Abstract
Background
The influences of genetic variants on functional clinical outcomes following stroke are unclear. In order to reliably quantify these influences, we undertook a comprehensive meta-analysis of outcomes after acute intracerebral haemorrhage (ICH) or ischaemic stroke (AIS) in relation to different genetic variants.
Methods
PubMed, PsycInfo, Embase and Medline electronic databases were searched up to January 2019. Outcomes, defined as favourable or poor, were assessed by validated scales (Barthel index, modified Rankin scale, Glasgow outcome scale and National Institutes of Health stroke scale).
Results
Ninety-two publications comprising 31,895 cases met our inclusion criteria. Poor outcome was observed in patients with ICH who possessed the APOE4 allele: OR =2.60 (95% CI = 1.25–5.41, p = 0.01) and in AIS patients with the GA or AA variant at the BDNF-196 locus: OR = 2.60 (95% CI = 1.25–5.41, p = 0.01) or a loss of function allele of CYP2C19: OR = 2.36 (95% CI = 1.56–3.55, p < 0.0001). Poor outcome was not associated with APOE4: OR = 1.02 (95% CI = 0.81–1.27, p = 0.90) or IL6-174 G/C: OR = 2.21 (95% CI = 0.55–8.86, p = 0.26) in patients with AIS.
Conclusions
We demonstrate that recovery of AIS was unfavourably associated with variants of BDNF and CYP2C19 genes whilst recovery of ICH was unfavourably associated with APOE4 gene.
Electronic supplementary material
The online version of this article (10.1007/s10072-019-04024-w) contains supplementary material, which is available to authorized users.
Keywords: Polymorphisms, POE, BDNF, Stroke outcomes
Introduction
After stroke, the majority of patients achieve fastest recovery by 3 months followed by a deceleration and plateauing thereafter [1, 2]. Whilst many factors may influence functional recovery including the size and location of the lesion, delay in treatment and age, these factors do not entirely explain the variability in outcome of stroke [2, 3].
Animal studies have demonstrated an influential role for genetics in post-stroke recovery [4]. A number of genes have been implicated which may impact anywhere from a molecular level upwards, culminating in anatomical changes seen with the degree of axonal sprouting and the strength of subsequent adaptive connections [5]. BDNF (brain-derived neurotrophic factor) is one such gene in which its higher concentrations being observed to correlate with favourable outcome in murine models [6].
A previous meta-analysis of stroke patients [7] has been conducted to quantify the specific role of the gene apolipoprotein E (APOE) but found no correlation with outcomes in patients recovering from AIS but appeared to associate with worse outcomes in those recovering from ICH (albeit with one study). Hitherto, there has been no attempt to quantify the roles of other genes. We have therefore conducted a meta-analysis to examine the associations of genetic variants with functional outcomes in patients recovering from AIS or ICH. To the best of our knowledge, this is the largest such study to date.
Methods
Literature search
We searched electronic databases including EMBASE, Medline, PsycINFO and PubMed up to and including January 2019 using search terms ‘cerebral infarct’, ‘stroke’, ‘brain vascular accident’, ‘cerebrovascular accident’ and ‘intracerebral haemorrhage’. Abbreviations (e.g. ICH) and MeSH terms were also searched. The terms ‘genetic’, ‘polymorphism’, ‘variant’, ‘variation’, ‘mutation’, ‘genotype’ or ‘phenotype’ were searched to identify genetic variants. Stroke outcomes were searched using the terms ‘NIHSS’, ‘Barthel’, ‘Rankin’, ‘Glasgow’, ‘Fugl-Meyer’, ‘FIM’ or ‘outcome’. These terms were combined using AND/OR Boolean operators. Additionally, references of all included publications were examined. If multiple publications on the same data were discovered, the largest dataset was selected. Non-English publications were also included.
Selection criteria
Studies describing patients with different genetic variants and their functional outcome at the latest available time point were included. Where multiple time points were measured, the final point was used. Where investigators did not detail rehabilitation strategies, it was assumed patients received rehabilitation according to local guidelines. Only studies in adults (aged ≥ 18 years) were selected. Monogenic stroke disorders were excluded as was transient ischaemic attacks and subarachnoid haemorrhage.
Statistical analysis
Genetic factors including GA or AA variant at the BDNF-196 locus, loss of function allele of CYP2C19, E4 allele and IL6 were used as determinant factors and Barthel index (BI), the modified Rankin scale (mRS), Glasgow Outcome Score (GOS) and the National Institutes of Health Stroke Scale (NIHSS) were used as stroke functional outcomes.
Only variants of the genes APOE, BDNF-196, CYP2C19 and IL6 were examined whilst the remaining genes and polymorphisms were not included due to insufficient number of studies or suitable data. McCarron et al. [8] did not use a validated scale whilst Broderick et al. [9] classified APOE-containing phenotypes differently to the other authors. Where necessary, data were presented with or without the inclusion of these two studies to see if the findings were substantially changed.
The scales used to assess functional outcome of stroke were variable, including the BI which assesses activities of daily living (range 0–100), mRS assesses global disability (range 0–6), GOS assesses the degree of recovery (range 1–5) and NIHSS assesses stroke severity (range 0–42). NIHSS and GOS are usually used immediately whilst mRS and BI are used in the days to months after stroke [10, 11]. As a result, only a handful of studies have been conducted to equate these scales [12–14]. In the present study, we relied on the authors’ interpretation of ‘favourable’ or ‘poor’ outcome based on the particular scale used in their studies. The majority of investigators defined ‘favourable’ outcome as mRS of 0–1 or 0–2 whilst some [8] defined ‘favourable’ outcome as being one in which the patient was at home at follow-up (mRS = 0–4) and ‘poor’ if dead or in care (mRS = 5–6). Similarly, the BI was defined as follows: ‘favourable’ = 60–100 and ‘poor’ = 0–90, NIHSS score: ‘favourable’ = 0–10 and ‘poor’ = 2–42 and GOS: ‘favourable’ = 5–8 and ‘poor’ = 1–4.
Analysis was performed using data analysed by Review Manager v5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Pooled odds ratios (OR) were calculated with 95% confidence intervals (CI) by the random effects model. Inter-study heterogeneity was examined by I2 index and studies were iteratively tested to reduce heterogeneity. Funnel plots were used to assess publication bias. Statistical significance threshold was accepted as p < 0.05.
Results
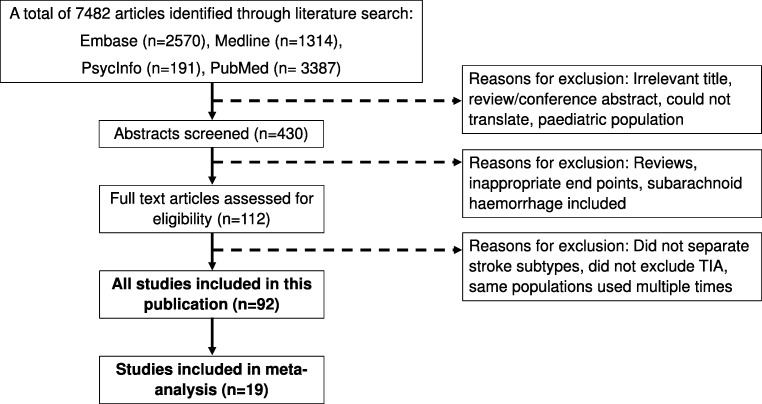
Our initial search strategy found 7482 studies, and a total of 92 (comprising 135 polymorphisms in 72 genes) met inclusion criteria (Fig. 1) [8, 9, 15–104]. Characteristics of study populations are described in Supplementary Table 1. and summary of the studies examining the associations between genetic factors and stroke outcomes is shown in Supplementary Table 2.
Fig. 1.
Flow chart of the screening process
APOE variants on functional outcome several months following AIS
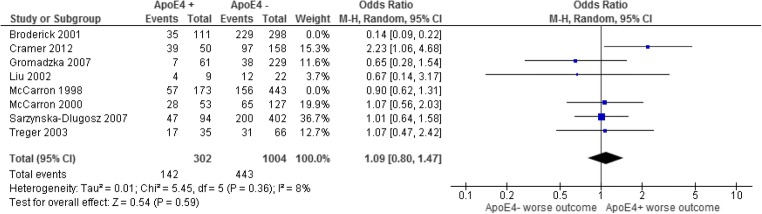
Six studies [26, 43, 59, 67, 82, 90] comprising 2185 patients (490 with at least one E4 allele and 1695 without) (Fig. 2) showed no association between outcome and the presence of at least one E4 allele: OR = 1.02 (0.81–1.27, p = 0.90). There was no evidence of inter-study heterogeneity (p = 0.42, I2 = 1%) or publication bias. Including studies by McCarron et al. [8] and Broderick et al. [9] changed the OR to 0.78 (0.41–1.47, p = 0.44).
Fig. 2.
The odds ratio of having a poor functional outcome up to several months after an AIS with the presence of the ApoE4 allele compared with those without. The random effects model was used to determine the 95% confidence intervals and significance. The blue squares represent the individual studies results and weighting whilst the black diamond shows the overall result
APOE variants on functional outcome up to several months after an ICH
Three studies [26, 34, 51] comprising 118 patients (40 with at least one E4 allele and 78 without) (Supplementary Fig. 1) revealed patients with ICH who possessed at least one E4 allele had increased risk of poor outcome: OR 2.45 (1.03–5.81, p = 0.04). There was no evidence of inter-study heterogeneity (p = 0.70, I2 = 0%) or publication bias. Including the study by McCarron 1998 [8] changed the OR to 2.60 (1.25–5.41, p = 0.01).
BDNF-196 variants on functional outcome up to 7 years following AIS
In six studies [26, 30, 33, 55, 89, 103] comprising 1241 cases (585 patients with a GG genotype and 656 without) (Fig. 3) showed patients with AIS who carried at codon 196 of the BDNF, the GA or AA genotypes were more likely to have poor outcome: OR 1.41 (1.02–1.94, p = 0.04). No heterogeneity (p = 0.30, I2 = 18%) or publication bias was observed. Excluding the study with longest follow-up of 7 years [89] increased the OR to 1.60 (1.08–2.37, p = 0.02).
Fig. 3.
Outcome up to 12 months following an AIS with the GG variant at the BDNF-196 locus versus GA/AA
Cytochrome P450 2C19 (CYP2C19) loss of function variants on functional outcome at 6 months after an AIS
CYP2C19 polymorphisms (poor metaboliser variants CYP2C19 *2, CYP2C19 *3 and CYP2C19 *17) were studied in three studies comprising 918 patients (446 carried a loss of function allele and 472 without) (Supplementary Fig. 3). Patients with AIS who carried a loss of function CYP2C19 allele were more likely to have poor outcome: OR = 2.36 (1.56–3.55, p < 0.0001). There was no significant heterogeneity (p = 0.96, I2 = 0%) or publication bias.
Interleukin 6-174 (IL-6) variants on functional outcome at 6 months in AIS
Two studies totalling 237 patients (144 with the GG variant and 93 without) (Supplementary Fig. 4) found no association between patients who carried the GC or CC genotype at codon 176 of IL6 gene and poor outcome: OR = 2.21 (0.55–8.86, p = 0.26).
Discussion
In this meta-analysis, the largest to date, we have shown that genetic influences on functional outcomes in stroke recovery differed between patients who sustained ICH and those who sustained AIS. On the one hand, patients with ICH who possessed APOE4 allele had a 2.6-fold increase in poor outcome; on the other hand, poor outcome was increased among patients with AIS who possessed poor metaboliser variants (CYP2C19 *2, CYP2C19 *3 and CYP2C19 *17) by 2.4-fold and those who carried BDNF GA or AA genotypes by 1.6-fold, but not in patients with AIS who possessed APOE4 or IL6 alleles. Our findings suggest that genetic factors may, in part, account for the variability in stroke recovery and could be served as prognostic markers in the management of stroke.
It is unclear how APOE4 influences poor outcomes in patients recovering from ICH but not in those recovering from AIS but may be linked to its relationship with coagulating ability. Weir et al. [105] have shown that among patients with ICH, compared with non-APOE4 allele carriers, carriers of APOE4 allele had higher partial thromboplastin time (PTT) ratios, i.e. greater propensity to bleed. The same study found that among patients with AIS, increasing APOE4 dose was associated with improved survival independent of stroke severity and PTT.
Similarly, underlying mechanisms on how BDNF-196 variants influence outcomes in patient with AIS remain uncertain. Physical exercise in rehabilitation is thought to increase BDNF levels resulting in a greater capacity for neuronal survival and plasticity after stroke [103, 106]. However, a meta-analysis in non-stroke patients has shown a lack of association between BDNF-196 genotype and serum BDNF levels [107]. Therefore, the association between BDNF-196 variation and outcomes is more complex than first thought. The methylation status of the BDNF gene may be important [55, 108] and additionally any purported effects are likely to be due to the interplay of multiple polymorphisms at different loci [107].
We observed that loss of function of CYP2C19 was also shown to associate with poor outcome in patient recovering from AIS. This relationship may be explained by a diminished ability of the enzyme to metabolise certain drugs used to treat AIS such as the antiplatelet agent clopidogrel [76, 100] resulting in reduced conversion of prodrug to the active form and efficacy. It has been shown that loss of function of CYP2C19 allele is time dependent [52]; therefore, the adverse effects on stroke recovery may eventually wear off. However, all these studies were conducted in Chinese patients; therefore, these findings may not be applicable to other populations.
It appears that genes involved with drug metabolism such as MDR1, COX2 and CYP2C19 [52, 62, 71, 76, 83, 85, 100] appear to have major influences on stroke outcome which may be explained by the inflammatory cascade in the recovery process of AIS. Similarly, variants in IL-1, IL-4, IL-6, IL-10 and IL-12 have also been shown to associate with increased levels of inflammatory markers, tissue damage and worse outcomes [18, 22, 42, 66, 97]. In addition, PAI1, FXIII and tPA gene polymorphisms, known to involve in the clotting pathways, can also impact on the degree of thrombosis and the efficacy of fibrinolysis after stroke [36, 41, 62]. Many more mutations in other genes have been identified that have yet to converge with the existing literature.
Limitations
As with all such analyses, a number of limitations need to be noted. Most papers focus solely on one or two genetic variations, yet it is likely that many such variants work in complex arrangements of gene-gene/gene-phenotype-environment interactions for eventual functional outcomes [100, 109]. For example, people with a family history of cardiovascular disease or risk factors such as hypercholesterolaemia and metabolic syndrome (increased central fat accumulation, hypertriglyceridaemia, low high-density lipoprotein cholesterol, hypertension and hyperglycaemia) may undergo behavioural changes such as intensive lifestyle modification.
The focus of our study was to examine the associations of genetic variants with functional outcomes in patients recovering from AIS or ICH. We recognise that the role of APOE4 allele is more complex, involving small vessel disease, Alzheimer’s disease, risk of ICH recurrence and cerebral amyloid angiopathy-related syndromes [110]. However, this topic is beyond the aim of our present study.
Many of the genes described herein appear to correlate with the occurrence and severity of stroke. A more severe stroke usually leads to a worse outcome but this information was not available for our analysis whilst mortality was not consistently included in a number of studies; thus, heterogeneity in individual studies was unavoidable. Like all meta-analyses, methodologies between studies often vary; in the present study, we recognise variations in rehabilitation methods, outcome measures, duration of stroke recovery and definition of outcomes varied between papers may influence the degree of association between genetic mutations and stroke recovery but we do not feel the direction of association would be affected. To minimise the variability in outcome measures between studies, we have categorised them as consistently as possible.
The possibility of publication bias cannot be discounted, although funnel plots did not show asymmetrical distribution and we have performed exhaustive literature searches to ensure the maximum coverage. There may be heterogeneity or lack of agreement across scales such that some patients with identical BI scores can have very different mRS scores, highlighting the subjectivity of the mRS in which bias could potentially be introduced. Furthermore, scales tend to vary at different rates after stroke; therefore, combining scales may only be useful after a certain time, but the ‘optimal time’ remains undetermined. In contrast to most scales which consist of a wide range of score, mRS suffers from its narrow range of only seven points (0–6). Whilst Govan et al. [14] created an equivalency between the BI, mRS, NIHSS and Scandinavian stroke scale, the remaining studies focused mainly on mRS and BI. To provide a degree of flexibility when combining the scales in the present study, equivalent categories were created, e.g. a score of 0–3 on the mRS is equated to 10–20 on the BI.
In conclusion, we have demonstrated that recovery of AIS was unfavourably associated with variants of BDNF and CYP2C19 genes whilst recovery of ICH was unfavourably associated with E4 allele of APOE gene. Our findings could be served as prognostic markers in the management of stroke.
Electronic supplementary material
(DOCX 85 kb)
Authors’ contributions
PS and PB reviewed the topic-related literature and performed the study concept and analysis design. NM performed the data collection and wrote the first draft, analysed, interpreted the data. TSH, PS, PB, RH and IL edited and TSH revised the manuscript. All authors checked, interpreted results and approved the final version.
Data Availability
No additional data are available.
Compliance with ethical standards
Competing interests
The authors declare that they have no conflicts of interest.
Ethical approval
This study does not require NHS Research Ethics Committee approval since it involves secondary analysis of anonymised data. This study was conducted in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Statement of human and animal rights
This article does not contain any studies with animals performed by any of the authors.
Provenance and peer review
Not commissioned; externally peer reviewed.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Thang S. Han, Phone: 01784443807, Email: thang.han@rhul.ac.uk
Pankaj Sharma, Email: pankaj.sharma@rhul.ac.uk.
References
- 1.Maguire J, Bevan S, Stanne T, Lorenzen E, Fernandez-Cadenas I, Hankey G, et al. GISCOME – Genetics of Ischaemic Stroke Functional Outcome network: a protocol for an international multicentre genetic association study. Eur Stroke J. 2017;2:229–237. doi: 10.1177/2396987317704547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van de Port IG, Kwakkel G, van Wijk I, Lindeman E. Susceptibility to deterioration of mobility long-term after stroke: a prospective cohort study. Stroke. 2006;37(1):167–171. doi: 10.1161/01.STR.0000195180.69904.f2. [DOI] [PubMed] [Google Scholar]
- 3.Kwakkel G, Kollen BJ. Predicting activities after stroke: what is clinically relevant? Int J Stroke. 2013;8(1):25–32. doi: 10.1111/j.1747-4949.2012.00967.x. [DOI] [PubMed] [Google Scholar]
- 4.Lindgren A, Maguire J. Stroke recovery genetics. Stroke. 2016;47(9):2427–2434. doi: 10.1161/STROKEAHA.116.010648. [DOI] [PubMed] [Google Scholar]
- 5.Overman JJ, Carmichael ST. Plasticity in the injured brain: more than molecules matter. Neuroscientist. 2014;20(1):15–28. doi: 10.1177/1073858413491146. [DOI] [PubMed] [Google Scholar]
- 6.MacLellan CL, Keough MB, Granter-Button S, Chernenko GA, Butt S, Corbett D. A critical threshold of rehabilitation involving brain-derived neurotrophic factor is required for poststroke recovery. Neurorehabil Neural Repair. 2011;25(8):740–748. doi: 10.1177/1545968311407517. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Gonzalez NA, Sudlow CL. Effects of apolipoprotein E genotype on outcome after ischaemic stroke, intracerebral haemorrhage and subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2006;77(12):1329–1335. doi: 10.1136/jnnp.2006.097543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MCarron MO, Muir KW, Weir CJ, Dyker AG, Bone I, Nicoll JA, et al. The apolipoprotein E epsilon4 allele and outcome in cerebrovascular disease. Stroke. 1998;29(9):1882–1887. doi: 10.1161/01.str.29.9.1882. [DOI] [PubMed] [Google Scholar]
- 9.Broderick J, Lu M, Jackson C, Pancioli A, Tilley BC, Fagan SC, Kothari R, Levine SR, Marler JR, Lyden PD, Haley EC, Brott T, Grotta JC, NINDS t-PA Stroke Study Group Apolipoprotein E phenotype and the efficacy of intravenous tissue plasminogen activator in acute ischemic stroke. Ann Neurol. 2001;49(6):736–744. doi: 10.1002/ana.1058. [DOI] [PubMed] [Google Scholar]
- 10.Harrison JK, McArthur KS, Quinn TJ. Assessment scales in stroke: clinimetric and clinical considerations. Clin Interv Aging. 2013;8:201–211. doi: 10.2147/CIA.S32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sulter G, Steen C, De Keyser J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke. 1999;30(8):1538–1541. doi: 10.1161/01.str.30.8.1538. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe CD, Taub NA, Woodrow EJ, Burney PG. Assessment of scales of disability and handicap for stroke patients. Stroke. 1991;22(10):1242–1244. doi: 10.1161/01.str.22.10.1242. [DOI] [PubMed] [Google Scholar]
- 13.Cioncoloni D, Piu P, Tassi R, Acampa M, Guideri F, Taddei S, Bielli S, Martini G, Mazzocchio R. Relationship between the modified Rankin Scale and the Barthel Index in the process of functional recovery after stroke. NeuroRehabilitation. 2012;30(4):315–322. doi: 10.3233/NRE-2012-0761. [DOI] [PubMed] [Google Scholar]
- 14.Govan L, Langhorne P, Weir CJ. Categorizing stroke prognosis using different stroke scales. Stroke. 2009;40(10):3396–3399. doi: 10.1161/STROKEAHA.109.557645. [DOI] [PubMed] [Google Scholar]
- 15.Aberg ND, Olsson S, Aberg D, Jood K, Stanne TM, Nilsson M, et al. Genetic variation at the IGF1 locus shows association with post-stroke outcome and to circulating IGF1. Eur J Endocrinol. 2013;169(6):759–765. doi: 10.1530/EJE-13-0486. [DOI] [PubMed] [Google Scholar]
- 16.Alberts MJ, Graffagnino C, McClenny C, DeLong D, Strittmatter W, Saunders AM, Roses AD. ApoE genotype and survival from intracerebral haemorrhage. Lancet. 1995;346(8974):575. doi: 10.1016/s0140-6736(95)91411-0. [DOI] [PubMed] [Google Scholar]
- 17.Appelboom G, Piazza M, Bruce SS, Zoller SD, Hwang B, Monahan A, Hwang RY, Kisslev S, Mayer S, Meyers PM, Badjatia N, Connolly ES. Variation in a locus linked to platelet aggregation phenotype predicts intraparenchymal hemorrhagic volume. Neurol Res. 2012;34(3):232–237. doi: 10.1179/1743132811Y.0000000080. [DOI] [PubMed] [Google Scholar]
- 18.Becker KJ, Dankwa D, Lee R, Schulze J, Zierath D, Tanzi P, Cain K, Dressel A, Shibata D, Weinstein J. Stroke, IL-1ra, IL1RN, infection and outcome. Neurocrit Care. 2014;21(1):140–146. doi: 10.1007/s12028-013-9899-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biffi A, Anderson CD, Jagiella JM, Schmidt H, Kissela B, Hansen BM, Jimenez-Conde J, Pires CR, Ayres AM, Schwab K, Cortellini L, Pera J, Urbanik A, Romero JM, Rost NS, Goldstein JN, Viswanathan A, Pichler A, Enzinger C, Rabionet R, Norrving B, Tirschwell DL, Selim M, Brown DL, Silliman SL, Worrall BB, Meschia JF, Kidwell CS, Broderick JP, Greenberg SM, Roquer J, Lindgren A, Slowik A, Schmidt R, Woo D, Rosand J, International Stroke Genetics Consortium APOE genotype and extent of bleeding and outcome in lobar intracerebral haemorrhage: a genetic association study. Lancet Neurol. 2011;10(8):702–709. doi: 10.1016/S1474-4422(11)70148-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouziana SD, Tziomalos K, Goulas A, Vyzantiadis TA, Panderi A, Etaatzitolios AI. Major adipokines and the -420C>G resistin gene polymorphism as predictors of acute ischemic stroke severity and in-hospital outcome. J Stroke Cerebrovasc Dis. 2018;27(4):963–970. doi: 10.1016/j.jstrokecerebrovasdis.2017.10.038. [DOI] [PubMed] [Google Scholar]
- 21.Cai H, Cai B, Sun L, Zhang H, Zhou S, Cao L, Guo H, Sun W, Yan B, Davis SM, Zhang Z, Liu X. Association between PTGS1 polymorphisms and functional outcomes in Chinese patients with stroke during aspirin therapy: interaction with smoking. J Neurol Sci. 2017;376:211–215. doi: 10.1016/j.jns.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 22.Chakraborty B, Chowdhury D, Vishnoi G, Goswami B, Kishore J, Agarwal S. Interleukin-6 gene -174 G/C promoter polymorphism predicts severity and outcome in acute ischemic stroke patients from North India. J Stroke Cerebrovasc Dis. 2013;22(5):683–689. doi: 10.1016/j.jstrokecerebrovasdis.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Chang WH, Park E, Lee J, Lee A, Kim YH. Association between brain-derived neurotrophic factor genotype and upper extremity motor outcome after stroke. Stroke. 2017;48(6):1457–1462. doi: 10.1161/STROKEAHA.116.015264. [DOI] [PubMed] [Google Scholar]
- 24.Cheng X, Xu J, Gu M, Wang M, Sun B, Li Z, Ni G, Wang G, Weng Z, Shi Y, Zhang Z, Liu X. Genetic variants in ALDH2 predict risk of ischemic stroke in a Chinese population. Gene. 2018;678:49–54. doi: 10.1016/j.gene.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Chon J, Kim HS, Yun DH, Yoo SD, Kim DH, Lee SA, Kim SK, Park HJ, Chung JH, Chung S, Yeo J. Association between a polymorphism (rs2071214) in Baculoviral IAP repeat containing 5 gene (BIRC5) and ischemic stroke in Korean population. Ann Rehabil Med. 2016;40(3):392–400. doi: 10.5535/arm.2016.40.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cramer SC, Procaccio V. GAIN Americas, GAIN International Study Investigators. Correlation between genetic polymorphisms and stroke recovery: analysis of the GAIN Americas and GAIN International Studies. Eur J Neurol. 2012;19(5):718–724. doi: 10.1111/j.1468-1331.2011.03615.x. [DOI] [PubMed] [Google Scholar]
- 27.Dardiotis E, Hadjigeorgiou GM, Dardioti M, Scarmeas N, Paterakis K, Aggelakis K, Komnos A, Tasiou A, Xiromerisiou G, Gabranis I, Zintzaras E, Papadimitriou A, Karantanas A. Alpha-1 antichymotrypsin gene signal peptide a/t polymorphism and primary intracerebral hemorrhage. Eur Neurol. 2008;59(6):307–314. doi: 10.1159/000121420. [DOI] [PubMed] [Google Scholar]
- 28.de Boer RGA, Spielmann K, Heijenbrok-Kal MH, van der Vliet R, Ribbers GM, van de Sandt-Koenderman WME. The role of the BDNF Val66Met polymorphism in recovery of aphasia after stroke. Neurorehabil Neural Repair. 2017;31(9):851–857. doi: 10.1177/1545968317723752. [DOI] [PubMed] [Google Scholar]
- 29.Diaz-Maroto Cicuendez I, Fernandez-Diaz E, Garcia-Garcia J, Jordan J, Fernandez-Cadenas I, Montaner J, et al. The UCP2-866G/A polymorphism could be considered as a genetic marker of different functional prognosis in ischemic stroke after recanalization. NeuroMolecular Med. 2017;19(4):571–578. doi: 10.1007/s12017-017-8470-x. [DOI] [PubMed] [Google Scholar]
- 30.Di Lazzaro V, Pellegrino G, Di Pino G, Corbetto M, Ranieri F, Brunelli N, et al. Val66Met BDNF gene polymorphism influences human motor cortex plasticity in acute stroke. Brain Stimul. 2015;8(1):92–96. doi: 10.1016/j.brs.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Husseini N, Hoffman BM, Bennett ER, Li YW, Williamson Taylor RA, Hailey CE, et al. Association of IL6ST (gp130) polymorphism with functional outcome following spontaneous intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2018;27(1):125–131. doi: 10.1016/j.jstrokecerebrovasdis.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 32.Ellul J, Markoula S, Marousi S, Galidi A, Kyritsis AP, Papathanasopoulos P, Georgiou I. Association of endothelial nitric oxide synthase polymorphism G894T with functional outcome in acute stroke patients. Neurol Res. 2011;33(8):835–840. doi: 10.1179/1743132811Y.0000000011. [DOI] [PubMed] [Google Scholar]
- 33.Essa H., Vasant D. H., Raginis-Zborowska A., Payton A., Michou E., Hamdy S. The BDNF polymorphism Val66Met may be predictive of swallowing improvement post pharyngeal electrical stimulation in dysphagic stroke patients. Neurogastroenterology & Motility. 2017;29(8):e13062. doi: 10.1111/nmo.13062. [DOI] [PubMed] [Google Scholar]
- 34.Fadel W, El-Seidy E, Abo-El-Safa A, Khalil M, Mohamed E, Fayed H et al (2012) Prognostic value of apolipoprotein E genotyping in primary intracerebral hemorrhage. Egypt J Neurol Psychiat Neurosurg 49(3):289–292
- 35.Fernandez-Cadenas I, Alvarez Sabin J, Molina CA, Ribo M, Rosell A, Penalba A, et al. ApoE genotype influences on efficacy and safety of thrombolytic treatment for ischemic stroke. Neurologia. 2006;21(4):176–180. [PubMed] [Google Scholar]
- 36.Fernandez-Cadenas I, Del Rio-Espinola A, Rubiera M, Mendioroz M, Domingues-Montanari S, Cuadrado E, et al. PAI-1 4G/5G polymorphism is associated with brain vessel reocclusion after successful fibrinolytic therapy in ischemic stroke patients. Int J Neurosci. 2010;120(4):245–251. doi: 10.3109/00207451003597169. [DOI] [PubMed] [Google Scholar]
- 37.French MA, Morton SM, Pohlig RT, Reisman DS. The relationship between BDNF Val66Met polymorphism and functional mobility in chronic stroke survivors. Top Stroke Rehabil. 2018;25(4):276–280. doi: 10.1080/10749357.2018.1437938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fridriksson J, Elm J, Stark BC, Basilakos A, Rorden C, Sen S, et al. BDNF genotype and tDCS interaction in aphasia treatment. Brain Stimul. 2018;11(6):1276–1281. doi: 10.1016/j.brs.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giannakopoulou E, Ragia G, Marousi S, Ellul J, Manolopoulos VG, Tavridou A. Association of monocyte chemoattractant protein-1 -2518A>G polymorphism with occurrence, severity, and outcome in ischemic stroke. Neurol Sci. 2013;34(8):1315–1320. doi: 10.1007/s10072-012-1235-2. [DOI] [PubMed] [Google Scholar]
- 40.Gomez-Sanchez JC, Delgado-Esteban M, Rodriguez-Hernandez I, Sobrino T, de la Ossa N P, Reverte S, et al. The human Tp53 Arg72Pro polymorphism explains different functional prognosis in stroke. J Exp Med. 2011;208(3):429–437. doi: 10.1084/jem.20101523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzalez-Conejero R, Fernandez-Cadenas I, Iniesta JA, Marti-Fabregas J, Obach V, Alvarez-Sabin J, et al. Role of fibrinogen levels and factor XIII V34L polymorphism in thrombolytic therapy in stroke patients. Stroke. 2006;37(9):2288–2293. doi: 10.1161/01.STR.0000236636.39235.4f. [DOI] [PubMed] [Google Scholar]
- 42.Gromadzka G, Sarzynska-Dlugosz I, Czlonkowska A. IL1RN intron 2 polymorphism caused by variable number tandem repeats is associated with 1-year outcome in patients with ischaemic stroke. J Neurol Neurosurg Psychiatry. 2007;78(2):183–186. doi: 10.1136/jnnp.2006.093104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gromadzka G, Baranska-Gieruszczak M, Sarzynska-Dlugosz I, Ciesielska A, Czlonkowska A. The APOE polymorphism and 1-year outcome in ischemic stroke: genotype-gender interaction. Acta Neurol Scand. 2007;116(6):392–398. doi: 10.1111/j.1600-0404.2007.00880.x. [DOI] [PubMed] [Google Scholar]
- 44.Gu M, Wang M, Cai B, Cheng X, Li Z, Sun B, et al. Chromosome 10q25 polymorphism is associated with susceptibility to large artery atherosclerotic stroke. Gene. 2018;691:18–23. doi: 10.1016/j.gene.2018.12.026. [DOI] [PubMed] [Google Scholar]
- 45.Guo J, Yu L, Zhang J, Chen N, Zhou M, He L. CRP gene polymorphism predicts post-stroke functional outcome in Han Chinese. Acta Neurol Scand. 2014;129(4):263–268. doi: 10.1111/ane.12180. [DOI] [PubMed] [Google Scholar]
- 46.He QS, Yang LF, Wang WB, Yuan B, Zhang LY, Guo XJ. Vascular endothelial growth factor gene is associated with hypertensive cerebellar hemorrhage and rehabilitative treatment. Genet Mol Res. 2015;14(3):9849–9857. doi: 10.4238/2015.August.19.18. [DOI] [PubMed] [Google Scholar]
- 47.He W, Lu M, Li G, Sun Z, Liu D, Gu L. Methylene tetrahydrofolate reductase (MTHFR) rs868014 polymorphism regulated by miR-1203 associates with risk and short term outcome of ischemic stroke. Cell Physiol Biochem. 2017;41(2):701–710. doi: 10.1159/000458429. [DOI] [PubMed] [Google Scholar]
- 48.He W, Wang Q, Gu L, Zhong L, Liu D. NOX4 rs11018628 polymorphism associates with a decreased risk and better short-term recovery of ischemic stroke. Exp Ther Med. 2018;16(6):5258–5264. doi: 10.3892/etm.2018.6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Helm EE, Tyrell CM, Pohlig RT, Brady LD, Reisman DS. The presence of a single-nucleotide polymorphism in the BDNF gene affects the rate of locomotor adaptation after stroke. Exp Brain Res. 2016;234(2):341–351. doi: 10.1007/s00221-015-4465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoy A, Leininger-Muller B, Poirier O, Siest G, Gautier M, Elbaz A, Amarenco P, Visvikis S. Myeloperoxidase polymorphisms in brain infarction. Association with infarct size and functional outcome. Atherosclerosis. 2003;167(2):223–230. doi: 10.1016/s0021-9150(02)00041-2. [DOI] [PubMed] [Google Scholar]
- 51.James ML, Blessing R, Bennett E, Laskowitz DT. Apolipoprotein E modifies neurological outcome by affecting cerebral edema but not hematoma size after intracerebral hemorrhage in humans. J Stroke Cerebrovasc Dis. 2009;18(2):144–149. doi: 10.1016/j.jstrokecerebrovasdis.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jia DM, Chen ZB, Zhang MJ, Yang WJ, Jin JL, Xia YQ, Zhang CL, Shao Y, Chen C, Xu Y. CYP2C19 polymorphisms and antiplatelet effects of clopidogrel in acute ischemic stroke in China. Stroke. 2013;44(6):1717–1719. doi: 10.1161/STROKEAHA.113.000823. [DOI] [PubMed] [Google Scholar]
- 53.Jing M, Li B, Hou X, Shoba J, Li C, Liang H, Zhang X, Liu E, Yang B, Meng X. OPN gene polymorphism and the serum OPN levels confer the susceptibility and prognosis of ischemic stroke in Chinese patients. Cell Physiol Biochem. 2013;32(6):1798–1807. doi: 10.1159/000356613. [DOI] [PubMed] [Google Scholar]
- 54.Keshavarz P, Saberi A, Sharafshah A, Asgari K, Rezaei S. Association of BDNF G196A gene polymorphism with ischemic stroke occurrence and its 6-month outcome in an Iranian population. Top Stroke Rehabil. 2016;23(4):254–260. doi: 10.1080/10749357.2016.1141491. [DOI] [PubMed] [Google Scholar]
- 55.Kim JM, Stewart R, Park MS, Kang HJ, Kim SW, Shin IS, Kim HR, Shin MG, Cho KH, Yoon JS. Associations of BDNF genotype and promoter methylation with acute and long-term stroke outcomes in an East Asian cohort. PLoS One. 2012;7(12):e51280. doi: 10.1371/journal.pone.0051280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee EJ, Oh MS, Kim JS, Chang DI, Park JH, Cha JK, Heo JH, Sohn SI, Kim DE, Kim HY, Kim J, Seo WK, Lee J, Park SW, Kim YJ, Lee BC. Serotonin transporter gene polymorphisms may be associated with poststroke neurological recovery after escitalopram use. J Neurol Neurosurg Psychiatry. 2018;89(3):271–276. doi: 10.1136/jnnp-2017-316882. [DOI] [PubMed] [Google Scholar]
- 57.Li Z, Luo S, Wang F, Zhen J, Sun H, Guo C. An association study between polymorphisms of the fractalkine receptor gene, CX3CR1, and cerebral infarction in the Han Chinese population. J Neurol Sci. 2012;320(1–2):12–15. doi: 10.1016/j.jns.2012.05.044. [DOI] [PubMed] [Google Scholar]
- 58.Liepert J, Heller A, Behnisch G, Schoenfeld A. Catechol-O-methyltransferase polymorphism influences outcome after ischemic stroke: a prospective double-blind study. Neurorehabil Neural Repair. 2013;27(6):491–496. doi: 10.1177/1545968313481282. [DOI] [PubMed] [Google Scholar]
- 59.Liu Y, Laakso MP, Karonen JO, Vanninen RL, Nuutinen J, Soimakallio S, Aronen HJ. Apolipoprotein E polymorphism and acute ischemic stroke: a diffusion- and perfusion-weighted magnetic resonance imaging study. J Cereb Blood Flow Metab. 2002;22(11):1336–1342. doi: 10.1097/01.WCB.0000033200.58646.B3. [DOI] [PubMed] [Google Scholar]
- 60.Lovkvist H, Jonsson AC, Luthman H, Jood K, Jern C, Wieloch T, et al. Variations in apolipoprotein D and sigma non-opioid intracellular receptor 1 genes with relation to risk, severity and outcome of ischemic stroke. BMC Neurol. 2014;14:191-014-0191-2. doi: 10.1186/s12883-014-0191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.MacLeod MJ, De Lange RP, Breen G, Meiklejohn D, Lemmon H, Clair DS. Lack of association between apolipoprotein E genoype and ischaemic stroke in a Scottish population. Eur J Clin Investig. 2001;31(7):570–573. doi: 10.1046/j.1365-2362.2001.00851.x. [DOI] [PubMed] [Google Scholar]
- 62.Maguire J, Thakkinstian A, Levi C, Lincz L, Bisset L, Sturm J, Scott R, Whyte S, Attia J. Impact of COX-2 rs5275 and rs20417 and GPIIIa rs5918 polymorphisms on 90-day ischemic stroke functional outcome: a novel finding. J Stroke Cerebrovasc Dis. 2011;20(2):134–144. doi: 10.1016/j.jstrokecerebrovasdis.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 63.Malueka RG, Dwianingsih EK, Sutarni S, Bawono RG, Bayuangga HF, Gofir A, Setyopranoto I. The D allele of the angiotensin-converting enzyme (ACE) insertion/deletion (I/D) polymorphism is associated with worse functional outcome of ischaemic stroke. Int J Neurosci. 2018;128(8):697–704. doi: 10.1080/00207454.2017.1412962. [DOI] [PubMed] [Google Scholar]
- 64.Manso H, Krug T, Sobral J, Albergaria I, Gaspar G, Ferro JM, et al. Variants of the matrix metalloproteinase-2 but not the matrix metalloproteinase-9 genes significantly influence functional outcome after stroke. BMC Med Genet. 2010;11:40-2350-11-40. doi: 10.1186/1471-2350-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marini S, Devan WJ, Radmanesh F, Miyares L, Poterba T, Hansen BM, Norrving B, Jimenez-Conde J, Giralt-Steinhauer E, Elosua R, Cuadrado-Godia E, Soriano C, Roquer J, Kourkoulis CE, Ayres AM, Schwab K, Tirschwell DL, Selim M, Brown DL, Silliman SL, Worrall BB, Meschia JF, Kidwell CS, Montaner J, Fernandez-Cadenas I, Delgado P, Greenberg SM, Lindgren A, Matouk C, Sheth KN, Woo D, Anderson CD, Rosand J, Falcone GJ, on behalf of the International Stroke Genetics Consortium 17p12 influences hematoma volume and outcome in spontaneous intracerebral hemorrhage. Stroke. 2018;49(7):1618–1625. doi: 10.1161/STROKEAHA.117.020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marousi S, Antonacopoulou A, Kalofonos H, Papathanasopoulos P, Karakantza M, Ellul J. Functional inflammatory genotypes in ischemic stroke: could we use them to predict age of onset and long-term outcome? Stroke Res Treat. 2011;2011:792923. doi: 10.4061/2011/792923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCarron MO, Muir KW, Nicoll JA, Stewart J, Currie Y, Brown K, et al. Prospective study of apolipoprotein E genotype and functional outcome following ischemic stroke. Arch Neurol. 2000;57(10):1480–1484. doi: 10.1001/archneur.57.10.1480. [DOI] [PubMed] [Google Scholar]
- 68.Mirowska-Guzel D, Gromadzka G, Czlonkowski A, Czlonkowska A. BDNF -270 C>T polymorphisms might be associated with stroke type and BDNF -196 G>A corresponds to early neurological deficit in hemorrhagic stroke. J Neuroimmunol. 2012;249(1–2):71–75. doi: 10.1016/j.jneuroim.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 69.Mirowska-Guzel D, Gromadzka G, Seniow J, Lesniak M, Bilik M, Waldowski K, et al. Association between BDNF-196 G>A and BDNF-270 C>T polymorphisms, BDNF concentration, and rTMS-supported long-term rehabilitation outcome after ischemic stroke. NeuroRehabilitation. 2013;32(3):573–582. doi: 10.3233/NRE-130879. [DOI] [PubMed] [Google Scholar]
- 70.Mirowska-Guzel D, Gromadzka G, Mendel T, Janus-Laszuk B, Dzierka J, Sarzynska-Dlugosz I, Czlonkowski A, Czlonkowska A. Impact of BDNF -196 G>A and BDNF -270 C>T polymorphisms on stroke rehabilitation outcome: sex and age differences. Top Stroke Rehabil. 2014;21(Suppl 1):S33–S41. doi: 10.1310/tsr21S1-S33. [DOI] [PubMed] [Google Scholar]
- 71.Munshi A. Genetic variation in MDR1, LPL and eNOS genes and the response to atorvastatin treatment in ischemic stroke. Hum Genet. 2012;131(11):1775–1781. doi: 10.1007/s00439-012-1202-2. [DOI] [PubMed] [Google Scholar]
- 72.Murthy SB, Levy AP, Duckworth J, Schneider EB, Shalom H, Hanley DF, Tamargo RJ, Nyquist PA. Presence of haptoglobin-2 allele is associated with worse functional outcomes after spontaneous intracerebral hemorrhage. World Neurosurg. 2015;83(4):583–587. doi: 10.1016/j.wneu.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 73.Olsson S, Jood K, Blomstrand C, Jern C. Genetic variation on chromosome 9p21 shows association with the ischaemic stroke subtype large-vessel disease in a Swedish sample aged </= 70. Eur J Neurol. 2011;18(2):365–367. doi: 10.1111/j.1468-1331.2010.03096.x. [DOI] [PubMed] [Google Scholar]
- 74.Park HK, Jo DJ. Polymorphisms of integrin, alpha 6 contribute to the development and neurologic symptoms of intracerebral hemorrhage in korean population. J Korean Neurosurg Soc. 2011;50(4):293–298. doi: 10.3340/jkns.2011.50.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Park HK, Kim MC, Kim SM, Jo DJ. Assessment of two missense polymorphisms (rs4762 and rs699) of the angiotensinogen gene and stroke. Exp Ther Med. 2013;5(1):343–349. doi: 10.3892/etm.2012.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qiu LN, Sun Y, Wang L, Han RF, Xia XS, Liu J, Li X. Influence of CYP2C19 polymorphisms on platelet reactivity and clinical outcomes in ischemic stroke patients treated with clopidogrel. Eur J Pharmacol. 2015;747:29–35. doi: 10.1016/j.ejphar.2014.11.037. [DOI] [PubMed] [Google Scholar]
- 77.Ragia G, Marousi S, Ellul J, Manolopoulos VG, Tavridou A. Association of functional VKORC1 promoter polymorphism with occurrence and clinical aspects of ischemic stroke in a Greek population. Dis Markers. 2013;35(6):641–646. doi: 10.1155/2013/769574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ramos-Araque Maria E., Rodriguez Cristina, Vecino Rebeca, Cortijo Garcia Elisa, de Lera Alfonso Mercedes, Sanchez Barba Mercedes, Colàs-Campàs Laura, Purroy Francisco, Arenillas Juan F., Almeida Angeles, Delgado-Esteban Maria. The Neuronal Ischemic Tolerance Is Conditioned by the Tp53 Arg72Pro Polymorphism. Translational Stroke Research. 2018;10(2):204–215. doi: 10.1007/s12975-018-0631-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ritarwan K, Amir D, Sembiring R, Sadewa A, Lelo A. Outcomes of ischemic stroke and -455 G/A beta fibrinogen gene polymorphism. Int J Pharm Res Health Sci. 2014;2(6):420. [Google Scholar]
- 80.Rodriguez C, Sobrino T, Agulla J, Bobo-Jimenez V, Ramos-Araque ME, Duarte JJ, et al. Neovascularization and functional recovery after intracerebral hemorrhage is conditioned by the Tp53 Arg72Pro single-nucleotide polymorphism. Cell Death Differ. 2017;24(1):144–154. doi: 10.1038/cdd.2016.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rodriguez C, Ramos-Araque ME, Dominguez-Martinez M, Sobrino T, Sanchez-Moran I, Agulla J, et al. Single-nucleotide polymorphism 309T>G in the MDM2 promoter determines functional outcome after stroke. Stroke. 2018;49(10):2437–2444. doi: 10.1161/STROKEAHA.118.022529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sarzynska-Dlugosz I, Gromadzka G, Baranska-Gieruszczak M, Ciesielska A, Czlonkowska A. APOE does not predict poor outcome 1 year after ischemic stroke. Neurol Res. 2007;29(1):64–69. doi: 10.1179/174313206X152528. [DOI] [PubMed] [Google Scholar]
- 83.Sharma V, Kaul S, Al-Hazzani A, Prabha TS, Rao PP, Dadheech S, et al. Association of C3435T multi drug resistance gene-1 polymorphism with aspirin resistance in ischemic stroke and its subtypes. J Neurol Sci. 2012;315(1–2):72–76. doi: 10.1016/j.jns.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 84.Sharma V, Dadheech S, Kaul S, Jyothy A, Munshi A. Association of ALOX5AP1 SG13S114T/A variant with ischemic stroke, stroke subtypes and aspirin resistance. J Neurol Sci. 2013;331(1–2):108–113. doi: 10.1016/j.jns.2013.05.024. [DOI] [PubMed] [Google Scholar]
- 85.Sharma V, Kaul S, Al-Hazzani A, Alshatwi AA, Jyothy A, Munshi A. Association of COX-2 rs20417 with aspirin resistance. J Thromb Thrombolysis. 2013;35(1):95–99. doi: 10.1007/s11239-012-0777-8. [DOI] [PubMed] [Google Scholar]
- 86.Shi J, He W, Wang Y, Hua J. Tagging functional polymorphism in 3’ untranslated region of methylene tetrahydrofolate reductase and risk of ischemic stroke. Cell Physiol Biochem. 2018;46(3):1019–1026. doi: 10.1159/000488833. [DOI] [PubMed] [Google Scholar]
- 87.Shiner CT, Pierce KD, Thompson-Butel AG, Trinh T, Schofield PR, McNulty PA. BDNF genotype interacts with motor function to influence rehabilitation responsiveness Poststroke. Front Neurol. 2016;7:69. doi: 10.3389/fneur.2016.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Song YL, Wang CJ, Wu YP, Lin J, Wang PL, Du WL, et al. Phosphodiesterase 4D polymorphisms associate with the short-term outcome in ischemic stroke. Sci Rep. 2017;7:42914. doi: 10.1038/srep42914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stanne TM, Tjarnlund-Wolf A, Olsson S, Jood K, Blomstrand C, Jern C. Genetic variation at the BDNF locus: evidence for association with long-term outcome after ischemic stroke. PLoS One. 2014;9(12):e114156. doi: 10.1371/journal.pone.0114156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Treger I, Froom P, Ring H, Friedman G. Association between apolipoprotein E4 and rehabilitation outcome in hospitalized ischemic stroke patients. Arch Phys Med Rehabil. 2003;84(7):973–976. doi: 10.1016/s0003-9993(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 91.Wang M, Gu M, Li Z, Sun B, Cheng X, Dai Z, Li S, Xiao L, Zhao M, Wang Z, Lin Y, Liu Y, Xu J, Zhang Z, Liu X. HDAC9 polymorphisms predict susceptibility, severity, and short-term outcome of large artery atherosclerotic stroke in Chinese population. J Mol Neurosci. 2019;67(1):165–171. doi: 10.1007/s12031-018-1221-0. [DOI] [PubMed] [Google Scholar]
- 92.Wang Y, Cai H, Zhou G, Zhang Z, Liu X. Effect of CYP2C19*2 and *3 on clinical outcome in ischemic stroke patients treated with clopidogrel. J Neurol Sci. 2016;369:216–219. doi: 10.1016/j.jns.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 93.Weinstein JR, Schulze J, Lee RV, Phillips H, Zierath D, Tanzi P, Shibata D, Cain KC, Becker KJ. Functional polymorphisms in toll-like receptor 4 are associated with worse outcome in acute ischemic stroke patients. Neuroreport. 2014;25(8):580–584. doi: 10.1097/WNR.0000000000000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu H, Weng Y, Zheng L, Li H, Gong Q, Fu Y, Zhao J. Polymorphism of the complement 5 gene is associated with large artery atherosclerosis stroke in Chinese patients. Arq Neuropsiquiatr. 2016;74(11):881–886. doi: 10.1590/0004-282X20160139. [DOI] [PubMed] [Google Scholar]
- 95.Wzorek J, Karpinski M, Wypasek E, Michalski M, Szczudlik A, Malinowski KP, et al. Alpha-2-antiplasmin Arg407Lys polymorphism and cryptogenic ischemic cerebrovascular events: Association with neurological deficit. Neurol Neurochir Pol. 2018;52(3):352–358. doi: 10.1016/j.pjnns.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 96.Xia C, Lin S, Yang J, He S, Li H, Liu M, You C. Genetic variations of COL4A1 gene and intracerebral hemorrhage outcome: a cohort study in a Chinese Han population. World Neurosurg. 2018;113:e521–e528. doi: 10.1016/j.wneu.2018.02.074. [DOI] [PubMed] [Google Scholar]
- 97.Yan J, MG J, AM P. Interleukin 6 promoter 174 G/C polymorphisms in acute ischemic stroke: G allele is protective but not associated with IL-6 levels or stroke outcome. J Neuroimmunol. 2016;293:22–27. doi: 10.1016/j.jneuroim.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 98.Yang J, Lin S, Zhou J, Wu B, Dong W, Arima H, Liu H, Zhang J, Li J, Liu M, Chengdu Stroke Registry and Nanjing First Hospital Stroke Registry investigators Genetic variations of MMP9 gene and intracerebral hemorrhage outcome: a cohort study in Chinese Han population. J Neurol Sci. 2014;343(1–2):56–59. doi: 10.1016/j.jns.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 99.Ye Z, Zhang H, Sun L, Cai H, Hao Y, Xu Z, Zhang Z, Liu X. GWAS-supported CRP gene polymorphisms and functional outcome of large artery atherosclerotic stroke in Han Chinese. NeuroMolecular Med. 2018;20(2):225–232. doi: 10.1007/s12017-018-8485-y. [DOI] [PubMed] [Google Scholar]
- 100.Yi X, Lin J, Wang Y, Zhou Q, Wang C, Cheng W, Chi L. Association of cytochrome P450 genetic variants with clopidogrel resistance and outcomes in acute ischemic stroke. J Atheroscler Thromb. 2016;23(10):1188–1200. doi: 10.5551/jat.33290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang J, Yin C, Zhang Y, Zhao L, Fu H, Feng J. The role of OLR1 polymorphisms in determining the risk and prognosis of ischemic stroke in a Chinese population. NeuroRehabilitation. 2013;32(2):391–396. doi: 10.3233/NRE-130860. [DOI] [PubMed] [Google Scholar]
- 102.Zhang Y, Liu S, Yue W, Shi Z, Guan Y, Li M, Ji Y, Li X. Association of apolipoprotein E genotype with outcome in hospitalized ischemic stroke patients. Medicine (Baltimore) 2017;96(50):e8964. doi: 10.1097/MD.0000000000008964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhao J, Wu H, Zheng L, Weng Y, Mo Y. Brain-derived neurotrophic factor G196A polymorphism predicts 90-day outcome of ischemic stroke in Chinese: a novel finding. Brain Res. 2013;1537:312–318. doi: 10.1016/j.brainres.2013.08.061. [DOI] [PubMed] [Google Scholar]
- 104.Zhao J, Bai Y, Jin L, Weng Y, Wang Y, Wu H, Li X, Huang Y, Wang S. A functional variant in the 3’-UTR of VEGF predicts the 90-day outcome of ischemic stroke in Chinese patients. PLoS One. 2017;12(2):e0172709. doi: 10.1371/journal.pone.0172709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weir CJ, McCarron MO, Muir KW, Dyker AG, Bone I, Lees KR, et al. Apolipoprotein E genotype, coagulation, and survival following acute stroke. Neurology. 2001;57(6):1097–1100. doi: 10.1212/wnl.57.6.1097. [DOI] [PubMed] [Google Scholar]
- 106.Mang CS, Campbell KL, Ross CJ, Boyd LA. Promoting neuroplasticity for motor rehabilitation after stroke: considering the effects of aerobic exercise and genetic variation on brain-derived neurotrophic factor. Phys Ther. 2013;93(12):1707–1716. doi: 10.2522/ptj.20130053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Terracciano A, Piras MG, Lobina M, Mulas A, Meirelles O, Sutin AR, Chan W, Sanna S, Uda M, Crisponi L, Schlessinger D. Genetics of serum BDNF: meta-analysis of the Val66Met and genome-wide association study. World J Biol Psychiatry. 2013;14(8):583–589. doi: 10.3109/15622975.2011.616533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, Fan G, Sun YE. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302(5646):890–893. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- 109.Manso H, Krug T, Sobral J, Albergaria I, Gaspar G, Ferro JM, Oliveira SA, Vicente AM. Evidence for epistatic gene interactions between growth factor genes in stroke outcome. Eur J Neurol. 2012;19(8):1151–1153. doi: 10.1111/j.1468-1331.2011.03625.x. [DOI] [PubMed] [Google Scholar]
- 110.Banerjee Gargi, Carare Roxana, Cordonnier Charlotte, Greenberg Steven M, Schneider Julie A, Smith Eric E, Buchem Mark van, Grond Jeroen van der, Verbeek Marcel M, Werring David J. The increasing impact of cerebral amyloid angiopathy: essential new insights for clinical practice. Journal of Neurology, Neurosurgery & Psychiatry. 2017;88(11):982–994. doi: 10.1136/jnnp-2016-314697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 85 kb)
Data Availability Statement
No additional data are available.