Abstract
Context
Boys with XXY have greater adiposity and a higher risk of cardiovascular disease. Infants with XXY have lower testosterone concentrations than typical boys, but no studies have evaluated adiposity in infants with XXY or the physiologic effects of giving testosterone replacement.
Objective
To determine the effect of testosterone on body composition in infants with XXY.
Design
Prospective, randomized trial.
Setting
Tertiary care pediatric referral center.
Participants
20 infants 6 to 15 weeks of age with 47,XXY.
Intervention
Testosterone cypionate 25 mg intramuscularly monthly for three doses vs no treatment.
Main Outcome Measures
Difference in change in adiposity (percent fat mass z scores); other body composition measures, penile length, and safety outcomes between treated and untreated infants; and comparison with typical infants.
Results
The increase in percent fat mass (%FM) z scores was greater in the untreated group than in the treated group (+0.92 ± 0.62 vs −0.12 ± 0.65, P = 0.004). Increases in secondary outcomes were greater in the testosterone-treated group for total mass, fat-free mass, length z score, stretched penile length, and growth velocity (P < 0.002 for all). At 5 months of age, adiposity in untreated infants with XXY was 26.7% compared with 23.2% in healthy male infants of the same age (P = 0.0037); there was no difference in %FM between the treated XXY boys and controls. Reported side effects were minimal and self-limited; no serious adverse events occurred.
Conclusions
Adiposity of untreated infants was 15% greater than that of male controls by 5 months of age. Testosterone treatment for infants with XXY resulted in positive changes in body composition.
Keywords: Klinefelter syndrome, XXY, mini-puberty, adiposity, sex chromosome aneuploidy, testosterone
The number of infants recognized to have sex chromosome aneuploidies has rapidly increased with the commercialization of noninvasive prenatal screening [1]. The most common sex chromosome aneuploidy, karyotype 47,XXY, also known as Klinefelter syndrome, occurs in 1 of every 600 male births [2]. The extra X chromosome affects testicular development, resulting in infertility and hypergonadotropic hypogonadism in adult men [3]. Boys with 47,XXY also have a higher risk of developmental delays, learning disabilities, and cardiometabolic disease [4]. Whether hypogonadism is present in boys with 47,XXY before puberty is debated [5]. Supporting evidence includes a higher prevalence of micropenis and cryptorchidism than in the general population, slower penile growth in infancy, and frequent hypotonia, all which may be attributable to relative testosterone deficiency in early life [3, 5].
The mini-puberty period describes the transient activation of the hypothalamic-pituitary-gonadal axis resulting in testicular testosterone production from ∼1 to 4 months of life [6]. Animal models and human studies suggest this is a critical window of programming with lifelong implications [7–13]. Although one study of 10 infants with XXY reported high normal testosterone, other studies assessing serum hormone concentrations in the mini-puberty period have concluded testosterone is on average lower for boys with 47,XXY [14–17]. Therefore, it is biologically plausible, and observational reports suggest, that testosterone treatment may be beneficial for cardiometabolism and neurodevelopment in 47,XXY [5, 18, 19]. However, there have been no intervention trials, particularly in infancy, to guide clinical management in this population. The aim of this study was to evaluate the immediate effects of a short course of testosterone treatment for infants with 47,XXY on physical outcomes, including body composition, growth velocity, and penile size.
1. Methods
A. Overall Study Design
This was a randomized clinical trial of testosterone cypionate IM injections compared with no treatment for 20 infants with nonmosaic karyotype 47,XXY. Study visits occurred at baseline and after completion of a 3-month course of treatment (25 mg IM every 28 days for a total of three doses). The primary outcome was change in percent fat mass (%FM) z scores between study visits. Secondary outcomes included change in total mass, fat mass (FM), fat-free mass (FFM), %FM, length z scores, and stretched penile length z scores, as well as parent-reported side effects. Body composition parameters for infants with XXY at the final study visit were also compared with those of healthy controls [20].
B. Setting, Recruitment, and Participants
The study took place at the University of Colorado/Children’s Hospital Colorado, home of the eXtraordinarY Kids Clinic and Research Program [21]. Participants were recruited through local and national advertisements, parent support groups, targeted social media groups, and genetic counseling offices. Study enrollment occurred from May 2015 through September 2017. Infants between 6 and 15 weeks of age were identified to have had an increased risk for aneuploidy prenatally through noninvasive prenatal screening and had a postnatal diagnostic karyotype from either cord blood or a venous sample to confirm nonmosaic 47,XXY karyotype before study enrollment. Additional inclusion criteria were gestational age ≥37 weeks, birth weight 2.5 to 97.5 percentile for gestational age, and no exogenous androgen exposure outside the study protocol. The study was approved by the local institutional review board (COMIRB 14-1720) and registered on ClinicalTrials.gov (NCT02408445), and the protocol is on file with the US Food and Drug Administration (Investigational New Drug file 124260). Written informed consent was provided from the parents of all participants.
C. Randomization
Block randomization into two blocks of 10 was used to allocate participants 1:1 to receive or not receive testosterone treatment. The parents and principal investigator were aware of the treatment status; however, coinvestigators assessing length, weight, body composition, and penile length remained blinded to treatment status throughout the study.
D. Intervention
All participants randomly assigned to treatment were given testosterone cypionate 200 mg/mL IM injection of 25 mg (0.125 mL) in the vastus lateralis at the end of the initial (enrollment) study visit and then every 4 weeks for a total of three doses. This dosage was selected because it is commonly used for the treatment of micropenis, with noted efficacy and minimal side effects [22].
E. Study Assessments
Prenatal, neonatal, and early infant history was obtained from parent report and review of medical records, including genetic test results confirming nonmosaic 47,XXY. All participants underwent a physical examination and body composition assessment at baseline and after 3 months. Length was measured to the nearest millimeter with a recumbent infant stadiometer. Length z scores were calculated according to the 2006 World Health Organization growth standards [23]. Whole body composition was assessed via air displacement plethysmography (PEA POD; COSMED USA, Inc., Concord, CA) [24]. FM and FFM to the nearest gram were estimated from total body mass and volume, and %FM was calculated by dividing the FM by the total body mass. Infants were measured twice for each study visit, and a third measurement was completed if the %FM differed by more than 5 percentage points. The first measurement was used in the analysis unless there were concerns for validity due to excessive infant movement. Because of the range of age at enrollment, age- and sex-derived %FM z scores were calculated from published norms [25]. Stretched penile length was measured to the nearest millimeter by a pediatric endocrinologist blinded to the treatment status. Testicular volume was estimated with a Prader orchidometer. Parents of the infants randomly assigned to receive treatment were asked to report any perceived side effects of testosterone throughout the study period, and adverse events were collected at the final study visit.
F. Statistical Analyses
Frequency distributions for each continuous variable were generated and assessed for normality visually and by the Shapiro-Wilk normality test; if assumptions were not met, nonparametric tests were used. Data points were examined for outliers and missing data, but because of the small sample size of this study, no data points were excluded. Baseline summary statistics for demographic variables, covariates known to affect body composition, and outcomes of interest were generated and reported as mean and SD unless otherwise noted. Individual change scores were calculated for primary and secondary outcomes as appropriate. For the primary outcome, Welch’s two-sided, two-tailed t test was used to compare the change in %FM z scores between treated and untreated groups, with an a priori level of significance set at 5%. With a sample size of 10 participants per group, we had 80% power to detect a difference in change scores of 0.75 between groups for our primary outcome. Secondary outcomes were compared between groups in a similar fashion, with Welch t tests or Mann-Whitney tests for continuous outcomes and Fischer exact tests for categorical outcomes. We did not adjust for multiple comparisons.
As a secondary exploratory analysis to assess how untreated infants with XXY compared with boys in the general population at 5 months of age, body composition parameters (%FM, FM, FFM, and total mass) at the final study visit were compared via Welch two-sided t tests for untreated boys with XXY and 296 male infants from the Healthy Start Study. In brief, the Healthy Start Study is a large cohort study of healthy mother-infant pairs recruited prenatally from the Denver metropolitan area and followed longitudinally to examine infant health and metabolism [26]. Although genetic testing was not specifically performed in these participants, given the prevalence of sex chromosome aneuploidies, this group of healthy boys is assumed to have a karyotype predominantly, if not exclusively, 46,XY. The Healthy Start Study protocol included body composition assessed with the same PEA POD equipment and identical methods at 4 to 6 months of age, and those data have been previously published [20]. Data were managed in Research Electronic Data Capture, and analysis was conducted in Prism GraphPad version 8.1.1.
2. Results
Baseline demographics and characteristics for participants by randomization status are shown in Table 1. Twenty male infants with a mean age of 72 ± 22 days enrolled, and all completed the study without protocol deviations. All mothers had cell-free fetal DNA screening revealing an increased risk for 47,XXY; the majority were screened because of advanced maternal age. No pregnancies were exposed to tobacco smoke, one pregnancy was complicated by gestational diabetes, and two pregnancies were induced because of maternal hypertension or preeclampsia. No infants had prenatal or perinatal complications, and there were no congenital malformations.
Table 1.
Demographics and Baseline Characteristics for Infants With XXY
| All (n = 20) | No Testosterone Treatment (n = 10) | Testosterone Treatment (n = 10) | P | |
|---|---|---|---|---|
| Pregnancy and birth characteristics | ||||
| Maternal age, y | 35.5 ± 3.5 | 35.1 ± 2.7 | 35.9 ± 4.3 | 0.62 |
| Race and ethnicity | 0.99 | |||
| Non-Hispanic White, n (%) | 17 (85%) | 9 (90%) | 8 (80%) | |
| Other, n (%) | 3 (15%) | 1 (10%) | 2 (20%) | |
| Reason for prenatal screening, n (%) | 0.58 | |||
| Advanced maternal age | 13 (65%) | 7 (70%) | 6 (60%) | |
| Elective | 6 (30%) | 3 (30%) | 3 (30%) | |
| In vitro fertilization | 1 (5%) | 0 (0%) | 1 (10%) | |
| Maternal weight gain in pregnancy, kg | 16.1 ± 5.6 | 16.2 ± 6.6 | 16.1 ± 4.9 | 0.94 |
| Gestational diabetes, n (%) | 1 (5%) | 1 (10%) | 0 (0%) | 0.99 |
| Gestational age at birth, wk | 39.3 ± 1.1 | 39.2 ± 1.0 | 39.3 ± 1.3 | 0.79 |
| Birth weight, kg | 3.22 ± 0.42 | 3.35 ± 0.39 | 3.09 ± 0.44 | 0.17 |
| Birth length, cm | 50.7 ± 1.9 | 51.1 ± 1.6 | 50.4 ± 2.1 | 0.42 |
| Infant characteristics at enrollment (baseline) | ||||
| Infant age, d | 72 ± 22 | 70 ± 23 | 73 ± 22 | 0.80 |
| Feeding method | 0.63 | |||
| Breastfed only, n (%) | 14 (70%) | 8 (80%) | 6 (60%) | |
| Formula or combination, n (%) | 6 (30%) | 2 (20%) | 4 (40%) | |
| Total body mass, kg | 5.4 ± 0.8 | 5.4 ± 0.7 | 5.3 ± 0.9 | 0.76 |
| Length, cm | 57.8 ± 2.9 | 58.6 ± 1.8 | 57.0 ± 3.6 | 0.21 |
| FFM, kg | 4.3 ± 0.5 | 4.3 ± 0.5 | 4.2 ± 0.5 | 0.55 |
| FM, kg | 1.1 ± 0.4 | 1.1 ± 0.4 | 1.1 ± 0.4 | 0.88 |
| %FM | 19.8 ± 5.1 | 19.7 ± 5.9 | 20.2 ± 4.6 | 0.75 |
| Stretched penile length, cm | 2.7 ± 0.5 | 2.8 ± 0.6 | 2.7 ± 0.3 | 0.43 |
Data presented are mean ± SD or number (%).
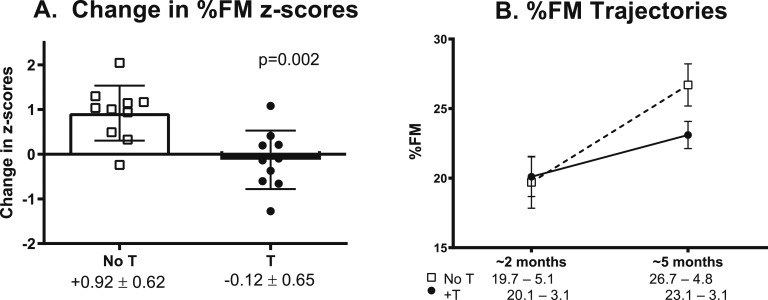
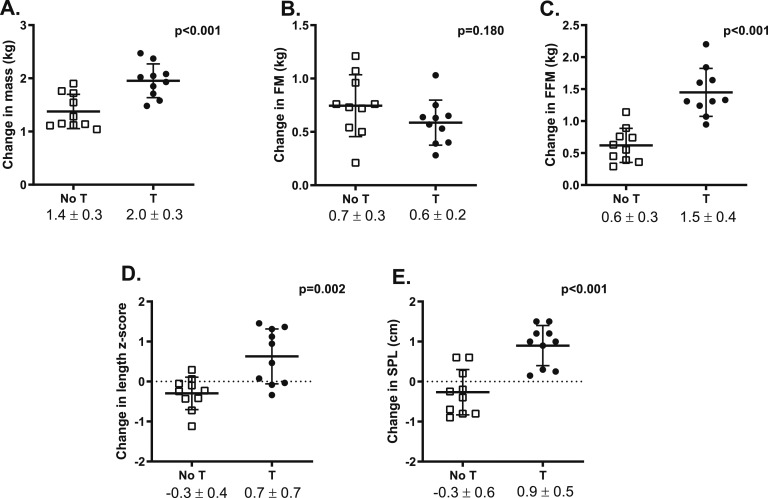
Baseline body composition parameters (FM, FFM, and %FM) were all within the normal ranges previously published for age and not significantly different between groups (Table 1) [25]. Figure 1 illustrates the primary outcome, change in %FM z scores. The difference in %FM z score change from before randomization was +1.04 in the untreated group compared with infants treated with testosterone (P = 0.002). Absolute %FM at the final visit was higher in the untreated infants, but this difference did not reach statistical significance (P = 0.061, Fig. 1). The difference in %FM between groups was attributable primarily to the larger increase in FFM in the testosterone-treated group rather than a direct difference in FM. Change in secondary outcomes were significantly different for total mass, FFM, length z score, and stretched penile length (Fig. 2). Growth velocity between the two study visits was 26.7 ± 2.5 cm/y in the untreated group and 34.2 ± 7.9 cm/y in the treated group (P < 0.001). Testicular volumes were unchanged from baseline to final visit and did not differ between groups (1.5 ± 0.6 mL for both groups).
Figure 1.
(A) Change in %FM z scores was significantly greater in untreated (open squares) than in testosterone-treated (closed circles) boys with XXY. Bars and error bars represent mean and SD, respectively, and symbols represent individual participants. (B) Absolute %FM was similar at baseline but higher in the untreated boys after 3 mo, although this difference did not reach statistical significance (P = 0.061). Error bars represent SEM. T, testosterone treatment.
Figure 2.
Change between the baseline (∼2 mo of age) and final visit (∼5 mo of age) in secondary outcomes including (A) total body mass, (B) FM, (C) FFM, (D) length, and (E) stretched penile length (SPL) between the untreated (open squares) and treated (black circles) infant boys with XXY. Bars and error bars represent the mean and SD, with symbols representing individual participants. T, testosterone treatment.
Summary data for FM, FFM, and total body mass for infants with XXY at the final study visit compared with published data from the Healthy Start Study are presented in Table 2. At a median age of 5 months, untreated boys with XXY had a %FM 3.5 percentage points greater than that of boys in the Healthy Start Study (P = 0.037, Fig. 3), representing a relative difference in adiposity of 15%. The %FM of untreated male infants was even higher than that of typical female infants (Fig. 3). There was no difference in %FM between the treated boys with XXY and healthy controls.
Table 2.
Body Composition Measures (Mean ± SD) at ∼5 Mo of Age in XXY and Healthy Start Study
| Typical Girlsa (n = 306) | Typical Boysa (n = 296) | XXY, No Testosterone Treatment (n = 10) | XXY, Testosterone Treatment (n = 10) | |
|---|---|---|---|---|
| Age, mo | 4.98 ± 0.92 | 4.89 ± 0.94 | 4.79 ± 0.87 | 5.05 ± 0.70 |
| Total body mass, kg | 6.57 ± 0.82 | 6.92 ± 0.81 | 6.78 ± 0.82 | 7.23 ± 0.89 |
| Length, cm | 63.1 ± 2.5 | 64.4 ± 2.6 | 64.0 ± 1.9 | 64.4 ± 2.3 |
| FFM, kg | 4.88 ± 0.53 | 5.29 ± 0.54 | 4.96 ± 0.61 | 5.57 ± 0.63 |
| FM, kg | 1.70 ± 0.50 | 1.63 ± 0.50 | 1.82 ± 0.43 | 1.69 ± 0.36 |
Reference data from the Healthy Start Study [20].
Figure 3.
Differences in %FM at 5 mo of age between typical girls and boys from the Healthy Start Study and infants with XXY, both untreated (white) and testosterone-treated (checkered). Bars and error bars represent means and SDs, respectively. T, testosterone treatment.
All testosterone injections were given per protocol and were well tolerated. In the treatment group, there was a total of three emergency department visits deemed to be not related to the intervention [final diagnoses: eczema (n = 1), viral upper respiratory illness (n = 1), and conjunctivitis (n = 1)]. No hospitalizations or surgeries were reported. Potential self-limited side effects endorsed by parents in the treatment group included noticing penile erections (n = 3), acne (n = 2), change in appetite (n = 1), stool pattern (n = 1), and sleep pattern (n = 1). There were no reports of pubic, axillary, or facial hair and no local reactions to the injections.
3. Discussion
This randomized, prospective testosterone intervention trial in infants with XXY demonstrates that a 3-month course of low-dose testosterone affected physical parameters in infants with XXY. In this study, change in adiposity (%FM z scores) was significantly greater for untreated infants than for treated infants. Testosterone treatment also resulted in increased FFM, growth velocity, and penile length, and side effects were minimal and self-limited.
Prenatal identification of sex chromosome aneuploidies, including XXY, has increased exponentially because of the increasing availability and use of cell-free fetal DNA prenatal screening [27]. Therefore, the demand for timely evidence-based clinical care is high. Testosterone treatment in infancy has emerged as a popular off-label treatment for boys with XXY, with observational reports of benefits in neurodevelopmental features [18, 19]. Animal models and limited clinical research have found that postnatal activation of the hypothalamic-pituitary-gonadal axis (known as the mini-puberty period of infancy) is a critical window of testosterone exposure during male development necessary for sexual differentiation of multiple tissues systemically [9, 11, 28]. The full implications of the mini-puberty period are still being studied, and rigorous research methods are desperately needed to guide standards of clinical care in XXY, as well as other populations that have impaired gonadal function in infancy [29]. However, no prospective, randomized trials of testosterone treatment in infants at risk for testosterone deficiency have been previously conducted.
Adiposity has consistently been shown to be approximately 1 SD higher in boys and men with XXY from childhood through adulthood [30, 31]. Given that testosterone production is impaired in XXY and is an anabolic steroid that promotes lean mass and decreases FM, testosterone deficiency is often assumed to be the cause of the body composition differences seen in XXY. Furthermore, both adiposity and testosterone deficiency have been correlated with adverse cardiometabolic health, including dyslipidemia, insulin resistance, and cardiac dysfunction [32, 33]. This study examined body composition in infants with XXY. Although adiposity was within the normal range at 2 months of age, by 5 months of age the 10 untreated infants with XXY already had a relative difference in adiposity of 15% more than healthy male infants. In a subanalysis of the Healthy Start Study, we found sex differences in body composition become more disparate in the first 5 months of life, potentially reflecting differences in hormone exposure during the mini-puberty period of infancy. It is possible the high adiposity seen throughout the lifespan in XXY originates from insufficient testosterone exposure during the mini-puberty period of infancy, although longitudinal studies would be needed to confirm this hypothesis. Testosterone treatment did prevent the adiposity gain the untreated infants experienced. Because of the short-term nature of the current study, it is unknown whether the positive effects of testosterone on body composition are sustained beyond the treatment period.
Sex differences in growth velocity and projected adult height were recently directly attributed to testosterone exposure in boys during the mini-puberty period [7]. Likewise, our study results confirm that infants with XXY who received testosterone had a more robust growth velocity (+7.5 cm/y) than infants who did not. Although growth velocity is not typically a targeted outcome in XXY, it is an important surrogate of the anabolic effects of testosterone and suggests the dosage of testosterone used in this study was effective. An increase in stretched penile length of ∼1 cm also supports the efficacy of the testosterone dosage. Importantly, consistent with previous reports in this population, penile lengths were below the mean for age at baseline, and treatment helped to normalize this outcome, whereas testicular volumes remained unchanged [3, 34]. Although changes in hormone concentrations were not assessed as part of this study because of the ethical and logistical challenges of serial blood draws in infants, there were no serious adverse events attributed to testosterone treatment, and side effects were minor and self-limited. Taken together, these results suggest that this dosing regimen was both effective and safe in this population. Although this study is too small and short-term to conclude that this dosing of testosterone should be used in clinical care, these results do provide the basis for a larger and longer confirmatory study of testosterone supplementation during mini-puberty for boys with XXY and other causes of neonatal and infantile hypogonadism.
Strengths of this study include the randomized, prospective design and rigorous assessment of body composition in infants. The sample size of this initial study is small, and therefore we could not adjust for factors known to contribute to differences in adiposity in infants, such as maternal weight gain, gestational age, and breastfeeding status; however, the randomized groups were well matched for these factors at baseline. We also were not powered to adjust for multiple comparisons in our analyses, and our findings should be considered exploratory. We have incorporated the results and experience of this study to develop a larger, more definitive randomized placebo-controlled trial of testosterone for infants with XXY (NCT03325647) that will allow confirmation of these findings in a larger sample size, assess additional outcomes including neurodevelopment, and determine whether immediate benefits to body composition are sustained beyond the initial treatment period.
In conclusion, a short course of testosterone treatment, 25 mg every 4 weeks for 3 doses, resulted in lower FM, higher FFM, greater growth velocity and penile length, and minimal side effects in infants with XXY. Testosterone treatment in infancy holds promise for boys with XXY and other forms of neonatal hypogonadism, and further investigation is needed to confirm these findings and assess additional clinically meaningful outcomes over time.
Acknowledgments
The authors thank the families who participated in this study, many of whom traveled for study visits. We thank Susan Howell, MS, CGC, for her contributions to recruitment and Natalie Nokoff, MD, for physical examinations. We also acknowledge the Association for X and Y Chromosome Variations (AXYS) for advertising the study on their website.
Financial Support: The study was funded primarily through pilot funds from the Children’s Hospital Colorado Department of Pediatric Endocrinology and the Colorado Clinical Translational Science Award [National Institutes of Health (NIH)/National Center for Advancing Translational Sciences UL1 TR002535] and support to S.M.D. through NIH/National Institute of Diabetes and Digestive and Kidney Diseases 2T32DK63687011A1 and NIH/Eunice Kennedy Shriver National Institute of Child Health and Human Development K23HD092588. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Clinical Trial Information: ClinicalTrials.gov no. NCT02408445 (registered 3 April 2015).
Additional Information
Disclosure Summary: S.M.D. has previously consulted for Antares Pharmaceuticals. N.R.T. and S.M.D. serve as volunteer members of the scientific advisory board of AXYS (Association for X and Y Chromosome Variations). The remaining authors have nothing to disclose.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Glossary
Abbreviations:
- FFM
fat-free mass
- FM
fat mass
- %FM
percent fat mass
References and Notes
- 1. Kornman L, Palma-Dias R, Nisbet D, Scott F, Menezes M, da Silva Costa F, McLennan A. Non-invasive prenatal testing for sex chromosome aneuploidy in routine clinical practice. Fetal Diagn Ther. 2018;44(2):85–90. [DOI] [PubMed] [Google Scholar]
- 2. Coffee B, Keith K, Albizua I, Malone T, Mowrey J, Sherman SL, Warren ST. Incidence of fragile X syndrome by newborn screening for methylated FMR1 DNA. Am J Hum Genet. 2009;85(4):503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davis SM, Rogol AD, Ross JL. Testis development and fertility potential in boys with Klinefelter syndrome. Endocrinol Metab Clin North Am. 2015;44(4):843–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davis S, Howell S, Wilson R, Tanda T, Ross J, Zeitler P, Tartaglia N. Advances in the interdisciplinary care of children with Klinefelter syndrome. Adv Pediatr. 2016;63(1):15–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fennoy I. Testosterone and the child (0–12 years) with Klinefelter syndrome (47XXY): a review. Acta Paediatr. 2011;100(6):846–850. [DOI] [PubMed] [Google Scholar]
- 6. Rey RA. Mini-puberty and true puberty: differences in testicular function. Ann Endocrinol (Paris). 2014;75(2):58–63. [DOI] [PubMed] [Google Scholar]
- 7. Kiviranta P, Kuiri-Hänninen T, Saari A, Lamidi ML, Dunkel L, Sankilampi U. Transient postnatal gonadal activation and growth velocity in infancy. Pediatrics. 2016;138(1):e20153561. [DOI] [PubMed] [Google Scholar]
- 8. Ghahramani NM, Ngun TC, Chen PY, Tian Y, Krishnan S, Muir S, Rubbi L, Arnold AP, de Vries GJ, Forger NG, Pellegrini M, Vilain E. The effects of perinatal testosterone exposure on the DNA methylome of the mouse brain are late-emerging. Biol Sex Differ. 2014;5(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Mello WG, de Morais SR, Dornelles RC, Kagohara Elias LL, Antunes-Rodrigues J, Bedran de Castro JC. Effects of neonatal castration and androgenization on sexual dimorphism in bone, leptin and corticosterone secretion. Bone. 2012;50(4):893–900. [DOI] [PubMed] [Google Scholar]
- 10. Swift-Gallant A, Coome LA, Ramzan F, Monks DA. Nonneural androgen receptors affect sexual differentiation of brain and behavior. Endocrinology. 2016;157(2):788–798. [DOI] [PubMed] [Google Scholar]
- 11. Dkhil MA, Al-Quraishy S, Abdel-Baki AA, Ghanjati F, Arauzo-Bravo MJ, Delic D, Wunderlich F. Epigenetic modifications of gene promoter DNA in the liver of adult female mice masculinized by testosterone. J Steroid Biochem Mol Biol. 2015;145:121–130. [DOI] [PubMed] [Google Scholar]
- 12. Alexander GM. Postnatal testosterone concentrations and male social development. Front Endocrinol (Lausanne). 2014;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, Mahurkar A, Russo SJ, Devine SE, McCarthy MM. Brain feminization requires active repression of masculinization via DNA methylation [published correction appears in Nat Neurosci. 2017;20:896] Nat Neurosci. 2015;18(5):690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cabrol S, Ross JL, Fennoy I, Bouvattier C, Roger M, Lahlou N. Assessment of Leydig and Sertoli cell functions in infants with nonmosaic Klinefelter syndrome: insulin-like peptide 3 levels are normal and positively correlated with LH levels. J Clin Endocrinol Metab. 2011;96(4):E746–E753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lahlou N, Fennoy I, Carel JC, Roger M. Inhibin B and anti-Müllerian hormone, but not testosterone levels, are normal in infants with nonmosaic Klinefelter syndrome. J Clin Endocrinol Metab. 2004;89(4):1864–1868. [DOI] [PubMed] [Google Scholar]
- 16. Aksglaede L, Petersen JH, Main KM, Skakkebaek NE, Juul A. High normal testosterone levels in infants with non-mosaic Klinefelter’s syndrome. Eur J Endocrinol. 2007;157(3):345–350. [DOI] [PubMed] [Google Scholar]
- 17. Lahlou N, Fennoy I, Ross JL, Bouvattier C, Roger M. Clinical and hormonal status of infants with nonmosaic XXY karyotype. Acta Paediatr. 2011;100(6):824–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Samango-Sprouse C, Stapleton EJ, Lawson P, Mitchell F, Sadeghin T, Powell S, Gropman AL. Positive effects of early androgen therapy on the behavioral phenotype of boys with 47,XXY. Am J Med Genet C Semin Med Genet. 2015;169(2):150–157. [DOI] [PubMed] [Google Scholar]
- 19. Samango-Sprouse CA, Sadeghin T, Mitchell FL, Dixon T, Stapleton E, Kingery M, Gropman AL. Positive effects of short course androgen therapy on the neurodevelopmental outcome in boys with 47,XXY syndrome at 36 and 72 months of age. Am J Med Genet A. 2013;161A(3):501–508. [DOI] [PubMed] [Google Scholar]
- 20. Sauder KA, Kaar JL, Starling AP, Ringham BM, Glueck DH, Dabelea D. Predictors of infant body composition at 5 months of age: the Healthy Start Study. J Pediatr. 2017;183:94–99 e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tartaglia N, Howell S, Wilson R, Janusz J, Boada R, Martin S, Frazier JB, Pfeiffer M, Regan K, McSwegin S, Zeitler P. The eXtraordinarY Kids Clinic: an interdisciplinary model of care for children and adolescents with sex chromosome aneuploidy. J Multidiscip Healthc. 2015;8:323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carrillo A. Disorders of sexual differentiation. In: Lifshitz F, ed. Pediatric Endocrinology. Vol 2. 5th ed New York, NY: Informa Healthcare USA; 2007. [Google Scholar]
- 23. WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85. [DOI] [PubMed] [Google Scholar]
- 24. Ma G, Yao M, Liu Y, Lin A, Zou H, Urlando A, Wong WW, Nommsen-Rivers L, Dewey KG. Validation of a new pediatric air-displacement plethysmograph for assessing body composition in infants. Am J Clin Nutr. 2004;79(4):653–660. [DOI] [PubMed] [Google Scholar]
- 25. Fields DA, Gilchrist JM, Catalano PM, Giannì ML, Roggero PM, Mosca F. Longitudinal body composition data in exclusively breast-fed infants: a multicenter study. Obesity (Silver Spring). 2011;19(9):1887–1891. [DOI] [PubMed] [Google Scholar]
- 26. Perng W, Ringham BM, Glueck DH, Sauder KA, Starling AP, Belfort MB, Dabelea D. An observational cohort study of weight- and length-derived anthropometric indicators with body composition at birth and 5 mo: the Healthy Start Study. Am J Clin Nutr. 2017;106(2):559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mennuti MT, Chandrasekaran S, Khalek N, Dugoff L. Cell-free DNA screening and sex chromosome aneuploidies. Prenat Diagn. 2015;35(10):980–985. [DOI] [PubMed] [Google Scholar]
- 28. Kuiri-Hänninen T, Sankilampi U, Dunkel L. Activation of the hypothalamic-pituitary-gonadal axis in infancy: minipuberty. Horm Res Paediatr. 2014;82(2):73–80. [DOI] [PubMed] [Google Scholar]
- 29. Copeland KC, Chernausek S. Mini-puberty and growth. Pediatrics. 2016;138(1):e20161301. [DOI] [PubMed] [Google Scholar]
- 30. Davis S, Cox-Martin M, Bardsley M, Kowal K, Zeitler P, Ross J.. Effects of oxandrolone on cardiometabolic health in boys with Klinefelter syndrome: a randomized controlled trial. J Clin Endocrinol Metab. 2017;102(1):176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gravholt CH, Jensen AS, Høst C, Bojesen A. Body composition, metabolic syndrome and type 2 diabetes in Klinefelter syndrome. Acta Paediatr. 2011;100(6):871–877. [DOI] [PubMed] [Google Scholar]
- 32. Andersen NH, Bojesen A, Kristensen K, Birkebaek NH, Fedder J, Bennett P, Christiansen JS, Gravholt CH. Left ventricular dysfunction in Klinefelter syndrome is associated to insulin resistance, abdominal adiposity and hypogonadism. Clin Endocrinol (Oxf). 2008;69(5):785–791. [DOI] [PubMed] [Google Scholar]
- 33. Bojesen A, Kristensen K, Birkebaek NH, Fedder J, Mosekilde L, Bennett P, Laurberg P, Frystyk J, Flyvbjerg A, Christiansen JS, Gravholt CH. The metabolic syndrome is frequent in Klinefelter’s syndrome and is associated with abdominal obesity and hypogonadism. Diabetes Care. 2006;29(7):1591–1598. [DOI] [PubMed] [Google Scholar]
- 34. Custer J, Rau R. Endocrinology. In: The Harriet Lane Handbook. 18th ed Amsterdam, The Netherlands: Elsevier; 2009:269–300. [Google Scholar]