Abstract
Background
Anti-cancer therapy is often a cause of premature ovarian insufficiency and infertility since the ovarian follicle reserve is extremely sensitive to the effects of chemotherapy and radiotherapy. While oocyte, embryo and ovarian cortex cryopreservation can help some women with cancer-induced infertility achieve pregnancy, the development of effective methods to protect ovarian function during chemotherapy would be a significant advantage.
Objective and rationale
This paper critically discusses the different damaging effects of the most common chemotherapeutic compounds on the ovary, in particular, the ovarian follicles and the molecular pathways that lead to that damage. The mechanisms through which fertility-protective agents might prevent chemotherapy drug-induced follicle loss are then reviewed.
Search methods
Articles published in English were searched on PubMed up to March 2019 using the following terms: ovary, fertility preservation, chemotherapy, follicle death, adjuvant therapy, cyclophosphamide, cisplatin, doxorubicin. Inclusion and exclusion criteria were applied to the analysis of the protective agents.
Outcomes
Recent studies reveal how chemotherapeutic drugs can affect the different cellular components of the ovary, causing rapid depletion of the ovarian follicular reserve. The three most commonly used drugs, cyclophosphamide, cisplatin and doxorubicin, cause premature ovarian insufficiency by inducing death and/or accelerated activation of primordial follicles and increased atresia of growing follicles. They also cause an increase in damage to blood vessels and the stromal compartment and increment inflammation. In the past 20 years, many compounds have been investigated as potential protective agents to counteract these adverse effects. The interactions of recently described fertility-protective agents with these damage pathways are discussed.
Wider implications
Understanding the mechanisms underlying the action of chemotherapy compounds on the various components of the ovary is essential for the development of efficient and targeted pharmacological therapies that could protect and prolong female fertility. While there are increasing preclinical investigations of potential fertility preserving adjuvants, there remains a lack of approaches that are being developed and tested clinically.
Keywords: fertility preservation, chemotherapy, follicle death, adjuvant therapy, ovarian reserve, protective therapies, cyclophosphamide, cisplatin, doxorubicin
Introduction
The effects of cancer and its treatment on female reproductive function are increasingly well documented. Overall, compared to the general population, women are 38% less likely to have a pregnancy after cancer diagnosis and treatment, with all diagnostic groups of cancer being associated with a reduction in the likelihood of subsequent pregnancies (Anderson et al., 2018). Given this situation, there is now a pressing need to develop methods to protect patients from the damaging effects of treatment. This review therefore assesses the effectiveness of potential treatments to protect the ovary against chemotherapy-induced damage, with the work to date reported for only pre-clinical studies, primarily animal models.
Over the last several decades, cervical cancer and breast cancer have had the greatest impact on reducing the number of pregnancies achieved, due to a combination of their high prevalence, the marked effect of their associated treatments and patient age. Recent years have seen improvements for these two particular malignancies and for other cancers, such as Hodgkin lymphoma, although some diagnoses have seen little, if any, change in their impact on subsequent chances of pregnancy (Anderson et al., 2018). While radiotherapy and surgery can have very significant adverse effects on female reproductive function, this review focuses on the adverse effects of chemotherapy and on possible approaches to reduce its impact. The adverse effects of chemotherapy on ovarian function have long been recognised (Fig. 1A) and there are increasingly detailed data documenting the effects of different regimens on short-term markers of ovarian function, longer-term fertility and risk of early menopause (Jayasinghe et al., 2018; van Dorp et al., 2018). The impact of estrogen deficiency as a result of loss of ovarian function on quality of life, bone function and cardiovascular and neurological health are also critical aspects of the longer-term effects of chemotherapeutic damage to the ovaries (European Society of Human Reproduction and Embryology, 2015; Lobo, 2017). This has led to the development of methods for female fertility preservation (Anderson et al., 2015). The advent of oocyte vitrification was a key advancement in the ability to successfully preserve postpubertal female fertility (Argyle et al., 2016), while ovarian tissue cryopreservation has had a growing evidence base with increasing documentation of success rates; it is feasible prior to puberty and is now recognised as an established rather than an experimental form of treatment in some countries (Donnez and Dolmans, 2017).
Figure 1.
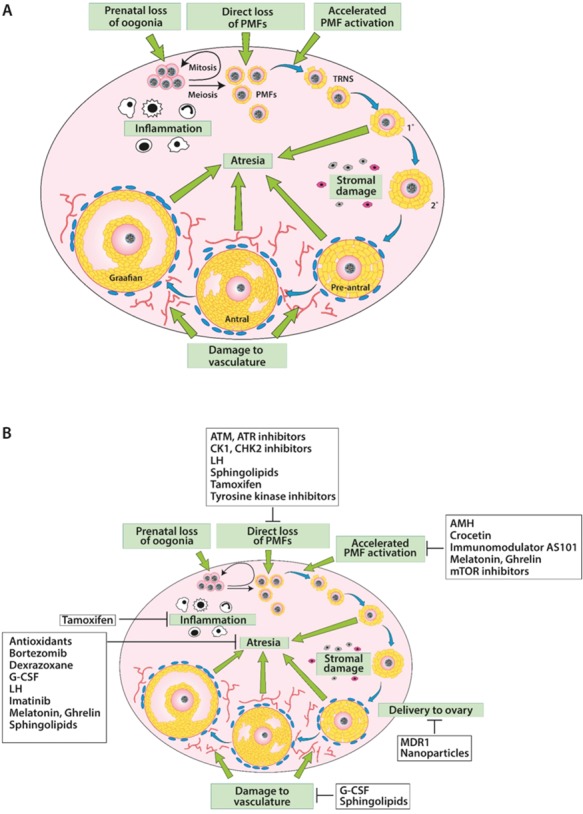
The damaging effects of chemotherapy drugs on the ovary and potential protectants against that damage. (A) Chemotherapy drugs can damage the ovary by inducing prenatal loss of oogonia, direct loss of primordial follicles, accelerated activation of primordial follicles, follicular atresia, stromal tissue damage, damage to the vasculature or inflammation. (B) Protectants examined to date have been shown to protect against all ovarian damage pathways other than that to stromal tissue. Other protectants are designed to reduce drug delivery to the ovary. PMF: primordial follicle; TRNS: transitional follicle.
Alongside the cryopreservation of gametes, a number of approaches have also been explored with the aim of protecting ovarian follicles and the oocytes they contain from chemotherapy-induced damage; these are reviewed in this paper.
The use of GnRH analogues to protect ovarian function has been the subject of a number of large randomised controlled trials, particularly in breast cancer, and these results have been widely analysed and discussed. It is now clear that this approach does reduce the prevalence of premature ovarian insufficiency (POI) in women treated for breast cancer (Lambertini et al., 2018) although it appears that relatively little ovarian function, as reflected by anti-Müllerian hormone (AMH), may be preserved by this approach (Leonard et al., 2017). The effectiveness of this approach in other cancers is not clear, with the largest trial in women with lymphoma showing no apparent benefit (Demeestere et al., 2016). Additionally, the benefits of this approach in terms of subsequent pregnancy and the avoidance of the consequences of POI, e.g., in bone health, remain to be clearly demonstrated in trials designed with those outcomes as a primary objective. As this approach has been extensively reviewed and is the subject of a number of meta-analyses (including Del Mastro et al., 2014; Lambertini et al., 2018; Vitek et al., 2014), it will not be discussed further here.
Methods
A thorough search was carried out for relevant articles in order to carry out an extensive study on the ovarian damage induced by chemotherapy and on new approaches to its protection. All authors contributed to the search and to establish the inclusion and exclusion criteria. As an analysis of published data, approval of an ethics committee is not relevant.
The research was performed using PubMed and Medline as sources, up to March 2019. The following keywords were used: chemotherapy, cyclophosphamide (CPM), cisplatin (CIS), doxorubicin (DOX), fertility preservation, adjuvant therapy, ovary, follicle death.
For the sections concerning the protective agents, the selection of the papers was performed using PRISMA guidelines (Moher et al., 2009), as described below and as reported in a PRISMA diagram (Fig. 2). The research was performed using PubMed and Medline as sources, up to March 2019. The following keywords were used: chemotherapy, alkylating agents, anthracycline, female, ovary, adjuvant therapy, fertoprotective therapy, fertility preservation, follicle death. All relevant articles were carefully evaluated. Initially, titles and abstracts were assessed to evaluate the eligibility of the studies. After this selection, the authors proceeded with the complete reading of the papers to identify those relevant for final inclusion. Reference lists of these papers were checked to identify other studies that should be included in this review. Only published articles, written in English and peer-reviewed, were included. The search included both animal and human studies. No restriction to any publishing year was applied, even though the search showed that relevant papers were dated starting from year 2000. Manuscripts were selected concerning protective agents towards CPM, cisplatin and doxorubicin on the ovary, while those concerning protective agents only against other chemotherapeutic drugs and radiotherapy were excluded from the review. Manuscripts describing no effect or adverse effects of the agents were included. Reports only presented as conference proceedings were excluded.
Figure 2.
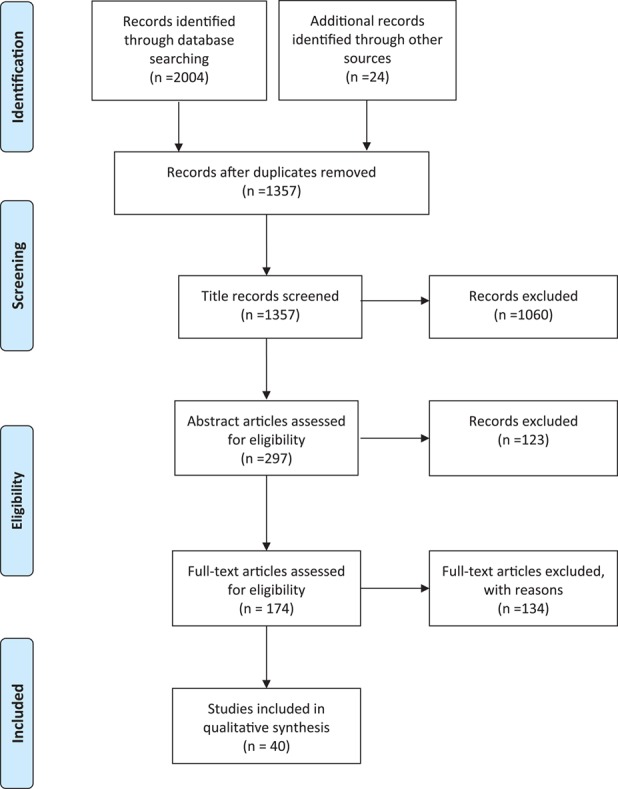
PRISMA flow diagram of literature search methodology for publications examining the effectiveness of potential fertility protective agents. Search results, study screening, and study inclusion, following a review of the literature carried out using PRISMA guidelines (Moher et al., 2009). Selected key words were searched in PubMed until March 2019, with 24 additional references identified through other sources, resulting in examination of 1357 papers. After the analysis of all publications for the inclusion and exclusion criteria, 40 articles from the initial search were used in this review.
Dynamics of the ovarian reserve
Underpinning the interpretation of the impact of chemotherapy on ovarian function are the established findings that oogonia enter meiosis during fetal life, and that the primordial follicle (PMF) pool is formed early on, in utero in humans and around the time of birth in rodents. These PMFs are located around the edge of the ovarian cortex, an area with relatively little vasculature (Delgado-Rosas et al., 2009). There is a steady rate of activation of PMFs throughout the reproductive lifespan, with its depletion resulting in the menopause. The biochemical pathways that regulate this activation of PMFs include growth factors acting through pathways, including the PI3K/PTEN/Akt pathway and the Hippo pathway (Ernst et al., 2017; Kawamura et al., 2013; Grosbois and Demeestere, 2018). The Akt pathway is of particular importance here, because it is modulated by both chemotherapy drugs and protectants, as discussed below. This pathway acts via FOXO3a, which in rodents is considered to be a key transcription factor in the maintenance of resting follicles, with activation of a PMF marked by translocation of FOXO3a from the nucleus to the cytoplasm (John et al., 2008; Chang et al., 2015). Activity of this PI3K/PTEN/Akt pathway within oocytes is a critical determinant of the size of the remaining PMF pool (Reddy et al., 2008). Inhibitory factors are also important, including AMH (Durlinger et al., 1999), but how these pathways and factors interact to provide precise regulation of the activation of PMFs remains unclear. Given the poor vasculature in the outer layer of the ovarian cortex where the PMFs are located (Fig. 1A), it is possible that PMFs are exposed to lower concentrations of chemotherapy drugs than the well-vascularised later-stage follicles, as the drugs primarily reaching PMFs via diffusion. The extent to which PMFs can be lost directly through atresia or apoptosis is also unclear, although genetic studies have documented a number of factors relating to apoptosis which appear to have major roles in the regulation of the size of the PMF pool. Some of these, such as the apoptotic regulator PUMA (Nguyen et al., 2018; Myers et al., 2014), may be future targets for intervention in the regulation of PMF loss, since they have particular roles in regulating DNA damage-induced oocyte death once follicle growth has been initiated, at least in the mouse (discussed more fully below), although its role in humans is not yet known. The great majority of follicles will become atretic at some stage during the follicular growth phase, and the high proliferation rate of granulosa cells in growing follicles makes them a sensitive target of many chemotherapy agents. Furthermore, the need to maintain chromosomal and DNA integrity in the oocyte, from PMF formation all the way through to subsequent ovulation and thus potentially for decades, also renders the oocyte itself a key site for the adverse effects of DNA-damaging chemotherapy agents.
Regulation of the rate at which PMFs undergo activation has an added complexity arising from the interaction between PMFs and the population of growing follicles. AMH produced by growing follicles is thought to have an important inhibitory effect on the rate of PMF activation (Durlinger et al., 1999), although evidence for this is much clearer in the rodent than in the human or in other larger mono-ovulatory mammalian species (Schmidt et al., 2005; Campbell et al., 2012). Inhibitory interactions between neighbouring PMFs slowing down the rate of activation have also been proposed (Da Silva-Buttkus et al., 2009); since the spatial distributed of PMFs is in clusters, particularly in the adult ovary (Gaytan et al., 2015), loss of some PMFs would reduce the inhibitory influence on other PMFs within the cluster. As such, loss of PMFs in chemotherapy-treated ovaries could be a result of a direct pathway whereby PMFs become damaged and die and/or due to an indirect pathway resulting in accelerated PMF activation, a loss that can be thought of as an accelerated version of ovarian ageing. Indirect loss of PMFs as a result of accelerated activation could be due to a loss of inhibitory factors from growing follicles that have undergone atresia (Kalich-Philosoph et al., 2013; Roness et al., 2013). It is important to bear in mind that chemotherapy drugs can also have damaging effects on the ovarian stroma and vasculature (Oktem and Oktay, 2007; Meirow et al., 2007), either of which can, in turn, impact negatively on the health of the follicular reserve, as well as impairing normal follicle development.
Together, these considerations of both direct and indirect effects on follicle pools at different stages of development provide a basis for interpretation of the effects of chemotherapy and thus approaches towards prevention of these adverse effects. Few such studies have examined effects on human ovary, with most using rodent models. As such, there is a pressing need for the effectiveness and safety of the most promising potential protectants to be determined in the human, a crucial step towards any subsequent clinical development. This will require studies to ensure that the protectants do not interfere with the efficacy of the cancer treatment and that they do not impair the developmental competence of oocytes or lead to the survival of oocytes with DNA damage in the form of genetic or epigenetic mutations (Clarke and Vieux, 2015; Kirsch-Volders et al., 2019).
Ovarian damage from chemotherapy and its potential protection
The effects of chemotherapy treatment on future fertility are relevant for both childhood cancers and those that affect women up to the age of menopause. The most common childhood cancers are leukaemia (around 26% of all registered cases), followed by central nervous system tumours and lymphomas. In young women, carcinomas (around 30% of cases), followed by lymphomas, melanomas and central nervous system tumours are most frequent, while in older pre-menopausal women, the relevant diseases are breast cancer (over 40% of cases), followed by melanoma, cervix and central nervous system tumours (https://seer.cancer.gov/statistics/; https://www.cancerresearchuk.org/health-professional/cancer-statistics/incidence/ageheading-Two; Cronin et al., 2018). Chemotherapy drugs are also used in the treatment of a wide range of non-malignant diseases, including auto-immune illnesses, with chemotherapy conditioning prior to stem cell transplant being increasingly used for haematological conditions, such as sickle cell anaemia.
With such an extensive group of diseases, treatment can involve administration of a wide range of chemotherapeutic classes and drugs, including alkylating agents (CPM, busulphan and others); alkylating-like platinum complexes (such as cisplatin [CIS]; anthracyclanes (including doxorubicin [DOX]); taxanes (docetaxel and others); topoisomerase inhibitors (such as etoposide); and vinca alkaloids (including vincristine).
The effects of chemotherapy drugs on female reproduction began to be reported in the 1970s, initially associated primarily with CPM treatment (see, for example, Miller et al., 1971; Fries et al., 1973; Koyama et al., 1977), with reports of amenorrhea, ovarian suppression and follicle destruction. From early in the development of chemotherapy drug use, administration of one drug alone became rare, with patients instead being treated with drug combinations. Given this, it has become increasingly difficult to determine which particular drug is responsible for the fertility-damaging effects, with relatively little known about the ovarian toxicity of some drugs. Nonetheless, there is strong clinical evidence that ovarian damage is particularly severe after administration of alkylating and alkylating-like agents (Chow et al., 2016; van Dorp et al., 2018; Meirow and Nugent, 2001), while DOX, which is used to treat a wide range of cancers, is amongst the non-alkylating agents most closely linked to female reproductive problems (Bar et al., 2003; Green et al., 2009; Bedoschi et al., 2016; Bedoschi et al., 2019). Consequently, many of the chemicals currently being investigated as potential protectants have been specifically tested to ameliorate damage induced by CPM, CIS or DOX. As such, the mechanism of action of, and potential protection against damage by, each of these three drugs are examined in detail below. It should be borne in mind that other drugs also have moderate or strong ovarian toxicity, particularly the alkylating agent busulphan (Chow et al., 2016). Some potential protectants, such as AMH, should, in theory, be effective against a wide range of chemotherapy drugs and are discussed separately.
Cyclophosphamide
Action of cyclophosphamide (CPM) and main uses in treatment
CPM is an alkylating agent, capable of covalently binding alkyl groups to DNA. Alkylating agents, originally derived from the mustard gas chemical warfare agents, were the first drugs shown to result in tumour regression. CPM itself is a pro-drug, metabolised by cytochrome P450 in the liver to form 4-hydroxycyclophosphamide, which is in turn converted to phosphoramide mustard and acrolein. Phosphoramide mustard is the primary active metabolite (Plowchalk and Mattison, 1991; Madden and Keating, 2014), inducing DNA crosslinking that leads to the formation of adducts that prevent DNA replication. Phosphoramide mustard also affects mitochondria, leading to a reduction in transmembrane potential and cytosolic cytochrome c accumulation. CPM is used in the treatment of a wide range of cancers, particularly the more frequent childhood cancers, melanomas and breast cancer, including the treatment of pregnant cancer patients (Hahn et al., 2006). It is also used in the treatment of auto-immune diseases, such as systemic lupus erythematosis and rheumatoid arthritis.
Effect of CPM on the ovary
Human ovary studies
CPM was the first chemotherapy drug to be linked to amenorrhea/POI and ovarian dysfunction (Miller et al., 1971; Fries et al., 1973; Koyama et al., 1977), and it continues to be regarded as one of the most gonadotoxic agents (Overbeek et al., 2017; Chemaitilly et al., 2017). Nevertheless, there are only a limited number of studies that have examined the effect of CPM on human ovarian tissue. From these, it is difficult to determine the precise effects of CPM on PMFs: while the PMF population has been shown to be reduced in size following CPM-exposure (Meng et al., 2014), there are reports both of direct damage to this follicle population (Li et al., 2014) and of a reduction in PMF numbers following accelerated activation, with the authors also suggesting that phosphoramide mustard itself may even directly induce accelerated PMF activation (Kalich-Philosoph et al., 2013; Lande et al., 2017). Direct effects have, however, been clearly demonstrated on PMFs in the mouse (see below) and on growing follicles in human ovarian tissue examined in vitro, with CPM inducing increased granulosa cell apoptosis and follicular atresia (Asadi Azarbaijani et al., 2015; Yuksel et al., 2015).
Non-human ovary studies
As with the clinical work, CPM was the first drug shown to induce ovarian damage and follicle loss in the mouse (Miller and Cole, 1970), and CPM exposure is regularly used as a pre-treatment to induce widespread follicular atresia in a variety of experiments where the research examines the physiology of an ovary devoid of follicles (see, for example, Goldman et al., 2017; Kano et al., 2017; Melekoglu et al., 2018; Tsuyoshi et al., 2015). For growing follicles, there is now a large body of evidence linking CPM exposure to follicle atresia and granulosa cell apoptosis (Jarrell et al., 1991; Ezoe et al., 2014; Piasecka-Srader et al., 2015; Yuksel et al., 2015; Chen et al., 2016). Exposure of growing follicles to CPM, particularly at earlier stages, has also been linked to embryo abnormalities and to malformation in the next generation in later pregnancies (Barekati et al., 2008; Meirow et al., 2001, respectively). The PMF pool is clearly affected by CPM exposure (Meirow et al., 1999; Kalich-Philosoph et al., 2013; Saleh et al., 2015; Chen et al., 2016; Goldman et al., 2017; Pascuali et al., 2018; Nguyen et al., 2018; Luan et al., 2019), although as with studies in the human ovary, it is not always clear whether PMF loss is due to direct damage and/or a result of indirect loss due to accelerated activation. Inflammation and vasculature damage have also been reported (Ezoe et al., 2014; Saleh et al., 2015; Luo et al., 2017; Pascuali et al., 2018). With CPM also administered to pregnant cancer patients, the effects of CPM on the developing ovary has also been investigated, with formation of fewer follicles formed (Fig. 1A) and acceleration of PMF activation (Ray and Potu, 2010; Comish et al., 2014). One recent study has demonstrated that maternal CPM exposure prior to conception can affect the competence of the offspring’s oocytes, which was associated with altered methylation of three imprinted genes (H19, Igf2r and Peg3) (Di Emidio et al., 2019).
Molecular pathways of CPM-induced ovarian damage
Most of our understanding of precisely how CPM induces ovarian damage has come from studies trying to prevent that damage (Table 1). As expected, given what is known about its action on cancer cells, there is clear evidence that CPM leads to an upregulation in apoptosis in the ovary, as is evident from the rapid induction of DNA breaks (Piasecka-Srader et al., 2015) and from a change in the expression levels of pro- and anti-apoptotic genes (Petrillo et al., 2011; Pascuali et al., 2018; Luan et al., 2019). Recent studies have demonstrated that the DNA damage-induced pro-apoptotic protein PUMA plays a key role in inducing oocyte apoptosis in rodents following CPM treatment (Nguyen et al., 2018), as is also the case following irradiation (Kerr et al., 2012). A decrease in production of antioxidant enzymes, such as superoxide dismutase, has also been shown (Khedr, 2015; Melekoglu et al., 2018). Work has also examined whether CPM stimulates the mTOR/PTEN/Akt pathway, resulting in accelerated PMF activation. Kalich-Philosoph et al. (2013) have shown induction of Akt, mTOR and FOXO3a within 24 h of CPM exposure. Increased phosphorylation of Akt and other parts in the pathway have also been shown by Goldman et al. (2017), although in that instance, the tissue was examined 7 days after the end of treatment. One study has now demonstrated that CPM specifically induces apoptosis in the oocytes of primordial follicles, while also not finding increased activation of such follicles (Luan et al., 2019). An inflammation response has been shown through increased levels of the pro-inflammatory cytokines IL-6 and -8 and TNFα alongside reduced levels of the anti-inflammatory IL-10, although this was in response to administration of both CPM and busulfan (Luo et al., 2017). CPM also leads, in mouse oocytes, to an increase in the expression of the cell redox state sensor SIRT1, as the result of the adaptive response to oxidative stress (Di Emidio et al., 2017). In fact, SIRT1 is involved in both inflammation and oxidative stress, by inhibiting NF-kB signalling and stimulating the expression of antioxidants through FoxO transcription factors, respectively (Xie et al., 2013; Salminen et al., 2013).
Table 1.
Molecular pathways involved in chemotherapy-induced ovarian damage.
Agents used to protect ovaries from CPM-induced damage
Potential protectants from CPM-induced damage have been investigated to determine protection against direct loss of PMFs, accelerated activation of PMFs, increased atresia of growing follicles and damage to the ovarian vasculature (Fig. 1B, Table II). At present, only Sphingosine-1-phosphate (S1P) and tamoxifen have been examined in the human ovary (see below).
Table II.
Agents used to protect ovaries from chemotherapy-induced damage.
| Protectant | Drug | Target action | Species | Reference |
|---|---|---|---|---|
| AMH/MIS | CPM | Accelerated PMF activation |
Mouse | Kano et al. 2016 |
| DOX | Sonigo et a.l 2018 | |||
| Carboplatin | ||||
| ATM inhibitors: | CIS | Direct loss of PMFs | Mouse | Tuppi et al. 2018 |
| ETP-46464 | DOX | Kim et al. 2018 | ||
| KU55399 | ||||
| ATR inhibitors: | CIS | Direct loss of PMFs | Mouse | Kim et al. 2018 |
| ETP-46464 | DOX | Luan et al. 2019 | ||
| AZD6738 | CPM | |||
| AS101 | CPM | Accelerated PMF activation | Mouse | Kalich-Philosoph et al. 2013 |
| Di Emidio et al. 2017 | ||||
| Bortezomib | DOX | Atresia | Mouse | Roti Roti et al. 2014 |
| Ceramide-1-phosphate | CPM | Direct loss of PMFs | Mouse | Pascuali et al. 2018 |
| Atresia | ||||
| Vascularization | ||||
| CHK2 inhibitors: | CIS | Direct loss of PMFs | Mouse | Rinaldi et al. 2017 |
| BML277 | DOX | Tuppi et al. 2018 | ||
| LY2603618 | CPM | Luan et al. 2019 | ||
| LY2606368 | ||||
| CK1 inhibitors: | CIS | Direct loss of PMFs | Mouse | Tuppi et al. 2018 |
| MK-8776 | DOX | |||
| CHIR-124 | ||||
| PMF670462 | ||||
| PMF4800567 | ||||
| PMF5006739 | ||||
| Crocetin | CPM | Accelerated PMF activation | Mouse | Di Emidio et al. 2017 |
| Dexrazoxane | DOX | Atresia | Mouse | Kropp et al. 2015 |
| Ghrelin | CIS | Accelerated PMF activation | Mouse | Jang et al. 2017 |
| G-CSF | CIS | Atresia | Mouse | Skaznik-Wikiel et al. 2013 |
| Vascularisation | Akdemir et al. 2014 | |||
| Imatinib | CIS | Direct loss of PMFs | Mouse | Kim et. 2013 |
| Atresia | Maiani et al. 2012 | |||
| Zamah et al. 2011 | ||||
| Rinaldi et al. 2017 | ||||
| Tuppi et al. 2018 | ||||
| Gonfloni et al. 2009 | ||||
| Kim et al. 2018 | ||||
| Luteinizing Hormone | CIS | Direct loss of PMFs | Mouse | Rossi et al. 2017 |
| Atresia | Tuppi et al. 2018 | |||
| MDR1 | CPM | Delivery to ovary | Mouse | Brayboy et al. 2013; 2017 |
| Salih 2011 | ||||
| Wang et al. 2018 | ||||
| Melatonin | CIS | Accelerated PMF activation | Mouse | Jang et al. 2016 |
| Mesna | CIS | Atresia | Rat | Li et al. 2013 |
| Mirtazapine | CIS | Atresia | Rat | Altuner et al. 2013 |
| mTORC inhibitors: | CPM | Accelerated PMF activation | Mouse | Adhikari et al. 2013 |
| Everolimus (RAD001) | CIS | Goldamn et al. 2017 | ||
| INK128 | Zhou et al. 2017 | |||
| Rapamycin | Tanaka et al. 2018 | |||
| Resveratrol | CIS | Atresia | Rat | Ozcan et al. 2015 |
| Sphingosine-1- phospate | CPM | Direct loss of PMFs | Mouse | Morita et al.2000 |
| Rat | Li et al. 2017 | |||
| Human | Li et al. 2014 | |||
| Meng et al. 2014 | ||||
| Sildenafil Citrate | CIS | Atresia | Rat | Taskin et al. 2015 |
| Tamoxifen | CPM | Direct loss of PMFs | Rat | Ting and Petroff 2010 |
| Inflammation | Human | Piasecka-Srader et al. 2015 | ||
| Sverrisdottir et al. 2009 | ||||
| Sverrisdottir et al. 2011 |
S1P and Ceramide 1 phosphate
Bioactive sphingolipids, S1P and ceramide 1 phosphate (C1P), produced through ceramide metabolism, are inhibitors of the ceramide-induced death pathway and regulate angiogenesis, vascular stability and permeability in a variety of tissues, in both physiological and pathological conditions (Bonnaud et al., 2007; Cuvillier et al., 1996). S1P prevents CPM-induced apoptotic follicle death in both human ovarian xenografts (Li et al., 2014; Meng et al., 2014) and isolated granulosa cells obtained from assisted reproduction techniques (Li et al., 2017). Intrabursal C1P administration ameliorates ovarian follicular and vascular development and restores fertility in CPM-treated mice, with reduced TUNEL staining and decreased cleaved caspase 3 and BAX expression, together clearly indicating a reduction in apoptosis in all follicles analysed (Pascuali et al., 2018).
AS101
The immunomodulator AS101 is a non-toxic, tellurium-based compound that modulates the PI3K/PTEN/Akt pathway, reducing the Akt and rpS6 phosphorylation that is induced by CPM (Makarovsky et al., 2003). It is used in clinical trials for neovascular age-related macular degeneration due to its immunomodulating characteristics and in the treatment of chemotherapy-induced thrombocytopenia and psoriasis. In vivo treatment of mice with AS101 lowers CPM-induced loss of PMFs, as well as apoptosis of granulosa cells in growing follicles, leading to improved reproductive outcomes (Kalich-Philosoph et al., 2013). Mice born to dams whose fertility is protected by AS101 treatment show no abnormalities, indicating that the quality of the rescued oocytes is also protected. Importantly, it has been demonstrated that not only does AS101 not interfere with the primary anti-neoplastic activity of CPM in vivo, but, in fact, it also may improve the efficiency of the anti-cancer activity of CPM, possibly due to reduced inflammation (Roness et al., 2016; Kalich-Philosoph et al., 2013; Kalechman et al., 1991; Roness et al., 2014).
Crocetin
Crocetin is a diapocarotenedioic acid, the main bioactive compound in saffron, which protects primordial and growing follicles from CPM-induced injury. Crocetin can also provide protection against toxicant-induced oxidative stress along with underlying disorders in numerous organs, tissues and cells (Xi et al., 2007). Crocetin administration (as with AS101) prevents an increase in SIRT1 expression, suggesting that preservation of the redox balance can help the ovary counteract CPM-induced ovarian damage (Di Emidio et al., 2017). Despite this, crocetin and AS101 were not able to fully counteract effects on oocyte imprinting in offspring (Di Emidio et al., 2019).
mTORC inhibitors
mTOR is a serine/threonine kinase and a metabolic sensor that regulates cell growth, proliferation, autophagy and survival (Wullschleger et al., 2006). Deletion of components of the mTOR pathway, including PTEN- and TSC1-negative regulators, can induce accelerated PMF activation in mice (Reddy et al., 2008; Adhikari et al., 2010; Chen et al., 2015). As such, in animal models, mTOR stimulators accelerate activation of PMFs, while mTOR inhibitors block the PMF-to-primary follicle transition (Sun et al., 2015). To determine if inhibition of the mTOR pathway could be used as a pharmacologic approach to prevent chemotherapy drug-induced POI, CPM-treated mice were administrated one of two mTOR complex (mTORC) inhibitors, either the clinically approved drug everolimus (RAD001) that inhibits mTORC1 or the experimental drug INK128 that inhibits mTORC1 and 2. Both treatments preserved the ovarian reserve, with ovaries containing PMF numbers that were comparable to those in control mice; furthermore, fertility was restored (Goldman et al., 2017). Similar results were obtained with the use of another inhibitor of mTORC1, rapamycin (Zhou et al., 2017; Adhikari et al., 2013).
Tamoxifen
The estrogen receptor modulator tamoxifen is widely used in the treatment of estrogen receptor-positive breast cancers and has been tested as an ovarian protectant against chemotherapy-induced damage. In cultured neonatal rat ovaries, tamoxifen treatment decreased CPM-induced follicle loss, probably through decreased expression of multiple genes related to inflammation, as well as tissue remodelling and vasodilation (Piasecka-Srader et al., 2015). Furthermore, tamoxifen was able to prevent CPM-induced toxicity in PMFs in vivo, protecting the fertility of treated rats (Ting and Petroff, 2010). However, when tamoxifen was given in a randomised-controlled trial to women treated with CPM, methotrexate and 5-fluorouracil, no effect was observed on their ovarian function (Sverrisdottir et al., 2011; Sverrisdottir et al., 2009).
Tyrosine kinase inhibitors
The use of inhibitors for the ATR-CHK2-p63 apoptotic pathway was effective in vitro in protecting primordial follicles from CPM-induced death (Luan et al., 2019).
Cisplatin
Action of CIS and main uses in treatment
CIS is a member of the platinum-based family of compounds. The ability of CIS to inhibit cell division was first identified in the 1960s, and it became the first FDA-approved platinum compound for cancer treatment in 1978 (Kelland, 2007). It is of heavy metal composition and acts as a DNA cross-linking agent that interferes with DNA repair mechanisms, blocks cell division, elicits DNA damage and triggers apoptotic cell death (Dasari and Tchounwou, 2014). The toxicity of CIS is particularly evident in tissues with a rapid cell turnover (Ozcelik et al., 2010). CIS interacts with several different cellular components, but DNA is its primary biological target (Roberts and Pera Jr, 1983). It exerts its cytotoxic mode of action through binding to DNA, by preferentially binding to N-7 positions of guanine and adenine to produce inter- and intra-strand DNA adducts (Kalil and McGuire, 2002). The CIS-induced DNA adducts are recognised by damage recognition proteins which transduce the DNA damage signal downstream and modulate several signal transduction pathways, such as Akt, c-ABL, ATR and MAPK/JNK/ERK (Siddik, 2003; Wang and Lippard, 2005). CIS-induced DNA damage activates cell cycle checkpoints, resulting in cell cycle arrest (Siddik, 2003). Cell cycle checkpoints are necessary to allow the cell repair mechanisms that enable the nucleotide excision repair complex to remove DNA adducts and promote cell survival. When DNA damage is minor, the DNA repair protein PARP detects the presence of DNA strand breaks and activates their repair; if, however, DNA damage is extensive and DNA repair is not completed, PARP activation can initiate cell death through apoptosis or necrosis (Soldani and Scovassi, 2002). In addition to binding covalently to DNA, CIS can also bind to nuclear and cytoplasmic proteins which can cause adverse toxic effects in patients (Kalil and McGuire, 2002; Chovanec et al., 2017), and it is possible that these mechanisms may add to its gonadotoxicity.
Effect of CIS on the ovary
Human ovary studies
There are limited clinical data available on the ovarian toxicity of CIS. Female patients treated with CIS have usually received it as part of a multiple-drug regimen, making it difficult to elucidate exact mechanisms of CIS-induced ovarian toxicity. CIS is generally considered to have moderate ovarian toxicity, although, to the best of our knowledge, only one clinical study has reported this, finding mild to moderate rates of amenorrhea and an increased risk of ovarian failure and infertility following treatment with a multi-drug regimen that included CIS (Maneschi et al., 1994). Others have found no detrimental effects on fertility following CIS treatment, particularly for germ cell tumours (Brewer et al., 1999; Weinberg et al., 2011), although effects on male fertility are clear (Chow et al., 2016). CIS-induced damage has been found in studies that have cultured human ovarian cortical pieces or granulosa cells in its presence, resulting in a decrease in follicle numbers and a reduction in steroidogenic activity (Bildik et al., 2015; Yuksel et al., 2015).
Non-human ovary studies
Most of the information on the ovarian toxicity of CIS has been derived from animal studies. There is a growing body of evidence demonstrating a loss of ovarian reserve and increased follicular atresia following CIS exposure in mouse and rat ovaries (Gonfloni et al., 2009; Yuksel et al., 2015; Kim et al., 2013; Morgan et al., 2013; Rossi et al., 2017; Nguyen et al., 2018), with immature oocytes being particularly susceptible (Rossi et al., 2017; Morgan et al., 2013). Studies examining effects of CIS exposure on follicles frequently report the loss of PMFs, through PMF death directly and/or indirectly due to accelerated activation; in either case, this would ultimately lead to POI (Yucebilgin et al., 2004; Chang et al., 2015). If CIS targets growing follicles, their death will result in decreased secretion of factors, such as AMH, that can inhibit PMF activation, and CIS has been shown to reduce circulating AMH and inhibin-α levels, corresponding with a decrease in the percentage of AMH-positive follicles (Yeh et al., 2006; Li et al., 2014; Ozcan et al., 2015; Yeh et al., 2008).
Molecular pathways of CIS-induced ovarian damage
Despite the therapeutic potential of CIS being discovered over 40 years ago, its mechanisms of action are still not fully understood. The proposed pathways to damage include CIS eliciting DNA damage that leads to the activation of apoptotic pathways through the p53 family of signalling and damage through oxidative stress and production of free radicals (Table 1). Exposure to CIS increases DNA damage in ovarian cells, resulting in apoptosis, as is evident from increased DNA damage, TUNEL and activation of apoptotic genes, such as caspase 3 (Bildik et al., 2015; Yuksel et al., 2015; Barberino et al., 2017; Jang et al., 2017). The tumour-suppressor p53 protein and members of its family play a crucial role in the degree to which CIS-induced DNA damage is repaired, ultimately determining cell fate. ATR, a kinase involved in checkpoint activation, is activated by CIS, and in turn can activate p53, as well as specific pathways of the MAPK cascade (Damia et al., 2001). The p53 homologue p63, specifically its isoform TAp63α, is expressed in dormant and growing oocytes (Suh et al., 2006). This protein is a key mediator of the DNA damage response within the ovary. CIS activates TAp63α thorough an ATR/CHEK1/CK1 pathway (Tuppi et al., 2018; Kim et al., 2018); this increases the expression of PUMA and NOXA, leading to apoptosis of those oocytes that are irreparably injured following CIS treatment, directly inducing follicle death (Kerr et al., 2012; Nguyen et al., 2018). CIS-exposure also leads to increased reactive oxygen species and a decrease in the ovarian antioxidant capacity (Ozcan et al., 2015). This has been correlated with decreased activities of the antioxidant enzyme superoxide dismutase (Bansod et al., 2017) and the thiol anti-oxidant glutathione (GSH) following CIS exposure (Li et al., 2013; Barberino et al., 2017). Exposure to CIS results in increased phosphorylation of key proteins in the PTEN/Akt/FOXO3a pathway, including PTEN, Akt, GSK3, FOXO3a and ERK, leading to nuclear export of FOXO3a which in turn suppresses its transcription activities, together leading to accelerated activation of PMFs (Jang et al., 2016; Chang et al., 2015).
Agents used to protect ovaries from CIS-induced damage
There is now a range of agents aiming to protect the ovary against CIS-induced damage, preventing direct loss of PMFs, accelerated PMF activation or follicular atresia (Fig. 1B, Table II). However, there are no published data on the potential of any of these to protect against damage in the human ovary.
Tyrosine kinase inhibitors
The p53 family member TAp63α is responsible for DNA damage-induced apoptosis of oocytes within PMFs. TAp63α is the only isoform of TAp63 expressed by the oocyte and becomes activated after DNA damage; tyrosine kinase signalling is involved in this key regulatory pathway (Suh et al., 2006). Recently, CHK2 and the executioner kinase CK1 have been identified as important for the elimination of mouse oocytes following double-stranded breaks in DNA (Bolcun-Filas et al., 2014; Tuppi et al., 2018), and their function is essential for downstream activation of TAp63 (Kehrloesser et al., 2018). In vitro, pharmacological inhibition of CHK2/CK1 was shown to be effective at rescuing oocytes from TAp63-mediated apoptosis induced by CIS or by DOX (see below) or following γ-irradiation (Rinaldi et al., 2017; Tuppi et al., 2018). The same results have been obtained using inhibitors of ATM and ATR (Tuppi et al., 2018; Kim et al., 2018), indicating that the TAp63 pathway could be an effective target for developing protective strategies in oncofertility. Pharmacological inhibitors of ATM, ATR, CHK2 and CK1 are already being used in preclinical and clinical trials (Rundle et al., 2017), potentially expediting their application to this field, while inhibitors of the downstream targets of TAp63α, NOXA or PUMA still need to be discovered.
Imatinib is an inhibitor of the oncogenic BCR-Abl tyrosine kinase enzyme and is itself used in the treatment of cancers, such as chronic myeloid leukaemia and gastro-intestinal stromal tumours. Data on the efficacy of imatinib against damage by CIS have been conflicting, with some studies finding protective effects (Gonfloni et al., 2009; Maiani et al., 2012; Kim et al., 2013), and others finding either no evidence of protection or even deleterious effects (Kerr et al., 2012; Zamah et al., 2011). All published studies have been carried out using the mouse as a model, and there are no data on effects on the human ovary. Recent work examining the mechanism of activation of TAp63α has demonstrated that imatinib is able to protect oocytes from CIS-induced death without interfering with TAp63α tetramerization (Tuppi et al., 2018). The same conclusion was reached in another study which generated a conditional knockout mouse with an oocyte-specific deletion of Abl1 and Abl2; ovaries from these mice treated with CIS show no protection against apoptosis (Kim et al., 2018). Imatinib is not an inhibitor of a specific tyrosine kinase signalling pathway, but can affect a range of pathways, including those downstream of c-Kit, the PDGF receptor and, to a lesser extent, c-Src (Seeliger et al., 2007). As tyrosine kinase signalling is involved in several key regulatory processes in the ovary, it could be that the mixed reports about the action of imatinib results from both beneficial and detrimental effects on the ovary due to its range of actions, and that inhibiting specific tyrosine kinase signalling pathways could provide the ovary with more effective protection.
Luteinizing Hormone
In addition to its cardinal roles in ovulation and induction and maintenance of the corpus luteum and its use in fertility programs for assisted reproduction techniques, recent research has shown that luteinizing hormone (LH) is also able to protect PMFs from the deleterious effects of CIS in prepubertal mice (Rossi et al., 2017). When CIS is administered concomitantly with LH, there is little degeneration of PMFs and mice maintain their fertility. LH is able to stimulate the DNA repair mechanisms and at the same time block CIS-induced apoptotic pathways in oocytes, an effect shown to be indirect, since the oocyte lacks LH receptors. LH does not counteract the activation/tetramerization of TAp63α (Rossi et al., 2017; Tuppi et al., 2018); its mechanism of action remains to be elucidated.
Melatonin and ghrelin
Melatonin (N-acetyl-5-methoxytryptamine) is a hormone synthesised and secreted mainly by the pineal gland (Venegas et al., 2012; Reiter et al., 2013; Reiter et al., 2014). Apart from its use in sleep disorders, melatonin can also reduce the side effects of anticancer drugs by removing hydrogen peroxide, singlet oxygen, superoxide anion radicals and peroxyl radicals (Casado-Zapico et al., 2010; Pariente et al., 2016). Its potential protective effect was first investigated in the male; in mouse testis, melatonin prevents CIS-induced testicular toxicity by scavenging free-radical products (Atessahin et al., 2006). More recently, protection has also been found in the female, where co-administration of CIS and melatonin has been shown to lead to a reduction in TUNEL in granulosa cells, resulting in reduced atresia of growing follicles and suppression of phosphorylation of the PTEN/AKT/FOXO3a pathway, thus limiting PMF activation (Jang et al., 2016).
Ghrelin is a hormonal peptide produced by cells in the gastrointestinal tract. Although it is known as the “hunger hormone,” increasing evidence supports a more complex role in the regulation of metabolism. As with melatonin, ghrelin has protective activity against CIS-induced testicular damage (Garcia et al., 2015; Whirledge et al., 2015). Oocytes within PMFs express receptors for both ghrelin and melatonin, and the combination has been tested for activity against CIS-induced damage (Jang et al., 2017). Co-administration of the two hormones blocks PMF loss by reducing PTEN and FOXO3a phosphorylation. Given the presence of their receptors in the oocyte, it is possible that these hormones have a direct action on PMFs, potentially affecting their capacity to remain in a quiescent state.
Antioxidants
Four different antioxidants, mesna, mirtazapine, resveratrol and sildenafil citrate, have been tested for their protective effects on the ovarian reserve of rats treated with CIS (Li et al., 2013; Ozcan et al., 2015; Taskin et al., 2015; Altuner et al., 2013). Mesna, sildenafil citrate and resveratrol prevented the loss of AMH-positive follicles; mirtazapine also improved fertility. The antioxidative enzymatic activity of superoxide dismutase and glutathione were increased after co-administration of mesna or mirtazapine with CIS. These results indicate that the maintenance of low levels of free radicals in the ovary is important to promote the survival of the ovarian reserve.
mTORC inhibitors
Everolimus prevents the loss of primordial follicle in cisplatin chemotherapy-induced treated mice, in which the activation of the PI3K/PTEN/AKT pathway was detected (Chang et al., 2015; Tanaka et al., 2018).
Doxorubicin
Action of DOX and main uses in treatment
The anthracycline antibiotic DOX (14-hydroxydaunomicyn) is a commonly used chemotherapy agent often known by its main trade names, Adriamycin or Rubex. It was originally isolated from Streptomyces peucetius in the 1970s and is now used to treat a large range of cancers, including breast, lung, gastric, ovarian, bladder, thyroid, leukaemia, sarcoma, lymphoma, neuroblastoma, Wilms’ tumours and paediatric cancers (Blum and Carter, 1974; Roti Roti et al., 2014; Xiao et al., 2017). The main and most widely recognised mechanism of DOX is the inhibition of the nuclear enzyme topoisomerase II. Topoisomerase II prevents supercoiling and twisting of the DNA during replication by producing double-strand nick and resealing of the cleaved DNA and is, therefore, particularly abundant in the G2/M cell cycle phase transition. DOX prevents topoisomerase II-DNA complex formation after the nicking phase, with an accumulation of DNA fragments which ultimately induces cell death. In addition, DOX stimulates the production of oxygen-free radicals and other reactive oxygen species (Tokarska-Schlattner et al., 2006) and is responsible for mitochondrial dysfunction (Clementi et al., 2003). Both free radical production and mitochondrial damage have a negative impact on cellular metabolism and membrane integrity, resulting in severe side effects. DOX also intercalates into DNA, impairing DNA replication and RNA and protein synthesis (Kiyomiya et al., 1998; Thorn et al., 2011; Paik et al., 1998; Zhang et al., 2017).
Effect of DOX on the ovary
Human ovary studies
Amongst other off-target effects, infertility has been reported in both adult and childhood patients as a consequence of regimens that include the use of DOX (Lipshultz et al., 2017). The probability of amenorrhea following treatments that includes the use of DOX ranges from 7% to 80%, primarily depending on the age of the woman at the time of exposure (Ben-Aharon et al., 2010; Letourneau et al., 2012; Knobf, 2006). However, as with many studies that investigate the effect of chemotherapy on fertility by examining time until menses resumption, these findings may not accurately reflect the full degree of gonadotoxic damage or the total effect on fertility lifespan, since full examination of either requires very long-term follow-up studies. Moreover, as with CPM and CIS, it is difficult to clearly isolate the role of a single drug, such as DOX when drugs are administered in combination. Only two studies have investigated the direct role of DOX on the human ovary (Li et al., 2014; Soleimani et al., 2011), with both studies using human ovarian cortex examined either in vitro and/or after xenotransplantation into immunocompromised mice. This work has also highlighted DOX-induced damage to the microvasculature as well as necrosis of the ovarian stromal tissue (Soleimani et al., 2011).
Non-human ovary studies
DOX affects cortical blood vessels in the mouse model, as well as in the human ovary, inducing fibrosis (Ben-Aharon et al., 2010). DOX has been shown to cross the follicle basement membrane and accumulate in the DNA and mitochondria of the oocyte (Bar-Joseph et al., 2010). A dose-dependent depletion of follicles, both primordial and early growing, has been found in many studies (Morgan et al., 2013; Perez et al., 1997; Roti Roti and Salih, 2012; Roti Roti et al., 2014; Aliotta et al., 2005; Tuppi et al., 2018). Later stages of follicle development are also affected by DOX treatment, resulting in a reduction in secondary and antral follicles. Furthermore, DOX decreases the ovulation rate, and although the ovulated oocytes appear morphologically normal, blastocyst number (Bar-Joseph et al., 2010), litter size (Kropp et al., 2015), and pup birth weight (Roti Roti et al., 2014) are reduced. The main DOX-induced damage in follicles appears to be in the mitotically active granulosa cells, with damage to oocytes being a consequence of follicle disruption (Bar-Joseph et al., 2010; Morgan et al., 2013; Roti Roti et al., 2012; Roti Roti et al., 2014; Wang et al., 2018). Ultimately, DOX exposure causes a reduction in fertile lifespan (Roti Roti et al., 2014).
Molecular pathways of DOX-induced ovarian damage
Apoptosis is the main, and certainly the most studied, process of cell death triggered by DOX in the ovary, although the exact pathway (s) is still somewhat unclear. Exploration of DOX-triggered apoptosis has made possible identification of several markers of programmed cell death in both human and animal model tissues (Table 1). PMFs in DOX-treated human ovarian cortex show increased expression of γH2AX and cleaved caspase 3 (Soleimani et al., 2011). Mouse granulosa and stromal/thecal cells isolated from growing follicles, as well as oocytes, have double-strand DNA breaks after DOX injection (Roti Roti et al., 2014; Roti Roti et al., 2012; Xiao et al., 2017). Double-strand breaks and γH2AX are also increased in antral follicles in marmoset monkey ovary exposed to DOX (Salih et al., 2015), as are metaphase II oocytes which undergo apoptosis after DOX exposure in vitro (Perez et al., 1997; Soleimani et al., 2011). As downstream effectors of the apoptotic pathway, the death regulating genes Bax and Bcl-2 appeared to be involved (Zhang et al., 2017), with oocytes collected from Bax-deficient animals resistant to DOX-induced apoptosis (Perez et al., 1997). As previously described, TAp63α becomes activated after oocyte DNA damage, and DOX can act on the TAp63 pathway to directly induce PMF loss (Tuppi et al., 2018). Granulosa cells exposed in vitro to DOX exhibit an increase in expression of p53 mRNA (Zhang et al., 2017), while the neonatal mouse ovary exhibits an increase in the apoptotic marker PARP (Morgan et al., 2013). As a late marker in the apoptotic pathway, TUNEL-positive cells are increased when the ovary is exposed to DOX (Ben-Aharon et al., 2010; Morgan et al., 2013; Roti Roti et al., 2014). Other molecular pathways have also been suggested to play a role in the gonadotoxicity caused by DOX. Accumulation of reactive oxygen species and superoxides, along with a decrease in mitochondrial membrane potential, have been observed in DOX-treated granulosa cells, suggesting involvement of oxidative stress mitochondrial-mediated apoptosis (Bar-Joseph et al., 2010; Zhang et al., 2017). Only preliminary indications are available as to whether the PI3K signalling pathway is activated by DOX, with AKT1 but not PTEN expression increased in marmoset ovary (Salih et al., 2015).
Agents used to protect ovaries from DOX-induced damage
Protectants against ovarian damage by DOX resulting in reduced activation of PMFs or follicular atresia have all been developed using the mouse as a model (Fig. 1B, Table II). However, none of these have been tested for their ability to protect against damage in the human ovary.
Sphingosine-1-phosphate
Morita et al. (2000) reported the first use of the sphingolipid S1P as a protector from oocyte apoptosis when induced by exposure to 0.1 Gy of ionizing radiation. To confirm the role of S1P, knockout mice lacking the acid sphingomyelinase gene were generated; in that mouse, germ cells were resistant to apoptosis and to DOX-induced DNA damage. In the human ovary, S1P prevents DOX-induced apoptotic follicle death in ovarian xenografts (Li et al., 2014; Meng et al., 2014).
Dexrazoxane
The iron-chelating EDTA derivative dexrazoxane can reduce DOX-induced DNA double-strand breaks, by chelating iron and inhibiting topoisomerase II. It is already used in cancer therapies, protecting the heart and skin from other off-targets effects of DOX without altering the effectiveness of DOX as a chemotherapeutic drug (Reichardt et al., 2018; Langer, 2014). In vitro and in vivo co-administration of DOX and dexrazoxane reduces ovarian DNA double-strand breaks and granulosa cell death, with dexrazoxane pre-treatment significantly restoring fertility in vivo (Kropp et al., 2015).
Bortezomib
Proteasome inhibitors have been developed as anti-cancer agents and several are either approved for clinical use to treat melanomas and lung cancers or currently in trials (Kouroukis et al., 2014). MG-132 and bortezomib both directly compete with DOX for proteasome binding, thus preventing nuclear accumulation of DOX in a leukemia cell line and also in cardiac cells (Amiri et al., 2004; Huang et al., 2012; Pajonk et al., 2005). Female mice treated in vivo with bortezomib show reduced DOX-induced DNA damage in all ovarian cell types, resulting in reduced preantral follicle atresia. Fertility studies have shown that bortezomib pretreatment leads to an increase in litter size and pup weight following DOX treatment, thus improving post-chemotherapy fertility (Roti Roti et al., 2014).
Tyrosine kinase inhibitors
Pharmacological inhibition of ATM, ATR, CHK2 or CK1 are all effective in vitro at rescuing oocytes from DOX-induced TAp63-mediated apoptosis (Rinaldi et al., 2017; Tuppi et al., 2018), although imatinib was not found to confer protection against DOX-induced damage to ovarian follicles (Morgan et al., 2013). The kinetics of DOX action are reported to be faster than that of CIS, probably because DOX directly induces double-strand breaks while CIS creates DNA adducts (Tuppi et al., 2018).
Protective agents acting independently of specific chemotherapy drug actions
Most of the research into potential protectants discussed above has been designed with the aim of protecting the ovary against a specific chemotherapy drug or drug class. Other potential protectants should, at least in theory, provide protection against a wide range of chemotherapy drugs, either by blocking a common damage pathway or by reducing the delivery of drugs to the ovary.
Protectants against specific damage pathways
Protection against increased PMF activation: AMH
AMH is primarily produced by granulosa cells of developing follicles and can inhibit activation of PMFs, at least in rodents. The situation in the human ovary is less clear, with publications showing both initiation and suppression of primordial follicle growth in response to AMH (Carlsson et al., 2006; Schmidt et al., 2005). The hypothesis that accelerated activation of PMFs, following the chemotherapy drug-induced death of growing follicles, is an important pathway leading to depletion of the PMF pool has led to the suggestion that AMH treatment might be an effective protectant against a wide range of drugs. Two recent studies have investigated this, examining the effectiveness of AMH at protecting the mouse ovary from CPM- and DOX-induced damage, along with damage from the platinum-containing agent carboplatin (Kano et al., 2017; Sonigo et al., 2018).
Kano and colleagues showed a significant protective effect of AMH administration from damage induced by each of CPM, DOX and carboplatin, particularly in terms of protecting the PMF pool. AMH appeared most effective against DOX-induced damage, but it is difficult to draw conclusions from this study about the comparative effectiveness of AMH against the different drugs, since the doses that were administered for each chemotherapy drug resulted in a lowering of the PMF pool to a different extent in each case (Kano et al., 2017). Nonetheless, this work highlights the importance of studying a range of chemotherapy drugs in this research.
More recently, Sonigo et al. (2018) also found that AMH reduced the loss of follicles induced by CPM, with protection against the reduction in number of oocytes that could be recovered following ovarian stimulation, although an effect on fertility was not shown. That study also provided data suggesting that AMH regulates FOXO3a phosphorylation and induces autophagy in ovaries. These results lead the authors to discuss the hypothesis that AMH reduces PMF loss induced by CPM through autophagy activation.
Both studies highlight the potential value of AMH as a protective agent in the field of oncofertility, with activity against several chemotherapeutic drugs. Additionally, since it is thought to be only involved in the regulation of reproductive function, it may not interfere with the therapeutic effects of chemotherapy.
Protection of ovarian blood vessels: Granulocyte-colony stimulating factor
Granulocyte-colony stimulating factor (G-CSF) is a cytokine that stimulates hematopoietic progenitor cell growth and has been successfully used alongside cancer therapy to overcome chemotherapy-induced myelosuppression (Ciurea et al., 2019). G-CSF increases microvessel density and decreases follicle loss in CPM- and busulfan-treated female mice compared to control groups: moreover, G-CSF can partially restore fertility (Skaznik-Wikiel et al., 2013). G-CSF, with or without stem cell factor, also extends the time until the onset of POI in mice treated with alkylating chemotherapy (Skaznik-Wikiel et al., 2013). An effect has also been seen against CIS in the rat, where all follicle counts (primordial, primary, secondary and tertiary) and serum AMH levels were significantly increased in the G-CSF-CIS treated group compared to the control group (Akdemir et al., 2014). Despite the clinical use of G-CSF in cancer treatment, making it a good ovarian protectant candidate to test clinically, there are no data on the effectiveness of G-CSF at protecting the human ovary against damage by chemotherapy drugs.
Protectants against chemotherapy drug delivery to the ovary
Upregulation of multidrug resistance gene 1
Multidrug resistance gene 1 (MDR1) is a phase three drug transporter enzyme involved in the metabolism, elimination and detoxification of chemotherapy drugs. By promoting the transport and efflux of various lipid-soluble anti-cancer agents, upregulation of MDR1 has been linked with resistance to chemotherapy (Fojo et al., 1987; Lepper et al., 2005). Retroviral transduction has been used to upregulate MDR1 in a granulosa cell line in order to reduce the uptake of chemotherapeutic agents into these cells. This upregulation of MDR1 was reported to protect granulosa cells from the toxic effects of both DOX and paclitaxel in a dose-dependent manner, with the MDR1-transduced granulosa cells showing significantly increased cell survival following treatment with either drug (Salih, 2011). These results are supported by other studies, where the inhibition of MDR transporters in human and mouse oocytes, as well as deletion of the gene in mice, has led to increased susceptibility to CPM toxicity (Brayboy et al., 2017; Wang et al., 2018; Brayboy et al., 2013).
Nanoparticles
A novel approach to ovarian protection, and indeed to reduce other toxicities, has been to encapsulate the chemotherapeutic drug arsenic trioxide inside nanoparticles that specifically target cancer cells (Ahn et al., 2013), with the result being decreased plasma levels and toxicity of these drugs. This strategy could be applied to other chemotherapeutic agents, to target more specifically target the cancer cells while protecting the gonads from damage.
Conclusion
The last decade has seen the development of a number of potential methods for protecting the ovary against damage from chemotherapy drugs (Fig. 1B, Table II). Such protectants are needed not only to preserve fertility but also to allow females to retain endocrine function and avoid the detrimental health consequences of POI. Most of that work has been carried out using rodent models, primarily the mouse. One strategy for such animal model studies, which has been used infrequently to date, is to examine protectants in an animal that has cancer (Qin et al., 2018), although this could be very effective. Surprisingly, few studies to date have examined the effectiveness of potential protectants directly in human ovarian tissue, although this strategy has been used to investigate the potential of S1P and tamoxifen to protect against CPM-induced damage. In the near future, it is vital for more studies to determine the effect of these drugs in the human ovary and/or for research to use large, monovulatory mammalian species as models. With that work, the hope then is that some of these protectants will be able to be taken into clinical trials. Clearly, any protectant will need to not only shield the ovary from damage but will also need to be shown not to interfere with the efficacy of the chemotherapy treatment. In light of that, potential protectants that are already administered to cancer patients are of particular interest here, including AS101, imatinib and G-CSF.
Notwithstanding our improved understanding of female reproduction in recent years, we still do not know what determines whether or not an individual PMF will undergo activation and initiate growth at a particular time. A number of potential protectants under current investigation involve manipulating the Akt pathway, stressing the importance of greater understanding of its role, including in the human ovary. The expression of p-Akt in mice pups is usually restricted to stromal cells and often the evaluation of p-Akt is by Western blotting on whole ovaries. Nevertheless, only recently, its co-expression in primary oocytes upon exposure to CPM with the activated apoptotic protein PARP was shown (Luan et al., 2019). We also have only a limited understanding of what constitutes a healthy oocyte, and it is of course vital that any clinically used protectant maintains the health and developmental competence of the oocyte. Therefore, the implications for the developmental competence of oocytes exposed to potential protectants, as well as chemotherapy agents, should be explored, taking into consideration the long reproductive lifespan of women, and the background deterioration of oocyte “quality” and repair mechanisms with age (Kujjo et al., 2011; Vandenbroucke et al., 2017; Momen et al., 2017). CPM treatment in particular is well established to result in abnormalities in rodent offspring following sub-sterilising dose exposure, although there is no evidence of increased risk of abnormalities or of genotoxicity in humans following maternal radiotherapy or chemotherapy exposure (Signorello et al., 2012; Winther et al., 2009; Winther et al., 2012; Vandenbroucke et al., 2017). Future studies will need to examine whether or not there is evidence of such effects after administration of protectants, as well as of chemotherapeutics. Epigenetic inheritance through the germ line or the potential for transgenerational effects should also be considered in future studies (Wei et al., 2015; Clarke and Vieux, 2015; Di Emidio et al., 2019). It is notable that most protectants under investigation focus on protecting against ovarian follicle death but it is also important to bear in mind that the stromal environment contributes to the health of follicles, and not focus research exclusively on direct effects on the follicles. At present, protecting the ovary against stromal damage appears to have had little attention (Fig. 1B), possibly one consequence of the majority of research using rodents as models. Overall, however, recent years have seen the development of many potential protectants, acting on a wide range of different pathways and with evidence for efficacy in the mouse model, offering hope for the subsequent development of clinically effective treatment.
Acknowledgement
Thanks to Ronnie Grant for help with the figure.
Authors’ roles
All authors contributed to the study design, literature analysis and drafting of the article, as well as critical reading and editing.
Funding
This work was supported by European Society of Human Reproduction and Embryology grant 2014-01, with some work undertaken in the MRC Centre for Reproductive Health, funded by MRC Centre grant MR/N022556/1. RAA has received fees and research support from Roche Diagnostics.
Conflict of interest
RAA reports personal fees and non-financial support from Roche Diagnostics, outside of the submitted work. All other authors confirm that they have no conflict of interest.
References
- Adhikari D, Risal S, Liu K, Shen Y. Pharmacological inhibition of mTORC1 prevents over-activation of the primordial follicle pool in response to elevated PI3K signaling. PLoS One 2013;8:e53810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari D, Zheng W, Shen Y, Gorre N, Hamalainen T, Cooney AJ, Huhtaniemi I, Lan ZJ, Liu K. Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum Mol Genet 2010;19:397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn RW, Barrett SL, Raja MR, Jozefik JK, Spaho L, Chen H, Bally MB, Mazar AP, Avram MJ, Winter JN et al. Nano-encapsulation of arsenic trioxide enhances efficacy against murine lymphoma model while minimizing its impact on ovarian reserve in vitro and in vivo. PLoS One 2013;8:e58491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akdemir A, Zeybek B, Akman L, Ergenoglu AM, Yeniel AO, Erbas O, Yavasoglu A, Terek MC, Taskiran D. Granulocyte-colony stimulating factor decreases the extent of ovarian damage caused by cisplatin in an experimental rat model. J Gynecol Oncol 2014;25:328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliotta JM, Passero M, Meharg J, Klinger J, Dooner MS, Pimentel J, Quesenberry PJ. Stem cells and pulmonary metamorphosis: new concepts in repair and regeneration. J Cell Physiol 2005;204:725–741. [DOI] [PubMed] [Google Scholar]
- Altuner D, Gulaboglu M, Yapca OE, Cetin N. The effect of mirtazapine on cisplatin-induced oxidative damage and infertility in rat ovaries. ScientificWorldJournal 2013;2013:327240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri KI, Horton LW, LaFleur BJ, Sosman JA, Richmond A. Augmenting chemosensitivity of malignant melanoma tumors via proteasome inhibition: implication for bortezomib (VELCADE, PS-341) as a therapeutic agent for malignant melanoma. Cancer Res 2004;64:4912–4918. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Brewster DH, Wood R, Nowell S, Fischbacher C, Kelsey TW, Wallace WHB. The impact of cancer on subsequent chance of pregnancy: a population-based analysis. Hum Reprod 2018;33:1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RA, Mitchell RT, Kelsey TW, Spears N, Telfer EE, Wallace WH. Cancer treatment and gonadal function: experimental and established strategies for fertility preservation in children and young adults. Lancet Diabetes Endocrinol 2015;3:556–567. [DOI] [PubMed] [Google Scholar]
- Argyle CE, Harper JC, Davies MC. Oocyte cryopreservation: where are we now? Hum Reprod Update 2016;22:440–449. [DOI] [PubMed] [Google Scholar]
- Asadi Azarbaijani B, Sheikhi M, Oskam IC, Nurmio M, Laine T, Tinkanen H, Makinen S, Tanbo TG, Hovatta O, Jahnukainen K. Effect of previous chemotherapy on the quality of cryopreserved human ovarian tissue in vitro. PLoS One 2015;10:e0133985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atessahin A, Sahna E, Turk G, Ceribasi AO, Yilmaz S, Yuce A, Bulmus O. Chemoprotective effect of melatonin against cisplatin-induced testicular toxicity in rats. J Pineal Res 2006;41:21–27. [DOI] [PubMed] [Google Scholar]
- Bansod S, Kageyama R, Ohtsuka T. Hes 5 regulates the transition timing of neurogenesis and gliogenesis in mammalian neocortical development. Development 2017;144:3156–3167. [DOI] [PubMed] [Google Scholar]
- Bar J, Davidi O, Goshen Y, Hod M, Yaniv I, Hirsch R. Pregnancy outcome in women treated with doxorubicin for childhood cancer. Am J Obstet Gynecol 2003;189:853–857. [DOI] [PubMed] [Google Scholar]
- Barberino RS, Menezes VG, Ribeiro A, Palheta RC Jr, Jiang X, Smitz JEJ, Matos MHT. Melatonin protects against cisplatin-induced ovarian damage in mice via the MT1 receptor and antioxidant activity. Biol Reprod 2017;96:1244–1255. [DOI] [PubMed] [Google Scholar]
- Barekati Z, Gourabi H, Valojerdi MR, Yazdi PE. Previous maternal chemotherapy by cyclophosphamide (cp) causes numerical chromosome abnormalities in preimplantation mouse embryos. Reprod Toxicol 2008;26:278–281. [DOI] [PubMed] [Google Scholar]
- Bar-Joseph H, Ben-Aharon I, Rizel S, Stemmer SM, Tzabari M, Shalgi R. Doxorubicin-induced apoptosis in germinal vesicle (GV) oocytes. Reprod Toxicol 2010;30:566–572. [DOI] [PubMed] [Google Scholar]
- Bedoschi G, Navarro PA, Oktay K. Chemotherapy-induced damage to ovary: mechanisms and clinical impact. Future Oncol 2016;12:2333–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedoschi GM, Navarro PA, Oktay KH. Novel insights into the pathophysiology of chemotherapy-induced damage to the ovary. Panminerva Med 2019;61:68–75. [DOI] [PubMed] [Google Scholar]
- Ben-Aharon I, Bar-Joseph H, Tzarfaty G, Kuchinsky L, Rizel S, Stemmer SM, Shalgi R. Doxorubicin-induced ovarian toxicity. Reprod Biol Endocrinol 2010;8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bildik G, Akin N, Senbabaoglu F, Sahin GN, Karahuseyinoglu S, Ince U, Taskiran C, Selek U, Yakin K, Guzel Y et al. GnRH agonist leuprolide acetate does not confer any protection against ovarian damage induced by chemotherapy and radiation in vitro. Hum Reprod 2015;30:2912–2925. [DOI] [PubMed] [Google Scholar]
- Blum RH, Carter SK. Adriamycin. A new anticancer drug with significant clinical activity. Ann Intern Med 1974;80:249–259. [DOI] [PubMed] [Google Scholar]
- Bolcun-Filas E, Rinaldi VD, White ME, Schimenti JC. Reversal of female infertility by Chk2 ablation reveals the oocyte DNA damage checkpoint pathway. Science 2014;343:533–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnaud S, Niaudet C, Pottier G, Gaugler MH, Millour J, Barbet J, Sabatier L, Paris F. Sphingosine-1-phosphate protects proliferating endothelial cells from ceramide-induced apoptosis but not from DNA damage-induced mitotic death. Cancer Res 2007;67:1803–1811. [DOI] [PubMed] [Google Scholar]
- Brayboy LM, Oulhen N, Long S, Voigt N, Raker C, Wessel GM. Multidrug resistance transporter-1 and breast cancer resistance protein protect against ovarian toxicity, and are essential in ovarian physiology. Reprod Toxicol 2017;69:121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayboy LM, Oulhen N, Witmyer J, Robins J, Carson S, Wessel GM. Multidrug-resistant transport activity protects oocytes from chemotherapeutic agents and changes during oocyte maturation. Fertil Steril 2013;100:1428–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer M, Gershenson DM, Herzog CE, Mitchell MF, Silva EG, Wharton JT. Outcome and reproductive function after chemotherapy for ovarian dysgerminoma. J Clin Oncol 1999;17:2670–2675. [DOI] [PubMed] [Google Scholar]
- Campbell BK, Clinton M, Webb R. The role of anti-Mullerian hormone (AMH) during follicle development in a monovulatory species (sheep). Endocrinology 2012;153:4533–4543. [DOI] [PubMed] [Google Scholar]
- Carlsson IB, Scott JE, Visser JA, Ritvos O, Themmen AP, Hovatta O. Anti-Mullerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum Reprod 2006;21:2223–2227. [DOI] [PubMed] [Google Scholar]
- Casado-Zapico S, Rodriguez-Blanco J, Garcia-Santos G, Martin V, Sanchez-Sanchez AM, Antolin I, Rodriguez C. Synergistic antitumor effect of melatonin with several chemotherapeutic drugs on human Ewing sarcoma cancer cells: potentiation of the extrinsic apoptotic pathway. J Pineal Res 2010;48:72–80. [DOI] [PubMed] [Google Scholar]
- Chang EM, Lim E, Yoon S, Jeong K, Bae S, Lee DR, Yoon TK, Choi Y, Lee WS. Cisplatin induces overactivation of the dormant primordial follicle through PTEN/AKT/FOXO3a pathway which leads to loss of ovarian reserve in mice. PLoS One 2015;10:e0144245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemaitilly W, Li Z, Krasin MJ, Brooke RJ, Wilson CL, Green DM, Klosky JL, Barnes N, Clark KL, Farr JB et al. Premature ovarian insufficiency in childhood cancer survivors: a report from the St. Jude Lifetime Cohort. J Clin Endocrinol Metab 2017;102:2242–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XY, Xia HX, Guan HY, Li B, Zhang W. Follicle loss and apoptosis in cyclophosphamide-treated mice: what’s the matter? Int J Mol Sci 2016;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Kang X, Wang L, Dong H, Wang C, Xiong Z, Zhao W, Jia C, Lin J, Zhang W et al. Rictor/mTORC2 pathway in oocytes regulates folliculogenesis, and its inactivation causes premature ovarian failure. J Biol Chem 2015;290:6387–6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chovanec M, Abu Zaid M, Hanna N, El-Kouri N, Einhorn LH, Albany C. Long-term toxicity of cisplatin in germ-cell tumor survivors. Ann Oncol 2017;28:2670–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow EJ, Stratton KL, Leisenring WM, Oeffinger KC, Sklar CA, Donaldson SS, Ginsberg JP, Kenney LB, Levine JM, Robison LL et al. Pregnancy after chemotherapy in male and female survivors of childhood cancer treated between 1970 and 1999: a report from the childhood cancer survivor study cohort. Lancet Oncol 2016;17:567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciurea SO, Al Malki MM, Kongtim P, Fuchs EJ, Luznik L, Huang XJ, Ciceri F, Locatelli F, Aversa F, Castagna L et al. The European Society for Blood and Marrow Transplantation (EBMT) consensus recommendations for donor selection in haploidentical hematopoietic cell transplantation. Bone Marrow Transplant 2019; doi: 10.1038/s41409-019-0499-z. [DOI] [PubMed] [Google Scholar]
- Clarke HJ, Vieux KF. Epigenetic inheritance through the female germ-line: the known, the unknown, and the possible. Semin Cell Dev Biol 2015;43:106–116. [DOI] [PubMed] [Google Scholar]
- Clementi ME, Giardina B, Di Stasio E, Mordente A, Misiti F. Doxorubicin-derived metabolites induce release of cytochrome C and inhibition of respiration on cardiac isolated mitochondria. Anticancer Res 2003;23:2445–2450. [PubMed] [Google Scholar]
- Comish PB, Drumond AL, Kinnell HL, Anderson RA, Matin A, Meistrich ML, Shetty G. Fetal cyclophosphamide exposure induces testicular cancer and reduced spermatogenesis and ovarian follicle numbers in mice. PLoS One 2014;9:e93311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin KA, Lake AJ, Scott S, Sherman RL, Noone AM, Howlader N, Henley SJ, Anderson RN, Firth AU, Ma J et al. Annual report to the nation on the status of cancer, part I: national cancer statistics. Cancer 2018;124:2785–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 1996;381:800–803. [DOI] [PubMed] [Google Scholar]
- Da Silva-Buttkus P, Marcelli G, Franks S, Stark J, Hardy K. Inferring biological mechanisms from spatial analysis: prediction of a local inhibitor in the ovary. Proc Natl Acad Sci U S A 2009;106:456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damia G, Filiberti L, Vikhanskaya F, Carrassa L, Taya Y, D'Incalci M, Broggini M. Cisplatinum and taxol induce different patterns of p53 phosphorylation. Neoplasia 2001;3:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 2014;740:364–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Mastro L, Ceppi M, Poggio F, Bighin C, Peccatori F, Demeestere I, Levaggi A, Giraudi S, Lambertini M, D’Alonzo A et al. Gonadotropin-releasing hormone analogues for the prevention of chemotherapy-induced premature ovarian failure in cancer women: systematic review and meta-analysis of randomized trials. Cancer Treat Rev 2014;40:675–683. [DOI] [PubMed] [Google Scholar]
- Delgado-Rosas F, Gaytan M, Morales C, Gomez R, Gaytan F. Superficial ovarian cortex vascularization is inversely related to the follicle reserve in normal cycling ovaries and is increased in polycystic ovary syndrome. Hum Reprod 2009;24:1142–1151. [DOI] [PubMed] [Google Scholar]
- Demeestere I, Brice P, Peccatori FA, Kentos A, Dupuis J, Zachee P, Casasnovas O, Van Den Neste E, Dechene J, De Maertelaer V et al. No evidence for the benefit of gonadotropin-releasing hormone agonist in preserving ovarian function and fertility in lymphoma survivors treated with chemotherapy: final Long-term report of a prospective randomized trial. J Clin Oncol 2016;34:2568–2574. [DOI] [PubMed] [Google Scholar]
- Di Emidio G, D’Aurora M, Placidi M, Franchi S, Rossi G, Stuppia L, Artini PG, Tatone C, Gatta V. Pre-conceptional maternal exposure to cyclophosphamide results in modifications of DNA methylation in F1 and F2 mouse oocytes: evidence for transgenerational effects. Epigenetics 2019; doi: 10.1080/15592294.2019.1631111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Emidio G, Rossi G, Bonomo I, Alonso GL, Sferra R, Vetuschi A, Artini PG, Provenzani A, Falone S, Carta G et al. The natural carotenoid crocetin and the synthetic tellurium compound AS101 protect the ovary against cyclophosphamide by modulating SIRT1 and mitochondrial markers. Oxid Med Cell Longev 2017;2017:8928604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnez J, Dolmans MM. Fertility preservation in women. N Engl J Med 2017;377:1657–1665. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Kramer P, Karels B, Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology 1999;140:5789–5796. [DOI] [PubMed] [Google Scholar]
- Ernst EH, Grondahl ML, Grund S, Hardy K, Heuck A, Sunde L, Franks S, Andersen CY, Villesen P, Lykke-Hartmann K. Dormancy and activation of human oocytes from primordial and primary follicles: molecular clues to oocyte regulation. Hum Reprod 2017;32:1684–1700. [DOI] [PubMed] [Google Scholar]
- European Society of Human Reproduction and Embryology Management of women with premature ovarian insufficiency, 2015
- Ezoe K, Daikoku T, Yabuuchi A, Murata N, Kawano H, Abe T, Okuno T, Kobayashi T, Kato K. Ovarian stimulation using human chorionic gonadotrophin impairs blastocyst implantation and decidualization by altering ovarian hormone levels and downstream signaling in mice. Mol Hum Reprod 2014;20:1101–1116. [DOI] [PubMed] [Google Scholar]
- Fojo AT, Ueda K, Slamon DJ, Poplack DG, Gottesman MM, Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A 1987;84:265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries JF, Sharp GC, McDevitt HO, Holman HR. Cyclophosphamide therapy in systemic lupus erythematosus and polymyositis. Arthritis Rheum 1973;16:154–162. [DOI] [PubMed] [Google Scholar]
- Garcia JM, Chen JA, Guillory B, Donehower LA, Smith RG, Lamb DJ. Ghrelin prevents cisplatin-induced testicular damage by facilitating repair of DNA double strand breaks through activation of p53 in mice. Biol Reprod 2015;93:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaytan F, Morales C, Leon S, Garcia-Galiano D, Roa J, Tena-Sempere M. Crowding and follicular fate: spatial determinants of follicular reserve and activation of follicular growth in the mammalian ovary. PLoS One 2015;10:e0144099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman KN, Chenette D, Arju R, Duncan FE, Keefe DL, Grifo JA, Schneider RJ. mTORC1/2 inhibition preserves ovarian function and fertility during genotoxic chemotherapy. Proc Natl Acad Sci USA 2017;114:3186–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonfloni S, Di Tella L, Caldarola S, Cannata SM, Klinger FG, Di Bartolomeo C, Mattei M, Candi E, De Felici M, Melino G et al. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat Med 2009;15:1179–1185. [DOI] [PubMed] [Google Scholar]
- Green DM, Sklar CA, Boice JD Jr, Mulvihill JJ, Whitton JA, Stovall M, Yasui Y. Ovarian failure and reproductive outcomes after childhood cancer treatment: results from the childhood cancer survivor study. J Clin Oncol 2009;27:2374–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosbois J, Demeestere I. Dynamics of PI3K and hippo signaling pathways during in vitro human follicle activation. Hum Reprod 2018;33:1705–1714. [DOI] [PubMed] [Google Scholar]
- Hahn KM, Johnson PH, Gordon N, Kuerer H, Middleton L, Ramirez M, Yang W, Perkins G, Hortobagyi GN, Theriault RL. Treatment of pregnant breast cancer patients and outcomes of children exposed to chemotherapy in utero. Cancer 2006;107:1219–1226. [DOI] [PubMed] [Google Scholar]
- Huang H, Liu N, Yang C, Liao S, Guo H, Zhao K, Li X, Liu S, Guan L, Liu C et al. HDAC inhibitor L-carnitine and proteasome inhibitor bortezomib synergistically exert anti-tumor activity in vitro and in vivo. PLoS One 2012;7:e52576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang H, Lee OH, Lee Y, Yoon H, Chang EM, Park M, Lee JW, Hong K, Kim JO, Kim NK et al. Melatonin prevents cisplatin-induced primordial follicle loss via suppression of PTEN/AKT/FOXO3a pathway activation in the mouse ovary. J Pineal Res 2016;60:336–347. [DOI] [PubMed] [Google Scholar]
- Jang H, Na Y, Hong K, Lee S, Moon S, Cho M, Park M, Lee OH, Chang EM, Lee DR et al. Synergistic effect of melatonin and ghrelin in preventing cisplatin-induced ovarian damage via regulation of FOXO3a phosphorylation and binding to the p27(Kip1) promoter in primordial follicles. J Pineal Res 2017;63:e12432. [DOI] [PubMed] [Google Scholar]
- Jarrell JF, Bodo L, YoungLai EV, Barr RD, O’Connell GJ. The short-term reproductive toxicity of cyclophosphamide in the female rat. Reprod Toxicol 1991;5:481–485. [DOI] [PubMed] [Google Scholar]
- Jayasinghe YL, Wallace WHB, Anderson RA. Ovarian function, fertility and reproductive lifespan in cancer patients. Expert Rev Endocrinol Metab 2018;13:125–136. [DOI] [PubMed] [Google Scholar]
- John GB, Gallardo TD, Shirley LJ, Castrillon DH. Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev Biol 2008;321:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalechman Y, Albeck M, Oron M, Sobelman D, Gurwith M, Horwith G, Kirsch T, Maida B, Sehgal SN, Sredni B. Protective and restorative role of AS101 in combination with chemotherapy. Cancer Res 1991;51:1499–1503. [PubMed] [Google Scholar]
- Kalich-Philosoph L, Roness H, Carmely A, Fishel-Bartal M, Ligumsky H, Paglin S, Wolf I, Kanety H, Sredni B, Meirow D. Cyclophosphamide triggers follicle activation and ‘burnout’; AS101 prevents follicle loss and preserves fertility. Sci Transl Med 2013;5: 185ra162. [DOI] [PubMed] [Google Scholar]
- Kalil NG, McGuire WP. Chemotherapy for advanced epithelial ovarian carcinoma. Best Pract Res Clin Obstet Gynaecol 2002;16:553–571. [DOI] [PubMed] [Google Scholar]
- Kano M, Sosulski AE, Zhang L, Saatcioglu HD, Wang D, Nagykery N, Sabatini ME, Gao G, Donahoe PK, Pepin D. AMH/MIS as a contraceptive that protects the ovarian reserve during chemotherapy. Proc Natl Acad Sci U S A 2017;114:E1688–E1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, Ho CH, Kawamura N, Tamura M, Hashimoto S et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci U S A 2013;110:17474–17479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrloesser S, Tuppi M, Dotsch V. CHK2 sets the stage for CK1 in oocyte quality control. Cell Death Differ 2018;25:1007–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer 2007;7:573–584. [DOI] [PubMed] [Google Scholar]
- Kerr JB, Hutt KJ, Michalak EM, Cook M, Vandenberg CJ, Liew SH, Bouillet P, Mills A, Scott CL, Findlay JK et al. DNA damage-induced primordial follicle oocyte apoptosis and loss of fertility require TAp63-mediated induction of Puma and Noxa. Mol Cell 2012;48:343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedr NF. Protective effect of mirtazapine and hesperidin on cyclophosphamide-induced oxidative damage and infertility in rat ovaries. Exp Biol Med (Maywood) 2015;240:1682–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Cordeiro MH, Serna VA, Ebbert K, Butler LM, Sinha S, Mills AA, Woodruff TK, Kurita T. Rescue of platinum-damaged oocytes from programmed cell death through inactivation of the p53 family signaling network. Cell Death Differ 2013;20:987–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Nair DM, Romero M, Serna VA, Koleske AJ, Woodruff TK, Kurita T. Transient inhibition of p53 homologs protects ovarian function from two distinct apoptotic pathways triggered by anticancer therapies. Cell Death Differ 2018;26:502–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch-Volders M, Pacchierotti F, Parry EM, Russo A, Eichenlaub-Ritter U, Adler ID. Risks of aneuploidy induction from chemical exposure: twenty years of collaborative research in Europe from basic science to regulatory implications. Mutat Res 2019;779:126–147. [DOI] [PubMed] [Google Scholar]
- Kiyomiya K, Matsuo S, Kurebe M. Proteasome is a carrier to translocate doxorubicin from cytoplasm into nucleus. Life Sci 1998;62:1853–1860. [DOI] [PubMed] [Google Scholar]
- Knobf MT. The influence of endocrine effects of adjuvant therapy on quality of life outcomes in younger breast cancer survivors. Oncologist 2006;11:96–110. [DOI] [PubMed] [Google Scholar]
- Kouroukis CT, Baldassarre FG, Haynes AE, Imrie K, Reece DE, Cheung MC, Cancer Care Ontario Hematology Disease Site G . Bortezomib in multiple myeloma: a practice guideline. Clin Oncol (R Coll Radiol) 2014;26:110–119. [DOI] [PubMed] [Google Scholar]
- Koyama H, Wada T, Nishizawa Y, Iwanaga T, Aoki Y. Cyclophosphamide-induced ovarian failure and its therapeutic significance in patients with breast cancer. Cancer 1977;39:1403–1409. [DOI] [PubMed] [Google Scholar]
- Kropp J, Roti Roti EC, Ringelstetter A, Khatib H, Abbott DH, Salih SM. Dexrazoxane diminishes doxorubicin-induced acute ovarian damage and preserves ovarian function and fecundity in mice. PLoS One 2015;10:e0142588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujjo LL, Chang EA, Pereira RJ, Dhar S, Marrero-Rosado B, Sengupta S, Wang H, Cibelli JB, Perez GI. Chemotherapy-induced late transgenerational effects in mice. PLoS One 2011;6:e17877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertini M, Moore HCF, Leonard RCF, Loibl S, Munster P, Bruzzone M, Boni L, Unger JM, Anderson RA, Mehta K et al. Gonadotropin-releasing hormone agonists during chemotherapy for preservation of ovarian function and fertility in premenopausal patients with early breast cancer: a systematic review and meta-analysis of individual patient-level data. J Clin Oncol 2018;36:1981–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande Y, Fisch B, Tsur A, Farhi J, Prag-Rosenberg R, Ben-Haroush A, Kessler-Icekson G, Zahalka MA, Ludeman SM, Abir R. Short-term exposure of human ovarian follicles to cyclophosphamide metabolites seems to promote follicular activation in vitro. Reprod Biomed Online 2017;34:104–114. [DOI] [PubMed] [Google Scholar]
- Langer SW. Dexrazoxane for the treatment of chemotherapy-related side effects. Cancer Manag Res 2014;6:357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R, Adamson D, Bertelli G, Mansi J, Yellowlees A, Dunlop J, Thomas GA, Coleman RE, Anderson RA, Anglo Celtic Collaborative Oncology G et al. GnRH agonist for protection against ovarian toxicity during chemotherapy for early breast cancer: the Anglo Celtic group OPTION trial. Ann Oncol 2017;28:1811–1816. [DOI] [PubMed] [Google Scholar]
- Lepper ER, Nooter K, Verweij J, Acharya MR, Figg WD, Sparreboom A. Mechanisms of resistance to anticancer drugs: the role of the polymorphic ABC transporters ABCB1 and ABCG2. Pharmacogenomics 2005;6:115–138. [DOI] [PubMed] [Google Scholar]
- Letourneau JM, Ebbel EE, Katz PP, Oktay KH, McCulloch CE, Ai WZ, Chien AJ, Melisko ME, Cedars MI, Rosen MP. Acute ovarian failure underestimates age-specific reproductive impairment for young women undergoing chemotherapy for cancer. Cancer 2012;118:1933–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Turan V, Lierman S, Cuvelier C, De Sutter P, Oktay K. Sphingosine-1-phosphate prevents chemotherapy-induced human primordial follicle death. Hum Reprod 2014;29:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Chen J, Fang X, Xia X. Sphingosine-1-phosphate activates the AKT pathway to inhibit chemotherapy induced human granulosa cell apoptosis. Gynecol Endocrinol 2017;33:476–479. [DOI] [PubMed] [Google Scholar]
- Li X, Yang S, Lv X, Sun H, Weng J, Liang Y, Zhou D. The mechanism of mesna in protection from cisplatin-induced ovarian damage in female rats. J Gynecol Oncol 2013;24:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipshultz ER, Holt GE, Ramasamy R, Yechieli R, Lipshultz SE. Fertility, cardiac, and orthopedic challenges in survivors of adult and childhood sarcoma. Am Soc Clin Oncol Educ Book 2017;37:799–806. [DOI] [PubMed] [Google Scholar]
- Lobo RA. Hormone-replacement therapy: current thinking. Nat Rev Endocrinol 2017;13:220–231. [DOI] [PubMed] [Google Scholar]
- Luan Y, Edmonds ME, Woodruff TK, Kim SY. Inhibitors of apoptosis protect the ovarian reserve from cyclophosphamide. J Endocrinol 2019;240:243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q, Yin N, Zhang L, Yuan W, Zhao W, Luan X, Zhang H. Role of SDF-1/CXCR4 and cytokines in the development of ovary injury in chemotherapy drug induced premature ovarian failure mice. Life Sci 2017;179:103–109. [DOI] [PubMed] [Google Scholar]
- Madden JA, Keating AF. Ovarian xenobiotic biotransformation enzymes are altered during phosphoramide mustard-induced ovotoxicity. Toxicol Sci 2014;141:441–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiani E, Di Bartolomeo C, Klinger FG, Cannata SM, Bernardini S, Chateauvieux S, Mack F, Mattei M, De Felici M, Diederich M et al. Reply to: Cisplatin-induced primordial follicle oocyte killing and loss of fertility are not prevented by imatinib. Nat Med 2012;18:1172–1174. [DOI] [PubMed] [Google Scholar]
- Makarovsky D, Kalechman Y, Sonino T, Freidkin I, Teitz S, Albeck M, Weil M, Geffen-Aricha R, Yadid G, Sredni B. Tellurium compound AS101 induces PC12 differentiation and rescues the neurons from apoptotic death. Ann N Y Acad Sci 2003;1010:659–666. [DOI] [PubMed] [Google Scholar]
- Maneschi F, Benedetti-Panici P, Scambia G, Salerno MG, D'Agostino G, Mancuso S. Menstrual and hormone patterns in women treated with high-dose cisplatin and bleomycin. Gynecol Oncol 1994;54:345–348. [DOI] [PubMed] [Google Scholar]
- Meirow D, Dor J, Kaufman B, Shrim A, Rabinovici J, Schiff E, Raanani H, Levron J, Fridman E. Cortical fibrosis and blood-vessels damage in human ovaries exposed to chemotherapy. Potential mechanisms of ovarian injury. Hum Reprod 2007;22:1626–1633. [DOI] [PubMed] [Google Scholar]
- Meirow D, Epstein M, Lewis H, Nugent D, Gosden RG. Administration of cyclophosphamide at different stages of follicular maturation in mice: effects on reproductive performance and fetal malformations. Hum Reprod 2001;16:632–637. [DOI] [PubMed] [Google Scholar]
- Meirow D, Lewis H, Nugent D, Epstein M. Subclinical depletion of primordial follicular reserve in mice treated with cyclophosphamide: clinical importance and proposed accurate investigative tool. Hum Reprod 1999;14:1903–1907. [DOI] [PubMed] [Google Scholar]
- Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update 2001;7:535–543. [DOI] [PubMed] [Google Scholar]
- Melekoglu R, Ciftci O, Eraslan S, Cetin A, Basak N. Beneficial effects of curcumin and capsaicin on cyclophosphamide-induced premature ovarian failure in a rat model. J Ovarian Res 2018;11:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Xu Z, Wu F, Chen W, Xie S, Liu J, Huang X, Zhou Y. Sphingosine-1-phosphate suppresses cyclophosphamide induced follicle apoptosis in human fetal ovarian xenografts in nude mice. Fertil Steril 2014;102:871–877e873. [DOI] [PubMed] [Google Scholar]
- Miller JJ 3rd, Cole LJ. Changes in mouse ovaries after prolonged treatment with cyclophosphamide. Proc Soc Exp Biol Med 1970;133:190–193. [DOI] [PubMed] [Google Scholar]
- Miller JJ 3rd, Williams GF, Leissring JC. Multiple late complications of therapy with cyclophosphamide, including ovarian destruction. Am J Med 1971;50:530–535. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momen NC, Ernst A, Arendt LH, Olsen J, Li J, Gissler M, Rasmussen F, Ramlau-Hansen CH. Maternal cancer and congenital anomalies in children - a Danish nationwide cohort study. PLoS One 2017;12:e0173355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan S, Lopes F, Gourley C, Anderson RA, Spears N. Cisplatin and doxorubicin induce distinct mechanisms of ovarian follicle loss; imatinib provides selective protection only against cisplatin. PLoS One 2013;8:43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita Y, Perez GI, Paris F, Miranda SR, Ehleiter D, Haimovitz-Friedman A, Fuks Z, Xie Z, Reed JC, Schuchman EH et al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat Med 2000;6:1109–1114. [DOI] [PubMed] [Google Scholar]
- Myers M, Morgan FH, Liew SH, Zerafa N, Gamage TU, Sarraj M, Cook M, Kapic I, Sutherland A, Scott CL et al. PUMA regulates germ cell loss and primordial follicle endowment in mice. Reproduction 2014;148:211–219. [DOI] [PubMed] [Google Scholar]
- Nguyen QN, Zerafa N, Liew SH, Morgan FH, Strasser A, Scott CL, Findlay JK, Hickey M, Hutt KJ. Loss of PUMA protects the ovarian reserve during DNA-damaging chemotherapy and preserves fertility. Cell Death Dis 2018;9:618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktem O, Oktay K. Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer 2007;110:2222–2229. [DOI] [PubMed] [Google Scholar]
- Overbeek A, Berg MH, Leeuwen FE, Kaspers GJ, Lambalk CB, Broeder E. Chemotherapy-related late adverse effects on ovarian function in female survivors of childhood and young adult cancer: a systematic review. Cancer Treat Rev 2017;53:10–24. [DOI] [PubMed] [Google Scholar]
- Ozcan P, Ficicioglu C, Yildirim OK, Ozkan F, Akkaya H, Aslan I. Protective effect of resveratrol against oxidative damage to ovarian reserve in female Sprague–Dawley rats. Reprod Biomed Online 2015;31:404–410. [DOI] [PubMed] [Google Scholar]
- Ozcelik B, Turkyilmaz C, Ozgun MT, Serin IS, Batukan C, Ozdamar S, Ozturk A. Prevention of paclitaxel and cisplatin induced ovarian damage in rats by a gonadotropin-releasing hormone agonist. Fertil Steril 2010;93:1609–1614. [DOI] [PubMed] [Google Scholar]
- Paik S, Bryant J, Park C, Fisher B, Tan-Chiu E, Hyams D, Fisher ER, Lippman ME, Wickerham DL, Wolmark N. erbB-2 and response to doxorubicin in patients with axillary lymph node-positive, hormone receptor-negative breast cancer. J Natl Cancer Inst 1998;90:1361–1370. [DOI] [PubMed] [Google Scholar]
- Pajonk F, Ophoven A, Weissenberger C, McBride WH. The proteasome inhibitor MG-132 sensitizes PC-3 prostate cancer cells to ionizing radiation by a DNA-PK-independent mechanism. BMC Cancer 2005;5:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariente R, Pariente JA, Rodriguez AB, Espino J. Melatonin sensitizes human cervical cancer HeLa cells to cisplatin-induced cytotoxicity and apoptosis: effects on oxidative stress and DNA fragmentation. J Pineal Res 2016;60:55–64. [DOI] [PubMed] [Google Scholar]
- Pascuali N, Scotti L, Di Pietro M, Oubina G, Bas D, May M, Gomez Munoz A, Cuasnicu PS, Cohen DJ, Tesone M et al. Ceramide-1-phosphate has protective properties against cyclophosphamide-induced ovarian damage in a mice model of premature ovarian failure. Hum Reprod 2018;33:844–859. [DOI] [PubMed] [Google Scholar]
- Perez GI, Knudson CM, Leykin L, Korsmeyer SJ, Tilly JL. Apoptosis-associated signaling pathways are required for chemotherapy-mediated female germ cell destruction. Nat Med 1997;3:1228–1232. [DOI] [PubMed] [Google Scholar]
- Petrillo SK, Desmeules P, Truong TQ, Devine PJ. Detection of DNA damage in oocytes of small ovarian follicles following phosphoramide mustard exposures of cultured rodent ovaries in vitro. Toxicol Appl Pharmacol 2011;253:94–102. [DOI] [PubMed] [Google Scholar]
- Piasecka-Srader J, Blanco FF, Delman DH, Dixon DA, Geiser JL, Ciereszko RE, Petroff BK. Tamoxifen prevents apoptosis and follicle loss from cyclophosphamide in cultured rat ovaries. Biol Reprod 2015;92:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowchalk DR, Mattison DR. Phosphoramide mustard is responsible for the ovarian toxicity of cyclophosphamide. Toxicol Appl Pharmacol 1991;107:472–481. [DOI] [PubMed] [Google Scholar]
- Qin Y, Iwase A, Murase T, Bayasula Ishida C, Kato N, Nakamura T, Osuka S, Takikawa S, Goto M et al. Protective effects of mangafodipir against chemotherapy-induced ovarian damage in mice. Reprod Biol Endocrinol 2018;16:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B, Potu BK. Ovarian folliculogenesis: detrimental effect of prenatal exposure to cyclophosphamide: a preliminary study. Bratisl Lek Listy 2010;111:369–372. [PubMed] [Google Scholar]
- Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hamalainen T, Peng SL et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 2008;319:611–613. [DOI] [PubMed] [Google Scholar]
- Reichardt P, Tabone MD, Mora J, Morland B, Jones RL. Risk-benefit of dexrazoxane for preventing anthracycline-related cardiotoxicity: re-evaluating the European labeling. Future Oncol 2018;14:2663–2676. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Rosales-Corral SA, Manchester LC, Tan DX. Peripheral reproductive organ health and melatonin: ready for prime time. Int J Mol Sci 2013;14:7231–7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Tamura H, Cruz MH, Fuentes-Broto L. Clinical relevance of melatonin in ovarian and placental physiology: a review. Gynecol Endocrinol 2014;30:83–89. [DOI] [PubMed] [Google Scholar]
- Rinaldi VD, Hsieh K, Munroe R, Bolcun-Filas E, Schimenti JC. Pharmacological inhibition of the DNA damage checkpoint prevents radiation-induced oocyte death. Genetics 2017;206:1823–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JJ, Pera MF Jr. DNA as a target for anticancer coordination compounds In Lippard SJ. (ed) Platinum, Gold, and Other Metal Chemotherapeutic Agents: Chemistry and Biochemistry. American Chemical Society, Washington, D.C.1983, 3–25. [Google Scholar]
- Roness H, Gavish Z, Cohen Y, Meirow D. Ovarian follicle burnout: a universal phenomenon? Cell Cycle 2013;12:3245–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roness H, Kalich-Philosoph L, Meirow D. Prevention of chemotherapy-induced ovarian damage: possible roles for hormonal and non-hormonal attenuating agents. Hum Reprod Update 2014;20:759–774. [DOI] [PubMed] [Google Scholar]
- Roness H, Kashi O, Meirow D. Prevention of chemotherapy-induced ovarian damage. Fertil Steril 2016;105:20–29. [DOI] [PubMed] [Google Scholar]
- Rossi V, Lispi M, Longobardi S, Mattei M, Rella FD, Salustri A, De Felici M, Klinger FG. LH prevents cisplatin-induced apoptosis in oocytes and preserves female fertility in mouse. Cell Death Differ 2017;24:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roti Roti EC, Leisman SK, Abbott DH, Salih SM. Acute doxorubicin insult in the mouse ovary is cell- and follicle-type dependent. PLoS One 2012;7:e42293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roti Roti EC, Ringelstetter AK, Kropp J, Abbott DH, Salih SM. Bortezomib prevents acute doxorubicin ovarian insult and follicle demise, improving the fertility window and pup birth weight in mice. PLoS One 2014;9:e108174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roti Roti EC, Salih SM. Dexrazoxane ameliorates doxorubicin-induced injury in mouse ovarian cells. Biol Reprod 2012;86:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundle S, Bradbury A, Drew Y, Curtin NJ. Targeting the ATR-CHK1 Axis in cancer therapy. Cancers (Basel) 2017;9:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh HS, Omar E, Froemming GR, Said RM. Tocotrienol preserves ovarian function in cyclophosphamide therapy. Hum Exp Toxicol 2015;34:946–952. [DOI] [PubMed] [Google Scholar]
- Salih SM. Retrovirus-mediated multidrug resistance gene (MDR1) overexpression inhibits chemotherapy-induced toxicity of granulosa cells. Fertil Steril 2011;95:1390–1396, e13911396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salih SM, Ringelstetter AK, Elsarrag MZ, Abbott DH, Roti EC. Dexrazoxane abrogates acute doxorubicin toxicity in marmoset ovary. Biol Reprod 2015;92:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Kaarniranta K, Kauppinen A. Crosstalk between oxidative stress and SIRT1: impact on the aging process. Int J Mol Sci 2013;14:3834–3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt KL, Kryger-Baggesen N, Byskov AG, Andersen CY. Anti-Mullerian hormone initiates growth of human primordial follicles in vitro. Mol Cell Endocrinol 2005;234:87–93. [DOI] [PubMed] [Google Scholar]
- Seeliger MA, Nagar B, Frank F, Cao X, Henderson MN, Kuriyan J. C-Src binds to the cancer drug imatinib with an inactive Abl/c-kit conformation and a distributed thermodynamic penalty. Structure 2007;15:299–311. [DOI] [PubMed] [Google Scholar]
- Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene 2003;22:7265–7279. [DOI] [PubMed] [Google Scholar]
- Signorello LB, Mulvihill JJ, Green DM, Munro HM, Stovall M, Weathers RE, Mertens AC, Whitton JA, Robison LL, Boice JD Jr. Congenital anomalies in the children of cancer survivors: a report from the childhood cancer survivor study. J Clin Oncol 2012;30:239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaznik-Wikiel ME, McGuire MM, Sukhwani M, Donohue J, Chu T, Krivak TC, Rajkovic A, Orwig KE. Granulocyte colony-stimulating factor with or without stem cell factor extends time to premature ovarian insufficiency in female mice treated with alkylating chemotherapy. Fertil Steril 2013;99:2045–2054e2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldani C, Scovassi AI. Poly (ADP-ribose) polymerase-1 cleavage during apoptosis: an update. Apoptosis 2002;7:321–328. [DOI] [PubMed] [Google Scholar]
- Soleimani R, Heytens E, Darzynkiewicz Z, Oktay K. Mechanisms of chemotherapy-induced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging (Albany NY) 2011;3:782–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonigo C, Beau I, Grynberg M, Binart N. AMH prevents primordial ovarian follicle loss and fertility alteration in cyclophosphamide-treated mice. FASEB J 2018;33:1278–1287. [DOI] [PubMed] [Google Scholar]
- Suh EK, Yang A, Kettenbach A, Bamberger C, Michaelis AH, Zhu Z, Elvin JA, Bronson RT, Crum CP, McKeon F. p63 protects the female germ line during meiotic arrest. Nature 2006;444:624–628. [DOI] [PubMed] [Google Scholar]
- Sun X, Su Y, He Y, Zhang J, Liu W, Zhang H, Hou Z, Liu J, Li J. New strategy for in vitro activation of primordial follicles with mTOR and PI3K stimulators. Cell Cycle 2015;14:721–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sverrisdottir A, Johansson H, Johansson U, Bergh J, Rotstein S, Rutqvist L, Fornander T. Interaction between goserelin and tamoxifen in a prospective randomised clinical trial of adjuvant endocrine therapy in premenopausal breast cancer. Breast Cancer Res Treat 2011;128:755–763. [DOI] [PubMed] [Google Scholar]
- Sverrisdottir A, Nystedt M, Johansson H, Fornander T. Adjuvant goserelin and ovarian preservation in chemotherapy treated patients with early breast cancer: results from a randomized trial. Breast Cancer Res Treat 2009;117:561–567. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Kimura F, Zheng L, Kaku S, Takebayashi A, Kasahara K, Tsuji S, Murakami T. Protective effect of a mechanistic target of rapamycin inhibitor on an in vivo model ofcisplatin-induced ovarian gonadotoxicity. Exp Anim 2018;67:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taskin MI, Yay A, Adali E, Balcioglu E, Inceboz U. Protective effects of sildenafil citrate administration on cisplatin-induced ovarian damage in rats. Gynecol Endocrinol 2015;31:272–277. [DOI] [PubMed] [Google Scholar]
- Thorn CF, Oshiro C, Marsh S, Hernandez-Boussard T, McLeod H, Klein TE, Altman RB. Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet Genomics 2011;21:440–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting AY, Petroff BK. Tamoxifen decreases ovarian follicular loss from experimental toxicant DMBA and chemotherapy agents cyclophosphamide and doxorubicin in the rat. J Assist Reprod Genet 2010;27:591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokarska-Schlattner M, Zaugg M, Zuppinger C, Wallimann T, Schlattner U. New insights into doxorubicin-induced cardiotoxicity: the critical role of cellular energetics. J Mol Cell Cardiol 2006;41:389–405. [DOI] [PubMed] [Google Scholar]
- Tsuyoshi H, Orisaka M, Fukuda S, Hattori K, Tsang BK, Yoshida Y. Protective effect of dienogest on chemotherapy-induced reduced fertility in female rats. Steroids 2015;93:1–7. [DOI] [PubMed] [Google Scholar]
- Tuppi M, Kehrloesser S, Coutandin DW, Rossi V, Luh LM, Strubel A, Hotte K, Hoffmeister M, Schafer B, De Oliveira T et al. Oocyte DNA damage quality control requires consecutive interplay of CHK2 and CK1 to activate p63. Nat Struct Mol Biol 2018;25:261–269. [DOI] [PubMed] [Google Scholar]
- Dorp W, Haupt R, Anderson RA, Mulder RL, Heuvel-Eibrink MM, Broeder E, Su HI, Falck Winther J, Hudson MM, Levine JM et al. Reproductive function and outcomes in female survivors of childhood, adolescent, and young adult cancer: a review. J Clin Oncol 2018;36:2169–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbroucke T, Verheecke M, Fumagalli M, Lok C, Amant F. Effects of cancer treatment during pregnancy on fetal and child development. Lancet Child Adolesc Health 2017;1:302–310. [DOI] [PubMed] [Google Scholar]
- Venegas C, Garcia JA, Escames G, Ortiz F, Lopez A, Doerrier C, Garcia-Corzo L, Lopez LC, Reiter RJ, Acuna-Castroviejo D. Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J Pineal Res 2012;52:217–227. [DOI] [PubMed] [Google Scholar]
- Vitek WS, Shayne M, Hoeger K, Han Y, Messing S, Fung C. Gonadotropin-releasing hormone agonists for the preservation of ovarian function among women with breast cancer who did not use tamoxifen after chemotherapy: a systematic review and meta-analysis. Fertil Steril 2014;102:808–815e801. [DOI] [PubMed] [Google Scholar]
- Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov 2005;4:307–320. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu M, Zhang J, Liu Y, Kopp M, Zheng W, Xiao S. Multidrug resistance protein 1 deficiency promotes doxorubicin-induced ovarian toxicity in female mice. Toxicol Sci 2018;163:279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Schatten H, Sun QY. Environmental epigenetic inheritance through gametes and implications for human reproduction. Hum Reprod Update 2015;21:194–208. [DOI] [PubMed] [Google Scholar]
- Weinberg LE, Lurain JR, Singh DK, Schink JC. Survival and reproductive outcomes in women treated for malignant ovarian germ cell tumors. Gynecol Oncol 2011;121:285–289. [DOI] [PubMed] [Google Scholar]
- Whirledge SD, Garcia JM, Smith RG, Lamb DJ. Ghrelin partially protects against cisplatin-induced male murine gonadal toxicity in a GHSR-1a-dependent manner. Biol Reprod 2015;92:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winther JF, Boice JD Jr, Frederiksen K, Bautz A, Mulvihill JJ, Stovall M, Olsen JH. Radiotherapy for childhood cancer and risk for congenital malformations in offspring: a population-based cohort study. Clin Genet 2009;75:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winther JF, Olsen JH, Wu H, Shyr Y, Mulvihill JJ, Stovall M, Nielsen A, Schmiegelow M, Boice JD Jr. Genetic disease in the children of Danish survivors of childhood and adolescent cancer. J Clin Oncol 2012;30:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 2006;124:471–484. [DOI] [PubMed] [Google Scholar]
- Xi L, Qian Z, Du P, Fu J. Pharmacokinetic properties of crocin (crocetin digentiobiose ester) following oral administration in rats. Phytomedicine 2007;14:633–636. [DOI] [PubMed] [Google Scholar]
- Xiao S, Zhang J, Liu M, Iwahata H, Rogers HB, Woodruff TK. Doxorubicin has dose-dependent toxicity on mouse ovarian follicle development, hormone secretion, and oocyte maturation. Toxicol Sci 2017;157:320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Zhang X, Zhang L. Negative regulation of inflammation by SIRT1. Pharmacol Res 2013;67:60–67. [DOI] [PubMed] [Google Scholar]
- Yeh J, Kim B, Liang YJ, Peresie J. Mullerian inhibiting substance as a novel biomarker of cisplatin-induced ovarian damage. Biochem Biophys Res Commun 2006;348:337–344. [DOI] [PubMed] [Google Scholar]
- Yeh J, Kim BS, Peresie J. Protection against cisplatin-induced ovarian damage by the antioxidant sodium 2-mercaptoethanesulfonate (mesna) in female rats. Am J Obstet Gynecol 2008;198:463.e461–463.e467. [DOI] [PubMed] [Google Scholar]
- Yucebilgin MS, Terek MC, Ozsaran A, Akercan F, Zekioglu O, Isik E, Erhan Y. Effect of chemotherapy on primordial follicular reserve of rat: an animal model of premature ovarian failure and infertility. Aust N Z J Obstet Gynaecol 2004;44:6–9. [DOI] [PubMed] [Google Scholar]
- Yuksel A, Bildik G, Senbabaoglu F, Akin N, Arvas M, Unal F, Kilic Y, Karanfil I, Eryilmaz B, Yilmaz P et al. The magnitude of gonadotoxicity of chemotherapy drugs on ovarian follicles and granulosa cells varies depending upon the category of the drugs and the type of granulosa cells. Hum Reprod 2015;30:2926–2935. [DOI] [PubMed] [Google Scholar]
- Zamah AM, Mauro MJ, Druker BJ, Oktay K, Egorin MJ, Cedars MI, Rosen MP. Will imatinib compromise reproductive capacity? Oncologist 2011;16:1422–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, He WH, Feng LL, Huang HG. Effect of doxorubicin-induced ovarian toxicity on mouse ovarian granulosa cells. Regul Toxicol Pharmacol 2017;86:1–10. [DOI] [PubMed] [Google Scholar]
- Zhou L, Xie Y, Li S, Liang Y, Qiu Q, Lin H, Zhang Q. Rapamycin prevents cyclophosphamide-induced over-activation of primordial follicle pool through PI3K/Akt/mTOR signaling pathway in vivo. J Ovarian Res 2017;10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]


