Abstract
Transient protein expression is an effective tool to rapidly unravel novel gene functions, such as transcriptional activity of promoters and sub-cellular localization of proteins. However, transient expression is not applicable to some species and varieties because of insufficient expression levels. We recently developed one of the strongest agroinfiltration-based transient protein expression systems for plant cells, termed ‘Tsukuba system.’ About 4 mg/g fresh weight of protein expression in Nicotiana benthamiana was obtained using this system. The vector pBYR2HS, which contains a geminiviral replication system and a double terminator, can be used in various plant species and varieties, including lettuces, eggplants, tomatoes, hot peppers, and orchids. In this study, we assessed the applicability of the Tsukuba system to several species of legumes, including Lotus japonicus, soybean Glycine max, and common bean Phaseolus vulgaris. The GFP protein was transiently expressed in the seedpods of all examined legume species, however, protein expression in leaves was observed only in P. vulgaris. Taken together, our system is an effective tool to examine gene function rapidly in several legume species based on transient protein expression.
Keywords: agroinfiltration, transient protein expression, Tsukuba system
Legumes such as the soybean (Glycine max) and the common bean (Phaseolus vulgaris) are economically important crops and account for approximately one third of the world’s main crop production. Legumes are globally important for human consumption and animal feed. Moreover, legumes are widely used in research in numerous scientific studies and in the industrial sectors. Because of their high content of protein, minerals, and dietary fiber, beans have become important dietary components that are frequently consumed and are considered as a meat-substitute. The advantages of beans for human nutrition and health have been explored more comprehensively in the past decade, leading to a globally increased consumption of beans. Most legumes have the ability to establish a symbiosis with nitrogen-fixing soil bacteria referred to as rhizobia (Suzaki et al. 2015). Further understanding and targeted application of this phenomenon may help achieve agricultural sustainability.
Transient gene expression system is useful to rapidly unravel novel gene functions, including assessments of transcriptional activity of promoters and sub-cellular localization of proteins. Moreover, examining transient gene expression is a more effective approach of investigating cell biology and physiology than the stable transgenic approach, which involves the time-consuming process of producing transformants. However, in some plants, transient protein expression has not been assessed due to insufficient expression levels. Protoplast transfection is also efficiently used for single-cell-based studies in many plant species, such as Arabidopsis thaliana (Yoo et al. 2007), maize (Sheen 2001), and rice (Zhang et al. 2011). This, however, requires a detailed protocol to prepare intact and highly competent protoplasts to introduce DNA.
Recently, we developed one of the most efficient transient protein expression systems in plant cells, termed ‘Tsukuba system.’ Using the Tsukuba system, about 4 mg protein per g fresh weight was produced from Nicotiana benthamiana. The vector pBYR2HS contains a geminiviral replication system and a double terminator (Yamamoto et al. 2018). Despite lower expression levels, compared with N. benthamiana, this vector can be used in several different plant species and varieties, including eggplants, peppers, lettuces, melons, orchids (Yamamoto et al. 2018), and in the wild type and several cultivars of tomato (Hoshikawa et al. 2019). In this study, we validated the applicability of this transient expression system to leguminous plants such as Lotus japonicus, soybean, and common bean using agroinfiltration.
The vector pBYR2HS-EGFP contains a geminiviral replication system with a tandem repeat of 35S promoter and a double terminator of the heat shock protein terminator and an extensin terminator (Yamamoto et al. 2018). Because the geminivirus has a broad host range within the dicotyledonous plants (Hefferon 2014), a transient expression system with pBYR2HS is presumably applicable to various leguminous plants, corresponding to results from previous studies on the Solanaceae (Hoshikawa et al. 2019). The duplicated 35S promoter was used in this vector, because duplication of 35S promoter produced more NPTII transcripts than single 35S promoter did (Kay et al 1987).
L. japonicus was proposed as a model legume due to its small size, large and abundant flowers and ease of pollination. Furthermore, this plant is amenable to transformation and regeneration from tissue culture (Handberg and Stougaard 1992). Therefore, it is important to establish a transient expression system in L. japonicus for rapid evaluation of gene function, such as sub-cellular protein localization. Studies on L. japonicus transformation have predominantly used Agrobacterium rhizogenes-mediated hairy root transformation where genes of interest can be transiently expressed specifically in the roots (Okamoto et al. 2013). Other transient gene expression systems such as those using protoplasts (Jia et al. 2018) and calli (Kimura et al. 2015) have been reported. These methods, however, involve numerous steps for the preparation and transformation of the respective material. Transient protein expression systems, in contrast, require fewer processing steps and are, thus, potential tools for further studies on L. japonicus.
A needle or a toothpick was used to punch holes in leaves of the L. japonicus varieties Miyakojima MG-20 and Gifu, between the leaf veins. A. tumefaciens GV3101 harboring pBYR2HS-EGFP was injected into the leaves using a 1 ml syringe without a needle. The Agrobacterium suspension was slowly injected into the seedpods using a 1 ml syringe with a 0.55×25 mm needle. Agroinfiltrated leaves and seedpods were incubated at 25°C under 16 h light and 8 h darkness for 3 days. After incubation, the seedpods were split in half. Infiltrated leaves and seedpods were exposed to a hand-held blue LED lamp in a dark room. Green fluorescent protein (GFP) emission of leaves and seedpods was captured using a regular digital camera with an SC-52 ultraviolet-absorbing filter (Table 1, Figure 1). No GFP emission was observed in L. japonicus leaves agroinfiltrated with pBYR2HS-EGFP (Figure 1A, C). Only red fluorescence derived from chlorophyll autofluorescence was detected in agroinfiltrated and non-transfected leaves (Figure 1A, C). Seedpods, in contrast, produced high GFP fluorescence (Figure 1B, D). These results indicate that high protein expression systems can be applied to seedpods of L. japonicus.
Table 1. Comparison of GFP expression in leaves and seedpods of several beans.
| Strain Name | Leaf | Seedpod | ||
|---|---|---|---|---|
| No infection | pBYR2HS-EGFP | No infection | pBYR2HS-EGFP | |
| L. japonicus Miyakojima MG-20 | — | — | — | +++ |
| L. japonicus Gifu B-129 | — | — | — | +++ |
| G. max cv. Sloan | — | — | — | + |
| G. max cv. Enrei | — | — | — | ++ |
| G. max cv. Fukuyutaka | — | — | — | + |
| P. vulgaris cv. RedHawk | — | ++ | — | +++ |
| P. vulgaris cv. Pueblo 152 | — | ++ | — | + |
| P. vulgaris cv. MLB 49 | — | ++ | — | ++ |
| P. vulgaris cv. Zorro | — | + | — | ++ |
+++, High expression level (GFP expression was detected over 80% of the infiltrated area); ++, medium expression level (over 50%); +, low expression level (over 20%); and —, no GFP emission. GFP expression was compared to negative control (no infection).
Figure 1. Transient GFP expression in leaves (A, C) and seedpods (B, D) of Lotus japonicus Miyakojima MG-20 (A, B) and Gifu B-129 (C, D) infiltrated with Agrobacterium tumefaciens containing pBYR2HS-EGFP. Leaves of two-month-old plants and about 2-cm length of green seedpods were used for infiltration. GFP was stimulated using a hand-held blue LED lamp, and emission was observed using an ultraviolet-absorbing filter (Fujifilm SC-52). No signal was detected in leaves and fruits of the negative control (NT). Autofluorescence was observed as red fluorescence due to excitation of chlorophyll by blue light. The size bar indicates 1 cm. Five or more samples were used for biological replication.
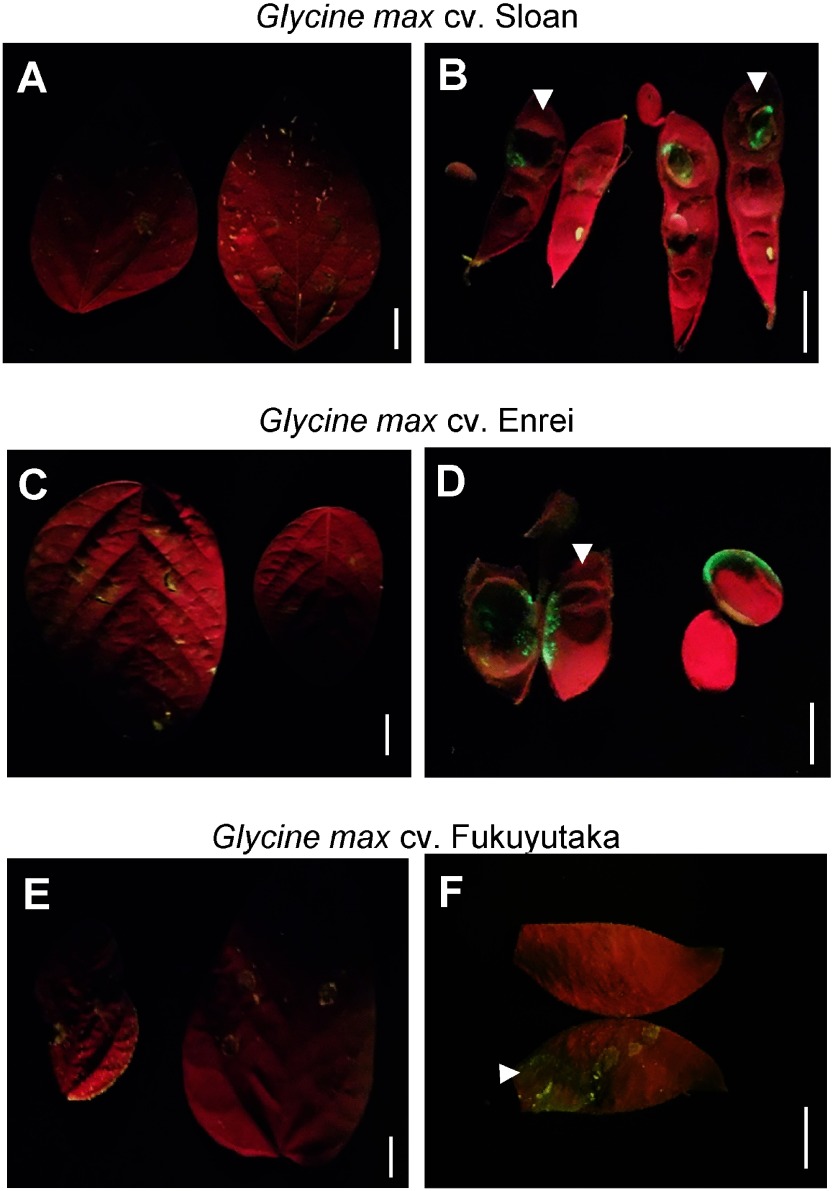
L. japonicus is considered a model legume; however, soybean (G. max) and the common bean (P. vulgaris) are important crop plants. Previous studies reported transient protein expression in soybean using particle bombardment (Wang et al. 1988) and agroinfiltration (King et al. 2015). According to the previous study (King et al. 2015), transient expression of β-glucuronidase in soybean leaves was achieved by sonication followed by vacuum infiltration and the reducing agent dithiothreitol was added to an infiltration buffer. Infiltration using a syringe is a considerably simpler method for agroinfiltration. GFP emission was detected in seedpods of L. japonicus (Figure 1B, D) using Tsukuba system, therefore syringe-infiltration with A. tumefaciens harboring pBYR2HS-EGFP was applied on the soybean cultivars Enrei, Fukuyutaka (from Soybean mini core collections; Kaga et al. 2012), and Sloan. Similar to L. japonicus, no GFP expression was detected in the leaves of any soybean cultivar (Figure 2A, C, E). In contrast, the seedpods of all examined soybean cultivars produced GFP emission (Figure 2B, D, F). The best results of syringe-infiltration using the high expression system pBYR2HS-EGFP were observed in the cultivar Enrei (Table 1, Figure 2D). In addition to seedpods, GFP expression was also observed in a wet seed of the cultivar Enrei (Figure 2D). It is well known that efficiency of Agrobacterium infection is dependent on the accessions in soybean (Sato et al. 2007). Thus, expression level of EGFP by using Tsukuba system is different in each accessions (Table 1).
Figure 2. GFP fluorescence was observed in seedpods but not in leaves of the soybean (Glycine max) cultivars Sloan (A, B), Enrei (C, D), and Fukuyutaka (E, F). Leaves of one-month-old plants and 3 to 5 cm length of green seedpods were investigated. Arrowheads indicate the place the needles were inserted into the seedpods. After insertion of needle, Agrobacterium solution was fulfilled in the internal space of the seedpod. GFP expression was not detected in leaves (A, C, E), but was observed in seedpods of these cultivars (B, D, F). The size bar indicates 1 cm. Five or more samples were used for biological replication.
Interestingly, GFP emission was observed in seedpods but not in leaves of L. japonicus and soybean. Due to the use of an agroinfiltration method, a type IV secretion system is required to transfer T-DNA into plant cells (Cascales and Christie 2003). Plant cell membranes in leaves of L. japonicus and soybean may inhibit type IV secretion systems. In a previous study, sonication followed by vacuum infiltration with the reducing agent dithiothreitol increased transient expression (King et al. 2015). Therefore, loosening of plant cell membranes may be necessary for delivering T-DNA to the nucleus. Another possibility is that defense to pathogen is different in each tissue. Production of the defensive plant hormone salicylic acid is induced by infection of Agrobacterium (Lee et al. 2009) and expression of bacterial vir genes is inhibited by salicylic acid (Yuan et al. 2007). Thus, transient protein expression level is increased in Arabidopsis expressing the NahG transgene, which converts salicylic acid to catechol, or in the sid2 mutant impaired in biosynthesis of salicylic acid (Rosas-Díaz et al. 2017). Taken together, difference of defense activation may cause difference of expression pattern. Many defense responsive genes have been predicted in soybean. According to RNA-seq data, 67 genes were selected as defense-related genes, whose expression level was significantly different between BLP (bacterial leaf pustule)-susceptible and BLP-resistant near-isogenic lines after Xanthomonas axonopodis pv. glycines (Kim et al. 2011). These genes were analyzed using the soybean eFP Browser (http://bar.utoronto.ca/efp_soybean/cgi-bin/efpWeb.cgi). Among 67 genes, expression level of 28 genes was less in green pods than in leaves (Supplementary Figure S1). Less defense activity in green pods may allow Agrobacterium infection.
GFP expression was detected in both leaves and seedpods of P. vulgaris cultivars RedHawk, Pueblo 152, MLB 49, and Zorro (Table 1, Figure 3). Sonication-based Agrobacterium-mediated transformation for leaf disc infiltration in P. vulgaris was reported previously (Nanjareddy et al. 2016). However, sonication is not required for agroinfiltration in P. vulgaris using the pBYR2HS-EGFP vector (Figure 3). Among the cultivars examined in this study, the cultivar RedHawk seemed to be a good cultivar for applying our transient expression system (Figure 3A, B).
Figure 3. GFP emission in leaves (A, C, E, G) and seedpods (B, D, F, H) of several cultivars of common bean (Phaseolus vulgaris), including RedHawk (A, B), Pueblo 152 (C, D), MLB 49 (E, F), and Zorro (G, H). Leaves of one-month-old plants and 5 to 10 cm length of green seedpods were investigated. Arrowheads indicate the place the needles were inserted to fulfill Agrobacterium solution in the internal space of the seedpods. The size bar indicates 1 cm. The highest level of GFP expression was detected in the P. vulgaris cultivar Red Hawk (Table 1). Five or more samples were used for biological replication.
Particle bombardment is also one of ways for transient protein expression. A soluble modified red-shifted GFP or a modified form of GFP [sGFP(S65T)] was able to be monitored after bombardment to soybean (Khalafalla et al. 2005; Ponappa et al. 1999). The transient expression of GUS was also detected in leaf tissue of bombarded cowpea (Finer et al 1992). The bombardment approach relies on the expensive biolistic equipment and agroinfiltration is a much cheaper method.
The high expression vector pBYR2HS harbors a geminiviral replication system with a double terminator (Yamamoto et al. 2018). This high expression system was successfully applied to legumes such as L. japonicus, soybean, and common bean. Transformation efficiency was substantially affected by the degree of compatibility of the target plant and Agrobacterium, and infection efficiency differed between cultivars. Therefore, different transient expression patterns were observed in this study. However, our high expression system can be applied to all species tested in this study. Considering that sequencing soybean genome can predict over 46,430 protein-coding genes, but most of these genes lack functional analysis (Schmutz et al. 2010), this system can be used to rapidly investigate gene functional characteristics, such as protein localization, and that it is a promising tool for research on legumes.
Acknowledgments
We thank Ms. Yuri Nemoto at the University of Tsukuba for technical support. This study was supported by a Grants-in-Aid for KAKENHI (JP16K07390, JP18H04773, JP19H04637) from MEXT, Japan, and a Cooperative Research Grant from the Plant Transgenic Design Initiative, Gene Research Center, University of Tsukuba.
Supplementary Data
References
- Cascales E, Christie PJ (2003) The versatile bacterial type IV secretion systems. Nat Rev Microbiol 1: 137–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finer JJ, Vain P, Jones MW, McMullen MD (1992) Development of the particle inflow gun for DNA delivery to plant cells. Plant Cell Rep 11: 323–328 [DOI] [PubMed] [Google Scholar]
- Handberg K, Stougaard J (1992) Lotus japonicus, an autogamous, diploid legume species for classical and molecular genetics. Plant J 2: 487–496 [Google Scholar]
- Hefferon KL (2014) DNA virus vectors for vaccine production in plants: Spotlight on geminiviruses. Vaccines (Basel) 2: 642–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshikawa K, Fujita S, Renhu N, Ezura K, Yamamoto T, Nonaka S, Ezura H, Miura K (2019) Efficient transient protein expression in tomato cultivars and wild species using agroinfiltration-mediated high expression system. Plant Cell Rep 38: 75–84 [DOI] [PubMed] [Google Scholar]
- Jia N, Zhu Y, Xie F (2018) An efficient protocol for model legume root protoplast isolation and transformation. Front Plant Sci 9: 670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaga A, Shimizu T, Watanabe S, Tsubokura Y, Katayose Y, Harada K, Vaughan DA, Tomooka N (2012) Evaluation of soybean germplasm conserved in NIAS genebank and development of mini core collections. Breed Sci 61: 566–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay R, Chan A, Daly M, McPherson J (1987) Duplication of CaMV 35S promoter sequences creates a strong enhancer for plant genes. Science 236: 1299–1302 [DOI] [PubMed] [Google Scholar]
- Khalafalla MM, Rahman SM, El-Shemy HA, Nakamoto Y, Wakasa K, Ishimoto M (2005) Optimization of particle bombardment conditions by monitoring of transient sGFP(S65T) expression in transformed soybean. Breed Sci 55: 257–263 [Google Scholar]
- Kim KH, Kang YJ, Kim DH, Yoon MY, Moon JK, Kim MY, Van K, Lee SH (2011) RNA-seq analysis of a soybean near-isogenic line carrying bacterial leaf pustule-resistant and -susceptible alleles. DNA Res 18: 483–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Cutler S, Isobe S (2015) A novel phenolic compound, chloroxynil, improves Agrobacterium-mediated transient transformation in Lotus japonicus. PLoS One 10: e0131626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JL, Finer JJ, McHale LK (2015) Development and optimization of agroinfiltration for soybean. Plant Cell Rep 34: 133–140 [DOI] [PubMed] [Google Scholar]
- Lee CW, Efetova M, Engelmann JC, Kramell R, Wasternack C, Ludwig-Müller J, Hedrich R, Deeken R (2009) Agrobacterium tumefaciens promotes tumor induction by modulating pathogen defense in Arabidopsis thaliana. Plant Cell 21: 2948–2962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanjareddy K, Arthikala MK, Blanco L, Arellano ES, Lara M (2016) Protoplast isolation, transient transformation of leaf mesophyll protoplasts and improved Agrobacterium-mediated leaf disc infiltration of Phaseolus vulgaris: Tools for rapid gene expression analysis. BMC Biotechnol 16: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto S, Yoro E, Suzaki T, Kawaguchi M (2013) Hairy root transformation in Lotus japonicus. Bio Protoc 3: e795 [Google Scholar]
- Ponappa T, Brzozowski AE, Finer JJ (1999) Transient expression and stable transformation of soybean using the jellyfish green fluorescent protein. Plant Cell Rep 19: 6–12 [DOI] [PubMed] [Google Scholar]
- Rosas-Díaz T, Cana-Quijada P, Amorim-Silva V, Botella MA, Lozano-Durán R, Bejarano ER (2017) Arabidopsis NahG plants as a suitable and efficient system for transient expression using Agrobacterium tumefaciens. Mol Plant 10: 353–356 [DOI] [PubMed] [Google Scholar]
- Sato H, Yamada T, Kita Y, Ishimoto M, Kitamura K (2007) Production of transgenic plants and their early seed set in Japanese soybean variety, Kariyutaka. Plant Biotechnol 24: 533–536 [Google Scholar]
- Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, Nelson W, Hyten DL, Song Q, Thelen JJ, Cheng J, et al. (2010) Genome sequence of the palaeopolyploid soybean. Nature 463: 178–183 [DOI] [PubMed] [Google Scholar]
- Sheen J (2001) Signal transduction in maize and Arabidopsis mesophyll protoplasts. Plant Physiol 127: 1466–1475 [PMC free article] [PubMed] [Google Scholar]
- Suzaki T, Yoro E, Kawaguchi M (2015) Leguminous plants: Inventors of root nodules to accommodate symbiotic bacteria. Int Rev Cell Mol Biol 316: 111–158 [DOI] [PubMed] [Google Scholar]
- Wang YC, Klein TM, Fromm M, Cao J, Sanford JC, Wu R (1988) Transient expression of foreign genes in rice, wheat and soybean cells following particle bombardment. Plant Mol Biol 11: 433–439 [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Hoshikawa K, Ezura K, Okazawa R, Fujita S, Takaoka M, Mason HS, Ezura H, Miura K (2018) Improvement of the transient expression system for production of recombinant proteins in plants. Sci Rep 8: 4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S-D, Cho Y-H, Sheen J (2007) Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565–1572 [DOI] [PubMed] [Google Scholar]
- Yuan ZC, Edlind MP, Liu P, Saenkham P, Banta LM, Wise AA, Ronzone E, Binns AN, Kerr K, Nester EW (2007) The plant signal salicylic acid shuts down expression of the vir regulon and activates quormone-quenching genes in Agrobacterium. Proc Natl Acad Sci USA 104: 11790–11795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Su J, Duan S, Ao Y, Dai J, Liu J, Wang P, Li Y, Liu B, Feng D, et al. (2011) A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods 7: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.