Abstract
Recently, gold-coated magnetic nanoparticles have drawn the interest of researchers due to their unique magneto-plasmonic characteristics. Previous research has found that the magneto-optical Faraday effect of gold-coated magnetic nanoparticles can be effectively enhanced because of the surface plasmon resonance of the gold shell. Furthermore, gold-coated magnetic nanoparticles are ideal for biomedical applications because of their high stability and biocompatibility. In this work, we synthesized Fe3O4@Au core-shell nanoparticles and coated streptavidin (STA) on the surface. Streptavidin is a protein which can selectively bind to biotin with a strong affinity. STA is widely used in biotechnology research including enzyme-linked immunosorbent assay (ELISA), time-resolved immunofluorescence (TRFIA), biosensors, and targeted pharmaceuticals. The Faraday magneto-optical characteristics of the biofunctionalized Fe3O4@Au nanoparticles were measured and studied. We showed that the streptavidin-coated Fe3O4@Au nanoparticles still possessed the enhanced magneto-optical Faraday effect. As a result, the possibility of using biofunctionalized Fe3O4@Au nanoparticles for magneto-optical biomedical assays should be explored.
Subject terms: Nanoscience and technology, Biomedical engineering
Introduction
Over the past few decades, nanotechnology has advanced rapidly. The special property of unique size of nanoparticles provides many advantages. Magnetic nanoparticles (MNPs) have been developed in many fields, such as biomedicine1–5, nano fluids6, magnetic resonance imaging7–9, and optics10. MNPs have recently attracted more attention for biomedical applications because of their magnetic and optical characteristics. MNPs can serve as drug carriers11, biosensors12,13, gene delivery systems14, etc.15.
Iron oxide MNPs are the most commonly used MNPs due to their superparamagnetic stability and biocompatibility16. The properties of Fe3O4 MNPs, such as size and shape, can be altered with different synthesis methods17 and as a result the MNPs can be specialized for different applications. Several synthesis routes have been reported including thermal decomposition18, co-precipitation19, and hydrothermal synthesis20. Each technique fits the demands of different biomedicine applications. Generally, the particle size of Fe3O4 MNPs is affected by pH variation21, temperature22, and stirring rate23 during the synthesis process.
To increase the stability and biocompatibility, the surface of the MNP generally needs to be modified with some noble metal or polymer. Several methods to modify MNPs made of iron oxide have been reported3,4,24,25. Gold, a noble metal with good biocompatibility, is commonly used in biomedical applications. Due to their biocompatibility gold coated MNPs have been developed and widely studied26,27. Moreover, gold coated MNPs simultaneously possess magnetic and plasmonic characteristics. Jain et al. reported that the magneto-optical Faraday effect in gold-coated iron oxide nanocrystals was enhanced due to surface plasmon resonance enhanced magneto-optics (SuPREMO)28. However, the nanoparticle surface generally needs to be modified with biomaterials or proteins for applications in biomedicine. It is well known that surface plasmon resonance (SPR) is very sensitive to the surface state of the nanoparticle. The SPR of a nanoparticle is highly responsive to small changes in the local refraction index29. Hence, the surface modification of biomaterials certainly alters the characteristics of the SPR.
In this work, we synthesized Fe3O4@Au core/shell magnetic nanoparticles and coated their surface with streptavidin (STA) to study how the addition of STA impacted the magneto-optical Faraday effect. STA is a widely used biomaterial for developing new biomedical methods because the conjugation of STA and biotin is very strong. It is commonly used to investigate the quantification process with biotin. We experimentally demonstrated that Fe3O4@Au core-shell MNPs were still able to enhance the magneto-optical Faraday rotation even after surface modification with STA. This result suggests that SuPREMO is a promising effect to exploit in biomedical assay techniques based on the magneto-optical effect, such as the Faraday immunoassay system30.
In our previous work30, we have demonstrated that the Faraday magneto-optical measurement with biofunctionalized magnetic nanoparticles (BMNs) results in a simple, convenient, and sensitive tool for assaying biomarkers. Due to the antibody–antigen interactions, BMNs conjugated with the biotargets to form large magnetic clusters over time. The magnetic characteristics of the BMNs reagent are altered as well. The Faraday rotation angle varies as a function of the size of the MNP. Therefore, we aim to observe the clustering process by measuring the Faraday effect of MNPs. Since SuPRMO MNPs possess the special characteristic of Faraday rotation enhancement, biofunctionalized SuPRMO MNPs are a potential reagent for increasing the sensitivity of the magneto-optical Faraday immunoassay technique.
Results and Discussions
X-ray diffraction (XRD) & UV-Vis spectrum
Figure 1a shows the powder X-ray diffraction (XRD) patterns of Fe3O4 and Fe3O4@Au core-shell MNPs. The diffraction angle of the (311) peak of the raw MNPs occurs at 35.46°, which means that the composition of the MNPs is magnetite before reducing the Au shell31. The XRD data showed that the synthesized particles are Fe3O4 with good crystallinity. After coating the MNPS with an Au shell, the XRD signals of the Fe3O4 core were shielded by the gold layer because of the heavy atom effect24. The absorbance of the synthesized particles was measured using ultraviolet-visible spectroscopy (UV-Vis) (U-2800A, HITACHI). The UV-Vis spectra (Fig. 1b) shows that the absorbance of pure Fe3O4 MNPs monotonically decreased with the wavelength of light. However, the Fe3O4@Au core-shell MNPs exhibited an absorption peak at a wavelength of approximately 538.5 nm due to the SPR effect of the gold layer. After the bonding of STA, the wavelength of the absorption peak of the Fe3O4@Au-STA MNPs increased to around 550 nm. The result clearly shows that the STA modification induces a red shift of the UV-Vis spectrum. This means that the refractive index of the STA does indeed alter the SPR condition of the Fe3O4@Au MNPs.
Figure 1.
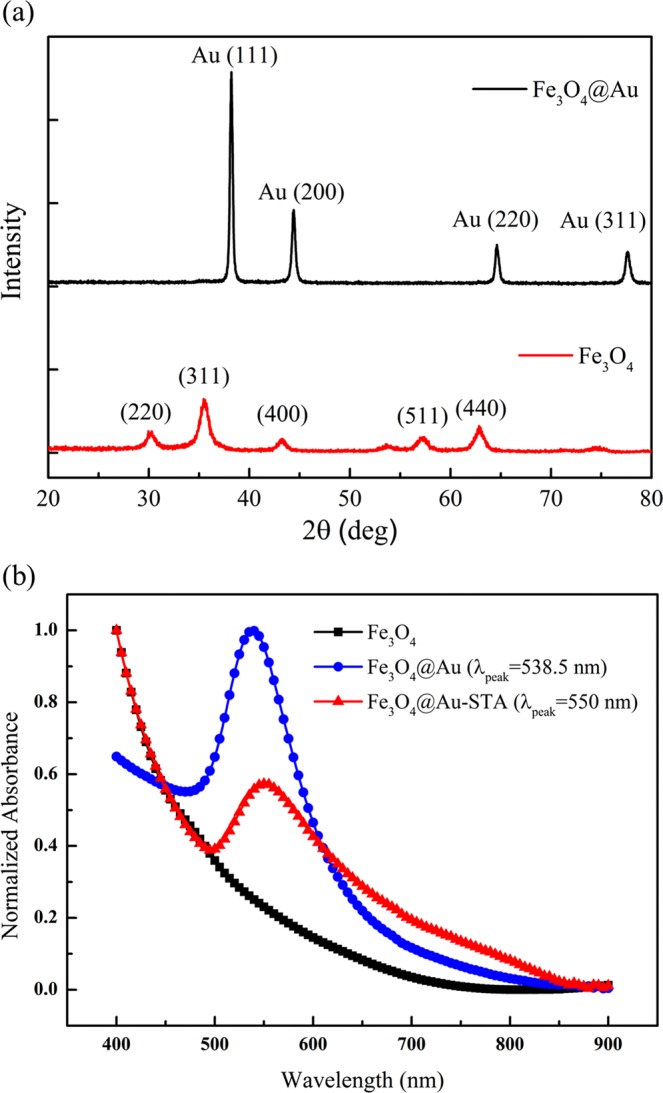
(a) Powder XRD patterns and (b) UV-Vis spectra of the Fe3O4, Fe3O4@Au core-shell and Fe3O4@Au-STA MNPs.
Dynamic light scattering
Figure 2 shows the hydrodynamic sizes of the Fe3O4, Fe3O4@Au core-shell, and Fe3O4@Au-STA MNPs measured by dynamic light scattering (DLS) (SZ-100Z, HORIBA). Table 1 shows the hydrodynamic diameter, polydispersity index (PI), and Zeta potential of the Fe3O4, Fe3O4@Au, and Fe3O4@Au-STA MNPs. The average hydrodynamic diameters of Fe3O4, Fe3O4@Au, and Fe3O4@Au-STA MNPs are 83.0 ± 22.4, 112.7 ± 29.3, and 132.8 ± 31.9 nm, respectively. As expected, the mean size and dispersion of the distribution of the MNPs increases as the amount of covering material increases. The Zeta potential data show that the Fe3O4, Fe3O4@Au, and Fe3O4@Au-STA are both stable in the solution.
Figure 2.
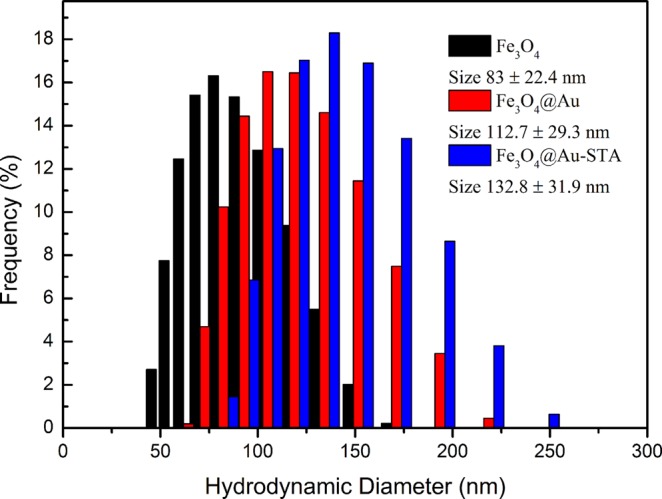
Distribution of the hydrodynamic diameter of the Fe3O4, Fe3O4@Au core-shell and Fe3O4@Au-STA MNPs.
Table 1.
Hydrodynamic diameter, polydispersity index (PI) and Zeta potential of the Fe3O4, Fe3O4@Au, and Fe3O4@Au-STA MNPs.
| MNPs | Size(nm) | PI | Zeta Potential(mV) |
|---|---|---|---|
| Fe3O4 | 83.0 ± 22.4 | 0.258 | −66.1 |
| Fe3O4@Au | 112.7 ± 29.3 | 0.300 | −57.8 |
| Fe3O4@Au-STA | 132.8 ± 31.9 | 0.338 | −46.8 |
High-resolution transmission electron microscopy (HRTEM)
Figure 3a presents a high-resolution transmission electron microscopy (HRTEM) (JEM-2010, JEOL Co. Ltd) image of the Fe3O4@Au core-shell MNPs. It shows that the shape of the synthesized MNPs was approximately spherical and their average size was about 66 nm. Figure 3b shows a magnified HRTEM image near the surface of a Fe3O4@Au core-shell MNP. The crystal structure of the gold shell on the Fe3O4 core can be clearly seen. The TEM image simultaneously shows the Au lattice near the particle surface and the Fe3O4 lattice at the core. The Fe3O4 lattice is relatively blurry because the electron beam has difficulty penetrating to the center of particle. The analysis of the energy-dispersive X-ray spectroscopy (EDS) (JEM-2010, JEOL Co. Ltd) for the Fe3O4@Au core-shell MNPs clearly revealed that the synthesized particles contained the elements Fe, O, and Au (Fig. 3c). The results of characteristic analysis proved that the Fe3O4@Au core-shell MNPs were successfully synthesized.
Figure 3.
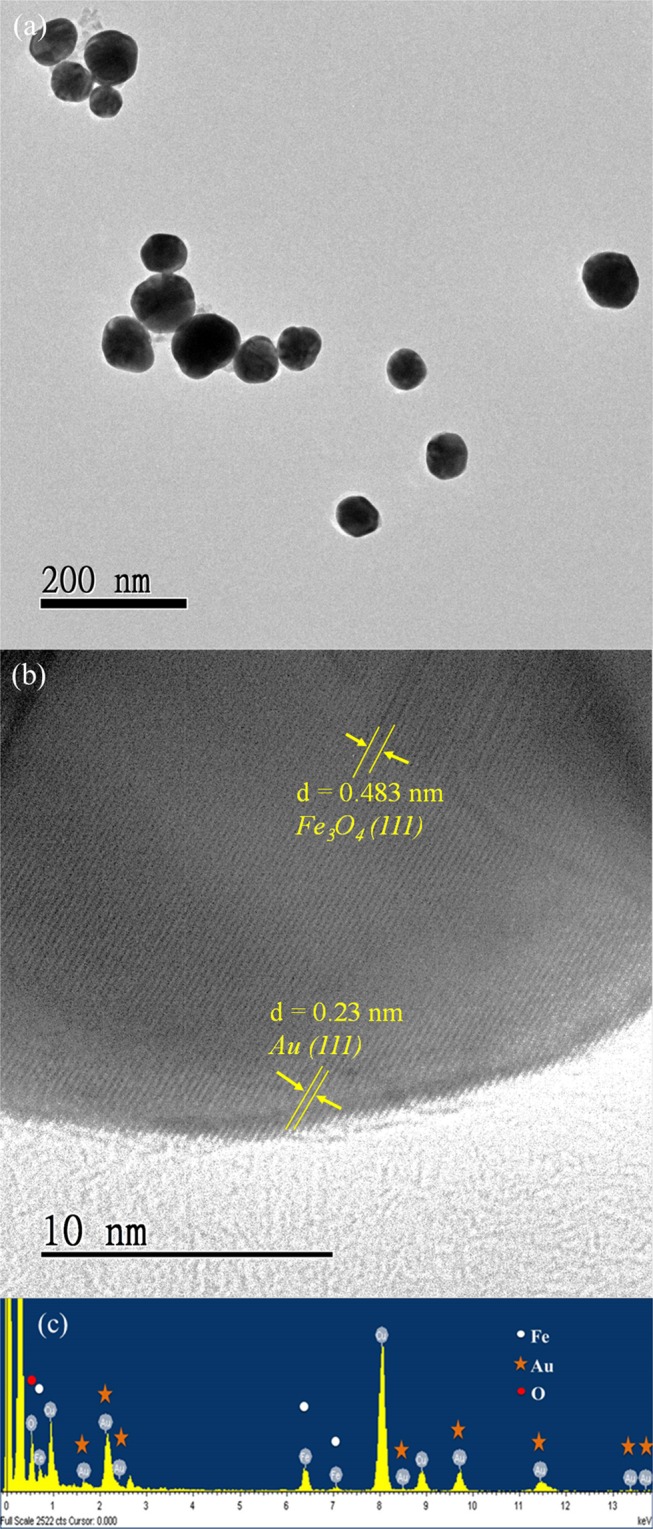
(a) TEM images of the Fe3O4@Au MNPs. The scale bar is 200 nm. (b) Magnified image of the gold shell on the Fe3O4 core. The scale bar is 10 nm. (c) EDS spectrum of the Fe3O4@Au MNPs.
Magnetization curve
Figure 4a shows the magnetization curve of the Fe3O4@Au MNP reagent measured by a SQUID magnetic property measurement system (MPMS, Quantum Design, Inc) at 300 K. The inset in Fig. 4a shows that there was no hysteresis in the magnetization curve even under a small magnetic field. Fe3O4@Au MNPs exhibited the characteristics of superparamagnetic material; noting that the magnetization shown is the magnetization of the MNP reagent, not the magnetization of the MNP powder. A 3-D Nanometer Scale Raman PL Microspectrometer (Tokyo Instruments, INC.) was used to determine whether STA was successfully coated on the surface of Fe3O4@Au MNPs. Figure 4b shows the Raman spectra of the Fe3O4@Au MNPs before and after coating STA. After STA was coated, peaks emerged in the region of 1200–1600 cm−1 with respect to the Raman signals of Fe3O4@Au MNPs. The marked peaks in Fig. 4b showed the presence of STA. Peaks at 1254 and 1279 cm−1 represent the amide III region and the peak at 1447 cm−1 represents the δ-CH2 and δ-CH3 bands. The Trp10, Trp7, Trp5, and Trp2 signals are at 1243, 1341, 1461, and 1580 cm−1, respectively32. All these peaks are the characteristic Raman signals of STA32 indicating that STA was successfully coated on the surface of the Fe3O4@Au MNPs.
Figure 4.
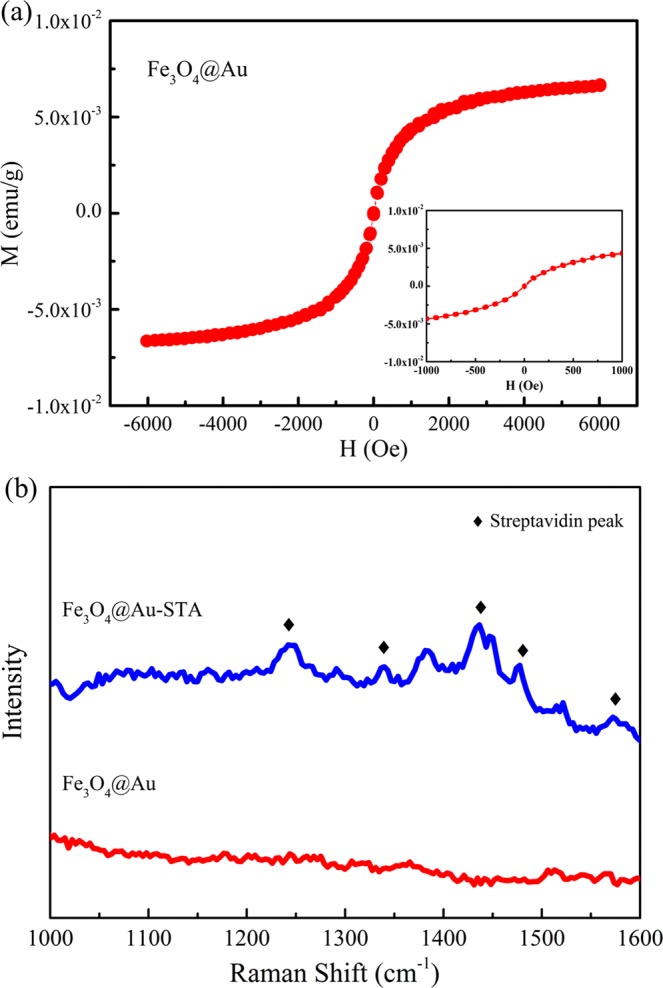
(a) Magnetic hysteresis curve of the Fe3O4@Au MNPs. The inset shows the magnetization of the Fe3O4@Au MNPs under a small magnetic field. (b) Raman spectra of the Fe3O4@Au MNPs before and after coating STA. The markers indicate the characteristic Raman peaks of STA.
Faraday rotation measurement
To confirm the Faraday rotation enhancement of the Fe3O4@Au-STA MNPs, we checked the magneto-optical characteristic of the pure STA reagent. Figure 5a is the Faraday rotation spectrum of pure STA reagent (100 μg/mL) as a function of the applied magnetic field. Clearly the magneto-optical Faraday effect of the pure STA reagent was extremely weak when the applied magnetic field was less than 100 gauss. Recalling that Fig. 1b revealed that the STA modification does indeed alter the SPR condition of the Fe3O4@Au NPs; Fig. 5b shows the Faraday rotation spectra of the Fe3O4@Au-STA and Fe3O4 MNPs reagents as a function of the applied magnetic field. To exclude the influence of magnetization on the Faraday rotations, the saturation magnetizations of the measured samples were controlled (Ms = 6.6 × 10−3 emu/g for both). Figure 5b shows that the Faraday rotation of the Fe3O4@Au-STA was larger than that of Fe3O4 for an applied magnetic field larger than 30 gauss. The gold layer of core shield MNPs can be seen as an optical cavity with multiple resonance modes. When the light at a corresponding frequency illuminates the cavity, the energy of that light is stored inside the cavity. The result is that the MNP inside cavity senses a stronger electromagnetic field than the MNP without a cavity. The enhanced interaction between the MNPs and light results in the larger Faraday rotation. This result proves that the Fe3O4@Au-STA MNPs still possessed the SuPREMO effect which enhances the Faraday rotation even after the STA coating was applied. The biomaterial modified magneto-plasmonic nanoparticle is promising for applications based on the magneto-optical Faraday effect. In our previous work30, we successfully developed a Faraday immunoassay technique based on the magneto-optical Faraday effect and biofunctionalized MNPs. Now, these experimental results suggest that biofunctionalized magneto-plasmonic nanoparticle can be exploited to improve sensitivity using the Faraday immunoassay technique.
Figure 5.
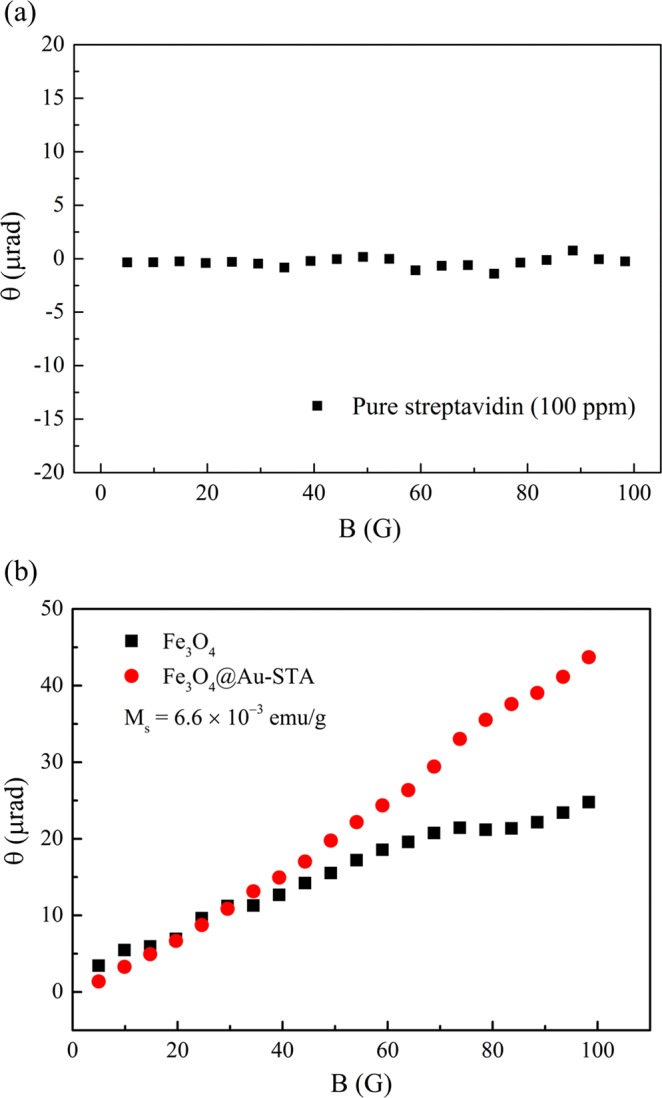
(a) Faraday rotation spectrum of the pure STA reagent with the concentration of 100 μg/mL. (b) Faraday rotation spectra of Fe3O4@Au-STA and Fe3O4 MNPs. The saturation magnetizations of the Fe3O4@Au-STA and Fe3O4 MNPs reagents both were 6.6 × 10−3 emu/g.
In summary, we synthesized the Fe3O4@Au core-shell MNPs and coated particle surfaces with STA. We observed that the Fe3O4@Au-STA MNPs still possess the Faraday rotation enhancement after conjugating the biomaterial on the surface of the gold layer. The experimental results imply that the biofunctionalized Fe3O4@Au core-shell MNPs still had the effect of SuPREMO and are promising for magneto-optical biomedical applications.
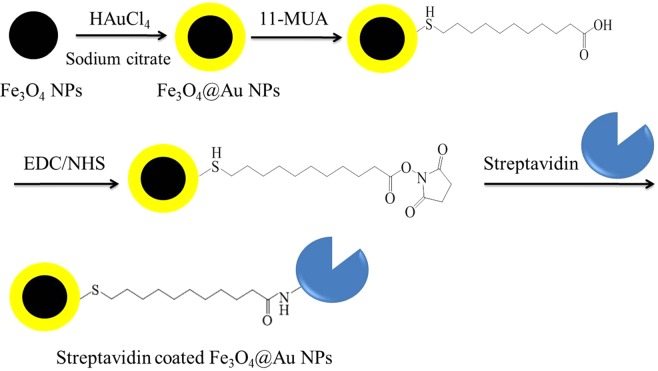
Methods Synthesis of the bio-functionalized core-shell Fe3O4@Au nanoparticles
In this study, iron oxide nanoparticles were prepared by co-precipitation of Fe(II) and Fe(III) first. An iron salt aqueous solution was combined with Ferric chloride (FeCl3.6H2O) and ferrous chloride (FeCl2.4H2O) at a ratio of 2:1. The iron salt aqueous solution was heated to 80 °C. Then, 28% NH4OH (W/V) was added to the iron salt aqueous solution and stirred for 30 min to form Fe3O4 nanoparticles. After that, Fe3O4 nanoparticles were obtained by magnetic separation. DI water was used to wash the precipitate.
Subsequently, the synthesized Fe3O4 MNPs were dispersed in 0.1 M (50 mL) Tetramethylammonium hydroxide (TMAOH) solution. Next, 0.1 mL Fe3O4/TMAOH solution and 100 mL DI water were stirred with sodium citrate (0.2 M, 3 mL) to replace surface hydroxide ions with citrate ions. Afterward, 1% (W/V) (0.5 mL) HAuCl4 solution and 0.2 M (0.2 mL) hydroxylamine hydrochloride (NH2OH·HCl) were iteratively added to the colloid to reduce the gold shells on the surface of Fe3O4 to form Fe3O4@Au core-shell MNPs33. The mixed colloid was continuously stirred during each iteration. In total 10 iterations were executed and every iteration took 20 minutes. Fe3O4@Au MNPs were obtained by centrifuging (6000 rpm, 30 min) and were then washed with DI water. The precipitate Fe3O4@Au MNPs were dispersed in ethanol (2 mL).
Surface modification was needed to bind STA onto the gold surface34. 11-mercaptoundecanoic acid (11-MUA) can be self-assembled on the gold surface of Fe3O4@Au MNPs and provides a carboxyl group for bioconjugation. Fe3O4@Au nanoparticles were added with 11-MUA (20 mM, 200 μL) and then continuously sonicated for 20 hours. We then centrifuged (12000 rpm, 10 min) the colloid and washed the precipitate with the ethanol. Afterwards, the precipitate was dissolved into PBS (0.001 M, 1 mL, pH 7.4). To activate the carboxyl groups on the gold surface prior to covalent coupling, N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (EDC) (20 mM, 200 μL) and n-hydroxysuccinimide (NHS) (40 mM, 200 μL) were added and continuously sonicated for 2 hours. Then the STA (1 mg/1 mL, 50 μL) was added and the mixed solution was sonicated for 1 hour to form Fe3O4@Au-STA MNPs. EDC and NHS enable the bioconjugation of carboxyl groups on the gold surface with the amino groups of STA molecules to form the streptavidin-coated Fe3O4@Au core-shell MNPs (Fe3O4@Au-STA). Finally, a liquid phase reagent containing Fe3O4@Au-STA MNPs was produced. Figure 6 shows the synthesis processes of Fe3O4@Au-STA MNPs.
Figure 6.
Flow chart of the synthesis process of streptavidin-coated Fe3O4@Au core-shell MNPs.
The Faraday rotation measurement setup
The Faraday rotation measurement was performed using AC magnetic fields and lock-in technique35,36. The light source was a diode-pumped solid-state laser with a wavelength of 532 nm. The frequency of the AC magnetic field was set at 813 Hz of which the environment noise was relatively lower. More details of the Faraday rotation measurement can be found in30. The measurement samples were prepared in liquid phase and encapsulated in sample holders made of glass. X-ray diffraction (XRD) was performed using BRUKER D8 SSS diffractometer with CuKα radiation.
Acknowledgements
The authors would like to acknowledge financial support funded by Ministry of Science and Technology of Taiwan under grants: MOST103-2112-M-005-005-MY3, MOST 106-2112-M-005-002, MOST 107-2112-M-005-012 and MOST 107-2314-B-005-002.
Author contributions
Conceptualization was deduced by K.-L.C.; most of the synthesis and measurements were done by S.-Y.W, C.-W.L., J.-M.C., Y.-J.L., and L.-W.C.; Formal Analysis, J.-M.C., Y.-J.L., and L.-W.C.; Investigation, C.-W.L.; Resources, C.-H.W., C.-C.J., and L.-M.W.; Writing-Original Draft Preparation, C.-W.L., and Y.-J.L.; Writing-Review & Editing, K.-L.C.; Supervision, K.-L.C.; Project Administration, K.-L.C.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pankhurst QA, Connolly J, Jones SK, Dobson J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003;36:167–181. doi: 10.1088/0022-3727/36/13/201. [DOI] [Google Scholar]
- 2.Berry CC, Curtis ASG. Functionalisation of magnetic nanoparticles for applications in biomedicine. J. Phys. D Appl. Phys. 2003;36:198–206. doi: 10.1088/0022-3727/36/13/203. [DOI] [Google Scholar]
- 3.Xu CJ, Sun SH. Monodisperse magnetic nanoparticles for biomedical applications. Polym. Int. 2007;56:821–826. doi: 10.1002/pi.2251. [DOI] [Google Scholar]
- 4.Xu C, et al. Nitrilotriacetic Acid-Modified Magnetic Nanoparticles as a General Agent to Bind Histidine-Tagged Proteins. J. Am. Chem. Soc. 2004;126:3392–3393. doi: 10.1021/ja031776d. [DOI] [PubMed] [Google Scholar]
- 5.Gao J, et al. Intracellular Spatial Control of Fluorescent Magnetic Nanoparticles. J. Am. Chem. Soc. 2008;130:3710–3711. doi: 10.1021/ja7103125. [DOI] [PubMed] [Google Scholar]
- 6.Abareshi M, Goharshadi EK, Zebarjad SM, Fadafan HK, Youssefi A. Fabrication, characterization and measurement of thermal conductivity of Fe3O4 nanofluids. J. Magn. Magn. Mater. 2010;332:3895–3901. doi: 10.1016/j.jmmm.2010.08.016. [DOI] [Google Scholar]
- 7.Sun C, Lee JSH, Zhang MQ. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008;60:1252–1265. doi: 10.1016/j.addr.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang YXJ, Hussain SM, Krestin GP. Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imaging. Eur. Radiol. 2001;11:2319–2331. doi: 10.1007/s003300100908. [DOI] [PubMed] [Google Scholar]
- 9.Chertok B, et al. Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors. Biomaterials. 2008;29:487–496. doi: 10.1016/j.biomaterials.2007.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radon A, Drygala A, Hawelek L, Lukowiec D. Structure and optical properties of Fe3O4 nanoparticles synthesized by co-precipitation method with different organic modifiers. Mater. Charact. 2017;131:148–156. doi: 10.1016/j.matchar.2017.06.034. [DOI] [Google Scholar]
- 11.Estelrich J, Escribano E, Queralt J, Busquets MA. Iron oxide nanoparticles for magnetically-guided and magnetically-responsive drug delivery. Int J Mol Sci. 2015;16:8070–8101. doi: 10.3390/ijms16048070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi X, et al. Enzymatic biosensors based on the use of metal oxide nanoparticles. Microchim. Acta. 2014;181:1–22. doi: 10.1007/s00604-013-1069-5. [DOI] [Google Scholar]
- 13.Baghayeri M, Zare EN, Lakouraj MM. A simple hydrogen peroxide biosensor based on a novel electro-magnetic poly(p-phenylenediamine)@Fe3O4 nanocomposite. Biosens. Bioelectron. 2014;55:259–265. doi: 10.1016/j.bios.2013.12.033. [DOI] [PubMed] [Google Scholar]
- 14.Jiang S, Eltoukhy AA, Love KT, Langer R, Anderson DG. Lipoid-coated iron oxide nanoparticles for efficient DNA and siRNA delivery. Nano Lett. 2012;13:1059–1064. doi: 10.1021/nl304287a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nidhin M, Indumathy R, Sreeram KJ, Nair BU. Synthesis of iron oxide nanoparticles of narrow size distribution on polysaccharide templates. Bull. Mater. Sci. 2008;31:93–96. doi: 10.1007/s12034-008-0016-2. [DOI] [Google Scholar]
- 16.Zhu N, et al. Surface modification of magnetic iron oxide nanoparticles. Nanomaterials. 2018;8:810. doi: 10.3390/nano8100810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahdavi M, et al. Synthesis, surface modification and characterisation of biocompatible magnetic iron oxide nanoparticles for biomedical applications. Molecules. 2013;18:7533–7548. doi: 10.3390/molecules18077533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma G, Jeevanandam P. Synthesis of self-assembled prismatic iron oxide nanoparticles by a novel thermal decomposition route. RSC Adv. 2013;3:189–200. doi: 10.1039/C2RA22004K. [DOI] [Google Scholar]
- 19.Petcharoen K, Sirivat A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B-Adv. 2012;177:421–427. doi: 10.1016/j.mseb.2012.01.003. [DOI] [Google Scholar]
- 20.Li H, et al. HEPES-involved hydrothermal synthesis of Fe3O4 nanoparticles and their biological application. RSC Adv. 2015;5:5059–5067. doi: 10.1039/C4RA12536C. [DOI] [Google Scholar]
- 21.Sun J, et al. Synthesis and characterization of biocompatible Fe3O4 nanoparticles. J Biomed Mater Res A. 2007;80:333–341. doi: 10.1002/jbm.a.30909. [DOI] [PubMed] [Google Scholar]
- 22.Mahdavi M, Ahmad M, Haron MJ, Rahman MZ, Fatehi A. Optimized conditions for graft copolymerization of poly(acrylamide) onto rubber wood fiber. BioResources. 2011;6:5110–5120. [Google Scholar]
- 23.Hua CC, et al. Size controlled synthesis and characterization of Fe3O4 nanoparticles by chemical coprecipitation method. Sains. Malaysiana. 2008;37:389–394. [Google Scholar]
- 24.Xu C, et al. Dopamine as A Robust Anchor to Immobilize Functional Molecules on the Iron Oxide Shell of Magnetic Nanoparticles. J. Am. Chem. Soc. 2004;126:9938–9939. doi: 10.1021/ja0464802. [DOI] [PubMed] [Google Scholar]
- 25.Gu H, Yang Z, Gao J, Chang CK, Xu B. Heterodimers of Nanoparticles: Formation at a Liquid-Liquid Interface and Particle-Specific Surface Modification by Functional Molecules. J. Am. Chem. Soc. 2005;127:34–35. doi: 10.1021/ja045220h. [DOI] [PubMed] [Google Scholar]
- 26.Xu ZC, Hou YL, Sun SH. Magnetic core/shell Fe3O4/Au and Fe3O4/Au/Ag nanoparticles with tunable plasmonic properties. J. Am. Chem. Soc. 2007;129:8698–8699. doi: 10.1021/ja073057v. [DOI] [PubMed] [Google Scholar]
- 27.Li J, et al. Hyaluronic acid-modified Fe3O4@Au core/shell nanostars for multimodal imaging and photothermal therapy of tumors. Biomaterials. 2015;38:10–21. doi: 10.1016/j.biomaterials.2014.10.065. [DOI] [PubMed] [Google Scholar]
- 28.Jain PK, Xiao Y, Walsworth R, Cohen AE. Surface Plasmon Resonance Enhanced Magneto-Optics (SuPREMO): Faraday Rotation Enhancement in Gold-Coated Iron Oxide Nanocrystals. Nano Lett. 2009;9:1644–1650. doi: 10.1021/nl900007k. [DOI] [PubMed] [Google Scholar]
- 29.Mock JJ, Smith DR, Schultz S. Local Refractive Index Dependence of Plasmon Resonance Spectra from Individual Nanoparticles. Nano Lett. 2003;3:485–491. doi: 10.1021/nl0340475. [DOI] [Google Scholar]
- 30.Chen KL, et al. A sensitive platform for in vitro immunoassay based on biofunctionalized magnetic nanoparticles and magneto-optical Faraday effect. Sens. actuators. B Chem. 2018;258:947–951. doi: 10.1016/j.snb.2017.11.144. [DOI] [Google Scholar]
- 31.Zhang X, Niu Y, Meng X, Li Y, Zhao J. Structural evolution and characteristics of the phase transformations between α-Fe2O3, Fe3O4 and γ-Fe2O3 nanoparticles under reducing and oxidizing atmospheres. Cryst. Eng. Comm. 2013;15:8166–8172. doi: 10.1039/c3ce41269e. [DOI] [Google Scholar]
- 32.Galarreta BC, Norton PR, Labarthet FL. SERS Detection of Streptavidin/Biotin Monolayer Assemblies. Langmuir. 2011;27:1494–1498. doi: 10.1021/la1047497. [DOI] [PubMed] [Google Scholar]
- 33.Lyon JL, Fleming DA, Stone MB, Schiffer P, Williams ME. Synthesis of Fe Oxide Core/Au Shell Nanoparticles by Iterative Hydroxylamine Seeding. Nano Lett. 2004;4:719–723. doi: 10.1021/nl035253f. [DOI] [Google Scholar]
- 34.Liu HL, Sonn CH, Wu JH, Lee KM, Kim YK. Synthesis of streptavidin-FITC-conjugated core–shell Fe3O4-Au nanocrystals and their application for the purification of CD4+ lymphocytes. Biomaterials. 2008;29:4003–4011. doi: 10.1016/j.biomaterials.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 35.Jain A, Kumar J, Zhou F, Li L, Tripathy S. A simple experiment for determining Verdet constants using alternating current magnetic fields. Am. J. Phys. 1999;67:714–717. doi: 10.1119/1.19358. [DOI] [Google Scholar]
- 36.Valev VK, Wouters J, Verbiest T. Precise measurements of Faraday rotation using ac magnetic fields. Am. J. Phys. 2008;76:626–629. doi: 10.1119/1.2894529. [DOI] [Google Scholar]