Background:
The inverted T (Wise pattern) mastectomy for patients with macromastia or significant breast ptosis has evolved along with generalized techniques for breast reconstruction. We present a review of Wise pattern breast reconstruction along with our technique for direct to implant reconstruction using dermal matrix.
Methods:
The literature was reviewed and an analysis of techniques and complications was performed. We present our series of patients incorporating dermal matrix and relatively large implants in direct to implant reconstruction.
Results:
Of 18 breasts reconstructed only 2 failed. One caused by flap necrosis secondary to smoking and one as a result of preoperative radiation.
Conclusion:
Wise pattern breast reconstruction using relatively large implants and dermal matrix in direct to implant reconstruction is a safe technique in selected patients with macromastia.
INTRODUCTION
Incisions and reconstructive techniques for restoring the female breast after mastectomy have greatly improved over the past several decades and now include nipple-sparing and skin-sparing mastectomies, microvascular autogenous tissue reconstruction, shaped implants, and the use of acellular dermal matrices. However, one of the more difficult challenges is reconstructing the breast in a patient with either macromastia or significant breast ptosis where the skin envelope exceeds the dimensions of currently available breast implants or the patient desires a smaller reconstructed breast. Those patients frequently are not candidates for nipple-sparing mastectomies due to the high risk of nipple ischemia or difficulty positioning the nipple in the appropriate place on the reconstructed breast mound. Toth and Lappert1 first used a modified Wise pattern in 16 patients with large or ptotic breasts to reduce the skin envelope and avoid the horizontal scar on the breast mound normally created by a mastectomy. Patients were reconstructed with either tissue expanders or autogenous transverse rectus abdominis muscle flaps. Over the years, clinicians have improved this technique with the use of de-epithelialized inferiorly based flaps, total muscle coverage, addition of dermal matrix, and the use of shaped implants. The purposes of this article are (1) to review and analyze the published literature on the use of Wise pattern breast reconstruction techniques, (2) to compare the results with standard implant-based reconstructions, and (3) to present our recent experience utilizing a modified direct to implant one stage Wise pattern reconstruction with dermal matrix and shaped implants. We compare our results to that reported with previously reported series.
MATERIALS AND METHODS
A PubMed review was performed of all articles using the key words/phrases: Wise pattern breast reconstruction; macromastia AND breast reconstruction, Wise pattern mastectomy, skin reducing mastectomy, dermal flap AND breast reconstruction, dermal flap AND mastectomy. The bibliography of each article that was relevant to the search was reviewed for additional papers that were not found in the PubMed search. A similar search was done on the website of Plastic and Reconstructive Surgery. These articles were then analyzed for the following data when provided: technique of reconstruction, numbers of patients, numbers of breasts reconstructed, single or 2-stage reconstruction (expander versus direct to implant), numbers and types of complications, failure rate, and average size of implant placed. We then analyzed our own data utilizing shaped implants, dermal pedicles and dermal matrix, both appraising what we learned with our procedure and comparing our results with that reported in the literature.
LITERATURE REVIEW
The literature review identified a total of 26 articles and 1 book chapter dealing with inverted T skin-sparing mastectomies and immediate reconstruction utilizing implants with either tissue expanders as a 2-stage procedure or a 1-stage direct to implant reconstruction (Table 1). These articles fell into several reconstructive patterns depending on the tissue utilized and the position of the implants. Several studies reported placing the implant directly beneath the skin and subcutaneous tissue flaps, whereas other investigators utilized a complete muscle coverage over the implant. Retaining an inferiorly based de-epithelialized flap with tissue that would otherwise be discarded with the Wise pattern allowed for a much larger tissue pocket and provided protection against necrosis of the tissue flaps at the inverted T junction. The de-epithelialized flap could either be left in place overlying the implant or sewn to the inferiorly released origin of the pectoralis major muscle underneath which was placed a tissue expander or implant. The addition of dermal matrix, sewn to the freed lower border of the pectoralis allows an even larger submuscular/subdermal matrix pocket to be created for a bigger implant while securing the lateral border of the implant to the chest wall thereby preventing its displacement toward the axilla. Alternatively, a portion of the serratus anterior could be elevated to serve this purpose.
Table 1.
Studies of Inverted T Mastectomy and Breast Reconstruction
| Study | Patients | Breasts | E (number) | DTI (number) | No. Implant Loss (%) | Necrosis, No Implant Loss (%) | Nicotine Necrosis |
|---|---|---|---|---|---|---|---|
| Carlson et al.2 (1997) | 68 | 68 | 0 | 68 | 0 | 18 (30) | 8 |
| Hammond et al.3 (2002) | 8 | 12 | 10 | 2 | 1 (8.3) | 1 (8.3) | Unspecified |
| Skoll and Hudson4 (2002) | 18 | 18 | 0 | 18 | 3 (0.16) | Unspecified | 1 |
| Prathap and Harland5 (2004) | 6 | 6 | 0 | 6 | 0 | 0 | 0 |
| della Rovere et al.6 (2008) | 10 | 18 | 18 | 0 | 1 (5.5) | 0 | 0 |
| Nava et al.7 (2006) | 28 | 30 | 0 | 30 | 4 (13) | 0 | 3 |
| Bayram et al.8 (2010) | 15 | 26 | 0 | 26 | 0 | 4 (15) | 1 |
| Derderian et al.9 (2009) | 20 | Unspecified | 0 | 20 | 0 | 5 | 2 |
| Ross10 (2012) | 10 | 20 | 0 | 20 | 0 | 1 (5) | 0 |
| Losken et al.11 (2010) | 27 | 34 | 0 | 34 | 3 (8) | 4 (12) | Unspecified |
| Colizzi et al.12 (2010) | 18 | 22 | 0 | 22 | 0 | 1 (4.5) | 1 |
| Nair et al.13 (2010) | 72 | 89 | 55 | 34 | 2 (2) | 1 (1) | Unspecified |
| Boneti et al.14 (2011) | 16 | Unspecified | 16 | Unspecified | Unspecified | Unspecified | |
| Irwin et al.15 (2013) | 64 | 104 | 0 | 104 | 4 (3.8) | 10 (9.6) | Unspecified |
| Dietz et al.16 (2012) | 43 | 43 | 43 | 0 | Unspecified | Unspecified | Unspecified |
| Clerico et al.17 (2012) | 3 | 5 | 0 | 5 | 0 | 2 (40) | Unspecified |
| Salgarello et al.18 (2012) | 14 | 16 | 0 | 16 | 1 (6.2) | 1 (6) | 0 |
| Gentileschi et al.19 (2013) | 23 | 23 | 0 | 23 | 0 | 1 (4) | 1 |
| Chang et al.20 (2013) | 6 | 11 | 0 | 11 | 0 | 1 (9) | Unspecified |
| Ladizinsky et al.21 (2013) | 110 | 170 | 72 | 98 | 2 (1.2) | 26 (15) | Unspecified |
| Demiri et al.22 (2017) | 50 | 65 | 65 | 0 | 4 (6.2) | 6 (9.2) | Unspecified |
| Serrurier et al.23 (2017) | 45 | 80 | 0 | 80 | 2 (0.025) | Unspecified | Unspecified |
| Santanelli et al.24 (2013) | 63 | 75 | 0 | 75 | 11 (1.3) | 9 (12) | 3 |
| King et al.25 (2014) | 16 | 19 | 4 | 15 | 0 | 3 (16) | Unspecified |
DTI, direct to implant; E, expanders.
Group 1: Subcutaneous Implant Position
One of the first described uses of the inferior dermal pedicle and Wise pattern in breast reconstruction was by Dr. John Bostwick in 1983.26 It was employed in subcutaneous mastectomies, sewing it to the free border of the elevated pectoralis muscle beneath which was placed an implant. In the original article by Toth and Lappert,1 a Wise pattern with relatively thick well-vascularized flaps was used but without mention of a dermal flap. Most of the patients in their series of 16 patients were reconstructed with transverse rectus abdominis muscle (TRAM) flaps underneath the inverted T skin flaps. Although the authors noted the concern for potential breakdown of the flaps in the confluence of incisions, they did not mention the loss of any of their expander reconstructions. In 2002, Skoll and Hudson4 reported a technique where a triangle of de-epithelialized skin between the vertical limbs of the Wise pattern was maintained and a 2 cm portion along the horizontal aspect of the inverted T pattern was also de-epithelialized, left attached to the unresected skin, and folded under the vertical and horizontal closures. The implant also was placed in the subcutaneous position. In that same year, the authors27 described a review of 18 of their patients, 11 of which had subcutaneous implant placement and 7 had submuscular implants (this included mobilization of the serratus and rectus fascia). The authors preferred the subcutaneous position because the muscle was too constricting while larger implants could be placed in the subcutaneous position. Of their 18 patients, there were 3 implants lost (16%) as a result of chemotherapy, smoking, and infection. In 2010, Bayram et al.8 described a procedure in 15 patients in utilizing an inverted T reconstruction following subcutaneous mastectomy for early breast cancer or for prophylaxis. They developed an inferiorly based dermal pedicle that maintained the nipple-areolar complex. The implant was placed in the subcutaneous plane above the pectoralis. There was no implant loss, although 4 breasts had either partial or total nipple/areolar loss (15%). Within this group of authors, only Hudson and Skoll27 reported on implant size which ranged from 120 to 550 cm3 with a mean of 300 cm3.
Group 2: Total Muscle Coverage
Total muscle coverage and utilization of the inverted T reconstruction was first published by Hudson and Skoll,27 although they soon switched to a subcutaneous implant position as it was less restricting and provided a better final appearance. In 2012, Salgarello et al.18 presented a series of 14 patients (16 breasts) in which the Wise pattern was used along with complete muscle/fascial coverage of anatomical gel implants. De-epithelialized flaps were not incorporated into the closure. Rather, the authors used pectoralis superiorly, superficial pectoralis fascia and subcutaneous tissue inferiorly, and lateral pectoral fascia and serratus muscle laterally. The limitation in pocket size with total muscle coverage would mandate either the use of tissue expanders in a 2-stage procedure or small implants. Two patients experienced skin flap necrosis (14.3%). Mean implant size was 416 cm3.
Group 3: Wise Pattern, Partial Muscle Coverage with Dermal Flap
The greatest number of published papers utilized a dermal flap along with partial coverage of either an implant or expander.3,5–7,10–17,19–25,28 What differentiated many of these reports was how the surgeons managed the portion of the device inferiorly or laterally not covered by the pectoralis muscle. Most investigators left this portion of the implant in the subcutaneous space without any additional coverage.5,10,11,13–15,17,20–25,28 Variations have included free nipple-areolar grafting,25 maintenance of a superiorly based residual disc of skin for future areolar nipple reconstruction,23 use of a Becker device expander implant,22 and nipple-sparing mastectomies.28 Before the use of acellular dermal matrix in breast reconstruction, those groups concerned with the migration of the breast implant included a portion of the de-epithelialized flap placed laterally12 or use of a portion of the serratus muscle or fascia to inhibit lateral implant migration.6,7,16,17,19
Skin necrosis at the inverted T junction was the common complication among those reports in which the data were analyzed and smoking seemed to be a significant contributing factor.7,16,19,21,22,24,26 Other findings contributing to necrosis were preoperative radiation15,22 and weight of the implant >468 g.24 Most studies reported that the presence of the de-epithelialized dermal flap prevented exposure and loss of the implant requiring only conservative wound management for healing,2,10,15,17,19–21,24,25 a skin graft,12,13,24 or a local flap11 or some other unspecified procedure.21
Major implant loss in the larger studies (>50 breasts) varied from 1.1%21 up to 4%.24 Higher percentages of implant loss were only observed in reports with fewer reconstructions (30 or less) (Table 1).
Seven reports addressed average implant size.7,10,12,15,19,23,24 Of these, the average was 440 cm3 with a range of 195 to 620 cm3.
Group 4: Partial Submuscular Coverage with Dermal Flap and Dermal Matrix
Derderian et al.9 first incorporated dermal matrix into their Wise pattern breast reconstructions. Depending on the size of the implant, the dermal matrix was either sutured to the inframammary fold inferiorly and the pectoralis superiorly or in larger reconstructions the serratus was also elevated and the dermal matrix was sutured directly to the de-epithelialized dermal flap. A total of 20 patients were reconstructed in this fashion. Breakdown at the T juncture occurred in 5 patients (25%). All were treated conservatively with wound care and none required implant removal. Two patients in the series smoked and both had complications. Mean implant size was 458 cm3.
A later study by Kilgo et al.29 compared a horizontal elliptical excision of excess skin to the inverted T reconstructions. Dermal matrix was used to provide additional support on either side of the de-epithelialized inferior-based flap in the later cases. These investigators only performed 2-stage reconstructions with tissue expanders. The complication rate between the 2 techniques was not statistically different except for an increase in tissue necrosis in the inverted T reconstructions. Despite that fact, there was no difference in implant loss. Final implant volumes were not reported although mean inflation size of the expanders in the inverted T group was 540.6 cm3.
Expanding on the procedure of Derderian et al.,9 we have reconstructed 18 breasts using the Wise pattern in conjunction with a dermal matrix sutured to the free border of the pectoralis major and laterally to the chest wall to secure the implant preventing lateral migration thereby avoiding the need for serratus elevation. Patients were chosen who had at least a D cup or significant breast ptosis.
Technique: Direct to Implant Reconstruction
The patient is marked in the standing position by drawing a line from the mid-clavicle or sternal notch to the areolar. The projection of the inframammary fold onto the line is marked as the new position for the eventual nipple/areolar reconstruction. From that point, diverging 7 cm lines are drawn to diverge a distance of 6–10 cm. In a breast reduction, the bottom width of these lines would then represent the width of the inferior pedicle of a breast reduction. However, for this procedure, we continue from the ends of these 7 cm lines to diverge further medially and laterally down to the inframammary fold thereby delineating a much wider dermal flap (Fig. 1B). The wider the flap, the more skin will be killed to form it and hence the greater the reduction in the skin envelope. Similarly, the wider the flap, the greater the protection for an underlying implant and dermal matrix. The inframammary fold is also marked. A circum-areolar mark is made once the patient is asleep.
Fig. 1.
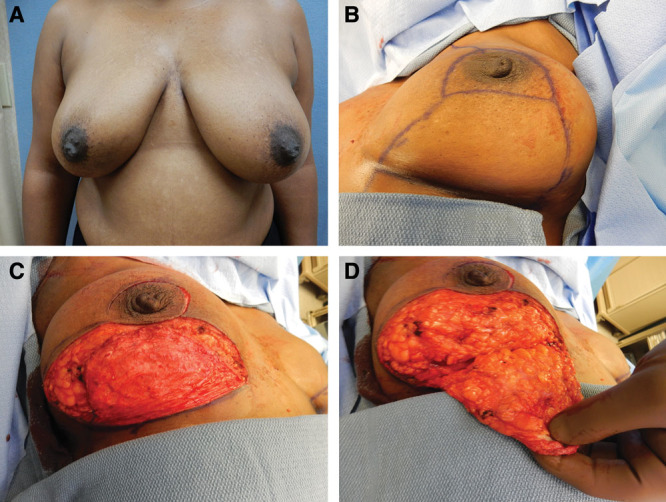
Steps in Wise pattern breast reconstruction. Preoperative frontal view (A). Markings for mastectomy and reconstruction (B). De-epithelialized inferiorly based flap (C). Elevation of dermal flap (D).
The plastic surgeon begins the procedure. All of the marks are scored. The wide-based dermal flap is de-epithelialized as in a breast reduction (Fig. 1C). After de-epithelialization, the dermal flap is created by full-thickness incisions around the periphery of the flap except the inframammary fold. The height of the flap ends just below the areolar complex. A full-thickness incision is made around the areolar. The dermal flap is then elevated with 1.5–2.0 cm of underlying fat down to the inframammary fold (Fig. 1D). At the fold, the dissection is deepened to the pectoralis/rectus fascia. The general surgeon now performs the mastectomy elevating the remaining skin flaps and resecting the breast along with the attached nipple/areolar complex.
Subsequently, a subpectoral pocket is created by identifying the lateral border of the pectoralis major muscle and detaching the origin of the muscle from the ribs and lateral inferior border of the sternum. Because a tissue expander is not employed, there must be some release of the muscle from the sternum to make a large enough medial pocket for the eventual implant. Thus far, we have not seen significant animation deformity. The perforated dermal matrix (AlloDerm, Allergan, Branchburg, N.J.) is then sutured to the inframammary fold and partially to the free border of the pectoralis medially (Fig. 2A). The final implant is then placed into the subdermal matrix/pectoralis pocket and the dermal matrix is sutured to the lateral chest wall to help hold the implant in position. The dermal pedicle is sutured over the dermal matrix to the pectoralis muscle (Fig. 2D) and the skin envelope is closed. Drains are placed before closure along the inframammary fold between the dermal flap and the dermal matrix and a second one along the lateral chest dissection. This procedure can yield a very close approximation to the preoperative size and shape of the breasts (Figs. 2–4).
Fig. 2.
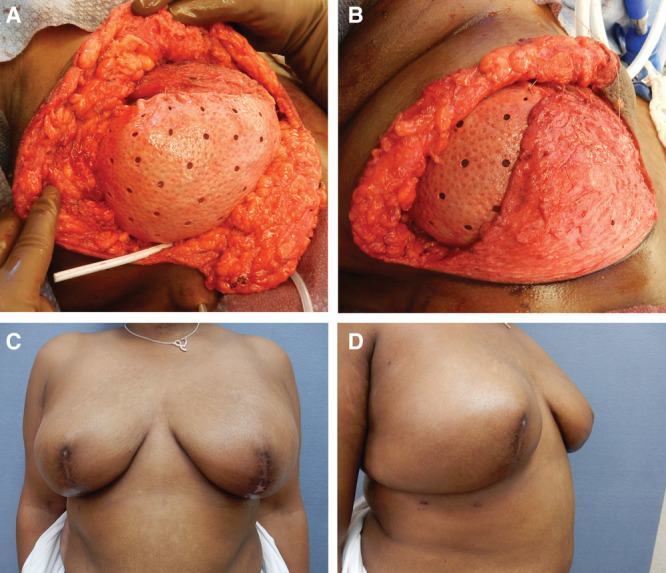
Steps and outcome of Wise pattern breast reconstruction. Dermal matrix sutured to pectoralis with underlying implant (A). Suture of dermal flap to pectoralis partially covering dermal matrix (B). Postoperative frontal (C) and lateral (D) views.
Fig. 4.
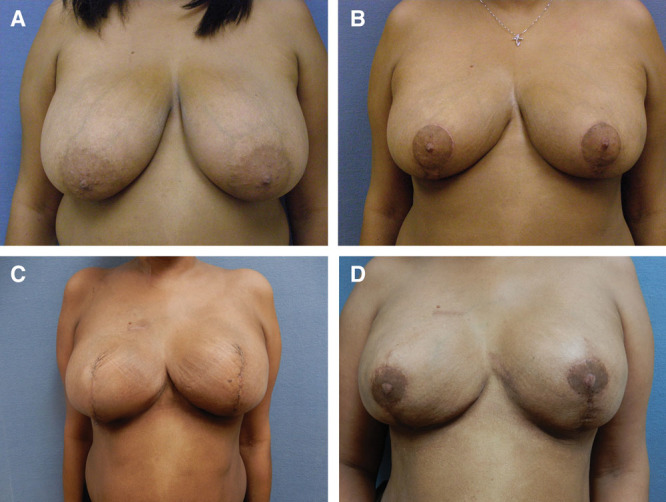
Before and after bilateral breast reconstruction with Wise inverted T pattern. (A). After bilateral breast reduction and development of left intraductal breast carcinoma (B). Following bilateral mastectomy and inverted T direct to implant reconstruction (C). After nipple-areolar reconstruction (D).
An alternative option in patients with large body mass indices (BMIs) is to place the implant, wrapped in dermal matrix above the muscle, and still incorporate the dermal flap to cover the dermal matrix underneath the T incision. This procedure will give somewhat more projection to the implant but requires hardy and thick skin/subcutaneous mastectomy flaps.
RESULTS
Average patient age was 57 (range 42–66), with an average weight of 87.3 kg (range of 58.5–136.4 kg) and average BMI of 30.91 (range of 19.6–44.39). The mean follow-up was 15.1 months (range of 2–34 months). All patients had direct to implant reconstructions. In 2 additional patients (3 breasts) with marked obesity (BMI 42.89 and 44.39), we elected to place the implants in a subcutaneous position. The rationale for this was the limited implant coverage afforded by the relatively small undersurface of the pectoralis muscle and need for a large implant to provide projection. In both cases, the implants were placed subcutaneously and covered on the anterior surface by dermal matrix. The dermal matrix helped hold the implant in position and potentially reduced the incidence of capsular contracture.9,29,30 The de-epithelialized inferiorly based flap then covers over the dermal matrix beneath the inverted T incision to protect the underlying implant and matrix from exposure. There were 2 implant failures (11.1%). One patient smoked heavily and the amount of necrosis of the flap was beyond the border of the protecting de-epithelialized skin, whereas the other had breast radiation therapy in combination with lumpectomy, and axillary dissection performed 20 years previously. The average size of implant placed was 561 cm3 (range 375–775), considerably larger than with other techniques.
DISCUSSION
Management of implant-based breast reconstruction in patients with large or ptotic breasts can be accomplished by horizontal skin reduction and placement of tissue expanders. This approach allows the eventual placement of relatively large implants in a two-stage operation. The utilization of the Wise inverted T pattern in patients with macromastia enables the plastic surgeon to avoid a mid breast horizontal incision with ensuing large dog-ears whose resection results in even a longer scar. Instead, the vertical scar of the inverted T is shorter and can be partially obscured by a nipple/areolar reconstruction. Furthermore, the de-epithelialized flap protects the underlying breast implant or dermal matrix from flap necrosis.10,11,18,20,21,25 Finally, this technique allows for a 1-stage implant breast reconstruction.
The primary weakness of the technique is the potential for flap necrosis in the junction of the horizontal and vertical limbs.10,11,18,20,21,25 Of those studies reporting outcomes in the current literature review, of a total of 954 breast reconstructions using the Wise pattern, there were 38 implant losses (3.98%). Failure was usually blamed on either skin necrosis or infection (Table 1). Of further interest was the finding that in those studies reporting on the incidence of nicotine use (284 breasts), 20 failures (5.8%) were associated with skin loss and the use of nicotine (Table 1). In some studies, smoking was the predominant cause of eventual implant loss.2,7,16,21,22,24 The presence of the de-epithelialized inferiorly based dermal flap protects the implant quite a bit, allowing some degree of flap loss without subsequent implant exposure.10,11,18,20,21,25 Depending on the amount of skin loss, the defect can be allowed to heal in secondarily following conservative wound management or be skin grafted.
One of the issues involved with implant placement is the prevention of migration of the device laterally toward the mid-axillary line. Several authors have blocked lateral migration with serratus muscle, dermis, or fascia.3,7,12,16,18,19,22 The problem with the use of dermis for this purpose is that it requires the sacrifice of additional skin coverage. The latter may then limit the size of the final implant. Our approach was to utilize dermal matrix sutured to the lateral chest wall to help hold the implant in position. The dermal matrix thereby serves 3 functions: it anchors the implant, it allows for a bigger implant pocket (average size of implants in the present study was 561 cm3), and may aid in decreasing capsular contracture.9,29,30 The dermal flap alone does not provide complete cover of the implant either medially or laterally. One could use 2 smaller pieces of acellular dermal matrix (ADM) to cover these areas. However, it is technically simpler to use 1 sheet to create the pocket with the pectoralis, placing the dermal flap over it.
The use of shaped cohesive gel implants may help to better define breast shape in the upper breast pole.11 However, smooth round devices can be employed with a plan for subsequent fat grafting as required. Even with the use of shaped devices, several of our patients required additional upper pole fat grafting. In the obese patient, thick skin flaps may obscure breast definition.11 In this situation, we elected to place the implant in the subcutaneous position, with a covering of dermal matrix and dermal flap to attempt to improve implant projection.
If one considers the loss of an implant as initial failure of this technique, of those 22 studies reporting this complication, the range varied from 0% to 20% with a mean of 4%. This compares very favorably with other reports in the literature for implant-based breast reconstructions.31–37 Our results are similarly consistent with smoking and preoperative radiation as significant risk factors.2,7,13,16,21,22,24 Besides the 2 flap failures, the only revisions were for additional fat grafting.
As we began using this technique, one of the questions that arose was how well this operation would hold up to postoperative radiation therapy. Three of our patients (all with subpectoral implants) sustained postoperative radiation therapy for positive lymph nodes. In each case, we noted firmer capsules around the implant (grades 2 and 3), some mild shrinkage of the soft tissues around the implant (amenable to fat grafting in all 3 cases), but relatively no major shift in implant position (Fig. 3).
Fig. 3.
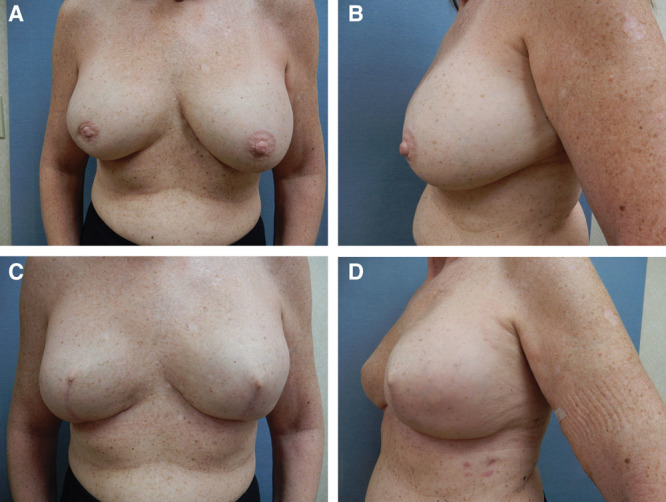
Before and after Wise pattern breast reconstruction. (A). Before bilateral mastectomy side view (B). After bilateral breast reconstruction and 1 year after radiation therapy to the right breast. Frontal view (C) and side view (D).
CONCLUSIONS
Utilization of the inverted T mastectomy pattern in combination with dermal matrix and a wide de-epthelialized dermal flap has allowed for a 1-stage, direct to implant reconstruction with a relatively large breast implant. The underlying dermal flap protects against skin flap breakdown and implant exposure. Based on our experience and literature review, this technique should be used with caution in patients who have had preoperative radiation or who smoke. The technique provides a complication rate well within the norm for breast reconstruction surgery. In the very obese patient, placement of the implant in the subcutaneous position with overlying dermal matrix and dermal flap is feasible due to the thickness of the skin flaps.
Footnotes
Published online 16 October 2019.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Toth BA, Lappert P. Modified skin incisions for mastectomy: the need for plastic surgical input in preoperative planning. Plast Reconstr Surg. 1991;87:1048–1053. [PubMed] [Google Scholar]
- 2.Carlson GW, Bostwick J, 3rd, Styblo TM, et al. Skin-sparing mastectomy. Oncologic and reconstructive considerations. Ann Surg. 1997;225:570–575; discussion 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammond DC, Capraro PA, Ozolins EB, et al. Use of a skin-sparing reduction pattern to create a combination skin-muscle flap pocket in immediate breast reconstruction. Plast Reconstr Surg. 2002;110:206–211. [DOI] [PubMed] [Google Scholar]
- 4.Skoll PJ, Hudson DA. Skin-sparing mastectomy using a modified Wise pattern. Plast Reconstr Surg. 2002;110:214–217. [DOI] [PubMed] [Google Scholar]
- 5.Prathap P, Harland RN. Wise pattern mastectomy with immediate breast reconstruction. Breast. 2004;13:502–505. [DOI] [PubMed] [Google Scholar]
- 6.della Rovere GQ, Nava M, Bonomi R, et al. Skin-reducing mastectomy with breast reconstruction and sub-pectoral implants. J Plast Reconstr Aesthet Surg. 2008;61:1303–1308. [DOI] [PubMed] [Google Scholar]
- 7.Nava MB, Cortinovis U, Ottolenghi J, et al. Skin-reducing mastectomy. Plast Reconstr Surg. 2006;118:603–610; discussion 611. [DOI] [PubMed] [Google Scholar]
- 8.Bayram Y, Kulahci Y, Irgil C, et al. Skin-reducing subcutaneous mastectomy using a dermal barrier flap and immediate breast reconstruction with an implant: a new surgical design for reconstruction of early-stage breast cancer. Aesthetic Plast Surg. 2010;34:71–77. [DOI] [PubMed] [Google Scholar]
- 9.Derderian CA, Karp NS, Choi M. Wise-pattern breast reconstruction: modification using AlloDerm and a vascularized dermal-subcutaneous pedicle. Ann Plast Surg. 2009;62:528–532. [DOI] [PubMed] [Google Scholar]
- 10.Ross GL. One stage breast reconstruction following prophylactic mastectomy for ptotic breasts: the inferior dermal flap and implant. J Plast Reconstr Aesthet Surg. 2012;65:1204–1208. [DOI] [PubMed] [Google Scholar]
- 11.Losken A, Collins BA, Carlson GW. Dual-plane prosthetic reconstruction using the modified Wise pattern mastectomy and fasciocutaneous flap in women with macromastia. Plast Reconstr Surg. 2010;126:731–738. [DOI] [PubMed] [Google Scholar]
- 12.Colizzi L, Lazzeri D, Agostini T, et al. Skin-reducing mastectomy: new refinements. J Plast Surg Hand Surg. 2010;44:296–301. [DOI] [PubMed] [Google Scholar]
- 13.Nair A, Jaleel S, Abbott N, et al. Skin-reducing mastectomy with immediate implant reconstruction as an indispensable tool in the provision of oncoplastic breast services. Ann Surg Oncol. 2010;17:2480–2485. [DOI] [PubMed] [Google Scholar]
- 14.Boneti C, Yuen J, Santiago C, et al. Oncologic safety of nipple skin-sparing or total skin-sparing mastectomies with immediate reconstruction. J Am Coll Surg. 2011;212:686–693; discussion 693. [DOI] [PubMed] [Google Scholar]
- 15.Irwin GW, Black A, Refsum SE, et al. Skin-reducing mastectomy and one-stage implant reconstruction with a myodermal flap: a safe and effective technique in risk-reducing and therapeutic mastectomy. J Plast Reconstr Aesthet Surg. 2013;66:1188–1194. [DOI] [PubMed] [Google Scholar]
- 16.Dietz J, Lundgren P, Veeramani A, et al. Autologous inferior dermal sling (autoderm) with concomitant skin-envelope reduction mastectomy: an excellent surgical choice for women with macromastia and clinically significant ptosis. Ann Surg Oncol. 2012;19:3282–3288. [DOI] [PubMed] [Google Scholar]
- 17.Clerico C, Ihrai T, Raoust I, et al. [Mastectomy and immediate breast reconstruction using a prosthesis and lower dermal flap: description of five cases]. Ann Chir Plast Esthet. 2012;57:606–611. [DOI] [PubMed] [Google Scholar]
- 18.Salgarello M, Visconti G, Barone-Adesi L, et al. Inverted-T skin-reducing mastectomy with immediate implant reconstruction using the submuscular-subfascial pocket. Plast Reconstr Surg. 2012;130:31–41. [DOI] [PubMed] [Google Scholar]
- 19.Gentileschi S, Bracaglia R, Garganese G, et al. Immediate definitive prosthetic reconstruction in patients with ptotic breasts. Ann Plast Surg. 2013;70:144–148. [DOI] [PubMed] [Google Scholar]
- 20.Chang LY, Hargreaves W, Segara D, et al. Experience in dermomyofascial pouch coverage of immediate implants following skin sparing reduction mastectomy. ANZ J Surg. 2013;83:135–138. [DOI] [PubMed] [Google Scholar]
- 21.Ladizinsky DA, Sandholm PH, Jewett ST, et al. Breast reconstruction with the Bostwick autoderm technique. Plast Reconstr Surg. 2013;132:261–270. [DOI] [PubMed] [Google Scholar]
- 22.Demiri E, Dionyssiou D, Sapountzis S, et al. Becker expander-based breast reconstruction following Wise pattern skin-reducing mastectomy: complication rates and risk factors. Aesthetic Plast Surg. 2017;41:304–311. [DOI] [PubMed] [Google Scholar]
- 23.Serrurier LC, Rayne S, Venter M, et al. Direct-to-implant breast reconstruction without the use of an acellular dermal matrix is cost effective and oncologically safe. Plast Reconstr Surg. 2017;139:809–817. [DOI] [PubMed] [Google Scholar]
- 24.Santanelli F, Longo B, Sorotos M, et al. Flap survival of skin-sparing mastectomy type IV: a retrospective cohort study of 75 consecutive cases. Ann Surg Oncol. 2013;20:981–989. [DOI] [PubMed] [Google Scholar]
- 25.King IC, Harvey JR, Bhaskar P. One-stage breast reconstruction using the inferior dermal flap, implant, and free nipple graft. Aesthetic Plast Surg. 2014;38:358–364. [DOI] [PubMed] [Google Scholar]
- 26.Bostwick J. Berger K. Total mastectomy with reduction of breast volume and skin (inverted T incision) pgs 642–651. In: Aesthetic and Reconstructive Breast Surgery. 1983St Louis, MO: C.V. Mosby Co.. [Google Scholar]
- 27.Hudson DA, Skoll PJ. Complete one-stage, immediate breast reconstruction with prosthetic material in patients with large or ptotic breasts. Plast Reconstr Surg. 2002;110:487–493; discussion 494. [DOI] [PubMed] [Google Scholar]
- 28.Peker F, Yuksel F, Karagoz H, et al. Breast reconstruction using de-epithelialized dermal flap after vertical-pattern skin-sparing mastectomy in macromastia. ANZ J Surg. 2015;85:64–68. [DOI] [PubMed] [Google Scholar]
- 29.Kilgo MS, Kaufman Gj, Shen AE, et al. A comparison of elliptical mastectomy to inverted-T pattern mastectomy in two-stage prosthetic breast reconstruction. Plast Recon Surg. 2015;136:426e–433e. [DOI] [PubMed] [Google Scholar]
- 30.Kim IK, Park SO, Chang H, Jin US. Inhibition Mechanism of acellular dermal matrix on capsule formation in expander-implant breast reconstruction after postmastectomy radiotherapy. Ann Surg Oncol. 2018;25:2279–2287. [DOI] [PubMed] [Google Scholar]
- 31.Hunsicker LM, Salzberg A. A cellular dermal matrix-assisted direct to implant breast reconstruction and capsular contracture: a 12 year experience. Plastic Recon Surg. 2014;134:4s, 82–1. 83. [DOI] [PubMed] [Google Scholar]
- 32.Basu CB, Jeffers L. The role of acellular dermal matrices in capsular contracture: a review of the evidence. Plast Reconstr Surg. 2012;130(5 Suppl 2):118S–124S. [DOI] [PubMed] [Google Scholar]
- 33.Alderman AK, Wilkins EG, Kim HM, et al. Complications in postmastectomy breast reconstruction: two-year results of the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg. 2002;109:2265–2274. [DOI] [PubMed] [Google Scholar]
- 34.Chun YS, Verma K, Rosen H, Lipsitz S, Morris D, Kenney P, Eriksson E. Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast Reconstr Surg. 2010;124:429, 436. [DOI] [PubMed] [Google Scholar]
- 35.Srinivasa DR, Garvey PB, Qi J, et al. Direct-to-implant versus two-stage tissue expander/implant reconstruction: 2-year risks and patient-reported outcomes from a prospective, multicenter study. Plast Reconstr Surg. 2017;140:869–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sorkin M, Qi J, Kim HM, et al. Acellular dermal matrix in immediate expander/implant breast reconstruction: a multicenter assessment of risks and benefits. Plast Reconstr Surg. 2017;140:1091–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kankam H, Hourston G, Forouhi P, Di Candia M, Wishart GC, Malata CM. Combination of acellular dermal matrix with a de-epithlialised dermal flap during skin-reducing mastectomy and immediate breast reconstruction. Ann R Coll Surg Engl. 2018:e1–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]


