Abstract
This systematic review and meta-analysis investigate the association between periodontitis and stroke. This review followed the methods established by the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines. Searches were conducted in five databases and two sources of grey literature. After the selection of the articles, a risk of bias evaluation was performed. Three meta-analyzes were performed: Assessing the overall association between stroke and periodontitis in case–control studies; Ischemic stroke and periodontitis in case–control studies; The association between stroke and periodontitis in cohort studies. Heterogeneity was assessed using the I2 index and the odds ratio was also calculated (p < 0.05). The Grading of Recommendations Assessment, Development and Evaluation (GRADE) was applied to evaluate the level of evidence. 2193 potentially relevant studies were identified, with 10 studies included in qualitative and quantitative analysis. All the articles were considered with low risk of bias and a low level of certainty. The results demonstrated a positive association between both disorders and increased risk for stroke among cohort studies (RR 1.88 [1.55, 2.29], p<0.00001, I2=0%) and for ischemic stroke events in case–control studies (RR 2.72 [2.00, 3.71], p<0.00001, I2= 4%). Periodontitis may represent a risk factor for stroke, especially in ischemic events. However, new studies with a robust design are necessary for a reliable conclusion.
Keywords: central nervous system, cerebrovascular disorders, stroke, periodontitis, periodontal attachment loss
Introduction
This cerebrovascular damage is characterized by brain vessels occlusion or blockage (ischemic stroke), as well as blood vessels rupture (hemorrhagic stroke).1 Based on the same mechanism of the ischemic stroke with a different period of occurrence, the transient ischemic attack is another type of cerebrovascular accident, with less than 24 hrs of duration.2 Moreover, other classifications or subtypes of these changes are described in the literature, such as subarachnoid hemorrhage, cerebral venous thrombosis, and spinal cord stroke.3 Although these events have different origins and pathogenesis, they all culminate in vascular changes that present themselves as one of the main death cause in the world.2,3 In this context, the presence of inflammatory oral diseases may also act as source of these vascular changes in a stroke condition.4
Among oral infections, periodontal diseases are considered as a huge variety of inflammatory conditions that affect the supporting structures of the teeth such as the gingiva, alveolar bone and periodontal ligament,5–8 which could result in tooth loss and contribute to systemic inflammation.8 Periodontitis, a type of periodontal disease, is defined as a chronic inflammatory disease of multifactorial origin, being related to plaques (biofilm),8 initiated by gram-negative bacteria that initiate an immune-inflammatory response of the host.9,10 It is characterized by the progressive destruction of the dental apparatus, causing the loss of the tissue support periodontal, with this leading to loss of clinical insertion (CAL) and alveolar bone loss, which can be evaluated through radiographic images, besides the presence of periodontal pockets and gingival bleeding.8
Periodontitis is associated with elevated levels of IL-6, C-Reactive Protein and TNF alpha in blood flow associated with systemic inflammation markers.11,12 The current evidence also shows that elevated levels of inflammatory markers are related to systemic diseases, such as Rheumatoid Arthritis,13 cardiovascular diseases,14 as well as to severe neurological deficits as dementia,15 Alzheimer’s Disease.16
The association between periodontal disease and stroke has been previously investigated in systematic reviews.17–19 However, the interaction among different types of cerebrovascular accidents has not been synthetized in a review capable to present the relevant clinical evidence. The aim of this systematic review and meta-analysis was to investigate the association between stroke and periodontitis in humans, considering the different types of cerebrovascular events.
Materials And Methods
Protocol And Registration
This systematic review was registered at PROSPERO under the code CRD42016033183 and performed according to PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) guidelines.20
Search Strategy, Eligibility Criteria And Data Extraction
The PECO strategy was followed in this systematic review. Observational studies in humans (P) exposed (E) and not exposed to periodontitis (C), in which the primary outcome was the risk of cerebrovascular accident, including hemorrhagic and ischemic attacks (O) (Supplementary material 1, Supplementary material 2). As an ischemic attack, the events of transient ischemic attack and ischemic stroke were considered in the evaluated studies.
Searches were conducted in the following electronic databases, without language restriction until september 2018: PubMed, Scopus, Web of Science, The Cochrane Library, LILACS, OpenGrey and Google Scholar. All publications presented in the databases contained a combination of controlled pre-defined MeSh and free terms related to cerebrovascular changes and periodontitis (Supplementary material 3). The terms previously defined were adapted to the rules of syntax of each bibliographic database.
After research in databases, all relevant citations were saved in a bibliographic reference manager (EndNote, x7 version, Thomson Reuters). Duplicated results were considered only once. After the importation to reference manager, the duplicates were removed, followed by exclusion of opinion articles, technical articles, guides and animal studies. The selection process was performed in two phases. The first phase includes the evaluation of titles and abstracts according to inclusion and exclusion criteria. In the phase two, the articles included in the first phase were evaluated according to full text by the same criteria. Additional citations were sought from the analysis of the reference list of all articles previously selected in phase two. The searches and selection process were conducted by three examiners (NCFF, KFBV and APCPSCA) and checked by a third examiner (RRL), in cases of disagreements.
After the selection, the data extraction was conducted from the included articles. A table was used to report the country, year of publication, study design, participant characteristics (source and sample size), age, periodontitis evaluation, results and statistical analysis.
In case of absence of information that makes data extraction or risk of bias evaluation impracticable, we attempted to contact the authors by e-mail. The contact consisted of sending a weekly email, for up to five consecutive weeks.
Quality Assessment Analysis
The papers that fulfilled the selection criteria were included for qualitative evaluation. The quality assessment of the included studies was made following a check-list established by Fowkes and Fulton21 and updated by Penoni et al22 (Supplementary material 4).
Risk Of Bias
After quality assessment evaluation, the studies were analyzed to determine the risk of bias, according to the possibility of “biased results,” “serious confounders,” and the “occurrence of chance”.21,22 Three questions were evaluated to determine the value of the study: “Are the results erroneously biased in a certain direction?” “Are there any serious confounding or other distorting influences?” and “Is it likely that the results occurred by chance?” If these 3 summary questions were answered with “no,” then there was a high probability that the research presented a low risk of bias.
Quantitative Synthesis Of The Results
The extracted data were analyzed using RevMan software (Review Manager v. 5.3, The Cochrane Collaboration; Copenhagen, Denmark) to assess the relationship between periodontal disease and stroke.
Three meta-analyses (MAs) were performed:
To evaluate the association between periodontitis and stroke in case–control studies, odds ratio (OR) and its 95% CI of the association between these two diseases (periodontitis and stroke) from included studies were extracted. Adjusted odds ratio was also used whenever possible; otherwise, crude odds ratio estimates were considered to measures effect as a log OR and the standard error of the log OR using generic inverse-variance weighting method. Combined results were presented as a pooled OR.
To evaluate the association between periodontitis and ischemic stroke in case–control studies, odds ratio (OR) and its 95% CI of the association between these two diseases (periodontitis and ischemic stroke) from included studies were extracted. Adjusted odds ratio was also used whenever possible; otherwise, crude odds ratio estimates were considered to measures effect as a log OR and the standard error of the log OR using generic inverse-variance weighting method. Combined results were presented as a pooled OR.
To evaluate the association between periodontitis and stroke in cohort studies, risk ratio (RR) and its 95% CI of the association between these two diseases (periodontitis and stroke) from included studies were extracted. Adjusted RR was also used whenever possible; otherwise, crude RR estimates were considered to measures effect as a log RR and the standard error of the log RR using generic inverse-variance weighting method. Combined results were presented as a pooled RR.
When necessary, the effect estimates were converted to OR or RR with the help of RevMan software tools.
If some of the necessary information was absent from any of the included studies, the authors were contacted (one contact by week, up to five weeks) to provide the missing data. After contacts, studies that remaining without sufficient data were excluded from the meta-analysis. Besides that, only studies that did not present methodological bias were included. Random effect model was applied when the studies were not functionally equivalent in which the objective was to generalize the results from the meta-analysis.23
Heterogeneity was tested using the I2 index and, if the heterogeneity was substantial or considerable (>50%), a sensitivity analyses were performed to verify the influence of each study on the pooled results.24
Level Of Evidence
The assessment of quality of the evidence was determined for the outcomes meta-analysis using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.25 Observational studies start as low evidence, and the quality of, or certainty in, the body of evidence decreases to very low quality if serious or very serious issues related to risk of bias, inconsistency, indirectness, imprecision and publication bias are present and increase if confounding factors and if the magnitude of effect is large or very large were detected. In this way, the quality of the evidence can range from very low, low, moderate and high.
Results
Study Selection
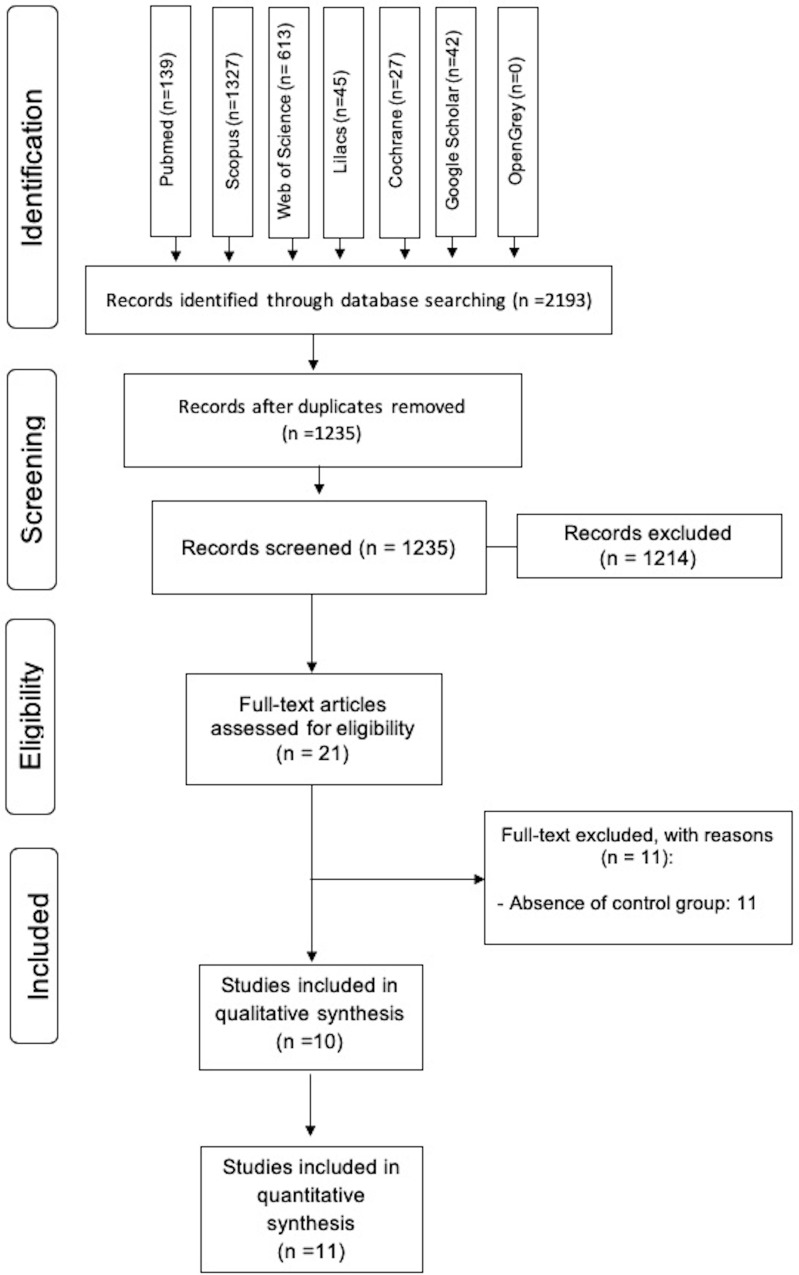
A total of 2193 articles was identified from the searches of databases, and 958 were excluded because they are duplicated. All 1235 titles and abstracts retrieved were analyzed according to the criteria of inclusion and exclusion, with an exclusion of 1214 studies. The remaining studies (n= 21) were evaluated by full-text. 11 studies were excluded in the full-text evaluation due to the absence of control group18,26–35 see supplementary material 5.
A total of ten studies was included and submitted to quality assessment (Figure 1).
Figure 1.
Flow diagram of literature searches and selection, according to the PRISMA statement.
Characteristics Of Included Studies
All the 10 articles included in this review were observational, 3 presented a prospective cohort design,36–38 while 7 were case-controlled studies.39–45 Only one study did not report a significant association between periodontitis and cerebrovascular changes.37 Among the studies, ischemic damages were the most evaluated, including ischemic stroke,38–40,43 transient ischemic attack,39 acute cerebral ischemia41 and lacunar infarct.45 Four studies investigated both hemorrhagic and non-hemorrhagic strokes.36,37,42,44
The Clinical Attachment Loss was the most used method of assessment for periodontitis identification36,39–42,44,45 (Table 1).
Table 1.
Summary Of Characteristics And Results Of The Included Studies
| Author, Year, Country; Study Design (Length Of Study In Years) | Participants | Age | Periodontitis Evaluation | Results | Statistical Analysis | |
|---|---|---|---|---|---|---|
| Source Of Sample | n | |||||
| (Holmlund et al, 201037); Sweden, cohort (12 years) | Department of Periodontology at Gavle County Hospital, Gavle, Sweden. | (7674)* | 51.7 ± 13.8 | Probing depth, periodontal severity index (PDSI) and number of deepened periodontal pockets (NDP). | No significant predictive power to future mortality after adjustment was observed for the periodontitis parameters. | Cox proportional hazard analyses |
| (Sen et al, 201838); United States, cohort (15 years) | ARIC (Atherosclerosis Risk in Communities) study, 4 communities in United States. | (15,792)* | 62.3± 5.6 | Periodontal profile classes (PPC): interproximal attachment level, probing depth, extent of bleeding on probing, gingival inflammation index, plaque index, presence/absence of full prosthetic crowns for each tooth, and tooth status presence. | 584 participants had incident ischemic strokes over a 15-year period. The 7 levels of PPC showed a trend toward an increased stroke risk (χ2 trend P<0.0001); the incidence rate for ischemic stroke/1000-person years was 1.29 for PPC-A (health), 2.82 for PPC-B, 4.80 for PPC-C, 3.81 for PPC-D, 3.50 for PPC-E, 4.78 for PPC-F, and 5.03 for PPC-G (severe periodontal disease). | Cox proportional hazard analyses |
| (Wu et al, 200036); United States, cohort (18 years) | First National Health and Nutrition Examination Survey, United States | (5434) 3634: no disease 1800: periodontitis | 25–74 years | CAL | Periodontitis is a significant risk factor for Cerebrovascular accidents and, in particular, nonhemorrhagic stroke ORs (95% CIs): 1.66 (1.15 to 2.39) | Cox proportional hazard model |
| (Abolfazli et al, 201141); Iran, c-c | General surgery ward and staff of Imam Hospital, Tabriz, Iran | (100)* | 53.3±13.01 | CAL | The more severe periodontitis (CAL≥6 mm) was observed among men in patients with cerebral ischemia (P=0.012). | Chi-squared test and Fisher’s test |
| (Diouf et al, 201544); Senegal, c-c | Fann National University Hospital, Senegal. | (220)* | Not informed | CAL | Periodontitis was significantly associated with stroke (OR= 1.58, 95% CI: 1.1–3.022; p< 0.001) | Logistic regression analysis |
| (Dörfer et al, 200439); Germany, c-c | Residents of the city of Heidelberg or the neighboring county, Germany. | (603)* | 18–75 years | CAL | Periodontitis is an independent risk factor for cerebral ischemia and acute exacerbation of inflammatory processes (p<0.001) ORs (95% CIs): 7.38 (1.55–15.03) | Logistic regression analysis |
| (Ghizoni et al, 201242); Brazil, c-c. | Neurosurgery Division of the Intensive Care Unit and Operative Dentistry Clinics of the Bauru School of Dentistry, University of São Paulo | (80)* | 30–80 years | Probing depth and CAL | Periodontal disease was specifically defined by the presence of at least one site showing a PPD≥4 mm, which resulted in 19 (95%) diseased patients in the test group and 17 (28.3%) patients in the control group, resulting in an unadjusted odds ratio of 48.06 (95% confidence interval:5.96 to 387.72, p<0.001). | chi-squared test, with a 95% confidence level. |
| (Leira et al, 201645); Spain, c-c. | Stroke Unit of the University Clinical Hospital of Santiago de Compostela, Spain. | (112)* | 68 (58–71) years | Probing depth and CAL | A strong association between Chronic periodontitis was reported (odds ratio 4.20; 95% confidence interval 1.81–10.20; p= 0.001). | Logistic regression analysis |
| (Palm et al, 201443); Germany, c-c. | Ludwigshafen, Germany. | (198)* | 68.2 ± 9.7 years | Questionnaires, evaluation of A. actinomycetemcomitans | The stroke group presented higher levels of A. Actinomycetemcomitans (p-0.003) | Wilcoxon signed-rank test |
| (Pradeep et al, 201040); India, c-c | Department of Neurology, Victoria Hospital and the National Institute of Mental Health and Neurosciences, Bangalore, India | (200)* | 33–68 years | CAL | The clinical attachment loss values of subjects with cerebrovascular accident (3.99 ± 1.21) were significantly higher when compared with those of the control group (3.18 ± 0.94, p<0.05) | Logistic regression analysis |
Note: *No information regarding number of participants with periodontitis and without periodontitis.
Abbreviations: c-c, case–control study; CAL, clinical attachment loss.
Risk Of Bias
After the quality and risk of bias assessments, it could be observed that all studies had methodological soundness with low susceptibility to bias (Table 2).
Table 2.
Risk Of Bias Assessment According To Fowkes And Fulton
| Guideline | Checklist | (Holmlund et al, 201037) | (Sen et al, 201838) | (Wu et al, 200036) | (Abolfazli et al, 201141) | (Diouf et al, 201544) | (Dörfer et al, 200439) | (Ghizoni et al, 201242) | (Leira et al, 201645) | (Palm et al, 201443) | (Pradeep et al, 201040) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study design appropriate to objectives? | Prevalence Cross-sectional | ||||||||||
| Prognosis Cohort | 0 | 0 | 0 | ||||||||
| Treatment Controled trial | |||||||||||
| Cause Cohort, case-control, cross-sectional | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Study sample representative? | Source of sample | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sampling method | 0 | 0 | 0 | + | 0 | 0 | + | + | 0 | + | |
| Sample size | 0 | 0 | 0 | + | 0 | + | + | + | 0 | + | |
| Entry criteria/exclusion | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Non-respondents | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Control group acceptable? | Definition of controls | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | + | 0 |
| Source of controls | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Matching/randomization | NA | NA | NA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Comparable characteristics | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Quality of measurements and outcomes? | Validity | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | + | 0 |
| Reproducibility | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Blindness | 0 | ++ | ++ | 0 | ++ | 0 | 0 | 0 | 0 | ++ | |
| Quality control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ++ | 0 | |
| Completeness | Compliance | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Drop outs | NA | NA | NA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Deaths | 0 | 0 | 0 | NA | NA | NA | NA | NA | NA | NA | |
| Missing data | NA | NA | NA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Distorting influences? | Extraneous treatments | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Contamination | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Changes over time | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Confounding factors | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Distortion reduced by analysis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Summary questions | Bias: Are the results erroneously biased in certain direction? | No | No | No | No | No | No | No | No | No | No |
| Confounding: Are there any serious confusing or other distorting influences? | No | No | No | No | No | No | No | No | No | No | |
| Chance: Is it likely that the results occurred by chance? | No | No | No | No | No | No | No | No | No | No |
However, some methodological limitations were identified among the included studies. Among the case–control studies included, problems were identified regarding the sampling method40–42,45 and sample size calculation.39,40,42,45,46 Moreover, four studies did not provide information regarding the blinding of the outcome evaluation by the accessors among the included studies.36,38,40,44
Quantitative Analysis
Considering the studies included in this systematic review, Abolfazili et al41 have two control groups and the result from both control groups were added and considered in these meta-analyses.
Dorfer et al44 and Holmlund et al37 presented OR and RR values from mild, moderate and severe periodontitis, respectively, while Sen et al38 presented RR values from mild and severe periodontitis. In these cases, a pooled OR and RR were calculated and employed in the first and second meta-analysis. Pooled RR was also calculated to Wu et al36 to obtain a pooled result including all stroke types (RR for cerebrovascular diseases not specified, RR for hemorrhagic stroke and RR for non-hemorrhagic stroke).
The results were presented separately for the three MA:
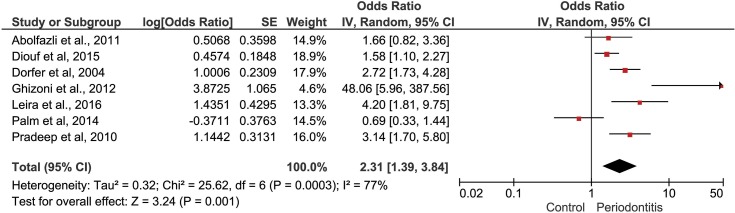
Stroke in case–control studies. In the first MA, seven studies were included.36,39–45 The overall heterogeneity was considerable (I2=77%). During sensitivity analysis, the heterogeneity ranges from 70% to 80% and, as no significant heterogeneity reduction was observed, none study was removed. The pooled results present data on the direction of association between periodontitis and stroke. Individuals with periodontitis had two times higher chance to suffer stroke (OR 2.31 [1.39, 3.84], p=0.001, I2= 77%) (Figure 2).
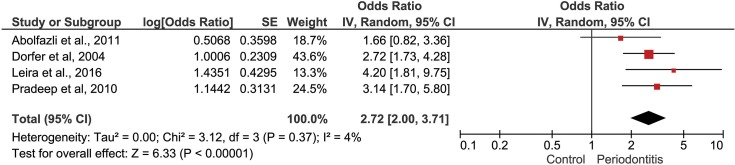
Ischemic stroke in case–control studies. In this second MA five studies were included.36,39–41,43,45 The overall heterogeneity was considerable (I2=72%). During sensitivity analysis, the heterogeneity ranges from 4% to 78% and, in attempt to reduce heterogeneity, Palm et al study was removed from this analysis. The pooled results present data on the direction of association between periodontitis and ischemic stroke. Individuals with periodontitis had two times higher chance to suffer ischemic stroke (OR 2.72 [2.00, 3.71], p<0.00001, I2= 4%) (Figure 3).
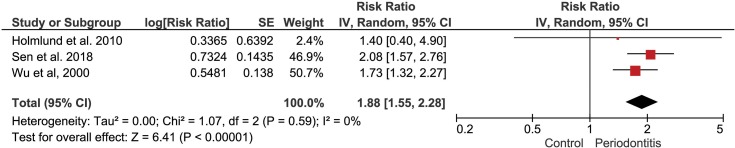
stroke in cohort studies. In this third MA, three studies were included36–38 and presented null heterogeneity (I2=0%). The pooled results present data on showed, again, association between periodontitis and stroke. Individuals with periodontitis had higher risk to suffer stroke (RR 1.88 [1.55, 2.28], p<0.00001) (Figure 4).
Figure 2.
Forest plot to CVA in case–control studies.
Figure 3.
Forest plot to Ischemic stroke in case–control studies.
Figure 4.
Forest plot to CVA in cohort studies.
Assessment Of The Level Of Evidence (GRADE)
Ten studies were used in the meta-analysis and were evaluated for the level of evidence.for the association between periodontitis and stroke in case–control studies, seven studies were included,36,39,40,42–45 although the large effect (OR> 2), this evidence was classified as low due to serious problems in “inconsistency” related to considerable overall heterogeneity. In the present study, it was found that the association between periodontitis and ischemic stroke in case–control studies39–41,43,45 the large effect (OR> 2), this evidence was classified as low due to serious problems in “imprecision” related to initial and final IC variation greater than 25%. In the evaluation of the Association between periodontitis and stroke in cohort studies, three studies were included.36,37 This evidence presented no serious problem and was qualified as low. Table 3 shows all the results found in the GRADE evaluation.
Table 3.
Evidence Profile: Association Between Periodontitis And Stroke
| Certainty Assessment | Summary Of Findings | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| № Studies | Risk Of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Overall certainty of evidence | Relative effect (95% CI) | Anticipated Absolute Effects | |
| Risk With Control | Risk Difference With Periodontitis | ||||||||
| Association between periodontitis and stroke in case–control studies | |||||||||
| 7 case-control studies | Not serious | Seriousa | Not serious | Not serious | Strong association | ⨁⨁◯◯ LOW | OR 2.31 (1.39 to 3.84) | Low | |
| 0 per 1.000 | 0 fewer per 1.000 (0 fewer to 0 fewer) | ||||||||
| Association between periodontitis and ischemic stroke in case–control studies | |||||||||
| 4 case-control studies | Not serious | Not serious | Not serious | Seriousb | Strong association | ⨁⨁◯◯ LOW | OR 2.72 (2.00 to 3.71) | Low | |
| 0 per 1.000 | 0 fewer per 1.000 (0 fewer to 0 fewer) | ||||||||
| Association between periodontitis and stroke in cohort studies | |||||||||
| 3 cohort studies | Not serious | Not serious | Not serious | Not serious | None | ⨁⨁◯◯ LOW | RR 1.88 (1.55 to 2.28) | 0 per 1.000 | 0 fewer per 1.000 (0 fewer to 0 fewer) |
Notes: aConsiderable heterogeneity (I2=70%). bInitial and final IC variation greater than 25%. Overall certainty of evidence: very low, low, moderate and high.
Abbreviations: CI, confidence interval; OR, odds ratio; RR, risk ratio.
Discussion
This systematic review has gathered scientific evidence based on the literature showing association between periodontitis and the various types of stroke. The results of the meta-analysis showed that individuals with periodontitis had a greater chance, about twice as much of suffering from some type of stroke, as to have ischemic stroke. Mixed results were observed between qualitative and quantitative analysis of the included studies. While most of the selected articles described an association between stroke and periodontitis in qualitative evaluation, this association was only detected among cohort studies and for ischemic stroke events in case–control studies. Even though a positive association was detected between both conditions in case–control studies, these results are questionable due to the high heterogeneity among the included studies.
Systematic reviews can summarize clinical questions by condensing much information on a critical evaluation of published studies, following a solid-stated method of development.24,47 When it is possible to perform them from randomized clinical trials, this type of study represents the best level of evidence available, even though this could be mutable according to the evaluated question.47 In a Systematic review, the performing of a meta-analysis can provide a quantitative evaluation of the problem investigated, comparing data of each study in a statistical analysis weighted in heterogeneity of studies evaluation.24
Besides the results, the quality of the identified articles in this review pointed absence of significant bias after the quality assessment evaluation (Table 2). Problems were observed in the methodological quality of some studies, mainly in the study sample, quality of measurements and blinding. In five articles, the sample size was marked as a problem due to the absence of a sample size calculation or lack of representativeness.39–42,45 The sampling method was pointed as problem in four studies.40–42,45 The blinding of the outcome was not performed in four of the included studies.36,38,40,44 The quality control was registered as a major problem in one study43 due to the absence of clinical assessment.
Even without compromising the major quality of the studies, these registered pitfalls must be avoided because they can induce to confounding effects on a trial.48 Due to its chronic and infectious origin, periodontitis may be associated as a source of mediators of inflammation into the blood circulation, which may contribute to exacerbate several diseases. This local inflammatory process may induce a systemic inflammatory state through mechanisms that include dissemination of pro-inflammatory cytokine and/or bacteria from the oral to extra-oral sites.49 In the present study, periodontitis was described as an independent risk factor of stroke. Ischemic or hemorrhagic events were both described in the selected studies, even though the ischemic events were more reported/investigated38–41,43,45 than both conditions.36,37,42,44
The presence of a wide spectrum inflammatory condition can increase the risk of a stroke episode in acute and chronic phases, in which inflammatory markers such as C‑Reactive protein, IL‑6 and lipoprotein‑associated phospholipase A2 were pointed as indicators of increase of stroke risk.31,50 Some of these factors, such as the IL-6 and the C-Reactive protein12,51 are also identified as biomarkers of periodontitis and are found on blood flow, suggesting a possible triggering of aggravation of stroke process.
Moreover, the presence of external inflammatory factors can also aggravate subsequent events that happen after the stroke.50 At this moment, an ischemic penumbral tissue surrounding the infarcted core is formed. A damaged tissue without electrical communication characterizes the penumbra area, but with sufficient cellular energy to its nutrition.52 However, due to this damage, this area is susceptible to secondary cell death, which occurs hours and days after the establishment of the primary center of the lesion.53 A systemic inflammation process was also associated with changes in microglia. It was suggested that this process could result on changes of phenotype in this tissue, resulting on the increase of neuroinflammation and neurodegeneration.54
The occurrence of cell death on the penumbra area can be enhanced by several factors, such as oxidative stress, reperfusion of tissue and microglial activity,31 which results on increased damage to stroke area, due to secondary neuronal death. The activation of these events can be triggered by a systemic inflammation,52,55 indicating that the periodontitis may represent a potential risk to aggravate the stroke condition.
In the present study, the selected articles (Table 3) focused on present an association between periodontitis and the incidence of stroke. Seven of the ten articles were case–control studies,39–45 only elucidating a static relationship between the evaluated conditions. Among the longitudinal studies, two presented periodontitis as a risk factor for stroke.36,38
In view of quantifying the systematic review, three meta-analyses were performed. The results show a positive association between stroke and the presence of periodontitis, with a risk of 2.31 (95% CI= 1.39–3.84; I2= 77%; p=0.0003) of suffering from a stroke in the periodontitis group of patients in case–control studies. However, these results must be questionable due to the high heterogeneity detected. The presence of an I2 index higher than 70% may indicate a lack of homogeneity among studies, invalidating the results presented. The study design (case-control) may be associated with the phenomena, including their retrospective design and the presence of bias related to the definition and selection of the sample inherent to this type of study (egger).
When these studies were subgrouped, and only the case–control studies which reported ischemic events were considered, a higher risk to present stroke among periodontitis patients (2.72 (95% CI= 2–3.71; I2= 4%; p<0.00001) was detected. Even though these results must be cautiously evaluated due to the type of study and a low evidence was attributed to them, these results may suggest an association between ischemic attacks and periodontal problems.
A previous meta-analysis evaluated the association among ischemic stroke and periodontitis19 and a similar RR (RR= 2.88) was presented by these authors, suggesting the association among two conditions. However, different inclusion criteria for periodontitis and cerebrovascular disease were adopted in the cited study. In our study, for the first time, we evaluated the prevalence of different types of cerebrovascular damages at the same time, comparing a healthy group and a periodontitis group.
Among the cohort studies, a greater risk of suffer cerebrovascular diseases (1.88 (95% CI= 1.55–2.28; I2= 0%; p<0.00001) was detected in periodontitis patients. Two of the three evaluated studies included only stroke cases associated with mortality,36,37 suggesting the need of further investigation of this association in prospective studies.
In our review, we tried to investigate the association of periodontitis and different types of cerebrovascular accident. Among the included studies, case-control and cohort studies were included and only 4 studies36,37,42,44 evaluated both conditions. Only one study evaluated the prevalence of periodontitis and hemorrhagic or non-hemorrhagic stroke and their association.36 So, we cannot state which condition is the most associated due to the limitations of the studies.
The suggested association between stroke and periodontitis highlights the systemic involvements of the diseases. Periodontitis has been associated with many conditions such as cardiovascular diseases, diabetes and obesity, rheumatoid arthritis,56 which shows the importance to correlate the identified risk factors and reinforce the needing of a multidisciplinary work on these conditions.
Due to the different classifications used for periodontitis regarding their severity among studies and the absence of enough numerical data, an assessment of the association between stroke risk and periodontitis was not performed.
Even though there is no reliable evidence that the treatment of periodontitis can reduce the chances of suffering from a stroke, due to the difficulties to collect and design a reliable study,57 the control of infectious diseases may represent a decrease in other risk factors also related to stroke.50 Moreover, the control of oral health in post-stroke patients has shown benefits related to oropharyngeal dysphagia and oral intake level.58
Therefore, oral care must be carried out in a preventive manner, avoiding the appearance of periodontal changes that may be the target of an increased predisposition to other diseases, such as stroke. The periodontal treatment can result in lower levels of oral bacteria and markers of inflammation, which may influence systemic disorders.59
In addition, in post-stroke patients, periodontal disease control may represent a way of trying to control the aggravation of the condition,60 especially in a hospital environment, where the control of oral health conditions is critical61 and may result in lower expenses in Public health at future times.
Despite the presented results, the studies included on this meta-analysis and systematic review present some limitations, including some methodological design problems, such as the lack of blinding and randomization; as well as the absence of the use of the same method to evaluate periodontitis. This compromise the establishment of a solid conclusion about the investigated problem. Most of the studies presented problems on sample18,33,39–43,62 and blinding process.18,30–32,36,40,42,44
Conclusion
This systematic review and meta-analysis suggest an increased risk of stroke in patients with periodontitis, especially in ischemic events. In addition, there is a strong association between both diseases. However, these results should be evaluated with caution due to the need for well-planned prospective studies for a more reliable conclusion, especially regarding the degree of severity of periodontitis and stroke, as well as to investigate the relationship of periodontitis in post-survival cases stroke.
Acknowledgments
The KFBV that analyzed all the studies and extracted the information from the eligible studies, analyzed the data and prepared the numbers and the table. The MBM and LCM that assisted in the quantitative analysis and evaluation of GRADE. AO RRL that was present at all stages of this review. This work was supported by Pró-Reitoria de Pesquisa da UFPA (PROPESP, UFPA, Brazil), Brazilian National Council for Scientific and Technological Development (CNPq). This study was financed in party by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) – Finance Cod 001.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lima RR, Santana LN, Fernandes RM, et al. Neurodegeneration and glial response after acute striatal stroke: histological basis for neuroprotective studies. Oxid Med Cell Longev. 2016;2016:3173564. doi: 10.1155/2016/3173564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colaianni V, Mazzei R, Cavallaro S. Copy number variations and stroke. Neurol Sci. 2016;37(12):1895–1904. doi: 10.1007/s10072-016-2658-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Hennerici MG. Classification of stroke subtypes. Cerebrovasc Dis. 2009;27(5):493–501. doi: 10.1159/000210432 [DOI] [PubMed] [Google Scholar]
- 4.Dziedzic T. Systemic inflammation as a therapeutic target in acute ischemic stroke. Expert Rev Neurother. 2015;15(5):523–531. doi: 10.1586/14737175.2015.1035712 [DOI] [PubMed] [Google Scholar]
- 5.Dye BA. Global periodontal disease epidemiology. Periodontol 2000. 2012;58(1):10–25. doi: 10.1111/j.1600-0757.2011.00413.x [DOI] [PubMed] [Google Scholar]
- 6.Eke PI, Dye BA, Wei L, et al. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J Periodontol. 2015;86(5):611–622. doi: 10.1902/jop.2015.140520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oppermann RV, Haas AN, Rosing CK, Susin C. Epidemiology of periodontal diseases in adults from Latin America. Periodontol 2000. 2015;67(1):13–33. doi: 10.1111/prd.12061 [DOI] [PubMed] [Google Scholar]
- 8.Papapanou PN, Sanz M, Buduneli N, et al. Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Periodontol. 2018;89 Suppl 1:S173–S182. doi: 10.1002/JPER.17-0721 [DOI] [PubMed] [Google Scholar]
- 9.Kamer AR, Craig RG, Dasanayake AP, Brys M, Glodzik-Sobanska L, de Leon MJ. Inflammation and Alzheimer’s disease: possible role of periodontal diseases. Alzheimers Dement. 2008;4(4):242–250. doi: 10.1016/j.jalz.2007.08.004 [DOI] [PubMed] [Google Scholar]
- 10.Teixeira FB, Saito MT, Matheus FC, et al. Periodontitis and alzheimer’s disease: a possible comorbidity between oral chronic inflammatory condition and neuroinflammation. Front Aging Neurosci. 2017;9(OCT). doi: 10.3389/fnagi.2017.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Passoja A, Puijola I, Knuuttila M, et al. Serum levels of interleukin-10 and tumour necrosis factor-alpha in chronic periodontitis. J Clin Periodontol. 2010;37(10):881–887. doi: 10.1111/j.1600-051X.2010.01602.x [DOI] [PubMed] [Google Scholar]
- 12.Gani DK, Mallineni SK, Ambalavanan, Ramakrishnan, Deepalakshmi EP. Estimation of the levels of C-reactive protein, interleukin-6, total leukocyte count, and differential count in peripheral blood smear of patients with chronic periodontitis in a South Indian population. West Indian Med J. 2012;61(8):826–831. [PubMed] [Google Scholar]
- 13.Kaur S, Bright R, Proudman SM, Bartold PM. Does periodontal treatment influence clinical and biochemical measures for rheumatoid arthritis? A systematic review and meta-analysis. Semin Arthritis Rheum. 2014;44(2):113–122. doi: 10.1016/j.semarthrit.2014.04.009 [DOI] [PubMed] [Google Scholar]
- 14.Zeng XT, Leng WD, Lam YY, et al. Periodontal disease and carotid atherosclerosis: a meta-analysis of 17,330 participants. Int J Cardiol. 2016;203:1044–1051. doi: 10.1016/j.ijcard.2015.11.092 [DOI] [PubMed] [Google Scholar]
- 15.Lee YT, Lee HC, Hu CJ, et al. Periodontitis as a modifiable risk factor for dementia: a nationwide population-based cohort study. J Am Geriatr Soc. 2017;65(2):301–305. doi: 10.1111/jgs.14449 [DOI] [PubMed] [Google Scholar]
- 16.Ide M, Harris M, Stevens A, et al. Periodontitis and cognitive decline in Alzheimer’s disease. PLoS One. 2016;11(3):e0151081. doi: 10.1371/journal.pone.0151081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janket SJ, Baird AE, Chuang SK, Jones JA. Meta-analysis of periodontal disease and risk of coronary heart disease and stroke. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95(5):559–569. doi: 10.1067/moe.2003.107 [DOI] [PubMed] [Google Scholar]
- 18.Lafon A, Tala S, Ahossi V, Perrin D, Giroud M, Béjot Y. Association between periodontal disease and non-fatal ischemic stroke: a case-control study. Acta Odontol Scand. 2014;72(8):687–693. doi: 10.3109/00016357.2014.898089 [DOI] [PubMed] [Google Scholar]
- 19.Leira Y, Seoane J, Blanco M, et al. Association between periodontitis and ischemic stroke: a systematic review and meta-analysis. Eur J Epidemiol. 2017;32(1):43–53. doi: 10.1007/s10654-016-0170-6 [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fowkes FG, Fulton PM. Critical appraisal of published research: introductory guidelines. BMJ. 1991;302(6785):1136–1140. doi: 10.1136/bmj.302.6785.1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Penoni DC, Fidalgo TK, Torres SR, et al. Bone density and clinical periodontal attachment in postmenopausal women: a systematic review and meta-analysis. J Dent Res. 2017;96(3):261–269. doi: 10.1177/0022034516682017 [DOI] [PubMed] [Google Scholar]
- 23.Borenstein M, Hedges L, Higgins JPT, Rothstein H. An Introduction to Meta-Analysis. Vol. 19; 2009. [Google Scholar]
- 24.Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. Versão 5.1.0. A Colaboração cochrane; 2011. Available from: http://handbook-5-1.cochrane.org Accessed October8, 2019.
- 25.Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 26.Elter JR, Offenbacher S, Toole JF, Beck JD. Relationship of periodontal disease and edentulism to stroke/TIA. J Dent Res. 2003;82(12):998–1001. doi: 10.1177/154405910308201212 [DOI] [PubMed] [Google Scholar]
- 27.Joshipura KJ, Hung HC, Rimm EB, Willett WC, Ascherio A. Periodontal disease, tooth loss, and incidence of ischemic stroke. Stroke. 2003;34(1):47–52. doi: 10.1161/01.str.0000052974.79428.0c [DOI] [PubMed] [Google Scholar]
- 28.Grau AJ, Becher H, Ziegler CM, et al. Periodontal disease as a risk factor for ischemic stroke. Stroke. 2004;35(2):496–501. doi: 10.1161/01.STR.0000110789.20526.9D [DOI] [PubMed] [Google Scholar]
- 29.Lee HJ, Garcia RI, Janket SJ, et al. The association between cumulative periodontal disease and stroke history in older adults. J Periodontol. 2006;77(10):1744–1754. doi: 10.1902/jop.2006.050339 [DOI] [PubMed] [Google Scholar]
- 30.Sim SJ, Kim HD, Moon JY, et al. Periodontitis and the risk for non-fatal stroke in Korean adults. J Periodontol. 2008;79(9):1652–1658. doi: 10.1902/jop.2008.080015 [DOI] [PubMed] [Google Scholar]
- 31.Jimenez M, Krall EA, Garcia RI, Vokonas PS, Dietrich T. Periodontitis and incidence of cerebrovascular disease in men. Ann Neurol. 2009;66(4):505–512. doi: 10.1002/ana.21742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim HD, Sim SJ, Moon JY, Hong YC, Han DH. Association between periodontitis and hemorrhagic stroke among Koreans: a case-control study. J Periodontol. 2010;81(5):658–665. doi: 10.1902/jop.2010.090614 [DOI] [PubMed] [Google Scholar]
- 33.Hashemipour MA, Afshar AJ, Borna R, Seddighi B, Motamedi A. Gingivitis and periodontitis as a risk factor for stroke: a case-control study in the Iranian population. Dent Res J (Isfahan). 2013;10(5):613–619. [PMC free article] [PubMed] [Google Scholar]
- 34.Holmlund A, Lampa E, Lind L. Oral health and cardiovascular disease risk in a cohort of periodontitis patients. Atherosclerosis. 2017;262:101–106. doi: 10.1016/j.atherosclerosis.2017.05.009 [DOI] [PubMed] [Google Scholar]
- 35.Beck JD, Moss KL, Morelli T, Offenbacher S. Periodontal profile class is associated with prevalent diabetes, coronary heart disease, stroke, and systemic markers of C-reactive protein and interleukin-6. J Periodontol. 2018;89(2):157–165. doi: 10.1002/JPER.17-0426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu TJ, Trevisan M, Genco RJ, Dorn JP, Falkner KL, Sempos CT. Periodontal disease and risk of cerebrovascular disease - The first national health and nutrition examination survey and its follow-up study. Arch Intern Med. 2000;160(18):2749–2755. doi: 10.1001/archinte.160.18.2749 [DOI] [PubMed] [Google Scholar]
- 37.Holmlund A, Holm G, Lind L. Number of teeth as a predictor of cardiovascular mortality in a cohort of 7,674 subjects followed for 12 years. J Periodontol. 2010;81(6):870–876. doi: 10.1902/jop.2010.090680 [DOI] [PubMed] [Google Scholar]
- 38.Sen S, Giamberardino LD, Moss K, et al. periodontal disease, regular dental care use, and incident ischemic stroke. Stroke. 2018;49(2):355–362. doi: 10.1161/STROKEAHA.117.018990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dörfer CE, Becher H, Ziegler CM, et al. The association of gingivitis and periodontitis with ischemic stroke. J Clin Periodontol. 2004;31(5):396–401. doi: 10.1111/j.1600-051x.2004.00579.x [DOI] [PubMed] [Google Scholar]
- 40.Pradeep AR, Hadge P, Raju PA, Shetty SR, Shareef K, Guruprasad CN. Periodontitis as a risk factor for cerebrovascular accident: a case-control study in the Indian population. J Periodontal Res. 2010;45(2):223–228. doi: 10.1111/j.1600-0765.2009.01220.x [DOI] [PubMed] [Google Scholar]
- 41.Abolfazli N, Ghoreishizadeh A, Ayramlu H, Ghavimi M, Ghoreishizadeh M, Salehsaber F. Periodontal disease and risk of cerebral ischemic stroke. J Neurol Sci. 2011;28(3):307–316. [Google Scholar]
- 42.Ghizoni JS, Taveira L, Garlet GP, et al. Increased levels of Porphyromonas gingivalis are associated with ischemic and hemorrhagic cerebrovascular disease in humans: an in vivo study. J Appl Oral Sci. 2012;20(1):104–112. doi: 10.1590/s1678-77572012000100019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palm F, Lahdentausta L, Sorsa T, et al. Biomarkers of periodontitis and inflammation in ischemic stroke: a case-control study. Innate Immun. 2014;20(5):511–518. doi: 10.1177/1753425913501214 [DOI] [PubMed] [Google Scholar]
- 44.Diouf M, Basse A, Ndiaye M, Cisse D, Lo CM, Faye D. Stroke and periodontal disease in Senegal: case-control study. Public Health. 2015;129(12):1669–1673. doi: 10.1016/j.puhe.2015.02.033 [DOI] [PubMed] [Google Scholar]
- 45.Leira Y, Lopez-Dequidt I, Arias S, et al. Chronic periodontitis is associated with lacunar infarct: a case-control study. Eur J Neurol. 2016;23(10):1572–1579. doi: 10.1111/ene.13080 [DOI] [PubMed] [Google Scholar]
- 46.Abd El-Aleem SA, Morales-Aza BM, Donaldson LF. Sensory neuropeptide mRNa up-regulation is bilateral in periodontitis in the rat: a possible neurogenic component to symmetrical periodontal disease. Eur J Neurosci. 2004;19(3):650–658. doi: 10.1111/j.1460-9568.2004.03179.x [DOI] [PubMed] [Google Scholar]
- 47.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fleming PS, Lynch CD, Pandis N. Randomized controlled trials in dentistry: common pitfalls and how to avoid them. J Dent. 2014;42(8):908–914. doi: 10.1016/j.jdent.2014.06.004 [DOI] [PubMed] [Google Scholar]
- 49.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15(1):30–44. doi: 10.1038/nri3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Esenwa CC, Elkind MS. Inflammatory risk factors, biomarkers and associated therapy in ischaemic stroke. Nat Rev Neurol. 2016;12(10):594–604. doi: 10.1038/nrneurol.2016.125 [DOI] [PubMed] [Google Scholar]
- 51.Pejcic A, Kesic LJ, Milasin J. C-reactive protein as a systemic marker of inflammation in periodontitis. Eur J Clin Microbiol Infect Dis. 2011;30(3):407–414. doi: 10.1007/s10096-010-1101-1 [DOI] [PubMed] [Google Scholar]
- 52.Vidale S, Consoli A, Arnaboldi M, Consoli D. postischemic inflammation in acute stroke. J Clin Neurol. 2017;13(1):1–9. doi: 10.3988/jcn.2017.13.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoffmann A, Zhu G, Wintermark M. Advanced neuroimaging in stroke patients: prediction of tissue fate and hemorrhagic transformation. Expert Rev Cardiovasc Ther. 2012;10(4):515–524. doi: 10.1586/erc.12.30 [DOI] [PubMed] [Google Scholar]
- 54.Perry VH, Teeling J. Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin Immunopathol. 2013;35(5):601–612. doi: 10.1007/s00281-013-0382-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alhadidi Q, Shah ZA. Cofilin mediates LPS-induced microglial cell activation and associated neurotoxicity through activation of NF-kappaB and JAK-STAT pathway. Mol Neurobiol. 2018;55(2):1676–1691. doi: 10.1007/s12035-017-0432-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Winning L, Linden GJ. Periodontitis and systemic disease: association or causality? Current Oral Health Rep. 2017;4(1):1–7. doi: 10.1007/s40496-017-0121-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li C, Lv Z, Shi Z, et al. Periodontal therapy for the management of cardiovascular disease in patients with chronic periodontitis. Cochrane Database Syst Rev. 2017;2017:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mituuti CT, Bianco VC, Bentim CG, de Andrade EC, Rubo JH, Berretin-Felix G. Influence of oral health condition on swallowing and oral intake level for patients affected by chronic stroke. Clin Interv Aging. 2015;10:29–35. doi: 10.2147/CIA.S62314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Darby IB, Mooney J, Kinane DF. Changes in subgingival microflora and humoral immune response following periodontal therapy. J Clin Periodontol. 2001;28(8):796–805. doi: 10.1034/j.1600-051x.2001.280812.x [DOI] [PubMed] [Google Scholar]
- 60.Ajwani S, Jayanti S, Burkolter N, et al. Integrated oral health care for stroke patients - a scoping review. J Clin Nurs. 2017;26(7–8):891–901. doi: 10.1111/jocn.13520 [DOI] [PubMed] [Google Scholar]
- 61.Terezakis E, Needleman I, Kumar N, Moles D, Agudo E. The impact of hospitalization on oral health: a systematic review. J Clin Periodontol. 2011;38(7):628–636. doi: 10.1111/j.1600-051X.2011.01727.x [DOI] [PubMed] [Google Scholar]
- 62.Grau AJ, Buggle F, Ziegler C, et al. Association between acute cerebrovascular ischemia and chronic and recurrent infection. Stroke. 1997;28(9):1724–1729. doi: 10.1161/01.str.28.9.1724 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. Versão 5.1.0. A Colaboração cochrane; 2011. Available from: http://handbook-5-1.cochrane.org Accessed October8, 2019.