Abstract
Development of opioid tolerance and dependence hinders the use of opioids for the treatment of chronic pain. In searching for the mechanism and potential intervention for opioid tolerance and dependence, we studied the action of two positive allosteric modulators of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR PAMs). In mice treated with morphine (100 mg/kg, s.c.), acute morphine tolerance and dependence developed in 4-6 h. Treatment with aniracetam, a well-established AMPAR PAM, was able to completely prevent and reverse the development of acute morphine antinociceptive tolerance to morphine. Partial, but significant, effects of aniracetam on acute morphine induced-physical dependence were also observed. Moreover, aniracetam significantly reversed the established morphine tolerance and dependence in a chronic model of opioid tolerance and dependence produced by intermittent morphine (10 mg/kg, s.c. for 5d). In addition, a new AMPAR PAM HJC0122 was found to have similar effects as aniracetam with a higher potency. These previously undisclosed actions of AMPAR PAMs are intriguing and may shed lights on understanding the APMA signaling pathway in opioid addiction. Moreover, these data suggest that AMPAR PAMs may have utility in preventing and treating opioid tolerance and dependence.
Keywords: AMPA, positive allosteric modulator, opioid tolerance, opioid dependence
1. Introduction
Opioids remain the most efficacious analgesics for acute ad severe pain. Although opioid analgesics are commonly prescribed for chronic pain treatment, their long-term efficacies are restrained by the rapid development of profound antinociceptive tolerance and physical dependence. Needs for dose escalation and association with feared complications in drug addiction surrender the clinical use of opioids in chronic pain to be problematic (Inturrisi, 2002; Jage, 2005). The molecular mechanisms underlying opioid tolerance and dependence are not fully elucidated.
The AMPA receptors are members of the ligand-gated ionotropic glutamatergic receptor family, playing a critical role in mediating fast excitatory synaptic transmission in the mammalian central nervous system (CNS) (Song and Huganir, 2002). The AMPA receptors are tetrameric assemblies of subunits GluR1-4 as two identical heterodimers, in which, GluR2 subunit is essential for the permeability of receptors to Ca2+ (Hollmann et al., 1991; Hume et al., 1991). Growing evidence suggests that regulation of the AMPA receptor is a critical component in the expression of postsynaptic forms of long-term potentiation and long-term depression, as well as homeostatic synaptic plasticity of excitatory synapses (Turrigiano, 2008; Lee and Kirkwood, 2011).
It has been reported that AMPA receptor antagonists LY293558 and LY300168 suppressed morphine withdrawal-induced activation of locus coeruleus neurons and behavioral signs of morphine withdrawal (Rasmussen et al., 1996; Rasmussen and Vandergriff, 2003). It was also reported that LY293558 attenuated analgesic tolerance to morphine, acute, but not chronic, morphine dependence produced by morphine pellet implantation (Kest et al., 1997; McLemore et al., 1997). Some of the AMPA receptor antagonists’ effect of antinociceptive tolerance may be due to their direct antinociceptive action as ACEA2085 potentiated morphine antinociception in response to an acute thermal stimulus (Nishiyama et al., 1998). Furthermore, mice deficient in AMPA GluR1 subunit showed reduced tolerance and less severe physical dependence (Vekovischeva et al., 2001; Aitta-aho et al., 2012).
More recently, another group of AMPA receptor modulators, the AMPA receptor positive allosteric modulators (AMPAR PAMs) has been developed to allosterically modulate AMPA receptor transmission (Reuillon et al., 2016). AMPAR PAMs are able to decrease receptor desensitization and/or deactivation and increase the magnitude and kinetics of AMPA receptor-mediated synaptic currents (Lynch, 2006; Arai and Kessler, 2007; Jardemark et al., 2012). Aniracetam, a clinically used AMPAR PAM as a cognition-enhancing drug, has been shown to selectively inhibit the spontaneous rapid desensitization of AMPA receptors (Lawrence et al., 2003; Vaglenova et al., 2008). HJC0122, a newly synthesized AMPAR PAM, was shown to display more potent effects as a neuroprotective agent to prevent neuroapoptosis (Chen et al., 2013). AMPAR PAMs have been shown to improve both positive and negative symptoms as well as cognitive impairment in schizophrenia (Tuominen et al., 2005; Jardemark et al., 2012) and enhance memory in Huntington’s disease (Simmons et al., 2009). Limited studies showed that AMPAR PAMs do not alter pain threshold or acute opioid analgesia (Ren et al., 2009; Oertel et al., 2010). AMPAkines CX546 and CX516, which are AMPAR PAMs, have been shown to attenuate hyperalgesia induced by spared nerve injury (SNI) and complete Freund’s Adjuvant (CFA) in the rat (Le et al., 2014).
No study, however, has investigated whether AMPAR PAMs are able to modulate the development or expression of opioid tolerance and/or dependence. In this study, we examined the effects of aniracetam and HJC0122 on antinociceptive tolerance to and physical dependence on morphine in two mouse models.
2. Materials and Methods
2.1. Materials
Morphine sulfate and naloxone were purchased from Hospira (Lake Forest, IL). HJC0122 ((2R)-Propane-2-sulfonic acid (2-{4-[2-(pyrrolidine-1-carbonyl)pyridin-4-yl]-phenyl}propyl) amide) were synthesized according to previously described (Chen et al., 2013). Other chemical reagents were obtained from Sigma (St. Louis, MO).
2.2. Animals
Male ICR mice (20-25 g, Harlan, Indianapolis, IN) were housed on a 14/10 h light/dark cycle (5:00AM on/7:00PM off) with access to food and water ad libitum. All experiments were performed after approval by the Animal Care and Use Committee of the University of Illinois and in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
2.3. Antinociception test
The tail-flick test was performed to assess basal nociception and morphine-induced antinociception as previously described (Tang et al., 2006; Hu et al., 2015). In brief, the distal one-third of mouse tail was immersed into a water bath maintained at 52 °C. The latency to a quick tail-flick response was recorded. Morphine-induced antinociception was determined 30 min after the injection of morphine (10 mg/kg s.c.) and expressed as the percentage of maximal possible effect (MPE) according to the following formula: %MPE= 100*(postdrug latency- predrug latency)/(cut off- predrug latency). A cut-off time of 12 sec was applied to prevent tissue damage.
2.4. Acute opioid tolerance and dependence
Mice were made acutely tolerant to and dependent on opioids by the treatment of morphine sulfate (100 mg/kg s.c., time=0)(Wang et al., 1999; Shen et al., 2013). Control mice received to an equal volume of saline. To assess tolerance to opioids, mice received test doses of morphine (1-10 mg/kg, s.c.) 4.5 h later and the antinociceptive effect was measured at 5 h. The presence of tolerance to morphine was demonstrated by a significant reduction of morphine-antinociceptive effect. Dependence to opioids was studied by naloxone-precipitated withdraw jumping. Mice were treated with naloxone (10 mg/kg i.p.) at 5 h and were immediately placed inside glass cylinders. Mice were observed for 15 min with the number of vertical jumps recorded. To prevent the development of morphine tolerance and dependence, AMPAR PAM aniracetam (5-50 mg/kg, i.p.) or HJC0122 (1, 10 mg/kg, i.p.) was given 30 min before the induction dose of morphine (100 mg/kg, s.c., time = −30 min). To reverse the established acute morphine tolerance and dependence, aniracetam (5-50 mg/kg, i.p.) or HJC0122 (1, 10 mg/kg, i.p.) was given 30min before the test dose of morphine (10 mg/kg, s.c.) or naloxone.
2.5. Rotarod test
To evaluate the possible locomotor impairment caused by aniracetam, a rotarod test was conducted as described previously (Chen et al., 2010; Yang et al., 2011). The rotarod apparatus (Model series 8; IITC, Woodland Hills, CA) consisted of a 1.25 inch-diameter rod powered by a motor with adjustable speeds, subdivided into five compartments. Mice were trained 24h previously by remaining on a fixed speed (4 rpm) rotarod for 60s. On the day of experiment, mice were retrained at the same speed and those that are unable to hold onto the rotarod for 60s or longer were excluded from further studies. Baseline was obtained 30min later by recording the latency of each mouse to fall off an accelerating rotarod (4-40 rpm over 300s). Mice were then treated with aniracetam (50 mg/kg, i.p.) or saline and retested on the accelerating rotarod (4-40 rpm over 300s) 0.5, 1, 2, 4 and 8 h later with a cut-off of 300s.
2.6. Chronic opioid tolerance and dependence
Groups of mice were treated with morphine (10 mg/kg, s.c.) or equal volume of saline twice a day for 5 days to induce chronic morphine tolerance and dependence (Yang et al., 2011). Morphine antinociception and tolerance was determined with a test dose of morphine (10 mg/kg, s.c.) on Day 6. On the same day, morphine dependence was examined by recording the number of withdrawal jumps precipitated by naloxone (10 mg/kg, i.p.). Aniracetam was administered 30 min before the test dose of morphine.
2.7. Data and Statistical Analysis
All data are presented as the mean ± S.E.M. Comparisons between treatment groups were analyzed using a one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. Statistical significance was established at the 95% confidence limit.
3. Results
3.1. Aniracetam prevents the development of acute opioid tolerance
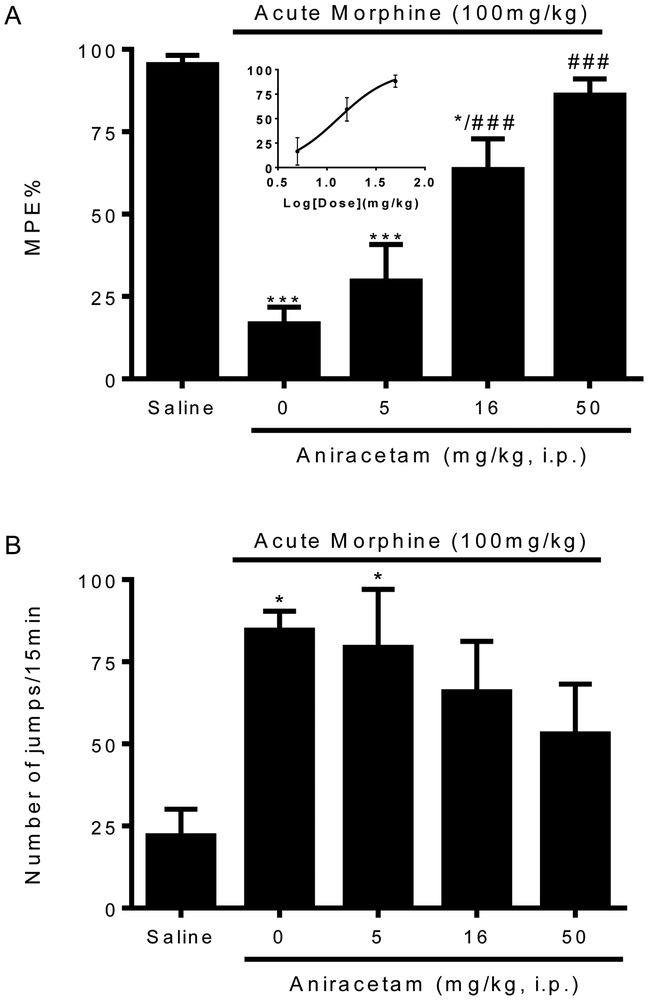
The effect of aniracetam on opioid tolerance and dependence was first assessed in a mouse model. Aniracetam is an AMPAR PAM that is clinically used for cognition-enhancing, with well-established AMPA-potentiation properties. To induce opioid tolerance and dependence, mice received a large dose of morphine (100 mg/kg, s.c.). After 4.5 h, morphine-treated mice exhibited a significant reduction of antinociception (16.7 ± 5.1 %MPE v.s. 95.4 ± 2.8 %MPE in saline-pretreated mice, p< 0.001, Fig.1A) produced by a test dose of morphine (10 mg/kg s.c.), indicative of the development of opioid antinociceptive tolerance. In the mice pretreated with aniracetam (50 mg/kg, i.p.) 30 min before the injection of morphine (100 mg/kg s.c.), morphine-antinociception tolerance was absent (86.1 ± 4.9 %MPE, p < 0.001 v.s. morphine alone, Fig. 1A). Pretreatment with aniracetam at a lower dose (16 mg/kg i.p.) was able to partially prevent morphine tolerance (63.5 ± 9.4 %MPE, p < 0.001 v.s. morphine alone, Fig. 1A). Aniracetam at the lowest dose used (5 mg/kg, i.p.) was ineffective (29.7 ± 11.1 %MPE, Fig. 1A). These data suggested that aniracetam prevented the development of acute morphine antinociceptive tolerance in a dose-dependent pattern, with an estimated EC50 around 13.0 mg/kg.
Figure 1. Effects of aniracetam in preventing acute opioid tolerance (A) and dependence (B).
Groups of eight mice were treated with aniracetam (5, 16, 50 mg/kg, i.p.) or saline (equal volume, i.p.) 30 minutes before administration of morphine (100 mg/kg, s.c.). Control mice received equal amount of saline (s.c.) instead of morphine. Opioid tolerance was monitored by significantly reduced antinociception induced by a test dose morphine (10 mg/kg, s.c.) 4.5 h later using the tail-flick test. Opioid dependence was tested by recording the number of naloxone-precipitated withdrawal jumps in 15 min. (A) Aniracetam at higher doses (16, 50 mg/kg, i.p.) significantly prevented the loss of morphine antinociception and reduced the development of acute opioid tolerance. EC50 was estimated from the dose response curve. (B) Aniracetam at all doses only displays partial effects in preventing opioid dependence. Data are expressed as the mean ± S.E.M. *P<0.05; ***P<0.001 compared with saline group; ###P<0.001 compared with morphine alone group.
Aniracetam’s effects on acute opioid dependence were tested by naloxone-precipitated withdraw jumping. Mice were given naloxone (10 mg/kg, i.p.) 4.5 h after large dose of morphine treatment, and the jumps were counted for 15 min. Morphine physical dependence was established in mice with the large dose of morphine treatment, which produced significantly more jumps than mice treated with saline (84.6 ± 5.7 v.s. 22.0 ± 8.2, p < 0.05, Fig. 1A). Pretreatment with Aniracetam (50 mg/kg, i.p.) only partially reduced the naloxone-precipitated jumps compared with morphine dependent mice pretreated with saline (53.0 ± 15.2, Fig. 1A). Aniracetam at lower doses (16, 5 mg/kg, i.p.) showed no effect on preventing the development of morphine dependence (Fig.1A). Taken together, we demonstrated that aniracetam prevented acute morphine tolerance and partially reduced acute morphine dependence.
3.2. Aniracetam has no effect on basal nociception, morphine antinociception, and locomotor activity.
To rule out the possibility that aniracetam produced an antinociception effect by itself or interfered with the antinociception induced by morphine, we tested the effect of aniracetam on basal nociception and morphine antinociception. Different groups of mice were given either aniracetam (50 mg/kg i.p.) alone or followed by the administration of morphine (1, 3, or 10 mg/kg s.c.). Aniracetam (50 mg/kg i.p.) neither affected basal nociception (Fig.2A) nor altered morphine-induced antinociception (Fig.2B). Another confounding factor in data interpretation was that aniracetam might cause locomotor impairment in mice. To account for this possibility, we further tested the effect of aniracetam on locomotor activity in a rotarod test. Administration of aniracetam (50 mg/kg, s.c.) to naïve mice did not affect locomotor coordination in the rotarod test (Fig.2C). These data suggested that the effect of Aniracetam at doses of up to 50 mg/kg on morphine tolerance and dependence was not attributed to altering basal nociception, interfering with morphine antinociception or impairing locomotor activity.
Figure 2. Effects of aniracetam on basal nociception (A), morphine antinociception (B) and locomotor activity (C).
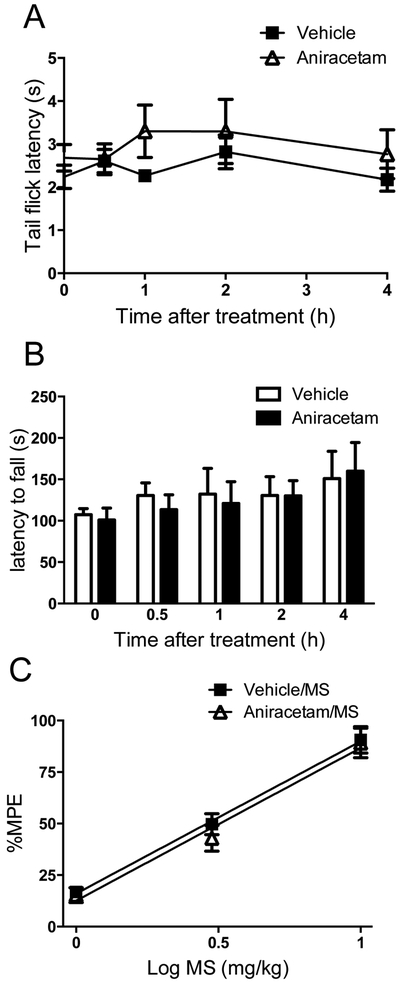
(A) Groups of mice received (i.p.) aniracetam (50 mg/kg) or equal volume of saline. The tail-flick test was carried out 0.5, 1, 2 and 4 h after the drug treatment. Aniracetam (50 mg/kg, i.p.) has no effect on basal nociception in mice.
(B) Mice received aniracetam (50 mg/kg, i.p.) or saline 30 min before morphine (1, 3, 10 mg/kg, s.c.). Aniracetam did not interfere with the antinociceptive effects produced by morphine.
(C) Mice locomotor activity was measured by the rotarod test, in which the latencies to fall from a rotation rod were recorded. Aniracetam (50 mg/kg, i.p.) did not impair the locomotor function of mice. Data are expressed as the mean ± S.E.M.
3.3. Aniracetam reversed acute opioid tolerance and partially blocked dependence.
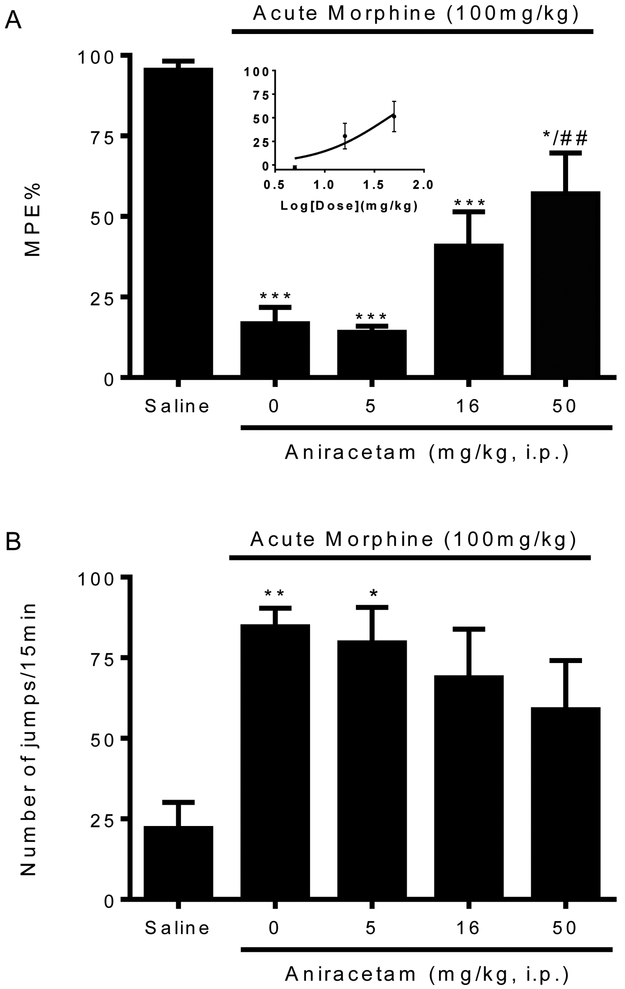
Given the preventive effect of aniracetam on acute morphine tolerance and dependence, we next investigated whether it can reverse the already established acute opioid tolerance and dependence. Mice treated with a large dose of morphine (100 mg/kg, s.c.) were treated with aniracetam (5, 16, 50 mg/kg, i.p.) 30 min before the testing dose of morphine (10 mg/kg, s.c.) or naloxone (10 mg/kg, i.p.). Aniracetam at the largest dose (50 mg/kg, i.p.) completely restored the antinociceptive effect of morphine (57.1 ± 12.6 %MPE, p < 0.01 v.s. morphine alone, Fig. 2A). At a lower dose, aniracetam produced partial effect on reducing the established acute morphine tolerance (40.8 ± 10.7 %MPE, Fig. 2A), and aniracetam at the lowest dose showed no effect (14.1 ± 1.8 %MPE, Fig. 2A). The EC50 of aniracetam reversing acute morphine tolerance is estimated to be 43.8 mg/kg. For the established morphine physical dependence, aniracetam produced a partial effect at all the doses (Fig. 2B). These results indicated that aniracetam is dose-dependently effective in reversing the acute opioid tolerance, and has a partial effect in reducing the acute opioid dependence.
3.4. Reversal of chronic opioid tolerance and dependence by aniracetam
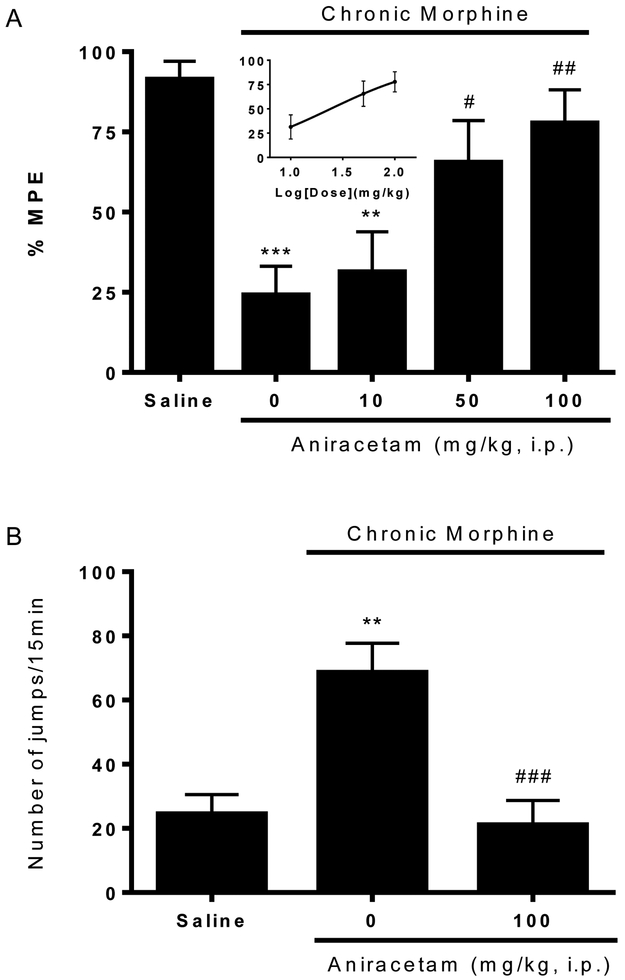
To confirm our observation with aniracetam in acute opioid tolerance and dependence, we used a chronic model to test the effect of aniracetam. Mice received morphine (10 mg/kg, s.c.) twice a day for five days. Prolonged treatment of morphine significantly reduced anti-nociceptive effect of a test dose of morphine (10 mg/kg, s.c.) given on Day 6 (24.2 ± 8.9 %MPE v.s. 91.4 ± 5.6 %MPE, p < 0.001, Fig. 4A), and also produced physical dependence presented by the naloxone precipitated withdraw jumps (68.6 ± 9.0 v.s. 24.5 ± 6.0, p < 0.01, Fig. 4B). Aniracetam (10, 50, 100 mg/kg, i.p.) were given 30 min before the test dose of morphine on Day 6. Larger doses of aniracetam (50, 100 mg/kg, i.p.) significantly restored the anti-nociception produced by the test dose of morphine (65.5 ± 13.0 %MPE, p<0.05 and and 77.8 ± 10.3 %MPE, p<0.01 v.s. morphine alone, respectively, Fig.4A), which showed the reversal of established chronic opioid tolerance with an estimated EC50 of 24.2 mg/kg. Also, aniracetam at a dose of 100 mg/kg (i.p.) significantly decreased the withdraw jumps precipitated by naloxone (21.1 ± 7.6, p<0.001 v.s. morphine alone, Fig. 4B), indicating the block of physical dependence. Here our results indicated that aniracetam reversed the established opioid tolerance and dependence produced by chronic exposure of morphine.
Figure 4. Reversal of chronic opioid (A) tolerance and (B) dependence by aniracetam.
Groups of eight mice were treated with morphine (10 mg/kg, s.c.) twice a day for 5 days to produce chronic opioid tolerance and dependence. Control group mice received equal numbers and volume of saline injections. Opioid tolerance and dependence were assessed on day 6 using the method described above. Aniracetam (10, 50, 100 mg/kg, i.p.) were given acutely to mice 30 min before the test dose of morphine on day 6. (A) Aniracetam dose-dependently restored morphine antinociception and reduced opioid tolerance. EC50 was estimated from the dose response curve. (B) Aniracetam at dose of 100 mg/kg significantly reduced naloxone-precipitated withdraw jumps and blocked opioid dependence. Data are expressed as the mean ± S.E.M. **P<0.01; ***P<0.001 compared with saline group; #P<0.05; ##P<0.01; ###P<0.001 compared with morphine alone group.
3.5. HJC0122 attenuated acute opioid tolerance and dependence
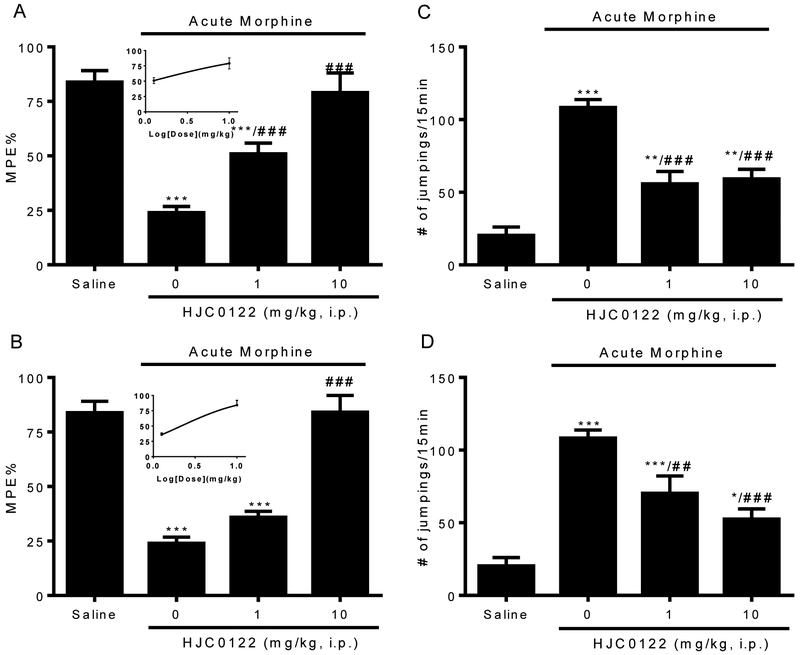
To further validate the effect of AMPAR PAMs in opioid tolerance and dependence, we studied HJC0122, a newer generation and more potent AMPAR PAM (Chen et al., 2013). Groups of mice were pretreated with HJC0122 (1, 10mg/kg) 30 min before induction dose of morphine (100mg/kg). Pretreatment with HJC0122 at both doses of 1 and 10mg/kg prevented the development of morphine antinociceptive tolerance (51.0 ± 4.9 %MPE by HJC0122 at 1 mg/kg, p<0.001; 79.1 ± 9.0 %MPE by HJC0122 at 10 mg/kg, p<0.001 v.s. 24.0 ± 2.82 %MPE morphine alone, Fig.5A). Mice pretreated with HJC0122 also showed significantly reduced naloxone-precipitated withdrawal jumps (55.8 ± 8.5 by HJC0122 at 1 mg/kg, p<0.001; 59.1 ± 6.7 by HJC0122 at 10 mg/kg, p<0.001, v.s. 108.3 ± 5.6 morphine alone, Fig.5B).
Figure 5. Effects of HJC0122 on preventing (A-B) and reversing (C-D) acute opioid tolerance and dependence.
Acute opioid tolerance and dependence were induced in mice by morphine (100mg/kg, s.c.). When mice were treated with HJC0122 (1, 10 mg/kg, i.p.) 30 min before the induction dose of morphine, HJC0122 significantly prevented the development of acute opioid (A) tolerance and (B) dependence. When mice received HJC0122 (1, 10 mg/kg, i.p.) 30 minutes before the test dose of morphine (10 mg/kg, s.c.), HJC0122 significantly reversed the established opioid (C) tolerance and (D) dependence in a dose dependent manner. Data are expressed as the mean ± S.E.M. *P<0.05; **P<0.01; ***P<0.001 compared with saline group; ##P<0.01; ###P<0.001 compared with morphine alone group.
When the mice were given HJC0122 acutely 30 min before the testing dose of morphine, HJC0122 at 10mg/kg completely restored the antinociceptive effect of morphine in mice with acute morphine tolerance (84.1 ± 7.6 %MPE, p<0.001 v.s. morphine alone, Fig.5C), and also significantly decreased the naloxone-precipitated withdrawal jumps (52.5 ± 7.1, p<0.001 v.s. morphine alone, Fig.5D). We found that HJC0122 not only prevented the development (Fig. 5A, B), but also reversed the established acute opioid tolerance and dependence (Fig. 5C, D). The estimated EC50 of preventing and reversing acute opioid tolerance for HJC0122 is 1.2 mg/kg and 2.2 mg/kg, respectively, which are about 10-20 fold lower than both of aniracetam at 13.0 mg/kg and 43.8 mg/kg. Taken together, our results suggest that AMPAR PAMs in general have effects in reducing opioid tolerance and dependence.
4. Discussion
In this study, we investigated the role of AMPAR PAMs in opioid tolerance and dependence. We found that aniracetam, a well-studied AMPAR PAM, and HJC0122, a newer generation modulator, attenuated morphine antinociceptive tolerance and physical dependence in two mouse models of opioid tolerance and dependence.
Development of tolerance and dependence lowered the efficacy and potency of opioid treatment in patients with chronic pain. The effects of AMPAR PAMs on opioid tolerance and dependence have not been investigated before. AMPAR PAMs, both aniracetam and HJC0122, tested in this study effectively prevented and also reversed the development of morphine acute and chronic tolerance and dependence in mice. The novel compound, HJC0122, with an effective dose of 10 mg/kg is more potent than aniracetam with a dose of 50-100 mg/kg intraperitoneally. A few previous studies have showed that AMPAR PAMs do not interfere with the threshold of acute pain (Ren et al., 2009; Oertel et al., 2010), and our results confirmed that aniracetam did not alter basal nociception or acute morphine antinociception. Importantly, aniracetam did not impair motor function in mice.
Previous studies reported that AMPA receptor antagonists could attenuate morphine tolerance and dependence (Kest et al., 1997; McLemore et al., 1997). Considering that AMPAR PAMs bind to AMPAR in an allosteric site to reduce the receptor deactivation kinetics and desensitization, the effect of these compounds on opioid tolerance and dependence is interestingly paradoxical. However, it is possible to be explained by their actions on AMPA receptors in descending inhibitory circuits, as it has been previously proposed (Le et al., 2014). The AMPA receptors constitute the periaqueductal gray-rostral ventromedial medulla (PAG-RVM) descending inhibitory pathway (van Praag and Frenk, 1990; Spinella et al., 1996). In PAG-RVM-spinal descending pathway, a well-known circuit for pain regulation (Fields et al., 1976; Basbaum and Fields, 1984; Heinricher et al., 2009), PAG neurons form glutamatergic projections through AMPA receptors on GABAergic cells in the RVM and lead to inhibition of dorsal horn neurons (Morgan et al., 2008). AMPA receptor signaling ensures the intact PAG-RVM-descending pathway (Guan et al., 2002). nucleus accumbens (NAc)-RVM circuit, which provides pain-induced analgesia, is another pain modulation pathway through AMPA receptor signaling (Gear et al., 1999; Ghalandari-Shamami et al., 2011). Recently, AMPAR PAMs have been shown to produce anti-hyperalgesic effects in both CFA inflammatory and SNI neuropathic pain models in rats (Le et al., 2014). This analgesic effect of AMPA PAMs may result from potentiation of AMPA receptors signaling in these descending inhibitory circuits.
On the other hand, pro-nociceptive effects and increased opioid tolerance and dependence may occur when transmission through AMPA receptors is enhanced in neurons of ACC (Xu et al., 2008), amygdala (Li and Neugebauer, 2004) and spinal dorsal horn (Hartmann et al., 2004). Increasing evidence supports that excessive transmission through glutamate receptors including AMPA receptors in the spinal cord contributes to the induction and maintenance of chronic pain (Hartmann et al., 2004; Park et al., 2008).
Thus, AMPA receptor signaling plays differential roles in opioid tolerance and dependence, depending on the CNS regions involved. In our study, aniracetam and HJC0122 were administered intraperitoneally. The systemically administration of AMPAR PAMs, may lead to the actions in the brain, spinal cord or periphery. Our data suggest that AMPAR PAMs in general was effective in reducing opioid tolerance and dependence as a net outcome. The distinct affinity of AMPAR PAMs for neurons in different brain regions may also contribute to this net effect. CX546 and CX516, two other AMPAR PAMs, both showed regionally different effect on synaptic transmission in the thalamus and the hippocampus (Xia et al., 2005). CX546 was reported to exhibit high binding affinity with NAc and brainstem (Montgomery et al., 2009). To obtain a more comprehensive idea of AMPAR PAMs’ effects, further studies are imperative to investigate the binding affinities of these AMPAR PAMs at the different regions in the CNS.
Besides opioid tolerance and dependence, another significant risk of opioid treatment is respiratory depression, which is the main reason for deaths due to opioid overdose (Paulozzi et al., 2006; Dahan et al., 2013). The management of feared complications of opioid treatment remains a major challenge in the clinic. Interestingly, some recent studies reported that AMPAR PAMs could stimulate respiratory rhythmogenesis, but only under the condition of ventilatory suppression, presenting these compounds as ideal drugs to selectively inhibit opioid-induced respiratory depression (Ren et al., 2006; Ren et al., 2009; Oertel et al., 2010). The selective counteracting effect of AMPAR PAMs on opioid-induced respiratory suppression was demonstrated in rats (Ren et al., 2006; Ren et al., 2009) and was also translated to humans (Oertel et al., 2010). With minimized risk of respiratory suppression and effects on attenuating the development of tolerance and dependence, AMPAR PAMs can be developed as ideal drugs for the combination use with opioid treatment.
In summary, we reported novel effects of AMPAR PAMs in attenuating opioid tolerance and dependence in two mouse models. Our findings may open a new avenue for preventing opioid tolerance and dependence, treating opioid addiction, and improving pain therapies by reducing opioid tolerance with novel AMPA receptor positive allosteric modulators. With their unique effects on suppressing opioid tolerance, dependence and stimulating depressed respiration, AMPAR PAMs have a very promising potential in the management of chronic pain in patients who have been previously exposed to opioids.
Figure 3. Effects of aniracetam in reversing acute opioid (A) tolerance and (B) dependence.
Separate groups of eight mice were treated with morphine (100 mg/kg, s.c.) to develop acute opioid tolerance and dependence. Aniracetam (5, 16, 50 mg/kg, i.p.) was administered 30 min before the test dose of morphine (10 mg/kg, s.c.). (A) Aniracetam (50 mg/kg, i.p.) significantly restored the antinociceptive effect of morphine. Lower doses of aniracetam (5, 16 mg/kg, i.p.) have partial effects on reducing acute opioid tolerance. EC50 was calculated based on the dose response curve. (B) Aniracetam (5, 16, 50 mg/kg, i.p.) partially reduced acute opioid dependence. Data are expressed as the mean ± S.E.M. *P<0.05; **P<0.01; ***P<0.001 compared with saline group; ##P<0.01 compared with morphine alone group.
Acknowledgement:
This work was supported in part by grants (R01 HL098141, R01 DA041809, P30 DA28821, and R01 DA038446) from the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The final peer-reviewed manuscript is subject to the NIH Public Access Policy.
Abbreviations:
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- PAM
positive allosteric modulator
- PAG-RVM
periaqueductal gray-rostral ventromedial medulla
- NAc
nucleus acumbens
- MPE
maximal possible effect
Contributor Information
Xiaoyu Hu, Department of Biopharmaceutical Sciences, University of Illinois, Chicago, IL 60612, USA.
Xuebi Tian, Department of Biopharmaceutical Sciences, University of Illinois, Chicago, IL 60612, USA.
Haijun Chen, Department of Pharmacology and Toxicology, and Center for Addiction Research, University of Texas Medical Branch, Galveston, TX 77555, USA.
Jia Zhou, Department of Pharmacology and Toxicology, and Center for Addiction Research, University of Texas Medical Branch, Galveston, TX 77555, USA.
Zaijie Jim Wang, Department of Biopharmaceutical Sciences, University of Illinois, Chicago, IL 60612, USA; Cancer Center, University of Illinois, Chicago, IL 60612, USA.
Reference
- Aitta-aho T, Moykkynen TP, Panhelainen AE, Vekovischeva OY, Backstrom P and Korpi ER (2012) Importance of GluA1 subunit-containing AMPA glutamate receptors for morphine state-dependency. PloS one 7:e38325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai AC and Kessler M (2007) Pharmacology of ampakine modulators: from AMPA receptors to synapses and behavior. Current drug targets 8:583–602. [DOI] [PubMed] [Google Scholar]
- Basbaum AI and Fields HL (1984) Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annual review of neuroscience 7:309–338. [DOI] [PubMed] [Google Scholar]
- Chen H, Wang CZ, Ding C, Wild C, Copits B, Swanson GT, Johnson KM and Zhou J (2013) A combined bioinformatics and chemoinformatics approach for developing asymmetric bivalent AMPA receptor positive allosteric modulators as neuroprotective agents. ChemMedChem 8:226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Yang C and Wang ZJ (2010) Ca2+/calmodulin-dependent protein kinase II alpha is required for the initiation and maintenance of opioid-induced hyperalgesia. The Journal of neuroscience : the official journal of the Society for Neuroscience 30:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan A, Overdyk F, Smith T, Aarts L and Niesters M (2013) Pharmacovigilance: a review of opioid-induced respiratory depression in chronic pain patients. Pain physician 16:E85–94. [PubMed] [Google Scholar]
- Fields HL, Anderson SD, Clanton CH and Basbaum AI (1976) Nucleus raphe magnus: a common mediator of opiate- and stimulus-produced analgesia. Transactions of the American Neurological Association 101:208–210. [PubMed] [Google Scholar]
- Gear RW, Aley KO and Levine JD (1999) Pain-induced analgesia mediated by mesolimbic reward circuits. The Journal of neuroscience : the official journal of the Society for Neuroscience 19:7175–7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghalandari-Shamami M, Hassanpour-Ezatti M and Haghparast A (2011) Intra-accumbal NMDA but not AMPA/kainate receptor antagonist attenuates WIN55,212-2 cannabinoid receptor agonist-induced antinociception in the basolateral amygdala in a rat model of acute pain. Pharmacology, biochemistry, and behavior 100:213–219. [DOI] [PubMed] [Google Scholar]
- Guan Y, Terayama R, Dubner R and Ren K (2002) Plasticity in excitatory amino acid receptor-mediated descending pain modulation after inflammation. The Journal of pharmacology and experimental therapeutics 300:513–520. [DOI] [PubMed] [Google Scholar]
- Hartmann B, Ahmadi S, Heppenstall PA, Lewin GR, Schott C, Borchardt T, Seeburg PH, Zeilhofer HU, Sprengel R and Kuner R (2004) The AMPA receptor subunits GluR-A and GluR-B reciprocally modulate spinal synaptic plasticity and inflammatory pain. Neuron 44:637–650. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Tavares I, Leith JL and Lumb BM (2009) Descending control of nociception: Specificity, recruitment and plasticity. Brain research reviews 60:214–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M, Hartley M and Heinemann S (1991) Ca2+ permeability of KA-AMPA--gated glutamate receptor channels depends on subunit composition. Science 252:851–853. [DOI] [PubMed] [Google Scholar]
- Hu X, Huang F, Szymusiak M, Liu Y and Wang ZJ (2015) Curcumin attenuates opioid tolerance and dependence by inhibiting Ca2+/calmodulin-dependent protein kinase II alpha activity. The Journal of pharmacology and experimental therapeutics 352:420–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume RI, Dingledine R and Heinemann SF (1991) Identification of a site in glutamate receptor subunits that controls calcium permeability. Science 253:1028–1031. [DOI] [PubMed] [Google Scholar]
- Inturrisi CE (2002) Clinical pharmacology of opioids for pain. The Clinical journal of pain 18:S3–13. [DOI] [PubMed] [Google Scholar]
- Jage J (2005) Opioid tolerance and dependence -- do they matter? European journal of pain 9:157–162. [DOI] [PubMed] [Google Scholar]
- Jardemark K, Marcus MM, Malmerfelt A, Shahid M and Svensson TH (2012) Differential effects of AMPA receptor potentiators and glycine reuptake inhibitors on antipsychotic efficacy and prefrontal glutamatergic transmission. Psychopharmacology 221:115–131. [DOI] [PubMed] [Google Scholar]
- Kest B, McLemore G, Kao B and Inturrisi CE (1997) The competitive alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate receptor antagonist LY293558 attenuates and reverses analgesic tolerance to morphine but not to delta or kappa opioids. The Journal of pharmacology and experimental therapeutics 283:1249–1255. [PubMed] [Google Scholar]
- Lawrence JJ, Brenowitz S and Trussell LO (2003) The mechanism of action of aniracetam at synaptic alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors: indirect and direct effects on desensitization. Molecular pharmacology 64:269–278. [DOI] [PubMed] [Google Scholar]
- Le AM, Lee M, Su C, Zou A and Wang J (2014) AMPAkines have novel analgesic properties in rat models of persistent neuropathic and inflammatory pain. Anesthesiology 121:1080–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK and Kirkwood A (2011) AMPA receptor regulation during synaptic plasticity in hippocampus and neocortex. Seminars in cell & developmental biology 22:514–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W and Neugebauer V (2004) Block of NMDA and non-NMDA receptor activation results in reduced background and evoked activity of central amygdala neurons in a model of arthritic pain. Pain 110:112–122. [DOI] [PubMed] [Google Scholar]
- Lynch G (2006) Glutamate-based therapeutic approaches: ampakines. Current opinion in pharmacology 6:82–88. [DOI] [PubMed] [Google Scholar]
- McLemore GL, Kest B and Inturrisi CE (1997) The effects of LY293558, an AMPA receptor antagonist, on acute and chronic morphine dependence. Brain research 778:120–126. [DOI] [PubMed] [Google Scholar]
- Montgomery KE, Kessler M and Arai AC (2009) Modulation of agonist binding to AMPA receptors by 1-(1,4-benzodioxan-6-ylcarbonyl)piperidine (CX546): differential effects across brain regions and GluA1-4/transmembrane AMPA receptor regulatory protein combinations. The Journal of pharmacology and experimental therapeutics 331:965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MM, Whittier KL, Hegarty DM and Aicher SA (2008) Periaqueductal gray neurons project to spinally projecting GABAergic neurons in the rostral ventromedial medulla. Pain 140:376–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama T, Yaksh TL and Weber E (1998) Effects of intrathecal NMDA and non-NMDA antagonists on acute thermal nociception and their interaction with morphine. Anesthesiology 89:715–722. [DOI] [PubMed] [Google Scholar]
- Oertel BG, Felden L, Tran PV, Bradshaw MH, Angst MS, Schmidt H, Johnson S, Greer JJ, Geisslinger G, Varney MA and Lotsch J (2010) Selective antagonism of opioid-induced ventilatory depression by an ampakine molecule in humans without loss of opioid analgesia. Clinical pharmacology and therapeutics 87:204–211. [DOI] [PubMed] [Google Scholar]
- Park JS, Yaster M, Guan X, Xu JT, Shih MH, Guan Y, Raja SN and Tao YX (2008) Role of spinal cord alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors in complete Freund's adjuvant-induced inflammatory pain. Molecular pain 4:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulozzi LJ, Budnitz DS and Xi Y (2006) Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiology and drug safety 15:618–627. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Kendrick WT, Kogan JH and Aghajanian GK (1996) A selective AMPA antagonist, LY293558, suppresses morphine withdrawal-induced activation of locus coeruleus neurons and behavioral signs of morphine withdrawal. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 15:497–505. [DOI] [PubMed] [Google Scholar]
- Rasmussen K and Vandergriff J (2003) The selective iGluR1-4 (AMPA) antagonist LY300168 attenuates morphine-withdrawal-induced activation of locus coeruleus neurons and behavioural signs of morphine withdrawal. Neuropharmacology 44:88–92. [DOI] [PubMed] [Google Scholar]
- Ren J, Ding X, Funk GD and Greer JJ (2009) Ampakine CX717 protects against fentanyl-induced respiratory depression and lethal apnea in rats. Anesthesiology 110:1364–1370. [DOI] [PubMed] [Google Scholar]
- Ren J, Poon BY, Tang Y, Funk GD and Greer JJ (2006) Ampakines alleviate respiratory depression in rats. American journal of respiratory and critical care medicine 174:1384–1391. [DOI] [PubMed] [Google Scholar]
- Reuillon T, Ward SE and Beswick P (2016) AMPA Receptor Positive Allosteric Modulators: Potential for the Treatment of Neuropsychiatric and Neurological Disorders. Current topics in medicinal chemistry 16:3536–3565. [DOI] [PubMed] [Google Scholar]
- Shen H, Hu X, Szymusiak M, Wang ZJ and Liu Y (2013) Orally administered nanocurcumin to attenuate morphine tolerance: comparison between negatively charged PLGA and partially and fully PEGylated nanoparticles. Molecular pharmaceutics 10:4546–4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DA, Rex CS, Palmer L, Pandyarajan V, Fedulov V, Gall CM and Lynch G (2009) Up-regulating BDNF with an ampakine rescues synaptic plasticity and memory in Huntington's disease knockin mice. Proceedings of the National Academy of Sciences of the United States of America 106:4906–4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song I and Huganir RL (2002) Regulation of AMPA receptors during synaptic plasticity. Trends in neurosciences 25:578–588. [DOI] [PubMed] [Google Scholar]
- Spinella M, Cooper ML and Bodnar RJ (1996) Excitatory amino acid antagonists in the rostral ventromedial medulla inhibit mesencephalic morphine analgesia in rats. Pain 64:545–552. [DOI] [PubMed] [Google Scholar]
- Tang L, Shukla PK, Wang LX and Wang ZJ (2006) Reversal of morphine antinociceptive tolerance and dependence by the acute supraspinal inhibition of Ca(2+)/calmodulin-dependent protein kinase II. The Journal of pharmacology and experimental therapeutics 317:901–909. [DOI] [PubMed] [Google Scholar]
- Tuominen HJ, Tiihonen J and Wahlbeck K (2005) Glutamatergic drugs for schizophrenia: a systematic review and meta-analysis. Schizophrenia research 72:225–234. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG (2008) The self-tuning neuron: synaptic scaling of excitatory synapses. Cell 135:422–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaglenova J, Pandiella N, Wijayawardhane N, Vaithianathan T, Birru S, Breese C, Suppiramaniam V and Randal C (2008) Aniracetam reversed learning and memory deficits following prenatal ethanol exposure by modulating functions of synaptic AMPA receptors. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 33:1071–1083. [DOI] [PubMed] [Google Scholar]
- van Praag H and Frenk H (1990) The role of glutamate in opiate descending inhibition of nociceptive spinal reflexes. Brain research 524:101–105. [DOI] [PubMed] [Google Scholar]
- Vekovischeva OY, Zamanillo D, Echenko O, Seppala T, Uusi-Oukari M, Honkanen A, Seeburg PH, Sprengel R and Korpi ER (2001) Morphine-induced dependence and sensitization are altered in mice deficient in AMPA-type glutamate receptor-A subunits. The Journal of neuroscience : the official journal of the Society for Neuroscience 21:4451–4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Bilsky EJ, Wang D, Porreca F and Sadee W (1999) 3-Isobutyl-1-methylxanthine inhibits basal muopioid receptor phosphorylation and reverses acute morphine tolerance and dependence in mice. European journal of pharmacology 371:1–9. [DOI] [PubMed] [Google Scholar]
- Xia YF, Kessler M and Arai AC (2005) Positive alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor modulators have different impact on synaptic transmission in the thalamus and hippocampus. The Journal of pharmacology and experimental therapeutics 313:277–285. [DOI] [PubMed] [Google Scholar]
- Xu H, Wu LJ, Wang H, Zhang X, Vadakkan KI, Kim SS, Steenland HW and Zhuo M (2008) Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 28:7445–7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Chen Y, Tang L and Wang ZJ (2011) Haloperidol disrupts opioid-antinociceptive tolerance and physical dependence. The Journal of pharmacology and experimental therapeutics 338:164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]