Abstract
Sleep impairments are a hallmark of acute bipolar disorder (BD) episodes and are present even in the euthymic state. Studying healthy subjects who are vulnerable to BD can improve our understanding of whether sleep impairment is a predisposing factor. Therefore, we investigated whether vulnerability to BD, dimensionally assessed by the hypomanic personality scale (HPS), is associated with sleep disturbances in healthy subjects. We analyzed participants from a population-based cohort who had completed the HPS and had either a 7-day actigraphy recording or a Pittsburgh sleep quality index (PSQI) assessment. In addition, subjects had to be free of confounding diseases or medications. This resulted in 771 subjects for actigraphy and 1766 for PSQI analyses. We found strong evidence that higher HPS scores are associated with greater intraindividual sleep variability, more disturbed sleep and more daytime sleepiness. In addition, factor analyses revealed that core hypomanic features were especially associated with self-reported sleep impairments. Results support the assumption of disturbed sleep as a possibly predisposing factor for BD and suggest sleep improvement as a potential early prevention target.
Subject terms: Predictive markers, Bipolar disorder, Pathogenesis, Human behaviour
Introduction
Sleep disturbances and increased daytime sleepiness occur in bipolar disorder (BD), including in the euthymic state1,2. Accumulating evidence suggests that impaired or reduced sleep and increased daytime sleepiness are not only symptoms of BD, but contribute to the disease process itself and to (hypo)manic behavior in particular. Experimental and longitudinal studies of patients, healthy subjects, and animals suggest that sleep deprivation can induce (hypo)mania3–9. Accordingly, cognitive behavioral therapy for inter-episode insomnia has resulted in a lower (hypo)manic relapse rate10, and stabilization of sleep and sleep–wake rhythms is an element of BD treatments11,12. Further, in a recent study13 daytime sleepiness predicted (hypo)manic relapse. Daytime sleepiness has been characterized as the behavioral expression of an unstable central nervous system (CNS)-arousal;14 and using electroencephalogram (EEG), unstable arousal has been demonstrated for BD, especially during mania15–18. The arousal regulation model of affective disorders and attention-deficit/hyperactivity disorder (ADHD)15,17 takes into consideration this unstable arousal in BD (and ADHD) and suggests that hypoarousal can partly cause (hypo)manic behavior. In an autoregulatory manner, the hyperactive and sensation-seeking behavior is seen as a compensatory arousal-stabilizing behavior, which can in turn increase sleep deficits, thus initiating a vicious circle contributing to mania15,17,18.
If impaired sleep is in fact a predisposing trait for BD, sleep improvement might be a target for early interventions. However, present findings on impaired sleep in euthymic BD leave it unclear to what extent these disturbances are a predisposition, a consequence of the disease process, or due to medication. For example, BD patients are often prescribed numerous medications19, many of which impact both arousal and sleep. Studying healthy subjects who are vulnerable to BD can improve our understanding of whether sleep impairment is a predisposing factor. Genetic high-risk studies have mostly shown that sleep in healthy offspring of BD patients is indeed impaired20–22. However, in addition to the small sample sizes in many of these studies, a further inherent limitation is the exclusive focus on a particular subgroup, namely offspring of relatives who have fallen ill. There is only one longitudinal study, which was not based on genetic high-risk offspring but on a healthy community sample, and which could nonetheless predict subsequent development of BD during a ten year follow up by poor sleep quality at baseline23. Moreover, this prediction of BD by poor sleep persisted when excluding subjects with a family history of affective disorders. However, results of this study were based on only 20 subjects who developed a full-blown BD. Missing data and a low probability of incident BD in healthy subjects not genetically at-risk are inherent problems of such longitudinal cohort studies. Thus, more data are needed. The psychometric high-risk approach24, which assesses BD risk in large populations via questionnaires such as the hypomanic personality scale (HPS)25, is one useful method. The HPS allows not only the identification of unusually high scorers (high risk subjects), but also the assessment of the total normal variance. The association of the entire continuum of bipolarity with sleep disturbances can then be examined. Such dimensional concepts of psychopathology have recently attracted much interest in the context of psychiatric genetics and the endophenotype approach26–28, and also the Research Domain Criteria Project (RDoC) of the National Institute of Mental Health (NIMH) pointed out the strength of dimensional assessments29.
The HPS assesses features of hypomanic episodes as described within the diagnostic systems, but also includes correlated traits such as social dominance. As the scale assesses a temperamental dimension, i.e., a personality trait rather than an acute episode, subjects are instructed to respond how they feel in general. Several studies have demonstrated the scale’s reliability and validity. The HPS is stable over time25,30, has been associated with psychiatric risk genes31,32, aggregates in families of afflicted patients33 and has discriminated bipolar patients from controls34,35. Cross-sectionally, HPS high scorers showed higher rates of (hypo)manic and depressed episodes and more psychosocial impairment and substance-use25,36,37. Longitudinally, the HPS predicted BD and hypomanic symptoms in addition to related disorders such as substance abuse38–40.
Further evidence for the role of hypomanic traits in BD have been presented by other authors such as Hagop S. Akiskal and Richard A. Depue41. Akiskal developed an interview (TEMPS-I42) and self-rating instrument (TEMPS-A43,44) in which the hyperthymic and cyclothymic temperaments measure comparable constructs to the HPS45,46. As is the case with the HPS, the TEMPS-A temperaments show long-term stability47, aggregate in families27,48, have been associated with psychiatric risk genes49 and have been used to discriminate and predict bipolar symptomatology48,50. One difference between the TEMPS-A and the HPS is that the TEMPS-A assesses a hyperthymic temperament separately from a cyclothymic temperament, whereas in the HPS both aspects are summed together in one total score. However, it has recently been suggested that the HPS should be separated into different subscales which may have divergent psychopathological correlates51–53. In addition to the research on affective temperament scales such as HPS and TEMPS-A, longitudinal studies have contributed further evidence that subthreshold hypomanic symptoms often precede BD39,54–58.
To date, only two small studies using student samples have assessed the association between sleep and HPS. In HPS high-scorers, greater intraindividual variability in sleep duration was observed via sleep diaries59 and actigraphy60. The latter study60 also showed shorter sleep duration and greater intraindividual variability in sleep efficiency in HPS high-scorers. As is usually the case, these two studies utilized the HPS total sum-score. However, in two recent studies51,53 HPS subscales were derived from factor analyses which differed in their associations with psychopathologically relevant traits51,53,61.
Objectives
The current study analyses the associations of objective and subjective sleep parameters and daytime sleepiness with a risk factor for BD, the HPS. In a large cohort of healthy subjects, we expect associations between the HPS and shorter and more disturbed sleep, increased daytime sleepiness and more night-to-night sleep-variability. In contrast to most prior research, we not only associate sleep with the HPS total score, but also with HPS subscales. To this end, we conducted the first factor analysis on the German translation62 of the HPS. We hypothesize that the associations of the HPS with sleep variables will differ with respect to the HPS subscales.
Materials and methods
Participants
The study sample was drawn from the LIFE-Adult study63, a population-based cohort comprised of 10,000 inhabitants of the city of Leipzig, Germany. Of the total sample, 3031 participants aged 60–82 years completed the HPS. Subjects had to be free of diseases or medications which could strongly impact sleep–wake behavior. Thus, participants with a history of stroke, multiple sclerosis, Parkinson’s disease, epilepsy, skull fracture, cerebral tumor, or meningitis were excluded, leaving 2788 subjects. Further excluded were individuals reporting current use of CNS-affecting drugs (leaving 2373 subjects). Based on data from structured clinical interviews for DSM-IV Axis I disorders, we selected subjects without a lifetime history of substance dependence, psychotic or BDs, and who were free of current affective or anxiety disorders (leaving 2087 subjects). In addition, participants were required to have available data from either the Pittsburgh sleep quality index (PSQI) assessment or an actigraphy recording for at least five nights. This resulted in a final sample of 771 subjects for actigraphy association analyses (372 female, Mage = 70.3 y), and 1766 subjects for PSQI association analyses (835 female, Mage = 69.6 y). Factor analyses of the HPS were conducted using all 2861 subjects with complete HPS responses (1371 female; age range: 60–82 years; Mage = 70.0). The study was approved by Leipzig University’s Ethics Committee (263-2009-14122009) and subjects gave written informed consent.
Objective and subjective sleep assessment
To obtain objective measurements of sleep, subjects wore the SenseWear Pro 3 Armband actigraph (BodyMedia; Pittsburgh, Pennsylvania) for an average of 6.9 days (range: 5–7 days). Analyses of night-sleep parameters were carried out as described in detail elsewhere64–66. Subjective ratings of sleep and sleep quality were obtained using the German version of the PSQI67 a self-rating instrument to assess sleep quality during the past 4 weeks.
Hypomanic personality scale (HPS)
The HPS, a self-rating scale used to assess BD risk25, was developed in undergraduates, which is reflected in some of the 48 items. As the current study administered the HPS to elderly subjects, four items from the German translation62 were deleted for reasons of compliance (see Supplementary Methods).
Statistical analysis
The factorial structure of the HPS was analyzed using the function irt.fa of R package psych (version 1.7.8) as described in Supplementary Methods. Remaining statistical analyses were performed using SPSS 22 (IBM; Armonk, New York). Associations between the sleep variables and the HPS were conducted for both the HPS total sum-score (HPS total) and factor scores for each subscale. We conducted partial Spearman correlations adjusting for sex and age. In order to confirm the results, we additionally compared the top and bottom decile HPS groups using Kruskal–Wallis tests.
Results
Factorial structure of the HPS
In the Supplementum, factor analysis results are described in detail and compared to the available factor solutions reported in two younger non-German samples51,68. Results revealed three factors (see Supplementary Figs. S1–S3), which were well in line with prior studies (see Supplementary Table S1). We labeled the first factor hypomanic core, as it was comprised of items with clearly hypomanic content. Items loading high on the second factor (social vitality) describe high self-confidence, social dominance, and leadership. The third factor appears to reflect a characteristic that has previously been recognized as “ordinariness”, as it describes balanced and controlled people who consider themselves as average persons68. Thus, we retained the term ordinariness.
Correlation analyses
Table 1 shows the Spearman correlations between the sleep parameters and HPS total as well as the subscales’ factor scores hypomanic core, social vitality, and ordinariness. In total, 46 out of 84 correlations reached the level of significance, with 40 of them remaining significant after multiple-test correction (see Supplementary Table S2). Accordingly, the quantile–quantile plot suggests that the distribution of observed p values considerably differs from a p value distribution under the null hypothesis (Fig. 1).
Table 1.
Partial Spearman correlations between hypomanic personality and sleep–wake variables
| HPS total sum-score | HPS subscale hypomanic core | HPS subscale social vitality | HPS subscale ordinariness | |||||
|---|---|---|---|---|---|---|---|---|
| rho | p | rho | p | rho | p | rho | p | |
| Actigraphy (n = 771) | ||||||||
| Means | ||||||||
| Sleep-onset latency | 0.038 | 0.294 | 0.042 | 0.240 | 0.054 | 0.134 | −0.035 | 0.329 |
| Sleep-onset time | −0.006 | 0.864 | −0.035 | 0.329 | 0.025 | 0.497 | 0.061 | 0.089 |
| Sleep-offset time | −0.013 | 0.721 | −0.041 | 0.252 | 0.007 | 0.851 | 0.056 | 0.118 |
| Sleep duration | −0.079 | 0.029* | −0.072 | 0.046* | −0.059 | 0.101 | 0.034 | 0.350 |
| NWAK | 0.078 | 0.030* | 0.069 | 0.056 | 0.069 | 0.056 | −0.011 | 0.757 |
| WASO | 0.103 | 0.004** | 0.091 | 0.011* | 0.080 | 0.027* | −0.046 | 0.203 |
| Sleep efficiency | −0.106 | 0.003** | −0.101 | 0.005** | −0.086 | 0.017* | 0.033 | 0.361 |
| Night-to-night variability | ||||||||
| Sleep-onset latency | 0.019 | 0.594 | −0.005 | 0.893 | 0.076 | 0.036* | −0.026 | 0.470 |
| Sleep-onset time | 0.122 | 7E−4** | 0.102 | 0.004** | 0.113 | 0.002** | 0.009 | 0.813 |
| Sleep-offset time | 0.110 | 0.002** | 0.119 | 9E−4** | 0.069 | 0.057 | −0.062 | 0.087 |
| Sleep duration | 0.098 | 0.006** | 0.092 | 0.011* | 0.097 | 0.007** | −0.003 | 0.931 |
| NWAK | 0.145 | 6E−5** | 0.109 | 0.002** | 0.152 | 2E−5** | −0.092 | 0.011** |
| WASO | 0.115 | 0.001** | 0.116 | 0.001** | 0.095 | 0.008** | −0.109 | 0.002** |
| Sleep efficiency | 0.106 | 0.003** | 0.095 | 0.008** | 0.103 | 0.004** | −0.092 | 0.011** |
| PSQI (n = 1766) | ||||||||
| Sleep-onset latencya | 0.013 | 0.576 | 0.078 | 0.001** | −0.080 | 8E−4** | −0.098 | 4E−5** |
| Bedtimea,b | 0.029 | 0.230 | 0.018 | 0.444 | 0.041 | 0.087 | 0.001 | 0.968 |
| Get-up timea | −0.022 | 0.348 | −0.023 | 0.344 | −0.020 | 0.413 | −0.013 | 0.581 |
| Sleep durationa | −0.038 | 0.114 | −0.095 | 7E−5** | 0.027 | 0.261 | 0.081 | 6E−4** |
| Sleep efficiencyc | 0.002 | 0.925 | −0.065 | 0.006** | 0.071 | 0.003** | 0.091 | 1E−4** |
| Daytime sleepinessa | 0.075 | 0.002** | 0.088 | 2E−4** | 0.052 | 0.028* | −0.086 | 3E−4** |
| PSQI scored | 0.063 | 0.008** | 0.158 | 3E−11** | −0.063 | 0.008** | −0.165 | 3E−12** |
Night-to-night variability is operationalized by intraindividual standard deviation (ISD) across a single subject’s multiple nights.
Note that hypomanic core, social vitality and ordinariness here refer to factor scores derived from factor analyses as described in the Methods of the Supplementum. Results were additionally confirmed by analyses with traditional sum scores (see Supplementary Table S4).
Effects of sex and age were partialled out. NWAK number of awakenings, WASO wake after sleep-onset time
*p < 0.05
**p < 0.01
aBased on the respective Pittsburgh sleep quality index (PSQI) item
bTime subject goes to bed
cQuotient of sleep duration and time in bed with the latter calculated from bedtime and get-up time
dPSQI total score calculated according the manual from all PSQI components; higher PSQI scores mean worse sleep quality
Fig. 1. Permutation-based quantile–quantile plot showing that the observed p values (blue circles) considerably differ from a random distribution under the null hypothesis (solid diagonal line).
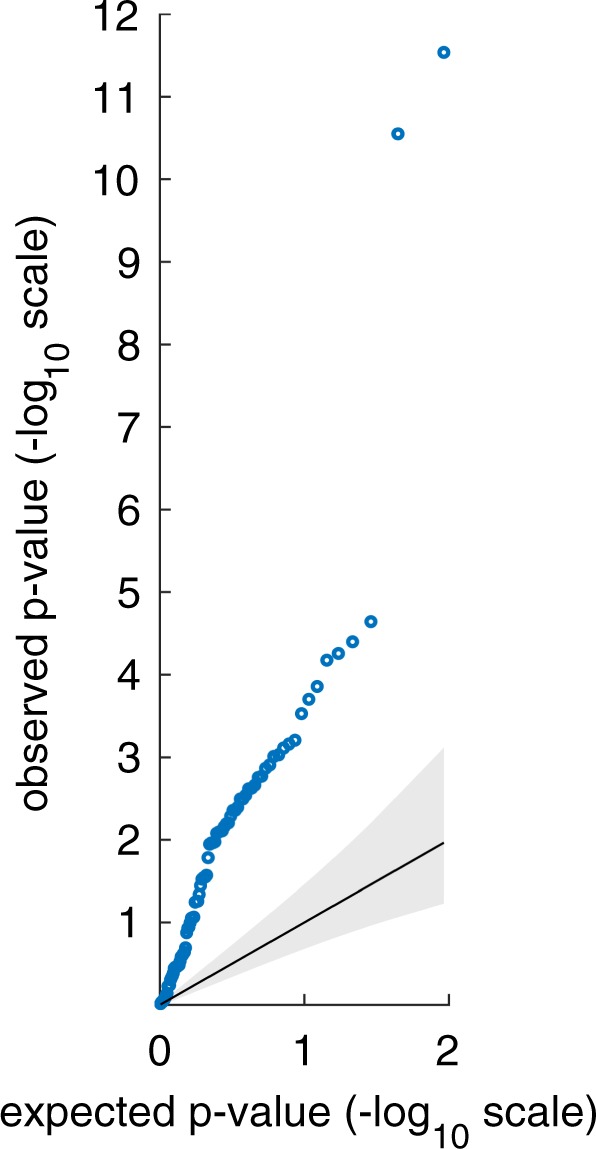
For the set of 84 observed p values (21 sleep variables × 4 personality scores), one million sets of 84 expected p values were derived after data permutation. During data permutation, original correlations within the domain of hypomanic personality variables and the domain of sleep variables were preserved while original correlations between the two domains were removed through random shuffling. Each set of p values was sorted in descending order. The solid diagonal line represents the mean expected p values at rank 1–84 plotted against themselves. The upper and lower bound of the gray area represent the 5th and 95th percentile of expected p values plotted against the mean expected p values. The blue circles represent the observed p values plotted against the mean expected p values
Objective sleep data
HPS total was significantly associated with shorter sleep duration, a greater number of awakenings, more time awake after sleep-onset (WASO) and lower sleep efficiency. At the subscale level, these sleep variables were also significantly associated in the same direction with hypomanic core, with the exception of number of awakenings, which narrowly missed the significance level. Associations of social vitality were similar, but only WASO and sleep efficiency reached significance. Ordinariness, in contrast, was not correlated with any of these actigraphic sleep variables.
HPS total was even more strongly associated with the night-to-night variability of the sleep parameters than with the means. HPS total, hypomanic core and social vitality were all associated with greater intraindividual variability in sleep-onset time, sleep duration, number of awakenings, WASO and sleep efficiency. In contrast, Ordinariness was associated with lower intraindividual variability in the number of awakenings, WASO, and sleep efficiency.
Subjective sleep data (PSQI)
HPS total correlated significantly with more daytime sleepiness and lower sleep quality. Even stronger associations with PSQI-variables were found for hypomanic core, which was associated with a longer latency of sleep-onset, shorter sleep duration, lower sleep efficiency, more daytime sleepiness, and lower sleep quality. The obtained correlation coefficients for hypomanic core were significantly higher than those of the HPS total (Supplementary Table S3), with the exception of daytime sleepiness which did not significantly differ in its positive associations with HPS total and hypomanic core.
In contrast to HPS total and hypomanic core, social vitality, and ordinariness were associated with better subjective sleep and accordingly, both scales’ correlation coefficients differed significantly from HPS total and hypomanic core (Table S3). Social vitality and ordinariness were associated with shorter sleep-onset latency, higher sleep efficiency, and better sleep quality. In addition, ordinariness also correlated with longer sleep duration and lower daytime sleepiness, while social vitality resembled hypomanic core in terms of its association with more daytime sleepiness.
Additional analyses for confirmation of correlation results
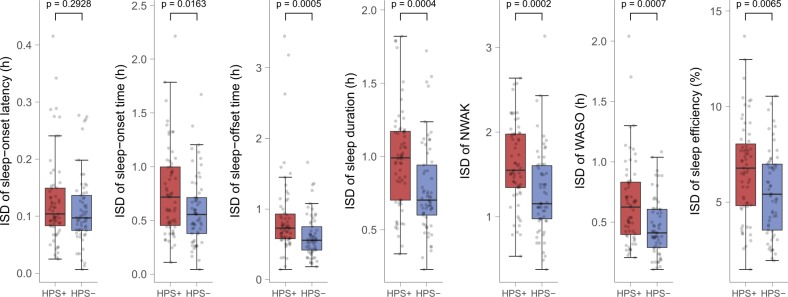
Further analyses were carried out to confirm that results were not dependent on the data-analytic method. First, all correlation analyses were repeated with unweighted sum-scores for each HPS subscale instead of factor scores, which resulted in comparable, albeit, as expected, somewhat weaker associations (Supplementary Table S4). Second, analyses were repeated using HPS decile extreme groups. Results resembled those of the correlation analyses (Supplementary Table S5), including the finding that the HPS groups differed more strongly concerning the intraindividual night-to-night variability of the sleep parameters than concerning the means. Figure 2 shows boxplots of the intraindividual sleep variability of actigraphic variables stratified by HPS extreme groups (see Supplementary Fig. S4 for boxplots of all sleep variables). With exception of sleep-onset latency, intraindividual variability for all variables was significantly higher in the top HPS decile group compared with the bottom decile group.
Fig. 2. Boxplots of intraindividual night-to-night variability of actigraphic sleep variables stratified by HPS extreme groups.
Intraindividual night-to-night variability is operationalized by intraindividual standard deviation (ISD) across a single subject’s multiple nights. Boxplots are stratified by top and bottom decile hypomanic personality scale (HPS) groups (HPS+, N = 63 vs. HPS−, N = 61). Boxes represent the interquartile range of each distribution (data between the lower and upper quartile), with the horizontal line corresponding to the median. Whiskers extend to the furthest observation within 1.5 times the interquartile range from the lower and upper quartile. Dots represent single data points, jittered horizontally to avoid overplotting
Discussion
The current study analyzed whether increased vulnerability to BD, as assessed by the HPS, is linked to more disturbed sleep in healthy subjects. Correlation analyses were conducted between objective as well as subjective sleep parameters and HPS subscales and total scale. Extreme group comparisons were also carried out to confirm results.
The correlation analyses as well as the extreme group comparisons revealed that a higher HPS total score is associated with worse sleep, greater night-to-night sleep variability and more daytime sleepiness. Thus, results confirm findings of impaired sleep in genetic high risk studies21.
Variability matters and HPS subscales differ in their sleep associations
A noteworthy finding is that the HPS was more strongly associated with the night-to-night variability than with the mean sleep variables. In line with this, irregularity in sleep/wake behavior has been reported for euthymic and manic patients2,69 and subjects with genetically or psychometrically operationalized heightened BD-risk2,21,59,60,70,71. In addition, another study found that greater sleep variability during euthymic state was associated with increased mania and depression severity over 12 months5. Thus, our results are in accordance with the role of social/circadian rhythm dysregulation in BD59,72.
Another finding is that the HPS subscales differed in their associations with perceived sleep (PSQI). First, the correlations of hypomanic core with sleep were significantly higher than the correlations of HPS total with sleep. Second, and in contrast to HPS total and hypomanic core, ordinariness, and social vitality showed associations in the opposite direction (i.e., they were associated with better perceived sleep). For ordinariness this was expected given the content of the scale. However, the association of social vitality with better perceived sleep is noteworthy, because the subscale is not only positively correlated with hypomanic core but also with objective sleep impairment. Such diametric associations of the positively correlated HPS facets are in line with the two available studies51,53 in which factor-analytic derived HPS subscales showed opposing associations with psychopathologically relevant traits. It might be that subjects scoring high on hypomanic core suffer more from impaired sleep, as the first factor (corresponding to hypomanic core in our analyses) has been found to be positively related to neuroticism51, whereas subjects scoring high on social vitality might not feel as impaired, given a negative association of social vitality with neuroticism51. Such reversed associations with HPS subscales could cancel each other out in predicting sleep when aggregated in the HPS total sum-score (as we demonstrated in a post-hoc linear regression analysis in Supplementary Results). Thus, the current study strongly supports the suggestion, recently made by several authors, of utilizing HPS subscales51–53,61.
In line with the findings of impaired objective sleep in both, hypomanic core and social vitality, both subscales were associated with increased daytime sleepiness. However, this increased sleepiness seems only to help subjects scoring high on social vitality (presumably low in neuroticism) to quickly fall asleep, as was seen in the negative correlation of social vitality with sleep-onset latency, whereas hypomanic core was associated with longer sleep-onset latency. Thus, hypomanic core more strongly resembles long sleep-onset latencies in (euthymic) BD1,2 and ADHD73,74, which may, despite high sleepiness, occur due to being too hyper and reluctant to fall asleep, rumination, or circadian alterations.
Sleep duration and daytime sleepiness
The finding that HPS total and hypomanic core were associated with shorter sleep duration is in line with studies on naturally short sleepers who showed more hypomanic, extraverted and impulsive traits75,76. The finding is also consistent with one small study on young HPS high-scorers and a study on children at genetic risk for BD. In both studies, shorter sleep duration was also reported60,77. These findings of shorter sleep contrast with the meta-analyses reporting longer sleep in euthymic BD compared to normal controls1,2. This might imply that long sleep duration is not a preexisting factor but occurs later as part of the disease. In addition, longer sleep could be merely a consequence of the disease, as patients might spend more time in bed13 as a consequence of sedating medications, unemployment or the instruction to get adequate sleep in order to avoid mania recurrence. Finally, the meta-analyses’ findings of longer sleep during euthymia when measured with actigraphy should be considered with caution, as sleep diaries did not show longer sleep but only longer time-in-bed2. It is known that actigraphs based on accelerometers tend to overestimate sleep duration in cases of insomnia symptomatology78. This might have contributed to the meta-analyses’ findings of longer sleep (as assessed by actigraphy) in euthymic BD compared to healthy controls. One can speculate that in the current study, because the applied SenseWear Armband uses not only an accelerometer, but also temperature and galvanic skin response sensors to estimate sleep, any potential overestimation of sleep duration might have been less pronounced.
The current study found that greater daytime sleepiness associates with HPS, which is in line with findings of higher sleepiness scores in euthymic BD patients2. It has been hypothesized that this sleepiness in BD reflects a trait-like arousal instability in some BD patients15,16, which would explain the impaired sustained attention in the euthymic state and unaffected relatives79. In addition, it has been speculated that the questionable efficacy and mania-inducing properties of antidepressants in BD might partly be attributable to their arousal-reducing properties, which could be problematic if habitual arousal is already low15,80,81. One might argue that increased sleepiness should result in longer sleep duration. However, it has been shown that in BD, longer sleep time and daytime sleepiness are independent presentations of hypersomnia13,82. Thus, the current findings agree with the assumption that hypoarousal plays a clinically relevant role in BD.
The association of HPS with shorter sleep and greater daytime sleepiness is in line with the arousal regulation model of affective disorders and ADHD, which posits that arousal instability due to short sleep or other reasons can contribute to (hypo)mania and ADHD15,17,83. As EEG is an excellent tool to assess arousal84, we have developed and validated the publically available Vigilance-Algorithm-Leipzig (VIGALL)14,85–89, which allows the assessment of brain arousal in resting-EEG recordings. Utilizing VIGALL, we demonstrated lower arousal during (hypo)mania18, and in genetic studies we demonstrated for the first time a link between arousal and ion channels90,91, which are of high relevance for BD92. ADHD shows symptom overlap with mania and high comorbidity with BD16,83. Correspondingly, several studies have also shown both impaired sleep and hypoarousal in ADHD, which may partly explain attention deficits, response to stimulants and compensatory hyperactive behavior15,83,93. As in mania, all factors which destabilize arousal such as sleep deficits worsen ADHD symptomatology, whereas sleep improvement is of therapeutic value15,17,83.
Prediction of HPS by sleep and covariates
For several reasons, our analyses focused only on sleep variables. Thus, we did not include variables on the diverse causes of disturbed sleep such as sleep apnea. One practical reason for this was that the LIFE cohort comprises only limited data on the causes of disturbed sleep. In fact, only two PSQI items provided information concerning sleep apnea and periodic limb movement disorder (with these two items not aggregated into the PSQI total score). Thus, systematically accounting for sleep disorders can only be done on a relatively weak basis. Most of all, we focused on sleep variables because disturbed sleep has been suggested as the final common pathway for a variety of triggers for manic episodes9. Sleep disturbances for a variety of reasons (e.g., life events, traveling, leisure activities, circadian dysregulation, drugs, sleep apnea, or periodic limb movement disorder) have been suggested to contribute to (hypo)mania12. Finally, we focused our analyses on sleep variables because our goal was to facilitate comparisons of our results for each given sleep variable, such as the WASO, with those from the available literature, particularly the meta-analyses of euthymic BD1,2.
Nonetheless, one might ask whether new insights would arise when all variables, including socioeconomic status, age, sex, and body mass index are included simultaneously in multivariable analyses. To investigate the relative importance of objective and subjective sleep variables while accounting for other covariates in predicting HPS scores, we carried out a series of regression analyses. In order to avoid overfitting and inflated R2 estimates, we pursued a tenfold cross-validation approach which involves evaluating the performance of a prediction model by applying the model to new data not used in training it. Results, which are outlined in the Supplemental in more detail, showed clear incremental predictive value of the sleep variables over the covariates in predicting HPS total, hypomanic core, and ordinariness (Supplementary Table S6). The incremental value of the sleep variables was most pronounced for hypomanic core, which is in line with hypomanic core being most strongly correlated with sleep in the correlation analyses. In contrast, there was no incremental predictive value of sleep variables on HPS social vitality. This finding could be due to the fact that a large proportion of variance in HPS social vitality was already explained by the covariate “socioeconomic status” (see Supplementary Fig. S5), which might be considered as further evidence that social vitality may be the healthier facet of the HPS51
Strengths and limitations
The HPS has mostly been utilized in samples of adolescents and young adults. To increase the appropriateness of the HPS in our elderly subjects, we deleted four items, thus limiting the comparability of our findings with other studies. However, the factorial structure of the shortened HPS in our sample of German elderly adults largely overlapped with those derived from the total HPS scale in younger samples51,53,68, suggesting that the utilized version reveals comparable construct estimates even across different national and age cohorts. Nonetheless, our HPS subscales and their differential associations with sleep variables need replication in other, particularly younger samples. Concerning our subscales’ differential association with BD, we are currently lacking data, but would hypothesize hypomanic core to be most strongly associated with BD. There is a large overlap of items from our hypomanic core subscale with the items showing the largest correlations with BD diagnoses in a study by Miller et al.34. All items from an HPS short version, comprising the six items with the highest BD-association34, are part of our hypomanic core subscale.
The current study demonstrated that the HPS is also applicable in subjects older than 60 years, which is not trivial given the strong decline of HPS scores with increasing age. For example, in our PSQI sample the mean of the HPS total score was M = 9.10 (SD = 5.37; mean extrapolated to a scale length of 48 items). The means and variances of younger samples are substantially higher. In the undergraduate sample by Eckblad and Chapman25, the mean was M = 21.74 (SD = 8.16). Similarly, in a German sample62 with an age range of 17–30 years, the mean was M = 18 (SD = 8.91). Thus, the current results are compelling in light of the low HPS variance in our sample, which may have made it more difficult to detect associations.
From another perspective, the older age of our sample may be both a limitation and a strength. The aim of our study was to associate the vulnerability factor HPS with sleep, and we were not interested in associations due to effects of current or former affective episodes or treatments. Therefore, we excluded subjects with acute affective episodes, psychotropic drugs, or a life time diagnosis of BD. In addition, our subjects are, due to their age, relatively unlikely to develop BD in the future. This means that, in contrast to studies in younger samples, we can also be quite sure that prodromes of emerging episodes have not biased our results. However, the age and exclusion criteria of the current sample imply that we may have analyzed a sample with superior health, not comparable to the younger samples in which the HPS has mostly been used. Notwithstanding, from a dimensional perspective on psychopathology, it is still reasonable to associate the entire “remaining” vulnerability factor variance with sleep. In addition, such a sample with superior health will still include subjects at high risk for BD. These subjects remained healthy, likely because of a lack of triggering factors or life-events or because of additional protective traits. It is remarkable that in this sample of likely superior health, the HPS is still associated with more impaired sleep and greater sleepiness.
The present study was well-powered and revealed strong evidence for a link between hypomanic temperament and sleep alterations. Nevertheless, observed effect sizes, albeit substantial in extreme group comparisons, were small in correlation analyses across all subjects. One reason for this might be that elderly subjects show low variances in HPS and wake–sleep behavior. Further, during the 7-day actigraphy assessment, sleep was undoubtedly influenced by numerous factors beyond the subject’s predisposition (e.g., common cold, sleep of the partner, and reactivity to the actigraph). Given these sources of error variance, the highly consistent results are very compelling.
Conclusions
The associations of the HPS with worse sleep were consistent, irrespective of the assessment modality and data analysis method. Sleep inter-night variability showed the most pronounced associations with HPS, thus further qualifying as a characteristic variable in BD21,70,94,95. As a further practical implication, this study supports the suggestion recently made by other authors51–53 that using HPS subscales will increase the diagnostic power.
The association of sleep alterations with HPS supports assumptions derived from prior studies that sleep disturbances may be a predisposing factor for BD. Analogous to prior studies in which sleep difficulties predicted mood episodes in genetic high risk off-spring70,77,96, one can hypothesize that sleep might predict conversion to BD in psychometric high-risk groups as assessed by the HPS. Early interventions to prevent psychiatric disorders or change their course are a cutting-edge topic in psychiatry57. Thus, improving sleep in HPS high-scorers may be a valuable early prevention approach, with sleep symptoms being easy to assess, modifiable, and largely free of social stigmatization.
Supplementary information
Acknowledgements
This publication is supported by LIFE—Leipzig Research Center for Civilization Diseases, Universität Leipzig. LIFE is funded by means of the European Union, by the European Regional Development Fund (ERDF) and by means of the Free State of Saxony within the framework of the excellence initiative. This publication was written within the framework of the cooperation between the German Depression Foundation and the “Deutsche Bahn Stiftung gGmbH”. We thank Dr. Elise Paul for proofreading the paper.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
These authors contributed equally: Tilman Hensch, David Wozniak
These authors jointly supervised to this work: Philippe Jawinski, Ulrich Hegerl
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tilman Hensch, Phone: ++49 341 97 24 587, Email: tilman.hensch@medizin.uni-leipzig.de.
Philippe Jawinski, Phone: ++49 30 2093 9391, Email: philippe.jawinski@hu-berlin.de.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41398-019-0632-1).
References
- 1.Geoffroy PA, et al. Sleep in patients with remitted bipolar disorders: a meta-analysis of actigraphy studies. Acta Psychiatr. Scand. 2015;131:89–99. doi: 10.1111/acps.12367. [DOI] [PubMed] [Google Scholar]
- 2.Ng TH, et al. Sleep-wake disturbance in interepisode bipolar disorder and high-risk individuals: a systematic review and meta-analysis. Sleep. Med. Rev. 2015;20:46–58. doi: 10.1016/j.smrv.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Barbini B, Bertelli S, Colombo C, Smeraldi E. Sleep loss, a possible factor in augmenting manic episode. Psychiatry Res. 1996;65:121–125. doi: 10.1016/S0165-1781(96)02909-5. [DOI] [PubMed] [Google Scholar]
- 4.Bauer M, et al. Temporal relation between sleep and mood in patients with bipolar disorder. Bipolar Disord. 2006;8:160–167. doi: 10.1111/j.1399-5618.2006.00294.x. [DOI] [PubMed] [Google Scholar]
- 5.Gruber J, et al. Sleep matters: sleep functioning and course of illness in bipolar disorder. J. Affect. Disord. 2011;134:416–420. doi: 10.1016/j.jad.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis KS, et al. Sleep loss as a trigger of mood episodes in bipolar disorder: individual differences based on diagnostic subtype and gender. Br. J. Psychiatry. 2017;211:169–174. doi: 10.1192/bjp.bp.117.202259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sylvia LG, et al. Sleep disturbance in euthymic bipolar patients. J. Psychopharmacol. 2012;26:1108–1112. doi: 10.1177/0269881111421973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wehr TA. Sleep loss: a preventable cause of mania and other excited states. J. Clin. Psychiatry. 1989;50(Suppl):8–16. [PubMed] [Google Scholar]
- 9.Wehr TA, Sack DA, Rosenthal NE. Sleep reduction as a final common pathway in the genesis of mania. Am. J. Psychiatry. 1987;144:201–204. doi: 10.1176/ajp.144.9.1248-a. [DOI] [PubMed] [Google Scholar]
- 10.Harvey AG, et al. Treating insomnia improves mood state, sleep, and functioning in bipolar disorder: a pilot randomized controlled trial. J. Consult Clin. Psychol. 2015;83:564–577. doi: 10.1037/a0038655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbini B, et al. Dark therapy for mania: a pilot study. Bipolar Disord. 2005;7:98–101. doi: 10.1111/j.1399-5618.2004.00166.x. [DOI] [PubMed] [Google Scholar]
- 12.Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am. J. Psychiatry. 2008;165:830–843. doi: 10.1176/appi.ajp.2008.08010077. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan KA, et al. Hypersomnia subtypes, sleep and relapse in bipolar disorder. Psychol. Med. 2015;45:1751–1763. doi: 10.1017/S0033291714002918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jawinski, P. et al. Recorded and reported sleepiness: the association between brain arousal in resting state and subjective daytime sleepiness. Sleep40, zsx099, 10.1093/sleep/zsx099 (2017). [DOI] [PubMed]
- 15.Hegerl U, Hensch T. The vigilance regulation model of affective disorders and ADHD. Neurosci. Biobehav. Rev. 2014;44:45–57. doi: 10.1016/j.neubiorev.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Hegerl U, Himmerich H, Engmann B, Hensch T. Mania and attention-deficit/hyperactivity disorder: common symptomatology, common pathophysiology and common treatment? Curr. Opin. Psychiatry. 2010;23:1–7. doi: 10.1097/YCO.0b013e328331f694. [DOI] [PubMed] [Google Scholar]
- 17.Hegerl, U., Sander C. & Hensch T. Arousal regulation in affective disorders. In: Systems Neuroscience in Depression (ed Frodl T.). (Academic Press, 2016).
- 18.Wittekind DA, et al. Early report on brain arousal regulation in manic vs depressive episodes in bipolar disorder. Bipolar Disord. 2016;18:502–510. doi: 10.1111/bdi.12440. [DOI] [PubMed] [Google Scholar]
- 19.Ghaemi SN, et al. Pharmacological treatment patterns at study entry for the first 500 STEP-BD participants. Psychiatr. Serv. 2006;57:660–665. doi: 10.1176/ps.2006.57.5.660. [DOI] [PubMed] [Google Scholar]
- 20.Maoz H, et al. Dimensional psychopathology in preschool offspring of parents with bipolar disorder. J. Child Psychol. Psychiatry. 2014;55:144–153. doi: 10.1111/jcpp.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melo MC, et al. Sleep and circadian alterations in people at risk for bipolar disorder: a systematic review. J. Psychiatr. Res. 2016;83:211–219. doi: 10.1016/j.jpsychires.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Pancheri C, et al. A systematic review on sleep alterations anticipating the onset of bipolar disorder. Eur. Psychiatry. 2019;58:45–53. doi: 10.1016/j.eurpsy.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Ritter PS, et al. Disturbed sleep as risk factor for the subsequent onset of bipolar disorder-Data from a 10-year prospective-longitudinal study among adolescents and young adults. J. Psychiatr. Res. 2015;68:76–82. doi: 10.1016/j.jpsychires.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Miller, G. A. The Behavioral High-Risk Paradigm in Psychopathology. (Springer, 1995).
- 25.Eckblad M, Chapman LJ. Development and validation of a scale for hypomanic personality. J. Abnorm. Psychol. 1986;95:214–222. doi: 10.1037/0021-843X.95.3.214. [DOI] [PubMed] [Google Scholar]
- 26.Burmeister M, McInnis MG, Zollner S. Psychiatric genetics: progress amid controversy. Nat. Rev. Genet. 2008;9:527–540. doi: 10.1038/nrg2381. [DOI] [PubMed] [Google Scholar]
- 27.Savitz JB, Ramesar RS. Personality: is it a viable endophenotype for genetic studies of bipolar affective disorder? Bipolar Disord. 2006;8:322–337. doi: 10.1111/j.1399-5618.2006.00309.x. [DOI] [PubMed] [Google Scholar]
- 28.Smoller JW, et al. Psychiatric genetics and the structure of psychopathology. Mol. Psychiatry. 2019;24:409–420. doi: 10.1038/s41380-017-0010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cuthbert BN. Research Domain Criteria: toward future psychiatric nosologies. Dialogues Clin. Neurosci. 2015;17:89–97. doi: 10.31887/DCNS.2015.17.1/bcuthbert. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hofmann BU, Meyer TD. Mood fluctuations in people putatively at risk for bipolar disorders. Br. J. Clin. Psychol. 2006;45:105–110. doi: 10.1348/014466505X35317. [DOI] [PubMed] [Google Scholar]
- 31.Johnson SL, Carver CS, Joormann J, Cuccaro M. A genetic analysis of the validity of the Hypomanic Personality Scale. Bipolar Disord. 2015;17:331–339. doi: 10.1111/bdi.12251. [DOI] [PubMed] [Google Scholar]
- 32.Ortega-Alonso A, et al. Genome-wide association study of psychosis proneness in the Finnish population. Schizophrenia Bull. 2017;43:1304–1314. doi: 10.1093/schbul/sbx006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyer TD, Hautzinger M. Hypomanic personality, social anhedonia and impulsive nonconformity: evidence for familial aggregation? J. Personal. Disord. 2001;15:281–299. doi: 10.1521/pedi.15.4.281.19183. [DOI] [PubMed] [Google Scholar]
- 34.Miller CJ, Johnson SL, Kwapil TR, Carver CS. Three studies on self-report scales to detect bipolar disorder. J. Affect. Disord. 2011;128:199–210. doi: 10.1016/j.jad.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rowland JE, et al. Cognitive regulation of negative affect in schizophrenia and bipolar disorder. Psychiatry Res. 2013;208:21–28. doi: 10.1016/j.psychres.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 36.Klein DN, Lewinsohn PM, Seeley JR. Hypomanic personality traits in a community sample of adolescents. J. Affect. Disord. 1996;38:135–143. doi: 10.1016/0165-0327(96)00005-5. [DOI] [PubMed] [Google Scholar]
- 37.Meyer TD, Hautzinger M. Screening for bipolar disorders using the Hypomanic Personality Scale. J. Affect. Disord. 2003;75:149–154. doi: 10.1016/S0165-0327(02)00042-3. [DOI] [PubMed] [Google Scholar]
- 38.Kwapil TR, et al. A longitudinal study of high scorers on the hypomanic personality scale. J. Abnorm. Psychol. 2000;109:222–226. doi: 10.1037/0021-843X.109.2.222. [DOI] [PubMed] [Google Scholar]
- 39.Walsh MA, DeGeorge DP, Barrantes-Vidal N, Kwapil TR. A 3-year longitudinal study of risk for bipolar spectrum psychopathology. J. Abnorm. Psychol. 2015;124:486–497. doi: 10.1037/abn0000045. [DOI] [PubMed] [Google Scholar]
- 40.Blechert J, Meyer TD. Are measures of hypomanic personality, impulsive nonconformity and rigidity predictors of bipolar symptoms? Br. J. Clin. Psychol. 2005;44:15–27. doi: 10.1348/014466504X19758. [DOI] [PubMed] [Google Scholar]
- 41.Depue RA, Krauss S, Spoont MR, Arbisi P. General behavior inventory identification of unipolar and bipolar affective conditions in a nonclinical university population. J. Abnorm. Psychol. 1989;98:117–126. doi: 10.1037/0021-843X.98.2.117. [DOI] [PubMed] [Google Scholar]
- 42.Akiskal HS, et al. TEMPS-I: delineating the most discriminant traits of the cyclothymic, depressive, hyperthymic and irritable temperaments in a nonpatient population. J. Affect. Disord. 1998;51:7–19. doi: 10.1016/S0165-0327(98)00152-9. [DOI] [PubMed] [Google Scholar]
- 43.Akiskal HS, Akiskal KK. TEMPS: temperament evaluation of Memphis, Pisa, Paris and San Diego. J. Affect. Disord. 2005;85:1–2. doi: 10.1016/j.jad.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 44.Akiskal HS, Akiskal KK, Haykal RF, Manning JS, Connor PD. TEMPS-A: progress towards validation of a self-rated clinical version of the temperament evaluation of the Memphis, Pisa, Paris, and San Diego Autoquestionnaire. J. Affect. Disord. 2005;85:3–16. doi: 10.1016/j.jad.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 45.Hensch T, Herold U, Brocke B. An electrophysiological endophenotype of hypomanic and hyperthymic personality. J. Affect. Disord. 2007;101:13–26. doi: 10.1016/j.jad.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 46.Walsh MA, Royal AM, Barrantes-Vidal N, Kwapil TR. The association of affective temperaments with impairment and psychopathology in a young adult sample. J. Affect. Disord. 2012;141:373–381. doi: 10.1016/j.jad.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 47.Kawamura Y, et al. Six-year stability of affective temperaments as measured by TEMPS-A. Psychopathology. 2010;43:240–247. doi: 10.1159/000313522. [DOI] [PubMed] [Google Scholar]
- 48.Solmi M, et al. A comparative meta-analysis of TEMPS scores across mood disorder patients, their first-degree relatives, healthy controls, and other psychiatric disorders. J. Affect. Disord. 2016;196:32–46. doi: 10.1016/j.jad.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 49.Greenwood TA, Akiskal HS, Akiskal KK, Kelsoe JR. Genome-wide association study of temperament in bipolar disorder reveals significant associations with three novel Loci. Biol. Psychiatry. 2012;72:303–310. doi: 10.1016/j.biopsych.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeGeorge DP, Walsh MA, Barrantes-Vidal N, Kwapil TR. A three-year longitudinal study of affective temperaments and risk for psychopathology. J. Affect. Disord. 2014;164:94–100. doi: 10.1016/j.jad.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 51.Schalet BD, Durbin CE, Revelle W. Multidimensional structure of the Hypomanic Personality Scale. Psychol. Assess. 2011;23:504–522. doi: 10.1037/a0022301. [DOI] [PubMed] [Google Scholar]
- 52.Stanton K, Gruber J, Watson D. Basic dimensions defining mania risk: a structural approach. Psychol. Assess. 2017;29:304–319. doi: 10.1037/pas0000337. [DOI] [PubMed] [Google Scholar]
- 53.Terrien S, Stefaniak N, Morvan Y, Besche-Richard C. Factor structure of the French version of the Hypomanic Personality Scale (HPS) in non-clinical young adults. Compr. Psychiatry. 2015;62:105–113. doi: 10.1016/j.comppsych.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 54.Alloy LB, et al. Progression along the bipolar spectrum: a longitudinal study of predictors of conversion from bipolar spectrum conditions to bipolar I and II disorders. J. Abnorm. Psychol. 2012;121:16–27. doi: 10.1037/a0023973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tijssen MJ, et al. Evidence that bipolar disorder is the poor outcome fraction of a common developmental phenotype: an 8-year cohort study in young people. Psychol. Med. 2010;40:289–299. doi: 10.1017/S0033291709006138. [DOI] [PubMed] [Google Scholar]
- 56.van Os J. The dynamics of subthreshold psychopathology: implications for diagnosis and treatment. Am. J. Psychiatry. 2013;170:695–698. doi: 10.1176/appi.ajp.2013.13040474. [DOI] [PubMed] [Google Scholar]
- 57.Vieta E, et al. Early intervention in bipolar disorder. Am. J. Psychiatry. 2018;175:411–426. doi: 10.1176/appi.ajp.2017.17090972. [DOI] [PubMed] [Google Scholar]
- 58.Hafeman DM, et al. Assessment of a person-level risk calculator to predict new-onset bipolar spectrum disorder in youth at familial risk. JAMA Psychiatry. 2017;74:841–847. doi: 10.1001/jamapsychiatry.2017.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meyer TD, Maier S. Is there evidence for social rhythm instability in people at risk for affective disorders? Psychiatry Res. 2006;141:103–114. doi: 10.1016/j.psychres.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 60.Ankers D, Jones SH. Objective assessment of circadian activity and sleep patterns in individuals at behavioural risk of hypomania. J. Clin. Psychol. 2009;65:1071–1086. doi: 10.1002/jclp.20608. [DOI] [PubMed] [Google Scholar]
- 61.Ford BQ, Mauss IB, Gruber J. Valuing happiness is associated with bipolar disorder. Emotion. 2015;15:211–222. doi: 10.1037/emo0000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meyer TD, Drüke B, Hautzinger M. Hypomane Persönlichkeit-Psychometrische Evaluation und erste Ergebnisse zur Validität der deutschen Version der Chapman-Skala. Z. Klin. Psychol. Psychother. 2000;29:35–42. doi: 10.1026//0084-5345.29.1.35. [DOI] [Google Scholar]
- 63.Loeffler M, et al. The LIFE-Adult-Study: objectives and design of a population-based cohort study with 10,000 deeply phenotyped adults in Germany. BMC Public Health. 2015;15:691. doi: 10.1186/s12889-015-1983-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spada J, et al. Genetic association of objective sleep phenotypes with a functional polymorphism in the neuropeptide S receptor gene. PLoS ONE. 2014;9:e98789. doi: 10.1371/journal.pone.0098789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spada J, et al. Genome-wide association analysis of actigraphic sleep phenotypes in the LIFE Adult Study. J. Sleep. Res. 2016;25:690–701. doi: 10.1111/jsr.12421. [DOI] [PubMed] [Google Scholar]
- 66.Jawinski P, et al. Time to wake up: no impact of COMT Val158Met gene variation on circadian preferences, arousal regulation and sleep. Chronobiol. Int. 2016;33:893–905. doi: 10.1080/07420528.2016.1178275. [DOI] [PubMed] [Google Scholar]
- 67.Hinz Andreas, Glaesmer Heide, Brähler Elmar, Löffler Markus, Engel Christoph, Enzenbach Cornelia, Hegerl Ulrich, Sander Christian. Sleep quality in the general population: psychometric properties of the Pittsburgh Sleep Quality Index, derived from a German community sample of 9284 people. Sleep Medicine. 2017;30:57–63. doi: 10.1016/j.sleep.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 68.Rawlings D, Barrantes-Vidal N, Claridge G, McCreery C, Galanos G. A factor analytic study of the Hypomanic Personality Scale in British, Spanish and Australian samples. Personal. Individ. Differ. 2000;28:73–84. doi: 10.1016/S0191-8869(99)00081-1. [DOI] [Google Scholar]
- 69.Scott J, et al. Activation in bipolar disorders: a systematic review. JAMA Psychiatry. 2017;74:189–196. doi: 10.1001/jamapsychiatry.2016.3459. [DOI] [PubMed] [Google Scholar]
- 70.Duffy Anne, Jones Steven, Goodday Sarah, Bentall Richard. Candidate Risks Indicators for Bipolar Disorder: Early Intervention Opportunities in High-Risk Youth. International Journal of Neuropsychopharmacology. 2015;19(1):pyv071. doi: 10.1093/ijnp/pyv071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoaki N, et al. Biological aspect of hyperthymic temperament: light, sleep, and serotonin. Psychopharmacology. 2011;213:633–638. doi: 10.1007/s00213-010-1999-0. [DOI] [PubMed] [Google Scholar]
- 72.Alloy LB, Nusslock R, Boland EM. The development and course of bipolar spectrum disorders: an integrated reward and circadian rhythm dysregulation model. Annu Rev. Clin. Psychol. 2015;11:213–250. doi: 10.1146/annurev-clinpsy-032814-112902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Díaz-Román A, Mitchell R, Cortese S. Sleep in adults with ADHD: systematic review and meta-analysis of subjective and objective studies. Neurosci. Biobehav. Rev. 2018;89:61–71. doi: 10.1016/j.neubiorev.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 74.Cortese S, Faraone SV, Konofal E, Lecendreux M. Sleep in children with attention-deficit/hyperactivity disorder: meta-analysis of subjective and objective studies. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48:894–908. doi: 10.1097/CHI.0b013e3181ac09c9. [DOI] [PubMed] [Google Scholar]
- 75.Monk TH, Buysse DJ, Welsh DK, Kennedy KS, Rose LR. A sleep diary and questionnaire study of naturally short sleepers. J. Sleep. Res. 2001;10:173–179. doi: 10.1046/j.1365-2869.2001.00254.x. [DOI] [PubMed] [Google Scholar]
- 76.Curtis, B. J., Williams P. G. & Anderson J. S. Objective cognitive functioning in self-reported habitual short sleepers not reporting daytime dysfunction: examination of impulsivity via delay discounting. Sleep41, zsy115, 10.1093/sleep/zsy115 (2018). [DOI] [PMC free article] [PubMed]
- 77.Egeland JA, et al. A 16-year prospective study of prodromal features prior to BPI onset in well Amish children. J. Affect. Disord. 2012;142:186–192. doi: 10.1016/j.jad.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 78.Lichstein KL, et al. Actigraphy validation with insomnia. Sleep. 2006;29:232–239. [PubMed] [Google Scholar]
- 79.Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J. Affect. Disord. 2009;113:1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 80.Hensch T, et al. Yawning in depression: worth looking into. Pharmacopsychiatry. 2015;48:118–120. doi: 10.1055/s-0035-1558013. [DOI] [PubMed] [Google Scholar]
- 81.Ulke C, et al. Brain arousal regulation in SSRI-medicated patients with major depression. J. Psychiatr. Res. 2018;108:34–39. doi: 10.1016/j.jpsychires.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 82.Kaplan KA, Gruber J, Eidelman P, Talbot LS, Harvey AG. Hypersomnia in inter-episode bipolar disorder: does it have prognostic significance? J. Affect. Disord. 2011;132:438–444. doi: 10.1016/j.jad.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Geissler J, Romanos M, Hegerl U, Hensch T. Hyperactivity and sensation seeking as autoregulatory attempts to stabilize brain arousal in ADHD and mania? Atten. Defic. Hyperact. Disord. 2014;6:159–173. doi: 10.1007/s12402-014-0144-z. [DOI] [PubMed] [Google Scholar]
- 84.Sander C, Hensch T, Wittekind DA, Bottger D, Hegerl U. Assessment of wakefulness and brain arousal regulation in psychiatric research. Neuropsychobiology. 2015;72:195–205. doi: 10.1159/000439384. [DOI] [PubMed] [Google Scholar]
- 85.Huang, J. et al. Evoked potentials and behavioral performance during different states of brain arousal. BMC Neurosci.18, 21, 10.1186/s12868-017-0340-9 (2017). [DOI] [PMC free article] [PubMed]
- 86.Huang J, et al. Test-retest reliability of brain arousal regulation as assessed with VIGALL 2.0. Neuropsychiatr. Electrophysiol. 2015;1:1–13. doi: 10.1186/s40810-015-0013-9. [DOI] [Google Scholar]
- 87.Olbrich S, et al. EEG-vigilance and BOLD effect during simultaneous EEG/fMRI measurement. Neuroimage. 2009;45:319–332. doi: 10.1016/j.neuroimage.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 88.Huang, J. et al. Impact of brain arousal and time-on-task on autonomic nervous system activity in the wake-sleep transition. BMC Neurosci.19, 10.1186/s12868-018-0419-y (2018). [DOI] [PMC free article] [PubMed]
- 89.Ulke C, et al. Coupling and dynamics of cortical and autonomic signals are linked to central inhibition during the wake-sleep transition. Sci. Rep. 2017;7:11804. doi: 10.1038/s41598-017-09513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jawinski Philippe, Kirsten Holger, Sander Christian, Spada Janek, Ulke Christine, Huang Jue, Burkhardt Ralph, Scholz Markus, Hensch Tilman, Hegerl Ulrich. Human brain arousal in the resting state: a genome-wide association study. Molecular Psychiatry. 2018;24(11):1599–1609. doi: 10.1038/s41380-018-0052-2. [DOI] [PubMed] [Google Scholar]
- 91.Jawinski P, et al. Brain arousal regulation in carriers of bipolar disorder risk alleles. Neuropsychobiology. 2015;72:65–73. doi: 10.1159/000437438. [DOI] [PubMed] [Google Scholar]
- 92.Harrison PJ, Geddes JR, Tunbridge EM. The emerging neurobiology of bipolar disorder. Trends Neurosci. 2018;41:18–30. doi: 10.1016/j.tins.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Strauss M, et al. Brain arousal regulation in adults with attention-deficit/hyperactivity disorder (ADHD) Psychiatry Res. 2018;261:102–108. doi: 10.1016/j.psychres.2017.12.043. [DOI] [PubMed] [Google Scholar]
- 94.Kaufmann CN, Gershon A, Eyler LT, Depp CA. Clinical significance of mobile health assessed sleep duration and variability in bipolar disorder. J. Psychiatr. Res. 2016;81:152–159. doi: 10.1016/j.jpsychires.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Scott J, Vaaler AE, Fasmer OB, Morken G, Krane-Gartiser K. A pilot study to determine whether combinations of objectively measured activity parameters can be used to differentiate between mixed states, mania, and bipolar depression. Int J. Bipolar Disord. 2017;5:5. doi: 10.1186/s40345-017-0076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Levenson JC, et al. Longitudinal sleep phenotypes among offspring of bipolar parents and community controls. J. Affect. Disord. 2017;215:30–36. doi: 10.1016/j.jad.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.