Abstract
The capacity to communicate via acoustic signals is prevalent across the animal kingdom, from insects to humans. What are the neural circuit mechanisms that underlie this ability? New methods for behavioral analysis along with an unparalleled genetic toolkit have recently opened up studies of acoustic communication in the fruit fly, Drosophila melanogaster. Its nervous system comprises roughly 100,000 neurons, yet flies are able to both produce and process time-varying sounds during courtship. Just as with more complex animals, sensory feedback plays an important role in shaping communication between the sexes. Here, we review recent work in Drosophila that has laid the foundation for solving the mechanisms by which sensory information dynamically modulates behavior.
Introduction
Communication is important for quality of life, and in many cases, survival. Unsurprisingly, animals have evolved numerous strategies for exchanging information with members of their own species, and some of the most elaborate are designed to attract a mate. Whether considering the complicated nest-building of the bowerbird [1], the multi-step mating dance of the jumping spider [2], or the aerial acrobatics of the hummingbird [3], males often go to extraordinary lengths to prove their suitability to a female. One such mating signal, common to many species, is song. In general, males produce the acoustic cue, while females silently arbitrate mating decisions [4–6] — although there are notable examples of both sexes vocalizing (e.g. in flies [7], songbirds [8], and mice [9]). Courtship songs range in complexity, from the stereotyped and repetitive chirp of the cricket [10] to the highly variable and multisyllabic song of the Bengalese finch [11]. Regardless of intricacy, males must produce these songs robustly and reliably in order to compete for a mate. How does the male nervous system produce the patterns present in song and how are these patterns processed by the female to drive mate choice? Solving these questions will reveal how neural circuits, and the computations they perform, mediate social interactions.
Studies of the mechanisms underlying either song production or perception have mostly focused on a small number of non-genetic model systems [12–14]. Recently, however, Drosophila melanogaster has emerged as a strong genetic model system for studies of acoustic communication. This is largely due to the development of (1) computational methods to automate the collection and analysis of sizable behavioral datasets and (2) an impressive genetic toolkit for targeting genes and neurons that play a role in sexually dimorphic behaviors. Such advances have revealed that males pattern their songs in accordance with the dynamics of female behaviour — demonstrating that (as with humans) Drosophila acoustic communication relies heavily on sensorimotor integration [15••]. Highly specific genetic manipulations have also begun to uncover the circuits and mechanisms that underlie acoustic behaviors. Here, we review these recent findings, their contributions to our understanding of acoustic communication, and the questions that remain unanswered.
Sensorimotor integration and the patterning of Drosophila song
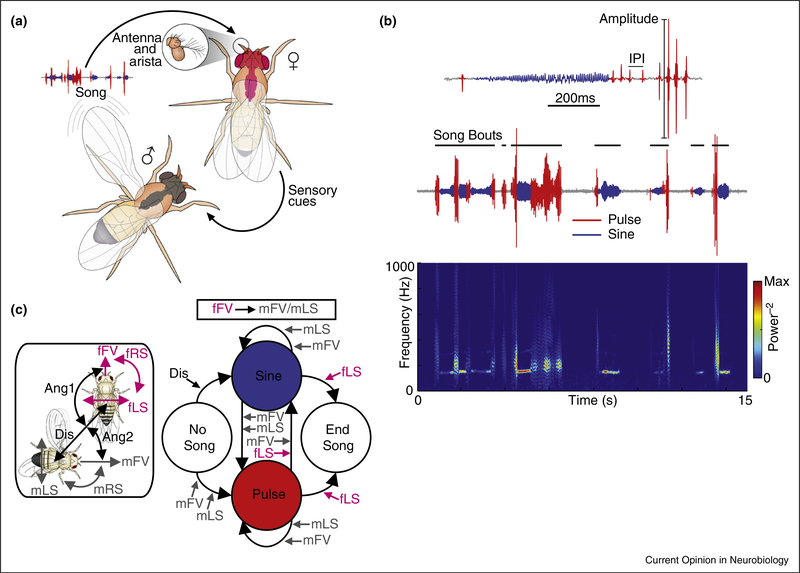
During courtship, male fruit flies chase females and generate courtship songs via wing vibration (Figure 1a); these songs are important for courtship to proceed to copulation [16]. While fly song has been investigated for more than fifty years [17], new methods have permitted a more thorough statistical analysis of Drosophila song patterns. These included optimization of hardware to detect the softest elements of song, parallelization of recordings on multiple microphones (facilitating high-throughput data collection), and development of software to automatically segment recorded song into its constituent elements [18] (Figure 2a). Males structure their songs into bouts (much like songbirds [4]), and most bouts consist of alternations between two modes, pulse and sine (Figure 1b). High-throughput analytic methods identified previously unobserved patterns in song (e.g. steady increases in sine mode frequency within song bouts), eliminated previously reported spurious song patterns (e.g. KH cycles — [19]), and have proved useful for beginning to map the genes [20] and neural circuits [21] underlying song production.
Figure 1.
(a) Schematic of Drosophila melanogaster acoustic communication. Central nervous systems are indicated in gray (male) and magenta (female) shading. (b) 15s example of fly song (middle) segmented into pulse (red) and sine (blue) modes (song bout structure is indicated), with accompanying spectrogram (bottom). A short song excerpt (top) highlights pulse amplitude and inter-pulse interval (IPI). (c) Left, schematic of fly movement features. Right, summary of the influence of movement features on song bout patterning, as revealed by GLM analysis. Modified with permission from [15••].
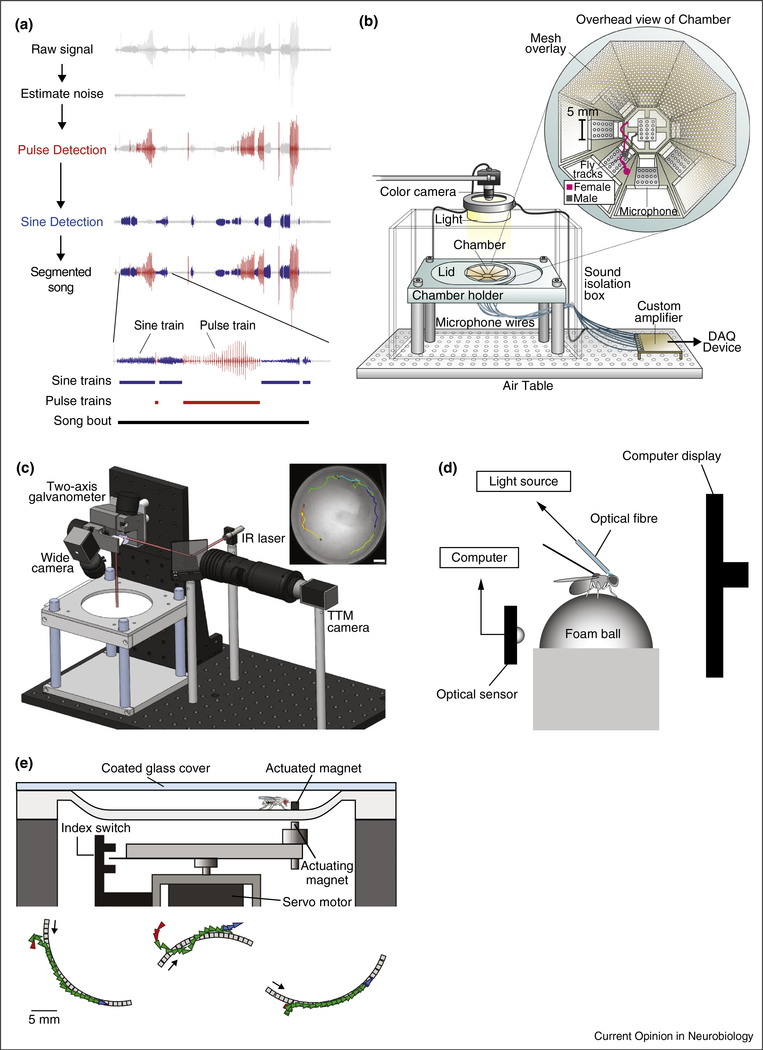
Figure 2.
(a) The stages of automated song segmentation. Modified with permission from [18]. (b) Drawing of setup for simultaneous tracking of flies and recording of song on multiple microphones. Modified with permission from [15••]. (c) Drawing of ‘FlyMAD’ apparatus for closed-loop thermogenetic activation of freely behaving flies: the fly is tracked throughout a 9 cm arena and galvanometer mirrors are adjusted to target the IR laser (red line) to the fly’s current location. Modified with permission from [32••]. (d) Sketch of fly-on-the-ball setup combining visual feedback and optogenetic neural activation. As the tethered fly walks, an optical sensor records ball movement and adjusts the image displayed accordingly. Optogenetic stimulation is targeted to the brain with an optical fiber. Modified with permission from [33••]. (e) Top, Schematic of ‘Flyatar’ apparatus wherein a remotely actuated fly dummy is used to elicit courtship behavior from the male. Bottom, three example chases identified by an automated behavior classifier. Modified with permission from [40••].
Males can sing hundreds of bouts, each lasting from ~50 ms to >30 s, before a mating decision occurs [15••,18]. Because courtship is innate, Drosophila song production was long considered a fixed action pattern — a behavioral sequence that is invariant to external factors once initiated [22]. This assumption was recently tested with a large dataset of tracked movements of male and female flies and simultaneously recorded male courtship song from an array of microphones in a large chamber (Figure 2b). Generalized linear models that took as inputs the parameterized motion tracks of the flies effectively predicted the patterning of male song: from bout initiation, through switches between song modes, to the termination of each bout (Figure 1c). This approach revealed that males are most sensitive to how far away the female is (her distance) and how fast she moves (her forward speed), and that this information sculpts song structure in real time. In other words, song emerges through a sequence of rapid sensorimotor transformations. More recent studies have now shown that males not only modulate song mode, but also the amplitude of acoustic signals, to compensate for changes in female distance, a behavior previously only documented in humans and songbirds [23•]. Together, these findings have revealed a new level of complexity in Drosophila social communication, likely arising from an equally intricate neural circuit.
The Drosophila song pathway
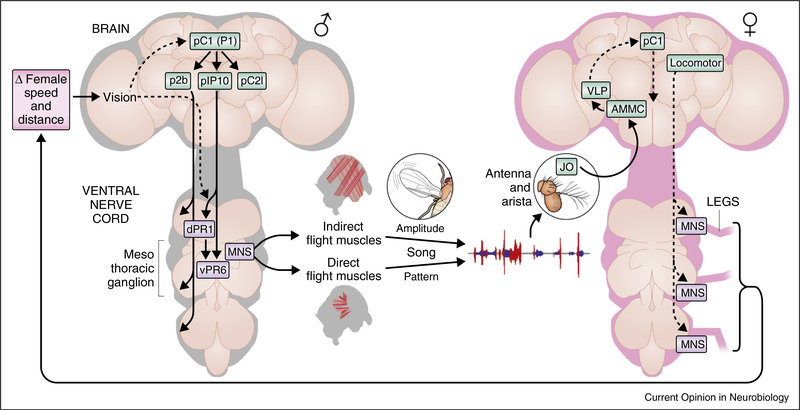
Two transcription factor-encoding genes, doublesex (dsx) and fruitless (fru), are known to specify the majority of sex-specific circuitry in the fruit fly (see also review in this issue: Auer and Benton) [24,25]. Optogenetic or thermogenetic activation of either fru or dsx neurons in males leads to song production or wing extension (often used as a proxy for song production), even in the absence of a female [26–28]. Of the ~2000 neurons expressing doublesex and/or fruitless, which are part of the song pathway? Intersectional genetic techniques have given researchers the ability to divide these neurons into subsets [29], and four of these neural classes (P1, pIP10, dPR1, and vPR6) have been causally linked to song production [28] (Figure 3). On the basis of their anatomy and the phenotypes generated via activation, it was postulated that the roughly 20 P1 neurons in the brain serve as ‘command neurons’ [30], triggering both sine and pulse song [15••] through downstream effectors, including the bilateral descending neurons, pIP10 [28] and P2b [31]. pIP10 in turn likely synapses onto dPR1, and both of these neurons innervate the mesothoracic ganglion of the ventral nerve cord (VNC) — where motor neurons driving the wing muscles reside. The vPR6 cluster, also located within the mesothoracic ganglion, is a likely downstream target of pIP10 and dPR1 and a putative component of the song central pattern generator: varying the amount of activation to vPR6 neurons causes a change in the rate at which song pulses are produced, a parameter known as the inter-pulse-interval (Figure 1b).
Figure 3.
Summary diagram of components of the song production (male, gray outline) and perception (female, magenta outline) pathways. Identified neurons and regions related to song production or perception in the brain (green) and ventral nerve cord (purple) are shown (JO — Johnston’s organ, AMMC — antennal mechanosensory and motor center, VLP — ventrolateral protocerebrum, MNS — motor neurons). Dashed arrows represent connections that are supported in the literature but for which the neural pathways remain unknown. For simplification, details of male song perception and putative male forward locomotor pathways are not shown.
This putative hierarchy has been supported by a number of subsequent studies. For example, artificial and acute activation of P1 — with either thermogenetic [32••] or optogenetic [33••] techniques (Figure 2c) — leads to wing extension that outlasts the activation period. Conversely, wing extension elicited through pIP10 activation ends immediately after the activation period. Another cluster of ~35 neurons in the male brain (pC2l) expresses dsx and not fru — activation of these neurons in tethered males generates wing vibration on only the contralateral side, while unilateral P1 activation can drive wing extension on either the left or right side of the male [34••]. These data collectively indicate that P1 neurons are the most upstream in the song pathway. However, because low-level activation of P1 (in combination with male olfactory cues) promotes aggression rather than courtship, P1 neurons likely have context-dependent functions in changing behavioral state [35•].
Downstream of the pathway outlined above, which motor neurons and muscles are involved in song production and patterning? Wing movements (and thereby song production) are controlled by two sets of thoracic muscles, the indirect and direct flight muscles (Figure 3). Of the direct muscles, hg1 (a dsx-expressing muscle) is uniquely enlarged in males, and the motor neurons that innervate this muscle are specifically required for production of sine song, whereas another motor neuron, ps1, is specifically required for aspects of pulse song production [21]. The indirect flight muscles appear to control the amplitude, rather than the pattern, of song [23•]. These data suggest that different neural circuits coordinate the production of sine versus pulse song, and that switching between these circuits, required to generate song bouts (Figure 1b), occurs at the level of the mesothoracic ganglion.
Vision and song patterning
Song pathway ‘command neurons’ should integrate courtship-relevant sensory signals to change behavioral state. Recent studies using the calcium indicator GCaMP to measure neural activity have shown that P1 neurons are active during visually induced chasing [34••], and respond to auditory [36] and pheromonal cues [34••,37,38]. However, increasing evidence suggests that vision is also of critical importance. For example, activating a subset of P1 neurons, or activating the majority of P1 neurons below threshold, is only able to drive wing extension when males are also presented with a visual stimulus — even if this stimulus is a square on a computer screen [34••], a rubber band [39] or a piece of wax [32••] (Figure 2d). In one study, the authors used an actuated magnet to simulate the female fly [40••] (Figure 2e). Males readily chased this ‘flyatar,’ and extended their wings, despite the absence of female pheromones (although coating the magnet with pheromones extended chase duration).
From these results, it is tempting to conclude that P1 represents the interface between sensory and motor pathways. However, new evidence suggests that the situation is more complicated. Constitutive activation at different levels of the song pathway (Figure 3) reveals that some visual information intersects the pathway downstream of P1 and pIP10 neurons, most likely in the VNC [23•]. In addition, other pathways of visual influence are indirect. Male self-motion (which is driven primarily by a visual estimate of female speed) strongly influences song patterning [15••]. Remarkably, this remains true even for artificially activated song produced in the absence of a female, indicating a neural link between the locomotor and song circuits. Thus, some sensory signals influence the decision to initiate song at the level of the P1 neurons while others modulate song patterning (e.g., switching between song modes or changing song amplitude) within the VNC or via interactions with, as of yet unmapped, locomotor circuits.
Despite the importance of vision for male song production, the visual neurons involved remain unknown. Neurons in the identified elementary motion detection (EMD) and looming pathways [41] affect the male’s ability to follow the female while singing, but silencing these neurons has little impact on the modulation of, for example, song amplitude with female distance [23•]. Candidates for neurons that carry distance information include small target motion detectors, so far only identified in larger insects [42]. Furthermore, while visual cues from the female influence male speed — and consequently song patterning — interpreting those cues requires knowledge of self-motion, as with visual processing during flight [43]. Once these two motor circuits (singing and walking) are mapped, it will be critical to determine how the visual and self-motor information streams are combined, and how contradictory cues are reconciled in order to correctly pattern song.
The influence of song on females
Males go to great lengths to produce songs matched to the dynamics of female motion — what aspects of song do females care about and how do they extract this information? One challenge to investigating female song perception is that, unlike male song, female receptivity is difficult to quantify. For example, the use of song playback assays (placing a wingless male with a female while playing synthetic or recorded song through a speaker and assessing copulation rates [44]), has led to conflicting results regarding which features of song females prefer [45–47]. More recently, with the advent of tracking software, experimenters have begun to use female speed to estimate song responses throughout courtship [15••,48••,49]. Such studies found that females either slow down or speed up in response to both sine and pulse song, dependent on their receptivity state [15••]. In addition, the average duration (or length) of song bouts (Figure 1b), extending over ~80 s, is the strongest predictor of female speed [48••]. Thus, as with male song patterning, female song processing can also be thought of as a continuous series of sensorimotor transformations.
Auditory processing begins in the Johnston’s organ (JO): a collection of ~480 primary sensory neurons activated in response to deflections of the auditory receiver, a structure known as the arista [50,51]. Song signals are processed by two of the five subsets of JO neurons [52] — these neurons project to the antennal mechanosensory and motor center (AMMC), and AMMC neurons in turn project largely to the ventrolateral protocerebrum (VLP) [53] (Figure 3). Whole cell patch clamp recordings of a subset of AMMC or VLP neurons (in immobilized females listening to artificial song) revealed surprisingly simple and stereotyped responses across neurons [48••,54,55]. These similarities facilitated the development of computational models to predict neural responses to natural song, and decoding these responses accurately predicted female speed [48••]. Whether these neurons are required for the female’s behavioral response to song remains to be determined, but neural silencing of two distinct AMMC neural types (not sampled in [48••]) reduced copulation rates [56•]. GCaMP imaging of those neurons’ responses suggested they are modestly tuned to the melanogaster conspecific inter-pulse interval (IPI). Thus neural tuning for short and long timescale song features appears to coexist within the AMMC and VLP. Short timescale features, like the IPI, likely indicate species identity, whereas long timescale features, like bout duration, may represent an individual male’s fitness (e.g., his ability to follow the female).
Which downstream neural circuits are responsible for extracting song information, integrating it over time, and producing a behavioral change? Although females do not produce the Fruitless protein, they generate a female-specific isoform of Doublesex, and doublesex-expressing neurons appear to be critical for regulating female courtship behaviors [57]. New genetic tools have identified two female-specific neural clusters, PC1 and PCd, which dramatically increase the probability of copulation when activated in female files, and produce a corresponding decrease in receptivity when silenced [58••]. The PC1 neurons also appear to respond (assessed via GCaMP imaging in immobilized flies) to both auditory and pheromonal stimuli, indicating they are involved in multimodal integration, akin to P1 neurons in the male. Interestingly, PC1 (female) and P1 (male) neurons are located in the same brain region and P1 neurons in the male also respond to auditory stimuli — in particular, they are tuned for conspecific pulse song parameters [36]. These data lead to the tantalizing hypothesis that similar neural architectures are responsible for song production in the male and perception in the female. This common neural elements hypothesis has been proposed in other systems [59,60] and would explain the apparent vestigial song production circuit in the female [26].
Conclusions
Drosophila melanogaster has emerged as a premier model for studies of sensorimotor integration during communication. Genetic and computational tools have revealed new complexity in courtship behavior (e.g., Drosophila male song causes changes in female locomotion, which in turn affect the patterning of song) and have identified components of both the song production and perception pathways. But significant work remains for a comprehensive understanding of the underlying neural circuit mechanisms. Ongoing work to map and characterize neural activity in the Drosophila visual [61] and locomotor [62] pathways will facilitate solving how sensory information (related to female movements) modulates activity within the song pathway of males. However, to date, only a limited number of song neurons have been identified — other neurons may have been missed in previous studies because either (1) they do not express the transcription factors fruitless or doublesex or (2) they do not elicit a behavioral phenotype when activated in isolation. Widening the search with new genetic tools that sparsely tile all neurons [63] may solve the prior, but activating smaller subsets of neurons may make it more unlikely to observe a behavioral phenotype. Thus, in vivo imaging and recording will certainly be required to characterize both song production pathways and female song processing pathways. Ideally, mapping neural circuits underlying sensorimotor integration should take place in behaving animals, but these experiments are challenging to execute [64]. One major obstacle for studying song patterning neurons is that accessing the ventral nerve cord for calcium imaging or whole-cell patch clamp electrophysiology necessitates opening up the thorax, which consequently prevents wing motion (and song production). As with other systems [65], it should be possible use fictive behavior as a proxy for song, but the development of new methods for non-invasive neural recording would certainly facilitate studies of sensorimotor integration. In addition, better measures of female behavior are required to interpret activity in her song processing pathways. It is now possible to automatically identify subtle behaviors in freely moving flies [66,67•]. These tools are well suited to finding a robust indicator of female receptivity — ideally one that can be observed in tethered flies during neural recordings. Despite the remaining challenges, the studies reviewed here have laid the groundwork for a complete dissection of the circuits and mechanisms underlying this example of complex social communication.
Acknowledgements
We thank Adam Calhoun and Richard Benton for comments on this manuscript. Figures 1a and 3 were illustrated by K. Ris-Vicari. PC was funded by an HHMI International Pre-Doctoral Fellowship and MM was funded by the Alfred P. Sloan Foundation, Human Frontiers Science Program, NSF CAREER award, NIH New Innovator Award, NSF BRAIN Initiative EAGER award, McKnight Foundation, and Klingenstein-Simons Foundation.
Footnotes
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Uy JA, Borgia G: Sexual selection drives rapid divergence in bowerbird display traits. Evolution 2000, 54:273–278. [DOI] [PubMed] [Google Scholar]
- 2.Richman DB, Jackson RR: A review of the ethology of jumping spiders (Araneae, Salticidae). Br Arachnol Soc 1992, 9:37. [Google Scholar]
- 3.Clark C: Courtship dives of Anna’s hummingbird offer insights into flight performance limits. Proc Biol Sci 2009, 276:3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brainard M, Doupe A: What songbirds teach us about learning. Nature 2002, 417:351–358. [DOI] [PubMed] [Google Scholar]
- 5.Ewing A, Bennet-Clark: The courtship songs of Drosophila. Behaviour 1968, 31:288–301. [Google Scholar]
- 6.Kelley D: Vocal communication in frogs. Curr Opin Neurobiol 2004, 14:751–757. [DOI] [PubMed] [Google Scholar]
- 7.LaRue KM, Clemens J, Berman GJ, Murthy M: Acoustic duetting in Drosophila virilis relies on the integration of auditory and tactile signals. Elife 2015:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fortune E, Rodríguez C, Li D, Ball G, Coleman M: Neural mechanisms for the coordination of duet singing in wrens. Science (New York, NY) 2011, 334:666–670. [DOI] [PubMed] [Google Scholar]
- 9.Neunuebel J, Taylor A, Arthur B, Egnor R: Female mice ultrasonically interact with males during courtship displays. eLife 2015, 4:e06203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bentley Hoy: The neurobiology of cricket song. Sci Am 1974, 231:34–50. [DOI] [PubMed] [Google Scholar]
- 11.Sakata J, Hampton C, Brainard M: Social modulation of sequence and syllable variability in adult birdsong. J Neurophysiol 2008, 99:1700–1711. [DOI] [PubMed] [Google Scholar]
- 12.Fee MS, Scharff C: The songbird as a model for the generation and learning of complex sequential behaviors. ILAR J 2010, 51:362–377. [DOI] [PubMed] [Google Scholar]
- 13.Ghazanfar A, Hauser M: The neuroethology of primate vocal communication: substrates for the evolution of speech. Trends Cogn Sci 1999, 3:377–384. [DOI] [PubMed] [Google Scholar]
- 14.Poulet J, Hedwig B: New insights into corollary discharges mediated by identified neural pathways. Trends Neurosci 2007:30. [DOI] [PubMed] [Google Scholar]
- 15.Coen P, Clemens J, Weinstein A, Pacheco D, Deng Y, Murthy M: Dynamic sensory cues shape song structure in Drosophila. Nature 2014, 507:233–237.•• Developed a behavioral assay to simultaneously record song and track fly movement. Song structure could be predicted from parametrized fly movements, demonstrating that song production is not a fixed action pattern and instead relies on continuous sensorimotor transformations.
- 16.Bennet-Clark HC, Ewing AW: Stimuli provided by courtship of male Drosophila melanogaster. Nature 1967, 215:669–671. [Google Scholar]
- 17.Shorey HH: Nature of the sound produced by Drosophila melanogaster during courtship. Science 1962, 137:677–678. [DOI] [PubMed] [Google Scholar]
- 18.Arthur B, Sunayama-Morita T, Coen P, Murthy M, Stern D: Multi-channel acoustic recording and automated analysis of Drosophila courtship songs. BMC Biol 2013, 11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stern D: Reported Drosophila courtship song rhythms are artifacts of data analysis. BMC Biol 2014, 12:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Philipsborn A, Jörchel S, Tirian L, Demir E, Morita T, Stern D, Dickson B: Cellular and behavioral functions of fruitless isoforms in Drosophila courtship. Curr Biol 2014, 24:242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shirangi T, Stern D, Truman J: Motor control of Drosophila courtship song. Cell Rep 2013, 5:678–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tinbergen N: The Study of Instinct. Clarendon Press/Oxford University Press; 1951. [Google Scholar]
- 23.Coen P, Xie M, Clemens J, Murthy M: Sensorimotor transformations underlying variability in song intensity during Drosophila courtship. Neuron 2016, 89:629–644.•Demonstrated that male flies modulate the intensity of their pulse song to compensate for changes in female distance and investigated the underlying sensorimotor transformations.
- 24.Rideout E, Dornan A, Neville M, Eadie S, Goodwin S: Control of sexual differentiation and behavior by the doublesex gene in Drosophila melanogaster. Nat Neurosci 2010:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stockinger P, Kvitsiani D, Rotkopf S, Tirián L, Dickson B: Neural circuitry that governs Drosophila male courtship behavior. Cell 2005:121. [DOI] [PubMed] [Google Scholar]
- 26.Clyne D, Miesenböck G: Sex-specific control and tuning of the pattern generator for courtship song in Drosophila. Cell 2008, 133:354–363. [DOI] [PubMed] [Google Scholar]
- 27.Pan Y, Robinett C, Baker B: Turning males on: activation of male courtship behavior in Drosophila melanogaster. PLOS ONE 2011:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Philipsborn A, Liu T, Yu J, Masser C, Bidaye S, Dickson B: Neuronal control of Drosophila courtship song. Neuron 2011, 69:509–522. [DOI] [PubMed] [Google Scholar]
- 29.Yu J, Kanai M, Demir E, Jefferis G, Dickson B: Cellular organization of the neural circuit that drives Drosophila courtship behavior. Curr Biol 2010, 20:1602–1614. [DOI] [PubMed] [Google Scholar]
- 30.Hedwig B: Control of cricket stridulation by a command neuron: efficacy depends on the behavioral state. J Neurophysiol 2000, 83:712–722. [DOI] [PubMed] [Google Scholar]
- 31.Kohatsu S, Koganezawa M, Yamamoto D: Female contact activates male-specific interneurons that trigger stereotypic courtship behavior in Drosophila. Neuron 2011, 69:498–508. [DOI] [PubMed] [Google Scholar]
- 32.Bath D, Stowers J, Hörmann D, Poehlmann A, Dickson B, Straw A: FlyMAD: rapid thermogenetic control of neuronal activity in freely walking Drosophila. Nat Methods 2014, 11:756–762.•• Established a method for closed-loop thermogenetic neural activation in freely moving flies by combining a real-time tracking system with galvanometer mirrors to target an infrared laser to flies. This system has the spatial resolution to separately target neurons in the head versus the thorax of the fly. Confirmed that P1 neural activation causes persistent courtship and wing extension, in this case towards an inanimate object.
- 33.Inagaki HK, Jung Y, Hoopfer ED, Wong AM, Mishra N, Lin JY, Tsien RY, Anderson DJ: Optogenetic control of Drosophila using a red-shifted channelrhodopsin reveals experience-dependent influences on courtship. Nat Methods 2014, 11:325–332.•• Established a method for optogenetic neural activation in freely moving flies via a red-shifted channelrhodopsin (ReaChR). Used this tool to demonstrate that P1 activation in males generated persistent wing extension that extended beyond the activation period, whereas pIP10 activation-induced wing extension ended at activation offset.
- 34.Kohatsu S, Yamamoto D: Visually induced initiation of Drosophila innate courtship-like following pursuit is mediated by central excitatory state. Nat Commun 2015:6.••Used a fly-on-the-ball setup combined with visual stimulation and opto-genetic activation of pC1 and pC2l neural clusters in males. Data support a hierarchy where pC1 neurons are upstream of pC2l in driving song production. Calcium responses in pC1 neurons were correlated with male pursuit of visual stimulus, indicating that PC1 receives visual and/or motor related inputs.
- 35.Hoopfer ED, Jung Y, Inagaki HK, Rubin GM, Anderson DJ: P1 interneurons promote a persistent internal state that enhances inter-male aggression in Drosophila. eLife 2016:4.• Optogenetically activated male P1 neurons at different intensities and found that the same subset of neurons could drive aggression or wing extension at low or high activation thresholds, respectively.
- 36.Zhou C, Franconville R, Vaughan A, Robinett C, Jayaraman V, Baker B: Central neural circuitry mediating courtship song perception in male Drosophila. eLife 2015:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kallman B, Kim H, Scott K: Excitation and inhibition onto central courtship neurons biases Drosophila mate choice. eLife 2015:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clowney, Iguchi S, Bussell J, Scheer E, Ruta V: Multimodal chemosensory circuits controlling male courtship in Drosophila. Neuron 2015, 87:1036–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pan Y, Meissner G, Baker B: Joint control of Drosophila male courtship behavior by motion cues and activation of male-specific P1 neurons. Proc Natl Acad Sci U S A 2012, 109:5–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agrawal S, Safarik S, Dickinson M: The relative roles of vision and chemosensation in mate recognition of Drosophila melanogaster. J Exp Biol 2014, 217:2796–2805.•• Demonstrated the sufficiency of visual cues for driving male courtship using an actuated magnet. Interestingly, bouts of chasing were of stereo-typed length, indicating the underlying neural circuits have an intrinsic timing in the absence of changes in sensory feedback.
- 41.Silies M, Gohl D, Clandinin T: Motion-detecting circuits in flies: coming into view. Annu Rev Neurosci 2013, 37:307–327. [DOI] [PubMed] [Google Scholar]
- 42.Nordström K: Neural specializations for small target detection in insects. Curr Opin Neurobiol 2012, 22:272–278. [DOI] [PubMed] [Google Scholar]
- 43.Kim A, Fitzgerald J, Maimon G: Cellular evidence for efference copy in Drosophila visuomotor processing. Nat Neurosci 2015, 18:1247–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.von Schilcher F: The role of auditory stimuli in the courtship of Drosophila melanogaster. Anim Behav 1976, 24:18–26. [Google Scholar]
- 45.Bennet-Clark HC, Ewing AW: Pulse interval as a critical parameter in the courtship song of Drosophila melanogaster. Anim Behav 1969, 17:755–759. [Google Scholar]
- 46.Talyn B, Dowse H: The role of courtship song in sexual selection and species recognition by female Drosophila melanogaster. Anim Behav 2004, 68:1165–1180. [Google Scholar]
- 47.Rybak F, Aubin T, Moulin B, Jallon J-M: Acoustic communication in Drosophila melanogaster courtship: are pulse- and sine-song frequencies important for courtship success? Can J Zool 2002, 80:987–996. [Google Scholar]
- 48.Clemens J, Girardin C, Coen P, Guan X-J, Dickson B, Murthy M: Connecting neural codes with behavior in the auditory system of Drosophila. Neuron 2015, 87:1332–1343.•• Established that females slow in response to song structure on long timescales. Used computational modelling, along with recordings from auditory neurons, to determine how these song features are represented in the brain and how neural representations are decoded to predict behavior.
- 49.Bussell J, Yapici N, Zhang S, Dickson B, Vosshall L: Abdominal-B neurons control Drosophila virgin female receptivity. Curr Biol 2014, 24:1584–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Göpfert MC, Robert D: The mechanical basis of Drosophila audition. J Exp Biol 2002, 205:1199–1208. [DOI] [PubMed] [Google Scholar]
- 51.Kamikouchi A, Shimada T, Ito K: Comprehensive classification of the auditory sensory projections in the brain of the fruit fly Drosophila melanogaster. J Comp Neurol 2006, 499:317–356. [DOI] [PubMed] [Google Scholar]
- 52.Kamikouchi A, Inagaki HK, Effertz T, Hendrich O, Fiala A, Göpfert MC, Ito K: The neural basis of Drosophila gravity-sensing and hearing. Nature 2009, 458:165–171. [DOI] [PubMed] [Google Scholar]
- 53.Lai J, Lo S-J, Dickson B, Chiang A-S: Auditory circuit in the Drosophila brain. Proc Natl Acad Sci 2012, 109:2607–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tootoonian S, Coen P, Kawai R, Murthy M: Neural representations of courtship song in the Drosophila brain. J Neurosci 2012, 32:787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lehnert BP, Baker AE, Gaudry Q, Chiang A-S, Wilson RI: Distinct roles of TRP channels in auditory transduction and amplification in Drosophila. Neuron 2013, 77:115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vaughan AG, Zhou C, Manoli DS, Baker BS: Neural pathways for the detection and discrimination of conspecific song in D. melanogaster. Curr Biol 2014, 24:1039–1049.• Identified two classes of AMMC interneurons (of 12 tested), aPN1 and aLN, which were each necessary for behavioral responses to song in both male and female flies.
- 57.Yamamoto D, Koganezawa M: Genes and circuits of courtship behaviour in Drosophila males. Nat Rev Neurosci 2013, 14:681–692. [DOI] [PubMed] [Google Scholar]
- 58.Zhou C, Pan Y, Robinett C, Meissner G, Baker B: Central brain neurons expressing doublesex regulate female receptivity in Drosophila. Neuron 2014, 83:149–163.••Used an intersectional genetic technique to define two clusters of female neurons (pC1 and pCd) that promote receptivity when activated. Further demonstrated that both clusters are activated by male-specific pheromones and one (pC1) responds to male courtship song.
- 59.Liberman A, Mattingly I: The motor theory of speech perception revised. Cognition 1985, 21:1–36. [DOI] [PubMed] [Google Scholar]
- 60.Hoy RR, Hahn J, Paul RC: Hybrid cricket auditory behavior: evidence for genetic coupling in animal communication. Science 1977, 195:82–84. [DOI] [PubMed] [Google Scholar]
- 61.Tuthill JC, Nern A, Holtz SL, Rubin GM, Reiser MB: Contributions of the 12 neuron classes in the fly lamina to motion vision. Neuron 2013, 79:128–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bidaye SS, Machacek C, Wu Y, Dickson BJ: Neuronal control of Drosophila walking direction. Science 2014, 344:97–101. [DOI] [PubMed] [Google Scholar]
- 63.Aso Y, Hattori D, Yu Y, Johnston RM, Iyer NA, Ngo T-TBT, Dionne H, Abbott LF, Axel R, Tanimoto H et al. : The neuronal architecture of the mushroom body provides a logic for associative learning. Elife 2014, 3:e04577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seelig J, Jayaraman V: Neural dynamics for landmark orientation and angular path integration. Nature 2015, 521:186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ahrens M, Li J, Orger M, Robson D, Schier A, Engert F, Portugues R: Brain-wide neuronal dynamics during motor adaptation in zebrafish. Nature 2012, 485:471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kabra M, Robie A, Rivera-Alba M, Branson S, Branson K: JAABA: interactive machine learning for automatic annotation of animal behavior. Nat Methods 2012, 10:64–67. [DOI] [PubMed] [Google Scholar]
- 67.Berman G, Choi D, Bialek W, Shaevitz J: Mapping the stereotyped behaviour of freely moving fruit flies. J Roy Soc Interface 2014, 11 20140672.•Developed an algorithm for mapping an animal’s actions into a low-dimensional space using the underlying structure of postural movement data and applied the method to videos of freely moving flies.