Abstract
Empathy relies on brain systems that support the interaction between an observer’s mental state and cues about the others’ experience. Beyond the core brain areas typically activated in pain empathy studies (insular and anterior cingulate cortices), the diversity of paradigms used may reveal secondary networks that subserve other more specific processes. A coordinate-based meta-analysis of fMRI experiments on pain empathy was conducted to obtain activation likelihood estimates along three factors and seven conditions: visual cues (body parts, facial expressions), visuospatial (first-person, thirdperson), and cognitive (self-, stimuli-, other-oriented tasks) perspectives. The core network was found across cues and perspectives, and common activation was observed in higher-order visual areas. Body-parts distinctly activated areas related with sensorimotor processing (superior and inferior parietal lobules, anterior insula) while facial expression distinctly involved the inferior frontal gyrus. Self- compared to other-perspective produced distinct activations in the left insula while stimulus- versus other-perspective produced distinctive responses in the inferior frontal and parietal lobules, precentral gyrus, and cerebellum. Pain empathy relies on a core network which is modulated by several secondary networks. The involvement of the latter seems to depend on the visual cues available and the observer's mental state that can be influenced by specific instructions.
Keywords: Pain empathy, fMRI, Meta-analysis, Activation Likelihood Estimate, Perspective-taking, Visual information
Introduction
Empathy is a multidimensional construct supported by distinct yet interacting neural networks (Decety & Jackson, 2004; Decety & Lamm, 2006; Shamay-Tsoory, 2011; Zaki & Ochsner, 2012; Morelli et al., 2015; De Waal & Preston, 2017; Tousignant et al., 2017). Researchers generally agree that empathy emerges from two major interacting components: an affective component and a cognitive component. The affective component of empathy, also labeled affective resonance (Decety & Meyer, 2008), affective empathy (Shamay-Tsoory, 2011; De Waal & Preston, 2017), experience sharing (Zaki & Ochsner, 2012) or affective sharing (Tousignant et al., 2017), can be broadly defined as vicariously experiencing other peoples’ sensorimotor states and emotions. The cognitive component of empathy, also known as cognitive empathy (De Waal & Preston, 2017), perspective taking (Decety & Jackson, 2006), self/other distinction (Tousignant et al., 2017) or mentalizing (Zaki & Ochsner, 2012), refers to the perspective of another person while maintaining a distinction between one’s own emotional state and that of the other. Thus, empathy is proposed by many to stem from the combined effect of these components and relies on various regulation mechanisms (Decety & Lamm, 2006; Decety et al., 2007; Eisenberg & Eggum, 2009; De Waal & Preston, 2017; Tousignant et al., 2017).
The neural correlates of empathy have been explored through a variety of functional neuroimaging paradigms in the last 15 years. The findings of this growing literature generally support the dichotomy between the affective and cognitive components of empathy (e.g. Nummenmaa et al., 2008; Shamay-Tsoory et al., 2009). On the one hand, tasks that aim to selectively engage the affective component of empathy are associated with several brain structures including the anterior cingulate cortex (ACC), thalamus, hypothalamus, amygdala, temporal pole, precentral gyrus (PreCG; i.e. primary motor cortex) and postcentral gyrus [PosCG; i.e. the primary and secondary somatosensory (SS) cortices; see Figure 5 in De Waal & Preston, 2017]. These brain structures are also associated with attentional, emotional, motivational and sensorimotor processes (Völlm et al., 2006; Lamm et al., 2007b; Nummenmaa et al., 2008). On the other hand, tasks that rely more on the cognitive component of empathy engage brain structures such as the ventromedial prefrontal cortex, dorsolateral prefrontal cortex, inferior parietal lobule (IPL), temporoparietal junction (TPJ), superior temporal gyrus (STG) and fusiform gyrus (see Figure 5 in De Waal & Preston, 2017). These structures are also associated with executive control, action representation, working memory and visuospatial processes (Völlm et al., 2006; Schnell et al., 2011; Bernhardt & Singer, 2012; De Waal & Preston, 2017). Additionally, tasks that engage the affective and the cognitive components of empathy have been associated with a common set of brain regions including the anterior midcingulate cortex (aMCC), supplementary motor area (SMA), cingulate motor area, anterior insula (AI) and inferior frontal gyrus (IFG; see Figure 5 in De Waal & Preston, 2017). This suggests that there might be a core network that is recruited across different empathy tasks. Additionally, differences in tasks used to probe empathy that can stem from differences of instructions or stimuli can also be associated with distinct and secondary networks (Bernhardt & Singer, 2012; Zaki & Ochsner, 2012; De Waal & Preston, 2017). The latter could be coherent with the fact that tasks tap differently in the affective or cognitive component of empathy.
Paradigms illustrating other’s pain (also termed vicarious pain, or more commonly referred to as pain empathy paradigms) are the most common method employed to examine neural networks underpinning empathy (for a discussion on the potential overinterpretation of the term empathy in such contexts, see Garcia-Larrea & Jackson, 2016). Typically, participants view static or dynamic visual stimuli of the limbs submitted to noxious stimulation (SS pain paradigms) or facial expressions of pain [emotional-communicative (EC) pain paradigms]. These various sensory and emotional visual stimuli may contribute to the differences found in the reported brain response across studies. For instance, Vachon-Presseau et al. (2012) showed that viewing facial expressions of pain, compared to the limbs submitted to noxious stimulation, triggered more activity in the midline frontal and parietal and amygdala, while the opposite contrast yielded more activity in sensorimotor regions. A recent meta-analysis (Xiong et al., 2019) confirmed that EC pain paradigms are related to structures in the frontal [i.e. IFG, middle frontal gyrus (MFG) and PreCG], temporal [i.e. middle temporal gyrus (MTG), STG and fusiform gyrus] and occipital [i.e. inferior (IOG) and middle occipital gyrus (MOG)] lobes, in addition to subcortical and limbic structures such as the thalamus, putamen, AI, amygdala and the anterior cingulate. Another factor that varies in the use of stimuli across SS pain paradigms is the visuospatial orientation that is either from one’s own (first-person) or from a protagonist’s (third-person) perspective. First-person perspective (1PP), compared to third-person perspective (3PP), is related to higher and faster subjective evaluations of the other’s pain and an increased modulation of brain activity in structures related to the affective component of empathy (Canizales et al., 2013; Vistoli et al., 2016). Finally, different types of instructions with regard to the cognitive perspective to adopt when viewing the stimuli are used across pain empathy paradigms. Indeed, participants are usually instructed to adopt either a self-perspective (i.e. to feel a person’s pain as if it was their own pain) or the other’s perspective (i.e. to imagine or to evaluate a person’s pain), or to focus on the stimulus without specific instruction about the perspective (i.e. to pay attention to the stimulus). For instance, Jackson et al. (2006a) showed that adopting a self-perspective is associated with higher pain ratings and involves more brain activity associated with the affective component of empathy [i.e. the secondary SS cortex (SII), ACC and insula], whereas taking the other’s perspective yielded specific increases in activation of structures related to the cognitive component of empathy (i.e. the PCC, precuneus and TPJ).
Differences in methodological choices (i.e. visual cues, visuospatial perspective, perspective-taking instructions) can influence the way the dimensions of empathy are solicited in participants and explain part of the variability across studies. However, very few studies have contrasted these conditions directly (e.g. Vachon-Presseau et al., 2012; Lamm et al., 2007b), and no study has previously tested all of these differences in methodology within the same study. Failure to contextualize results within such methodological variations can pave the way for misleading conclusions about between-study divergence (Coll & Jackson, 2016) or oversimplified interpretations of empathy processes (De Waal & Preston, 2017). It thus remains difficult to draw an integrative view of how visual cues, visuospatial perspective and perspective taking influence the brain response during pain empathy. Previous meta-analyses partly addressed this issue (Lamm et al., 2011; Timmers et al., 2018). For instance, Lamm et al. (2011) conducted an image-based meta-analysis that compared SS pain paradigms to abstract-cue paradigms (i.e. abstract symbols indicating that another person is receiving a noxious stimulus). Since this 2011 meta-analysis, the number of functional magnetic resonance imaging (fMRI) investigations on pain empathy has exploded, reaching more than 200 studies. Paradigms have consequently varied greatly in terms of visual stimuli and instructions used to solicit empathy. In a recent meta-analysis, Timmers et al. (2018) examined differences between paradigms using either stimuli of facial expressions of pain or noxious stimulations applied to a body limb and differences between paradigms that oriented the participant’s perspective either toward the self (labeled perceptual/affective paradigms) or the other (labeled cognitive/evaluative paradigms). However, other methodological differences, such as visuospatial perspective, have not been addressed. In addition, results indicated that SS compared to EC pain paradigms were related distinctively to the bilateral MFG, the bilateral IPL and the right superior parietal lobule (SPL), whereas no specific region was found to be associated with the EC compared to the SS pain paradigms. This result is surprising given that several empirical studies (e.g. Danziger et al., 2009; Vachon-Presseau et al., 2012), as well as qualitative reviews and theoretical papers (e.g. Decety & Jackson, 2004; Tremblay et al., 2018; De Vignemont & Singer, 2006), support the functional dissociation between SS and EC pain cues during pain communication (e.g. Hadjistavropoulos et al., 2011) and empathy (e.g. De Waal & Preston, 2017). The unexpected finding could be explained by the definition of the different conditions, which are relatively broad and in which different constructs overlap.
The objectives of the current activation likelihood estimation (ALE) meta-analysis on pain empathy studies were 2-fold: first, to provide an up-to-date quantitative map of brain region and networks (the core network) involved in empathy for pain and, second, to address between-study methodological differences through a factorial- and theoretical-based framework (secondary networks). Between-study differences were systematically and quantitatively compared according to three factors and seven conditions that stem from the choice of methods for each included study. Importantly, these factors/conditions can also be organized in terms of their reliance on the different components of empathy proposed in contemporary neurocognitive models: visual cues (body parts submitted to noxious stimulations and facial expressions of pain), visuospatial perspective (first-person visual perspective and third-person visual perspective) and cognitive perspective (self-, stimuli- and other-oriented tasks).
Methods
Studies and coordinates selection
To select fMRI studies using pain empathy paradigms, a systematic literature search was conducted between the 5th and 31th of January 2018 inclusively. Articles were obtained through online databases without any timeline restriction. Selected keywords were ‘pain’ in conjunction with ‘fMRI’ or ‘MRI’ or ‘magnetic resonance imaging’ or ‘Imaging’ in conjunction with ‘empathy’ or ‘empathic’ or ‘empathie’ or ‘facial expression’ or ‘vicarious’. This initial search led to a total of 717 articles across PubMed (n = 245), Embase (n = 243), Medline (n = 119), PsychINFO (n = 91) and CINAHL (n = 19) databases. A preselection of articles was done based on title and abstract by the authors Josiane Jauniaux and Ali Khatibi independently. Only empirical fMRI studies assessing vicarious pain and/or empathy published in peer-reviewed journals were included. Quantitative or qualitative reviews were excluded. A total of 193 potential studies were identified.
The identified studies were then independently inspected and counter-verified by authors Josiane Jauniaux and Ali Khatibi according to the following selection criteria: (i) studies using visual stimuli; (ii) studies on healthy populations; (iii) studies on clinical populations, health professionals, children, seniors and long-term mindfulness meditation practitioners reporting results from a control group separately; (iv) studies reporting MNI or Talairach coordinates; and (v) studies reporting results from regions of interest or whole-brain analysis. Studies that had first-hand pain, namely, studies applying nociceptive stimulations on participants and a pain empathy condition, but for which the pain empathy condition was not conducted simultaneously with the first-hand pain condition were also included. Studies using different conditions, for instance, showing pictures of individuals of different ethnicities, level of attractiveness or degree of familiarity, were considered as well. Studies using different conditions and/or tasks, for example, induced hypnotic analgesia, exposing participants to violent video games and induced stress, were included. To reduce the number of potential confounding variables, studies that used complex visual scenes (e.g. complex social scenarios, sports situations, abstract cues) or auditory stimuli (e.g. narratives or sounds to indicate that pain was being administered) were excluded. Studies using paradigms to induce social exclusion or during which participants needed to inflict pain upon someone were also excluded. Finally, studies using the same original data set as a previous published paper were removed. In sum, a total of 94 studies met the inclusion and exclusion criteria. See Figure 1 for a full overview of the study selection process.
Fig. 1.
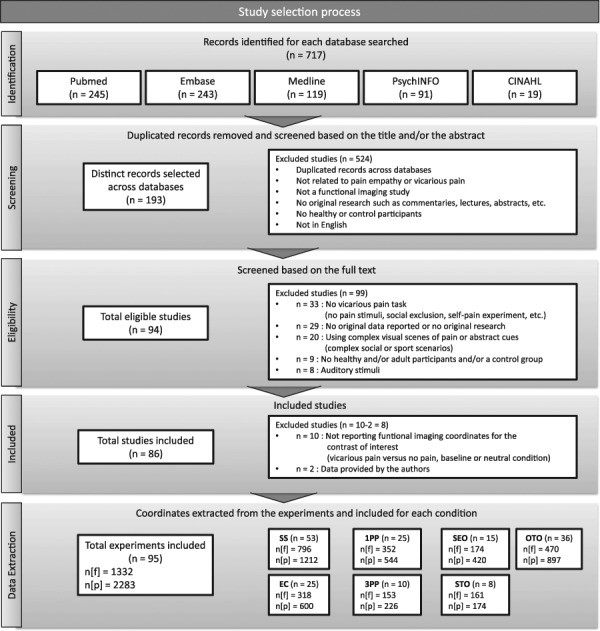
Diagram outlining the study selection process. n, number of studies or experiments; n [f], number of foci; n [p], number of participants.
Among those, fMRI coordinates were extracted from only 84 studies. Ten studies met the inclusion criteria but did not report the results for the contrast of either vicarious pain > no pain or vicarious pain > baseline. The findings for three of these studies could be included after the authors agreed to provide the data from this contrast. The final sample included 86 studies; 95 experiments; 2283 participants, about 46% of whom were women (at least 1061 women; 4 studies did not report the women/men ratio); and 1332 foci. Participants were all adults from the general population and were aged between 19 and 49 years (mean, 21.89 years; s.d., 2.96 years). Age and standard deviation averages were calculated based on the number of studies in which the age (73 studies) and standard deviation (63 studies) were reported and were weighted for the number of subjects in each study. Of these, not all studies reported standard deviation. Thus, the calculations are based on the available information.
Experiments were categorized into three factors and seven conditions based on methodological variations and the processes involved. Two factors involved largely bottom-up (i.e. stimulus-driven) processes: visual cue and visuospatial perspective. The third factor engaged more top-down (i.e. instruction-driven and/or task dependent) processes, namely, the self/other cognitive perspective. For each of these factors, a set of corresponding conditions were identified, described in detail hereinafter.
Visual cue refers to nature of the visual information available from the other’s pain experience. Across paradigms, the visual cue presented is mainly SS (i.e. limbs exposed to noxious stimulations) or EC (i.e. social affective cues such as facial expressions of pain) in nature. Thus, the experiments were divided based on these two types of visual information forming two conditions. Some studies used both stimuli of the limbs in pain and facial expressions of pain within the same experimental condition in their experiment; these studies were taken into account in the general analysis for pain empathy but were not included in either the individual SS or EC condition.
Visuospatial perspective refers to the spatial orientation of visual information, or the observer’s point of view of the other’s pain. Differences in visuospatial perspectives exist mainly across SS paradigms. Therefore, for this factor, only studies using SS paradigms were included. Stimulation of the limbs is generally presented from one’s own perspective (1PP; 0–45° angle) or a protagonist’s perspective (3PP; 180° angle). Thus, studies using an SS paradigm were divided based on these two visuospatial perspectives, leading to two conditions. Some studies used stimuli of the limbs presented from several visuospatial perspectives; these studies were included in the general analysis for pain empathy but were not added into the 1PP or 3PP condition. In the cases when researchers did not clearly report which visuospatial perspective was used for their stimuli, a short survey was sent to them in order to obtain this information (see Supplementary Material for more details). Following the authors’ responses, 11 experiments were added to the factor visuospatial perspective. In total, 35 experiments were included in this factor.
Lastly, the self/other cognitive perspective, which relates to the cognitive and effortful process of taking either the self perspective or the other’s perspective, has been manipulated through explicit instructions across paradigms. Three conditions were identified for this factor, namely, the self-perspective [self-oriented (SEO) tasks], the other perspective [other-oriented (OTO) tasks], or the neutral perspective [stimuli-oriented (STO) tasks]. More precisely, studies for which an instruction was given to the participants who oriented their attention toward a self-perspective were included in the SEO condition. The instructions could be as follows: to rate/evaluate/judge how they (the participants) felt empathic for the person, to share the emotional feelings of the person, to empathize with the person, to experience the feeling of the person, to indicate if they experience pain while viewing the person in pain or to explicitly take their own perspective. Studies using a photo cue (an image of the participant) to instruct the participants to adopt their perspective were also included in the SEO condition. Studies for which an instruction was given to the participants who oriented their attention toward the other perspective were included in the OTO condition. The instructions could be as follows: to rate/evaluate/judge the perceived unpleasantness and/or the intensity of the other’s pain, to evaluate if the person was suffering from pain or not, to put themselves into the other’s perspective, to imagine how the person feels, to imagine the emotions of the person or to put themselves into the perspective of an observer. Studies using a photo cue (a photo of a stranger) to indicate to the participants to adopt another perspective were also included in the OTO condition. Finally, studies for which an instruction was provided to the participants who oriented their attention toward the stimuli were included in the STO condition. The instructions could be as follows: to view the stimuli attentively, to passively view the stimuli, to carefully look at the stimuli, to watch the stimuli, to pay attention to the stimuli and then, in some cases, to press on a button when viewing the fixation cross. In some studies, the instruction given to the participants were not clearly reported. These studies were not included in any of the specific self/other cognitive perspective conditions but were taken into account in the general pain empathy analysis. Some researchers have used visual stimuli of the limbs and/or facial expressions of pain while using different instructions in their studies. In order to reduce the heterogeneity across studies included in the factor self/other cognitive perspective and to better isolate the effect of the cognitive perspective, at first, only studies using an SS paradigm were included in this factor. Then, analyses were carried out a second time, adding studies using an EC paradigm in order to examine whether the introduction of visual stimuli of facial expressions of pain would change the pattern of results.
Table 1 reports all studies included in the meta-analysis and their corresponding conditions. Table 2 reports the number of experiments, participants and foci by factors and conditions. See Supplementary Table 1 for more methodological details regarding each study.
Table 1.
List of references included in the meta-analysis and their corresponding factors and conditions
Studies in parentheses used an EC paradigm and were added in the self/other pain cognitive perspective factor.
Table 2.
Description of the sample: number of selected studies, experiments, foci and participants in each factor and their associated conditions
| Factors and their associated conditions | N of studies | N of experiments | N of foci | N of participants |
|---|---|---|---|---|
| Pain visual cues | 75 | 78 | 1114 | 1812 |
| SS | 52 | 53 | 796 | 1212 |
| EC | 23 | 25 | 318 | 600 |
| Pain visuospatial perspectives | 32 | 35 | 505 | 770 |
| 1PP | 23 | 25 | 352 | 544 |
| 3PP | 9 | 10 | 153 | 226 |
| Self/other cognitive perspectives taking | 52 (69) | 59 (77) | 805 (1014) | 1491 (1922) |
| SEO | 15 (17) | 15 (17) | 174 (202) | 420 (475) |
| STO | 8 (13) | 8 (13) | 161 (266) | 174 (284) |
| OTO | 29 (39) | 36 (47) | 470 (546) | 897 (1163) |
| All pain empathy studies | 86 | 95 | 1332 | 2283 |
The number in parentheses refers to the number of studies, experiments, foci and participants; included studies are those that used SS and EC pain paradigms for the self/other pain cognitive perspective taking factor.
Coordinate-based meta-analysis: ALE
The ALE method used in this study consists of modeling the uncertainty in localization of activation foci using Gaussian probability density distributions (Eickhoff et al., 2009, 2012). Gaussian distributions quantitatively adjust for the spatial uncertainty resulting from between-participant and between-template variance of the neuroimaging foci in order to model the coordinates (Turkeltaub et al., 2012). The width of these Gaussian functions is computed based on the number of participants in each experiment (Turkeltaub et al., 2012). The resulting ALE value is an estimate of the probability that at least one of the foci in the studies coordinates is truly located at a given voxel value (Turkeltaub et al., 2012). For more details about methodological procedures to compute ALE analyses, see the User Manuel for Ginger ALE (Fox et al., 2013).
For the current study, the coordinate-based meta-analysis was performed using the latest version of the GingerAle software (version 2.3.6) available on the BrainMap web site (http://www.brainmap.org/ale/) (Eickhoff et al. 2017). From the selected studies, stereotactic coordinates from the main effect of vicarious pain > no pain or vicarious pain > baseline, independently from other variables (i.e. effect of a task, condition or group), were extracted and were used in order to conduct a general ALE map for all experiments (i.e. global pain empathy map). Due to the very small number of studies reporting decreases in activation (i.e. negative blood oxygen level-dependant contrasts), only increases in activation across and between studies were examined, and deactivation responses were excluded. Coordinates originally reported in Talairach space were converted to the MNI space using the Lancaster (icbm2tal) transformation (Lancaster et al., 2007; Laird et al., 2011) implemented in Ginger ALE. It should be noted that the conventional terminology of the ALE method was used in this paper: the word ‘experiment’ refers to a single experimental contrast analysis, whereas the term ‘study’ refers to an empirical article reporting one or more experiments.
Conjunction and subtraction analyses were then conducted. To do so, pooled ALE maps were computed. A pooled map was generated for the visual cue factor by merging SS with EC conditions data sets. A pooled map was generated for the visuospatial perspective by merging 1PP with 3PP conditions data sets. Three pooled maps were generated for the self/other cognitive perspective by merging the following conditions: SEO with OTO, SEO with STO and OTO with STO. These pooled maps were computed a second time by adding studies using an EC paradigm. The following conjunction and subtraction analyses were performed for each factor:
-
1)
Visual cue
SS ∩ EC
SS > EC
EC > SS
-
2)
Visuospatial perspective
1PP ∩ 3PP
1PP > 3PP
3PP > 1PP
-
3)
Self/other cognitive perspective taking
SEO ∩ STO (with and without studies using an EC paradigm)
SEO > STO (with and without studies using an EC paradigm)
STO > SEO (with and without studies using an EC paradigm)
OTO ∩ STO (with and without studies using an EC paradigm)
OTO > STO (with and without studies using an EC paradigm)
STO > OTO (with and without studies using an EC paradigm)
SEO ∩ OTO (with and without studies using an EC paradigm)
SEO > OTO (with and without studies using an EC paradigm)
OTO > STO (with and without studies using an EC paradigm)
Statistical significance was assessed using the cluster-level inference (Eickhoff et al., 2012, 2017). A cluster-forming threshold of an uncorrected P value of 0.001 and a cluster-level inference threshold of 0.05 (permutation test) were applied for each factor (i.e. each pooled map) and for each of the seven conditions (i.e. each single ALE map). Contrasts and conjunction analyses were run with an uncorrected P value of 0.001 at first and then with a voxel-wise false discovery rates (FDRpNs) of 0.05 and 0.01 as the cluster-forming threshold to improve sensitivity to strong but focal activation. For all these analysis, a large mask size and the random-effect Turkeltaub nonadditive method were applied to minimize within-experiment and within-group effects (Turkeltaub et al., 2012). Images were created using MRIcron software (http://www.mccauslandcenter.sc.edu/mricro/mricron). Broadman areas were determined using XJView (http://www.alivelearn.net/xjview/) from the coordinates found in the result files provided by the GingerAle software.
Results
Pain empathy
The ALE analysis across all pain empathy experiments revealed peak values in several brain regions classically found to be related to empathy neural networks. Regions consistently activated included frontal brain areas, including the IFG bilaterally (BA 44/45, extending dorsally to BA9 in the left hemisphere; BA 44 extending dorsally to BA6 in the right hemisphere), the left superior frontal gyrus (SFG; the SMA) and the left aMCC. In the parietal lobes, the following regions were consistently activated across all pain empathy experiments: the bilateral IPL (anterior/dorsal to the TPJ) and the right SPL. Activation was also observed in the AI, posterior insula (PI) and fusiform gyrus bilaterally, and the right anterior lobe of the cerebellum. Subcortical regions including the thalamus, the amygdala and the lentiform nucleus/striatum also showed consistent activation across pain empathy experiments. Finally, activations were found in temporal–occipital regions, such as the left IOG, inferior temporal gyrus [ITG; in the extrastriate body area (EBA)/occipital face area (OFA)] and the right MOG. Results are shown in Figure 2, and coordinates for all peak activations and ALE values are reported in Table 3.
Fig. 2.
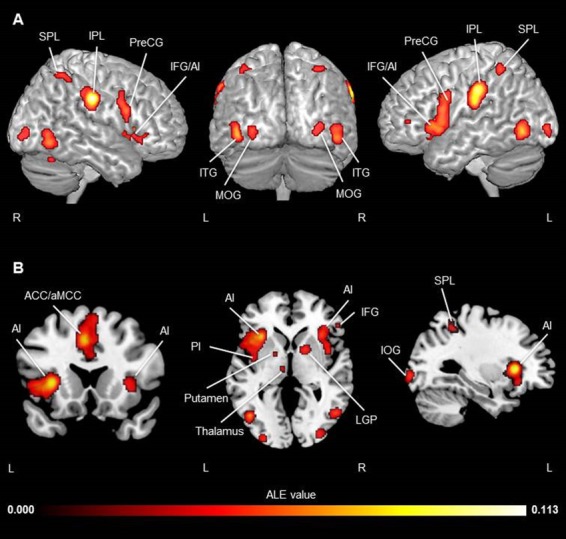
Activation likelihood clusters across all pain empathy experiments for the pain > no pain condition. Regions consistently activated during pain empathy resulting from an ALE meta-analysis of the pain > no pain condition of 95 experiments in fMRI pain empathy studies. ALE map is superimposed on the template brain ch2better.nii.gz in MNI coordinate space using MRIcron software. (A) Three-dimensional view. (B) Coronal (y = 20), axial (z = 3) and sagittal (x = −33) views. Thresholds: cluster-forming FDRpN <0.01 and cluster-level inference <0.05. See Table 3 for peak coordinates and ALE values.
Table 3.
Significant activation likelihood clusters across all pain empathy experiments
| Cluster no. | Hemisphere | BA | Label | Cluster center | Volume (mm3) |
Hemisphere | BA | Label | Cluster foci | ALE value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | |||||||||
| 1 | Left | 13 | AI | −43 | 14 | 7 | 12 088 | Left | 13 | AI | −32 | 22 | 4 | 0.1044 |
| Left | 44 | IFG | −50 | 10 | 4 | 0.0596 | ||||||||
| Left | 44 | IFG | −52 | 10 | 16 | 0.0570 | ||||||||
| Left | 6 | IFG | −58 | 10 | 28 | 0.0518 | ||||||||
| Left | 6 | PreCG | −52 | 6 | 28 | 0.0518 | ||||||||
| Left | Claustrum | −32 | 24 | −8 | 0.0421 | |||||||||
| Left | 13 | AI | −38 | −2 | 14 | 0.0419 | ||||||||
| Left | 13 | AI | −42 | −4 | 2 | 0.0408 | ||||||||
| 2 | Left | 32 | aMCC | −2 | 19 | 43 | 7912 | Left | 32 | aMCC | −4 | 22 | 40 | 0.0877 |
| Right | 6 | SFG (SMA) | 4 | 14 | 60 | 0.0504 | ||||||||
| Left | 6 | SFG (SMA) | 0 | 18 | 54 | 0.0487 | ||||||||
| 3 | Left | 40 | IPL | −58 | −24 | 32 | 4424 | Left | 40 | IPL | −58 | −26 | 36 | 0.0998 |
| Left | 40 | IPL | −58 | −22 | 26 | 0.0880 | ||||||||
| 4 | Right | 40 | IPL | 61 | −24 | 32 | 4080 | Right | 40 | IPL (SII) | 62 | −20 | 34 | 0.1125 |
| 5 | Right | 13 | AI | 38 | 19 | 1 | 2824 | Right | 13 | AI | 34 | 22 | 4 | 0.0580 |
| Right | 13 | AI | 42 | 6 | 0 | 0.0454 | ||||||||
| 6 | Right | 37 | Fusiform | 51 | −64 | −6 | 2168 | Right | 37 | MOG | 52 | −64 | −8 | 0.0595 |
| 7 | Left | 37 | Fusiform | −45 | −69 | −4 | 2064 | Left | 37 | ITG/fusiform | −44 | −70 | −4 | 0.0710 |
| 8 | Right | 9 | IFG | 55 | 11 | 26 | 1864 | Right | 44 | IFG | 58 | 12 | 24 | 0.0560 |
| Right | 6 | IFG (vPMC) | 52 | 8 | 30 | 0.0512 | ||||||||
| Right | 6 | PreCG | 52 | 8 | 40 | 0.0346 | ||||||||
| 9 | Left | 40 | IPL | −37 | −48 | 51 | 1520 | Left | 40 | IPL/intraparietal | −36 | −48 | 56 | 0.0492 |
| Left | 40 | IPL/intraparietal | −42 | −36 | 42 | 0.0400 | ||||||||
| Left | 40 | IPL/intraparietal | −34 | −44 | 48 | 0.0376 | ||||||||
| 10 | Left | Thalamus | −11 | −13 | 7 | 840 | Left | Thalamus | −12 | −12 | 8 | 0.0518 | ||
| 11 | Right | 7 | SPL | 35 | −50 | 57 | 824 | Right | 7 | SPL | 34 | −54 | 58 | 0.0445 |
| Right | 7 | SPL | 38 | −46 | 56 | 0.0372 | ||||||||
| 12 | Right | Lentiform nucleus | 17 | 6 | 2 | 776 | Right | Striatum/lentiform nucleus | 16 | 6 | 2 | 0.0502 | ||
| 13 | Right | 18 | MOG | 33 | −88 | −1 | 696 | Right | 18 | MOG | 34 | −88 | 0 | 0.0434 |
| 14 | Left | 18 | IOG | −31 | −94 | −3 | 624 | Left | 18 | IOG | −30 | −94 | −2 | 0.0483 |
| 15 | Right | Cerebellum | 35 | −62 | −26 | 208 | Right | Cerebellum | 34 | −62 | −26 | 0.0394 | ||
| 16 | Left | 10 | IFG | −44 | 44 | 5 | 88 | Left | 10 | IFG | −44 | 44 | 4 | 0.0339 |
| 17 | Left | Amygdala | −20 | −8 | −15 | 80 | Left | Amygdala | −20 | −8 | −16 | 0.0363 | ||
| 18 | Right | PI | 41 | −6 | −9 | 80 | Right | PI | 42 | −6 | −8 | 0.0354 | ||
| 19 | Left | Lentiform nucleus | −17 | 1 | 1 | 72 | Left | LGP | −18 | 2 | 2 | 0.0336 | ||
| 20 | Right | Amygdala | 21 | −5 | −15 | 48 | Right | Amygdala | 20 | −6 | −14 | 0.0347 | ||
| 21 | Right | 45 | IFG | 53 | 33 | −1 | 40 | Right | 45 | IFG | 52 | 32 | 0 | 0.0334 |
| 22 | Right | Thalamus | 10 | −13 | 9 | 24 | Right | Thalamus | 10 | −14 | 8 | 0.0326 | ||
| 23 | Right | 6 | MFG | 6 | 0 | 56 | 8 | Right | 6 | SFG (SMA) | 6 | 0 | 56 | 0.0313 |
Higher ALE values are associated with greater probability of activation across experiments. Abbreviations: SI, primary SS cortex; vPMC, ventral premotor cortex. Thresholds: cluster-forming threshold FDRpN <0.01 and a cluster-level inference <0.05. Note that for the general ALE analysis across all experiments, only a cluster-forming threshold of FDRpN of 0.01 is reported because the analysis revealed too many very large clusters to be interpretable with a cluster-forming threshold of P-uncorrected <0.001 or FDRpN <0.05.
Visual cues
The ALE map for SS alone showed significant clusters in the following regions: bilateral AI, MFG, PreCG, IFG, MOG, claustrum and thalamus; left ACC, aMCC, IPL, IOG, SFG, claustrum and putamen; and right PosCG, SPL, lateral globus pallidus (LGP) and fusiform gyrus (see Figure 3A and Table S2 in Supplementary Material for peak activation coordinates and ALE values). The ALE map for EC alone showed significant clusters of activation in the bilateral IFG and ITG, left thalamus (ventral anterior and ventral lateral nucleus), AI, ACC, amygdala and right MTG (see Figure 3B; peak coordinates and ALE values are reported in Supplementary Table S3). The conjunction analysis for SS and EC revealed consistent activity in the bilateral AI, bilateral ITG (EBA/OFA) and left ACC. The subtraction analysis showed activations for SS compared to EC in the bilateral IPL, SPL and AI/claustrum, left PI, and right PosCG and precuneus. The IFG was activated bilaterally for EC compared to SS (see Figure 3 and Table 4 for peak coordinates and ALE values). In summary, SS and EC pain paradigms were related to common [i.e. left ACC, bilateral AI and ITG (EBA/OFA, BA37)] and distinct [i.e. SS: bilateral IPL (BA40), AI and SPL (BA7); EC: bilateral IFG (BA44/45)] activations.
Fig. 3.
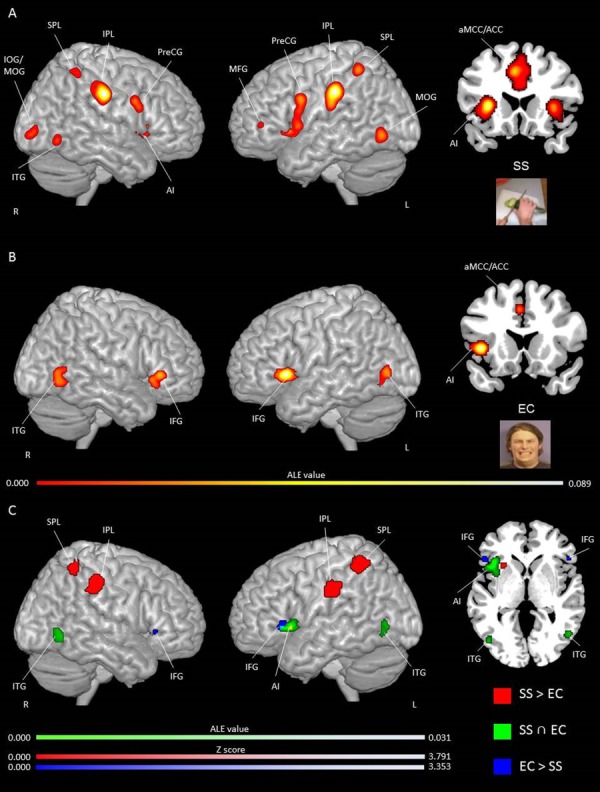
Activation likelihood clusters for pain empathy experiments using SS or EC visual pain information during a pain > no pain condition. Regions consistently, distinctively and commonly activated during SS and EC visual pain information. (A) ALE single map for SS (n = 53) in three-dimensional and coronal (y = 22) view. See Table S1 for peak coordinates and ALE values. (B) ALE single map for EC (n = 25) in three-dimensional and coronal views (y = 16). See Table S2 for peak coordinates and ALE values. (C) Regions commonly and distinctly activated during SS and EC resulting from conjunction and subtraction analyses. ALE clusters specifically related to SS in red and to EC in blue, and commonly activated in green in three-dimensional and axial (z = 1) views. ALE maps are superimposed on the template brain ch2better.nii.gz in MNI coordinate space using MRIcron software. Thresholds: cluster-forming P-uncorrected <0.001 and cluster-level inference <0.05.
Table 4.
Significant activation likelihood clusters for conjunctions and subtractions analyses for the pain visual cue factor
| Cluster no. | Hemisphere | BA | Label | Cluster center | Volume (mm3) | Hemisphere | BA | Label | Cluster foci | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | |||||||||
| SS ∩ EC | ALE value | |||||||||||||
| Clusters found with a P-uncorrected < 0.001 threshold | ||||||||||||||
| 1 | Left | 13 | AI | −41 | 18 | 1 | 2168 | Left | 13 | AI | −38 | 22 | 2 | 0.0311 |
| 2 | Right | 19 | ITG | 50 | −34 | −5 | 960 | Right | 19 | ITG | 50 | −62 | −4 | 0.0201 |
| 3 | Left | 32 | ACC | −3 | 15 | 41 | 624 | Left | 32 | ACC | −2 | 14 | 40 | 0.0244 |
| 4 | Left | 37 | ITG | −45 | −71 | −1 | 496 | Left | 37 | ITG | −46 | −72 | 2 | 0.0197 |
| Left | 37 | ITG | −44 | −70 | −4 | 0.0150 | ||||||||
| 5 | Right | 13 | AI | 40 | 24 | −4 | 8 | Right | 13 | AI | 40 | 24 | −4 | 0.0141 |
| 6 | Right | 13 | AI | 42 | 22 | −2 | 8 | Right | 13 | AI | 42 | 22 | −2 | 0.0125 |
| No additional cluster found with a voxel-wise FDRpN < 0.05 threshold | ||||||||||||||
| SS > EC | Z score | |||||||||||||
| Clusters found with a P-uncorrected < 0.001 threshold | ||||||||||||||
| 1 | Left | 7 | SPL | −35 | −35 | 55 | 3088 | Left | 7 | SPL | −35 | −48 | 56 | 3.7190 |
| 2 | Right | 40 | IPL | 56 | −29 | 43 | 2224 | Right | 40 | IPL | 56 | −31 | 44 | 3.7180 |
| Right | 2 | PosCG | 60 | −24 | 49 | 3.7540 | ||||||||
| Right | 2 | PosCG | 52 | −22 | 44 | 3.3528 | ||||||||
| 3 | Left | 40 | IPL | −59 | −22 | 34 | 1960 | Left | 40 | IPL | −59 | −22 | 34 | 3.7190 |
| 4 | Right | 7 | Precuneus | 37 | 37 | 59 | 728 | Right | 7 | Precuneus | 35 | −50 | 62 | 3.7190 |
| Right | 7 | Precuneus | 36 | −50 | 57 | 3.3528 | ||||||||
| Right | 40 | SPL | 41 | −49 | 55 | 3.2389 | ||||||||
| 5 | Left | Claustrum | −28 | −28 | 5 | 536 | Left | AI/claustrum | −27 | 17 | 3 | 3.7190 | ||
| 6 | Right | 13 | AI | 32 | 36 | 9 | 32 | Right | 13 | AI | 36 | 22 | 9 | 3.0902 |
| Additional cluster found with a voxel-wise FDRpN < 0.05 threshold | ||||||||||||||
| 2 | Left | 40 | IPL | −36 | −47 | 55 | 1392 | Left | 40 | IPL | −36 | −47 | 55 | 3.7190 |
| 4 | Left | PI | −28 | 18 | 5 | 272 | Left | PI | −28 | 17 | 5 | 3.5401 | ||
| 9 | Right | 40 | IPL | 38 | −48 | 60 | 8 | Right | 40 | IPL | 38 | −48 | 60 | 3.2389 |
| EC > SS | Z score | |||||||||||||
| Clusters found with a P-uncorrected < 0.001 threshold | ||||||||||||||
| v1 | Left | 45 | IFG | −51 | 25 | 1 | 216 | Left | 45 | IFG | −50 | 25 | 1 | 3.3528 |
| 2 | Right | 45 | IFG | 51 | 26 | −1 | 40 | Right | 45 | IFG | 52 | 26 | 0 | 3.3528 |
| No additional cluster found with a voxel-wise FDRpN < 0.05 threshold | ||||||||||||||
Higher ALE values or Z scores are associated with greater probability of activation across experiments.
Visuospatial perspective
The ALE single map for 1PP yielded convergence of increased activity in the bilateral IPL and SPL, right PI, IFG, claustrum, MFG, PreCG and MOG, and left AI, PreCG, ITG, aMCC/ACC and putamen (see Figure 4A and Supplementary Table S4 for peak coordinates and ALE values). The ALE single map for 3PP yielded consistent activations in the bilateral PosCG and left AI, ACC, MOG and IPL (see Figure 4A and Supplementary Table S5 for peak coordinates and ALE values). Conjunction analysis for both perspectives revealed bilateral activity in the PosCG, left AI, ACC and IPL (Figure 4B). Subtraction analysis revealed no specific clusters for the 1PP (1PP > 3PP) or 3PP (3PP > 1PP; see Table 5 for the peak activation coordinates and ALE values). Overall, based on the ALE single maps, the 1PP and 3PP conditions showed a similar pattern of activations. However, the 1PP condition was associated with a greater extent of activations in structures related to the affective and cognitive components of empathy (i.e. bilateral SPL, right IPL, PI, SPL, IFG, claustrum, and MOG, left ITG and putamen). Both visuospatial perspectives commonly recruited structures implicated in the affective component of empathy and self/other distinction (i.e. left AI, ACC, and IPL and bilateral PosCG).
Fig. 4.
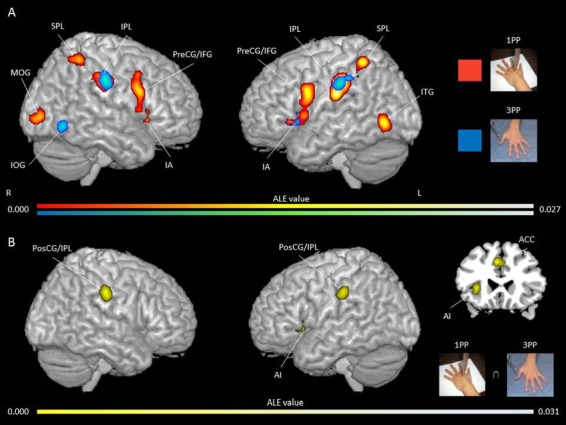
Activation likelihood clusters for pain empathy experiments using SS pain information presented from a 1PP or 3PP during a pain > no pain condition. Regions consistently, distinctively and commonly activated during SS paradigm presented from a 1PP or 3PP. (A) ALE single map for 1PP (n = 25) in red and ALE single map for 3PP (n = 10) in blue in three-dimensional view. See Table S3 for peak coordinates and ALE values for 1PP. See Table S4 for peak coordinates and ALE values for 3PP. (B) Clusters commonly activated for both visuospatial perspectives in yellow in three-dimensional and coronal (z = 23) views. See Table 5 for peak coordinates and ALE values. Maps are superimposed on the template brain ch2better.nii.gz in MNI coordinate space using MRIcron software. Thresholds for single maps: cluster-forming P-uncorrected <0.001 and cluster-level inference <0.05. Threshold for the conjunction map: cluster-forming P-uncorrected <0.001.
Table 5.
Significant activation likelihood clusters for conjunctions and subtractions analyses for the pain visuospatial perspective factor
| Cluster no. | Hemisphere | BA | Label | Cluster center | Volume (mm3) | Hemisphere | BA | Label | Cluster foci | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | |||||||||
| 1PP ∩ 3PP | ALE value | |||||||||||||
| Clusters found with a P-uncorrected < 0.001 threshold | ||||||||||||||
| 1 | Right | 2 | PosCG | 64 | −21 | 36 | 1552 | Right | 2 | PosCG | 62 | −22 | 38 | 0.0221 |
| 2 | Left | 13 | AI | −33 | −21 | 3 | 1408 | Left | 13 | AI | −32 | 20 | 4 | 0.0272 |
| 3 | Left | 2 | PosCG | −57 | −25 | 36 | 1208 | Left | 2 | PosCG | −56 | −26 | 38 | 0.0202 |
| 4 | Left | 32 | ACC | −3 | 24 | 39 | 920 | Left | 32 | ACC | −4 | 24 | 38 | 0.0215 |
| 5 | Left | 40 | IPL | −39 | −38 | 43 | 56 | Left | 40 | IPL | −40 | −38 | 42 | 0.0134 |
| Additional cluster found with a voxel-wise FDRpN < 0.05 threshold | ||||||||||||||
| 2 | Right | 2 | PosCG | 62 | −22 | 37 | 16 | Right | 2 | PosCG | 62 | −22 | 38 | 0.0221 |
| 1PP > 3PP | Z score | |||||||||||||
| No cluster found with a P-uncorrected < 0.001 threshold | ||||||||||||||
| No additional cluster found with a voxel-wise FDRpN < 0.05 threshold | ||||||||||||||
| 3PP > 1PP | Z score | |||||||||||||
| No cluster found with a P-uncorrected < 0.001 threshold | ||||||||||||||
| No additional cluster found with a voxel-wise FDRpN < 0.05 threshold | ||||||||||||||
Higher ALE values or Z scores are associated with greater probability of activation across experiments.
Self/other cognitive perspective taking
For the SEO condition, the single ALE map showed consistent activation in the left IFG, IPL, ACC, aMCC, AI, PreCG, MFG and claustrum, and right IPL and MOG (Figure 5 and Supplementary Table S6 for peak coordinates and ALE values). Several clusters of activation were found for the SEO condition when studies using an EC paradigm were added, namely, the bilateral IPL and claustrum, and left AI, IFG, PreCG, aMCC, ACC and MFG. Refer to Table S7 in Supplementary Material for peak coordinates and ALE values. For the OTO condition, the single ALE map revealed consistent activation in the bilateral AI, IFG, MFG and fusiform gyrus; left PI, IPL, caudate head, ACC, claustrum and IOG; and right SPL, aMCC, culmen and PosCG (see Figure 5 and Supplementary Table S8 for peak coordinates and ALE values). When adding studies using an EC paradigm, the analysis revealed a similar pattern, but with more regions of activation. Specifically, in this analysis, the bilateral AI, IFG, SPL, SFG and IPL, left ACC, claustrum, and fusiform gyrus and right MOG, were recruited (see Supplementary Table S9). For the STO condition, the single ALE map showed consistent activations in the bilateral IPL and additionally in the right PosCG and IOG, and left IFG, PreCG, ITG and fusiform (Figure 5 and Table S10). When adding studies using an EC paradigm, several additional clusters were found, including in the bilateral IPL, left AI, ITG, IFG and claustrum, and right PosCG, IOG, fusiform gyrus and SFG (see Supplementary Table S11 for peak coordinate and ALE values).
Fig. 5.
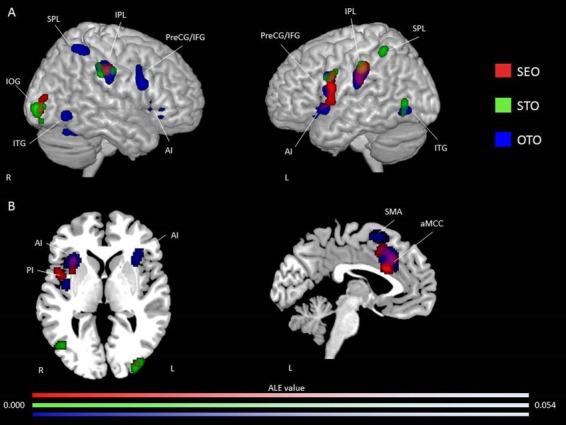
Activation likelihood clusters for pain empathy experiments using SS pain information and instructions to adopt either a self-perspective or another person’s perspective, or to focus on the stimuli during a pain > no pain condition. (A) Regions consistently activated during SS pain information and instructions either to adopt a self-perspective (SEO; n = 15) in red or to focus on the stimuli (STO; n = 8) in green and another person’s perspective (OTO; n = 36) in blue resulting from three-single ALE meta-analysis presented in three-dimensional view. See Tables S5, S7 and S9 for peak coordinates and ALE values for SEO, OTO and STO, respectively. (B) Axial (z = 74) and sagittal (x = 88) views. ALE maps are superimposed on the template brain ch2better.nii.gz in MNI coordinate space using MRIcron software. Thresholds: cluster-forming P-uncorrected <0.001 and cluster-level inference <0.05.
When running the conjunction and subtraction analyses, results showed common and distinct patterns of activation for certain conditions. Conjunction analyses showed consistent activities in the bilateral IPL, left PreCG and right MOG for the SEO and STO (Figure 6A); the bilateral IPL and left ACC, AI, MFG, IFG and SFG for SEO and OTO (Figure 6A); and the bilateral IPL, left ITG, PreCG and fusiform, and right PosCG for the OTO and STO (Figure 6A). The contrast STO > OTO was associated with activations in the right IOG, cerebellum and IPL, and left PreCG and IFG (Figure 6B). Other contrasts did not show significant activation (i.e. SEO > STO, STO > SEO, OTO > SEO, SEO > OTO and OTO > STO; see Table 6 for peak coordinates and ALE values for each of these analyses). When adding studies using an EC paradigms in the analyses for the self/other cognitive perspective factor, the conjunction analyses revealed consistent activity in the bilateral IPL, left PreCG, IFG and AI for the SEO and STO conditions; the bilateral IPL and left ACC, AI, claustrum, MFG and IFG for SEO and OTO conditions; and the bilateral IPL, left ITG, IFG and AI, and right PosCG and SFG for the OTO and STO conditions. The subtraction analysis STO > OTO was associated with consistent activation in the bilateral anterior lobe of the cerebellum, right IOG and SFG, and left IFG. The subtraction analysis SEO > OTO showed specific activations in the left AI for the SEO condition. Other contrasts did not show significant activations (i.e. SEO > STO, STO > SEO, OTO > SEO and OTO > STO). See Table 7 for peak coordinates and ALE values for each of these analyses.
Fig. 6.
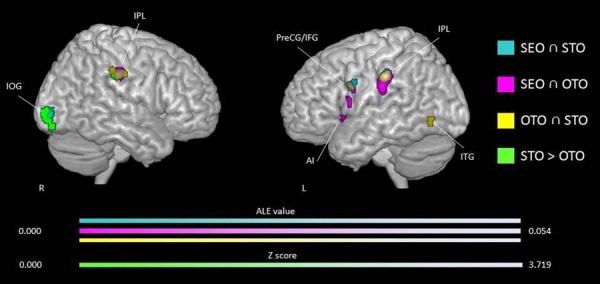
Common and distinct activation likelihood clusters for pain empathy experiments using SS pain information and instructions to adopt either a self-perspective or another person’s perspective, or to focus on the stimuli during the pain > no pain condition. Regions commonly and distinctively activated during SS pain information and instructions to adopt either a self-perspective or another person’s perspective, or to focus on the stimuli resulting from conjunction and subtraction analyses. Conjunction analysis for studies using instructions to adopt a self-perspective (SEO) and to focus on the stimuli (STO) in cyan, a SEO and another person’s perspective (OTO) in violet, and an OTO and an STO in yellow in three-dimensional view. Subtraction analysis between STO and OTO in green. See Table 6 for peak coordinates and ALE values for panels A, B and C. Maps are superimposed on the template brain ch2better.nii.gz in MNI coordinate space using MRIcron software. Threshold for the conjunction and subtraction maps: cluster-forming P-uncorrected <0.001.
Table 6.
Significant activation likelihood clusters for conjunctions and subtractions analyses for the self/other cognitive perspective taking factor
| Cluster no. | Hemisphere | BA | Label | Cluster center | Volume (mm3) | Hemisphere | BA | Label | Cluster foci | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | |||||||||
| SEO ∩ STO | ALE value | |||||||||||||
| Clusters found with a P-uncorrected < 0.001 threshold | ||||||||||||||
| 1 | Left | 40 | IPL | −58 | −26 | 38 | 1000 | Left | 40 | IPL | −58 | −26 | 38 | 0.0262 |
| 2 | Right | 40 | IPL | 62 | −23 | 37 | 712 | Right | 40 | IPL | 62 | −24 | 36 | 0.0164 |
| 3 | Left | 6 | PreCG | −51 | 6 | 29 | 592 | Left | 6 | PreCG | −50 | 6 | 28 | 0.0162 |
| 4 | Right | 18 | MOG | 33 | −89 | −1 | 368 | Right | 18 | MOG | 32 | −88 | −4 | 0.0137 |
| Right | 18 | MOG | 36 | −88 | 4 | 0.0127 | ||||||||
| No additional cluster found with a voxel-wise FDRpN < 0.05 threshold | ||||||||||||||
| SEO > STO | Z score | |||||||||||||
| No cluster found with a P-uncorrected < 0.001 threshold | ||||||||||||||
| No cluster found with a voxel-wise FDRpN < 0.05 threshold | ||||||||||||||
| STO > SEO | Z score | |||||||||||||
| No cluster found with a P-uncorrected < 0.001 threshold | ||||||||||||||
| No cluster found with a voxel-wise FDRpN < 0.05 threshold | ||||||||||||||
| SEO ∩ OTO | ALE value | |||||||||||||
| Clusters found with a P-uncorrected < 0.001 threshold | ||||||||||||||
| 1 | Left | 40 | IPL | −58 | −24 | 32 | 1960 | Left | 40 | IPL | −58 | −26 | 38 | 0.0261 |
| Left | 40 | IPL | −58 | −22 | 28 | 0.0212 | ||||||||
| 2 | Left | 32 | ACC | −4 | 21 | 38 | 1824 | Left | 32 | ACC | −4 | 24 | 36 | 0.0236 |
| Left | 32 | ACC | −2 | 20 | 32 | 0.0198 | ||||||||
| Left | 32 | MFG | −4 | 14 | 46 | 0.0169 | ||||||||
| 3 | Left | 13 | AI | −33 | 20 | 5 | 1120 | Left | 13 | AI | −34 | 20 | 6 | 0.0246 |
| Left | Claustrum | −34 | 14 | 0 | 0.0138 | |||||||||
| 4 | Right | 40 | IPL | 61 | −23 | 37 | 912 | Right | 40 | IPL | 62 | −24 | 38 | 0.0206 |
| 5 | Left | 9 | IFG | −53 | 7 | 27 | 312 | Left | 9 | IFG | −52 | 8 | 28 | 0.0157 |
| Left | 9 | IFG | −52 | 6 | 24 | 0.0155 | ||||||||
| 6 | Left | 44 | IFG | −55 | 9 | 13 | 304 | Left | 44 | IFG | −56 | 8 | 12 | 0.0183 |
| 7 | Left | 32 | ACC | −4 | 26 | 26 | 8 | Left | 32 | ACC | −4 | 26 | 26 | 0.0104 |
| 8 | Left | 6 | SFG | −2 | 14 | 52 | 8 | Left | 6 | SFG | −2 | 14 | 52 | 0.0113 |
| No additional cluster found with a voxel-wise FDRpN < 0.05 threshold | ||||||||||||||
| SEO > OTO | Z score | |||||||||||||
| No cluster found with a P-uncorrected < 0.001 threshold | ||||||||||||||
| No cluster found with a voxel-wise FDRpN < 0.05 threshold | ||||||||||||||
| OTO > SEO | Z score | |||||||||||||
| No cluster found with a P-uncorrected < 0.001 threshold | ||||||||||||||
| No cluster found with a voxel-wise FDRpN < 0.05 threshold | ||||||||||||||
| OTO ∩ STO | ALE value | |||||||||||||
| Clusters found with a P-uncorrected < 0.001 threshold | ||||||||||||||
| 1 | Right | 40 | IPL | 59 | −23 | 37 | 1232 | Right | 40 | IPL | 62 | −26 | 34 | 0.0182 |
| Right | 2 | PosCG | 56 | −18 | 36 | 0.0143 | ||||||||
| Right | 40 | IPL | 56 | −30 | 40 | 0.0143 | ||||||||
| 2 | Left | 40 | IPL | −58 | −25 | 37 | 896 | Left | 40 | IPL | −58 | −26 | 38 | 0.0271 |
| 3 | Left | 37 | ITG | −44 | −68 | −6 | 360 | Left | 37 | ITG | −44 | −70 | −2 | 0.0147 |
| Left | 19 | Fusiform | −44 | −68 | −6 | 0.0131 | ||||||||
| 4 | Left | 6 | PreCG | −52 | 8 | 28 | 232 | Left | 6 | PreCG | −50 | 6 | 26 | 0.0150 |
| No additional cluster found with a voxel-wise FDRpN < 0.05 threshold | ||||||||||||||
| OTO > STO | Z score | |||||||||||||
| No cluster found with a P-uncorrected < 0.001 threshold | ||||||||||||||
| No cluster found with a voxel-wise FDRpN < 0.05 threshold | ||||||||||||||
| STO > OTO | Z score | |||||||||||||
| Clusters found with a P-uncorrected < 0.001 threshold | ||||||||||||||
| 1 | Right | 18 | Cerebellum | 32 | −92 | −5 | 1104 | Right | Cerebellum | 31 | −92 | −8 | 3.7190 | |
| Right | 18 | IOG | 34 | −92 | 2 | 3.3528 | ||||||||
| 2 | Left | 6 | PreCG | −39 | 4 | 30 | 240 | Left | 6 | PreCG | −37 | 7 | 26 | 3.3528 |
| Left | 6 | PreCG | −39 | 3 | 32 | 3.1560 | ||||||||
| 3 | Right | IPL | −65 | −29 | 35 | 56 | Right | 40 | IPL | 66 | −28 | 34 | 3.7190 | |
| 4 | Left | IFG | −46 | 6 | 32 | 8 | Left | 6 | IFG | −46 | 6 | 32 | 3.0902 | |
| 5 | Left | 9 | PreCG | −46 | 4 | 34 | 8 | Left | 6 | PreCG | −46 | 4 | 34 | 3.1560 |
| No additional cluster found with a voxel-wise FDRpN < 0.05 threshold | ||||||||||||||
Higher ALE values or Z scores are associated with greater probability of activation across experiments.
Table 7.
Significant activation likelihood clusters for conjunctions and subtractions analyses for the self/other cognitive perspective taking factor with studies using an SS or an EC paradigm
| Cluster no. | Hemisphere | BA | Label | Cluster center | Volume (mm3) |
Hemis phere |
BA | Label | Cluster foci | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | |||||||||
| SEO ∩ STO | ALE value | |||||||||||||
| Clusters found with a P-uncorrected < 0.001 threshold | ||||||||||||||
| 1 | Left | 40 | IPL | −58 | −26 | 38 | 920 | Left | 40 | IPL | −58 | −26 | 38 | 0.0262 |
| 2 | Right | 40 | IPL | 62 | −23 | 37 | 608 | Right | 40 | IPL | 62 | −24 | 36 | 0.0192 |
| 3 | Left | 6 | PreCG | −51 | 7 | 27 | 552 | Left | 6 | PreCG | −50 | 6 | 28 | 0.0164 |
| Left | 44 | IFG | −52 | 10 | 18 | 0.0143 | ||||||||
| 4 | Left | 13 | AI | −32 | 21 | 6 | 512 | Left | 13 | AI | −30 | 22 | 46 | 0.0198 |
| −58 | −26 | 38 | Left | 13 | AI | −36 | 20 | 4 | 0.0143 | |||||
| No additional cluster found with an FDRpN < 0.05 threshold | ||||||||||||||
| SEO > STO | Z score | |||||||||||||
| No cluster found with a P-uncorrected < 0.001 threshold | ||||||||||||||
| No cluster found with a voxel-wise FDRpN < 0.05 threshold | ||||||||||||||
| STO > SEO | Z score | |||||||||||||
| No cluster found with a P-uncorrected < 0.001 threshold | ||||||||||||||
| No cluster found with a voxel-wise FDRpN < 0.05 threshold | ||||||||||||||
| SEO ∩ OTO | ALE value | |||||||||||||
| Clusters found with a P-uncorrected < 0.001 threshold | ||||||||||||||
| 1 | Left | 40 | IPL | −58 | −25 | 32 | 2176 | Left | 40 | IPL | −58 | −26 | 38 | 0.0262 |
| Left | 40 | IPL | −58 | −22 | 28 | 0.0226 | ||||||||
| 2 | Left | 32 | ACC | −4 | 22 | 37 | 1976 | Left | 32 | ACC | −4 | 26 | 36 | 0.0241 |
| Left | 32 | ACC | −4 | 22 | 30 | 0.0209 | ||||||||
| Left | 32 | MFG | −4 | 14 | 46 | 0.0169 | ||||||||
| 3 | Left | 13 | AI | −33 | 20 | 5 | 1088 | Left | 13 | AI | −34 | 20 | 6 | 0.0248 |
| Left | Claustrum | −36 | 12 | 0 | 0.0154 | |||||||||
| 4 | Right | 40 | IPL | 62 | −23 | 37 | 936 | Right | 40 | IPL | 62 | −24 | 38 | 0.0206 |
| 5 | Left | 44 | IFG | −55 | 9 | 11 | 384 | Left | 44 | IFG | −54 | 8 | 12 | 0.0189 |
| 6 | Left | 13 | AI | −40 | −2 | 13 | 168 | Left | 13 | AI | −38 | −2 | 14 | 0.0129 |
| 7 | Left | Claustrum | −40 | 3 | 0 | 32 | Left | Claustrum | −40 | 4 | 0 | 0.0127 | ||
| 8 | Left | Claustrum | −40 | 0 | 2 | 24 | Left | Claustrum | −40 | 0 | 2 | 0.0117 | ||
| 9 | Left | 13 | AI | −42 | 4 | −2 | 8 | Left | 13 | AI | −42 | 4 | −2 | 0.0109 |
| 10 | Left | 13 | AI | −38 | 10 | 0 | 8 | Left | 13 | AI | −38 | 10 | 0 | 0.0126 |
| No additional cluster found with a cluster-level inference < 0.05 and an FDRpN < 0.01 threshold | ||||||||||||||
| SEO > OTO | Z score | |||||||||||||
| Clusters found with a P-uncorrected < 0.001 threshold | ||||||||||||||
| 1 | Left | 13 | AI | −41 | 7 | 5 | 56 | Left | 13 | AI | −40 | 6 | 5 | 3.3528 |
| 2 | Left | 13 | AI | −36 | 8 | 6 | 8 | Left | 13 | AI | −36 | 8 | 6 | 3.1560 |
| No additional cluster found with an FDRpN < 0.05 threshold | ||||||||||||||
| OTO > SEO | Z score | |||||||||||||
| No cluster found with a P-uncorrected < 0.001 threshold | ||||||||||||||
| No cluster found with a voxel-wise FDRpN < 0.05 threshold | ||||||||||||||
| OTO ∩ STO | ALE value | |||||||||||||
| Clusters found with a P-uncorrected < 0.001 threshold | ||||||||||||||
| 1 | Right | 2 | PosCG | 60 | −22 | 36 | 1272 | Right | 2 | PosCG | 60 | −20 | 36 | 0.0223 |
| Right | 40 | IPL | 56 | −30 | 40 | 0.0144 | ||||||||
| 2 | Left | 13 | AI | −35 | 21 | 4 | 1000 | Left | 13 | AI | −42 | 20 | 2 | 0.0205 |
| Left | Claustrum | −28 | 22 | 6 | 0.0200 | |||||||||
| 3 | Left | 40 | IPL | −58 | −26 | 38 | 840 | Left | 40 | IPL | −58 | −26 | 38 | 0.0300 |
| 4 | Left | 37 | ITG | −45 | −70 | −4 | 600 | Left | 37 | ITG | −44 | −70 | −2 | 0.0212 |
| 5 | Right | 6 | SFG | 5 | 14 | 58 | 464 | Right | 6 | SFG | 6 | 14 | 58 | 0.0218 |
| 6 | Left | 44 | IFG | −52 | 10 | 16 | 8 | Left | 44 | IFG | −52 | 10 | 16 | 0.0128 |
| No additional cluster found with an FDRpN < 0.05 threshold | ||||||||||||||
| OTO > STO | Z score | |||||||||||||
| No cluster found with a P-uncorrected < 0.001 threshold | ||||||||||||||
| No cluster found with a voxel-wise FDRpN < 0.05 threshold | ||||||||||||||
| STO > OTO | Z score | |||||||||||||
| Clusters found with a P-uncorrected < 0.001 threshold | ||||||||||||||
| 1 | Right | Cerebellum | 32 | −91 | −8 | 1048 | Right | Cerebellum | 33 | −91 | −8 | 3.7190 | ||
| Right | 18 | IOG | 32 | −96 | 0 | 3.5401 | ||||||||
| 2 | Left | Cerebellum | −36 | −65 | −16 | 136 | Left | Cerebellum | −37 | −65 | −15 | 3.7190 | ||
| 3 | Left | 13 | IFG | −46 | 25 | 2 | 136 | Left | 13 | IFG | −45 | 26 | 1 | 3.7190 |
| 4 | Right | 6 | SFG | 8 | 15 | 54 | 112 | Right | 6 | SFG | 8 | 16 | 54 | 3.7190 |
| No additional cluster found with an FDRpN < 0.05 threshold | ||||||||||||||
Higher ALE values or Z scores are associated with greater probability of activation across experiments.
In summary, based on the single ALE maps, results showed that other-oriented tasks were associated with a greater extent of activations in the core (i.e. left AI and right aMCC) and secondary (i.e. left MFG, right IFG, SPL and bilateral fusiform gyrus) brain networks related to pain empathy compared to self-oriented tasks. Other-oriented tasks were associated with a great extent of activation in the core (i.e. left AI/ACC) and secondary (i.e. left MFG, right PosCG, IFG, SPL and fusiform gyrus) compared to stimuli-oriented tasks. Self-oriented tasks were associated with a greater extent of activations in the core (i.e. left AI) and secondary (i.e. IFG) networks compared to stimuli-oriented tasks. In addition, the three types of tasks were related to common activations in the bilateral IPL. Other- and self-oriented tasks commonly activated the left AI and ACC. Finally, distinct activations were found for stimuli- compared to other-oriented tasks in frontoparietal (i.e. right IPL and left PreCG and IFG) and occipital (right IOG) structures in addition to the cerebellum when considering only SS pain paradigms. Self- compared to other-oriented task was related to distinct activations in the left AI when considering both SS and EC pain paradigms.
Discussion
The neural correlates of empathy and its components have been explored using a number of pain empathy paradigms involving different stimuli, instructions and tasks. Failure to consider the underlying processes and these methodological variations in the study of empathy oversimplifies the interpretation of neuroimaging studies and can give a misleading impression of the results obtained with these diverse experimental conditions. The objectives of the current work were to provide a general quantitative map of brain structures involved in empathy based on previous fMRI studies on pain empathy and to replicate how empathy may reveal a core network, as previously found (Lamm et al., 2011; Timmers et al., 2018). Moreover, it aimed to explore secondary networks in empathy which may depend on specific properties of the stimuli and tasks used across these different studies. At first, a general coordinate-based ALE meta-analysis on 95 fMRI pain empathy experiments was conducted. Studies were then categorized based on their methodological variations, leading to three factors and seven conditions: pain visual cues (i.e. body parts in noxious situations and facial expressions of pain), pain visuospatial perspectives (i.e. IPP and 3PP), and self/other cognitive perspectives taking (i.e. self-, stimuli- and other-oriented tasks). ALE conjunction and subtraction analyses were carried out in order to investigate whether secondary networks could be related to these specific conditions.
Empathy and its related processes
It is generally accepted that empathy is supported by two major components, an affective and a cognitive component (Decety & Jackson, 2004; Decety & Lamm, 2006; De Waal & Preston, 2017; Morelli et al., 2015; Shamay-Tsoory, 2011; Zaki & Ochsner, 2012). The current research, which quantitatively synthesized almost a hundred fMRI experiments, supports this conceptualization, showing that pain observations recruit several structures throughout the brain networks associated with these empathy components. Indeed, the general analysis showed the engagement of frontal (i.e. IFG), parietal (i.e. SPL, IPL), sublobar (i.e. AI/PI, thalamus), limbic (i.e. aMCC/ACC, amygdala) and subcortical (i.e. cerebellum) structures, which are typically associated with the affective component of empathy (Decety & Jackson, 2004; De Waal & Preston, 2017; Fan et al., 2011; Lamm et al., 2011; Tousignant et al., 2017). The study also showed activation of other parietal (i.e. IPL) and temporal (i.e. fusiform gyrus and ITG) structures, which are typically associated with the cognitive component of empathy (Decety & Jackson, 2004; Fan et al., 2011; De Waal & Preston, 2017; Tousignant et al., 2017). Although empathy can be divided into these two major components, more rudimentary processes may underlie these ‘umbrellas components’ (De Waal & Preston, 2017). The combined activation of the structures underlying these processes is likely to facilitate a fully empathic experience, as discussed hereinafter.
An affective representation: at the core of pain empathy
The current research shows that pain empathy relies on a core network of structures that include the AI and the aMCC/ACC. These regions were found to be consistently recruited across a variety of pain paradigms. Similar to Lamm et al.’s, 2011 meta-analysis, the present analysis reveals a large number of clusters relating to activation of the AI and aMCC/ACC (69 and 49 clusters out of 95 related to the AI and aMCC/ACC, respectively). In addition, the AI and aMCC/ACC were consistently and commonly activated across different visual cues, visuospatial perspectives and self- and other-oriented perspective-taking instructions. These results also support those of other meta-analyses that found activation in the AI and aMCC/ACC during empathy for non-pain-related conditions (Fan et al., 2011; Bzdok et al., 2012; Timmers et al., 2018) and for empathy for non-pain-negative affective states (Timmers et al., 2018). Activity in these structures has been suggested to be associated with the affective/perceptual component of pain experience (Peyron et al., 2000; Rainville, 2002; Garcia-Larrea & Peyron, 2013), as well as with the affective-resonance component of empathy (Decety & Jackson, 2004; Lamm et al., 2011). Accordingly, our results of combined activation of the AI and aMCC/ACC are in line with the hypothesis of a shared neural representation related to the affective component of pain. It should be noted, however, that the meta-analysis of Bzdok et al. (2012) showed that the amygdala, the rostal ACC and the posterior cingulate cortex were also commonly activated across the selected studies, results that were not replicated in the present study. The coordinated-based meta-analysis of Bzdok et al. (2012) differed from the current analysis in that it was conducted with paradigms that employed visual, textual or auditory stimuli of social interactions during which participants watched passively or evaluated various dimensions of the others’ emotional states. Although results from this previous meta-analysis diverge partly from the current results, it appears that the AI and the aMCC/ACC are consistently identified as part of what we call the core network of empathy. Additional structures may be recruited depending on the specific affective modality, visual information or perspective with which an observer is to empathize.
Although the AI and the aMCC/ACC are suggested to be at the basis of an affective shared neural representation during empathy, evidence from other research fields suggests that these structures are also implicated in a variety of other functions. Although the AI and the ACC have been consistently shown to be related to nociceptive stimulation, it was alternatively proposed to view these areas being part of a functional system involved in detecting, orienting attention toward and reacting to salient sensory events (Legrain et al., 2011). Indeed, a neuroimaging review (Uddin, 2015) and meta-analysis (Uddin et al., 2014) suggest that the insula is a key node of the ‘salience network’ relating to the capacity to detect relevant stimuli in the environment. Additionally, research shows that the AI contributes to interoceptive representations and subjective awareness of the body’s various states (Craig, 2003, 2009). Moreover, in relation to pain, a large body of evidence shows a posterior–anterior functional dissociation within the insula. The posterior parts of the insula are suggested to integrate nociceptive afferents (Yarkoni et al., 2011), encode pain intensity (Frot et al., 2014; Uddin, 2015), and may be related to preconscious pain perception (Bastuji et al., 2016). In contrast, the anterior parts of the insula are suggested to underpin evaluative aspects of pain sensation (Frot et al., 2014; Uddin, 2015) and conscious voluntary reactions (Bastuji et al., 2016). Furthermore, a review suggests a caudal–anterior and lateralized functional organization of the insula for pain experience and pain observation (Jackson et al., 2006b). Within the left insula, the caudal to the mid-areas would be more related to pain experience, whereas the anterior areas would be preferentially associated with pain empathy. Within the right insula, pain experience would be related to the full caudal–anterior spectrum. The anterior areas would be associated only in part with pain empathy. Since our results showed consistent activation in the anterior and caudal insula/PI bilaterally and more frequently in the AI, one may conclude that the insula cortices, in particular the anterior parts, may play an important role in detecting salient cues related to the other’s pain in order to evoke a conscious interoceptive image of the other’s pain sensation and intensity.
An ongoing debate challenges the previous assumptions about the specific function of the dorsal ACC (dACC) in pain (Wager et al., 2016). A considerable body of evidence suggests that the dACC is responsive to pain (Lieberman & Eisenberger, 2015), pain empathy (Yesudas & Lee, 2015) and both (Lamm et al., 2011). However, it would be misleading to say that this structure is selective to pain experience and/or pain observation, as it is also responsive to viewing or experiencing negative emotions (Zaki et al., 2016) and other cognitive functions, such as attention (Fox et al., 2006; Yeo et al., 2011), inhibition (Wager et al., 2005), and language, motor, learning and memory (Wager et al., 2016), to name only a few. However, neuroscientists seem to agree on one point: the dACC ‘subserves survival-relevant functions’ (Lieberman & Eisenberger (2015); Wager et al., 2016). More precisely, the dACC has been linked to the attentional control that serves to regulate cognitive processes and to adjust our behaviors in accordance with internal goals (Shenhav et al., 2016). Based on a large-scale quantitative analysis of fMRI data, Lieberman & Eisenberger (2015) proposed that the dACC serves an alarm-like function for goal-related conflicts requiring attention. During pain, the role of the dACC may therefore be to integrate basic affective and cognitive processes in order to assign and organize, in an attentional control manner, other brain functions and behaviors to assure survival. In addition, several studies show that brain responses in the AI and the ACC correlate with prosocial behaviors (Hein et al., 2010; Masten et al., 2011; Rameson et al., 2012). Our study suggests that such processes might take part in pain empathy, as the dACC and the AI were consistently activated during pain empathy. Perhaps the AI and ACCs constitute a network that, firstly, allows an internal, somatovisceral and conscious representation of the other’s pain and, secondly, coordinates other brain functions to select adaptive behaviors such as empathic responses and prosocial behaviors.
A somatosensorimotor representation
The discovery of mirror neurons located in the ventral premotor cortex (F5) in nonhuman primates (Gallese et al., 1996) prompted affective and social neuroscientists to propose the perception–action hypothesis, which stipulates that the perception of others’ actions automatically activates the observer’s brain representations of these actions (Preston & De Waal, 2002). More specifically, according to this hypothesis, a ‘mirror mechanism would be at the basis of brain mechanisms that transform sensory representations of others’ behaviour into one’s own motor or visceromotor representations concerning that behaviour’ (Rizzolatti & Sinigaglia, 2016). Indeed, substantial evidence shows that observation of others’ actions is related to a network of cortical structures typically activated when executing those actions in human (see Caspers et al., 2010; Grosbras et al., 2012; Molenberghs et al., 2012, for meta-analyses). These include the IPL, the ventral premotor cortex and the caudal part of the IFG (see Figure 2 in Rizzolatti & Sinigaglia, 2016). Additional activations in the SPL (Rizzolatti & Sinigaglia, 2016) and SMA (Mukamel et al., 2010) have also been associated with action execution and action observation. In line with this prediction, our results show activation of such networks during pain empathy, with consistent activation observed in the IPL, ventral premotor cortex, and caudal part of the IFG, SPL and SMA. The experience of pain is associated with expected or observable actions, such as the withdrawal of a hand from a noxious object, which might engage this ‘mirroring’ system at the basis of the perception–action coupling mechanism. In addition to an internal representation of the motor aspects of the other’s pain, an SS representation of the observed pain might also be triggered during pain empathy. Indeed, our general map of pain empathy showed consistent activations in the SS cortices and thalamus, brain areas related to the capacity to discriminate sensory characteristics of direct pain experience, such as the intensity, quality and localization (Buschnell et al., 1999; Peyron et al., 2000; Morisson et al., 2013). Thus, a shared neural representation of the other’s SS pain characteristics might unfold during pain observation. Taken together, our results support the view that mirroring mechanisms might be at the origin of the activation of an embodied somatosensorimotor representation of the other’s pain engaged during pain observation.
Processing social nonverbal communication cues
In addition to internal affective and somatosensorimotor representations of the other’s pain, the current results suggest that visual processing of social nonverbal communication cues is performed through both shared and partly distinct channels during pain observation. Indeed, our general ALE map revealed consistent activations in the ITG, more specifically in the EBA and the OFA. Moreover, the conjunction analysis revealed that these structures were commonly activated in paradigms using SS (i.e. limbs submitted to noxious stimulation) and EC cues (i.e. facial expression of pain). The EBA/OFA are structures classically related to visual perception of human body parts (Peelen & Downing, 2007). Furthermore, the general ALE map revealed activation in the amygdala, a structure associated with relevant biological movements, for instance, faces and bodies expressing emotions (Adolphs et al., 1994; Adolphs, 2001; Atkinson & Adolphs, 2005). The amygdala is also well known to be related to negative emotions such as fear and anxiety (Davis, 1992), and a recent study showed that when instructed to rate their own affective responses to others’ emotional faces, variations in participants’ amygdala activation were related to the variations in their subjective responses (Seara-Cardoso et al., 2015). The body and the face are important agents of communication during social interactions, as they both allow us to express and communicate emotions (Peelen & Downing, 2007). As consistent and concurrent activities of the EBA/OFA and amygdala were found in the current work, these regions may work in concert to detect and process socioemotional nonverbal communication cues during pain empathy. From an evolutionary point of view, the processing of socioemotional cues has an adaptive function of signaling potential dangers to prepare fight-or-flight responses (Khatibi et al., 2015). When empathizing, the detection of socioemotional cues might be a basic process relevant in order to understand others’ EC cues, for instance, of fear and/or anxiety, associated with the pain experience and to initiate, if needed, fight-or-flight responses.
Our general ALE map across all selected fMRI studies on pain empathy provides quantitative meta-analytic evidence that several brain structures are implicated during pain observation; these brain structures subserve internal somatosensorimotor and affective representations of the other’s pain, in addition to processing relevant social nonverbal communication cues. However, these results do not indicate if some of these structures could be a part of secondary networks involved in the processing of specific aspects of the other’s pain experience or are related to different cognitive processes in the observers, such as taking different perspectives. ALE conjunction and subtraction analyses were performed to examine more closely how different visual pain cues and observers’ perspectives might be related to specific networks.
Secondary networks implicated during pain empathy
Visual cues
In addition to a core empathy network, secondary networks related to specific pain visual cues have been identified. Indeed, subtraction analysis revealed that SS cues (i.e. observation of body parts in painful situations) were related to activations in the SPL and IPL, in addition to the AI bilaterally. Pain empathy studies show that SS pain information generally involves action understanding (inferior parietal/ventral premotor cortices) and SS processes to a greater extent compared to other types of pain visual information, such as abstract cues (Lamm et al., 2011) or EC pain information (Timmers et al., 2018). In addition, as mentioned earlier, the AI is a key node permitting interoceptive and subjective representations of pain experience/observation and encoding evaluative aspects of pain. The results of the current work extend these findings and establish a distinct neural network engaged by the observation of SS pain cues during pain empathy. This type of pain visual cues seems to engage action understanding, SS sensation processes, and embodied conscious pain representation, and thereby probably engage a somatosensorimotor pain representation to a greater extent compared to EC pain cues.
In contrast, EC pain cues (i.e. facial expression of pain) were related to specific activations in the bilateral IFG (BA 45/47). The present results reveal new findings not reported in a recent ALE meta-analysis also examining differences between EC and SS pain cues (Timmers et al., 2018). Indeed, the study by Timmers et al. (2018) did not show distinct higher ALE values for EC pain cues compared to SS pain cues, as was found in the present study. The divergence of results may be related to differences in the number and selection of studies. Timmers et al. (2018) included 48 experiments that used an SS pain paradigm and 22 experiments that used an EC pain paradigm for their ALE contrasts analyses. In our work, we included 53 experiments in the SS pain condition and 25 experiments in the EC pain condition for our ALE contrasts analyses. In addition, the included experiments were not extracted from exactly the same studies. For instance, in the current work, some studies were excluded during the selection of the studies process, as they used complex visual scenes as stimulus or were not reporting results from a healthy adult population, and consequently were not included in the EC pain condition, which were included in Timmers et al. (2018). Inversely, some studies were included in the current meta-analysis (e.g. Budell et al., 2010; Chiesa et al., 2017), which were not in Timmers et al. (2018). It is, however, difficult to determine why these studies were not included in Timmers et al. (2018), as the inclusion/exclusion criteria for each condition (e.g. EC pain paradigm) were not specified in details. Thereby, a direct comparison of results of both meta-analyses based on the selected studies for each condition is hazardous. Another explanation could be related to differences in the choice of statistical thresholds. In Timmers et al. (2018), images were thresholded to a corrected P < 0.05 level using a cluster-level inference (5000 permutations, initial cluster-forming threshold of FDR P < 0.01). In the current work, images were thresholded at first to an FDRpN P < 0.05 and then to an uncorrected P < 0.001 using a cluster-level inference (5000 permutations, initial cluster-forming threshold of FDR P < 0.01). Clusters were found in the bilateral IFG with the uncorrected P < 0.001, whereas no cluster was found with an FDRpN P < 0.05. Thereby, differences in results between Timmers et al. (2018) meta-analysis and current meta-analysis might be related to differences in thresholds. We cannot exclude that the distinct activation found in the bilateral inferior frontal lobule for the EC pain cue compared to somatosensory pain cue might be a false positive (as an uncorrected P < 0.001 increases the rate of false positives). However, the large number of empirical studies (e.g. Danziger et al., 2009; Vachon-Presseau et al., 2012), as well as qualitative reviews and theoretical papers (e.g. Decety & Jackson, 2004; Tremblay et al., 2018; De Vignemont & Singer, 2006) supporting the functional dissociation between SS and EC pain cues during pain communication (e.g. Hadjistavropoulos et al., 2011) and empathy (e.g. De Waal & Preston, 2017), instills confidence in the present findings. The absence of effect found in Timmers et al. (2018) might be related to a false negative due to a more conservative statistical control. A role for the IFG in the distinctive processing of EC cues appears highly plausible based on the available literature.
There are many studies supporting the multifunctional role of the IFG in human nonverbal communication during interpersonal interactions, including through intentional body movements (emblematic gestures; Lindenberg et al., 2012) and facial expression of pain (see Xiong et al., 2019, for a review). Motor mirroring processes located in the IFG may allow human to understand others’ communicative gestures, including facial expressions (Iacoboni et al., 1999; Heiser et al., 2003; Koski et al., 2002). As the IFG is a structure classically related to speech production (Damasio, 1992), as well as face imitation (Budell et al., 2010; Carr et al., 2003; Iacoboni et al., 1999), social-affective researchers have suggested that there may be a common evolutionary root between speech production and gesture recognition, which would permit the understanding of others’ communicational cues during nonverbal interactions (Liakakis et al., 2011) and more broadly during empathy (Decety & Chaminade, 2003; Seitz et al., 2008).
Our results are also consistent with other studies showing the involvement of the IFG during empathy (Carr et al., 2003; Liakakis et al., 2011). In particularly, the left IFG is found to be related to the evaluation of emotional face expressions (Seitz et al., 2008). In Budell et al. (2010, 2015), the evaluation and production of meaningful facial expressions of pain were related to a greater activation in the IFG compared to the discrimination and imitation of motor components of the same facial expressions. In another study, a group of patients with lesions in the either left (n = 3) or right (n = 5) IFG (BA 44/45) showed altered emotional empathic abilities, as measured by the two affective scales (personal distress and empathic concern) of the Interpersonal Reactivity Index (Davis, 1980). These results suggest that the IFG not only contributes to understanding others by imitation but also subserves an affective/evaluative aspect of the other’s pain experience. In sum, our results are in line with previous studies supporting the important role of the IFG in the evaluative/affective component of the other’s pain when the pain experience is communicated through sociocommunicative cues. Facial expressions of pain may be a symbolic and abstract gesture of the affective pain experience shared by others.
Taken together, results of the current work expand upon previous findings and add robustness to the neural dissociation related to distinct pain visual cues. Our results, which are based on a large data set of neuroimaging literature, establish a neural dissociation between SS and EC pain information during pain empathy. SS cues activated brain areas associated with somatosensorimotor resonance (SPL, IPL, AI), whereas EC cues activated distinctly regions related to social nonverbal communication, such as gestures and affective resonance (IFG).
Visuospatial perspective
Unlike visual pain information, differences in visuospatial perspectives do not seem to recruit specific neural networks, although differences in activation were found in a number of brain regions. Indeed, based on the subtraction analyses, no specific clusters were found to be consistently activated for the 1PP or the 3PP. However, when examining the single ALE map of both perspectives separately, some differences can be observed. A first-person compared to a third-person visuospatial perspective seems to evoke a larger number of brain activations related to somatosensorimotor resonance (SPL, IFG, premotor and SMA cortices) and visual perception of body parts (ITG/EBA), suggesting that a 1PP results in a more robust embodied self-pain representation. This result is consistent with previous studies documenting the effect of the visuospatial perspective on neural response during pain observation. Pain assessment responses are faster (Vistoli et al., 2016), pain evaluation is higher (Canizales et al., 2013), and brain activity is greater (Canizales et al., 2013; Vistoli et al., 2016) when viewing limbs in noxious situations from a 1PP compared to a 3PP. In sum, a 1PP seems to facilitate pain somatosensorimotor resonance, but such differences should be interpreted with caution given the absence of significant effect in the contrast analyses. Future brain imaging research should further examine how activity within those areas relates to relevant behavioral responses to pain in self and others.
Cognitive perspective
To date, studies on cognitive perspective during pain observation are inconsistent and limited. On the one hand, studies show that imagining oneself in pain during pain empathy paradigms is related to higher self-ratings (Jackson et al., 2006a; Cheng et al., 2010) and enhanced activation in brain structures related to SS resonance (SII) and affective resonance (ACC and insula) (Jackson et al., 2006a; Lamm et al., 2007b; Cheng et al., 2010). For instance, Lamm et al. (2007b) noted a higher signal change associated with a self-oriented perspective in the middle insula and PI compared to adopting the perspective of a sufferer. On the other hand, in the study by Vistoli et al. (2016), no effect of the instruction was identified at either the behavioral or neural level, regardless of the visuospatial perspective. The current study provided a meta-analytic examination of the effect of instructions designed to orient the participant’s perspective toward the self- or the other’s pain.
Based on the subtraction analysis, the current study showed no specific activation related to a self- or other-oriented perspective considering only paradigms using SS pain visual cues. However, when considering paradigms using SS and EC visual cues, specific activations were found in the left AI for the self- compared to other-oriented perspective. A self-oriented instruction seems to rely more on an embodied pain representation compared to other-oriented instruction, but this effect is found only when both SS and EC pain cues are used. As shown earlier, the present study tend to support the idea that SS compared to EC pain visual information enhance activity in regions related to pain somatosensorimotor resonance (i.e. superior parietal lobule, IPL, and AI) to a greater extent. Perhaps, when using an instruction designed to modulate the participant’s perspective on top of viewing SS visual cues only, the effect of instructions is too small to be detected.
The current research also demonstrates that instructing participants to direct their attention toward the stimulus (stimulus-oriented perspective) during pain empathy was related to activations in specific regions compared to an instruction orienting the participants’ attention toward the others, namely, in the anterior lobe of the cerebellum, MOG/IOG, IPL and PreCG/IFG. The attention network model of Corbetta & Shulman (2002) suggests a large-scale dorsal frontoparietal network that embodies orienting attention processes to the external environment by sending top-down biasing signal to sensory input. Indeed, if a person directs his or her attention toward a particular sensory modality, information processing is greatly facilitated for the corresponding stimuli and suppressed for the nonattended stimuli (Posner, 1980). Moreover, activation can increase in the bilateral inferior frontal areas and in the anterior core of the dorsal network during tasks where participants are required to detect a target presented in a stream of frequent and standard stimuli (see Kim, 2014, for a quantitative fMRI meta-analysis). Here, the higher ALE values in occipital and frontoparietal areas for stimulus-oriented compared to other-oriented instructions may reflect a stronger exteroceptive attentional focus in the former condition. Rather than allowing participants to process pain stimuli according to how they naturally attend to the other’s emotional state, a stimulus-oriented instruction could shift the participants’ attention more toward the visual features of the stimuli. In other words, using a stimuli-oriented instruction during a pain empathy task seems to increase top-down attentional control subserved by the dorsal frontoparietal network.
Finally, when comparing the single ALE maps for the self- and other-oriented perspectives, a larger number of brain regions related to the pain empathy network are observed for the other-oriented perspective. A possible explanation may be related to the greater variability in the instructions verbatim used to orient the participants’ perspective and spontaneous strategies adopted by participants to orient their perspective. Across the studies using a self-oriented instruction, some provided more precise instructions than others on how to adopt a self-oriented perspective. For example, some studies requested participants to ‘try to experience the feelings of the person whose body part is shown in the pictures’ (e.g. Gao et al., 2017). This type of formulation is more specific and may help participants accomplish the task more adequately and minimize heterogeneity across participants. Other studies using a self-oriented instruction mentioned to ‘take their own perspective’ (e.g. Van Der Heiden et al., 2013). This type of instruction is more abstract and may be interpreted differently between participants. On the other hand, studies using other-oriented instructions usually used either a pain discrimination task or a pain evaluation task. In these studies, instructions are generally more precise and clear. This type of task may engage participants more in the other’s pain experience and therefore in a more embodied pain experience.
Another explication could be that some studies included a behavioral measurement (e.g. rating the level of unpleasantness and/or of intensity of the pain or rating the amount of the pain on a visual analog scale or a Likert scale) while others not, and this is not perfectly distributed across our conditions over the whole sample of studies. Also, for studies that included a behavioral measurement, the rating period could be before, during or after the scanning session and thereby be or not part of the targeted period of interest. From a set of 15 experiments that used an SS paradigm included in the self-perspective condition, 47% were asking the participants to rate something during the scanning session. From a set of 17 experiments that used either an SS or an EC paradigm included in the self-perspective condition, 47% were using rating during the scanning session. From the set of 36 experiments that used an SS paradigm included in the other-perspective condition, 67% were using rating during the scanning session. From the set of 47 experiments that used either an SS paradigm or an EC paradigm included in the other-perspective condition, 72% were using rating during the scanning session. All these differences related to the behavioral measures (i.e. the moment of rating, the type of rating, the instructions of the ratings, etc.) are all possible confounding variables and could have influenced the processes (e.g. motor, anticipation, action, monitoring, etc.) at play during the conditions. See column Behavioral Measures in Supplementary Table 1 for more details on these methodological differences.
Limitations
Several efforts have been made to carry out an exhaustive search and to obtain a maximum of experiments in each condition. Nevertheless, three conditions had a low number of experiments for the ALE quantitative analysis (n = 10 for third-person visuospatial perspective, n = 15 for self-oriented cognitive perspective, n = 8 for stimuli-oriented cognitive perspective). Eickhoff et al. (2016) suggested that an ALE meta-analysis requires at least 20 experiments to achieve a reasonable power. Meanwhile, the results in Bossier et al. (2018) indicate a relatively good balance between type I and II errors, with conditions involving only 10 experiments. An up-to-date meta-analysis might be necessary in the near future to strengthen or perhaps to nuance the available findings and interpretations once additional fMRI studies of pain empathy are conducted for these more specific conditions. Another explanation for the lack of significant results for some of the analyses conducted, in particular the subtraction analyses, can possibly stem from the methodological discrepancies among the experimental designs across studies. Indeed, other factors might contribute to the heterogeneity across studies: methodological factors, such as the inclusion or not of a behavioral measure (i.e. to rate different aspects of the pain) during the scanning session, characteristics of the targets depicted in the stimuli and/or sociodemographic characteristics of participants.
Conclusions
In conclusion, undertaking a quantitative meta-analysis of neuroimaging studies on pain empathy and comparing the influence of different pain empathy paradigms on the brain response are essential steps to gain an improved understanding the neurobiological basis of human empathy. Synthesizing the abundance of fMRI reports on pain empathy allows for improved sample size, statistical power and validity compared to considering only results from single neuroimaging studies. The current meta-analysis demonstrates that empathy recruits different neural networks associated with affective resonance, somatosensorimotor resonance and the processing of nonverbal socioemotional communicational cues. Moreover, this work provides evidence that differences in emotional visual information, visuospatial perspective and cognitive perspective taking involve the brain networks underpinning empathy to varying degrees. In addition, results suggest that pain empathy paradigms are particularly efficient in recruiting brain structures that are related to the affective components of empathy (i.e. AI and aMCC/ACC), as well as the regions related to pain somatosensorimotor resonance (i.e. IPL, SPL and IFG). This highlights the need to develop other paradigms that would particularly solicit other components of empathy, such as the cognitive component of empathy, or emotion regulation processes in the context of pain, as this would help to broaden our understanding of empathy processes and their related neural correlates. Finally, although different neural networks implicated in empathy have been identified, it remains unclear how these networks are interrelated. As empathy emanates from several interacting components, fMRI studies using global multivoxel pattern analysis and investigating connectivity patterns would be relevant in order to yield stronger interpretations of the interrelations between these networks.
Conflict of interest
The authors certify that they have no actual or potential conflicts of interest regarding the research reported in this article.
Supplementary Material
Acknowledgments
The authors are grateful to the researchers who kindly responded to their request for information on their studies and who provided additional results. The authors are also grateful to the members of the laboratory who helped revise the manuscript, especially Stephanie Sutliff.
References
- Adolphs R. (2001). The neurobiology of social cognition. Current Opinion in Neurobiology, 11, 231–9. [DOI] [PubMed] [Google Scholar]
- Adolphs R., Tranel D., Damasio H., Damasio A. (1994). Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature, 372(6507), 669–72. [DOI] [PubMed] [Google Scholar]
- Akitsuki Y., Decety J. (2009). Social context and perceived agency affects empathy for pain: an event-related fMRI investigation. NeuroImage, 47(2), 722–34. [DOI] [PubMed] [Google Scholar]
- Atkinson A.P., Adolphs R. (2005). Visual emotion perception: mechanisms and processes In: Barett L.F., Niedenthal P.M., Winkielman P., editors. Emotion and Consciousness, New York: Guilford Press, pp. 150–82. [Google Scholar]
- Azevedo R.T., Macaluso E., Avenanti A., Santangelo V., Cazzato V., Aglioti S.M. (2013). Their pain is not our pain: brain and autonomic correlates of empathic resonance with the pain of same and different race individuals. Human Brain Mapping, 34(12), 3168–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo R.T., Macaluso E., Viola V., Sani G., Aglioti S.M. (2014). Weighing the stigma of weight: an fMRI study of neural reactivity to the pain of obese individuals. NeuroImage, 91, 109–19. [DOI] [PubMed] [Google Scholar]
- Bastuji H., Frot M., Perchet C., Magnin M., Garcia-Larrea L. (2016). Pain networks from the inside: spatiotemporal analysis of brain responses leading from nociception to conscious perception. Human Brain Mapping, 37(12), 4301–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benuzzi F., Lui F., Duzzi D., Nichelli P.F., Porro C.A. (2008). Does it look painful or disgusting? Ask your parietal and cingulate cortex. The Journal of Neuroscience, 28(4), 923–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlingeri M., Gallucci M., Danelli L., Forgiarini M., Sberna M., Paulesu E. (2016). Guess who's coming to dinner: brain signatures of racially biased and politically correct behaviors. Neuroscience, 332, 231–41. [DOI] [PubMed] [Google Scholar]
- Bernhardt B.B., Singer T. (2012). The neural basis of empathy. Annual Review of Neuroscience, 35, 1–23. [DOI] [PubMed] [Google Scholar]
- Bos P.A., Montoya E.R., Hermans E.J., Keysers C., Van Honk J. (2015). Oxytocin reduces neural activity in the pain circuitry when seeing pain in others. NeuroImage, 113, 217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossier H., Seurinck R., Kühn S., Banaschewski T., Barker J.G., Bokde W.L.A., Martinot J–L, Lemaitre H., Paus T., Millenet S., Moerkerke B., 2018. The influence of study-level inference models and study set size on coordinate-based fMRI meta-analyses. Frontiers in Neuroscience 11:745, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M., Jha A.P., Bylsma L.M., Fabian S.A., Solomon P.E., Prkachin K.M. (2005). Viewing facial expressions of pain engages cortical areas involved in the direct experience of pain. NeuroImage, 25(1), 312–9. [DOI] [PubMed] [Google Scholar]
- Braboszcz C., Brandao-Farinelli E., Vuilleumier P. (2017). Hypnotic analgesia reduces brain responses to pain seen in others. Scientific Reports, 7(9778), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budell L., Jackson P., Rainville P. (2010). Brain responses to facial expressions of pain: emotional or motor mirroring? NeuroImage, 53(1), 355–63. [DOI] [PubMed] [Google Scholar]
- Budell L., Kunz M., Jackson P.L., Rainville P. (2015). Mirroring pain in the brain: emotional expression versus motor imitation. PLoS One, 10(2), 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschnell M.C., Duncan G.H., Hofbauer R.K., Ha B., Chen J.I., Carrier B. (1999). Pain perception: is there a role for primary somatosensory cortex? Proceedings of the National Academy of Sciences, 96, 7705–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D., Schilbach L., Vogeley K., Schneider K., Laird A.R., Langner R., Eickhoff S.B. (2012). Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Structure & Function, 217(4), 783–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canizales D.L., Voisin J.I.A., Michon P.E., Roy M.A., Jackson P.L. (2013). The influence of visual perspective on the somatosensory steady-state response during pain observation. Frontiers in Human Neuroscience, 7(849), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Contreras-Huerta L.S., McFadyen J., Cunnington R. (2015). Racial bias in neural response to others' pain is reduced with other-race contact. Cortex, 70, 68–78. [DOI] [PubMed] [Google Scholar]
- Carr L., Iacoboni M., Dubeau M.C., Mazziotta J.C., Lenzi G.L. (2003). Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proceedings of the National Academy of Sciences of the United States of America, 100, 5497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S., Zilles K., Laird A.R., Eickhoff S.B. (2010). ALE meta-analysis of action observation and imitation in the human brain. NeuroImage, 50, 1148–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.C., Chen C.C., Decety J., Cheng Y. (2014). Aging is associated with changes in the neural circuits underlying empathy. Neurobiology of Aging, 35(4), 827–36. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Lin C.P., Liu H.L., Hsu Y.Y., Lim K.E., Hung D., Decety J. (2007). Expertise modulates the perception of pain in others. Current Biology, 17(19), 1708–13. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Chen C., Lin C.P., Chou K.H., Decety J. (2010). Love hurts: an fMRI study. NeuroImage, 51(2), 923–9. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Chen C., Decety J. (2017). How situational context impacts empathic responses and brain activation patterns. Frontiers in Behavioral Neuroscience, 11(165), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon B.K., Im D.-M., Harada T., et al. (2013). Cultural modulation of the neural correlates of emotional pain perception: the role of other-focusedness. Neuropsychologia, 51, 1177–86. [DOI] [PubMed] [Google Scholar]
- Chiao J.Y., Mathur V.A., Harada T., Lipke T. (2009). Neural basis of preference for human social hierarchy versus egalitarianism. Ann. N.Y Academy of Sciences, 1167, 174–81. [DOI] [PubMed] [Google Scholar]
- Chiesa P.A., Liuzza M.T., Macaluso E., Aglioti S.M. (2017). Brain activity induced by implicit processing of other’s pain and pleasure. Human Brain Mapping, 38, 5562–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christov-Moore L., Iacoboni M. (2016). Self–other resonance, its control and prosocial inclinations: brain–behavior relationships. Human Brain Mapping, 37(4), 1544–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christov-Moore L., Conway P., Iacoboni M., 2017. Deontological dilemma response tendencies and sensorimotor representations of harm to others. Front. Intefr. Neurosci. 11. 34, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll M.-P., Jackson P.L. (2016). Beyond action: shared representations in non-motor domains In: Obhi S.S., Cross E.S., editors. Shared Representations: Sensorimotor Foundations of Social Life, Cambridge, U.K.: Cambridge University Press, pp. 59–85. [Google Scholar]
- Coll M.-P., Grégoire M., Eugène F., Jackson P.L. (2017). Neural correlates of prosocial behavior towards persons in pain in healthcare providers. Biological Psychology, 128, 1–10. [DOI] [PubMed] [Google Scholar]
- Contreras-Huerta L.S., Baker K.S., Reynolds K.J., Batalha L., Cunnington R. (2013). Racial bias in neural empathic responses to pain. PLoS One, 8(12), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. (2002). Control of goal-directed andstimulus-driven attention in the brain. Nature Reviews. Neuroscience, 3, 201–15. [DOI] [PubMed] [Google Scholar]
- Corradi-Dell'Acqua C., Hofstetter C., Vuilleumier P. (2011). Felt and seen pain evoke the same local patterns of cortical activity in insular and cingulate cortex. The Journal of Neuroscience, 31(49), 17996–8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini M., Galata G., Romani L.G., Aglioti S.M. (2008). Empathic neural reactivity to noxious stimuli delivered to body parts and non-corporeal objects. The European Journal of Neuroscience, 28(6), 1222–30. [DOI] [PubMed] [Google Scholar]
- Craig A.D. (2003). Interoception: the sense of the physiological condition of the body. Current Opinion in Neurobiology, 13(4), 500–5. [DOI] [PubMed] [Google Scholar]
- Craig A.D. (2009). How do you feel now? The anterior insula and human awareness. Nature Reviews. Neuroscience, 10(1), 59–70. [DOI] [PubMed] [Google Scholar]
- Cui F., Abdelgabar A.-R., Keysers C., Gazzola V. (2015). Responsibility modulates pain-matrix activation elicited by the expressions of others in pain. NeuroImage, 114, 371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio A.R. (1992). Aphasia. N. Engl. J. Med., 326, 531–9. [DOI] [PubMed] [Google Scholar]
- Danziger N., Faillenot I., Peyron R. (2009). Can we share a pain we never felt? Neural correlates of empathy in patients with congenital insensitivity to pain. Neuron, 61(2), 203–12. [DOI] [PubMed] [Google Scholar]
- Davis M.H. (1980). A multidimensional approach to individual differences in empathy. Journal Supplemental Abstract Service Catalog of Selected Documents in Psychology, 10, 85. [Google Scholar]
- Davis M.H. (1992). The role of the amygdala in fear and anxiety. Annual Review of Neuroscience, 15, 353–75. [DOI] [PubMed] [Google Scholar]
- De Vignemont F., Singer T. (2006). The empathic brain: how, when and why? Trends in Cognitive Sciences, 10(10), 435–41. [DOI] [PubMed] [Google Scholar]
- De Waal F.B.M., Preston S.D. (2017). Mammalian empathy: behavioural manifestations and neural basis. Nature Reviews. Neuroscience, 8, 498–509. [DOI] [PubMed] [Google Scholar]
- Decety J., Chaminade T. (2003). Neural correlates of feeling sympathy. Neuropsychologia, 41, 127–38. [DOI] [PubMed] [Google Scholar]
- Decety J., Jackson P.L. (2004). The functional architecture of human empathy. Behavioral and Cognitive Neuroscience Reviews, 2, 71–100. [DOI] [PubMed] [Google Scholar]
- Decety J., Jackson P.L. (2006). A social-neuroscience perspective on empathy. Current Directions in Psychological Science, 15(2), 54–8. [Google Scholar]
- Decety J., Lamm C. (2006). Human empathy through the lens of social neuroscience. ScientificWorldJournal, 6, 1146–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J., Meyer M. (2008). From emotion resonance to empathic understanding: a social developmental neuroscience account. Development and Psychopathology, 20(4), 1053–80. [DOI] [PubMed] [Google Scholar]
- Decety J., Michalska K.J. (2010). Neurodevelopmental changes in the circuits underlying empathy and sympathy from childhood to adulthood. Develop. Sci., 13(6), 886–99. [DOI] [PubMed] [Google Scholar]
- Decety J., Jackson P.L., Brunet E. (2007). The cognitive neuropsychology of empathy. Empathy in Mental Illness, 239–744. [Google Scholar]
- Decety J., Echols S., Correll J. (2009). The blame game: the effect of responsibility and social stigma on empathy for pain. Journal of Cognitive Neuroscience, 22(5), 985–97. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Laird A.R., Grefkes C., Wang L.E., Zilles K., Fox P.T. (2009). Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping, 30(9), 2907–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S.B., Bzdok D., Laird A.R., Kurth F., Fox P.T. (2012). Activation likelihood estimation meta-analysis revisited. NeuroImage, 59(3), 2349–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S.B., Nichols T.E., Laird A.R., et al. (2016). Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. NeuroImage, 137, 70–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S.B., Laird A.R., Fox P.M., Lancaster J.L., Fox P.T. (2017). Implementation errors in the gingerALE software: description and recommendations. Human Brain Mapping, 38(1), 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N., Eggum N.D. (2009). Empathic responding: sympathy and personal distress. The Social Neuroscience of Empathy., 6, 71–83. [Google Scholar]
- Enzi B., Amirie S., Brüme M. (2016). Empathy for pain-related dorsolateral prefrontal activity is modulated by angry face perception. Experimental Brain Research, 234, 3335–45. [DOI] [PubMed] [Google Scholar]
- Fan Y., Duncan N.W., Greck M., Northoff G., 2011. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neurosci. Bioehav. Rev. 35(3), 903–11. [DOI] [PubMed] [Google Scholar]
- Fan Y.T., Chen C., Chen S.C., Decety J., Cheng Y. (2014). Empathic arousal and social understanding in individuals with autism: evidence from fMRI and ERP measurements. Social Cognitive and Affective Neuroscience, 9(8), 1203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C., Li Z., Feng X., Wang L., Tian T., Luo Y.J. (2016). Social hierarchy modulates neural responses of empathy for pain. Social Cognitive and Affective Neuroscience, 11(3), 485–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourie M.M., Stein D.J., Solms M., Gobodo-Madikizela P., Decety J. (2017). Empathy and moral emotions in post-apartheid South Africa: an fMRI investigation. Social Cognitive and Affective Neuroscience, 881-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Corbetta M., Snyder A.Z., Vincent J.L., Raichle M.E. (2006). Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proceedings of the National Academy of Sciences of the United States of America, 103(26), 10046–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox P.T., Laird A.R., Eickhoff S.B., Lancaster J.L., Fox M., Uecker A.M., Ray K.L. (2013). User Manual for GingerALE 2.3, San Antonio, TX: UT Health Science left San Antonio. [Google Scholar]
- Frot M., Faillenot I., Maugière F. (2014). Processing of nocieceptive input from posterior to anterior insula in humans. Human Brain Mapping, 35, 5486–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino J., Yamasaki N., Miyata J., et al. (2014). Altered brain response to others′ pain in major depressive disorder. Journal of Affective Disorders, 165, 170–5. [DOI] [PubMed] [Google Scholar]
- Gallese V., Fadiga L., Fogassi L., Rizzolatti G. (1996). Action recognition in the premotor cortex. Brain, 119, 593–609. [DOI] [PubMed] [Google Scholar]
- Gao X., Pan W., Li C., Weng L., Yao M., Chen A. (2017). Long-time exposure to violent video games does not show desensitization on empathy for pain: an fMRI study. Frontiers in Psychology, 8(650), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Larrea L., Jackson P.L. (2016). Pain and the Conscious Brain, Lippincott Williams & Wilkins. [Google Scholar]
- Garcia-Larrea L., Peyron R. (2013). Pain matrices and neuropathic pain matrices: a review. Pain, 154, S29–43. [DOI] [PubMed] [Google Scholar]
- Grice-Jackson T., Critchley H.D., Banissy M.J., Ward J. (2017). Consciously feeling the pain of others reflects atypical functional connectivity between the pain matrix and frontal-parietal regions. Front. Hum. Neursci., 11(507), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosbras M.H., Beaton S., Eickhoff S.B. (2012). Brain regions involved in human movement perception: a quantitative voxel-based meta-analysis. Human Brain Mapping, 33, 431–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X., Han S. (2007). Attention and reality constraints on the neural processes of empathy for pain. NeuroImage, 36(1), 256–67. [DOI] [PubMed] [Google Scholar]
- Gu X., Liu X., Guise K.G., Naidich T.P., Hof P.R., Fan J. (2010). Functional dissociation of the frontoinsular and anterior cingulate cortices in empathy for pain. The Journal of Neuroscience, 30(10), 3739–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X., Liu X., Van Dam N.T., Hof P.R., Fan J. (2013). Cognition–emotion integration in the anterior insular cortex. Cerebral Cortex, 23(1), 20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X., Eilam-Stock T., Zhou T., et al. (2015). Autonomic and brain responses associated with empathy deficits in autism spectrum disorder. Human Brain Mapping, 36(9), 3323–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Zheng L., Zhang W., et al. (2012). Empathic neural responses to others’ pain depend on monetary reward. 30(10), 3227–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Zheng L., Wang H., et al. (2013). Exposure to violence reduces empathetic responses to other’s pain. Brain and Cognition, 82(2), 187–91. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N., Zürcher N.R., Rogier O., et al. (2014). Emotional contagion for pain is intact in autism spectrum disorders. Translational Psychiatry, 4(e343), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjistavropoulos T., Craig K.D., Duck S., et al. (2011). A biopsychosocial formulation of pain communication. Psychological Bulletin, 137(6), 910. [DOI] [PubMed] [Google Scholar]
- Han S., Fan Y., Xu X., et al. (2009). Empathic neural responses to others’ pain are modulated by emotional contexts. Human Brain Mapping, 30(10), 3227–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., He K., Wu B., et al. (2017). Empathy for pain motivates actions without altruistic effects: evidence of motor dynamics and barin activity. Social Cognitive and Affective Neuroscience, 12(6), 893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein G., Silani G., Preuschoff K., Batson C.D., Singer T. (2010). Neural responses to ingroup and outgroup members' suffering predict individual differences in costly helping. Neuron, 68(1), 149–60. [DOI] [PubMed] [Google Scholar]
- Heiser M., Iacoboni M., Maeda F., Marcus J., Mazziotta J.C. (2003). The essential role of Broca’s area in imitation. The European Journal of Neuroscience, 17, 1123–8. [DOI] [PubMed] [Google Scholar]
- Hu Y., Cui Z., Fan M., Pei Y., Wang Z. (2018). Effects of acute alcohol intoxication on empathic neural responses for pain. Frontiers in Human Neuroscience, 11(640), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M., Woods R.P., Brass M., Bekkering H., Mazziotta J.C., Rizzolatti G. (1999). Cortical mechanisms of human imitation. Science, 286, 2626–8. [DOI] [PubMed] [Google Scholar]
- Jackson P.L., Meltzoff A.N., Decety J. (2005). How do we perceive the pain of others? A window into the neural processes involved in empathy. NeuroImage, 24(3), 771–9. [DOI] [PubMed] [Google Scholar]
- Jackson P.L., Brunet E., Meltzoff A.N., Decety J. (2006a). Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia, 44(5), 752–61. [DOI] [PubMed] [Google Scholar]
- Jackson P.L., Rainville P., Decety J. (2006b). To what extent do we share the pain of others? Insight from the neural bases of pain empathy. Pain, 125(1), 5–9. [DOI] [PubMed] [Google Scholar]
- Jackson P.L., Latimer M., Eugène F., MacLeod E., Hatfield T., Vachon-Presseau E., Michon P.-E. (2017). Empathy in paediatric intensive care nurses part 2: neural correlates. Journal of Advanced Nursing, 73(11), 2686–95. [DOI] [PubMed] [Google Scholar]
- Jankowiak-Siuda K., Rymarczyk K., Żurawski Ł., Jednoróg K., Marchewka A. (2015). Physical attractiveness and sex as modulatory factors of empathic brain responses to pain. Frontiers in Behavioral Neuroscience, 9(236), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatibi A., Schrooten M., Bosmans K., Volders S., Viaeyen J.W.S., Van den Bussche E. (2015). Sub-optimal presentation of painful facial expressions enhances readiness for action and pain perception following electrocutaneous stimulation. Frontiers in Psychology, 6(913), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. (2014). Involvement of the dorsal and ventral attention networks in oddball stimulus processing: a meta-analysis. Hum. Brain Map., 35(5), 2265–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski L., Wohlschläger A., Bekkering H., Woods R.P., Dubeau M.C., Mazziotta J.C., et al. (2002). Modulation of motor and premotor activity during imitation of targetdirected actions. Cerebral Cortex, 12, 847–55. [DOI] [PubMed] [Google Scholar]
- Krach S., Kamp-Becker I., Einhäuser W., et al. (2015). Evidence from pupillometry and fMRI indicates reduced neural response during vicarious social pain but not physical pain in autism. Human Brain Mapping, 36, 4730–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird A.R., Eickhoff S.B., Fox P.M., et al. (2011). The BrainMap strategy for standardization, sharing, and meta-analysis of neuroimaging data. BMC Research Notes., 4, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C., Decety J. (2008). Is the extrastriate body area (EBA) sensitive to the perception of pain in others? Cerebral Cortex, 18(10), 2369–73. [DOI] [PubMed] [Google Scholar]
- Lamm C., Nusbaum H.C., Meltzoff A.N., Decety J. (2007a). What are you feeling? Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain. PLoS One, 12(e1292), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C., Batson C.D., Decety J. (2007b). The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. Journal of Cognitive Neuroscience, 19(1), 42–58. [DOI] [PubMed] [Google Scholar]
- Lamm C., Meltzoff A.N., Decety J. (2010). How do we empathize with someone who is not like us? A functional magnetic resonance imaging study. Journal of Cognitive Neuroscience, 22(2), 362–76. [DOI] [PubMed] [Google Scholar]
- Lamm C., Decety J., Singer T. (2011). Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage, 54(3), 2492–502. [DOI] [PubMed] [Google Scholar]
- Lancaster J.L., Tordesillas-Gutiérrez D., Martinez M., et al. (2007). Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Human Brain Mapping, 28(11), 1194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen H.R., Siebner H.R., Haren T., Madsen K., Grønlund R., Hulme O., Henningsson S. (2014). Variation in the oxytocin receptor gene is associated with behavioral and neural correlates of empathic accuracy. Frontiers in Behavioral Neuroscience, 8(423), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.J., Song H.J., Decety J., et al. (2013). Do patients with fibromyalgia show abnormal neural responses to the observation of pain in others? Neuroscience Research, 75(4), 305–15. [DOI] [PubMed] [Google Scholar]
- Legrain V., Iannetti G.D., Plaghki L., Mouraux A. (2011). The pain matrix reloaded: a salience detection system for the body. Progress in Neurobiology, 93(1), 111–24. [DOI] [PubMed] [Google Scholar]
- Li X., Liu Y., Luo S., Wu B., Wu X., Han S. (2015). Mortality salience enhances racial in-group bias in empathic neural responses to others’ suffering. NeuroImage, 118, 376–85. [DOI] [PubMed] [Google Scholar]
- Liakakis G., Nickel J., Seitz R.J. (2011). Diversity of the inferior frontal gyrus—a meta-analysis of neuroimaging studies. Behav. Brain Resear., 225, 341–7. [DOI] [PubMed] [Google Scholar]
- Lieberman M.D., Eisenberger N.I. (2015). The dorsal anterior cingulate cortex is selective for pain: results from large-scale reverse inference. Proceedings of the National Academy of Sciences of the United States of America, 112(49), 15250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberg R., Uhlig M., Scherfeld D., Schlaug G., Seitz R.J. (2012). Communication with emblematic gestures: shared and distinct neural correlates of expression and reception. Human Brain Mapping, 33(4), 812–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S., Shi Z., Yang X., Wang X., Han S. (2014). Reminders of mortality decrease midcingulate activity in response to others’ suffering. Social Cognitive and Affective Neuroscience, 9(4), 477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S., Li B., Ma Y., Zhang W., Rao Y., Han S. (2015). Oxytocin receptor gene and racial ingroup bias in empathy-related brain activity. NeuroImage, 110, 22–31. [DOI] [PubMed] [Google Scholar]
- Ma Y., Wang C., Han S. (2011). Neural responses to perceived pain in others predict real-life monetary donations in different socioeconomic contexts. NeuroImage, 57(3), 1273–80. [DOI] [PubMed] [Google Scholar]
- Masten C.L., Morelli S.A., Eisenberger N.I. (2011). An fMRI investigation of empathy for ‘social pain’ and subsequent prosocial behavior. NeuroImage, 55(1), 381–8. [DOI] [PubMed] [Google Scholar]
- Mazzola V., Latorre V., Petito A., et al. (2010). Affective response to a loved one's pain: insula activity as a function of individual differences. PLoS One, 5,12(e15268), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenberghs P., Cunnington R., Mattingley J.B. (2012). Brain regions with mirror properties: a meta-analysis of 125 human fMRI studies. Neuroscience and Biobehavioral Reviews, 36, 341–9. [DOI] [PubMed] [Google Scholar]
- Morelli S.A., Rameson L.T., Lieberman M.D. (2014). The neural components of empathy: predicting daily prosocial behavior. Social Cognitive and Affective Neuroscience, 9(1), 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli S.A., Lieberman M.D., Zaki J. (2015). The emerging study of positive empathy. Social and Personality Psychology Compass, 9(2), 57–68. [Google Scholar]
- Moriguchi Y., Decety J., Ohnishi T., et al. (2007). Empathy and judging other's pain: an fMRI study of alexithymia. Cerebral Cortex, 17(9), 2223–34. [DOI] [PubMed] [Google Scholar]
- Morisson I., Tipper S.P., Fenton-Adams W.L., Bach P. (2013). “Feeling” others’ painful actions: the sensorimotor integration of pain and action information. Human Brain Mapping, 34, 1982–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison I., Downing P.E. (2007). Organization of felt and seen pain responses in anterior cingulate cortex. NeuroImage, 37(2), 642–51. [DOI] [PubMed] [Google Scholar]
- Morrison I., Lloyd D., Di Pellegrino G., Roberts N. (2004). Vicarious responses to pain in anterior cingulate cortex: is empathy a multisensory issue? Cognitive, Affective, & Behavioral Neuroscience, 4(2), 270–8. [DOI] [PubMed] [Google Scholar]
- Morrison I., Peelen M.V., Downing P.E. (2007). The sight of others’ pain modulates motor processing in human cingulate cortex. Cerebral Cortex, 17(9), 2214–22. [DOI] [PubMed] [Google Scholar]
- Mukamel R., Ekstrom A.D., Kaplan J., Iacoboni M., Fried I. (2010). Single-neuron responses in humans during execution and observation of actions. Current Biology, 20, 750–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll-Hussong M., Otti A., Wohlschlaeger A.M., et al. (2013). Neural correlates of deficits in pain-related affective meaning construction in patients with chronic pain disorder. Psychosomatic Medicine, 75(2), 124–36. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L., Hirvonen J., Parkkola R., Hietanen J.K. (2008). Is emotional contagion special? An fMRI study on neural systems for affective and cognitive empathy. NeuroImage, 43(3), 571–80. [DOI] [PubMed] [Google Scholar]
- Patil I., Calo M., Fornasier F., Cushman F., Silani G. (2017). The behavioral and neural basis of empathic blame. Scientific Reports, 7(1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelen M.V., Downing P.E. (2007). The neural basis of visual body perception. Nature Reviews. Neuroscience, 8(8), 636–48. [DOI] [PubMed] [Google Scholar]
- Peyron R., Laurent B., Gardia-Larrea L. (2000). Functional imaging of brain responses to pain. A review and meta-analysis. Neurophysiologie Clinique, 30(5), 263–88. [DOI] [PubMed] [Google Scholar]
- Posner M. (1980). Orienting of attention. Quart. J. Exp. Psychol., 32, 3–25. [DOI] [PubMed] [Google Scholar]
- Preis M.A., Schmidt-Samoa C., Dechent P., Kroener-Herwig B. (2013). The effects of prior pain experience on neural correlates of empathy for pain: an fMRI study. Pain, 154(3), 411–8. [DOI] [PubMed] [Google Scholar]
- Preston S.D., Waal F.B.M., 2002. Empathy: its ultimate and proximate bases. The Behavioral and Brain Sciences 25, 1–72. [DOI] [PubMed] [Google Scholar]
- Quio-Tasserit E., Corradi-Dell’Acqua C., Vuilleumier P. (2017). The good, the bad, and the suffering. Transient emotional episodes modulate the neural circuits of pain and empathy. Neuropsychologia, 1–18. [DOI] [PubMed] [Google Scholar]
- Rainville P. (2002). Brain mechanisms of pain affect and pain modulation. Current Opinion in Neurobiology, 12, 195–204. [DOI] [PubMed] [Google Scholar]
- Rameson L.T., Morelli S.A., Lieberman M.D. (2012). The neural correlates of empathy: experience, automaticity, and prosocial behavior. Journal of Cognitive Neuroscience, 24(1), 235–45. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Sinigaglia C. (2016). The mirror mechanism: basic principal of brain function. Nature Reviews. Neuroscience, 17(12), 757–65. [DOI] [PubMed] [Google Scholar]
- Ruckmann J., Bodden M., Jansen A., Kircher T., Dodel R., Rief W. (2015). How pain empathy depends on ingroup/outgroup decisions: a functional magnet resonance imaging study. Psychiat. Res. Neuroim., 234(1), 57–65. [DOI] [PubMed] [Google Scholar]
- Saarela M.V., Hlushchuk Y., Williams A.C.D.C., Schürmann M., Kalso E., Hari R. (2007). The compassionate brain: humans detect intensity of pain from another’s face. Cerebral Cortex, 17(1), 230–7. [DOI] [PubMed] [Google Scholar]
- Schnell K., Bluschke S., Konradt B., Walter H. (2011). Functional relations of empathy and mentalizing: an fMRI study on the neural basis of cognitive empathy. NeuroImage, 54(2), 1743–54. [DOI] [PubMed] [Google Scholar]
- Seara-Cardoso A., Viding E., Lickley R.A., Sebastian C.L. (2015). Neural responses to others’ pain vary with psychopathic traits in healthy adult males. Cognitive, Affective, & Behavioral Neuroscience, 15(3), 578–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz R.J., Scherfeld D., Friederichs S., Popp K., Wittsack H.J., Azari N.P., et al. (2008). Valuating other people’s emotional face expression: a combined fMRI and EEG study. Neurosci., 152, 713–22. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory S.G. (2011). The neural bases for empathy. The Neuroscientist, 17(1), 18–24. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory S.G., Aharon-Peretz J., Perry D. (2009). Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain, 132(3), 617–27. [DOI] [PubMed] [Google Scholar]
- Sheng F., Liu Q., Li H., Fang F., Han S. (2014). Task modulations of racial bias in neural responses to others’ suffering. NeuroImage, 88, 263–70. [DOI] [PubMed] [Google Scholar]
- Shenhav A., Cohen J.D., Botvinick M.M. (2016). Dorsal anterior cingulate cortex and the value of control. Nature Neuroscience, 19(10), 1286–91. [DOI] [PubMed] [Google Scholar]
- Simon D., Craig K.D., Miltner W.H., Rainville P. (2006). Brain responses to dynamic facial expressions of pain. Pain, 126(1–3), 309–18. [DOI] [PubMed] [Google Scholar]
- Tamm S., Nilsonne G., Schwatz J., et al. (2017). The effect of sleep restriction on empathy for pain: an fMRI study in younger and older adults. Sci.Rep., 7(1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers I., Park A.L., Fischer M.D., Kronman C.A., Heathcote L.C., Hernandez J.M., Simons L.E. (2018). Is empathy for pain unique in its neural correlates? A meta-analysis of neuroimaging studies of empathy. Frontiers in Behavioral Neuroscience, 12, 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomava L., Majdandzic J., Hummer A., Windischberger C., Heinrichs M., Lamm C. (2017). Increased neural responses to empathy for pain might explain how acute stress increases prosociality. Social Cognitive and Affective Neuroscience, 12(3), 401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tousignant B., Eugène F., Jackson P.L. (2017). A developmental perspective on the neural bases of human empathy. Infant Behavior & Development, 48, 5–12. [DOI] [PubMed] [Google Scholar]
- Tremblay M.P.B., Meugnot A., Jackson P.L. (2018). The neural signature of empathy for physical pain … not quite there yet! In: Social and Interpersonal Dynamics in Pain, Cham: Springer, pp. 149–72. [Google Scholar]
- Turkeltaub P.E., Eickhoff S.B., Laird A.R., Fox M., Wiener M., Fox P. (2012). Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Human Brain Mapping, 33(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q. (2015). Salience processing and insular cortical function and dysfunction. Nature Reviews. Neuroscience, 16(1), 55–61. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q., Kinnison J., Pessoa L., Anderson M.L. (2014). Beyond the tripartite cognition–emotion–interoception model of the human insular cortex. Journal of Cognitive Neuroscience, 26(1), 16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushida T., Ikemoto T., Tanaka S., et al. (2008). Virtual needle pain stimuli activates cortical representation of emotions in normal volunteers. Neuroscience Letters, 439(1), 7–12. [DOI] [PubMed] [Google Scholar]
- Vachon-Presseau E., Roy M., Martel M.O., et al. (2012). Neural processing of sensory and emotional-communicative information associated with the perception of vicarious pain. NeuroImage, 63(1), 54–62. [DOI] [PubMed] [Google Scholar]
- Van Der Heiden L., Scherpiet S., Konicar L., Birbaumer N., Veit R. (2013). Inter-individual differences in successful perspective taking during pain perception mediates emotional responsiveness in self and others: an fMRI study. NeuroImage, 65, 387–94. [DOI] [PubMed] [Google Scholar]
- Vistoli D., Achim A.M., Lavoie M.A., Jackson P.L. (2016). Changes in visual perspective influence brain activity patterns during cognitive perspective-taking of other people's pain. Neuropsychologia, 85, 327–36. [DOI] [PubMed] [Google Scholar]
- Völlm B.A., Taylor A.N., Richardson P., et al. (2006). Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. NeuroImage, 29(1), 90–8. [DOI] [PubMed] [Google Scholar]
- Wager T.D., Sylvester C.Y.C., Lacey S.C., Nee D.E., Franklin M., Jonides J. (2005). Common and unique components of response inhibition revealed by fMRI. NeuroImage, 27(2), 323–40. [DOI] [PubMed] [Google Scholar]
- Wager T.D., Atlas L.Y., Botvinick M.A., et al. (2016). Pain in the ACC? Proceedings of the National Academy of Sciences of the United States of America, 113(18), E2474–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Wu B., Liu Y., Wu X., Han S. (2015). Challenging emotional prejudice by changing self-concept: priming independent self-construal reduces racial in-group bias in neural responses to other’s pain. Social Cognitive and Affective Neuroscience, 10(9), 1195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong R.C., Fu X., Wu L.Z., Zhang C.H., Wu H.X., Shi Y., Wu W. (2019). Brain pathways of pain empathy activated by pained facial expressions: a meta-analysis of fMRI using the activation likelihood estimation method. Neural Regeneration Research, 14(1), 172–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Zuo X., Wang X., Han S. (2009). Do you feel my pain? Racial group membership modulates empathic neural responses. The Journal of Neuroscience, 29(26), 8525–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T., Poldrack R.A., Nichols T.E., Van Essen D.C., Wager T.D. (2011). Large-scale automated synthesis of human functional neuroimaging data. Nature Methods, 8(8), 665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo B.T., Krienen F.M., Sepulcre J., et al. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesudas E.H., Lee T.M. (2015). The role of cingulate cortex in vicarious pain. BioMed Research International, 1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J., Ochsner K.N. (2012). The neurscience of empathy; progress, pitfalls and promise. Nature Neuroscience, 15(5), 675–80. [DOI] [PubMed] [Google Scholar]
- Zaki J., Wager T.W., Singer T., Keysers C., Gazzola V. (2016). The anatomy of suffering: understanding the relationship between nociceptive and empathic pain. Trends in Cognitive Sciences, 20(4), 249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L., Wang Q., Cheng X., et al. (2016a). Perceived reputation of others modulates empathic neural responses. Experimental Brain Research, 234(1), 125–32. [DOI] [PubMed] [Google Scholar]
- Zheng L., Zhang F., Wei C., et al. (2016b). Decreased empathic responses to the ‘lucky guy’ in love: the effect of Intrasexual competition. Frontiers in Psychology, 7(660), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


