Abstract
In analogy to the appreciation of humor, that of tickling is based upon the re-interpretation of an anticipated emotional situation. Hence, the anticipation of tickling contributes to the final outburst of ticklish laughter. To localize the neuronal substrates of this process, functional magnetic resonance imaging (fMRI) was conducted on 31 healthy volunteers. The state of anticipation was simulated by generating an uncertainty respecting the onset of manual foot tickling. Anticipation was characterized by an augmented fMRI signal in the anterior insula, the hypothalamus, the nucleus accumbens and the ventral tegmental area, as well as by an attenuated one in the internal globus pallidus. Furthermore, anticipatory activity in the anterior insula correlated positively with the degree of laughter that was produced during tickling. These findings are consistent with an encoding of the expected emotional consequences of tickling and suggest that early regulatory mechanisms influence, automatically, the laughter circuitry at the level of affective and sensory processing. Tickling activated not only those regions of the brain that were involved during anticipation, but also the posterior insula, the anterior cingulate cortex and the periaqueductal gray matter. Sequential or combined anticipatory and tickling-related neuronal activities may adjust emotional and sensorimotor pathways in preparation for the impending laughter response.
Keywords: affective touch, anticipation, anterior insula, fMRI, tickle, periaqueductal gray matter
Introduction
The anticipation of a sensation of tickling is considered to be an integral part of the mechanism underlying ticklish laughter (Ramachandran, 1998). Since the tickling that induces laughter is applied particularly to vulnerable bodily parts, it is initially anticipated as a potential threat. It may be reappraised as harmless during the actual stimulation, which is frequently associated even with positive feelings (Provine, 2004). This circumstance is exemplified by a child’s reaction to the menace of tickling, which involves an attempt to escape from it, screaming or laughter. Hence, the anticipation of tickling appears to trigger neuronal processes that are relevant during laughter. Investigations appertaining to the neuronal responses of humor and laughter have progressively targeted the focus of interest (Wild et al., 2003; Lauterbach et al., 2013; Scott et al., 2014), not least regarding their association with physiological parameters (Lackner et al., 2014) and the impact of affect-regulating pathways (Mobbs et al., 2003). As yet, the inter-dependency of laughter and the related anticipatory processes has not been a subject of study.
According to James (1890), the expectation of an externally applied stimulus involves the same centers in the brain as those that are related to its actual experience. During the act of stimulation, this mechanism would help to improve the efficiency with which the information is processed. For example, the primary sensorimotor cortices that are involved in the anticipation of touch and of tickling are similar to those that are implicated in the actual sensory experience (Carlsson et al., 2000). The sense of touch mediates not only a discriminatory but also an affective dimension (Olausson et al., 2002), which, if sufficiently potent, triggers an emotional reaction. This response involves changes in heart rate, blood pressure, respiration and vocalization. In the case of tickling, vocalization is manifested as laughter. Hence, the affective response to tickling and the related anticipatory processes may be deep-seated in the same brain centers.
Emotional reactions are driven by the so-called emotional motor system—an ancient, involuntarily regulated pathway that complements the voluntary system [reviewed by Holstege and Subramanian (2016)]. The midbrain periaqueductal gray matter (PAG) is an important relay in this system; it is immediately implicated in the expression of emotions, including the control and the co-ordination of the motor neurons that are involved in laryngeal and respiratory movements (Holstege, 2014). There is broad scientific concordance in considering the PAG as pivotal in triggering vocalization in various species, including humans [(Jurgens, 1998, 2009; Belyk and Brown, 2016), see also Figure 1]. Laryngeal and respiratory vocal effectors are also controlled by the volitional motor system, notably, in humans, by newly developed primary motor regions exhibiting monosynaptic connections [(Fitch, 2011; Simonyan and Horwitz, 2011; Belyk and Brown, 2017), see also Figure 1]. This situation permits the production of a flexible vocal repertoire including speech and song. By contrast, core processes for laughter as a spontaneous form of emotional vocalization appear to be seated in subcortical regions. This tenet is supported by the observation that laughter is vividly produced in infants at an early age, namely, by the fourth month. Furthermore, patients with bilateral damage at sites of the motor cortex that are associated with vocal control lose the ability to sing and speak while retaining the capacity to produce non-verbal emotional utterances, such as moans, cries and laughter (Groswasser et al., 1988). The PAG is strongly interconnected with numerous brain regions that are associated with the limbic system, particularly with the lateral hypothalamus (Behbehani et al., 1988), which is intrinsically coupled with the emotional motor system (Holstege, 1992). Investigations in the squirrel monkey suggest that the hypothalamus furnishes the most substantial input to implement vocalizations that accompany emotional states (Dujardin and Jurgens, 2006). In the rat, the lateral hypothalamus is involved in vocalizations that are related to the expression of positive emotions (Burgdorf et al., 2007), notably to those that occur in the context of tickling and play (Roccaro-Waldmeyer et al., 2016). In humans, the lateral hypothalamus is activated during the processing of humor (Watson et al., 2007; Schwartz et al., 2008) and of ticklish laughter (Wattendorf et al., 2013). Both the hypothalamus and the PAG receive projections from the insula (Reep and Winans, 1982); the latter represents a primary cortical relay for tactile afferents that mediate affective information (Olausson et al., 2002). The functions of the anterior insula (AI) are believed to be implicated in the triggering of human laughter (Watson et al., 2007; Holstege and Subramanian, 2016). Interestingly in this context, the AI not only senses the physiological condition of the body and related feelings, but also estimates the impact of an upcoming stimulation on this bodily state (Craig, 2002, 2009). Furthermore, the AI is believed to form a part of a limbic-related processing network that produces an affective state in response to the emotional significance of a stimulus, which is then automatically relayed to regulate emotional behavior [(Phillips et al., 2003), see also Figure 1]. Along these lines, activity in this cortical region occurs during the experience of tickling or a pleasant touch and during the anticipation of these sensations (Lovero et al., 2009; Lucas et al., 2015). However, a possible involvement of downstream regions, such as the hypothalamus and the PAG, has not been considered thus far. Our own investigations have permitted us to demonstrate activity that is associated with ticklish laughter in the AI/hypothalamic/PAG axis (Wattendorf et al., 2013; Wattendorf et al., 2016). However, in these former studies, no attempt was made to locate the sites of activity that are associated with the preceding anticipatory processes. It was with this aim in view that the present study was conducted. The partial brain volume targeted included the three aforenamed key nodes of the emotional motor system. By focusing on the AI, the hypothalamus and the PAG, we have now distinguished the processes of anticipation from those that are relevant during tickling.
Figure 1.
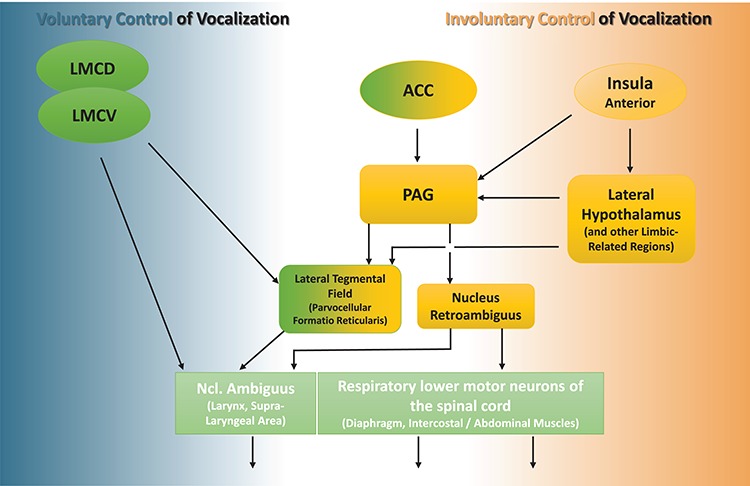
Simplified model of efferent pathways for vocal control in humans. Brain regions are indicated in yellow when bearing reference to, or representing the emotional motor pathway, in green when being assigned to the voluntary motor pathway, and in lime-green when relating to the effectors for vocal output. The PAG is crucial for the control of emotional vocal expression via its connections with the nucleus ambiguus (laryngeal effectors) and the nucleus retroambiguus [respiratory/vocal coordination (Jurgens, 2002; Holstege and Subramanian, 2016; Belyk and Brown, 2017)]. The PAG-activity is driven by the hypothalamus, which furnishes the main input for spontaneous vocal reactions (Dujardin and Jurgens, 2006). The PAG is also a target of efferences from the ACC. The ACC is an evolutionarily older cortical site, which appears to support a directed expression of vocalization (Jurgens, 1998). In humans, the functions of the dorsal ACC include emotional intonations that are superimposed on the production of volitional speech (Barrett et al., 2004; Aziz-Zadeh et al., 2010; Belyk and Brown, 2016; Dichter et al., 2018). The AI cortex senses the current affective state and automatically relays the corresponding information to the emotional motor system (Phillips et al., 2003). The ventral laryngeal motor cortex (LMCV) is the homologue of the non-human primate LMC, whereas the dorsal laryngeal motor cortex (LMCD) is unique in the human primary motor cortex. These primary motor cortical regions extend the potential of voluntary vocal control, permitting flexible speech and song, notably via the reticular formation of the lower brainstem and by direct connections with laryngeal effectors in the nucleus ambiguus (Fitch, 2011; Simonyan and Horwitz, 2011; Belyk and Brown, 2017).
List of Abbreviations
A condition anticipation of tickling
ACC anterior cingulate gyrus
AI anterior insula
AID anterior insula, dorsal
AIV anterior insula, ventral
BOLD Blood-oxygen-level-dependent
EPI echo planar imaging
C condition foot contact (touching)
fMRI functional magnetic resonance imaging
FWE family-wise error
PGI globus pallidus internal segment
PAG periaqueductal gray matter
HYP hypothalamus
LMCD laryngeal motor cortex, dorsal
LMCV laryngeal motor cortex, ventral
MI mid insula
MNI Montreal Neurological Institute
NAC nucleus accumbens
PI posterior insula
ROI region of interest
SVC small volume correction
SPM statistical parametric mapping
T tickling
Thalamus A thalamus nuclei anteriores
Thalamus CM thalamus nucleus centromedianus
Thalamus VA thalamus nucleus ventralis anterior
Thalamus VL thalamus nucleus ventralis lateralis
Thalamus VP thalamus nucleus ventralis posterior
Materials and methods
Subjects
Among the 43 healthy participants, 31 (21 females and 10 males; mean age: 24.3 years; age range: 20–29 years) were included in the functional magnetic resonance imaging (fMRI) study. Three individuals were discarded due to technical constraints during the acquisition process. Nine individuals were excluded from the evaluation because their head movements consistently exceeded the limit of head motion, as revealed by the statistical parametric mapping (SPM) re-alignment procedure (see Supplementary Information: Participants). The informed consent of all participants was obtained, and the procedure was approved by the Ethical Committee of the University Hospital of Greifswald, Germany (BB63/10).
Behavioral data
An fMRI-adapted fiber-optic microphone (MR confon, Magdeburg, Germany) was used to record laughter during the scanning procedure. The audacity® software 2.1.1 (Audacity Team, www.audacityteam.org) was implemented to evaluate the intensity of the audible signal. Laughter always involves a strong expiratory component, which, in its weaker form, may be produced without articulation. The occurrence of a strong expiration without an accompanying audible vocalization was thus classified as an expiratory bout of laughter. Bursts of laughter that consisted of only one audible articulation were defined as weak; those involving several (>1) audible articulations that were produced in rapid succession were defined as strong. A further subdivision of the latter category was not possible, since strong bouts of laughter often ended in undefined audible phenomena. According to this approach, the intensity of laughter was assessed in an escalating form, which may reflect the underlying neuronal processes: in primates, the intensity of a single burst of vocalization is correlated with neuronal activity in the PAG, which is the critical region of its initiation (Larson, 1991). Existing publications report on the correlation between the physical magnitude of the specific sensory stimuli and the exponential neuronal responses in the lower range of the perceived phenomenon (Molski, 2011). Considering that in the present investigation we frequently reported expiratory responses without an accompanying audible vocalization (weak reaction, see Results), we here simplified the predicted exponential trend of the neuronal response that was elicited and coded the different intensities of bouts of laughter with exponential weights (expiratory laughter = 1×, weak laughter = 2×, strong laughter = 4×). For each participant, maximally 15 scores for the 15 tickling events were determined in this way. Calculation of the sum of these scores permitted a classification of the total vocal response that was produced during the tickling periods in terms of both the frequency and the intensity of the audible events. This value was used for the correlation analysis of this parameter with the anticipatory or the tickling-related activity. After the fMRI session, the participants completed a questionnaire in which they were requested to rate the mean sensation of tickling and the perceived pleasantness on a visual analogue scale, ranging from 1–10, with 1 being the lowest score (no sensation of tickle or pleasantness) and 10 the highest. Statistical analyses were conducted using SPSS 21 (IBM) and Excel (Microsoft).
Experimental fMRI design
We investigated the selected brain volume in the framework of an event-related paradigm with the following three conditions: (i) tickling of the right foot (T) by a friend or his/her partner. In this condition, the participant was encouraged not to suppress the audible vocalizations (Wattendorf et al., 2013). (ii) Monotonous foot contacts (C). (iii) Anticipation (A), which precedes the T or the C stimulus (Figure 2). Upcoming episodes of T or C were visually indicated by the same ‘smiley face’. To prevent habituation of the person being tickled, the T and the C stimulations were executed with a variable delay (jitter) of up to 4.9 s (mean: 2.45 s; SD: 1.5), which represents the anticipatory activity (A). A jittering of successive stimuli also improves the evaluation accuracy in event-related designs (Dale, 1999). A red bar, which was visible only to the tickler, was randomly superimposed on the left or the right side of the screen, at the end of the anticipatory period, and served as the signal to begin the episode of tickling (T) or touching (C). Each of these two conditions (T and C) was presented 15 times, each with a duration of 6.2 s and alternated with a baseline condition (11.2 s) in which participants had to look at a fixation cross. Technical and methodological difficulties are frequently encountered during the testing of a paradigm that is associated with physical motions. To minimize head movements and the associated artefacts in the fMRI signal, the participants held a wooden barbecue stick between their teeth, which did not interfere with the production of laughter (Wattendorf et al., 2013; Wattendorf et al., 2016). Since this procedure involves an exertion of the facial muscles during the entire scanning session, it may account for confounding brain activity due to smiling (Hennenlotter et al., 2005) during T.
Figure 2.
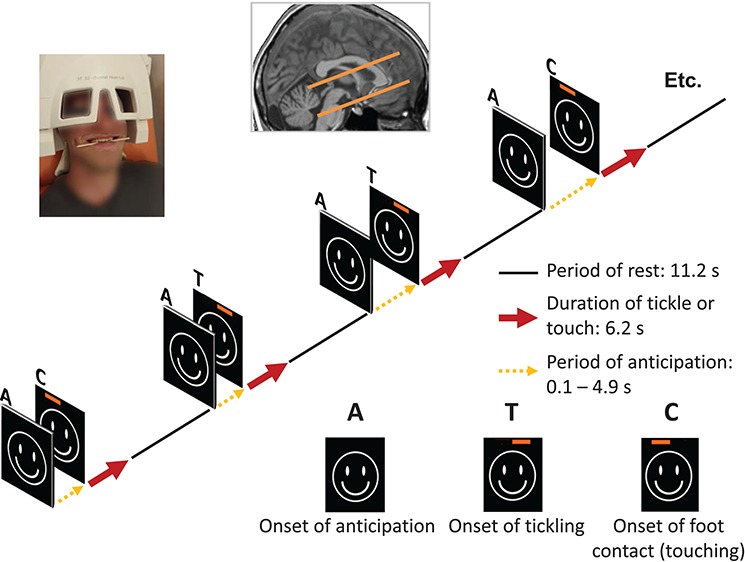
Experimental design. During the fMRI-scanning procedure, the participants experienced two different sensory stimulations, which were randomly applied: simple contact (C) or tickling (T) of the right foot. A visual cue signalized the upcoming stimulation, which followed only after a variable delay—the phase of anticipation (A)—of 0.1 to 4.9 s. To secure the unpredictability of the situation to the tickled person, the nature of the stimulation (tickling or touching) was signalized to the tickler alone by a superimposed red bar on the screen at the onset. The corresponding neuronal activity was measured in a defined brain volume, which included the AI, the hypothalamus and the PAG. A picture of a participant has been included, demonstrating the experimental set-up that was used.
Acquisition of data
Imaging was performed on a 3 T Scanner (VERIO, Siemens, Erlangen, Germany) with a 12-channel head-coil. Functional images were acquired using a T2*-weighted echo planar imaging (EPI) sequence [repetition time (TR): 2 s; echo time (TE): 30 ms; flip angle [α]: 70°], which embraced the anterior insula, the hypothalamus and the PAG in 20 interleaved axial slices (resolution: 1.73 × 1.73 × 2 mm3; matrix: 64 × 64 × 20 voxels; total acquisition time: 608 s). The frontal and the occipital poles were not included in this brain volume (see Figure 1). A phase-oversampling strategy was implemented to avoid folding artifacts. To minimize susceptibility artifacts, images were additionally tilted by 30o relative to the anterior/posterior commissure [AC-PC line (Robinson et al., 2004)]. For a gradient-echo field mapping, 34 phase and magnitude images were acquired [TR: 488 ms; TE(1): 4.92 ms; TE(2): 7.38 ms; [α]: 60ο; resolution: 1.73 × 1.73 × 2 mm3]. A T1-weighted, three-dimensional magnetization-prepared rapid gradient echo (MPRAGE) sequence (TR: 1900 ms; TE: 2.52 ms; [α]: 9°; voxel size: 1 × 1 × 1 mm3; matrix: 256 × 256 × 176 voxels; 176 sagittal slices) was used to obtain structural images of the brain anatomy.
Analysis of data
Data were analyzed using SPM8 software (Wellcome Department of Cognitive Neuroscience, London, England) (Friston et al., 1995), running on Matlab version R2012a (MathWorks Inc, Natick, MA).
The pre-processing procedure involved an unwarping of the geometrically distorted functional images (EPIs) in the phase-encoding direction using the FieldMap toolbox for SPM8. After slice-timing correction, each of the 304 individual volumes was re-aligned to the one which displayed half-maximal displacement (as indicated by the translation or rotation parameters with the highest maximal deviation) to correct for motion artifacts. Slow-signal drifts were eliminated using a temporal high-pass filter (128 s). Each EPI was co-registered with the T1-weighted anatomical image. The co-registered T1-image was segmented and normalized to the Montreal Neurological Institute (MNI) template; EPIs were resliced at 1.73 × 1.73 × 2 mm3. The resulting EPIs were smoothed with a 6 × 6 × 6 mm Gaussian Kernel filter (full-width at half maximum).
First-level analysis: to control for variance due to motions of the head, movement parameters that were estimated during the re-alignment procedure were introduced as regressors into the general linear model. The fMRI data were additionally adjusted for artifacts by a reduced weighting of motion-contaminated volumes using the RobustWLS toolbox (Diedrichsen and Shadmehr, 2005). An event-related analysis was conducted to separately identify the regions of the brain that were activated by the anticipation (A) and the tickling (T) stimuli in each subject. A reaction and neuronal conduction time of 400 ms after the request to effectively tickle was included in the modeling, which is shorter than the period that was implemented in our previous investigation (1 s), in which the participants could not prepare in advance (Wattendorf et al., 2013). We proceeded in the same way for the evaluation of the C condition. Contrast images were calculated to describe the comparisons T > A and T > C.
Second-level analysis: a hypothesis-driven SPM analysis was undertaken in a region of interest (ROI) that comprised the bilateral anterior insular cortex (AI), the midbrain and the bilateral hypothalamus. The anterior insular cortex (AI) was included with a view to relating regional activation patterns with the monitored and corresponding affective state (Phillips et al., 2003). The midbrain and the hypothalamus were included with a view to revealing the impact thereon of the behavioral reaction (Dujardin and Jurgens, 2006). The AI was defined according to data that are presented in the Anatomical Atlas of Neuromorphometrics [SPM (http://neuromorphometrics.com)]. The midbrain/hypothalamus was defined according to the Atlas of the Automated Anatomic Labeling (AAL) toolbox (Tzourio-Mazoyer et al., 2002). Functional maps appertaining to the conditions anticipation (A), tickling (T) and monotonous foot contacts (C) as well as to T vs A and T vs C were analyzed in the predefined ROI volume, based on a small volume correction (SVC) at the voxel level. Voxels were considered to be significant if they survived a family-wise-error (FWE)-corrected threshold (P < 0.05) that was adjusted for the small volume. With a view to classifying activities in accordance with the results of our previous investigation on ticklish laughter (Wattendorf et al., 2013), the described evaluations were complemented with an explorative analysis of the brain volume which has been measured. Thus, in addition to activity in the AI, the hypothalamus and the PAG, that in the mid- and the posterior portions of the insular cortex, the basal ganglia, the thalamus, the anterior lobe of the cerebellum and portions of the parietal, the temporal and the frontal lobes were also evaluated. In this instance, the results for T, A and C, as well as for T vs A and T vs C, were reported at a significance threshold of P < 0.05, which was FWE-corrected (voxel level) for the multiple comparisons involving the measured brain volume.
A linear-regression analysis (implemented in SPM8) permitted a correlation between each participant’s total (intensity-weighted) vocal output in response to tickling and the corresponding beta values (effect size) of the anticipation (A) and the tickling (T) events. The results are reported for the two aforementioned ROIs (anterior insula and midbrain/hypothalamus) after an SVC analysis with a corrected threshold of P (FWE) < 0.05 at the voxel level that was adjusted for the small volume.
The anatomical locations of significant areas of activation were identified using the SPM Anatomy Toolbox [(version 21 (Eickhoff et al., 2005)] and the Atlas of the Human Brain (Mai et al., 2004). The posterior and the mid, as well as the dorsal and the ventral anterior regions of the insular cortex, were distinguished according to the labeling patterns that were reported by Wager and Barrett (2004). To assess a potential impact of motion artifacts on the BOLD signal, scan-to-scan frame displacements (calculated by the sum of the six motion parameters for head movements combined over all volumes) were introduced as the regressor [see (Power et al., 2012; Yan et al., 2013)] in a further analysis of the A and the T conditions (P < 0.001, uncorrected).
Results
Behavior
Six of the 31 participants manifested an audible response to each of the 15 episodes of tickling; four exhibited no reaction whatsoever (median: 11). The intensity of an event of ticklish laughter was gauged as ‘strong’ (number of events: 0–15; median: 3), ‘weak’ (number of events: 0–4; median: 0) or ‘expiratory’ (number of events: 0–12; median: 3). The differences in intensity were taken into account in calculating the amount of laughter that was produced by each participant (see Methods). The average aggregated score per subject was 24.2 (‘strong’ laughter: 4.71, weighted as 18.84; ‘weak’ laughter: 0.81, weighted as 1.62; ‘expiratory’ laughter: 3.74). With respect to this parameter, no significant differences were observed between males and females (Mann–Whitney test; P = 0.44). The participants rated the stimulus as ‘pleasant’ [mean score: 7.18 (SD: ±1.9) of 10; no significant gender differences] and as ‘rather ticklish’ [mean score: 5.35 (SD: ±2.1) of 10; no significant gender differences]. Ticklishness correlated with the amount of laughter that was produced (Pearson’s test: r = 0.477, P = 0.007).
FMRI results
The brain regions that were activated in anticipation of tickling (A) and those that were associated with tickling (T) could be clearly distinguished by their extent of activation in the insular cortex of both hemispheres. A was associated with activation in the anterior insula (AI), whereas T involved additional activity in its posterior (PI) and mid (MI) portions (Figures 3 and 4, Table 1, Supplementary Table S2). The anticipatory situation (A) was associated with significant activation in additional sites: the bilateral nucleus accumbens (extending into the putamen in the left hemisphere), the posterior lateral hypothalamus in the left hemisphere and the ventral tegmental area. During tickling (T), activity was likewise detected in the nucleus accumbens (extending from the putamen and the pallidum) and the lateral hypothalamus, but also in the thalamus, the amygdala, the anterior cingulate cortex (ACC), the PAG, the brainstem tegmentum and the cerebellum in both hemispheres. The activity in the PAG appears to be localized in two longitudinally oriented columns ventrolateral (Bandler and Shipley, 1994) to the midbrain aqueduct (see Supplementary Figure S2). Activation of the ACC was significant in the direct comparisons T > A and T > C and that of large portions of the pallidum in the comparison T > A (see Supplementary Table S2). A closer inspection of the data confirmed an attenuated fMRI signal in the bilateral internal segments of the globus pallidus during A (24-10-4; z = 6.30/-24-8-6; z = 6.26, see Supplementary Figure S3). An analysis of the activity relating to monotonous foot contacts (C) revealed the response to tickling to be distinct from non-ticklish touch (see Supplementary Figure S1, Tables S1 and S2). In particular, the involvement of subcortical regions was specific for the tickling but not for the touching condition. By contrast, during both forms of somatosensory stimulation, the right posterior superior temporal sulcus was involved, which points to the social dimension of both situations (Beauchamp, 2015).
Figure 3.
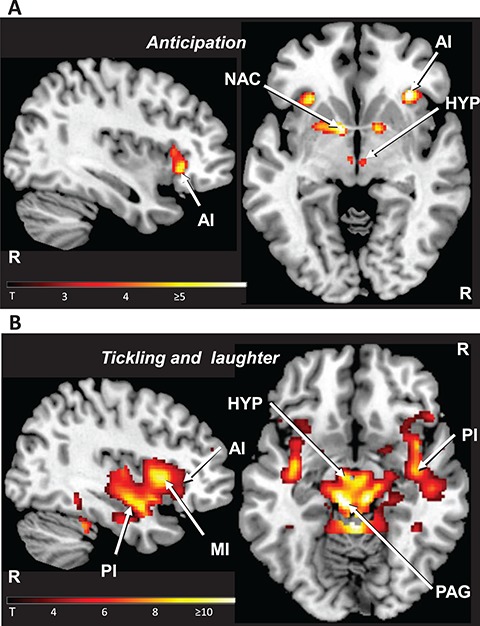
Group-related activity during tickling and its anticipation. Panel A: the anticipation of the affective stimulation (A) involved the AI, the nucleus accumbens (NAC) and the hypothalamus (HYP). In the illustrated example, the level of significance for threshold activity was set at P < 0.001 (uncorrected). Panel B: tickling and the accompanying laughter (T) activated the mid (MI) and the posterior (PI) regions of the insula, the hypothalamus (HYP) and the PAG (P < 0.001; uncorrected). Activation of the nucleus accumbens as well as of the anterior insular cortex is not represented. The right hemisphere (R) is indicated.
Figure 4.
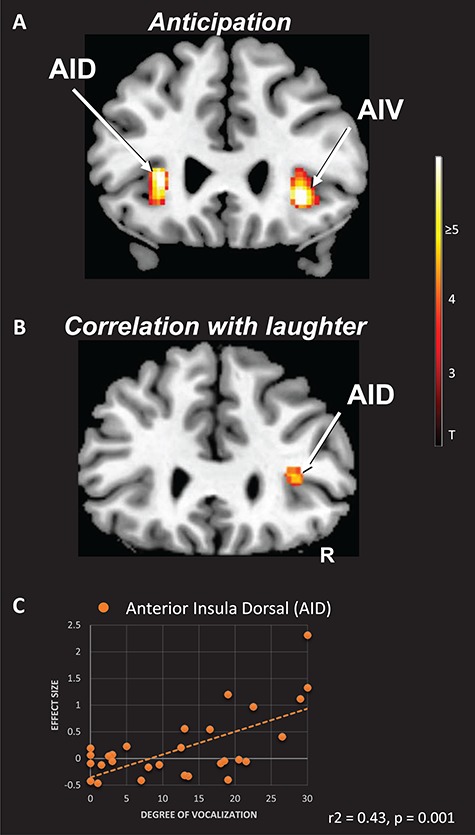
Anticipatory and tickling-associated activity correlating with laughter. Panel A: the AI cortex of both hemispheres was activated during the anticipation of tickling (A). In the illustrated example (P < 0.001; uncorrected), activation of the AI in the right hemisphere involved not only the ventral (AIV), but also the dorsal (AID) portions. Panel B: anticipatory (A) activity in the anterior dorsal insula (AID) predicts the laughter reaction during tickling (P < 0.001; uncorrected). Panel C: the scatterplot shows the relationship between the beta values (effect size) that were derived from the peak of activity in the AID of the right hemisphere and the degree of laughter (summed score of the weighted laughter bursts) that was emitted during tickling (r2 = 0.43, P = 0.001). The right hemisphere (R) is indicated.
Table 1.
Main effects of tickling (T) and of anticipation (A) and correlating activity of (A) and of (T) with the laughter that was produced during (T)
| Activated brain region |
MNI Coordinates (peak x y z)/ Cluster size (voxels) |
Z-score | |||||
|---|---|---|---|---|---|---|---|
| Main effects | Side | T | A | T | A | ||
| Periaqueductal gray ext. into Midbrain tegmentum Cond. (A): ventral tegmental area |
R | 9-32-22 | 10357 | 0-25-22 | 6.92 | 3.94* | |
| L | −5-31-10 | 10357 | 7.54 | ||||
| Lateral hypothalamus | R | 5-15-12 | 10357 | 6.57 | |||
| L | −5-13-12 | 10357 | −5-15-8 | 6.58 | 3.87* | ||
| Posterior insula (gyri longi insulae) |
R | 38-1-16 | 10357 | 6.57 | |||
| L | −38-6-16 | 578 | 6.67 | ||||
| Mid-insula (gyri breves insulae) | R | 40 13 2 | 10357 | 6.96 | |||
| L | −42 4 2 | 10357 | 6.69 | ||||
| Dorsal anterior insula (gyri breves insulae) |
R | a | |||||
| L | −31 25 6 | 10357 | −30 25 6 | 64 | 5.52 | 5.45 | |
| Ventral anterior insula (gyri breves insulae) |
R | 33 18-8 | 33 26-4 | 37 | 3.97* | 5.58 | |
| L | −35 23-8 | 4.25* | |||||
| Inferior frontal gyrus/lateral orbital gyrus/piriform cortex | R | 29 11-16 | 10357 | 4.82 | |||
| L | −30 9-16 | 39 | 5.74 | ||||
| Inferior frontal gyrus (pars opercularis) |
R | 50 16-2 | 10357 | 6.85 | |||
| L | −50 18-4 | 10357 | 5.50 | ||||
| Thalamus (VA, VL, VP,CM, A) |
R | 12-24 4 | 10357 | 5.55 | |||
| L | −17-15 6 | 10357 | 5.96 | ||||
| Pallidum/putamen | R | 15 2-4 | 10357 | 5.50 | |||
| L | −17-5 0 | 10357 | 5.70 | ||||
| Nucleus accumbens | R | 10357 | 10 7-2 | 13 | a | 4.98 | |
| L | 10357 | −11 4-4 | 44 | a | 5.79 | ||
| Amygdala/hippocampus | R | 29-6-24 | 10357 | 5.18 | |||
| L | −29-8-24 | 578 | 5.66 | ||||
| Cerebellum (lobus anterior) | R/L | 0-43-14 | 10357 | 7.43 | |||
| Post. superior temporal sulcus | R | 52-20-6 | 10357 | 6.58 | |||
| Laughter correlation | |||||||
| Dorsal anterior insula | R | 34 30 8 | 3.98* | ||||
| Midbrain tegmentum extending to periaqueductal gray |
R | 10-29-6 | 46 | 3.73* | |||
FMRI-activation sites were thresholded at P < 0.05 (FWE) corrected for multiple comparisons over the measured brain volume (voxel level). Moreover, according to the a priori hypothesis, a small volume correction (SVC) analysis was performed centered on the anterior insula, the hypothalamus and the midbrain (voxel level; P < 0.05; FWE corrected). Activations that were significant with the SVC analysis only are marked with *. Significant activations without a local maximum are indicated with a. Cluster sizes referring to multiple brain regions are italicized. Coordinates of maximal activation are displayed in MNI stereotactic space.
The laughter score correlated positively with anticipatory activity in the dorsal AI on the right side (Figure 4, Table 1). In the right midbrain tegmentum, it was related to episodes of tickling (Table 1).
The quality control revealed that when the individual summed motion-frame-displacement values were implemented in a second-level regression analysis, only a few voxel values attained statistical significance for condition T (cerebellum: z = 3.52, voxels = 6; putamen: z = 3.22, voxels = 6) and none for condition A. Thus, a major confounding effect of motion on the results can be excluded.
Discussion
In our previous studies (Wattendorf et al., 2013; Wattendorf et al., 2016), the experimental set-up did not differentiate between anticipation of tickling (A) and tickling itself (T). It now appears that activity in the AI and in the hypothalamus is associated with A and with T, while that in the mid-posterior portions of the insula (MI, PI) and in the PAG is primarily involved in the T-condition. These findings accord with those of previous investigations addressing the brain representation of affective touch (Lovero et al., 2009; Lucas et al., 2015), namely, as appertaining to the distribution of anticipatory and stimulus-related activity in the insular cortex. However, in these former studies, no activity was revealed in the hypothalamus and the PAG, two subcortical centers that participate in an imminent behavioral response (Mobbs et al., 2007). It is conceivable that, during the anticipation of tickling, an internal representation of the expected consequences is used and combined in a modified reaction to the actual stimulation.
The anticipation of tickling is associated with neuronal activity in the anterior insula
The posterior insular cortex contains primary interoceptive afferences that the anterior portions integrate with cognitive and limbic-related information (Craig, 2002; Critchley, 2005; Craig, 2009). This process represents the basis of emotional experiences and has been associated with a broad spectrum of functional conditions (Kurth et al., 2010). The anterior insular cortex (AI) is believed to comprise a portion of the neuronal network that produces an affective state corresponding to an emotional stimulation (Phillips et al., 2003). Interestingly in this context, an anticipation of the averse consequences of a stimulus invariably leads to an activation of the AI and, at the same time, is associated with autonomous excitement and behavioral changes (Etkin and Wager, 2007). Indeed, the prediction of an affective state in the absence of a peripheral input appears to be a key role of the AI (Paulus and Stein, 2006). This observation may also apply to tickling or a pleasant touch: although activity in the AI has been observed during the sensory stimulation itself (Carlsson et al., 2000; Morrison, 2016), this region appears to be specifically implicated in paradigms that signal an upcoming experience of the related bodily sensations (Lovero et al., 2009). Our data support the implication that the AI is involved in the anticipation of tickling (A). The functions that involve its right ventral portion permit the drawing of further conclusions.
One of the characteristics of tickling is that during the act itself, it is re-interpreted as a harmless stimulus. The continuous up-dating of information appertaining to the actual situation is assumed to be supported by the von Economo neurons (VEN) in the ventral portion of the AI (von Economo, 1926), which have been implicated in the rapid, highly integrated representations of an emotional experience (Craig, 2009). In a previous study, the mechanism that underlies the recognition and the re-appraisal of humorous stimuli has indeed been explained by this process (Watson et al., 2007). The ventral AI [agranular, see (Evrard et al., 2014)] is functionally involved in an affective state that is generated according to an emotional rather than a cognitive input (Kurth et al., 2010). This region appears to be the seat of feelings that are not defined or interpreted (Wager and Barrett, 2004), such as those that are associated with the experience of one of the basic emotions (Damasio et al., 2000) or a state of intensified drive [e.g. the craving for food (Pelchat et al., 2004)]. The ventral AI also participates in the regulation of peripheral physiological changes that are related to affective states (Mutschler et al., 2009). Notably, neuronal activity that is restricted to the right ventral AI signalizes higher sympathetic arousal (Critchley et al., 2000), as gauged, for example, by the concomitant change in skin conductance that occurs during the experience of positive emotions (Kuniecki et al., 2003). Moreover, in the rat, efferent sympathetic projections from the agranular and the dysgranular insular cortices have been traced (Cechetto and Chen, 1990; Yasui et al., 1991). This discovery serves as more than circumstantial evidence of the insula’s involvement in regulation via the sympathetic nervous system, which has been verified in humans: unexpected cardiac events following insular stroke are deemed to be associated with the sympathetic role of the right AI (Tokgozoglu et al., 1999). Hence, during the anticipation of tickling, the dextroventral AI possibly acts as a dynamic point of reference (Gu et al., 2013) in the automatic regulation of the laughter circuitry.
The anticipation of tickling is associated with neuronal activity in the lateral hypothalamus, the nucleus accumbens and the ventral tegmental area
An involvement of the lateral hypothalamus in human laughter was first suggested by findings in pathological situations (Martin, 1950), relating, in particular, to those in which its tuberolateral portions were lesioned (Valdueza et al., 1994) and has been confirmed by the results of our previous fMRI study (Wattendorf et al., 2013). The hypothalamus is anatomically connected to several regions of the brainstem, notably to the PAG (Holstege, 1987). It is deemed to stimulate the PAG to implement the motoric patterns of affective vocalizations (Dujardin and Jurgens, 2006). However, our investigation revealed that, in the anticipation of tickling (A) the posterior lateral hypothalamus, but not the PAG was activated. In this context, it is of interest that a substantial proportion of the neurons in the lateral hypothalamic area of rats and monkeys respond specifically to appetitive or aversive situations themselves rather than to associated sensory or motoric processes (Nakamura and Ono, 1986; Noritake and Nakamura, 2011). Also, the results of imaging studies in healthy individuals have confirmed the function of the (lateral) hypothalamus to be associated with the processing of the emotional valence of a stimulus (Karlsson et al., 2010). Interestingly, experiments in the squirrel monkey have revealed stimulation of the PAG to evoke changes in the intensity, but not in the specific acoustic parameters, such as rhythm or the articulation of vocalizations (Larson, 1991; Dusterhoft et al., 2004). This finding is compatible with a role of this region in initiating and gating rather than in elaborating the vocal response. Hence, any anticipatory influence of the hypothalamus on the PAG (e.g. on the intensity of the vocal response) would become manifest only during the actual vocalization. Moreover, during the anticipatory phase, respiration is being imperceptibly adjusted to prepare for laughter, a process that always occurs in the post-inspiratory expiratory phase (Dutschmann et al., 2014). By its influence on respiration (Redgate, 1963), the lateral hypothalamus could play an indirect role in regulating the laryngeal voice box, perhaps via vocal pattern generators in the parvicellular reticular formation (Van Daele and Cassell, 2009). In addition, our findings reveal the anticipation of tickling to involve the nucleus accumbens and the ventral tegmental area. These regions support not only the processing of emotionally salient or rewarding events in humans (Carter et al., 2009), but also the development of responses to predictive cues, at least in rats (Yun et al., 2004; Roitman et al., 2005).
Laughter-associated anticipatory activity involves the anterior insula
In those participants who manifested higher anticipatory activity in the dorsal AI of the left hemisphere, laughter was consistently more pronounced during the act of tickling (T). This finding implies an influence at a level of stimulus-encoding that relates to cognitive appraisal. In their meta-analysis, Wager and Barrett (2004) reported emotional situations in which undifferentiated affective states need to be translated into plans of action to be powerful predictors of activity in the dorsal AI [dysgranular, see (Evrard et al., 2014)]. This region is activated in situations that implicate specific avoidance or approach strategies, for example, those in which an individual is attentive to painful (Kong et al., 2006) or other incentive external stimuli (Anders et al., 2004). Analogously, in our study, those participants who attached more importance to, or consistently anticipated the sensory stimulation, were probably more ticklish and accordingly more prone to laughter. As an example, an investigation of the neuronal correlates of touch by Lovero et al. (2009) revealed anticipatory activity in the AI to be correlated with that in a more mid-to-posterior region that responds during stimulation. This process would thus directly enhance the sensory response, if coupled, in terminal fields for primary interoceptive afferents.
Tickling with accompanying laughter activates an emotional and sensorimotor network
In contrast to the anticipatory phase (A), the act of tickling itself (T) was associated with a more extensive pattern of activation in regions of the brain that included the PAG, the amygdala, the basal ganglia, the lateral hypothalamus and the insula. As far as this finding goes, our results confirm those of our earlier investigation on ticklish laughter (Wattendorf et al., 2013). However, in the present study, with an adapted paradigm, a more discriminative analysis was possible. Firstly, A and T likewise activated the anterior insular cortex (AI), whereas only the latter condition involved its mid-posterior portions (MI, PI). Evidence deriving from a variety of species indicates that the PI receives primary interoceptive information from the ventral posterior thalamus (Dum et al., 2009). This region not only associates the incoming information with subjective evaluation in the AI (Craig, 2009), but also projects directly to the same posterior thalamic region, as well as to the dorsolateral striatum (Cechetto and Saper, 1987; Chikama et al., 1997). In humans, decision-making processes related to actions that are relatively automatic or habitual are mediated by neuronal circuitries involving the sensorimotor cortices and the dorsolateral striatum/putamen (Balleine et al., 2007). Moreover, the suppression of activity in the internal segment of the globus pallidus during A, suggests that the inhibitory input from this region to the anterior and the ventrolateral thalamus (Haber, 2016) was restricted and that the motor-related functions of these thalamic regions were probably disinhibited. Hence, more direct and automatic processes may be implemented in the evocation of laughter. In this respect, our findings accord with existing data, which afford evidence that an involuntary (Ruch and Ekman, 2001) and ‘reflex-like’ (Martin, 1950) mechanism underlies the outburst of laughter in humans.
Secondly, we found that T activated the ACC, which has both autonomic and emotional regulatory functions (Mai and Paxinos, 2012). Referring to research on vocalizations in the squirrel monkey, the ACC is believed to initiate directed but not reflexive vocal behavior via its projections to the PAG (Jurgens, 2009). In humans, this region has been implicated in the volitional control of the affective components of vocalization (Hage and Nieder, 2016), for example, when angry manifestations are produced on command (Fruhholz et al., 2015). In our previous investigation, this region was not activated during T. However, in the present study, the entire epoch of T was investigated, whereas in the previous one, only the early response to T was considered. It is conceivable that during the ongoing stimulation, an awareness of the social dimension of the situation sets in, therewith involving control functions in the ACC. This social awareness may serve to regulate the magnitude of the vocal reaction (Larson, 1991) or, more subtly, to signalize social status by context-dependent frequency modulations (Pisanski et al., 2016). The extent to which ticklish laughter involves obligatory control by the cortical centers is currently unknown.
In the present study, the neuronal processes that are initiated by the anticipation of tickling could, for the first time, be distinguished from those that are evoked by the act of tickling itself. It is conceivable that in a natural (non-experimental) setting, with its accompanying menacing gestural components, the equivalent anticipatory activity would be more potent and specific, eliciting vocalizations such as screaming and laughter. Moreover, some of the participants may have exhibited a tendency to react in a purely expiratory manner to T whereas others may have vocalized intensively when exposed to a comparable stimulation. To improve the characterization of the triggering processes of ticklish laughter, future fMRI studies could investigate, in parallel, multiple physiological parameters, such as changes in skin conductance, with a view to correlating them with the rated audible expressions.
Conclusions
The pattern of activity that characterizes the anticipation of tickling was distinct from the one that is manifested during effective stimulation. This finding modifies and extends James’ contention (1890) that the same brain regions are involved during the expectation and the experience of an externally applied stimulus. Anticipation of tickling apparently evokes regulatory processes that are directly and indirectly coded in the emotional and the sensorimotor systems. In this way, the two pathways can be adjusted for laughter.
Funding
The present work was funded by the Swiss National Foundation (grant no. 31003A-144036).
Supplementary Material
Acknowledgement
We would like to thank Andrea Samson and Michael Mouthon for their helpful suggestions during the elaboration of the manuscript.
References
- Anders S., Lotze M., Erb M., Grodd W., Birbaumer N. (2004). Brain activity underlying emotional valence and arousal: a response-related fMRI study. Human Brain Mapping, 23(4), 200–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz-Zadeh L., Sheng T, and Gheytanchi A. (2010) Common Premotor Regions for the Perception and Production of Prosody and Correlations with Empathy and Prosodic Ability, Plos One, 5(1), e8759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine B.W., Delgado M.R., Hikosaka O. (2007). The role of the dorsal striatum in reward and decision-making. Journal of Neuroscience, 27(31), 8161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandler R., Shipley M. (1994). Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends in Neurosciences, 17(9), 379–389. [DOI] [PubMed] [Google Scholar]
- Barrett J., Pike G. B. and Paus T. (2004) The role of the anterior cingulate cortex in pitch variation during sad affect, European Journal of Neuroscience, 19(2), 458–64. [DOI] [PubMed] [Google Scholar]
- Beauchamp M.S. (2015). The social mysteries of the superior temporal sulcus. Trends in Cognitive Sciences, 19(9), 489–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behbehani M.M., Park M.R., Clement M.E. (1988). Interactions between the lateral hypothalamus and the periaqueductal gray. Journal of Neuroscience, 8(8), 2780–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyk M., Brown S. (2016). Pitch underlies activation of the vocal system during affective vocalization. Social Cognitive and Affective Neuroscience, 11(7), 1078–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyk M., Brown S. (2017). The origins of the vocal brain in humans. Neuroscience and Biobehavioral Reviews, 77, 177–93. [DOI] [PubMed] [Google Scholar]
- Burgdorf J., Wood P.L., Kroes R.A., Moskal J.R., Panksepp J. (2007). Neurobiology of 50-kHz ultrasonic vocalizations in rats: electrode mapping, lesion, and pharmacology studies. Behavioural Brain Research, 182(2), 274–83. [DOI] [PubMed] [Google Scholar]
- Carlsson K., Petrovic P., Skare S., Petersson K., Ingvar M. (2000). Tickling expectations: neural processing in anticipation of a sensory stimulus. Journal of Cognitive Neuroscience, 12(4), 691–703. [DOI] [PubMed] [Google Scholar]
- Carter R.M., Maclnnes J.J., Huettel S.A., Adcock R.A. (2009). Activation in the VTA and nucleus accumbens increases in anticipation of both gains and losses. Frontiers in Behavioral Neuroscience, 3(21), pp. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechetto D.F., Chen S.J. (1990). Subcortical sites mediating sympathetic responses from insular cortex in rats. American Journal of Physiology, 258(1), R245–55. [DOI] [PubMed] [Google Scholar]
- Cechetto D.F., Saper C.B. (1987). Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. Journal of Comparative Neurology, 262(1), 27–45. [DOI] [PubMed] [Google Scholar]
- Chikama M., McFarland N.R., Amaral D.G., Haber S.N. (1997). Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. Journal of Neuroscience, 17(24), 9686–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A.D. (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience, 3(8), 655–66. [DOI] [PubMed] [Google Scholar]
- Craig A. (2009). How do you feel—now? The anterior insula and human awareness. Nature Reviews Neuroscience, 10(1), 59–70. [DOI] [PubMed] [Google Scholar]
- Critchley H.D. (2005). Neural mechanisms of autonomic, affective, and cognitive integration. Journal of Comparative Neurology, 493(1), 154–66. [DOI] [PubMed] [Google Scholar]
- Critchley H.D., Elliott R., Mathias C.J., Dolan R.J. (2000). Neural activity relating to generation and representation of galvanic skin conductance responses: a functional magnetic resonance imaging study. Journal of Neuroscience, 20(8), 3033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M. (1999). Optimal experimental design for event-related fMRI. Human Brain Mapping, 8(2–3), 109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio A.R., Grabowski T.J., Bechara A., et al. (2000). Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience, 3(10), 1049–56. [DOI] [PubMed] [Google Scholar]
- Dichter B. K., Breshears J. D., Leonard M. K. and Chang E. F. (2018) The Control of Vocal Pitch in Human Laryngeal Motor Cortex, Cell, 174(1), 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J., Shadmehr R. (2005). Detecting and adjusting for artifacts in fMRI time series data. NeuroImage, 27(3), 624–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin E., Jurgens U. (2006). Call type-specific differences in vocalization-related afferents to the periaqueductal gray of squirrel monkeys (Saimiri sciureus). Behavioural Brain Research, 168(1), 23–36. [DOI] [PubMed] [Google Scholar]
- Dum R.P., Levinthal D.J., Strick P.L. (2009). The spinothalamic system targets motor and sensory areas in the cerebral cortex of monkeys. Journal of Neuroscience, 29(45), 14223–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusterhoft F., Hausler U., Jurgens U. (2004). Neuronal activity in the periaqueductal gray and bordering structures during vocal communication in the squirrel monkey. Neuroscience, 123(1), 53–60. [DOI] [PubMed] [Google Scholar]
- Dutschmann M., Jones S.E., Subramanian H.H., Stanic D., Bautista T.G. (2014). The physiological significance of postinspiration in respiratory control. Breathing, Emotion and Evolution, 212, 113–30. [DOI] [PubMed] [Google Scholar]
- von Economo C. (1926). A new type of special cells of lobus cinguli and lobus insulae. Zeitschrift fur die Gesamte Neurologie und Psychiatrie, 100, 704–10. [Google Scholar]
- Eickhoff S.B., Stephan K.E., Mohlberg H., et al. (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage, 25(4), 1325–35. [DOI] [PubMed] [Google Scholar]
- Etkin A., Wager T.D. (2007). Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry, 164(10), 1476–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard H.C., Logothetis N.K., Craig A. (2014). Modular architectonic organization of the insula in the Macaque monkey. Journal of Comparative Neurology, 522(1), 64–97. [DOI] [PubMed] [Google Scholar]
- Fitch (2011). The evolution of syntax: an exaptationist perspective. Frontiers in Evolutionary Neuroscience, 3, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Holmes A.P., Worsley K.J., Poline J.-P., Frith C.D., Frackoviak R.S.J. (1995). Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping, 2, 189–210. [Google Scholar]
- Fruhholz S., Klaas H.S., Patel S., Grandjean D. (2015). Talking in fury: the cortico-subcortical network underlying angry vocalizations. Cerebral Cortex, 25(9), 2752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groswasser Z., Korn C., Groswasserreider I., Solzi P. (1988). Mutism associated with buccofacial apraxia and bihemispheric lesions. Brain and Language, 34(1), 157–68. [DOI] [PubMed] [Google Scholar]
- Gu X.S., Hof P.R., Friston K.J., Fan J. (2013). Anterior insular cortex and emotional awareness. Journal of Comparative Neurology, 521(15), 3371–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S. (2016). Perspective on basal ganglia connections as described by Nauta and Mehler in 1966: where we were and how this paper effected where we are now. Brain Research, 1645, 4–7. [DOI] [PubMed] [Google Scholar]
- Hage S.R., Nieder A. (2016). Dual neural network model for the evolution of speech and language. Trends in Neurosciences, 39(12), 813–29. [DOI] [PubMed] [Google Scholar]
- Hennenlotter A., Schroeder U., Erhard P., et al. (2005). A common neural basis for receptive and expressive communication of pleasant facial affect. NeuroImage, 26(2), 581–91. [DOI] [PubMed] [Google Scholar]
- Holstege G. (1987). Some anatomical observations on the projections from the hypothalamus to brain-stem and spinal-cord—an HRP and autoradiographic tracing study in the cat. Journal of Comparative Neurology, 260(1), 98–126. [DOI] [PubMed] [Google Scholar]
- Holstege G. (1992). The emotional motor system. European Journal of Morphology, 30(1), 67–79. [PubMed] [Google Scholar]
- Holstege G. (2014). The periaqueductal gray controls brainstem emotional motor systems including respiration. Central Nervous System Control of Respiration, 209, 379–405. [DOI] [PubMed] [Google Scholar]
- Holstege G., Subramanian H.H. (2016). Two different motor systems are needed to generate human speech. Journal of Comparative Neurology, 524(8), 1558–77. [DOI] [PubMed] [Google Scholar]
- James W. (1890). Principles of Psychology, New York: Henry Holt. [Google Scholar]
- Jurgens U. (1998). Neuronal control of mammalian vocalization, with special reference to the squirrel monkey. Naturwissenschaften, 85(8), 376–88. [DOI] [PubMed] [Google Scholar]
- Jurgens U. (2002) Neural pathways underlying vocal control, Neuroscience and Biobehavioral Reviews, 26(2), 235–258. [DOI] [PubMed] [Google Scholar]
- Jurgens U. (2009). The neural control of vocalization in mammals: a review. Journal of Voice, 23(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Karlsson K.A.E., Windischberger C., Gerstl F., Mayr W., Siegel J.M., Moser E. (2010). Modulation of hypothalamus and amygdalar activation levels with stimulus valence. NeuroImage, 51(1), 324–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J., White N.S., Kwong K.K., et al. (2006). Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Human Brain Mapping, 27(9), 715–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuniecki M., Urbanik A., Sobiecka B., Kozub J., Binder M. (2003). Central control of heart rate changes during visual affective processing as revealed by fMRI. Acta Neurobiologiae Experimentalis, 63(1), 39–48. [DOI] [PubMed] [Google Scholar]
- Kurth F., Zilles K., Fox P.T., Laird A.R., Eickhoff S.B. (2010). A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Structure & Function, 214(5–6), 519–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner H.K., Weiss E.M., Hinghofer-Szalkay H., Papousek I. (2014). Cardiovascular effects of acute positive emotional arousal. Applied Psychophysiology and Biofeedback, 39(1), 9–18. [DOI] [PubMed] [Google Scholar]
- Larson C.R. (1991). On the relation of PAG neurons to laryngeal and respiratory muscles during vocalization in the monkey. Brain Research, 552(1), 77–86. [DOI] [PubMed] [Google Scholar]
- Lauterbach E.C., Cummings J.L., Kuppuswamy P.S. (2013). Toward a more precise, clinically-informed pathophysiology of pathological laughing and crying. Neuroscience and Biobehavioral Reviews, 37(8), 1893–916. [DOI] [PubMed] [Google Scholar]
- Lovero K.L., Simmons A.N., Aron J.L., Paulus M.P. (2009). Anterior insular cortex anticipates impending stimulus significance. NeuroImage, 45(3), 976–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M.V., Anderson L.C., Bolling D.Z., Pelphrey K.A., Kaiser M.D. (2015). Dissociating the neural correlates of experiencing and imagining affective touch. Cerebral Cortex, 25(9), 2623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai J.K., Paxinos G. (2012). The Human Nervous System, London: Elsevier. [Google Scholar]
- Mai J.K., Assheuer J., Paxinos G. (2004). Atlas of the Human Brain, San Diego: Elsevier Academic Press. [Google Scholar]
- Martin J.P. (1950). Fits of laughter (sham mirth) in organic cerebral disease. Brain, 73(4), 453–64. [DOI] [PubMed] [Google Scholar]
- Mobbs D., Greicius M.D., Abdel-Azim E., Menon V., Reiss A.L. (2003). Humor modulates the mesolimbic reward centers. Neuron, 40(5), 1041–8. [DOI] [PubMed] [Google Scholar]
- Mobbs D., Petrovic P., Marchant J.L., et al. (2007). When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science, 317(5841), 1079–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molski M. (2011). Extended Stevens’ power law. Physiology & Behavior, 104(5), 1031–6. [DOI] [PubMed] [Google Scholar]
- Morrison I. (2016). ALE meta-analysis reveals dissociable networks for affective and discriminative aspects of touch. Human Brain Mapping, 37(4), 1308–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutschler I., Wieckhorst B., Kowalevski S., et al. (2009). Functional organization of the human anterior insular cortex. Neuroscience Letters, 463(2), 166. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Ono T. (1986). Lateral hypothalamus neuron involvement in integration of natural and artificial rewards and cue signals. Journal of Neurophysiology, 55(1), 163–81. [DOI] [PubMed] [Google Scholar]
- Noritake A., Nakamura K. (2011). Neuronal modulation in appetitive and aversive contexts in the primate lateral hypothalamus. Neuroscience Research, 71, E371-E. [Google Scholar]
- Olausson H., Lamarre Y., Backlund H., et al. (2002). Unmyelinated tactile afferents signal touch and project to insular cortex. Nature Neuroscience, 5(9), 900–4. [DOI] [PubMed] [Google Scholar]
- Paulus M.P., Stein M.B. (2006). An insular view of anxiety. Biological Psychiatry, 60(4), 383–7. [DOI] [PubMed] [Google Scholar]
- Pelchat M.L., Johnson A., Chan R., Valdez J., Ragland J.D. (2004). Images of desire: food-craving activation during fMRI. NeuroImage, 23(4), 1486–93. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Drevets W.C., Rauch S.L., Lane R. (2003). Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biological Psychiatry, 54(5), 504–14. [DOI] [PubMed] [Google Scholar]
- Pisanski K., Cartei V., McGettigan C., Raine J., Reby D. (2016). Voice modulation: a window into the origins of human vocal control? Trends in Cognitive Sciences, 20(4), 304–18. [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59(3), 2142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provine R.R. (2004). Laughing, tickling, and the evolution of speech and self. Current Directions in Psychological Science, 13(6), 215–8. [Google Scholar]
- Ramachandran V.S. (1998). The neurology and evolution of humor, laughter, and smiling: the false alarm theory. Medical Hypotheses, 51(4), 351–4. [DOI] [PubMed] [Google Scholar]
- Redgate E.S. (1963). Central factors in neural regulation—hypothalamic influence on respiration. Annals of the New York Academy of Sciences, 109(2), 606. [DOI] [PubMed] [Google Scholar]
- Reep R.L., Winans S.S. (1982). Efferent connections of dorsal and ventral agranular insular cortex in the hamster, Mesocricetus-Auratus. Neuroscience, 7(11), 2609–35. [DOI] [PubMed] [Google Scholar]
- Robinson S., Windischberger C., Rauscher A., Moser E. (2004). Optimized 3 T EPI of the amygdalae. NeuroImage, 22(1), 203–10. [DOI] [PubMed] [Google Scholar]
- Roccaro-Waldmeyer D.M., Babalian A., Muller A., Celio M.R. (2016). Reduction in 50-kHz call-numbers and suppression of tickling-associated positive affective behaviour after lesioning of the lateral hypothalamic parvafox nucleus in rats. Behavioural Brain Research, 298, 167–80. [DOI] [PubMed] [Google Scholar]
- Roitman M.F., Wheeler R.A., Carelli R.M. (2005). Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron, 45(4), 587–97. [DOI] [PubMed] [Google Scholar]
- Ruch W., Ekman P. (2001). The Expressive Pattern of Laughter, Tokyo: Word Scientific Publisher. [Google Scholar]
- Schwartz S., Ponz A., Poryazova R., et al. (2008). Abnormal activity in hypothalamus and amygdala during humour processing in human narcolepsy with cataplexy. Brain, 131, 514–22. [DOI] [PubMed] [Google Scholar]
- Scott S.K., Lavan N., Chen S., McGettigan C. (2014). The social life of laughter. Trends in Cognitive Sciences, 18(12), 618–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K., Horwitz B. (2011). Laryngeal motor cortex and control of speech in humans. The Neuroscientist, 17(2), 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokgozoglu S.L., Batur M.K., Topcuoglu M.A., Saribas O., Kes S., Oto A. (1999). Effects of stroke localization on cardiac autonomic balance and sudden death. Stroke, 30(7), 1307–11. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage, 15(1), 273–89. [DOI] [PubMed] [Google Scholar]
- Valdueza J.M., Cristante L., Dammann O., et al. (1994). Hypothalamic hamartomas—with special reference to gelastic epilepsy and surgery. Neurosurgery, 34(6), 949–58. [DOI] [PubMed] [Google Scholar]
- Van Daele D.J., Cassell M.D. (2009). Multiple forebrain systems converge on motor neurons innervating the thyroarytenoid muscle. Neuroscience, 162(2), 501–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Barrett L.F. (2004) From affect to control: functional specialization of the insula in motivation and regulation. Online publication at http://www.apa.org/pubs/databases/psycextra (c.f. http://dx.doi.org/10.1101/102368).
- Watson K.K., Matthews B.J., Allman J.M. (2007). Brain activation during sight gags and language-dependent humor. Cerebral Cortex, 17(2), 314–24. [DOI] [PubMed] [Google Scholar]
- Wattendorf E., Westermann B., Fiedler K., Kaza E., Lotze M., Celio M.R. (2013). Exploration of the neural correlates of ticklish laughter by functional magnetic resonance imaging. Cerebral Cortex, 23(6), 1280–9. [DOI] [PubMed] [Google Scholar]
- Wattendorf E., Westermann B., Lotze M., Fiedler K., Celio M.R. (2016). Insular cortex activity and the evocation of laughter. Journal of Comparative Neurology, 524(8), 1608–15. [DOI] [PubMed] [Google Scholar]
- Wild B., Rodden F.A., Grodd W., Ruch W. (2003). Neural correlates of laughter and humour. Brain, 126, 2121–38. [DOI] [PubMed] [Google Scholar]
- Yan C.G., Cheung B., Kelly C., et al. (2013). A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage, 76(1), 183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui Y., Breder C.D., Saper C.B., Cechetto D.F. (1991). Autonomic responses and efferent pathways from the insular cortex in the rat. Journal of Comparative Neurology, 303(3), 355–74. [DOI] [PubMed] [Google Scholar]
- Yun I.A., Wakabayashi K.T., Fields H.L., Nicola S.M. (2004). The ventral tegmental area is required for the behavioral and nucleus accumbens neuronal firing responses to incentive cues. Journal of Neuroscience, 24(12), 2923–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


