Abstract
Purpose
In previous research, we have found that LMP1-specific chimeric antigen (HELA/CAR) T cells can specifically recognize and kill LMP1-positive NPC cells. However, the tumor-inhibitory effectiveness of HELA/CART cells needs to be enhanced.
Methods
We created two CARs that contain the T cell receptor-ζ (TCR-ζ) signal transduction domain with the CD28 and CD137 (4-1BB) or CD134 (OX-40) intracellular domains in tandem (HELA/137CAR or HELA/134CAR). Then, the tumor-inhibitory functions of two new CAR-T cells were investigated, both in vitro and in vivo.
Results
The results showed that, after short-term expansion, primary human T cells were subjected to lentiviral gene transfer, resulting in large numbers of cells with >80% CAR expression. All CART cells were effective in killing SUNE1-LMP1 and C1R-neo cells, while HELA/137CART cells produced greater quantities of IFN-γ and IL-2 than HELA/CART cells. However, the level of IL-2 not INF-γ secreted by HELA/134CART cells was increased under the stimulation of LMP1 antigen. In an LMP1-positive NPC mouse xenograft model, HELA/137CART cells exhibited better antitumor activity and longer survival time in vivo compared with HELA/CAR T cells.
Conclusion
The findings suggest that CD137 and CD28 is a better costimulatory signaling domain than CD28 only for optimizing tumor-inhibitory roles.
Keywords: chimeric antigen receptors, LMP1, EBV, CD137
Introduction
Latent Epstein–Barr virus (EBV) infection is associated with a heterogeneous group of malignancies, including Burritt’s lymphoma, Hodgkin’s disease, nasopharyngeal carcinoma (NPC), gastric adenocarcinoma, and lymphoproliferative disease (LPD).1 EBV-specific CTLs have been used successfully to treat EBV-LPD2,3 but have shown less efficacy for other EBV-associated malignancies, mostly due to the downregulation of EBV proteins. The EBV latent membrane protein 1 (LMP1) is essential for EBV-mediated transformation and tumorigenesis, and it is widely expressed in multiple human malignancies, including NPC, EBV-positive Hodgkin’s disease, and peripheral T/NK-cell lymphomas.4,5 As LMP1 is highly limited to EBV-associated cancer cells, LMP1 has been identified as an ideal target for EBV-positive malignancies.6,7
The genetic engineering of T cells to express chimeric antigen receptors (CARs) has emerged as a promising strategy for cancer treatment. CARs combine an antigen recognition domain of a specific antibody with the signaling domains of the TCR CD3ζ chain or/and a costimulatory domain that can trigger T-cell activation in a manner similar to endogenous T-cell receptors. The costimulatory domains, including the intracellular domain of CD28 and a tumor necrosis factor receptor (TNFR) family member such as CD134 (OX-40) and CD137 (4-1BB), can remarkably augment cytokine secretion, promote CAR-T cell survival and enhance the CAR-T cell killing function in preclinical animal models of cancer and in solid tumors.8,9
In our previous research, we have reported that HELA/CAR T cells can specifically recognize and kill LMP1-positive NPC cells.10 In this study, we constructed two 3rd-generation CARs, HELA/137CAR and HELA/134CAR, by adding CD134 or CD137 signaling domains between the CD28 and CD3ζ domains. Compared to HELA/CAR, HELA/137CAR showed superior antitumor activity and long-persistence properties in an LMP1-positive NPC xenograft model.
Materials And Methods
Cell Lines And Culture
Human tumor cell lines consisting of SUNE1, LMP1-overexpression SUNE1-LMP1 and C666-1 cells (NPC cell line) were kindly provided by Novartis Pharmaceuticals Co., Ltd. EBV-LMP1-negative HNE2 cells (NPC cell line) and HNE2-LMP1 (cell line constantly expressing LMP1 after the introduction of full-length LMP1 cDNA into HNE2 cells) were purchased from Xiangya Central Experiment Laboratory, EBV-negative Ramos cells (Burkitt’s lymphoma cell line), EBNA-positive Daudi cells (Burkitt’s lymphoma cell line) and Raji cells (Burkitt’s lymphoma cell line) and RPMI 6666 cells (Hodgkin’s lymphoma cell line), neomycin drug-resistant C1R-neo cells (B-cell lymphoblastoid cell line) were obtained from American Type Culture Collection (ATCC) (USA), and B95-8 cells (The EBV-producing marmoset B-cell line) were kindly provided by Dr. Song (Baylor College of Medicine, USA), and the EBV-transformed LCL was prepared by infecting human B cells with EBV. Human tumor cells and T cells were cultured in RPMI-1640 (GIBCO, Invitrogen), supplemented with 2mM L-glutamine, 100 U/mL penicillin, 100μg/mL streptomycin, and 10% heat-inactivated defined fetal calf serum. All cells were maintained at 37°C in a humidified atmosphere of 5% CO2.
Generation And Expression Of Recombinant Anti-LMP1 CARs
The LMP1-scFv-CH2CH3-CD28-CD134-CD3ζ (HELA/134CAR) and LMP1-scFv-CH2CH3-CD28-CD137-CD3ζ (HELA/137CAR) cells were generated as follows. The DNA coding for the endodomains of CD134 (aa 241-277) and CD137 (aa 214-255) was synthesized and subcloned into the HELA/CAR vector between the CD28 and CD3ζ sequences by GenScript.11,12 Lentiviral transduction of T cells with recombinant receptors was described in detail previously, and receptor expression was monitored by flow cytometric analyses.8,13 Peripheral blood mononuclear cells (PBMCs) derived from healthy donors were collected and processed by Ficoll-Hypaque density-gradient centrifugation in Jiangsu Blood Center. Written informed consent was obtained from all the participants enrolled this study and the whole protocol was approved by the Ethics Committee of Nanjing Medical University.
Lentivirus Production And Transduction Of T Cells
To produce lentiviral supernatant, Lenti-XTM 293 T cells (Clontech, USA) were co-transfected with HELA/134CAR or HELA/137CAR vector, plasmid psPAX2 encoding the sequence for lentiviral envelope expression, and plasmid pMD2.G containing the sequence for VSV-G using 293fectionTM transfection reagent (Life technologies, USA). Supernatants containing the lentivirus were collected 48 and 72 hrs later and concentrated 60-fold by ultracentrifugation (Amicon Ultra 100 kD, Millipore, USA).
Anti-CD3/CD28 activated T cells were transduced with lentiviral vectors as previously described.14 Briefly, peripheral blood mononuclear cells were isolated via discontinuous density-gradient centrifugation on diluted Ficoll-Paque PLUS (GE Healthcare Life Sciences, USA). Peripheral blood mononuclear cells (1×106 per well) in a non-tissue culture-treated 24-well plate (BD Biosciences, USA) were coated with CD3 (eBioscience, USA) and CD28 monoclonal antibodies (eBioscience, USA) at a final concentration of 1 μg/mL. On day 2, cells were harvested for lentiviral transduction. For transduction, we precoated another non-tissue culture-treated 24-well plate with 0.5 mL RetroNectin (20 μg/mL) in PBS for 2 hrs at room temperature (Takara, JP). Wells were washed with HBSS (Sigma, USA) and incubated for 2 hrs at 32°C with lentivirus. Subsequently, 5×105 T cells per well were transduced with lentivirus in the presence of 100 units/mL hIL-2 (eBioscience, USA). After 48 to 72 hrs, cells were removed and expanded with 50 units/mL of hIL-2 for 10 to 15 days before use. T cells transduced with HELA/CAR, HELA/134CAR, HELA/137CAR, or control lentivirus were used as HELA/CART, HELA/134CART, HELA/137CART, and Mock cells. T cells without infection were employed as control cells.
Flow Cytometry
We used a FACS Calibur instrument (BD) and FlowJo software for all flow cytometric analyses (>10,000 events). In all cases, negative controls included isotype antibodies. Cells were washed once with PBS before the addition of antibodies. After 30 mins of incubation at 4°C in the dark, the cells were washed once and resuspended in FACS buffer (eBioscience, USA) before analysis.
T cells were analyzed with anti-CD8 PE, anti-CD4 APC, and anti-CD3 PerCP (eBioscience, USA), and tumor cell lines were analyzed with anti-LMP1 F(ab)2 and anti-Fab FITC (Jackson ImmunoResearch, USA). CAR vectors also coded ZsGreen fluorescent protein, which can be detected by flow cytometry. The percentage of T cells positive for ZsGreen fluorescent protein indicated the lentivirus transfection efficiency. The surface expression of CAR was confirmed using APC anti-human IgG Fc antibody (BioLegend) and R-PE-protein L to detect scFv expression of CAR (Celltechgen technology) as previously described.15
Cytotoxicity Assays
A slightly modified version of a previously published flow cytometry cytotoxicity assay was used.15 In this assay, NPC cell lines (SUNE1-LMP1 and SUNE1) or B lymphoma cell lines (C1R-neo and Ramos) were labeled at 1×106 cells/mL with 5 μM Cell Proliferation Dye eFluor® 670 (670) in PBS (eBioscience, USA). The cells were mixed and incubated at 37°C for 30 mins, then washed and suspended in cytotoxicity medium (RMPI1640 + 3% bovine serum albumin [BSA]). CAR T cells were added at the indicated E:T (effector:target) ratios. Four hours after co-incubation, 50 μg/mL propidium iodide (PI; Sigma) was added to stain for dead cells, and the cells were immediately analyzed by flow cytometry. Internal controls included single color-stained target cells and PI and double-stained targets in the absence of transduced T cells to determine the percentage of target cells undergoing spontaneous lysis. The percentages of labeled target cells that were PI+ vs PI- were determined for each condition. Dead target cells were defined as double positive for 670 and PI. Gated 670 cells were analyzed for the presence of PI. The percentage of specific tumor cell lysis was calculated using the following equation: (PI+ cells/total number of 670+ cells) ×100%, corrected for the number of spontaneously lysed targets.
Detection Of IFN-γ, IL-2, And Cell Proliferation
Lymphoma C1R-neo cells were washed and suspended in T cell culture medium at 1 × 106 cells/mL without IL-2. We then plated 1×105 target cells in a 96-well round bottom plate. In the absence of IL-2, effector cells and target cells were mixed at a ratio of 1:1 in T cell culture medium at a concentration of 1×106 cells/mL, and the assay was performed in triplicate. The plate was incubated at 37°C for 24 hrs. The concentrations of IFN-γ and IL-2 in supernatant were determined by an ELISA kit according to the manufacturer’s instructions (eBioscience). The number of CART or control T cells were determined directly by counting manually using hemocytometer every day, and then the multiple cell proliferation was calculated.
Xenograft Model Construction And Treatment
BALB/c nude mice were purchased from Model Animal Research Center of Nanjing University and kept in SPF conditions at Huadong Medical Institute of Biotechniques. All the animal experiments were performed under protocols approved by the Ethics Committee for Animal Research in Nanjing Medical University. For the SUNE1-LMP1 xenograft model, mice subcutaneously injected with 5×106 SUNE1-LMP1 cells were sacrificed after the tumor diameter was approximately 0.5 cm; the tumors were excised rapidly, cut aseptically into 1 mm3 pieces, and inoculated subcutaneously into the flanks of experimental mice. After inoculation, tumor sizes were measured every 10 days with Vernier calipers; volumes were calculated by the following formula: 1/2 × length × (width)2. Tumors were allowed to grow for 10 days, and the tumor burden reached approximately 100 mm3. The mice were randomly assigned to six different groups (N=6/group). The animals were injected with 4×106 T cells/100 μL on days 10, 20, and 30 intravenously through the tail.
Immunohistochemistry
Tumor sections were incubated with monoclonal rabbit anti-CD3ζ antibody (ab188850, Abcam) overnight at 4°C. After washing, the sections were incubated with biotin-labeled goat anti-rabbit antibody for 30 mins at room temperature and were subsequently incubated with streptavidin-conjugated horseradish peroxidase (HRP). Sections were colorized with 3,3-diaminobenzidine (DAB) chromogen solution and counterstained with hematoxylin. All sections were immunostained at the same time and under the same conditions. The results were examined by two investigators in a blinded manner, and a detailed protocol of IHC evaluation was described previously.16
Statistical Analysis
The data are reported as the means±SEM. Statistical analysis was performed by the use of unpaired Student’s t-test (tumor volume). Student’s t-test was used to evaluate differences in cytokine secretion and specific cytolysis. GraphPad Prism 5.0 (GraphPad Software) was used for the statistical calculations. p<0.05 was considered significant.
Results
Efficient Generation Of CAR T Cells Using Lentiviral Gene Transfer
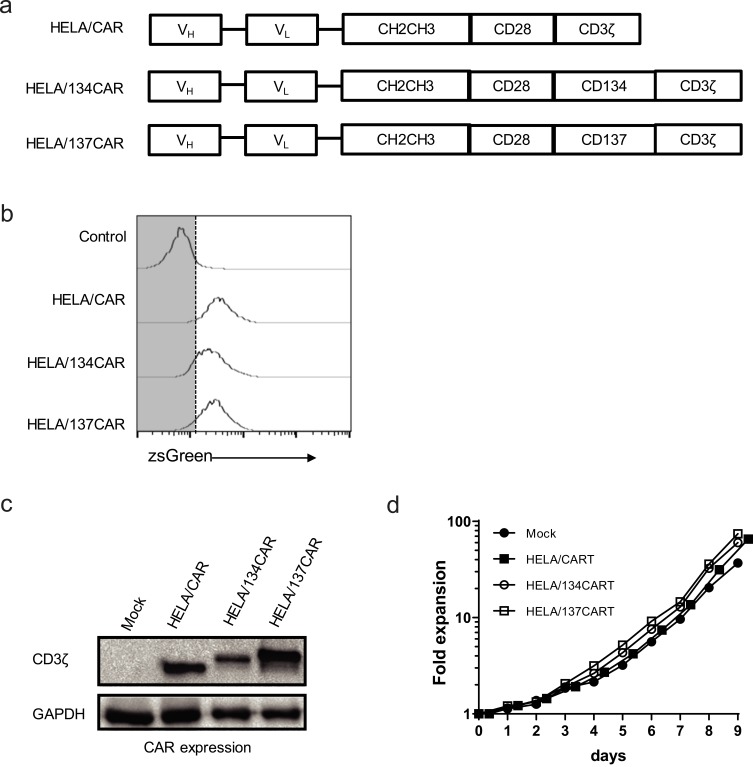
Based on the HELA/CAR structure, the intracellular regions of CD134 and CD137 were inserted between CD28 and CD3ζ of HELA/CAR T cells to construct 3rd-generation HELA/134CAR T cells and HELA/137CAR T cells targeting LMP1 (Figure 1A).
Figure 1.
Lentiviral gene transfer combined with αCD3/αCD28 antibody-mediated activation of T cells permits the generation of large numbers of αLMP1-specific chimeric antigen receptor (CAR+) T cells. (A) Schematic diagram showing the αLMP1-specific CAR used in this study. (B) αLMP1-specific CAR surface expression in primary human CD3+ T cells by detecting ZsGreen expression. Expression was examined 8 days after transduction with the indicated CAR-encoding lentiviral vector at an MOI of ~50. (C) CD3ζ expression analyzed by Western blot analysis of αLMP1-specific CART cells. (D) In vitro expansion of CD3+ T cells following activation with αCD3/αCD28 antibodies and transduction of the indicated CAR on day 2. Data are representative of 3–5 independent experiments, PBMC isolated from different individuals.
By using lentiviral vectors and transductions at a multiplicity of infection of 50, the CARs which could be expressed over 85% in primary T cells were measured by flow cytometric analysis of ZsGreen, human IgFc or scFv expression (Figure 1B and Supplementary Figure S1A). The expression of anti-LMP1 CAR was also detected by Western blot analysis (Figure 1C). There was over 50-fold expansion of CAR+ T cells, which could be achieved after transduction and growth in ~10 days (Figure 1D). The expression levels of the CAR in primary lymphocytes were stable for at least 6 weeks after transduction (Supplementary Figure S1B).
HELA/134CAR T cells (HELA/134CART) and HELA/137CAR T cells (HELA/137CART) are effective in killing LMP1-positive tumor cells.
There were significant differences in LMP1 expression in different lymphoma cells and NPC cell lines (Supplementary Figure S2). In this study, the LMP1-positive NPC cell line SUNE1-LMP1, the LMP1-positive lymphoma cell line C1R-neo, the LMP1-negative NPC cell line SUNE1, and the LMP1-negative lymphoma cell line Ramos were selected as target cells.
To enhance the functionality of the anti-LMP1 CART cells, we introduced the signal transduction domains of CD134 or CD137 in the HELA/CAR (Figure 1A). In the results of the SUNE1-LMP1 cytotoxicity assays, HELA/CART, HELA/134CART, and HELA/137CART were able to more effectively lyse SUNE1-LMP1 cells than were control T cells at target cell ratios of 20:1, 10:1, 5:1, and 2:1. However, there was no significant difference in the killing rate of SUNE1-LMP1 between HELA/CART, HELA/134CART, and HELA/137CART (Figure 2A and B). CAR-triggered cytotoxicity is antigen-specific, with only negligible lysis of Ramos cells that lack the expression of the LMP1 (Figure 2C), but these CART cells were effective at killing C1R-neo cells (Figure 2C). Moreover, the cytotoxic activity of HELA/CAR co-cultured with SUNE1 cells is demonstrated in Supplementary Figure S3.
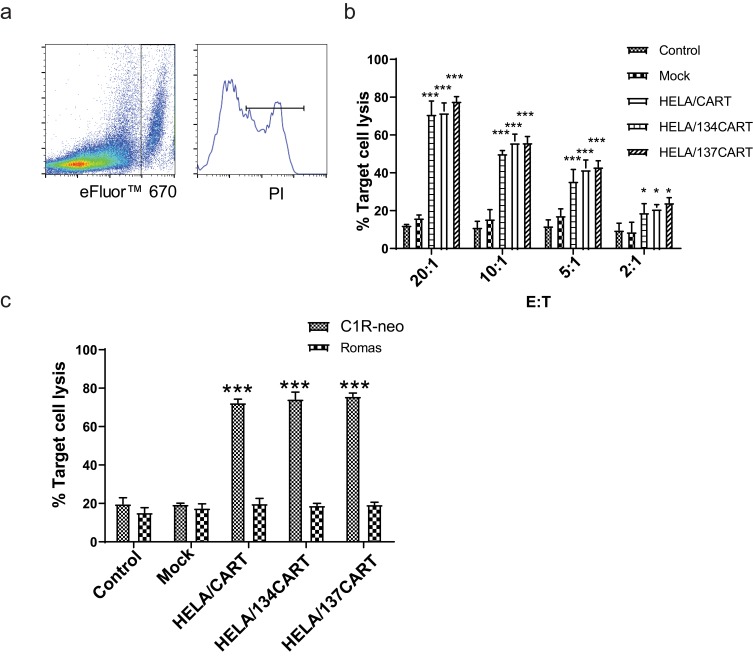
Figure 2.
Cytotoxic activity of CART cells co-cultured with SUNE1-LMP1 and C1R-neo cells. On day 10, transduced cells were transferred into IL-2 free medium for 24 hrs, the HELA/CAR T cells were incubated with SUNE1-LMP1 or C1R-neo cells at the indicated E:T ratio for 4 hrs, and killing of target cells was quantified using a FACS-based cytotoxicity assay. Mean values ±SEM calculated from three independent experiments are shown. (A) Representative images of FACS-based cytotoxicity assay. (B) SUNE1 cells that express LMP1 (SUNE-LMP1) were used as target cells for the cytotoxicity assay. Effector cells were added at the ratio indicated. The killing rates of HELA/CART, HELA/134CART, and HELA/137CART cells to SUNE1-LMP1 cells were significantly higher than that of control T or mock cells, but cytotoxicity of the three CART cells is similar. (C) C1R-neo and Ramos cells were used as target cells for the cytotoxicity assay. All of CART cells showed superior killing effectiveness to C1R-neo cell line compared with controls. In comparison, these CART cells rarely exerted killing role on Ramos cells. *p<0.05; ***p<0.001.
The Effects Of The Costimulatory Domain On The Cytokine Secretion Of CAR-Driven T Cells
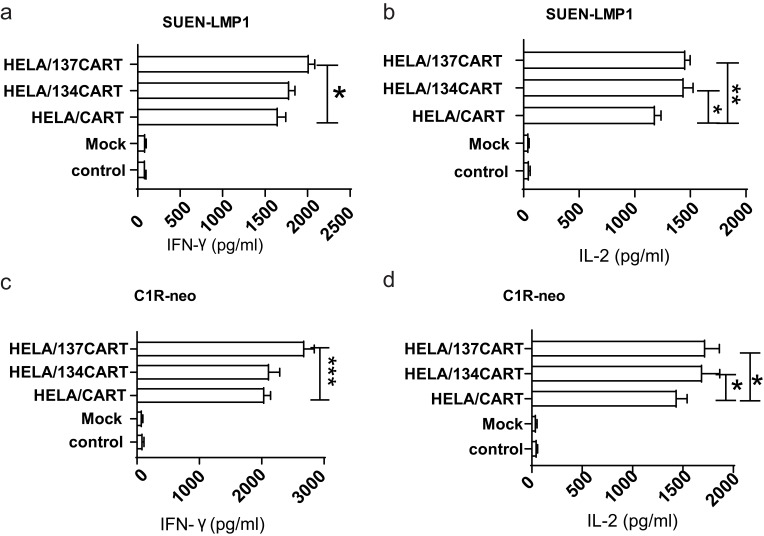
Following CAR activation with SUNE-LMP1 cells, T cells expressing CARs produced abundant quantities of interferon-γ (IFN-γ) and interleukin-2 (IL-2) compared with mock and control T cells (Figure 3). In addition, HELA/137CART cells produced larger quantities of IFN-γ (2022 pg/mL vs 1656 pg/mL, p < 0.05) but not HELA/134CART cells (1790 pg/mL vs 1656 pg/mL, p = 0.45) compared with HELA/CART cell (Figure 3A). In comparison, both HELA/134CART and HELA/137CART cells secreted increasing IL-2 (1445 pg/mL vs 1187 pg/mL, p < 0.05 and 460 pg/mL vs 1187 pg/mL, p < 0.05) (Figure 3B) comparing to HELA/CART cells, but there was no significant difference between HELA/134CART and HELA/137CART cells in IL-2 secretion (p > 0.05). When CARs were stimulated with LMP1-negative SUNE cells, all of the CART cells and mock or control T cells secreted similar concentrations of IFN-γ and IL-2 (Supplementary Figure S4A and B). When these CART cells incubated with LMP1-positive B-cell lymphoblastoid cell line CIR-neo and LCL, IFN-γ and IL-2 expression were similar when CART cells incubated with SUNE-LMP1 cell line (Figure 3C and D and Supplementary Figure S4C and S4D). There was no visible difference of IFN-γ and IL-2 expression when HELA/CART cell co-cultured with LMP1-negative cells compared with the control groups (Supplementary Figure S4A and B). These findings confirm that the addition of costimulatory domains into CARs could modulate cytokine secretion.
Figure 3.
CART cells produced IFN-γ and IL-2. (A and C) HELA/CAR T cells released significant amounts of IFN-γ when co-cultured with SUNE1-LMP1 and C1R-neo cells. The results are expressed as the mean±SEM and reflect data from three biological replicates. The x-axis indicates the concentration of IFN-γ. (B and D) HELA/CAR T cells released significant amounts of IL-2 when co-cultured with SUNE1-LMP1 cells and C1R-neo cells. The results are expressed as the mean±SEM and reflect data from three biological replicates. The x-axis indicates the concentration of IL-2. *p<0.05; **p<0.01, ***p<0.001.
HELA/137CART Inhibits Tumor Growth More Effectively Than HELA/CART In Vivo
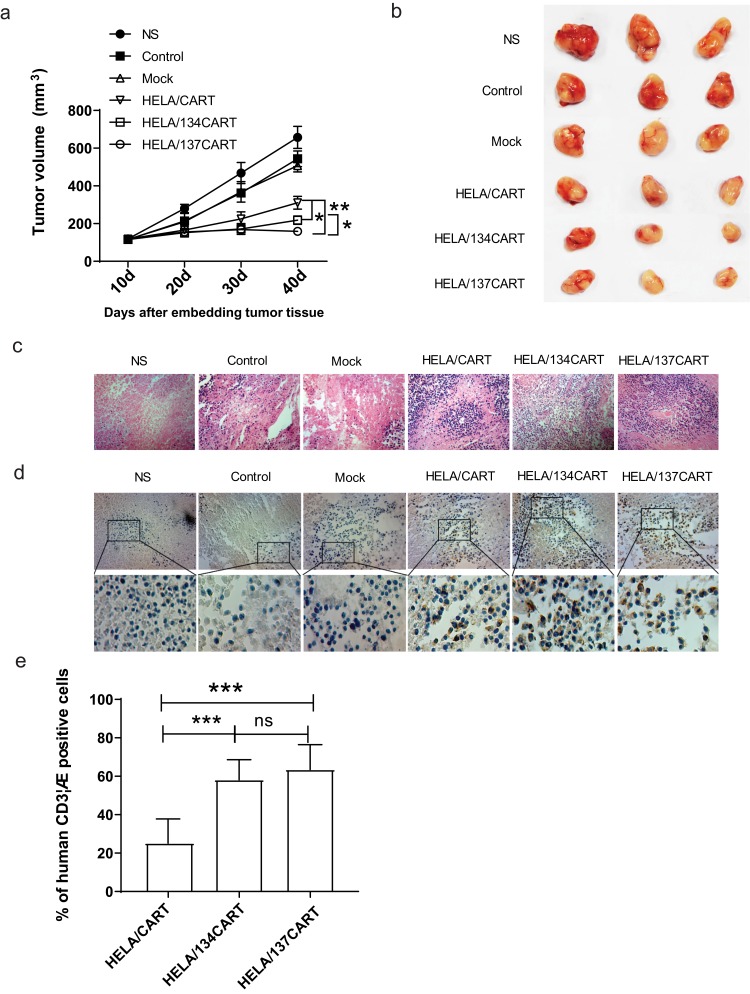
CART cells targeting LMP1 were able to effectively inhibit the growth of SUNE1-LMP1 subcutaneously in nude mice. Forty days after the tumor cells were inoculated subcutaneously, the HELA/137CART group was able to inhibit tumor growth more effectively than the HELA/CART and HELA/134CART groups, while the HELA/134CART group performed better than the HELA/CART group (Figure 4A and B). On day 44, all mice were sacrificed. The subcutaneous tumors were paraffin-embedded and stained with H&E. The results showed that compared with the control group and the control T cells group, the tumor tissue necrosis area of the HELA/CART, HELA/134CART, and HELA/137CART groups was larger, and more lymphocyte infiltration was detected in the necrotic tumor tissue. The control group also showed tumor tissue necrosis in some areas, but the tissue necrosis range was small, and there was no lymphocyte infiltration (Figure 4C). Anti-human CD3ζ antibody was further used to detect lymphocytes infiltrating into the tumor by immunohistochemistry. IHC results showed that HELA/CART, HELA/134CART, and HELA/137CART could migrate to tumor tissue, which implies that CART cells were able to infiltrate and kill LMP1-positive tumor cells (Figure 4D). ImageJ analysis showed that the expression of CD3ζ in HELA/134CART and HELA/137CART was significantly higher than in HELA/CART. In comparison, no difference of CD3ζ expression was noticed between HELA/137CART and HELA/134CART (Figure 4E).
Figure 4.
Antitumor activity of HELA/CART cells in a xenograft model in vivo. (A) BALB/c nude mice were inoculated with SUNE1-LMP1 cells overexpressing LMP1 and allowed to develop established tumors over 10 days. The results are expressed as the mean tumor volume (mm3) (mean±SD) for the six groups (N=6/group). The HELA/137CART group was able to inhibit tumor growth more effectively than the HELA/CART and HELA/134CART groups. *p<0.05; **p<0.01. (B) Images of representative tumor samples from sacrificed mice are shown. (C) The tumor samples were paraffin-embedded and stained with H&E. The tumor tissue necrosis area of the HELA/CART, HELA/134CART, and HELA/137CART treatment groups was larger and showed more lymphocyte infiltration, compared with control groups. (D) Immunohistochemical (IHC) staining for anti-human CD3ζ was performed on tumor samples. HELA/134CART and HELA/137CART cells were more likely to infiltrate into tumors than HELA/CART cells. (E) The percent of human CD3ζ-positive cells in tumor tissue. The ratios of HELA/134CART and HELA/137CART groups are high than HELA/CART group. ns, no significant difference; ***p<0.001.
Discussion
CART cells can bind to the surface of targeted tumor cells with high affinity and specificity and perform the cytotoxic activities of T cells in a non-MHC-restricted manner. Human T lymphocytes modified with CARs can recognize tumor-associated antigens and have been acknowledged as a revolutionary method for cancer immunotherapy. Although anti-CD19 CART has recently achieved impressive clinical breakthrough in patients with CD19-positive hematologic malignancies,17,18 the results of CART cell treatment in solid tumors have been unsatisfactory.
One limitation of CART cell therapy is that TAAs (tumor-associate antigens) are not specific to tumors but are also expressed by normal cells, leading to on-target, off-tumor toxicity. Since LMP1 is specifically expressed on EBV-malignant tumors, LMP1-targeted CART therapy can theoretically achieve promising treatment outcomes for LMP1-positive tumors.19 In addition, previous studies have characterized the incorporation of CD137/CD134 domains in CART therapy, which could dramatically enhance the therapeutic effectiveness.9,20,21 Previously, we have demonstrated that a HELA/CAR T cell caused specific cell lysis in LMP1-positive NPC. In this study, we created two novel HELA/137CART and HELA/134CART by adding CD134 or CD137 co-stimulatory domains on the basis of the previous HELA/CART. Although there was no significant difference in the killing rate of LMP1-positive tumor cells between HELA/CART, HELA/134CART, and HELA/137CART, the production of IFN-γ and IL-2 was significantly higher in HELA/134CART and HELA/137CART than in HELA/CART, implying the critical potential of tumor-inhibitory activity of HELA/134CART and HELA/137CART. As expected, HELA/134CART and HELA/137CART showed better tumor-inhibitory effect in xenograft tumors than HELA/CART. Furthermore, the tumor volume of HELA/137CART group is smaller than HELA/134CART group. Several reports showed that chimeric receptors with CD134 or CD137 exerted more powerful and specific cytotoxicity against tumor cells.21–23 However, few reports compared the effect of CD134 and CD137 tandem with CD28. CD134 and CD137 are two important members of the tumor necrosis factor (TNF)-nerve growth factor (NGF) receptor family, which have been acknowledged as critical costimulatory molecules for cellular activation.24 For example, CD137 was substantial for T-cell persistence and expansion.25,26 Nevertheless, CD134 and CD28 CART cell could induce activation-induced cell death (AICD) and reduce antitumor efficiency in vivo in spite of producing higher INF-γ9.
Another obstacle of CART therapy is short duration time. To exert the activity of tumor eradication, the survival time of CART cells is required to be at least one week.27 Unfortunately, CART cells are often eliminated before full action.28,29 It has been reported that CD137 plays an important role in T cells proliferation and survival through the activation of the AKT target of rapamycin pathway and the upregulation of the anti-apoptotic genes.30 Kawalekar et al also showed the pivotal role of CD137-related signaling in inducing serial metabolic regulation, including the elevation of respiratory capacity, fatty acid oxidation, and mitochondrial biogenesis, leading to the enhancement of T cell differentiation and survival.31 In our study, the IHC result demonstrated that HELA/137CART and HELA/134CART could still be significantly detected in xenograft tumors after 44 days, unlike HELA/CAR. Our results are in agreement with the previous findings and highlight the crucial implications of HELA/137CART for immunosurveillance and tumor eradication.
Moreover, previous studies generally used retrovirus or electroporation to introduce the chimeric receptor.32 In this study, we employed lentiviral gene transfer, which permits highly efficient generation of CART cells, with >70% successful gene transfer. We also used retroviral vectors to express HELA/CAR, but these exhibited significant silencing of CAR expression along with cell expansion in vitro (data not shown).
To sum up, the generation of HELA/137CART exhibits LMP1-specific tumor-inhibitory effectiveness and produces more IFN-γ and IL-2 than HELA/CART cells in response to LMP1 antigen. Our findings underline a novel and promising strategy for immunotherapy for LMP1-positive tumors.
Acknowledgements
This work is supported by the grants from the National Natural Science Foundation of China (No. 81773268); the Innovation team and key open project of Networking and Collaboration Centre for Cancer Personalized Medical Research of Nanjing Medical University (JX21817902/005); the Young Scientist Project of Jiangsu Provincial Commission of Health and Family Planning (No. QNRC2016535); the Natural Science Foundation of Jiangsu Province (BK20181489).
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Ethical Statement
The study was approved by the Ethics Committee for Animal Research in Nanjing University Medical School (IRB number: 20120607).
Author Contributions
ZQF designed the study; XJT, QT and YM performed the in vitro experiments; XJT, XCH and LZJ performed the in vivo experiments; XJT, QT and JZ analyzed the data; XJT and YM drafted the manuscript; ZQF and JZ supervised the study. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Dr Xiaochen Huang reports grants from Jiangsu cancer Hospital, during the conduct of the study. The authors declare that they have no other competing interests in this work.
References
- 1.Taylor GS, Long HM, Brooks JM, Rickinson AB, Hislop AD. The immunology of Epstein-Barr virus-induced disease. Annu Rev Immunol. 2015;33:787–821. [DOI] [PubMed] [Google Scholar]
- 2.Doubrovina E, Oflaz-Sozmen B, Prockop SE, et al. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood. 2012;119(11):2644–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heslop HE, Slobod KS, Pule MA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115(5):925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang LW, Jiang S, Gewurz BE. Epstein-Barr Virus LMP1-mediated oncogenicity. J Virol. 2017;91:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kieser A, Sterz KR. The Latent Membrane Protein 1 (LMP1). Curr Top Microbiol Immunol. 2015;391:119–149. [DOI] [PubMed] [Google Scholar]
- 6.Tsao SW, Tsang CM, To KF, Lo KW. The role of Epstein-Barr virus in epithelial malignancies. J Pathol. 2015;235(2):323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsao SW, Tramoutanis G, Dawson CW, Lo AK, Huang DP. The significance of LMP1 expression in nasopharyngeal carcinoma. Semin Cancer Biol. 2002;12(6):473–487. [DOI] [PubMed] [Google Scholar]
- 8.Milone MC, Fish JD, Carpenito C, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17(8):1453–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hombach AA, Rappl G, Abken H. Arming cytokine-induced killer cells with chimeric antigen receptors: CD28 outperforms combined CD28-OX40 “super-stimulation”. Mol Ther. 2013;21(12):2268–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang X, Zhou Y, Li W, et al. T cells expressing a LMP1-specific chimeric antigen receptor mediate antitumor effects against LMP1-positive nasopharyngeal carcinoma cells in vitro and in vivo. J Biomed Res. 2014;28(6):468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pule MA, Straathof KC, Dotti G, Heslop HE, Rooney CM, Brenner MK. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005;12(5):933–941. [DOI] [PubMed] [Google Scholar]
- 12.Wilkie S, Picco G, Foster J, et al. Retargeting of human T cells to tumor-associated MUC1: the evolution of a chimeric antigen receptor. J Immunol. 2008;180(7):4901–4909. [DOI] [PubMed] [Google Scholar]
- 13.Song DG, Ye Q, Poussin M, Harms GM, Figini M, Powell DJ Jr. CD27 costimulation augments the survival and antitumor activity of redirected human T cells in vivo. Blood. 2012;119(3):696–706. [DOI] [PubMed] [Google Scholar]
- 14.Lee HJ, Lee YS, Kim HS, et al. Retronectin enhances lentivirus-mediated gene delivery into hematopoietic progenitor cells. Biologicals. 2009;37(4):203–209. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg JE, Sherwood SW, Clayberger C. A novel method for measuring CTL and NK cell-mediated cytotoxicity using annexin V and two-color flow cytometry. J Immunol Methods. 1999;224(1–2):1–9. [DOI] [PubMed] [Google Scholar]
- 16.Huang J, Zhu H, Wang X, et al. The patterns and expression of KDR in normal tissues of human internal organs. J Mol Histol. 2011;42(6):597–603. [DOI] [PubMed] [Google Scholar]
- 17.Rotolo A, Karadimitris A, Ruella M. Building upon the success of CART19: chimeric antigen receptor T cells for hematologic malignancies. Leuk Lymphoma. 2017;1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer J, Paret C, El Malki K, et al. CD19 isoforms enabling resistance to CART-19 immunotherapy are expressed in B-ALL patients at initial diagnosis. J Immunother. 2017;40(5):187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen R, Zhang D, Mao Y, et al. A human Fab-based immunoconjugate specific for the LMP1 extracellular domain inhibits nasopharyngeal carcinoma growth in vitro and in vivo. Mol Cancer Ther. 2012;11(3):594–603. [DOI] [PubMed] [Google Scholar]
- 20.Tammana S, Huang X, Wong M, et al. 4-1BB and CD28 signaling plays a synergistic role in redirecting umbilical cord blood T cells against B-cell malignancies. Hum Gene Ther. 2010;21(1):75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imai C, Mihara K, Andreansky M, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18(4):676–684. [DOI] [PubMed] [Google Scholar]
- 22.Zhong XS, Matsushita M, Plotkin J, Riviere I, Sadelain M. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol Ther. 2010;18(2):413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hombach AA, Abken H. Costimulation by chimeric antigen receptors revisited the T cell antitumor response benefits from combined CD28-OX40 signalling. Int J Cancer. 2011;129(12):2935–2944. [DOI] [PubMed] [Google Scholar]
- 24.Arch RH, Thompson CB. 4-1BB and Ox40 are members of a tumor necrosis factor (TNF)-nerve growth factor receptor subfamily that bind TNF receptor-associated factors and activate nuclear factor kappaB. Mol Cell Biol. 1998;18(1):558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carpenito C, Milone MC, Hassan R, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009;106(9):3360–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song DG, Ye Q, Carpenito C, et al. In vivo persistence, tumor localization, and antitumor activity of CAR-engineered T cells is enhanced by costimulatory signaling through CD137 (4-1BB). Cancer Res. 2011;71(13):4617–4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gade TP, Hassen W, Santos E, et al. Targeted elimination of prostate cancer by genetically directed human T lymphocytes. Cancer Res. 2005;65(19):9080–9088. [DOI] [PubMed] [Google Scholar]
- 28.Cartellieri M, Bachmann M, Feldmann A, et al. Chimeric antigen receptor-engineered T cells for immunotherapy of cancer. J Biomed Biotechnol. 2010;2010:956304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamers CH, Willemsen R, van Elzakker P, et al. Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood. 2011;117(1):72–82. [DOI] [PubMed] [Google Scholar]
- 30.Starck L, Scholz C, Dorken B, Daniel PT. Costimulation by CD137/4-1BB inhibits T cell apoptosis and induces Bcl-xL and c-FLIP(short) via phosphatidylinositol 3-kinase and AKT/protein kinase B. Eur J Immunol. 2005;35(4):1257–1266. [DOI] [PubMed] [Google Scholar]
- 31.Kawalekar OU, O’Connor RS, Fraietta JA, et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity. 2016;44(2):380–390. [DOI] [PubMed] [Google Scholar]
- 32.Park JR, Digiusto DL, Slovak M, et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 2007;15(4):825–833. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.