Abstract
Adolescent idiopathic scoliosis (AIS) is a three-dimensional (3D) deformity of the spinal column in pediatric population. The primary cause of scoliosis remains unknown. The lack of such understanding has hampered development of effective preventive methods for management of this disease. A long-held assumption in pathogenesis of AIS is that the upright spine in human plays an important role in induction of scoliosis. Here, the variations in the sagittal curve of the scoliotic and non-scoliotic pediatric spines were used to study whether specific sagittal curves, under physiological loadings, are prone to 3D deformation leading to scoliosis. To this end, finite element models of the S shaped elastic rods, which their curves were derived from the radiographs of 129 sagittal spinal curves of adolescents with and without scoliosis, were generated. Using the mechanics of deformation in elastic rods, this study showed that the 3D deformation patterns of the two-dimensional S shaped slender elastic rods mimics the 3D patterns of the spinal deformity in AIS patients with the same S shaped sagittal spinal curve. On the other hand, the rods representing the non-scoliotic sagittal spinal curves, under the same mechanical loading, did not twist thus did not lead to a 3D deformation. This study provided strong evidence that the shape of the sagittal profile in individuals can be a leading cause of the 3D spinal deformity as is observed in the AIS population.
Subject terms: Paediatric research, Pathogenesis, Biomedical engineering
Introduction
Adolescent idiopathic scoliosis (AIS) manifests as a three-dimensional (3D) deformity of the spinal column that occurs around puberty. Several factors such as genetics1,2, biomechanics3, morphology of the vertebral bones, intervertebral disc, and muscle physiology4–9, hormones10,11, environmental factors12,13, and neural system deficiencies14,15 are considered as the underlying cause of the spinal deformity in AIS. While each of these factors explained part of the pathogenesis of this disease, none have proved to be its main or primary cause. As experimental studies suggest, the role of the mechanical loading of the upright spine in pathogenesis of the disease cannot be ignored16–21.
The unique shape of the human spine has been hypothesized to relate to the induction of scoliosis3. The dorsal loading of the upright spine in human was proposed to result in spinal instability and has been suggested as the biomechanical causation of scoliosis22. A shift in the position of the center of mass around puberty results in asymmetric loading of the thorax23. This causes rotation of the spinal segments away from the sagittal plane which in turn results in an increased differentiated loading of the anterior and posterior aspects of the vertebrae, causing asymmetric growth and bone deformation under the Hueter-Volkmann law24,25. While the mechanism of deformity progression in AIS is widely accepted, the underlying mechanisms that trigger instability in the spine and excessive rotation, leading to scoliosis in 2–4% of the pediatric population, are not well explained.
The mechanics of deformation in elastic rods are explored experimentally and analytically26–29. These concepts have been successfully explained the physics of various topological transformation in DNA and proteins (e.g., from a loop to a twisted loop)30–32. In a previous topological classification of the spinal curves in AIS patients33,34, two distinct axial deformation patterns of the scoliotic spines were observed: if a non-scoliotic spine projects into a straight line from the top view (axial view), the scoliotic spine has either a V shaped or a S shaped projection34. The V shaped projection can be conceived as a loop (by connecting the two ends) whereas the S shaped projection presents a twisted loop or a lemniscate shape. Figure 1 demonstrates these two different projection patterns. As these two axial projections (Fig. 1) were associated with different sagittal curves in the scoliotic classification34, the question was raised that whether the variation in the sagittal curves of the pediatric spine dictates the patterns of spinal deformation and can be the leading cause of the 3D spinal deformity i.e. scoliosis.
Figure 1.
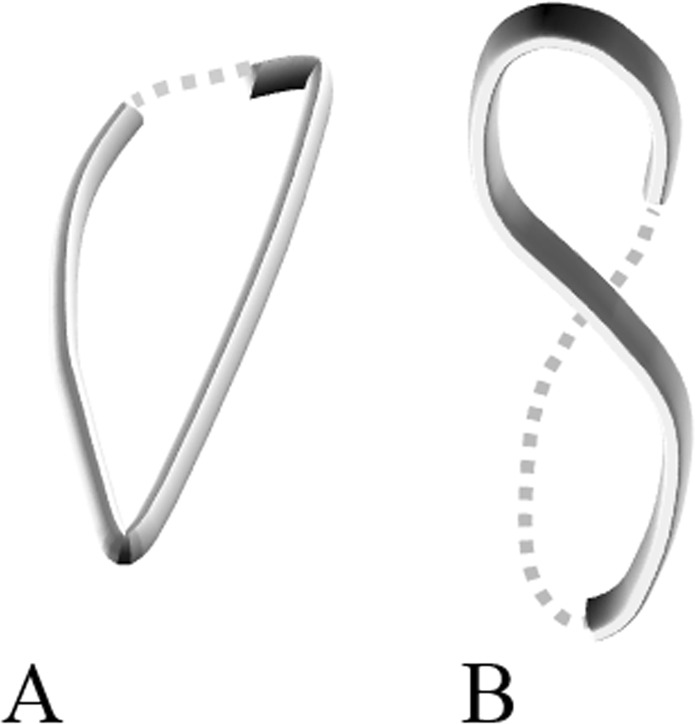
Two different axial projections of the scoliotic spines33 (A) V shaped projection that can be extrapolated to a closed loop and (B) S shaped projection that can be extrapolated to a twisted loop (lemniscate).
In this study, finite element analysis (FEA) of two-dimensional (2D) S shaped elastic rods was conducted to determine the deformation patterns of the rods as a function of variations in the 2D curve geometry, while the mechanical loading and material properties were the same across the models. The geometry of the 2D S shaped rods were determined from the two-view spinal X-rays of 126 right thoracic AIS patients and three age- and sex-matched non-scoliotic adolescents. It was hypothesized that the patterns of 3D deformation of 2D S shaped elastic rods (spine sagittal profiles) relate to the 3D patterns of the spinal deformity in the AIS patients with the same sagittal curves.
Results
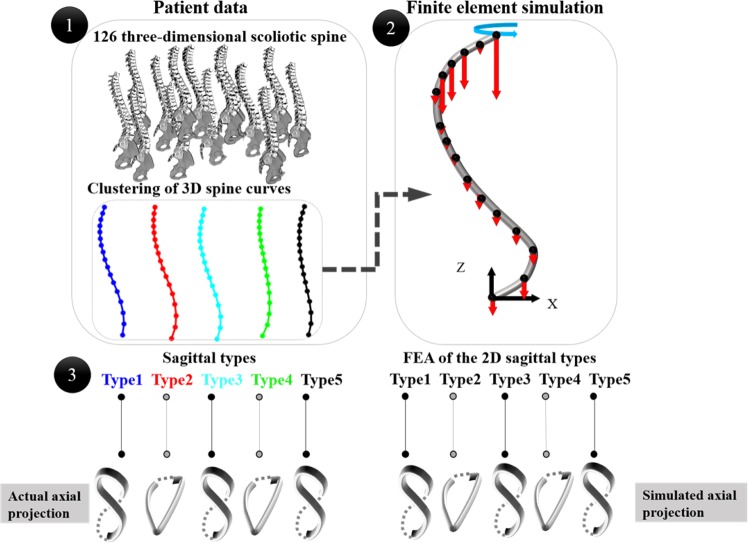
The study’s workflow is shown in Fig. 2. The study steps include: 1- classification of 126 scoliotic 3D spinal curves34,35, 2- developing the finite element models of the curved elastic rods using the S shaped sagittal spinal curve of the scoliotic cluster centers and the average of three non-scoliotic spines. 3- Comparing the patterns of deformation between the actual spinal deformity in the patient subtypes and the simulated curved rods deformations.
Figure 2.
The schematic of the study workflow: 1- Clustering of 126 right thoracic AIS and identifying the sagittal subtypes. 2- Finite element analysis (FEA) of the curved elastic rod – the geometry of the rods were determined from the AIS sagittal subtypes. The red arrows show the magnitude of the gravitational load at each vertebral level (Supplementary Materials-Table 1S). The blue arrow shows the direction of the torsion moment. 3- Comparison between the patterns of 3D deformation of the spine in AIS subtypes and the curved (S shaped) elastic rods with the same 2D curvature as the AIS sagittal subtypes.
Classification and sagittal subtypes
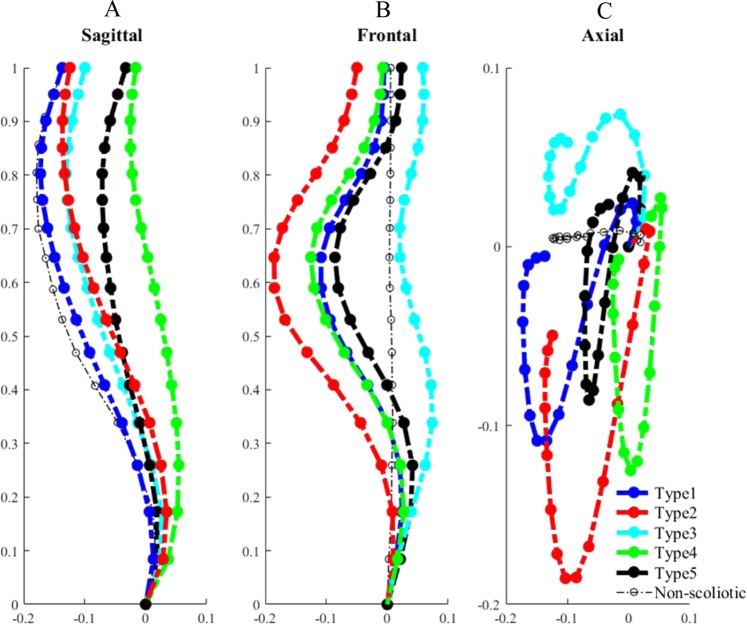
Figure 3 shows the five subtypes of the 126 right thoracic AIS patients resulted from the clustering of the 3D spinal curves: Type 1: n = 30, Type 2: n = 29, Type 3: n = 18, Type 4: n = 26, and Type 5: n = 23. The five subtypes’ sagittal, frontal, and axial views of the spinal curves are shown in Fig. 3A–C, respectively. The average of the non-scoliotic spinal curves is also superimposed on Fig. 3. The axial and frontal projections of the non-scoliotic spine were close to a straight line (Fig. 3B,C).
Figure 3.
Five right thoracic scoliotic subtypes and the average of non-scoliotic spinal profiles in (A) Sagittal, (B) Frontal, (C) Axial views. The X, Y, Z axes show unite-less normalized distances.
The five scoliotic subtypes were divided into two subgroups based on the patterns of axial projections: Group I included Types 2 and 4 that have a loop shaped axial projection (Fig. 3C). In these patients the kyphotic (concave) segment of the curve was shorter than the lordotic (convex) section (6 ± 2 vertebrae versus 11 ± 2) and the inflection point of the curve moves to the mid thoracic section (see Methods section). Group II included Types 1, 3, and 5 that have a lemniscate axial projection (Fig. 3C). In these patients the kyphotic part of the curve was longer than the lordotic section (12 ± 2 versus 5 ± 2 vertebrae) and the inflection point of the sagittal curve moves to the lower thoracic/ upper lumbar area. In the non-scoliotic cohort the kyphotic section is longer than the lordotic section (12 ± 2 versus 4 ± 1 vertebrae), but the inflection point is slightly lower compared to the position of the inflection point in Group II.
Finite element analysis and 3D deformation
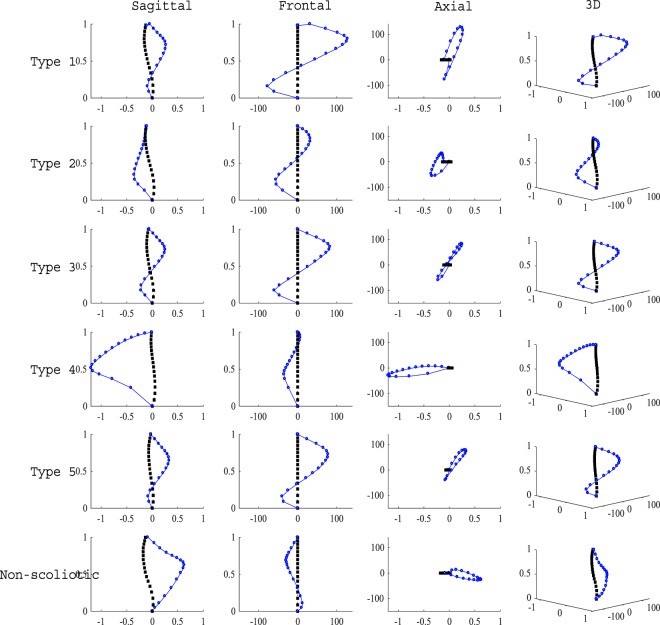
Figure 4 shows the results of the FEA of the five sagittal subtypes and the non-scoliotic spines. The final shape of the deformed rod (blue dotted line) was superimposed on the original shape of the rods (black dotted line), in the frontal, sagittal, axial, and 3D views (Fig. 4). All rods developed two curves in the frontal planes under the aforementioned loading (Fig. 2). The axial shape of the deformed rods with the geometry of the Types 2 and 4 scoliotic subtypes (Group I) was loop shaped. The axial shape of the deformed rods with the geometry of the Types 1, 3, and 5 scoliotic subtypes (Group II) were lemniscate. The axial shape of the “non-scoliotic” deformed elastic rod was loop shaped but deformed in the opposite direction of the axial projection of the Group I curves.
Figure 4.
The initial (black line) and final curvature (blue line) of the elastic rods representing the five scoliotic sagittal subtypes and non-scoliotic spine under gravitational loading and torsion in frontal, sagittal, axial, and 3D views. The axes show the isotropic normalized lengths in the Z-direction for both initial and deformed curves.
The axial deformations (differences between the final and initial positions of each point on the rod) of the rods representing the sagittal subtypes and non-scoliotic spines are superimposed in Fig. 5 to allow a better comparison between the deformation patterns of the Group I AIS (Types 2 and 4), Group II AIS (Types 1, 3, and 5), and the average of the three non-scoliotic adolescents. The deformations are normalized to the maximum deformation in the Z direction. Group I rods showed a looped shaped axial deformation (also Supplementary Materials Fig. 3S-A) and Group II rods showed a lemniscate axial deformation (also Supplementary Materials Fig. 3S-B). The axial deformation of the non-scoliotic rod was distinctively different from Group I and Group II (also Supplementary Materials Fig. 3S-C). The ratio of the average displacement in the X direction to the Y direction was: Type1 = 0.004, Type2 = 0.05, Type3 = 0.004, Type4 = 0.06, Type5 = 0.006, and non-scoliotic = −0.01.
Figure 5.
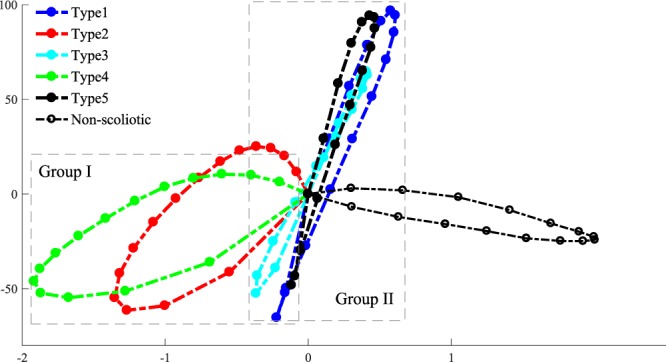
The superimposed axial projection of the normalized 3D deformation of the S shaped rods. The X and Y axes show unite-less normalized distances.
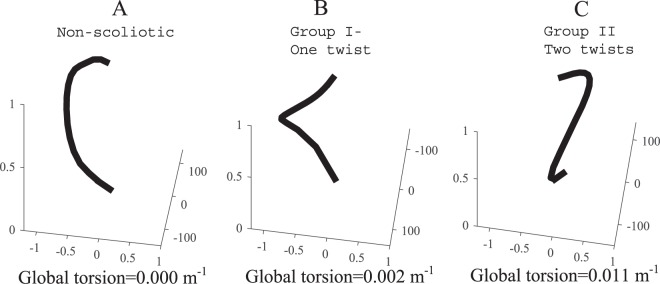
The average deformation shape of the rods in non-scoliotic, Group I, and Group II are shown in Fig. 6. In the non-scoliotic curve, the rod deformation remained in one plane and no twist in the rod was observed (Fig. 6A). The curved rods in Group I (Types 2 and 4) showed one twist in the (Fig. 6B). Rods in Group II (Types 1, 3, 5 models) showed two twists (Fig. 6C). The global torsion of the non-scoliotic deformed curved (Fig. 6A), average Group I deformed curve (Fig. 6B), and average Group II deformed curve (Fig. 6C) were −3.4e-4(m−1), 0.0015(m−1), and 0.0112(m−1), respectively.
Figure 6.
Normalized average 3D deformation of the elastic rods in the “non-scoliotic”, Group I, and Group II rods. The X, Y, Z axes show unit-less normalized distances. The global torsion for each curve is shown65.
The 3D deformation of the additional S shaped rods, resulted from interpolating the sagittal curves in Group I (Types 2 and 4), sagittal curves in Group II (Types 1, 3, and 5), and the three non-scoliotic curves are presented in the Supplementary Materials.
Pattern correlation between the scoliotic subtypes and non-scoliotic deformation of the S shaped rods
The results of the pattern correlation of the 3D deformation (X, Y, Z coordinates) of the rods between Types 1–5 and non-scoliotic 3D deformations are shown in Table 1. The 3D deformations in Group I were strongly correlated; Types 2 and 4, r = 0.89, 95%CI [0.71–0.88], p = 0.00. The 3D deformations in Group II were strongly correlated; Types 1 and 3 and Types 1 and 5, r = 0.99, 95%CI [0.99–0.99], p = 0.00, and Types 3 and 5 r = 0.98, 95%CI [0.96–0.98], p = 0.00. The correlation between the 3D deformation in Groups I and II was non-significant (Types 1 and 4, Types 3 and 4, and Types 4 and 5) or moderately correlated (Types 1 and 2, Types 2 and 3, and Types 2 and 5) (Table 1). 3D deformation of the AIS clusters were significantly correlated to the 3D deformation of the non-scoliotic spines, however the correlation was not as strong as the within AIS groups correlations (Table 1).
Table 1.
Pattern correlation of the 3D rod deformation between the scoliotic subtypes and the non-scoliotic group.
| 1 | 2 | 3 | 4 | 5 | Non-scoliotic | Types |
|---|---|---|---|---|---|---|
| 1 | 0.72 [0.55–0.83], p = 0.00 | 0.99 [0.99–0.99], p = 0.00 | 0.18 [−0.09, 0.43], p = 0.81 | 0.99 [0.99–0.99], p = 0.00 | 0.64 [0.61, 0.89], p = 0.00 | 1 |
| 1 | 0.73 [0.69–0.89], p = 0.00 | 0.89 [0.71–0.88], p = 0.00 | 0.69 [0.51–0.81], p = 0.00 | 0.73 [0.62–0.91], p = 0.00 | 2 | |
| 1 | 0.33 [0.06–0.56], p = 0.83 | 0.98 [0.96–0.98], p = 0.00 | 0.70 [0.61,0.88], p = 0.00 | 3 | ||
| 1 | 0.15 [−0.13,0.41], p = 0.72 | 0.65 [0.60,0.85], p = 0.00 | 4 | |||
| 1 | 0.61 [0.49,0.80], p = 0.00 | 5 | ||||
| 1 | Non-scoliotic |
Discussion
The pathogenesis of the spinal deformity development in AIS is not well understood. Here, concepts in mechanics of elastic rods were used to show that the variations in the sagittal curve relate to the 3D deformation patterns of the spine in AIS. Finite element models of the 2D curved elastic rods, representing different sagittal types in 126 AIS patients34 and three non-scoliotic adolescents, showed that deformation pattern of the curved elastic rods changes as a function of the rod’s curvature and associates with the patterns of 3D spinal deformity in AIS patients with the same sagittal curves. This finding provided evidence that the sagittal curve of the spine is a leading cause of spinal deformity in AIS.
Several biomechanical factors have been related to the mechanism of the spinal deformity development in AIS. Slender vertebrae and discs in AIS patients, compared to the non-scoliotic age-matched adolescents, is believed to increase the spinal flexibility and lead to spinal deformity in AIS36. The sagittal profile in AIS patients was reported to be more posteriorly inclined which increases the posteriorly directed shear force in AIS compared to the age-matched non-scoliotic adolescents contributing to the spinal instbility37. Morphological parameters of the vertebrae and discs were related to the progression rate of scoliosis38. It has been also suggested that the mechanical loading of the upright spine can be the primary cause of scoliosis22. The axial loading of the spine is higher in quadrupeds than bipedal’s spines39. In bipedals a less axially supported spine, which probably contributes to easier upright locomotion40,41, may be impacted by other spinal loadings e.g. shear and make the spine less stable22. The current analysis showed, in an elastic rod, without considering the differences in the spinal components (discs, vertebrae)36,38 and without generalizing the vertebral alignment37, and under the same external loadings, some sagittal subtypes do not twist and deform in only one plane (Fig. 6A) whereas other sagittal curve types are prone to scoliotic-like 3D deformation with one or two twists in the spine (Fig. 6B,C). The 2D shape of the rod changed the acting forces and moments on the rod resulting in variation in the deformation patterns, underlining the role of the spinal sagittal curve in development of spinal deformity.
The classification of the 3D centerlines in this study resulted in identifying a clear axial classification of right thoracic AIS: loop and Lemniscate shapes (previously presented as V and S shaped, respectively)34 (Fig. 1). These shapes are also shown in other biological and mechanical systems28,30–32. We also showed these two axial curve patterns, within the right thoracic cohort, can guide the fusion level as they determine the location of the changes in the axial rotation of the vertebrae thus the number of true 3D spinal curves42. These findings may allude to the fact that the patterns of deformation of the spine in the transverse view, as determined in this study, can explain important differences between the scoliotic curves and can be used as an axial classification of the scoliotic curve.
3D elastic deformation has been observed in many natural and physiological systems such as proteins and DNAs30–32. A looped DNA can twist and transform to a lemniscate shape as a function of bending rigidity of the strands when the system’s energy attains a critical level32,43,44. The fundamentals of these deformations are explained analytically using Kirchhoff’s theory of elastic rods44,45. In analytical examination of the spinal deformity in AIS, mechanical phenomenon such as 2D buckling of the rods have been used to explain the mechanics of spinal deformation previously36,38,46–53. However, considering the 3D deformation of the spine in AIS, the mechanics of 2D buckling fails to explain essential aspects of the curve progression. By taking into account the sagittal curve of the spine, both bending and compression stresses apply on the spine as a result of the gravitation load. Here, a torsional moment also was included to simulate the previously reported shift in the center of mass at the onset of curve development in AIS patients23. This torsion was essential to break the symmetry of the system and initiate the 3D deformation. The result of the current simulation shifts the current beliefs about the mechanism of the spinal deformation in AIS from 2D buckling to 3D elastic deformation under bending and twisting. Understanding the mechanism of spinal deformity development in AIS is the first step in efficient management of the disease that aims to prevent the deformity development or reverse the deformation process.
Modeling the spine as elastic rods assists with understanding the intra-patient differences, which can be useful in clinical management of the scoliosis. As it is shown before, different deformation patterns in elastic rods can be observed by changing the mechanical loading, material stiffness, boundary conditions, or geometry of the rod28,29. It has been shown that a gradual increase in the twist and torsional energy can change the coiling pattern resulting in a twist in the rod28. This phenomenon can explain the differences between the curve patterns in Group I and Group II. Group I patients present with one twist in the curve, smaller torsion, and larger lateral deviation as opposed to the Group II patients with two twists, smaller lateral deviation (coiling radius), and high torsion34 (Fig. 6). As it was shown previously, due to the differences between Group I (Type 2 and 4) and Group II (Types 1, 3, and 5), the patients’ response to the spinal fusion surgery may vary, thus different surgical fusion lengths should be selected for these two groups: Longer fusion in Group I patients is required to stabilize the longer curve as opposed to the patients in Group II where spontaneous correction of the second curve can be expected as a response to the primary curve derotation and correction42. By showing the relationship between the sagittal curve types and deformation patterns in elastic rods, the importance of the spinal sagittal curve correction in both conservative and surgical treatments of AIS were emphasized which was in line with the previous observations35,54–57. Reversing the 3D spinal deformation by derotation and frontal translation of the spine in AIS does not adequately address the condition as here we showed that variation in the sagittal profile could initiate the deformity and is the underlying cause of the deformity. As the variation in sagittal profiles is an important factor in 3D deformation of the spine (Fig. 6), it stands to reason that adjustments in the sagittal plane as part of the curve correction process, both conservatively and surgically, are critical.
Quantitative differences between the simulated deformations of the curved elastic rods and the deformation of the spine in patient groups with the same sagittal curve were expected. Due to the simplifications of the model (e.g., the isotropic homogeneous material properties, ignoring the differential stiffness between the upper and lower sections of the rod (thoracic and lumbar), assuming a zero angle at the L5 (sacral slope)) quantitative differences in the simulated rods and actual 3D spinal deformation were observed; yet patterns of deformations were similar. Moreover, T1 was fixed in the axial plane while in reality both frontal and sagittal imbalances are shown as part of the curve progression mechanism and the postural compensation of the trunk 8,58. This study purposefully used a reduced order model of the spine to test the theoretical role of the spinal sagittal shape in 3D scoliotic deformation without including any other assumption on intra-patient variations.
The mechanics of the 3D deformation of elastic rods showed that the 3D deformation patterns of the spine can be a characteristic of the S shaped curvature of the sagittal spine. However, it is important to notice that this finding does not refute the possibility of forming such sagittal profiles or variation in the mechanical loading (deformation) in individuals as a result of variations in the discs and ligaments material properties or width and height of the vertebral body. Genetics can tie into the development of various sagittal alignment as it may impact the discs, ligaments, and muscles biomechanics, growth rate, or simply the postural differences between individuals. Both rapid growth of the spine and rapid rise of estrogen level during puberty52,53,59,60, and the link between the estrogen and increased ligament laxity61,62 can be linked to the onset of the spinal deformation at that age particularly in the female patients. The current analysis only investigated the role of the sagittal profile in development of 3D spinal deformities. The factors leading to variations in the loading characteristics of the sagittal profile, related to the geometry, growth rate, and mechanical properties of the spine, remain to be explored. Finally, in the current study only right thoracic curves, the most common type of AIS, were included. Future studies that include other curve types are warranted.
In conclusion, the finding of this study reduced the question of why scoliosis happens to why variation in the sagittal spinal profile occurs in the pediatric population. With large dataset on the pediatric population before the onset of the spinal deformity development, the current analysis can be used as a risk assessment tool to predict the risk of spinal deformity development in the pediatric population. This can open a new line of research that aims to prevent the spinal deformity development by determining the spinal loading patterns (the sagittal alignment and flexibility of the immature spine) at a higher risk of 3D deformation in pre-scoliotic pediatric population. Conservative methods to protect the spine during this phase can be explored. This new approach can introduce a changing paradigm in the clinical care of idiopathic scoliosis by shifting the clinical care from the deformity correction to prevention in this patient population.
Methods
Classification and identifying unique sagittal profiles in AIS patients
The institutional review board (IRB) at the Children’s Hospital of Philadelphia approved all the study procedures as detailed in the method section. The research process was performed in accordance with the relevant guidelines and regulations underlined in the approval. As the study was performed retrospectively, a waiver of consent/parental agreement was granted by the IRB.
A total of 126 right thoracic AIS patients were included retrospectively. The inclusion criteria were adolescent between 12–16 years old, a main thoracic curve (most common AIS curve type) exceeding 20° Cobb angle with an apex above T11-T12 intervertebral disc, no previous spinal surgery, no neuromuscular disorders, and no musculoskeletal conditions other than scoliosis. Three non-scoliotic adolescents were included as the control cohort. The non-scoliotic subjects were verified by both clinical examination and two-view radiographs of the spine and did not develop scoliosis at the follow-ups after growth spurt.
3D reconstructions of the orthogonal spinal radiographs were generated for all the subjects using the method described in Humbert et al.63. A previously validated algorithm was used to extract the centroid of the vertebral bodies64. A K-mean classification, with a prior knowledge of the number of clusters33, was used to classify the isotropically normalized 3D spline (X, Y, Z coordinates) resulted from connecting the vertebral bodies centroids35. The sagittal profiles of the non-scoliotic spines were also isotropically normalized to the unit height.
Data preparation
The average sagittal profiles of each AIS cluster was used to create the geometry of the 2D curved (S shaped) elastic rods in the finite element models. The sagittal profiles of the clusters were grouped based on their axial projection patterns; Group I: loop shaped projection and Group II: twisted loop or lemniscate projection (Fig. 1). Additional sagittal profiles were generated by interpolating between the sagittal profiles of the clusters in Group I and between the sagittal profiles of the clusters in Group II. This step was added to include additional S shaped curves and test the sensitivity of the 3D deformation patterns of the S shaped rods to variations in the sagittal curves. The global torsion of the average deformed curves in the non-scoliotic groups, Group I, and Group II were calculated by the method described in65 Based on the sagittal view, the number of vertebrae was counted in the two sections of the spine with different curvatures i.e. spinal convexity and concavity in the sagittal plane. The point at which the sign of the curvature changed or the center of the section over which the sign of the curvature changed was considered as the inflection point.
Finite element models
2D S shaped elastic rods with unit length, circular cross section with a radius of 1e-3, Young modulus of 1000 PA, and a Poisson ratio of 0.3, with isotropic material properties were generated. A small torsion around the Z-axis, +1e-4 Pa, was applied to simulate the asymmetric trunk weight distribution23. The X, Y, Z displacement of the rod at L5 level were fixed. The X and Y displacements at T1 were fixed but the vertical (Z direction) displacement was allowed. The gravity was applied at T1-L5 vertebral centers according to the previously validated experimental data for FEA of the spine66–69 (Table 1.S- Supplementary Material). The FEA was performed for the rods with the sagittal geometry of the scoliotic subtypes and the interpolated sagittal curves, and for the average of the non-scoliotic curves and the individual non-scoliotic spines. The 3D deformation was normalized to the maximum deformation in the Z direction.
Pattern correlation of the deformed rods
The 3D shapes of the deformed 2D S shaped rods were compared between the models in Group I and Group II as well as between the non-scoliotic model and the two AIS groups. 3D pattern correlation related the X, Y, Z coordinates of the deformed rods between the models by calculating the covariance matrix of the 3D deformation patterns to compare the inter- and intra-group correlations between the 3D deformations.
Supplementary information
Acknowledgements
I would like to acknowledge professors Dennis DeTurck, Samuel Kadoury, Pedro Ponte-Castaneda, and Prashant Purohit for insightful conversations.
Data availability
Non-patient related data that support the findings of this study are available from the author upon reasonable request.
Code availability
The executable code is available from the author upon reasonable request for non-commercial purposes only.
Competing interests
The author declares no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-53068-7.
References
- 1.Nada D, et al. Association of Circulating YKL-40 Levels and CHI3L1 Variants with the Risk of Spinal Deformity Progression in Adolescent Idiopathic Scoliosis. Sci Rep. 2019;9:5712. doi: 10.1038/s41598-019-41191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hassan A, et al. Adolescent idiopathic scoliosis associated POC5 mutation impairs cell cycle, cilia length and centrosome protein interactions. PLoS One. 2019;14:e0213269. doi: 10.1371/journal.pone.0213269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarwark JF, Castelein RM, Maqsood A, Aubin CE. The Biomechanics of Induction in Adolescent Idiopathic Scoliosis: Theoretical Factors. J Bone Joint Surg Am. 2019;101:e22. doi: 10.2106/JBJS.18.00846. [DOI] [PubMed] [Google Scholar]
- 4.Wren TA, Ponrartana S, Aggabao PC, Poorghasamians E, Gilsanz V. Association Between Vertebral Cross-sectional Area and Vertebral Wedging in Children and Adolescents: A Cross-sectional Analysis. J Bone Miner Res. 2017;32:2257–2262. doi: 10.1002/jbmr.3210. [DOI] [PubMed] [Google Scholar]
- 5.Begon M, Scherrer SA, Coillard C, Rivard CH, Allard P. Three-dimensional vertebral wedging and pelvic asymmetries in the early stages of adolescent idiopathic scoliosis. Spine J. 2015;15:477–486. doi: 10.1016/j.spinee.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe K, et al. The Influence of Lumbar Muscle Volume on Curve Progression After Skeletal Maturity in Patients With Adolescent Idiopathic Scoliosis: A Long-Term Follow-up Study. Spine Deform. 2018;6:691–698 e691. doi: 10.1016/j.jspd.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Shu CC, Melrose J. The adolescent idiopathic scoliotic IVD displays advanced aggrecanolysis and a glycosaminoglycan composition similar to that of aged human and ovine IVDs. Eur Spine J. 2018;27:2102–2113. doi: 10.1007/s00586-018-5515-2. [DOI] [PubMed] [Google Scholar]
- 8.Pasha S, Sankar WN, Castelein RM. The Link Between the 3D Spino-pelvic Alignment and Vertebral Body morphology in Adolescent Idiopathic Scoliosis. Spine Deform. 2019;7:53–59. doi: 10.1016/j.jspd.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 9.Pasha S, et al. Three-dimensional spinopelvic relative alignment in adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2014;39:564–570. doi: 10.1097/BRS.0000000000000193. [DOI] [PubMed] [Google Scholar]
- 10.Silva RTE, et al. Role of Different Hormones in the Pathogenesis and Severity of Adolescent Idiopathic Scoliosis. Acta Ortop Bras. 2017;25:15–17. doi: 10.1590/1413-785220172501168600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yun YH, et al. Influence of growth hormone treatment on radiographic indices of the spine: propensity-matched analysis. J Orthop Surg Res. 2017;12:130. doi: 10.1186/s13018-017-0630-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayes T, Anadio JM, Sturm PF. Prevalence of Vitamin D Deficiency in Pediatric Patients With Scoliosis Preparing for Spinal Surgery. Spine Deform. 2017;5:369–373. doi: 10.1016/j.jspd.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Ji XR, et al. Change of selenium in environment and risk of adolescent idiopathic scoliosis: a retrospective cohort study. Eur Rev Med Pharmacol Sci. 2013;17:2499–2503. [PubMed] [Google Scholar]
- 14.Picelli A, et al. Do adolescents with idiopathic scoliosis have body schema disorders? A cross-sectional study. J Back Musculoskelet Rehabil. 2016;29:89–96. doi: 10.3233/BMR-150602. [DOI] [PubMed] [Google Scholar]
- 15.Pasha S, Baldwin K. Are we simplifying balance evaluation in adolescent idiopathic scoliosis? Clin Biomech (Bristol, Avon) 2018;51:91–98. doi: 10.1016/j.clinbiomech.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Braun JT, et al. Experimental scoliosis in an immature goat model: a method that creates idiopathic-type deformity with minimal violation of the spinal elements along the curve. Spine (Phila Pa 1976) 2003;28:2198–2203. doi: 10.1097/01.BRS.0000085095.37311.46. [DOI] [PubMed] [Google Scholar]
- 17.Machida M, et al. Pathogenesis of idiopathic scoliosis. Experimental study in rats. Spine (Phila Pa 1976) 1999;24:1985–1989. doi: 10.1097/00007632-199910010-00004. [DOI] [PubMed] [Google Scholar]
- 18.O’Kelly C, et al. The production of scoliosis after pinealectomy in young chickens, rats, and hamsters. Spine (Phila Pa 1976) 1999;24:35–43. doi: 10.1097/00007632-199901010-00009. [DOI] [PubMed] [Google Scholar]
- 19.Machida M, et al. Pathological mechanism of idiopathic scoliosis: experimental scoliosis in pinealectomized rats. Eur Spine J. 2005;14:843–848. doi: 10.1007/s00586-004-0806-1. [DOI] [PubMed] [Google Scholar]
- 20.Cheung KM, et al. The effect of pinealectomy on scoliosis development in young nonhuman primates. Spine (Phila Pa 1976) 2005;30:2009–2013. doi: 10.1097/01.brs.0000179087.38730.5d. [DOI] [PubMed] [Google Scholar]
- 21.Gorman KF, Breden F. Idiopathic-type scoliosis is not exclusive to bipedalism. Med Hypotheses. 2009;72:348–352. doi: 10.1016/j.mehy.2008.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castelein RM, van Dieen JH, Smit TH. The role of dorsal shear forces in the pathogenesis of adolescent idiopathic scoliosis–a hypothesis. Med Hypotheses. 2005;65:501–508. doi: 10.1016/j.mehy.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 23.de Reuver, S. et al. The Changing position of the Center of Mass of the Thorax During Growth in Relation to Pre-Existent Vertebral Rotation. Spine (Phila Pa 1976) (2018). [DOI] [PubMed]
- 24.Stokes IA, Spence H, Aronsson DD, Kilmer N. Mechanical modulation of vertebral body growth. Implications for scoliosis progression. Spine (Phila Pa 1976) 1996;21:1162–1167. doi: 10.1097/00007632-199605150-00007. [DOI] [PubMed] [Google Scholar]
- 25.Stokes IA. Analysis and simulation of progressive adolescent scoliosis by biomechanical growth modulation. Eur Spine J. 2007;16:1621–1628. doi: 10.1007/s00586-007-0442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cosserat, E. & Cosserat, F. Theory of Deformable Bodies. Scientific Library A. Hermann and Sons (1909).
- 27.Mahadevan, L. & Keller, J. B. Coiling of flexible ropes. Proceedings of the Royal Society of London. Series A: Mathematical, Physical and Engineering Sciences452 (1996).
- 28.Jawed MK, Da F, Joo J, Grinspun E, Reis PM. Coiling of elastic rods on rigid substrates. PNSA. 2014;111:14663–14668. doi: 10.1073/pnas.1409118111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su T, Liu J, Terwagne D, Reis PM, Bertoldi K. Buckling of an elastic rod embedded on an elastomeric matrix: planar vs. non-planar configurations. Soft Matter. 2014;10:6294–6302. doi: 10.1039/C4SM00952E. [DOI] [PubMed] [Google Scholar]
- 30.Dittmore A, Silver J, Neuman KC. Kinetic Pathway of Torsional DNA Buckling. J Phys Chem B. 2018;122:11561–11570. doi: 10.1021/acs.jpcb.8b07504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brahmachari S, Dittmore A, Takagi Y, Neuman KC, Marko JF. Defect-facilitated buckling in supercoiled double-helix DNA. Phys Rev E. 2018;97:022416. doi: 10.1103/PhysRevE.97.022416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benham CJ, Mielke SP. DNA mechanics. Annu Rev Biomed Eng. 2005;7:21–53. doi: 10.1146/annurev.bioeng.6.062403.132016. [DOI] [PubMed] [Google Scholar]
- 33.Pasha S. 3D spinal and rib cage predictors of brace effectiveness in adolescent idiopathic scoliosis. BMC Musculoskelet Disord. 2019;20:384. doi: 10.1186/s12891-019-2754-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pasha S, Hassanzadeh P, Ecker M, Ho V. A hierarchical classification of adolescent idiopathic scoliosis: Identifying the distinguishing features in 3D spinal deformities. PLoS One. 2019;14:e0213406. doi: 10.1371/journal.pone.0213406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pasha S, Flynn J. Data-driven Classification of the 3D Spinal Curve in Adolescent Idiopathic Scoliosis with an Applications in Surgical Outcome Prediction. Sci Rep. 2018;8:16296. doi: 10.1038/s41598-018-34261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen H, et al. The Height-Width-Depth Ratios of the Intervertebral Discs and Vertebral Bodies in Adolescent Idiopathic Scoliosis vs Controls in a Chinese Population. Sci Rep. 2017;7:46448. doi: 10.1038/srep46448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlosser TP, Vincken KL, Rogers K, Castelein RM, Shah SA. Natural sagittal spino-pelvic alignment in boys and girls before, at and after the adolescent growth spurt. Eur Spine J. 2015;24:1158–1167. doi: 10.1007/s00586-014-3536-z. [DOI] [PubMed] [Google Scholar]
- 38.Nault ML, et al. Three-dimensional spinal morphology can differentiate between progressive and nonprogressive patients with adolescent idiopathic scoliosis at the initial presentation: a prospective study. Spine (Phila Pa 1976) 2014;39:E601–606. doi: 10.1097/BRS.0000000000000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith T. The use of a quadruped as an in vivo model for the study of the spine – biomechanical considerations. European Spine J. 2002;11:137–144. doi: 10.1007/s005860100346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castillo, E. R. & Lieberman, D. E. Shock attenuation in the human lumbar spine during walking and running. J of Experimental Biology, 221 (2018). [DOI] [PubMed]
- 41.Gracovetsky S. An hypothesis for the role of the spine in human locomotion: A challenge to current thinking. J Biomed Eng. 1985;7:205–216. doi: 10.1016/0141-5425(85)90021-4. [DOI] [PubMed] [Google Scholar]
- 42.Pasha, S. & Baldwin, K. Surgical outcome differences between the 3D subtypes of right thoracic adolescent idiopathic scoliosis. Eur Spine J (2019). [DOI] [PubMed]
- 43.Teng X, Hwang W. Elastic Energy Partitioning in DNA Deformation and Binding to Proteins. ACS Nano. 2016;10:170–180. doi: 10.1021/acsnano.5b06863. [DOI] [PubMed] [Google Scholar]
- 44.Huang Z. Modulating DNA configuration by interfacial traction: an elastic rod model to characterize DNA folding and unfolding. J Biol Phys. 2011;37:79–90. doi: 10.1007/s10867-010-9200-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao Y, Huang Z, Wang S. An elastic rod model to evaluate effects of ionic concentration on equilibrium configuration of DNA in salt solution. J Biol Phys. 2014;40:179–192. doi: 10.1007/s10867-014-9344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karnezis IA. Flexural-torsional buckling initiates idiopathic scoliosis. Med Hypotheses. 2011;77:924–926. doi: 10.1016/j.mehy.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 47.Lucas DB. Mechanics of the spine. Bull Hosp Joint Dis. 1970;31:115–131. [PubMed] [Google Scholar]
- 48.Dickson RA. The etiology and pathogenesis of idiopathic scoliosis. Acta Orthop Belg. 1992;58(Suppl 1):21–25. [PubMed] [Google Scholar]
- 49.Azegami Hideyuki, Murachi Shunji, Kitoh Junzoh, Ishida Yoshito, Kawakami Noriaki, Makino Mitsunori. Etiology of Idiopathic Scoliosis. Clinical Orthopaedics and Related Research. 1998;357:229–236. doi: 10.1097/00003086-199812000-00029. [DOI] [PubMed] [Google Scholar]
- 50.Roaf R. The basic anatomy of scoliosis. J Bone Joint Surg Br. 1966;48:786–792. doi: 10.1302/0301-620X.48B4.786. [DOI] [PubMed] [Google Scholar]
- 51.Noshchenko A, et al. Predictors of spine deformity progression in adolescent idiopathic scoliosis: A systematic review with meta-analysis. World J Orthop. 2015;6:537–558. doi: 10.5312/wjo.v6.i7.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siu King Cheung C, et al. Abnormal peri-pubertal anthropometric measurements and growth pattern in adolescent idiopathic scoliosis: a study of 598 patients. Spine (Phila Pa 1976) 2003;28:2152–2157. doi: 10.1097/01.BRS.0000084265.15201.D5. [DOI] [PubMed] [Google Scholar]
- 53.Nicolopoulos KS, Burwell RG, Webb JK. Stature and its components in adolescent idiopathic scoliosis. Cephalo-caudal disproportion in the trunk of girls. J Bone Joint Surg Br. 1985;67:594–601. doi: 10.1302/0301-620X.67B4.4030857. [DOI] [PubMed] [Google Scholar]
- 54.Pasha S, Ilharreborde B, Baldwin K. Sagittal Spinopelvic Alignment After Posterior Spinal Fusion in Adolescent Idiopathic Scoliosis: A Systematic Review and Meta-analysis. Spine (Phila Pa 1976) 2019;44:41–52. doi: 10.1097/BRS.0000000000002736. [DOI] [PubMed] [Google Scholar]
- 55.Pasha S, Flynn JM, Sankar WN. Outcomes of selective thoracic fusion for Lenke 1 adolescent idiopathic scoliosis: predictors of success from the sagittal plane. Eur Spine J. 2018;27:2223–2232. doi: 10.1007/s00586-018-5553-9. [DOI] [PubMed] [Google Scholar]
- 56.Pasha, S. 3D Spinal and Ribcage Predictors of Brace Effectiveness in Adolescent Idiopathic Scoliosis. BMC Musculoskeletal Disorders (2019). [DOI] [PMC free article] [PubMed]
- 57.Cobetto N, et al. 3D correction of AIS in braces designed using CAD/CAM and FEM: a randomized controlled trial. Scoliosis Spinal Disord. 2017;12:24. doi: 10.1186/s13013-017-0128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McCance SE, Denis F, Lonstein JE, Winter RB. Coronal and sagittal balance in surgically treated adolescent idiopathic scoliosis with the King II curve pattern. A review of 67 consecutive cases having selective thoracic arthrodesis. Spine (Phila Pa 1976) 1998;23:2063–2073. doi: 10.1097/00007632-199810010-00005. [DOI] [PubMed] [Google Scholar]
- 59.Grivas TB, Vasiliadis E, Mouzakis V, Mihas C, Koufopoulos G. Association between adolescent idiopathic scoliosis prevalence and age at menarche in different geographic latitudes. Scoliosis. 2006;1:9. doi: 10.1186/1748-7161-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burwell RG. Aetiology of idiopathic scoliosis: current concepts. Pediatr Rehabil. 2003;6:137–170. doi: 10.1080/13638490310001642757. [DOI] [PubMed] [Google Scholar]
- 61.Wild CY, Steele JR, Munro BJ. Why do girls sustain more anterior cruciate ligament injuries than boys?: a review of the changes in estrogen and musculoskeletal structure and function during puberty. Sports Med. 2012;42:733–749. doi: 10.1007/BF03262292. [DOI] [PubMed] [Google Scholar]
- 62.Chidi-Ogbolu N, Baar K. Effect of Estrogen on Musculoskeletal Performance and Injury Risk. Front Physiol. 2018;9:1834. doi: 10.3389/fphys.2018.01834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Humbert L, De Guise JA, Aubert B, Godbout B, Skalli W. 3D reconstruction of the spine from biplanar X-rays using parametric models based on transversal and longitudinal inferences. Med Eng Phys. 2009;31:681–687. doi: 10.1016/j.medengphy.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 64.Pasha, S. et al. Application of Low-dose Stereoradiography in In Vivo Vertebral Morphologic Measurements: Comparison With Computed Tomography. J Pediatr Orthop (2017). [DOI] [PubMed]
- 65.Kadoury S, Shen J, Parent S. Global geometric torsion estimation in adolescent idiopathic scoliosis. Med Biol Eng Comput. 2014;52:309–319. doi: 10.1007/s11517-013-1132-8. [DOI] [PubMed] [Google Scholar]
- 66.Pearsall DJ, Reid JG, Livingston LA. Segmental inertial parameters of the human trunk as determined from computed tomography. Ann Biomed Eng. 1996;24:198–210. doi: 10.1007/BF02667349. [DOI] [PubMed] [Google Scholar]
- 67.Liu YK, Laborde JM, Van Buskirk WC. Inertial properties of a segmented cadaver trunk: their implications in acceleration injuries. Aerosp Med. 1971;42:650–657. [PubMed] [Google Scholar]
- 68.Clin J, Aubin CE, Lalonde N, Parent S, Labelle H. A new method to include the gravitational forces in a finite element model of the scoliotic spine. Med Biol Eng Comput. 2011;49:967–977. doi: 10.1007/s11517-011-0793-4. [DOI] [PubMed] [Google Scholar]
- 69.Pasha, S., Aubin, C. E., Parent, S., Labelle, H. & Mac-Thiong, J. M. Biomechanical loading of the sacrum in adolescent idiopathic scoliosis. Clin Biomech (Bristol, Avon)29, 296–303, J.clinbiomech.2013.12.004 (2014). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Non-patient related data that support the findings of this study are available from the author upon reasonable request.
The executable code is available from the author upon reasonable request for non-commercial purposes only.