined as the resistance to motion. It is measured by static friction (the force that must be overcome to start moving the object) and dynamic friction (the force needed to keep a surface in motion at a constant velocity).
Different implant surfaces can have different values of friction and wear and these aspects can result in different behavior with the skin and soft tissues. Authors have postulated that all implants produce a variable degree of implant debris over time.44 In fact, friction between the implant surface and the overlying tissues can cause chronic inflammation. Friction and wear can lead to the breaking off of tiny silicone particles from the implant surface, which can also aggravate inflammation through macrophage digestion.
Surface Area
SA has been utilized as an important parameter for evaluating different surfaces. The SA for a 1-cm2 disk taken from the implant shell in the most common silicone surfaces available usually ranges from 85 to 551 mm2, and the surface texture ranges from 8 to 602% greater than that of a flat surface (79 mm2).42 Atlan et al42 found the highest SA values for the Nagor Nagotex, Polytech POLYtxt, and Polytech Microthane surfaces (>300 mm2); results for the Allergan Biocell, Sientra True, and Eurosilicone Cristalline surfaces ranged from 200 to 300 mm2, while the Allergan Smooth, Motiva SilkSurface, and Motiva VelvetSurface textures had the lowest SA results, ranging from 80 to 100 mm2. Some studies have found increasing evidence that the inflammatory process, bacterial attachment, and subsequent biofilm formation are significantly impacted by surface topography and SA.13,35,37,45,46 Surfaces with dimensional aspects much smaller than microbial cells and lower SA have been reported to inhibit attachment by reducing the contact area between bacteria cells and the surface.45–48 Compared with smooth surfaces, textured implant surfaces offer greater SA to integrate into the surrounding soft tissues via the inflammatory process and also permit tissue ingrowth and bacteria adhesion.22,27,35
Roughness
Surface roughness is one of the most relevant parameters, defined as variations in the height of the surface relative to a reference plane. Average roughness (Ra) usually measures microscopic peaks and valleys and represents the arithmetic average of the absolute values for the roughness profile ordinates.38,39,41 At this time, Ra is the most frequently used parameter in engineering practice and can be measured either along a single line profile or along a set of parallel line profiles (surface maps).35,38,39,41 It is usually defined by 1 of the 2 statistical height descriptors advocated by the American National Standards Institute and the ISO.35,40
Alterations in the surface Ra of implants influence cell response by increasing the SA of the implant adjacent to soft tissues, thereby improving cell attachment.35,37,41,45,46 Despite its importance in terms of surface evaluation, sometimes Ra is not an adequate differential for surfaces, since it does not differentiate between “spiky” and “scratched” surfaces, which exhibit the same Ra. Additional parameters are required for this purpose such as Rp (maximum peak height), Rv (maximum valley depth), and Ry (maximum peak-to-valley roughness height).35,38,39,41,46
Recently Ra has been frequently associated with SA, and both parameters have been implicated in cell adhesion.7,37 James et al compared biofilm formation on the surface of implants with varying SA-Ra values.37 The Ra of each material was assessed using noncontact profilometry, and bacterial attachment and biofilm formation were tested using a CDC biofilm reactor and CLSM for the presence of Staphylococcus epidermidis, Pseudomonas aeruginosa, and Ralstonia pickettii. These authors reported significantly greater bacterial attachment and biofilm formation on implants with higher SA-Ra values than those with lower values. Their CLSM findings also confirmed the formation of thicker biofilms on the implants with rougher surface textures.
Skewness/Kurtosis
Two other height descriptors are used besides Ra: skewness (Sku) and kurtosis (Ku).38,49 Both are not measured parameters per se but rather statistical tools for evaluating the location and variability of a data set.50 Statistically Sku is an evaluation of symmetry (or more precisely, lack of symmetry) and also measures the sharpness of the roughness profile.38,50 Sku represents the degree of distortion from a symmetrical normal distribution. A symmetrical distribution will have a Sku of zero.35,50 In general, an implant surface with a positive Sku pattern implies that the mean and median will be greater than the mode and presents more peaks than valleys. Contrarily, a negative Sku pattern signifies that the mean and median will be less than the mode and is associated with more valleys than peaks (Figs. 2 and 3).
Fig. 2.
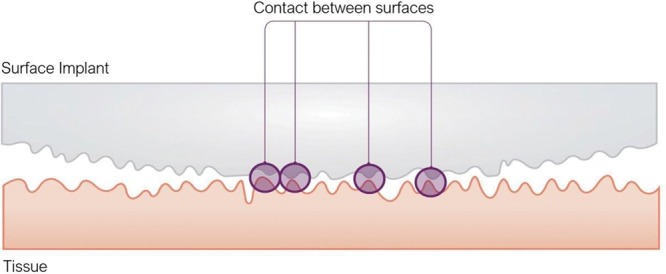
Schematic representation of different Sku patterns: positive Sku equates with a predominance of peaks than valleys. Negative Sku equates with a predominance of valleys over peaks.
Fig. 3.
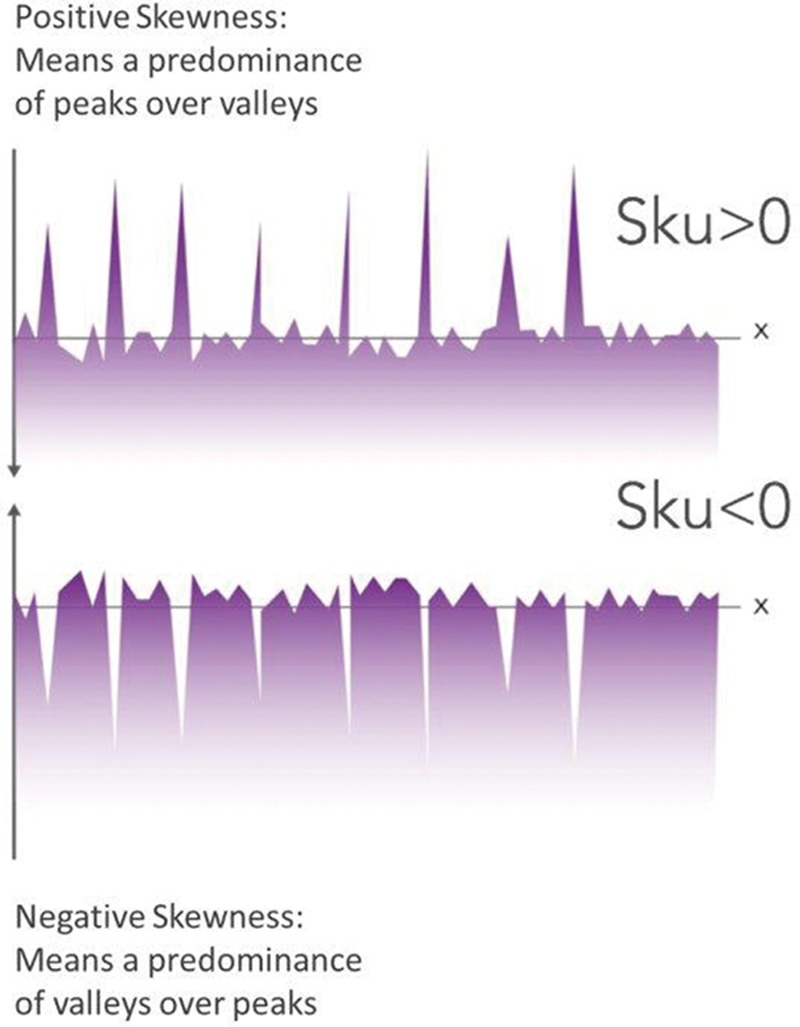
Comparative average Sku (± SD (SD)) coefficients for commercially available breast implants, measured by non-contact profilometry (uSurf Mobile). Results property of Establishment Labs.
Ku, in turn, measures whether the data are heavy-tailed or light-tailed relative to normal distribution; in other words, data sets with high Ku values tend to have heavy tails, or outliers, while data sets with low Ku values tend to have light tails, or lack of outliers.38,39,50 A rough surface can be described more accurately if the Ku parameter is included in the surface evaluation.35,38,39 This parameter is a descriptor of the “peakedness” of the surface and is even more sensitive to isolated peaks and isolated valleys than Sku.39,50
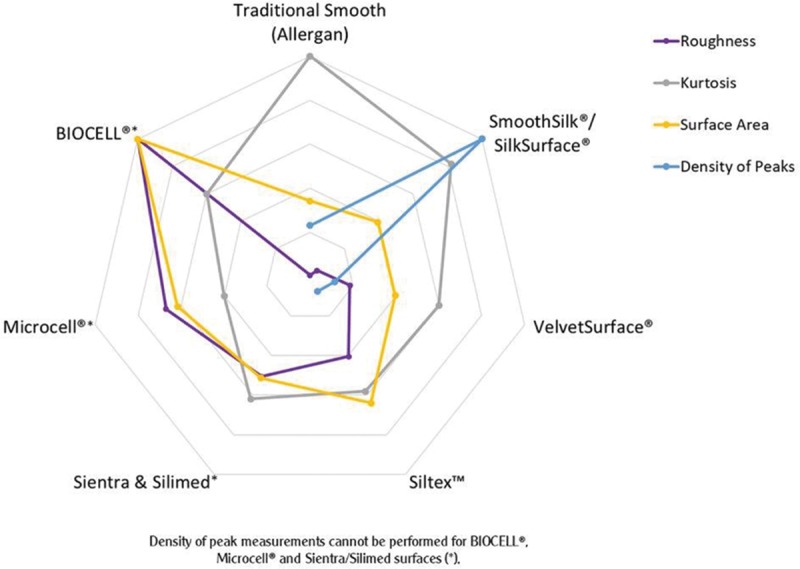
Sku and Ku are other secondary parameters that are also theoretically relevant in assessing the quality of a silicone implant surface. Table I and Figure 4 demonstrate comparatively the data related to Ra, Sku, SA, and density of peaks between the different surfaces available in the current market.
Fig. 4.
Spider web diagram showing surface parameters (roughness, Ku, SA, and density of peaks) of different commercially available silicone breast implant surfaces measured according to ISO 25178-2:2012 and ISO 14607:2018 with a non-contact profilometer uSurf Mobile, over 4 mm2. Results property of Establishment Labs.
Breast Implant Surface Characteristics
Recent experimental and clinical research has demonstrated that surface topographies influence cell attachment.7,35,37,45,46 Furthermore, the inflammatory and bacterial responses to the implant can be greatly modified or even controlled by the nature and texture of the implant surface.36
Smooth and Nano Surfaces
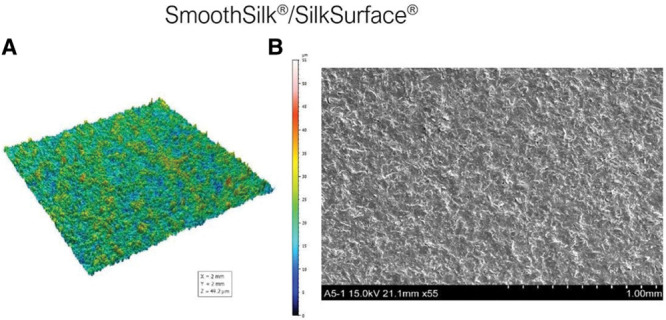
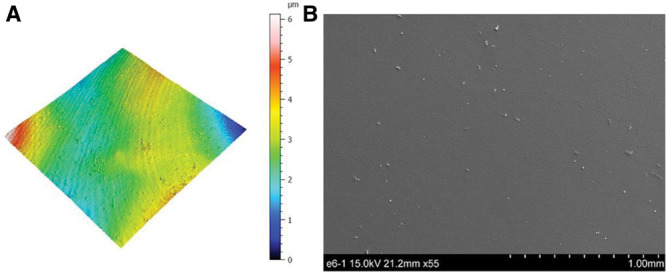
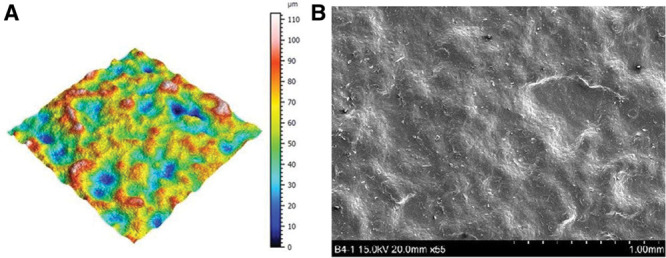
Smooth surfaces usually appear to be externally smooth at low magnification, but differential interference contrast microscopy reveals an irregular surface pattern. Barr et al48 observed ripples on smooth implants using scanning electron and light microscopy; the ripples became more evident at higher magnifications and in electron microscopy, with average widths of ~5 μm between ripples (Fig. 5). A smooth surface usually produces a nonadherent dense capsule, with highly aligned and organized collagen fibers.22,42,51 ISO 14607 also classifies the SilkSurface as smooth; this product has an average of 49,000 contact points per cm2 which are 16 μm (16,000 nm) deep, with smaller and shallower depressions representing the smallest surface available compared with previous “micro” or “macro” surfaces (Fig. 6, Table I).35,52 This surface was classified as a “nanosurface” before the new ISO definition and has proven to be a consistent surface with Ra of 3,600 ± 400 nm (or 3.6 μm) measured using a noncontact usurf mobile profilometer (Nanofocus, Oberhausen, Germany) (Fig. 7).52 The previous term “nano” is a semantic issue and a question of view and perspective, since there is no consensus about the limits of the micro or nanoscale. Based on current knowledge, this surface is better defined as smooth and in accordance to the classification proposed by ISO 2018 based on roughness parameters.
Fig. 5.
(A) Traditional smooth texture 3D topography view taken with uSurf Mobile Profilometer. Per ISO 25178-2:2012. (B) Traditional smooth texture, at a scale of 1 mm and ×55 magnification. Results property of Establishment Labs.
Fig. 6.
Surfaces classification according to ISO 14607:2018 (±SD). Measurements performed according to ISO 25178-2:2012 and ISO 14607:2018 with a noncontact profilometer uSurf Mobile, over 4 mm2. Results property of Establishment Labs.
Fig. 7.
(A) SmoothSilk/SilkSurface 3D topography view taken with uSurf Mobile Profilometer per ISO 25178-2:2012. (B) SmoothSilk/SilkSurface scanning electron microscopy image at a scale of 1 mm and ×55 magnification. Results property of Establishment Labs.
Table 1.
Average Surface Metrology Parameters of Silicone Breast Implant Surfaces Measured According to ISO 14607:2018
| Parameter (±SD) | Traditional Smooth (Allergan) | SmoothSilk/SilkSurface | VelvetSurface | Siltex | Sientra and Silimed | Microcell | BIOCELL |
|---|---|---|---|---|---|---|---|
| Roughness (Ra) (µm) | 0.3 ± 0.2 | 3.2 ± 0.6 | 14.6 ± 2.5 | 32.0 ± 5.0 | 40.0 ± 6.0 | 53.0 ± 9.0 | 79.0 ± 14.0 |
| Density of peaks (peaks/cm2) | 5,996 ± 6,105 | 25,820 ± 5,766 | 2,897 ± 980 | 2,008 ± 1,605 | NA | NA | NA |
| Ku (Sku) | 5.0 ± 2.0 | 4.1 ± 0.6 | 3.0 ± 0.7 | 2.9 ± 0.3 | 3.1 ± 0.4 | 2.0 ± 0.1 | 3.0 ± 1.0 |
| SA (mm2) | 1.0 ± 0.0 | 1.15 ± 0.04 | 1.16 ± 0.02 | 1.87 ± 0.29 | 1.5 ± 0.2 | 1.8 ± 0.2 | 2.92 ± 0.52 |
| ISO 14607:2018 Surface classification | Smooth | Smooth | Microtextured | Microtextured | Microtextured | Macrotextured | Macrotextured |
Density of peak measurements cannot be performed for BIOCELL, Microcell, and Sientra/Silimed surfaces (NA). Results property of Establishment Labs.
Previous studies reported that this topography seems to influence foreign body response by reducing the planar arrangement of fibroblasts and promoting optimum adhesion based on stable focal contacts.7,35,51 Some studies evaluating different breast implant surfaces observed similar capsule morphology across groups of surfaces, with larger textures showing disorganized alignment of collagen fibers.42 However, the tissue along the implant-tissue interface for textures with the smallest SA values (Allergan Smooth, Motiva SilkSurface, and Motiva VelvetSurface) was mostly flat, with the collagen fibers of the capsule aligned parallel to the surface.42,52
Micro and Macro Surfaces
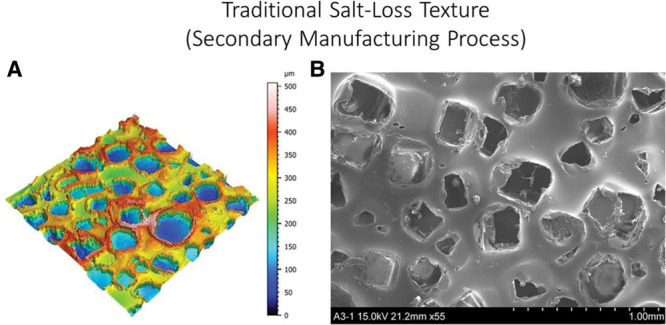
Most commercially available breast implants feature some type of elastomer surface alteration to increase surface roughness. In fact, several surface modifications to increase roughness have emerged over the past decades, such as the Siltex and Biocell surfaces.22,23 The Biocell depressions are irregular, since they are created by salt with different particle sizes, ranging from 600 to 800 μm (0.6–0.8 mm) in diameter and from 150 to 200 μm (0.15–0.2 mm) in depth; an edge raised 70–90 μm around each of these depressions increases the total depth (Fig. 8).27,35 The Siltex surface comprises micronodules ranging from 40 to 100 μm in height and 70 to 150 μm wide, with more regular distribution and average density of 30 nodules per 1.5 mm2 (Fig. 9).27
Fig. 8.
(A) Traditional “Salt-loss” texture (secondary manufacturing process), 3D topography view taken with uSurf Mobile Profilometer. Per ISO 25178-2:2012. (B) Cavities dimensions’ measurements of the “salt-loss” macrotexture, at a scale of 1 mm and ×55 magnification. Results property of Establishment Labs.
Fig. 9.
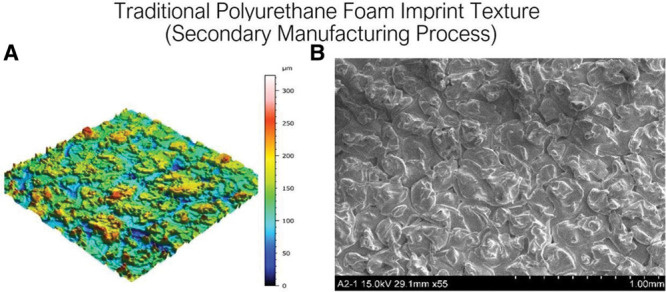
(A) Traditional Polyurethane Foam Imprint Texture (secondary manufacturing process), “PU foam imprint” texture 3D topography view taken with uSurf Mobile Profilometer. Per ISO 25178-2:2012. (B) “Negative imprint with foam” texture, at a scale of 1 mm and ×55 magnification. Results property of Establishment Labs.
Textured surfaces were followed by the introduction of microtexturization, featuring surfaces with more miniaturized roughness, into clinical practice. The Microthane implant shell cover (Polytech Health & Aesthetics, Dieburg, Germany) is made of medical-grade micropolyurethane foam and has a mean surface roughness of 1,500 μm.53 Barr et al documented through scanning electron and light microscopy that the micropolyurethane surface has the deepest structure of all textured surfaces, with total depth of ~1,500 μm and PUC outer of ~1,000 μm in depth.48
The VelvetSurface introduced in the 2000s by Motiva features 1,800–2,200 contact points per cm2 of 40–100 μm depth (40,000–100,000 nm) (Figs. 6 and 10, Table I).35 Atlan et al observed different patterns of capsular formation in a series of micro- and macrotexture surfaces;42 these authors reported that the Polytech MESMO, Mentor Siltex, and Allergan Microcell surfaces had small tissue projections scattered along the interface, which added a small degree of disorganization to the collagen fiber alignment. Meanwhile, the same group found that the Allergan Biocell, Sientra True, Eurosilicone Cristalline, Nagor Nagotex, Polytech Polytxt, and Polytech Microthane surfaces produced larger, more prominent tissue projections that resulted in irregular arrangement of collagen fibers, creating a more disorganized capsule morphology. The capsule morphology of Polytech’s Microthane surface contained a high degree of fragments of the texture material embedded throughout the capsule tissue greater than textured silicone implants.42
Fig. 10.
(A) VelvetSurface texture 3D topography view taken with uSurf Mobile Profilometer. per ISO 25178-2:2012. (B) VelvetSurface texture, at a scale of 1 mm and ×55 magnification. Results property of Establishment Labs.
Potential Influence of Surface Roughness on the Inflammatory Process and Cellular and Bacterial Adherence
Cellular attachment to any implant surface is an elaborate process which is mainly controlled by interaction between cellular factors (such as appendages or extracellular materials) that can permit cell sensing of the surface and chemical factors related to the implant surface such as topography (roughness/area), surface charge/energy, and wettability.53 In some situations, cells cannot be modified to make them less likely to adhere to surfaces. In this scenario, it is more appropriate to make implant surfaces less hospitable to cellular adhesion, and consequently surface modification is a promising strategy for preventing cell adhesion and the formation of biofilms on medical devices.7,19,35,37,45,46 In other specific situations, some bacteria can be modified to make them less likely to adhere to surfaces.54 Thus, increasing research is being developed toward precision antimicrobial therapeutics that target key virulence determinants of specific bacteria, while leaving the remainder of the host microbiota preserved.54,55 In this direction, there are vaccines and nonantibiotic therapeutics that can specifically target bacteria to prevent adhesion and biofilm formation.54
Previous histological studies have demonstrated that macrotexture surfaces have the deepest/largest pores or even nodules, and consequently allow more tissue ingrowth.27 Smooth surfaces have a smooth and irregular structure with no pores, which limits the number of sites for tissue ingrowth and consequently reduces the possibility of tissue adherence to the implant.22,51 Some studies evaluated the strength of the interaction between the implant shell and fibrous capsule according to the peak force required to separate the surrounding tissue from the shell.42 In this study, this peak force generally increased proportional to the increasing complexity of the surface texture. The peak force required for separation was ~0.3 N for Allergan Smooth and Motiva VelvetSurface, and 0.9–1.9 N for Sientra True and Allergan Biocell. The adherence force required for separation was significantly greater for the Polytech Microthane surface than for the other textures.42
Several recent studies have assessed the potential influence of surface characteristics on the inflammatory process and cellular adhesion.7,19,35,37,45,46,51,56,57 For example, Barr et al48,51 investigated the biocompatibility of silicone surfaces via cell-surface interaction, creating well-defined topographies containing numerous micron-sized pillars, pores, and grooves and evaluating how fibroblasts reacted and aligned to these surfaces. Their findings indicated that fibroblast adhesion and the reactions these cells have to silicone can be manipulated to enhance biointegration between the implant and breast tissue.
Concerning bacterial adhesion, James et al evaluated bacterial attachment and biofilm formation on the surface of implants with varying SA-Ra values using a CDC biofilm reactor.37 According to the authors, a significantly greater biofilm formation on implants with higher SA-Ra values was noted when compared with those with lower SA-Ra values. Walker et al58, in an in vitro study, evaluated the role of bacteria (Staphylococcus epidermidis) to bind both abiotic surfaces and host factors to form a biofilm. For this purpose, the authors identifed matrix proteins that S. epidermidis may exploit to infect various breast implant surfaces. According to the authors, textured surfaces support greater bacterial biofilm formation at baseline, while the addition of collagen significantly increases biomass on all surfaces evaluated. They observed that S. epidermidis isolated from implants all encoded SdrF, an expression and carriage of polysaccharide intercellular adhesin and serine-aspartate repeat proteins, which bind collagen. According to these results, these strains had a clear affinity for Type I collagen, forming dense, highly structured biofilms in its presence.
Valencia-Lazcano et al56 observed significant numbers of fibroblasts attached to textured surfaces in comparison with smooth surfaces, and that surfaces with greater roughness and reduced hydrophilicity produced greater cell adhesion. In a recent article, Kyle et al discussed the manufacturing techniques and properties of breast implant surfaces by reproducing extracellular matrix topography in polydimethylsiloxane.57 These authors found that polydimethylsiloxane with replicated acellular dermal matrix (ADM) surfaces promoted cell adhesion and proliferation compared with commercially available implant surfaces; vinculin and collagen 1 also were upregulated in fibroblasts on biomimetic surfaces, while IL8, TNFa, TGFb1, and HSP60 were downregulated.
The research on new silicone implant surfaces produced with additive surface modification techniques that are associated with lower SA and Ra values has shown promising outcomes in terms of reducing bacterial growth and promoting guided tissue integration.35 Understanding how surface properties affect the inflammatory cell response and biofilm formation is critically important in designing implants that can provide satisfactory solutions to minimize clinical complications in the long term. New technologies may provide important tools for designing a new generation of breast implants with the potential for enhanced antimicrobial properties, minimizing the inflammatory response and reducing bacterial adhesion.
Current Classifications for Different Silicone Implant Surfaces
In 2019, a number of different classifications systems were proposed to attempt to functionally stratify smooth and textured implants that are commercially available (Table II).59 Each classification system has a slight variation in order of manufacturers. The most widely utilized classification by manufacturers and government authorities is the ISO classification based upon SA and roughness and last updated in May 2018. The ISO 14607 norm addresses requirements for breast implants related to safety, design attributes, materials, manufacturing, packaging, sterilization, and information supplied by the manufacturer. ISO stratifies textures by Ra as determined by scanning electron microscopy into categories of smooth, microtextured, and macrotextured. The recent publication of ISO 14607:2018 states that any implant with roughness below 10 μm (measured according to ISO 25178-2:2012) is now classified as a smooth surface. Implants with surface roughness values of 10–50 μm and >50 μm are now classified as microtexture and macrotexture, respectively.40 Barr et al51 proposed a classification system based upon roughness, surface wettability, and macrophage polarization using scanning electron microscopy and laser confocal microscopy. Jones et al7 and James et al37 classifications include a propensity for bacterial adherence in their grading scheme, which they describe as a shortcoming of ISO. To date, none of these classification systems have yet to be clinically validated in a prospective manner to determine which is best predictive for infection risks or CC. Clinical trials are warranted to differentiate texture classifications between manufacturers. In April 2019, the French National Agency for Medicines and Health Products Safety (ANSM) imposed a ban of sale on what they classified as macrotextured, restricting further sales of Allergan Microcell and Biocell, Eurosilicone, Nagor, and Polytech implants.
Table 2.
Summary of Smooth and Textured Implant Classifications
| ISO 2018Ra by SEM | ANSM 2018Ra by SEM | Barr/Bayat 2017Ra by SEM and LCM | Atlan 2018SA by X-ray CT | Jones/Deva 2018SEM, SA/Roughness by MicroCT | James/Kinney 2018 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bact Adhes, SA/Roughness by Profilometry | |||||||||||
| Smooth <10 μm | All smooth, Motiva silk | Smooth | All smooth | Nano-texture (Smooth) < 5 μm | All Smooth | Smooth/nanotexture 80–100 mm2 | All smooth, Motiva Silk and Velvet | 1 Minimal | All smooth, Motiva Silk/Velvet | Smooth | All smooth Motiva Silk/Velvet |
| Microtextured 10–50 μm | Motiva Velvet, B-Lite, Allergan Microcell/ BRST, Mentor Siltex, Sientra True | Microtextured | Arion Micro, Sebbin Micro, Motiva Silk/Velvet | Meso-texture (Sub-cellular) <15 μm Micro-texture <75 μm and >10 μm |
Motiva silk Porous: Sientra True, Eurosilicone, PIP Non-porous: Mentor Siltex, Polytech PU, Motiva Velvet |
Microtextured 100–200 mm2 | Mentor Siltex, Allergan Microcell/BRST | 2 Low | Mentor Siltex, Nagor | Rough | Allergan Biocell, Mentor Siltex |
| Macrotextured >50 μm | Allergan Biocell, Silimed PU, Polytech PU | Macrotextured | Allergan Microcell/Biocell, Mentor Siltex, Eurosilicone Micro, Nagor, Polytech Silimed |
Macro-texture >75 μm | Porous: Biocell, Sebbin Non-porous: none | Macrotextured 200–300 mm2 Macrotexture-Plus >300 mm2 |
Allergan Biocell, Sientra True, Eurosilicone Nagor, Polytech |
3 Intermed 4 High |
Allergan Biocell, Eurosilicone Polytech PU, Surgitek PU Silimed PU |
||
| Based upon ISO-14607:2018 | By ANSM per ISO-14607:2007 | Peer reviewed scientific publications | |||||||||
SA is a measure of the total area that the outer surface topography of an implant occupies and that interfaces with the patient. Surface roughness is a measure of the average height of the peaks and valleys of an implant surface.
Adapted with permission from Clemens MW. Bridging the knowledge gap: Commentary on the epidemiology of Breast Implant Associated Large Cell Lymphoma in Australia and New Zealand. Plast Reconstr Surg. 2019.
Bact adhes, bacterial adhesion; LCM, Laser confocal microscopy; SEM, scanning electron microscopy.
Potential Influences of Different Surfaces on BIA-ALCL Risk
The etiology of BIA-ALCL is obscure; however, one of the main theories is related to the chronic inflammatory stimulation leading to T-cell proliferation in patients who are genetically susceptible. One theory proposes that a biofilm formation, associated with host factors and their immune response, activate T-lymphocytes and trigger polyclonal proliferation and, in rare cases, monoclonal proliferation and the development of BIA-ALCL.60 Some previous studies have described a higher rates of biofilm formation in textured implants compared with smooth implants.7,13,37 In addition, an in vitro study observed a direct association between biofilm formation in contaminated textured implants and the number of lymphocytes, specifically the subtype CD4+ T cells.13,60
BIA-ALCL became an emerging cancer entity, with unquestionable association with breast implants, notably, those with a textured surface.7,8,13,14,33–35,61,68 Although >600 cases of BIA-ALCL have been confirmed in different countries, all clinical cases with a clinical history have described textured implants.14,61 Despite the fact that >30 BIA-ALCL event reports with smooth surfaces were acknowledged by the US FDA, histories were either mixed smooth/textured or no clinical history available. There are no proved BIA-ALCL cases associated with only smooth implants with no previous history of textured implant in any case report, published series, or registry with an accurate clinical history.14,33,34,62 The FDA confirms that “BIA-ALCL is predominantly associated with textured surface implants.”63
In a recent study, Loch-Wilkinson et al14 have evaluated potential implant-specific risks of BIA-ALCL and observed that all cases were associated with textured surfaces. In addition, a more recent update performed by the same group observed that the majority (85%) were related to higher SA textures and the observed risk was 1:2,832 for polyurethane, 1:3,345 for Biocell, and 1:86,029 for Siltex-textured implants. The risk of BIA-ALCL was ~25.7 times higher for Biocell and 30.3 times higher with polyurethane surfaces, compared with microtextured implants.64 Based on the author’s proposed classification, the authors evaluated the risk of BIA-ALCL with SA and observed no cases with grade 1 smooth/textured surfaces, and found 78.9% of cases were associated with grade 3 or 4 textured implants. The authors theorize that a higher SA and roughness are possibly related to an increase in the attachment and growth of bacteria to an implant surface.61,64 Similarly, in a retrospective review of confirmed cases of BIA-ALCL in the United States, a strong association with textured implants was noted, with an incidence 67.6 times of BIA-ALCL when compared with smooth implants.62 Currently, Allergan Biocell failed to have renewal of its European CE mark and is restricted from sale in 37 countries, including Europe, Israel, Singapore, Canada, and South Africa. Note, neither the U.S. FDA nor any government authority advocates for the preventive replacement or even removal of textured implants in patients without confirmed diagnosis of BIA-ALCL.63
Scientific evidence is beginning to confirm a multifactorial etiology, including the risk factors previously mentioned: infection, genetic propensity, and the behavior of the highest SA implants, which may more promptly to stimulate a chronic inflammatory reaction and trigger BIA-ALCL.
Future Prospects for the Present and New Implant Surfaces in Breast Surgery
Hallmarks of Plastic Surgery include innovation, research, and adaptation. As plastic surgeons, we are trained to assess a clinical need and arrive at a solution. As we enter this era once again focused on the safety and efficacy of silicone breast implants, the scientific community needs to come together to find a solution and come up with answers that are accurate, factual, and reproducible. Although there are factors affecting outcomes that are outside of our control such as genetic susceptibility to certain malignancies or illness, as well as the specific host response to a breast implant, we are able to control other postulated factors such as the degree of texture, roughness, and SA of these devices. Innovation and continued investigation will hopefully lead to improved devices and optimized surgical techniques that can potentially lead to improved patient safety.
Currently in the United States, all breast implants remain available that include anatomic and round as well as smooth or textured surface devices. In addition, any physician, regardless of their training or specialty, can implant these breast devices. Regulatory agencies around the world are slowly dictating what devices can be used; however, they have not mandated who can insert them. As a specialty, plastic surgeons should be diligent about what we implant into patients, the specific technique of breast implantation, and make sure that patients are well informed regarding the long-term risks of these devices including anaplastic large-cell lymphoma and Breast implant Illness.
New devices will become available that may have unique properties that are centered on improving long-term safety and efficacy. In fact, Barr et al pointed out that surfaces with 5-μm2 projections demonstrated to reduce the planar arrangement of fibroblasts observed in CC and nanoscale islands of 35 nm have been shown to increase optimum adhesion compared with smooth controls.22,63 However, the promotion of these devices should be based on scientific evidence and not marketing claims, sensationalism, or pseudo-science. The focus should always be on patient safety. As such, there is a clear pathway for newer breast implants with surface characteristics that may be able to improve patient outcomes.
CONCLUSIONS
The process of implant surface texturization is complex, varied, and unique to each breast implant manufacturer. Differences in surface characteristics create variable biocompatibility which leads to direct measurable effects upon inflammation. Many complications and adverse sequelae of implants, such as infection, CC, and BIA-ALCL, may be in part related to the biocompatibility of implant surfaces. This has critical implications for a surgeon in implant selection as well as for manufacturers in the future development of novel surfaces.
ACKNOWLEDGMENT
Some figures had editorial support from Establishment Labs.
Footnotes
Published online 9 October 2019.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. A.M.M. serves as a consultant/board member for Establishment Labs, Holdings, Inc., and has shares of stocks in the company. M.W.C. was a clinical investigator for Mentor Corporation (Athena trial), a clinical investigator for Establishment Labs (U.S. Food and Drug Administration safety trial), and former consultant for Allergan Corporation (2012–2015). M.Y.N. is a consultant for Allergan (Irvine, CA) and Chief Surgical Officer for PolarityTE (Salt Lake City, UT).
REFERENCES
- 1.American Society of Plastic Surgeons (ASPS). 2017 Plastic surgery statistics report. https://www.plasticsurgery.org/documents/News/Statistics/2017/plastic-surgery-statistics-report-2017.pdf. Accessed November 9, 2018.
- 2.International Society of Plastic and Regenerative Surgeons (ISPRES). ISAPS Statistics. https://www.isaps.org/blog/isaps-statistics.
- 3.Young VL, Watson ME. Breast implant research: where we have been, where we are, where we need to go. Clin Plast Surg. 2001;28:451–483, vi. [PubMed] [Google Scholar]
- 4.Chang EI, Hammond DC. Clinical results on innovation in breast implant design. Plast Reconstr Surg. 2018;142(4S The Science of Breast Implants):31S–38S. [DOI] [PubMed] [Google Scholar]
- 5.Spear SL, Murphy DK, Slicton A, et al. ; Inamed Silicone Breast Implant U.S. Study Group. Inamed silicone breast implant core study results at 6 years. Plast Reconstr Surg. 2007;120(7 suppl 1):8S–16S; discussion 17S. [DOI] [PubMed] [Google Scholar]
- 6.Adams WP., Jr. Capsular contracture: what is it? What causes it? How can it be prevented and managed? Clin Plast Surg. 2009;36:119–26, vii. [DOI] [PubMed] [Google Scholar]
- 7.Jones P, Mempin M, Hu H, et al. The functional influence of breast implant outer shell morphology on bacterial attachment and growth. Plast Reconstr Surg. 2018;142:837–849. [DOI] [PubMed] [Google Scholar]
- 8.Wong CH, Samuel M, Tan BK, et al. Capsular contracture in subglandular breast augmentation with textured versus smooth breast implants: a systematic review. Plast Reconstr Surg. 2006;118:1224–1236. [DOI] [PubMed] [Google Scholar]
- 9.Barnsley GP, Sigurdson LJ, Barnsley SE. Textured surface breast implants in the prevention of capsular contracture among breast augmentation patients: a meta-analysis of randomized controlled trials. Plast Reconstr Surg. 2006;117:2182–2190. [DOI] [PubMed] [Google Scholar]
- 10.Poeppl N, Schreml S, Lichtenegger F, et al. Does the surface structure of implants have an impact on the formation of a capsular contracture? Aesthetic Plast Surg. 2007;31:133–139. [DOI] [PubMed] [Google Scholar]
- 11.Asplund O, Gylbert L, Jurell G, et al. Textured or smooth implants for submuscular breast augmentation: a controlled study. Plast Reconstr Surg. 1996;97:1200–1206. [DOI] [PubMed] [Google Scholar]
- 12.Tandon VJ, DeLong MR, Ballard TN, et al. Evolving trends in textured implant use for cosmetic augmentation in the united states. Plast Reconstr Surg. 2018;142:1456–1461. [DOI] [PubMed] [Google Scholar]
- 13.Hu H, Jacombs A, Vickery K, et al. Chronic biofilm infection in breast implants is associated with an increased T-cell lymphocytic infiltrate: implications for breast implant-associated lymphoma. Plast Reconstr Surg. 2015;135:319–329. [DOI] [PubMed] [Google Scholar]
- 14.Loch-Wilkinson A, Beath KJ, Knight RJW, et al. Breast implant-associated anaplastic large cell lymphoma in australia and new zealand: high-surface-area textured implants are associated with increased risk. Plast Reconstr Surg. 2017;140:645–654. [DOI] [PubMed] [Google Scholar]
- 15.Khavanin N, Clemens MW, Pusic AL, et al. Shaped versus round implants in breast reconstruction: a multi-institutional comparison of surgical and patient-reported outcomes. Plast Reconstr Surg. 2017;139:1063–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pajkos A, Deva AK, Vickery K, et al. Detection of subclinical infection in significant breast implant capsules. Plast Reconstr Surg. 2003;111:1605–1611. [DOI] [PubMed] [Google Scholar]
- 17.Del Pozo JL, Tran NV, Petty PM, et al. Pilot study of association of bacteria on breast implants with capsular contracture. J Clin Microbiol. 2009;47:1333–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danino MA, Nizard N, Paek LS, et al. Do bacteria and biofilm play a role in double-capsule formation around macrotextured implants? Plast Reconstr Surg. 2017;140:878–883. [DOI] [PubMed] [Google Scholar]
- 19.Feng G, Cheng Y, Wang SY, et al. Bacterial attachment and biofilm formation on surfaces are reduced by small-diameter nanoscale pores: how small is small enough? NPJ Biofilms Microbiomes. 2015;2:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang DT, Jones JA, Meyerson H, et al. Lymphocyte/macrophage interactions: biomaterial surface-dependent cytokine, chemokine, and matrix protein production. J Biomed Mater Res A. 2008;87:676–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cronin TD, Gerow FJ. Augmentation mammaplasty: a new “natural feel” prosthesis. In: Transactions of the Third International Congress of Plastic Surgery. 1964:Washington, DC: American Society of Plastic Surgeons:13–18. [Google Scholar]
- 22.Barr S, Bayat A. Breast implant surface development: perspectives on development and manufacture. Aesthet Surg J. 2011;31:56–67. [DOI] [PubMed] [Google Scholar]
- 23.Maxwell GP, Gabriel A. Breast implant design. Gland Surg. 2017;6:148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashley FL. A new type of breast prosthesis. Preliminary report. Plast Reconstr Surg. 1970;45:421–424. [DOI] [PubMed] [Google Scholar]
- 25.Batich C, Williams J, King R. Toxic hydrolysis product from a biodegradable foam implant. J Biomed Mater Res. 1989;23(A3 suppl):311–319. [DOI] [PubMed] [Google Scholar]
- 26.Hester TR, Jr, Ford NF, Gale PJ, et al. Measurement of 2,4-toluenediamine in urine and serum samples from women with même or replicon breast implants. Plast Reconstr Surg. 1997;100:1291–1298. [DOI] [PubMed] [Google Scholar]
- 27.Danino AM, Basmacioglu P, Saito S, et al. Comparison of the capsular response to the biocell RTV and mentor 1600 siltex breast implant surface texturing: a scanning electron microscopic study. Plast Reconstr Surg. 2001;108:2047–2052. [DOI] [PubMed] [Google Scholar]
- 28.Lista F, Ahmad J. Evidence-based medicine: augmentation mammaplasty. Plast Reconstr Surg. 2013;132:1684–1696. [DOI] [PubMed] [Google Scholar]
- 29.Schaub TA, Ahmad J, Rohrich RJ. Capsular contracture with breast implants in the cosmetic patient: saline versus silicone–a systematic review of the literature. Plast Reconstr Surg. 2010;126:2140–2149. [DOI] [PubMed] [Google Scholar]
- 30.Hall-Findlay EJ. Breast implant complication review: double capsules and late seromas. Plast Reconstr Surg. 2011;127:56–66. [DOI] [PubMed] [Google Scholar]
- 31.Bengtson B, Brody GS, Brown MH, et al. ; Late Periprosthetic Fluid Collection after Breast Implant Working Group. Managing late periprosthetic fluid collections (seroma) in patients with breast implants: a consensus panel recommendation and review of the literature. Plast Reconstr Surg. 2011;128:1–7. [DOI] [PubMed] [Google Scholar]
- 32.Miranda RN, Medeiros LJ, Ferrufino-Schmidt MC, et al. Pioneers of breast implant-associated anaplastic large cell lymphoma: history from case report to global recognition. Plast Reconstr Surg. 2019;143(3S A Review of Breast Implant-Associated Anaplastic Large Cell Lymphoma):7S–14S. [DOI] [PubMed] [Google Scholar]
- 33.Nava MB, Adams WP, Jr, Botti G, et al. MBN 2016 aesthetic breast meeting BIA-ALCL consensus conference report. Plast Reconstr Surg. 2018;141:40–48. [DOI] [PubMed] [Google Scholar]
- 34.Clemens MW, Brody GS, Mahabir RC, et al. How to diagnose and treat breast implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg. 2018;141:586e–599e. [DOI] [PubMed] [Google Scholar]
- 35.Munhoz AM, Santanelli di Pompeo F, De Mezerville R. Nanotechnology, nanosurfaces and silicone gel breast implants: current aspects. Case Reports Plast Surg Hand Surg. 2017;4:99–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cappellano G, Ploner C, Lobenwein S, et al. Immunophenotypic characterization of human T cells after in vitro exposure to different silicone breast implant surfaces. PLoS One. 2018;13:e0192108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.James GA, Boegli L, Hancock J, et al. Bacterial adhesion and biofilm formation on textured breast implant shell materials. Aesthetic Plast Surg. 2019;43:490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitehouse DJ. Handbook of Surface Metrology. 1994Bristol, England: Institute of Physics Publishing. [Google Scholar]
- 39.Bhushan B. Principles and Applications of Tribology. 1999New York, NY: Wiley. [Google Scholar]
- 40.Non-Active Surgical Implants – Mammary Implants Particular Requirements. Geneva, Switzerland: International Organization for Standardization; ISO 14607:2018 [Google Scholar]
- 41.Bhushan B. Surface roughness analysis and measurement techniques. In: Modern Tribology Handbook. 2001Taylor & Francis Group Ed. [Google Scholar]
- 42.Atlan M, Nuti G, Wang H, et al. Breast implant surface texture impacts host tissue response. J Mech Behav Biomed Mater. 2018;88:377–385. [DOI] [PubMed] [Google Scholar]
- 43.Jin ZM, Stoneb M, Inghamc E, et al. Biotribology. Curr Orthop. 2006;20:32–40. [Google Scholar]
- 44.Hallab NJ, Samelko L, Hammond D. The inflammatory effects of breast implant particulate shedding: comparison with orthopedic implants. Aesthet Surg J. 2019;39(Supplement_1):S36–S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsu LC, Fang J, Borca-Tasciuc DA, et al. Effect of micro- and nanoscale topography on the adhesion of bacterial cells to solid surfaces. Appl Environ Microbiol. 2013;79:2703–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitehead KA, Colligon J, Verran J. Retention of microbial cells in substratum surface features of micrometer and sub-micrometer dimensions. Colloids Surf B Biointerfaces. 2005;41:129–138. [DOI] [PubMed] [Google Scholar]
- 47.Chang WR, Kim IJ, Manning DP, et al. The role of surface roughness in the measurement of slipperiness. Ergonomics. 2001;44:1200–1216. [DOI] [PubMed] [Google Scholar]
- 48.Barr S, Hill E, Bayat A. Current implant surface technology: an examination of their nanostructure and their influence on fibroblast alignment and biocompatibility. Eplasty. 2009;9:e22. [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas TR. Characterization of surface roughness. Precis Eng. 1981;3:97–104. [Google Scholar]
- 50.Hansson KN, Hansson S; Skewness and kurtosis: Important Parameters in the Characterization of Dental Implant Surface Roughness—A Computer Simulation. International Scholarly Research Network - ISRN Materials Science. 2011;305312,1–6. [Google Scholar]
- 51.Barr S, Hill EW, Bayat A. Functional biocompatibility testing of silicone breast implants and a novel classification system based on surface roughness. J Mech Behav Biomed Mater. 2017;75:75–81. [DOI] [PubMed] [Google Scholar]
- 52.Munhoz AM. The functional influence of breast implant outer shell morphology on bacterial attachment and growth. Plast Reconstr Surg. 2019. 144(1):143e–144e. [DOI] [PubMed] [Google Scholar]
- 53.www.polytech-health-aesthetics.com/index.php/en/products/mammary implants/implant-surfaces.
- 54.Spaulding CN, Klein RD, Schreiber HL, IV, et al. Precision antimicrobial therapeutics: the path of least resistance? NPJ Biofilms Microbiomes. 2018;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olson PD, Kuechenmeister LJ, Anderson KL, et al. Small molecule inhibitors of staphylococcus aureus rnpa alter cellular mrna turnover, exhibit antimicrobial activity, and attenuate pathogenesis. Plos Pathog. 2011;7:e1001287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valencia-Lazcano AA, Alonso-Rasgado T, Bayat A. Characterisation of breast implant surfaces and correlation with fibroblast adhesion. J Mech Behav Biomed Mater. 2013;21:133–148. [DOI] [PubMed] [Google Scholar]
- 57.Kyle DJ, Oikonomou A, Hill E, et al. Development and functional evaluation of biomimetic silicone surfaces with hierarchical micro/nano-topographical features demonstrates favourable in vitro foreign body response of breast-derived fibroblasts. Biomaterials. 2015;52:88–102. [DOI] [PubMed] [Google Scholar]
- 58.Walker JN, Pinkner CL, Lynch AJL, et al. Deposition of host matrix proteins on breast implant surfaces facilitates Staphylococcus epidermidis biofilm formation: in vitro analysis. Aesthet Surg J. 2019:sjz099 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 59.Clemens MW. Discussion: the epidemiology of breast implant-associated anaplastic large cell lymphoma in australia and new zealand confirms the highest risk for grade 4 surface breast implants. Plast Reconstr Surg. 2019;143:1295–1297. [DOI] [PubMed] [Google Scholar]
- 60.Hu H, Johani K, Almatroudi A, et al. Bacterial biofilm infection detected in breast implant-associated anaplastic large-cell lymphoma. Plast Reconstr Surg. 2016;137:1659–1669. [DOI] [PubMed] [Google Scholar]
- 61.Collett DJ, Rakhorst H, Lennox P, et al. Current risk estimate of breast implant-associated anaplastic large cell lymphoma in textured breast implants. Plast Reconstr Surg. 2019;143(3S A Review of Breast Implant-Associated Anaplastic Large Cell Lymphoma):30S–40S. [DOI] [PubMed] [Google Scholar]
- 62.Doren EL, Miranda RN, Selber JC, et al. U.S. epidemiology of breast implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg. 2017;139:1042–1050. [DOI] [PubMed] [Google Scholar]
- 63.Green AM, Jansen JA, van der Waerden JP, et al. Fibroblast response to microtextured silicone surfaces: texture orientation into or out of the surface. J Biomed Mater Res. 1994;28:647–653. [DOI] [PubMed] [Google Scholar]
- 64.Magnusson M, Beath K, Cooter R, et al. The epidemiology of breast implant-associated anaplastic large cell lymphoma in australia and new zealand confirms the highest risk for grade 4 surface breast implants. Plast Reconstr Surg. 2019;143:1285–1292. [DOI] [PubMed] [Google Scholar]
- 65.Wong CH, Samuel M, Tan BK, et al. Capsular contracture in subglandular breast augmentation with textured versus smooth breast implants: a systematic review. Plast Reconstr Surg. 2006;118:1224–1236. [DOI] [PubMed] [Google Scholar]
- 66.Sforza M, Zaccheddu R, Alleruzzo A, et al. Preliminary 3-year evaluation of experience with silksurface and velvetsurface motiva silicone breast implants: a single-center experience with 5813 consecutive breast augmentation cases. Aesthet Surg J. 2018;38(suppl_2):S62–S73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Quirós MC, Bolaños MC, Fassero JJ. Six-year prospective outcomes of primary breast augmentation with nano surface implants. Aesthet Surg J. 2019;39:495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.American Society of Plastic Surgeons. BIA-ALCL physician resources. http://www.plasticsurgery.org/alcl. Accessed June 10, 2019.


