Abstract
Late-life depression (LLD) is an affective disorder that is highly prevalent among older people. Cognitive reserve (CR) refers to an active process that facilitates the flexibility and efficiency of the neural networks to compensate for impairments that emerge in consequence of brain pathology. The current functional magnetic resonance imaging study investigated whether and how CR affects emotional regulation, level of depression severity and neural activity associated with affective control during emotional Stroop (eStroop) task. Altogether, 90 older people participated in this study, 50 of whom suffered from LLD. We used years of education and verbal fluency capacity as proxies for CR. Clinical participants with relatively higher CR presented with milder degrees of depression, better eStroop performance and stronger neural activity in the middle frontal gyrus (MFG) involved with exercising affective control. Results of the mediation analysis indicated that both education and verbal fluency significantly mediated the association between the depression severity and MEG activity. These results suggest a negative association between CR and age-related clinical symptoms of emotional dysregulation. Our neurobehavioral findings provide supportive evidence that CR implies efficiency of top-down emotional regulation and operates as a protective factor against emotional and cognitive vulnerability in the aging brain.
Keywords: cognitive reserve, emotional regulation, fMRI, depression, mediation, aging
Introduction
Late-life depression (LLD) is one of the most common psychiatric disorders afflicting the growing geriatric population and is associated with cognitive, affective and somatic abnormalities in individuals aged 60 years and older (Blazer, 2003; Fiske et al., 2009; Desseilles et al., 2011; Elliott et al., 2011). There is evidence to suggest that the severity of depressive symptomatology in LLD is a causal factor associated with neurocognitive decline, even when patients enter the euthymic phase or achieve remission (Paterniti et al., 2002; Bhardwaj et al., 2010; O'Shea et al., 2015; Yao & Meng, 2015). Recent cross-sectional and longitudinal studies, however, have failed to demonstrate a significant relationship between LLD symptoms and cognitive impairments (Ganguli et al., 2006; Becker et al., 2009). The discrepancy between these results suggests the presence of underlying mediators related to cognitive functioning in LLD, particularly individual differences in susceptibility to cognitive and functional decline in the context of age-related brain pathology (Barnett et al., 2006; Stern, 2007; O'Shea et al., 2015).
Cognitive reserve (CR) refers to an active process that involves facilitating the flexibility and efficiency of neural networks, to compensate for impairments emerging as a consequence of brain damage or pathology (Stern, 2002, 2007; Stern et al., 2018b). A growing body of evidence supports the CR-related hypothesis that people with higher CR cope with age- and disease-related neurocognitive changes better than those with lower CR (Steffener & Stern, 2012; Stern, 2012) and, moreover, that levels of CR predict cognitive decline progression in individuals with neurological conditions (Tucker & Stern, 2011; Stern, 2012; Soldan et al., 2015). For example, Alzheimer’s disease (AD) patients who had more education and demonstrated greater engagement in leisure activities (i.e. higher CR) suffered less neurocognitive decline (Scarmeas & Stern, 2004; Wilson et al., 2013). By contrast, low CR would operate as a vulnerability factor for increased clinical symptoms of disease and functional limitations (Barnett et al., 2006; Phillips et al., 2008; Meng & D’Arcy, 2012). These results suggest that CR may act as a protective mechanism by facilitating cognitive performance and decreasing age-related and disease-associated clinical symptoms consequent to brain pathology (Stern, 2007, 2009, 2012). There is increasing interest in CR’s mode of impacting the relationship between age-related neurological diseases (e.g. AD) and cognitive decline; however, it remains unclear whether CR has analogous effects in the context of psychiatric conditions, especially among the at-risk elderly population (Butters et al., 2000, 2008; Tucker & Stern, 2011; Giogkaraki et al., 2013; Watson & Joyce, 2015; Deschamps, 2018).
Previous behavioral studies have used multiple measures of CR (e.g. educational attainment, occupational complexity and verbal ability) to demonstrate that levels of CR in later life impact the risk of depression (Turner & Lloyd, 1999; Spitznagel et al., 2006; Ladin, 2008). In these researches, higher CR appears to act as a protective factor against the symptoms of psychiatric conditions, including major depressive disorder (Turner & Lloyd, 1999; Barnett et al., 2006; Venezia et al., 2018). These findings suggest that CR may be encoded more generally, rather than in disorder-specific ways, for the elderly population. Given that CR mechanisms may allow people to cope more effectively with a variety of age- and disease-related forms of cognitive decline and functional changes, it is worth noting that CR is relevant to both the pathological and normal aging process. Despite the failure of some investigations to demonstrate CR’s positive effects on cognitive performance in individuals with LLD (Bhalla et al., 2005), a recent study reported that reading ability acts as a CR moderator to significantly influence the association between depressive symptoms and executive functioning in individuals with LLD (O'Shea et al., 2015). Moreover, LLD patients with lower CR showed a greater loss of total brain volume than those with higher CR (O'Shea et al., 2015), suggesting that the protective effects of CR on cognitive functions and brain structure are generally obtained in the context of age-related psychiatric disorders.
Although there is considerable research focusing on the behavioral impacts of CR on age-related psychiatric disorders and on individual differences in cognitive performance, the underlying neural mechanisms in LLD are poorly understood. Focusing solely on studies reliant on functional magnetic resonance imaging (fMRI) furnishes an opportunity to examine the neural signatures and mechanisms by which CR scaffolds neural function and maintains optimal cognitive performance in the face of aging-associated brain neurodegradation. The concept of neural compensation is often invoked in fMRI studies in regard to neural mechanisms involved in the response to age- and disease-related brain pathology (Barulli & Stern, 2013; Stern et al., 2018b). Neural compensation refers to an inter-individual variability in the capacity to compensate for brain pathology by making more efficient use of neural networks and recruiting alternative brain circuits (Park & Reuter-Lorenz, 2009; Grady, 2012; Huang et al., 2012; Stern, 2012; Korsnes & Ulstein, 2014; Cabeza et al., 2018). Evidence from recent functional neuroimaging studies reveals that higher CR was associated with greater activation in task-related brain regions in elderly patients with AD (Scarmeas et al., 2004; Sheline et al., 2006). Moreover, a recent meta-analysis study indicated that elderly patients with AD and higher CR exhibited greater activation in the anterior cingulate cortex (ACC) during cognitive tasks relative to those with lower CR (Colangeli et al., 2016). These findings suggest that elderly individuals with higher levels of CR are more capable of recruiting alternative and/or additional brain networks to respond to cognitive changes due to pathological aging, especially within the prefrontal regions (Korsnes & Ulstein, 2014; Scarmeas & Stern, 2004; Stern et al., 2018b).
There is compelling evidence to suggest that CR levels may be linked to the resilience and adaptability of the brain to cope with age- and disease-related cognitive declines. However, it is unclear whether and how CR modulates emotional regulation, measured as the level of depression severity and affective control, and neural mechanisms associated with emotional control in geriatric depression. This fMRI study investigated whether individual differences in educational attainment and verbal capacity (the most widely used proxies for CR) modified the adverse influence of age-related psychiatric conditions on the severity of depression, cognitive performance and neural processing of emotional-cognitive control in LLD. Specifically, we hypothesized that the known adverse age-related psychiatric symptoms and cognitive impairment would be attenuated among individuals with higher CR who suffer from LLD. The cognitive and neural processes of emotional-cognitive control were assessed using the emotional Stroop (eStroop) task, which heavily taps into selective attention, inhibition of emotional responses and conflict resolution (Dalgleish & Watts, 1990; Williams et al., 1996; Ben-Haim et al., 2016; Song et al., 2017). Individuals with LLD showed altered emotion-related activities in prefrontal-ACC-insula circuitries, and this has been identified as reflecting aberrant cognitive control mechanisms for processing emotional information (Fales et al., 2008, 2009; Frodl, 2016). We then predicted that CR would modulate the neural activation patterns of localization involved in cognitive control and emotional awareness during the eStroop task, which involve the dorsolateral prefrontal cortex (DLPFC), ACC and the anterior insula (AI). Given that post-hoc mediation analysis provides a decent way of investigating the role of intermediate variables which play a role in the relationship between two other variables (Na et al., 2017; Fan et al., 2018), we applied this approach to our efforts to identify the associations of clinical and neurological variables which may be interrelated in LLD, CR, depression symptoms and brain function.
Materials and methods
Participants
Participants included 55 older adults with LLD (LLD group) and 40 age-matched healthy elderly controls (HEC group). Participants from both the LLD and the HEC group were over 60 years of age. These LLD-suffering individuals were recruited from the Chang Gung Memorial Hospital and had all experienced their first major depressive episode of their lifetime at age >50 years. The diagnosis of unipolar major depressive disorder was confirmed by the Structural Clinical Interview recommended in the Diagnostic and Statistical Manual of Mental Disorders Fifth Edition (APA, 2013). Antidepressant use was maintained during this study due to ethical reasons but all such medications usage was unchanged for at least 2 weeks prior to the scan (Wong et al., 2016; Lam et al., 2018). The HEC individuals, matched in terms of age and gender, were recruited from advertisements and screened for major psychiatric and neurological illnesses by a physician. All participants had normal or corrected-to-normal visual acuity and were right-handed, as assessed by the Edinburgh Inventory for Handedness (Oldfield, 1971). Participants afflicted with significant physical or neurologic illness or substance abuse/dependence in the past 12 months were excluded. Table 1 displays our participants’ demographic information. This study was approved by the Institutional Review Board in Chang Gung Memorial Hospital, and informed consent was obtained from all participants.
Table 1.
Demographic and clinical variables of the study participants
| HEC group (N = 40) | LLD group (N = 55) | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | P value | |
| Age (yrs) | 68.10 | 5.30 | 66.36 | 5.42 | 0.12 |
| Gender (M/F) | 15/25 | 17/38 | 0.50 | ||
| Education (yrs) | 10.56 | 4.82 | 7.27 | 2.52 | <0.001 |
| MMSE | 27.58 | 1.80 | 28.18 | 1.50 | 0.07 |
| HAMD | 4.95 | 3.82 | 11.66 | 6.41 | <0.001 |
| HAMA | 6.32 | 4.12 | 13.34 | 8.48 | <0.001 |
| GDS | 3.10 | 2.84 | 7.37 | 3.55 | <0.001 |
Abbreviations: M/F, Male/Female; MMSE, Mini-Mental State Examination; HAMD, Hamilton Depression Rating Scale; HAMA, Hamilton Anxiety Rating Scale; GDS, Geriatric Depression Scale.
Psychological measures
Before fMRI scanning, all participants underwent a battery of assessments on psychological measures. The Chinese version of the Mini-Mental State Examination (MMSE) was used to assess participants’ general cognitive function (Folstein et al., 1975; Shyu & Yip, 2001). The reliability and validity of the MMSE have been validated among elderly individuals with psychiatric conditions (McDowell, 2006). All participants scored >24. The Chinese version of the 15-item self-reported Geriatric Depression Scale (GDS) assessment was adopted to measure the severity of depression among the participants (Sheikh & Yesavage, 1986; Chan, 1996). Additionally, clinician-rated measures of the Chinese version of the Hamilton Anxiety Rating Scale (HAMA) and the Hamilton Depression Rating Scale (HAMD) were used to assess anxiety and depression symptoms, respectively (Hamilton, 1960, 1969; Zheng et al., 1988). The GDS has well-established and robust psychometric validity and reliability properties among older adults (Hamilton, 1960, 1969; Zheng et al., 1988).
CR measures
Educational level and verbal fluency capacity, two commonly used proxies of CR, were collected as measures of CR in this study (Barulli & Stern, 2013; Stern, 2013; Stern et al., 2018a). Educational level was defined in terms of the number of years of formal education. Verbal fluency capacity was determined by a short test of verbal functioning (Lezak et al., 2012) and a set test used to detect age-related and individual differences in the language domain (Isaacs & Kennie, 1973; Federmeier et al., 2010). All participants were asked to produce as many words as possible from four successive categories (animals, colors, fruits and towns), each of which was given a 60-second timed response window. The verbal fluency scores were obtained by averaging the total number of correctly named items.
EStroop fMRI task
Participants performed a modified version of the color–word eStroop task during fMRI scanning (Chan et al., 2010; Price et al., 2012). In the eStroop task, participants were instructed to indicate the ink color of target words that are either emotionally salient or neutral. The target word is evocative of a neutral emotion (e.g. ‘motivation’), a positive emotion (e.g. ‘joy’) or a negative emotion word (e.g. ‘sad’). Participants were required to indicate via button press whether the ink color of each upper target word matches the color meaning of a lower word as accurately and quickly as possible. Responses must be made within 2 seconds, and every trial lasts for 3 seconds. The task consists of six blocks of each of the experimental condition (i.e. neutral, positive, negative) repeated twice, with 12 trials in each block which are intermixed with a 12-seccond baseline condition (i.e. fixation). Each block was delivered in pseudo-random order.
Behavioral analysis
To investigate differences between LLD and HEC on psychometric measures, a 2 × 3 analysis of variance (ANOVA) was conducted using group (LLF and HEC) as an independent variable and Hamilton depression (HAMD) and anxiety (HAMA) rating scales and GDS as dependent variables. To examine differences in the eStroop accuracy and reaction time (RT) between LLD and HEC, repeated measures 2 × 3 ANOVA was undertaken using group (LLD vs HEC) as between-group factor, experimental condition (positive vs negative vs neutral) as within-group factor, and mean accuracy rates (%) and mean RT (milliseconds) as dependent variables. Post-hoc t tests were carried out to follow up on significant main and interaction effects.
MRI data acquisition and processing
Structural and functional MRI sessions were conducted on a clinical 3-T MRI scanner with an 8-channel head coil (Discovery MR 750, GE Healthcare). A total of 128 image volumes were acquired during the eStroop task-evoked fMRI using a T2*-weighted echo planar imaging (EPI) sequence with TR/TE/flip angle = 3000 ms/30 ms/90°. Thirty-six contiguous axial slices were acquired with a slice thickness of 4 mm, a 64 × 64 matrix and a 3.44 mm × 3.44 mm in-plane resolution. Structural MRI images were acquired with a brain volume imaging sequence (TR/TE/flip angle = 8.2 ms/3.2 ms/12°, 1 mm isotropic resolution).
SPM8 software (Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK) implemented in MATLAB software (The MathWorks, Natick, MA, USA) was used to preprocess the fMRI data. The first four volumes of each fMRI session were discarded to allow for T1 equilibration effects. The remaining images underwent preprocessing, including head motion correction, normalization to the EPI template with a resampled voxel size of 2 × 2 × 2 mm and smoothing with an isotropic 8 mm full-width half-maximum Gaussian kernel. The data were high-pass filtered with a frequency cutoff at 128 seconds. We excluded volumes in which participant head motion exceeded 3 mm or 3° (three healthy older controls and five LLD).
eStroop fMRI analysis
A general linear model was used to examine the differences between each eStroop condition and to compare them at the group level. Three regressors were employed to model the onset of outcome presentation. At the first level, a voxel-by-voxel multiple regression analysis of the expected blood oxygenation level-dependent (BOLD) response for each of the active conditions and the null event was applied. The entire duration of the individual blocks was convolved with a canonical hemodynamic response function for each participant. Movement parameters from the realignment output were included as nuisance regressors. Then, each effect of interest was tested across all participants. Linear contrasts were applied to the parameter estimates so obtained. We created images of parameter estimates for the two contrasts (positive vs neutral and negative vs neutral) in each participant. This was a factorial design with one between-group factor (group: LLD vs HEC) and one within-group factor (experimental condition: positive vs negative). Therefore, we performed a two-way mixed ANOVA on fMRI data at the group level analyses. All significant results of whole-brain analysis were determined based on a voxel-wise height threshold of P < 0.001 and multiple comparison correction at a spatial extent threshold of familywise-error (FWE) P < 0.05. Activations were overlaid on a representative high-resolution structural T1-weighted image from a single subject from the SPM 8 canonical image set, co-registered to the Montreal Neurological Institute (MNI) space.
The group-level activation regions were served as prior knowledge for the following region-of-interest (ROI) analysis. Prior ROIs, determined from previous fMRI studies that have been identified to be critical for cognitive control and emotional processing during eStroop-like tasks (Chan et al., 2010; Price et al., 2012), were created via conjunctions of both anatomically defined and functionally defined ROIs for each participant (Fan et al., 2014). The anatomical ROIs were identified using the Wake Forest University Pickatlas Tool (Maldjian et al., 2003) based on the Automated Anatomical Labeling Atlas (Tzourio-Mazoyer et al., 2002). A total of four ROIs were created, including middle frontal gyrus (MFG), DLPFC, ACC and AI. Mean BOLD response magnitude for emotional words vs neutral words contrast for each participant within each ROIs were extracted using the MarsBaR software (Brett et al., 2002). A false discovery rate (FDR) procedure was additionally employed on the FWE-corrected P values to further correct for the number of ROIs. Correlation analyses with FDR correction were conducted to further characterize the significant signals and to assess the relationship between the hemodynamic responses to the eStroop task, CR and severity of depression. The corrected statistical threshold was set at P < 0.05, two-tailed. These determined the associated between hypothetically selected ROIs, GDS scores, years of education and verbal fluency scores.
Mediation analysis
Based on a standard three-variable path model with a bootstrap test, a single-level version of the mediation path model was used to gain deeper insight into the linkage between clinical outcomes and brain hemodynamic response. The path coefficients in this mediator model and the bootstrap 95% confidence intervals (CI) were estimated using the Preacher and Hayes Indirect Mediation Analysis tool for SPSS 20 for total and specific indirect effects of independent variable on dependent variable through mediator (5000 bootstrap samples). As recommended, the indirect effect was regarded as statistically significant if the 95% CI did not include zero.
Results
Demographic and behavioral analyses
The participants’ demographic and clinical variables are listed in Table 1. The age, gender ratio and MMSE score did not differ between LLD and HEC groups. The independent t test showed significant differences between groups with respect to HAMD [t(91) = −5.87, P < 0.001, d = 1.05], HAMA [t(91) = −4.82, P < 0.001, d = 1.27] and GDS [t(90) = −6.22, P < 0.001, d = 1.32]. We performed a 2 × 3 ANOVA on the eStroop accuracy data with group (LLD vs HEC) as a between-subject factor and condition (positive vs negative vs neutral) as a within-subject factor. The group had a significant effect on accuracy [F(1, 93) = 5.45, P = 0.02, η2 = 0.055]. Post hoc tests indicated that individuals with HEC displayed higher rate of accuracy than those with LLD. No effect is found on neither the condition [F(2, 186) = 1.38, P = 0.25, η2 = 0.015] nor the group-by-condition interaction [F(2, 186) = 0.88, P = 0.42, η2 = 0.009]. In addition, the same group x condition ANOVA on the mean RT data of the eStroop task revealed a significant main effect of group [F(1, 93) = 6.36, P = 0.01, η2 = 0.064], indicating that the LLD group had increased latencies in response to all neutral and emotional word stimuli, relative to the HEC group. Moreover, a significant main effect of condition was observed [F(2, 186) = 7.88, P = 0.001, η2 = 0.078]. Both positive and negative words showed longer RT relative to neutral words. No interaction between group and condition was observed [F(2, 186) = 2.35, P = 0.10, η2 = 0.025].
eStroop fMRI results
An initial direct comparison of the emotional words vs neutral words condition (collapsed across group) yielded significant activations in several frontal and parietal regions, including bilateral MFG, right AI, left posterior insula, right ACC, right IFG, left DLPFC, bilateral fusiform gyrus, bilateral lingual gyrus, right supramarginal gyrus, left angular gyrus, left postcentral gyrus and left middle temporal gyrus. All of these regions have previously been reported in several studies using similar interference paradigms (Ochsner & Gross, 2005; Blasi et al., 2007; Korsnes & Ulstein, 2014). The direct comparison of emotional vs neural words contrast between the two groups clearly demonstrates that healthy controls (HEC) showed greater activation in bilateral MFG, left DLPFC and left angular gyrus relative to LLD. In contrast, LLD recruited greater neural activation in right AI, right ACC, right inferior frontal gyrus, bilateral fusiform gyrus, bilateral lingual gyrus, right supramarginal gyrus, left postcentral gyrus, left posterior insula, left middle temporal gyrus and left culmen relative to HEC (Table 2 and Figure 1). Neither condition nor group × condition interaction was significant.
Table 2.
Regions showing activations to GROUP effects
| Brain area | MNI coordinates | Z score | Cluster size (mm3) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| HEC group > LLD group | |||||
| Dorsolateral frontal gyrus | –20 | −8 | 48 | 4.01 | 92 |
| MFG | −30 | 4 | 38 | 4.03 | 137 |
| MFG | 34 | −2 | 34 | 4.81 | 212 |
| Angular gyrus | −36 | −60 | 26 | 4.77 | 257 |
| LLD group > HEC group | |||||
| Supramarginal gyrus | 48 | −34 | 28 | 3.59 | 24 |
| Postcentral gyrus | −48 | −16 | 26 | 3.81 | 29 |
| AI | 46 | 2 | 10 | 4.40 | 182 |
| Posterior insula | −42 | −16 | 10 | 4.20 | 84 |
| ACC | 4 | 40 | 8 | 3.74 | 50 |
| Calcarine | 28 | −58 | 6 | 4.08 | 95 |
| Inferior frontal gyrus | 46 | 30 | 0 | 4.83 | 218 |
| Middle temporal gyrus | −40 | −62 | −4 | 3.67 | 40 |
| Lingual gyrus | 16 | −50 | −8 | 3.86 | 745 |
| Lingual gyrus | −20 | −54 | −12 | 3.63 | 142 |
| Fusiform gyrus | 24 | −66 | −12 | 4.42 | 745 |
| Cerebellum/Culmen | −10 | −38 | −16 | 3.93 | 118 |
| Fusiform gyrus | −36 | −70 | −16 | 3.70 | 128 |
Fig. 1.
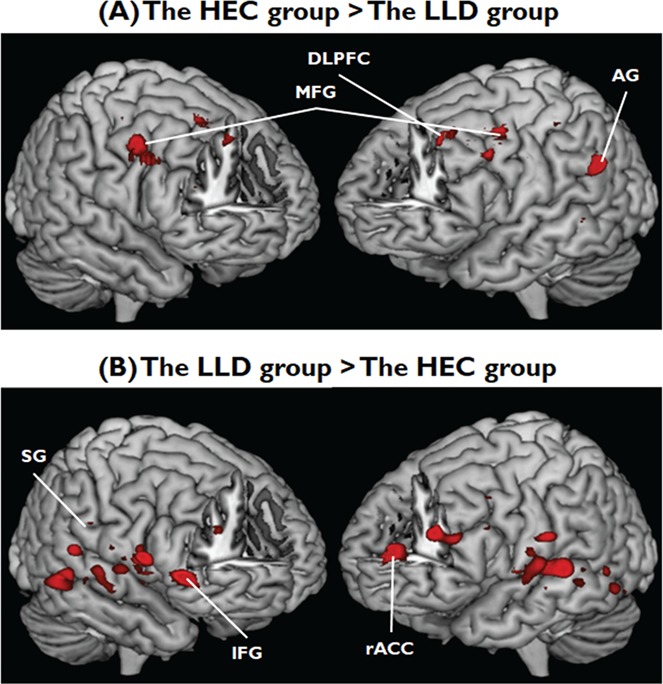
Hemodynamic responses to the eStroop tasks between two groups. (A) The intensity of hemodynamic responses to perceiving positive and negative words that were higher in individuals with HEC than those with LLD; (B) the intensity of hemodynamic responses that were higher in individuals with LLD than those of HEC. FEW-corrected P < 0.05. Abbreviations: HEC, healthy elderly controls; LLD, late-life depression, DLPFC, dorsolateral prefrontal cortex; MFG, middle frontal gyrus; AG, angular gyrus; SG, supramarginal gyrus; IFG, inferior frontal gyrus; rACC, rostral anterior cingulate cortex.
ROI analyses
Regarding the ROI results, significant main effects of group on left MFG [F(1, 93) = 8.91, P = 0.004, η2 = 0.087], left DLPFC [F(1, 93) = 8.37, P = 0.005, η2 = 0.083], right ACC [F(1, 93) = 7.54, P = 0.007, η2 = 0.075] and right AI [F(1, 93) = 11.53, P = 0.001, η2 = 0.11] were observed. Post hoc analysis revealed that HEC showed greater activation within the left MFG [t(93) = 2.99, P = 0.004, d = 0.62] and the left DLPFC [t(93) = 2.89, P = 0.005, d = 0.59], whereas LLD showed greater activation in right ACC [t(93) = 2.75, P = 0.007, d = 0.57] and right AI [t(93) = 3.40, P = 0.001, d = 0.71] when processing emotional words. Neither condition nor interaction between group and condition was significant in all ROIs.
Brain-behavioral association between CR, neural activities, eStroop performance and psychometric measures
The correlation analysis indicated that verbal fluency scores positively correlated with the overall accuracy (r = 0.37, P < 0.01) and negatively correlated with the mean RT (r = −0.27, P = 0.04) of the eStroop task for LLD but not HEC. These results suggest that individuals with LLD and higher levels of CR could be associated with better cognitive control during the emotional interference task.
The correlation analysis revealed that accuracy for negative emotional words positively correlated with brain activation in the left MFG (r = 0.28, P = 0.04) for the LLD group. This indicated that individuals with LLD who had higher accuracy rates for interference of negative words could be associated with greater cognitive-control activation in MFG. For the HEC group, we did not find significant correlations between negative word accuracy and hemodynamic response for any ROI.
When the two groups were combined, lower GDS ratings correlated with a stronger activation within the left MFG in response to both emotional words (r = −0.22, P = 0.04) (Figure 2A). Moreover, years of education and verbal fluency scores both positively correlated with activation in the left MFG (education: r = 0.29, P < 0.01; verbal fluency: r = 0.23, P = 0.02) (Figure 2B and C). These results revealed that individuals from both the LLD and the HEC groups who had less severe depressive symptoms and higher CR had stronger MFG activation during the eStroop task. However, we did not find any significant correlation among CR, severity of depression and the hemodynamic responses in other ROIs.
Fig. 2.
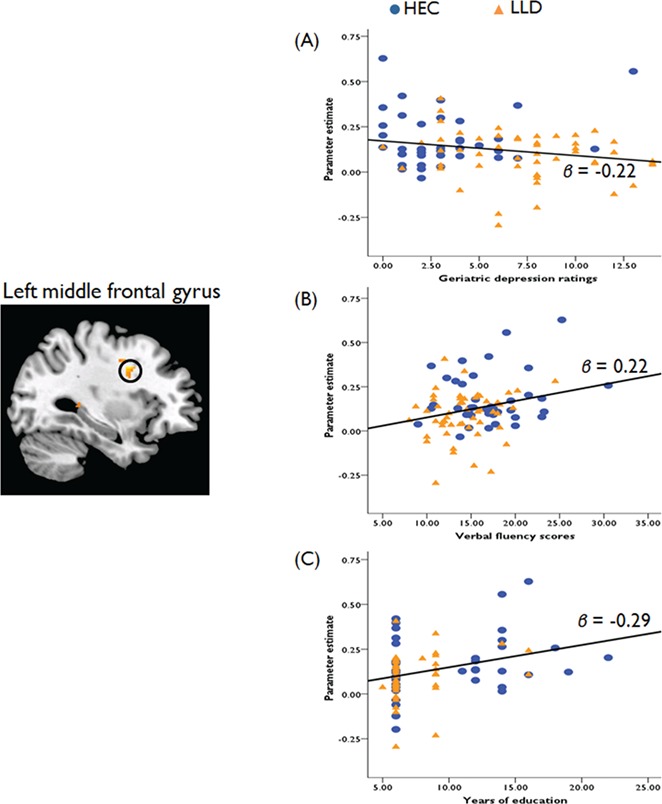
Correlations between hemodynamic responses, severity of depression and two measures of the CR. (A) Geriatric depression ratings are negatively correlated with hemodynamic responses in the MFG (β = −0.22). Both (B) verbal fluency scores and years of education (C) are positively correlated with the MFG activation (verbal fluency, β = 0.22; years of education, β = 0.29).
When the two groups were combined, we further observed a significant positive relationship for HAMA ratings (r = 0.22, P = 0.04). In both the LLD and the HEC groups, more anxiety symptoms were associated with greater activation of AI in response to the interference of emotional words. We did not find any significant correlation among anxiety ratings and the hemodynamic responses in other ROIs.
Mediation analysis
As both GDS ratings and verbal fluency correlated with activation of left MFG and better verbal fluency capacity was associated with lower severity of depression (r = −0.28, P < 0.01), we performed a bootstrapped mediation analysis to investigate the associations between these variables. This analysis revealed that it was no longer significant after verbal fluency was integrated into the model (path c1’; b = −4.10, SE = 2.78, P = 0.14) while the association between severity of depression and activation in left MFG was statistically significant (path c1; b = −5.78, SE = 2.75, P = 0.038). The bias-corrected 95% CI for the specific indirect effect of MFG activation on depression symptoms through verbal fluency yielded a lower limit of −4.46 and an upper limit of −0.18 (upper part of Figure 3). Since zero was not included in the CI, it can be concluded that the association between the severity of depression and the MFG response to emotional words during the eStroop task was significantly mediated by verbal function in all elderly participants.
Fig. 3.
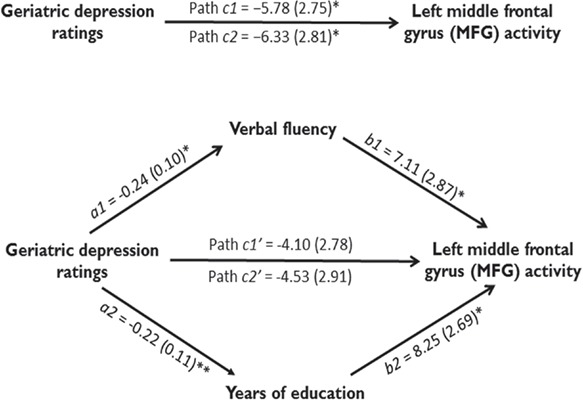
Mediation analyses results. Path diagram showing the relationships between severity of depression, verbal fluency, education and the hemodynamic response in the path model. The predictor region in severity of depression (geriatric depression ratings) is shown at left, which also predicts verbal fluency and years of education (a1 and a2 paths), respectively. The lines are labeled with path coefficients, and standard errors are shown in parentheses. The mediator factors (verbal fluency and years of education) connections to the left MFG activation in response to emotional words are the b1 and b2 paths. They are calculated controlling for severity of depression and for the mediator factor, as is standard in mediation models. *P < 0.05, **P < 0.01, two-tailed.
Given that both GDS and years of education correlated with activation of left MFG and that more years of education correlated with lower severity of depression (r = −0.27, P = 0.01), we then performed a mediation analysis. The results showed that it was no longer significant after integrating years of education into the model (path c2’; b = −4.53, SE = 2.91, P = 0.12) while the association between severity of depression and left MFG activation was statistically significant (path c2; b = −6.33, SE = 2.81, P = 0.027). The significance of the mediating effect was confirmed by the 95% CI (range: −4.39 to −0.08; lower part of Figure 3). More education was associated with lower severity of depression and increased MFG activation with perceiving the interference of emotional words. The results suggest that education also served as a mediator for the severity of depression–MFG relation in patients with later life depression.
Discussion
The novelty of this study derives from its status as the first to establish a relationship between CR, severity of depression, cognitive performance and its neural correlates among geriatric depression. We found that verbal fluency capacity positively correlated with eStroop performance in LLD and severity of depression negatively correlated with the activity within the eStroop task-evoked prefrontal regions. As we expected, elderly individuals with high CR (i.e. more years of education and higher verbal fluency scores) showed lower severity of depression and increased neural activity in MFG than those of individuals with lower CR. Moreover, mediation analysis showed that both education and verbal fluency significantly mediated the association between severity of depression and neural activity associated with processing cognitive control of emotional information within the MFG.
Here, we found that both the LLD and HEC groups had an increased eStroop RT for both positive and negative words relative to neutral words. In addition, individuals with LLD showed longer RT and lower accuracy rates across different conditions relative to healthy elderly. These results conflict with the schema-based models of depression, which suggest that individuals with depression should be associated with an attentional bias toward depression-relevant stimuli or mood-congruent materials (Beck, 1979; Bower, 1981). The qualitative reviews conclude that the eStroop interference for negative words in depression is equivocal (Gotlib et al., 1996; Mogg & Bradley, 2005) and a quantitative meta-analysis provides further evidence that there are no significant differences in the interference effects between negative and positive stimuli among individuals in the depressed group (Epp et al., 2012). In the present study, our fMRI results squared with our behavioral findings and showed that there was no significant neural activation associated with processing negative emotional words relative to positive emotional words in the LLD group. Moreover, the altered brain activations were evident for both positive and negative emotional words in LLD relative to HEC. Altogether, our results and these studies suggest that emotional context insensitivity is the key finding in major depressive disorder, which is characterized by reduced emotional reactivity to both positively and negatively valenced stimuli (Blazer, 2003; Bylsma et al., 2008; Fiske et al., 2009; Desseilles et al., 2011; Elliott et al., 2011; Epp et al., 2012).
We found that LLD relative to HEC exhibited increased activation within AI and ACC but decreased MFG and DLPFC activation in response to the emotional interference stimuli. The results are consistent with previous functional neuroimaging studies (Fales et al., 2008; Mitterschiffthaler et al., 2008; Fales et al., 2009; Sliz & Hayley, 2012). These findings may imply that individuals with LLD have difficulty disengaging from emotional information and that they display aberrant cognitive control mechanisms during emotional interference tasks (Fales et al., 2008, 2009; Frodl, 2016). There is behavioral evidence that the processing of emotion on cognitive functioning includes two mutually influential components: top-down and bottom-up components (Ochsner & Gross, 2005; Phillips et al., 2008). Accumulating neuroimaging evidence has also revealed that the network of affective processing areas (e.g. amygdala and AI) can modulate subjective awareness of emotions and facilitate perceptual processing by biasing attention through bottom-up mechanisms (LeDoux, 2000; Ongur & Price, 2000). Moreover, the prefrontal regions (e.g. DLPFC, MFG and ACC) that regulate emotional responses and engage in attentional and complex cognitive operations during interference tasks appear to serve as top-down control areas (Ochsner & Gross, 2005; Blasi et al., 2007; Kanske et al., 2011; Song et al., 2017). A recent brain connectivity study indicates that individuals with geriatric depression failed to demonstrate activity that was synchronized between affective and cognitive control networks (Gazzaley et al., 2008; Wang et al., 2015). In addition, the connectivity strength between the fronto-limbic networks significantly and negatively correlated with severity of depression (Drevets et al., 2008; Dannlowski et al., 2009). Here, there is a negative correlation between severity of depression and hemodynamic response within MFG but not within AI during the eStroop may reflect top-down suppressive processing in those with lower severity of depression. Additional research is required to probe the relationship between severity of depression and effective connectivity within these networks in geriatric depression.
According to the concept of CR, pre-existing cognitive capacities or various aspects of life experience may provide a reserve that protects against severity of symptoms or cognitive impairment as a consequence of brain pathology (Butters et al., 2000, 2008; Stern, 2007, 2012). Our data are largely consistent with that anticipated in the hypothesis advanced by Butters et al. (2000), which predicts that depressed elderly individuals with lower CR may not withstand the toxic effect of depression on cognition. Our data provide supportive evidence for this conception and show that the elderly with higher levels of CR (verbal fluency capacity) associated with reduced depression symptoms and better eStroop performance (more accurate and shorter RT). Although these findings are inconsistent with a recent cohort study that indicated that those individuals with higher CR showed greater decrements in cognitive performance as severity of depression increased (O'Shea et al., 2015), certainly the different research design types (cross-sectional vs longitudinal), the characteristics of sampling and the differential assessment of depressive symptoms may account for the discrepant findings (Spitznagel et al., 2006; Tucker & Stern, 2011). LLD would have had more opportunity for exposure to educational, cultural, social and physical environments than those with schizophrenia during adolescence and young adult years (Forcada et al., 2015). Therefore, the CR model may better apply to geriatric depression (Butters et al., 2000, 2008).
Our behavioral and neuroimaging findings provide evidence substantiating the protective effects of CR on cognition in the context of geriatric depression. These results strongly suggest that CR should be considered and controlled when using neuroimaging techniques to investigate age-related neurocognitive decline and assess relapse in psychiatric disorders (Butters et al., 2008; Deschamps, 2018). We observed that more years of education and better verbal fluency capacity positively correlated with increased MFG activation in response to emotional interferences and cognitive control. This finding supports the notion postulated by Stern et al. that individuals with higher levels of CR are able to utilize alternative or additional neural mechanisms to cope with brain pathology (i.e. neural compensation) when pathology alters the task-related processing networks (Park & Reuter-Lorenz, 2009; Stern, 2009, 2012; Cabeza et al., 2018). MFG and other prefrontal regions have been shown to be involved in regulating the task-irrelevant emotional stimuli on executive control (Decety & Lamm, 2006). The fMRI research found that elder patients with AD who have higher CR showed enhanced activity in the prefrontal regions activity relative to those with lower CR during the cognitive tasks (Scarmeas et al., 2004; Sheline et al., 2006; Colangeli et al., 2016). Moreover, the association of left MFG with Stroop performance was observed in the LLD group. We speculated that such finding may reflect a compensatory mechanism that LLD recruited more neural resources for reorganization of the neural substrates underlying emotional eStroop task for optimal performance (e.g. Park & Reuter-Lorenz, 2009; Stern, 2012; Cabeza et al., 2018). Alternatively, the LLD may employ more strategy selection abilities to regulate affective information that lead to additional neural effort for optimizing task performance (Barulli et al., 2013; Stern et al., 2018).
Our results further indicate that differences in CR can modulate brain activity in MFG aimed at processing the executive control of emotional information. Although the mediation analysis approach utilized in this study does not constitute a formal test of CR as postulated by Stern et al. (2008), the study pushes forward our understanding to assess the effects of CR on the association between severity of depression and functional brain activity among healthy and pathological aging when neuroimaging data are applied. We found that CR mediates the association between severity of depression and brain activation in cognitive control across individuals with HEC and LLD. The elderly with more years of education and better verbal fluency capacity showed decreased depressive symptoms as well as enhanced functional activation within MFG during eStroop task. Given the equivocal results of previous research into the link between the psychiatric conditions and cognitive dysfunction (Ganguli et al., 2006; Becker et al., 2009; Bhardwaj et al., 2010; O'Shea et al., 2015; Yao & Meng, 2015), these results may help to explain this discrepancy. They indicate that individual differences in CR could impact the very nature of the observed relationship. Importantly, findings from the effectiveness studies suggest that CR can modulate the outcome of neurorehabilitation programs (Legendre et al., 2003; Martin et al., 2015; Mondini et al., 2016). The current study, combining correlation and mediation analyses illuminates the mode by which the proxies for CR influence the severity of depression and the cognition-related brain activity. Specifically, the proxies of CR may be used to assess individual differences in vulnerability to neurocognitive decline and as a critical marker for treatment responses and prognosis after pathological aging.
The associations between CR proxies and psychiatric measures were demonstrated when the two groups were combined in our medial analysis model. We did not find such correlations in each separate group. This result suggests that CR may be encoded more generally rather than in disorder-specific way for elderly populations. Notably, CR is relevant not just to the pathological aging process but also to the normal aging process. CR mechanisms may allow people to cope more effectively with a variety of age- and disease-related cognitive decline and functional changes (Stern, 2007, 2012). This study, however, has some limitations. First, we cannot exclude the possibility that the effects of psychotropic medications could have confounded our findings. Given our ethical concerns and to prevent neural and psychological changes, we only recruited individuals with LLD who were taking medications that had not changed at least for 2 weeks prior to the MRI scanning. In addition, late-life anxiety is often comorbid with geriatric depression (Beekman et al., 2000) and the present findings observed that higher anxiety ratings correlated with increased AI activation in response to emotional words stimuli. After controlling for severity of anxiety, the significant differences in signal change within all ROIs between individuals with LLD and those with HEC remained. Thus, the impact of comorbid anxiety is unlikely to bias the observed effects in this study. Moreover, longitudinal studies and/or different version of eStroop tasks (e.g. face-word eStroop) are required to determine the evidence required to support the use of CR approaches in clinical practice.
In closing, our behavioral and neuroimaging results clearly demonstrate that CR mediates neural modulation of emotional control and regulation in people with LLD. Participants that had more years of education and better verbal fluency capacity were associated with reduced severity of depression and improved facilitation of neural top-down control processing over emotional information. This study complements existing literature on the role of CR in both healthy and pathological aging, and further provides probative evidence that CR operates as a protective mechanism at both behavioral and neural levels to increase the resilience and adaptability of the brain to cope with geriatric depression and its attendant deficits.
Funding
This study was supported by medical research grants CMRPG3C0041/42 and CRRPG2G0061 from Chang Gung Memorial Hospital to C.L.; and The University of Hong Kong May Endowed Professorship in Neuropsychology awarded to T.M.C.L.; and the Center for Intelligent Drug Systems and Smart Biodevices (IDS2B) from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan to C.M.H. and Y.T.F.
Conflict of interest. None declared.
References
- APA (2013). Diagnostic and Statistical Manual of Mental Disorders (DSM-5), Washington: Author. [DOI] [PubMed] [Google Scholar]
- Barnett J.H., Salmond C.H., Jones P.B., Sahakian B.J. (2006). Cognitive reserve in neuropsychiatry. Psychological Medicine, 36(8), 1053–64doi:10.1017/S0033291706007501. [DOI] [PubMed] [Google Scholar]
- Barulli D., Stern Y. (2013). Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends in Cognitive Sciences, 17(10), 502–9doi:10.1016/j.tics.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T. (1979). Cognitive Therapy and the Emotional Disorders, London: Penguin. [Google Scholar]
- Becker J.T., Chang Y.F., Lopez O.L., Dew M.A., Sweet R.A., Barnes D., Reynolds C.F. (2009). Depressed mood is not a risk factor for incident dementia in a community-based cohort. The American Journal of Geriatric Psychiatry, 17(8), 653–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekman A.T., de E., van Balkom A.J., Deeg D.J., van Dyck R., van Tilburg W. (2000). Anxiety and depression in later life: co-occurrence and communality of risk factors. The American Journal of Psychiatry, 157(1), 89–95doi:10.1176/ajp.157.1.89. [DOI] [PubMed] [Google Scholar]
- Ben-Haim M.S., Williams P., Howard Z., Mama Y., Eidels A., Algom D. (2016). The emotional Stroop task: assessing cognitive performance under exposure to emotional content. Journal of visualized experiments: JoVE, 112, 53720.doi:10.3791/53720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla R.K., Butters M.A., Zmuda M.D., Seligman K., Mulsant B.H., Pollock B.G., Reynolds C.F. 3rd. (2005). Does education moderate neuropsychological impairment in late-life depression? International Journal of Geriatric Psychiatry, 20(5), 413–7doi:10.1002/gps.1296. [DOI] [PubMed] [Google Scholar]
- Bhardwaj A., Wilkinson P., Srivastava C., Sharma M. (2010). Cognitive deficits in euthymic patients with recurrent depression. The Journal of Nervous and Mental Disease, 198(7), 513–5doi:10.1097/NMD.0b013e3181e4c5ba. [DOI] [PubMed] [Google Scholar]
- Blasi G., Goldberg T.E., Elvevag B., Rasetti R., Bertolino A., Cohen J., Mattay V.S. (2007). Differentiating allocation of resources and conflict detection within attentional control processing. The European Journal of Neuroscience, 25(2), 594–602doi:10.1111/j.1460-9568.2007.05283.x. [DOI] [PubMed] [Google Scholar]
- Blazer D.G. (2003). Depression in late life: review and commentary. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 58(3), 249–65. [DOI] [PubMed] [Google Scholar]
- Bower G.H. (1981). Mood and memory. The American Psychologist, 36(2), 129–48. [DOI] [PubMed] [Google Scholar]
- Brett M., Anton J.L., Valabrgue R., Poline J.-B. (2002). Region of interest analysis using an SPM toolbox In: 8th International Conference on Functional Mapping of the Human Brain, June 2–6, 2002, Sendai, Japan, Vol. 13.
- Butters M.A., Becker J.T., Nebes R.D., Zmuda M.D., Mulsant B.H., Pollock B.G., Reynolds C.F. 3rd. (2000). Changes in cognitive functioning following treatment of late-life depression. The American Journal of Psychiatry, 157(12), 1949–54doi:10.1176/appi.ajp.157.12.1949. [DOI] [PubMed] [Google Scholar]
- Butters M.A., Young J.B., Lopez O., Aizenstein H.J., Mulsant B.H., Reynolds C.F. 3rd, Becker J.T. (2008). Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues in Clinical Neuroscience, 10(3), 345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylsma L.M., Morris B.H., Rottenberg J. (2008). A meta-analysis of emotional reactivity in major depressive disorder. Clinical Psychology Review, 28(4), 676–91doi:https://doi.org/10.1016/j.cpr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Cabeza R., Albert M., Belleville S., Craik F.I.M., Duarte A., Grady C.L., Rajah M.N. (2018). Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nature Reviews. Neuroscience, 19(11), 701–10doi:10.1038/s41583-018-0068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A.C. (1996). Clinical validation of the Geriatric Depression Scale (GDS): Chinese version. Journal of Aging and Health, 8(2), 238–53doi:10.1177/089826439600800205. [DOI] [PubMed] [Google Scholar]
- Chan S.-C., Raine A., Lee T.M.C. (2010). Attentional bias towards negative affect stimuli and reactive aggression in male batterers. Psychiatry Research, 176(2), 246–9doi:10.1016/j.psychres.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Colangeli S., Boccia M., Verde P., Guariglia P., Bianchini F., Piccardi L. (2016). Cognitive reserve in healthy aging and Alzheimer’s disease: a meta-analysis of fMRI studies. American Journal of Alzheimer's Disease and Other Dementias, 31(5), 443–9doi:10.1177/1533317516653826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish T., Watts F.N. (1990). Biases of attention and memory in disorders of anxiety and depression. Clinical Psychology Review, 10(5), 589–604doi:10.1016/0272-7358(90)90098-U. [Google Scholar]
- Dannlowski U., Ohrmann P., Konrad C., Domschke K., Bauer J., Kugel H., Suslow T. (2009). Reduced amygdala-prefrontal coupling in major depression: association with MAOA genotype and illness severity. The International Journal of Neuropsychopharmacology, 12(1), 11–22doi:10.1017/S1461145708008973. [DOI] [PubMed] [Google Scholar]
- Decety J., Lamm C. (2006). Human empathy through the lens of social neuroscience. ScientificWorldJournal, 6, 1146–63doi:10.1100/tsw.2006.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschamps T. (2018). The cognitive reserve should be controlled when using neuroimaging to assess relapse in major depressive disorder. JAMA Psychiatry, 75(9), 973.doi:10.1001/jamapsychiatry.2018.1477. [DOI] [PubMed] [Google Scholar]
- Desseilles M., Schwartz S., Dang-Vu T.T., Sterpenich V., Ansseau M., Maquet P., Phillips C. (2011). Depression alters "top-down" visual attention: a dynamic causal modeling comparison between depressed and healthy subjects. NeuroImage, 54(2), 1662–8doi:10.1016/j.neuroimage.2010.08.061. [DOI] [PubMed] [Google Scholar]
- Drevets W.C., Price J.L., Furey M.L. (2008). Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Structure & Function, 213(1–2), 93–118doi:10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R., Zahn R., Deakin J.F., Anderson I.M. (2011). Affective cognition and its disruption in mood disorders. Neuropsychopharmacology, 36(1), 153–82doi:10.1038/npp.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epp A.M., Dobson K.S., Dozois D.J., Frewen P.A. (2012). A systematic meta-analysis of the Stroop task in depression. Clinical Psychology Review, 32(4), 316–28doi:10.1016/j.cpr.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Fales C.L., Barch D.M., Rundle M.M., Mintun M.A., Mathews J., Snyder A.Z., Sheline Y.I. (2009). Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. Journal of Affective Disorders, 112(1–3), 206–11doi:10.1016/j.jad.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fales C.L., Barch D.M., Rundle M.M., Mintun M.A., Snyder A.Z., Cohen J.D., Sheline Y.I. (2008). Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biological Psychiatry, 63(4), 377–84doi:10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y.T., Chen C., Chen S.C., Decety J., Cheng Y. (2014). Empathic arousal and social understanding in individuals with autism: evidence from fMRI and ERP measurements. Social Cognitive and Affective Neuroscience, 9(8), 1203–13doi:10.1093/scan/nst101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y.T., Fang Y.W., Chen Y.P., Leshikar E.D., Lin C.P., Tzeng O.J.L., Huang C.M. (2018). Aging, cognition, and the brain: effects of age-related variation in white matter integrity on neuropsychological function. Aging & Mental Health, 1–9doi:10.1080/13607863.2018.1455804. [DOI] [PubMed] [Google Scholar]
- Federmeier K.D., Kutas M., Schul R. (2010). Age-related and individual differences in the use of prediction during language comprehension. Brain and Language, 115(3), 149–61doi:10.1016/j.bandl.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiske A., Wetherell J.L., Gatz M. (2009). Depression in older adults. Annual Review of Clinical Psychology, 5, 363–89doi:10.1146/annurev.clinpsy.032408.153621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–98. [DOI] [PubMed] [Google Scholar]
- Forcada I., Mur M., Mora E., Vieta E., Bartres-Faz D., Portella M.J. (2015). The influence of cognitive reserve on psychosocial and neuropsychological functioning in bipolar disorder. European Neuropsychopharmacology, 25(2), 214–22doi:10.1016/j.euroneuro.2014.07.018. [DOI] [PubMed] [Google Scholar]
- Frodl T. (2016). Systems Neuroscience in Depression, Academic Press. [Google Scholar]
- Ganguli M., Du Y., Dodge H.H., Ratcliff G.G., Chang C.C.H. (2006). Depressive symptoms and cognitive decline in late life: a prospective epidemiological study. Archives of General Psychiatry, 63(2), 153–60. [DOI] [PubMed] [Google Scholar]
- Gazzaley A., Clapp W., Kelley J., McEvoy K., Knight R.T., D'Esposito M. (2008). Age-related top-down suppression deficit in the early stages of cortical visual memory processing. Proceedings of the National Academy of Sciences of the United States of America, 105(35), 13122–6doi:10.1073/pnas.0806074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giogkaraki E., Michaelides M.P., Constantinidou F. (2013). The role of cognitive reserve in cognitive aging: results from the neurocognitive study on aging. Journal of Clinical and Experimental Neuropsychology, 35(10), 1024–35doi:10.1080/13803395.2013.847906. [DOI] [PubMed] [Google Scholar]
- Gotlib I.H., Roberts J.E., Gilboa E. (1996). Cognitive interference in depression In: Sarason I.G., Pierce G.R., Sarason B.R., editors. Cognitive Interference: Theories, Methods, and Findings, Hillsdale, NJ, England: Lawrence Erlbaum Associates Inc., pp.347–77. [Google Scholar]
- Grady C. (2012). The cognitive neuroscience of ageing. Nature Reviews. Neuroscience, 13(7), 491–505doi:10.1038/nrn3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. (1960). A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry, 23(1), 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. (1969). Diagnosis and rating of anxiety. British Journal of Psychiatry, Special Publication No, 3, 76–9. [Google Scholar]
- Huang C.M., Polk T.A., Goh J.O., Park D.C. (2012). Both left and right posterior parietal activations contribute to compensatory processes in normal aging. Neuropsychologia, 50(1), 55–66doi:10.1016/j.neuropsychologia.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs B., Kennie A.T. (1973). The set test as an aid to the detection of dementia in old people. The British Journal of Psychiatry, 123(575), 467–70. [DOI] [PubMed] [Google Scholar]
- Kanske P., Heissler J., Schonfelder S., Bongers A., Wessa M. (2011). How to regulate emotion? Neural networks for reappraisal and distraction. Cerebral Cortex, 21(6), 1379–88doi:10.1093/cercor/bhq216. [DOI] [PubMed] [Google Scholar]
- Korsnes M.S., Ulstein I.D. (2014). Cognitive effects of late life depression: review of neuropsychological findings. Journal of Behavioral and Brain Science, 4, 141–57. [Google Scholar]
- Ladin K. (2008). Risk of late-life depression across 10 European Union countries: deconstructing the education effect. Journal of Aging and Health, 6, 653–70. [DOI] [PubMed] [Google Scholar]
- Lam C.L.M., Liu H.L., Huang C.M., Wai Y.Y., Lee S.H., Yiend J., Lee T.M.C. (2018). The neural correlates of perceived energy levels in older adults with late-life depression. Brain Imaging and Behavior, [Epub ahead of print], doi:10.1007/s11682-018-9940-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J.E. (2000). Emotion circuits in the brain. Annual Review of Neuroscience, 23, 155–84doi:10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Legendre S.A., Stern R.A., Solomon D.A., Furman M.J., Smith K.E. (2003). The influence of cognitive reserve on memory following electroconvulsive therapy. The Journal of Neuropsychiatry and Clinical Neurosciences, 15(3), 333–9doi:10.1176/jnp.15.3.333. [DOI] [PubMed] [Google Scholar]
- Lezak M.D., Howieson D.B., Bigler E.D., Tranel D. (2012). Neuropsychological assessment, 5th edn, New York: Oxford University Press. [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19(3), 1233–9. [DOI] [PubMed] [Google Scholar]
- Martin D.M., Galvez V., Loo C.K. (2015). Predicting retrograde autobiographical memory changes following electroconvulsive therapy: relationships between individual, treatment, and early clinical factors. The International Journal of Neuropsychopharmacology, 18(12), 1–8doi:10.1093/ijnp/pyv067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell I. (2006). Measuring Health: A Guide to Rating Scales and Questionnaires, 3rd edn, New York: Oxford University Press. [Google Scholar]
- Meng X., D'Arcy C. (2012). Education and dementia in the context of the cognitive reserve hypothesis: a systematic review with meta-analyses and qualitative analyses. PLoS One, 7(6), e38268doi:10.1371/journal.pone.0038268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitterschiffthaler M.T., Williams S.C., Walsh N.D., Cleare A.J., Donaldson C., Scott J., Fu C.H. (2008). Neural basis of the emotional Stroop interference effect in major depression. Psychological Medicine, 38(2), 247–56doi:10.1017/S0033291707001523. [DOI] [PubMed] [Google Scholar]
- Mogg K., Bradley B.P. (2005). Attentional bias in generalized anxiety disorder versus depressive disorder. Cognitive Therapy and Research, 29(1), 29–45. [Google Scholar]
- Mondini S., Madella I., Zangrossi A., Bigolin A., Tomasi C., Michieletto M., Mapelli D. (2016). Cognitive reserve in dementia: implications for cognitive training. Frontiers in Aging Neuroscience, 8, 84.doi:10.3389/fnagi.2016.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na J., Huang C.-M., Park D.C. (2017). When age and culture interact in an easy and yet cognitively demanding task: older adults, but not younger adults, showed the expected cultural differences. Frontiers in Psychology, 8, 457, doi:10.3389/fpsyg.2017.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9(5), 242–9doi:10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Ongur D., Price J.L. (2000). The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex, 10(3), 206–19. [DOI] [PubMed] [Google Scholar]
- O'Shea D.M., Fieo R.A., Hamilton J.L., Zahodne L.B., Manly J.J., Stern Y. (2015). Examining the association between late-life depressive symptoms, cognitive function, and brain volumes in the context of cognitive reserve. International Journal of Geriatric Psychiatry, 30(6), 614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D.C., Reuter-Lorenz P. (2009). The adaptive brain: aging and neurocognitive scaffolding. Ann. Rev. of Psy., 60, 173–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterniti S., Verdier-Taillefer M.H., Dufouil C., Alperovitch A. (2002). Depressive symptoms and cognitive decline in elderly people. Longitudinal study. The British Journal of Psychiatry, 181, 406–10. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Ladouceur C.D., Drevets W.C. (2008). A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry, 13(9), 829, 833–57doi:10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R.B., Siegle G., Mohlman J. (2012). Emotional Stroop performance in older adults: effects of habitual worry. The American Journal of Geriatric Psychiatry, 20(9), 798–805doi:10.1097/JGP.0b013e318230340d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N., Habeck C.G., Zarahn E., Anderson K.E., Park A., Hilton J., Stern Y. (2004). Covariance PET patterns in early Alzheimer's disease and subjects with cognitive impairment but no dementia: utility in group discrimination and correlations with functional performance. NeuroImage, 23(1), 35–45doi:10.1016/j.neuroimage.2004.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N., Stern Y. (2004). Cognitive reserve: implications for diagnosis and prevention of Alzheimer’s disease. Current Neurology and Neuroscience Reports, 4(5), 374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh J.I., Yesavage J.A. (1986). Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clinical Gerontologist: The Journal of Aging and Mental Health, 5(1–2), 165–73doi:10.1300/J018v05n01_09. [Google Scholar]
- Sheline Y.I., Barch D.M., Garcia K., Gersing K., Pieper C., Welsh-Bohmer K., Doraiswamy P.M. (2006). Cognitive function in late life depression: relationships to depression severity, cerebrovascular risk factors and processing speed. Biological Psychiatry, 60(1), 58–65doi:10.1016/j.biopsych.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Shyu Y.I., Yip P.K. (2001). Factor structure and explanatory variables of the Mini-Mental State Examination (MMSE) for elderly persons in Taiwan. Journal of the Formosan Medical Association, 100(10), 676–83. [PubMed] [Google Scholar]
- Sliz D., Hayley S. (2012). Major depressive disorder and alterations in insular cortical activity: a review of current functional magnetic imaging research. Frontiers in Human Neuroscience, 6, 323.doi:10.3389/fnhum.2012.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldan A., Pettigrew C., Lu Y., Wang M.C., Selnes O., Albert M., Team B.R. (2015). Relationship of medial temporal lobe atrophy, APOE genotype, and cognitive reserve in preclinical Alzheimer's disease. Human Brain Mapping, 36(7), 2826–41doi:10.1002/hbm.22810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S., Zilverstand A., Song H., d’Oleire Uquillas F., Wang Y., Xie C., Zou Z. (2017). The influence of emotional interference on cognitive control: a meta-analysis of neuroimaging studies using the emotional Stroop task. Scientific Reports, 7(1), 2088–8doi:10.1038/s41598-017-02266-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitznagel M.B., Tremont G., Brown L.B., Gunstad J. (2006). Cognitive reserve and the relationship between depressive symptoms and awareness of deficits in dementia. The Journal of Neuropsychiatry and Clinical Neurosciences, 18(2),186–90. [DOI] [PubMed] [Google Scholar]
- Steffener J., Stern Y. (2012). Exploring the neural basis of cognitive reserve in aging. Biochimica et Biophysica Acta, 1822(3), 467–73doi:10.1016/j.bbadis.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. (2002). What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society, 8(3), 448–60. [PubMed] [Google Scholar]
- Stern Y. (2007). Cognitive Reserve: Theory and Applications, New York: Taylor & Francis. [Google Scholar]
- Stern Y. (2009). Cognitive reserve. Neuropsychologia, 47(10), 2015–28doi:10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. (2012). Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurology, 11(11), 1006–12doi:10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. (2013). Cognitive reserve: implications for assessment and intervention. Folia Phoniatrica et Logopaedica, 65(2), 49–54doi:10.1159/000353443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y., Arenaza-Urquijo E.M., Bartres-Faz D., Belleville S., Cantilon M., Chetelat G., Conceptual Frameworks W. (2018a). Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer's & Dementia, [Epub ahead of print], doi:10.1016/j.jalz.2018.07.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y., Gazes Y., Razlighi Q., Steffener J., Habeck C. (2018b). A task-invariant cognitive reserve network. NeuroImage, 178, 36–45doi:10.1016/j.neuroimage.2018.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker A.M., Stern Y. (2011). Cognitive reserve in aging. Current Alzheimer Research, 8(4), 354–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R.J., Lloyd D.A. (1999). The stress process and the social distribution of depression. Journal of Health and Social Behavior, 40, 374–404. [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Joliot M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage, 15(1), 273–89doi:10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Venezia R.G., Gorlyn M., Burke A.K., Oquendo M.A., Mann J.J., Keilp J.G. (2018). The impact of cognitive reserve on neurocognitive performance in major depressive disorder. Psychiatry Research, 270, 211–8doi:10.1016/j.psychres.2018.09.031. [DOI] [PubMed] [Google Scholar]
- Wang L., Chou Y.H., Potter G.G., Steffens D.C. (2015). Altered synchronizations among neural networks in geriatric depression. BioMed Research International, 2015, 343720.doi:10.1155/2015/343720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson A., Joyce E. (2015). Cognitive reserve and neuropsychiatric disorders. Current Opinion in Behavioral Sciences, 4, 142–6doi:10.1016/j.cobeha.2015.05.003. [Google Scholar]
- Williams J.M., Mathews A., MacLeod C. (1996). The emotional Stroop task and psychopathology. Psychological Bulletin, 120(1), 3–24. [DOI] [PubMed] [Google Scholar]
- Wilson R.S., Boyle P.A., Yu L., Barnes L.L., Schneider J.A., Bennett D.A. (2013). Life-span cognitive activity, neuropathologic burden, and cognitive aging. Neurology, 81(4), 314–21doi:10.1212/WNL.0b013e31829c5e8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong N.M., Liu H.L., Lin C., Huang C.M., Wai Y.Y., Lee S.H., Lee T.M. (2016). Loneliness in late-life depression: structural and functional connectivity during affective processing. Psychological Medicine, 46(12), 2485–99doi:10.1017/S0033291716001033. [DOI] [PubMed] [Google Scholar]
- Yao P., Meng C. (2015). Longitudinal causal inference of cognitive function and depressive symptoms in elderly people. Epidemiology, Biostatistics and Public Health, 12(3), e11262. [Google Scholar]
- Zheng Y.P., Zhao J.P., Phillips M., Liu J.B., Cai M.F., Sun S.Q., Huang M.F. (1988). Validity and reliability of the Chinese Hamilton Depression Rating Scale. The British Journal of Psychiatry, 152, 660–4. [DOI] [PubMed] [Google Scholar]


