Abstract
African Americans have double the prevalence of Alzheimer’s disease (AD), as compared to European Americans. However, the underlying causes of this health disparity are due to a multitude of environmental, lifestyle, and genetic factors that are not yet fully understood. Here, we review the effects of the two largest genetic risk factors for AD in African Americans: Apolipoprotein E (APOE) and ABCA7. We will describe the direct effects of genetic variation on neural correlates of cognitive function and report the indirect modulating effects of genetic variation on modifiable AD risk factors, such as aerobic fitness. As a means of integrating previous findings, we present a novel schematic diagram to illustrate the many factors that contribute to AD risk and impaired cognitive function in older African Americans. Finally, we discuss areas that require further inquiry, and stress the importance of racially diverse and representative study populations.
Keywords: African American (AA), APOE ε4, ABCA7, aerobic fitness, cognitive function, cognitive decline, Alzheimer’s disease
Introduction
African Americans are at an elevated risk of cognitive decline and memory loss, with double the prevalence of Alzheimer’s disease (AD) as compared to European Americans (Logue et al., 2011; Barnes and Bennett, 2014; Alzheimer’s Association, 2019). The underlying causes of this health disparity are not sufficiently understood. Apolipoprotein E (APOE) and ABCA7, two genes involved in lipid metabolism, are the strongest heritable contributors to AD in African Americans (Reitz et al., 2013). However, the influence of genetic risk on environmental and behavioral risk factors, and their combined effects on AD biomarkers in African Americans, is yet to be determined. Furthermore, little is known about the neural substrates of cognition in older African Americans and how they relate to genetic risk factors for AD.
Here, we review recent work outlining two distinct ways genetic risk impacts AD biomarkers in African Americans. First, we examine the direct effects of genetic variation on neural correlates of cognitive function, such as activation and functional connectivity from functional magnetic resonance imaging (fMRI) studies. Second, we discuss the indirect effects of genetics on brain structure and function, via interaction with modifiable risk factors for AD, specifically aerobic fitness.
African Americans are at an increased risk of cardiovascular disease (Obisesan et al., 2012), which has been established as an important predictor for AD (Izquierdo-Porrera and Waldstein, 2002). Management or improvement of cardiovascular risk factors through increased aerobic fitness and exercise can reduce the risk for cognitive decline and dementia (Baumgart et al., 2015). Consistent with this, low levels of physical activity is one of the most prevalent risk factors for AD (Norton et al., 2014; Cass, 2017). In particular, African Americans have lower rates of physical activity as compared to European Americans (Gothe and Kendall, 2016; Benjamin et al., 2019). As such, aerobic fitness and exercise may be more viable modifiable factors to attenuate the risk for AD in African Americans.
It is important to delineate the difference between AD as determined by neuroimaging, biofluid biomarkers, or autopsy, as compared to the clinical diagnosis of Alzheimer’s and related dementias. However, to remain aligned with the terminology of the original cited works, throughout this review we refer to both instances as AD.
Direct Effects of Genetics
APOE
The APOE ε4 allele is one of the strongest genetic risk factors for AD (Potter and Wisniewski, 2012). APOE functions to regulate lipid metabolism in the brain by mediating the uptake of lipoproteins; in particular, it modulates the clearance of amyloid-β (Aβ; Di Paolo and Kim, 2011). Both dysfunctional cholesterol processing and Aβ aggregation have been implicated in AD pathogenesis (Schultz et al., 2017). In European Americans, the APOE ε4 allele has been associated with 2–3 times the risk of AD in heterozygotes and 12 times the risk in homozygotes (Michaelson, 2014). African Americans have a higher frequency of the APOE ε4 allele (Logue et al., 2011; Barnes and Bennett, 2014), and ε4 homozygosity is highly associated with AD in African-ancestry groups (Hendrie et al., 2014). However, the results are inconsistent for heterozygotic carriers (Farrer et al., 1997), with some studies suggesting that APOE ε4 may have less predictive impact on AD outcomes in African-ancestry populations, including African Americans (Rajabli et al., 2018). Despite the mixed nature of these findings, APOE ε4 has been associated with increased risk of late onset AD (LOAD) in African Americans (OR = 2.31; 95% increased risk; Reitz et al., 2013).
APOE ε4 has also been linked to episodic memory-related dysfunction in the medial temporal lobe (MTL; Bookheimer et al., 2000; Filippini et al., 2009; Dennis et al., 2010; Michaelson, 2014), one of the earliest brain regions impacted by the progression of AD. APOE ε4 genotype and amyloid-induced synaptic pathology have been related to accelerated rates of AD pathology within the MTL (Potter and Wisniewski, 2012), particularly in hippocampal sub-regions in both rodent and human models (Palmer and Good, 2011).
Pattern separation—the ability to independently represent and store similar experiences by reducing mnemonic interference (Leal and Yassa, 2018)—relies on MTL function. As such, one way to characterize decline into mild cognitive impairment (MCI) and AD is by a shift away from pattern separation towards pattern completion, which is mediated by dysfunctional hippocampal hyperactivity (Yassa et al., 2011b). Impaired mnemonic discrimination is associated with atypical hyperactivation in the dentate gyrus (DG) and CA3 hippocampal subfields (Dickerson et al., 2005; Yassa et al., 2011a,b; Reagh et al., 2017) in healthy older adults (Toner et al., 2009; Stark et al., 2013) and those with MCI (Yassa et al., 2010; Bakker et al., 2012, 2015; Tran et al., 2017).
Research examining the impact of APOE ε4 genotype on MTL function, via performance on a mnemonic discrimination task, has yielded mixed results in different racial populations with varying degrees of cognitive impairment. A study in MCI patients reported no differences in hippocampal hyperactivation or mnemonic discrimination based on APOE ε4 status (Tran et al., 2017). Conversely, AD patients that were homozygotic carriers of the APOE ε4 allele performed worse on challenging mnemonic discriminations (Wesnes et al., 2014). When examining spatial mnemonic discrimination across cognitively impaired and unimpaired older adults, impaired ε4 carriers performed worse than unimpaired carries and either group of non-carriers (Sheppard et al., 2016).
These previous studies were primarily conducted in European American cohorts and/or did not report the specific racial breakdown of their subject pools. In a population of cognitively healthy older African Americans, there were APOE ε4-related impairments in mnemonic discrimination, coincident with hyperactivity in the left DG/CA3 and the CA1. Although the overall effect of APOE ε4 on AD outcomes in African Americans remains unclear (Farrer et al., 1997; Tang et al., 2001; Hendrie et al., 2014; Rajabli et al., 2018), this result may suggest that APOE ε4-related hippocampal dysfunction can manifest in healthy older African Americans and may be an indicator of future disease status.
While APOE ε4 is associated with a moderately increased risk for progression from MCI to AD-type dementia (Elias-Sonnenschein et al., 2011), it may not alter the disease progression during the preclinical period (Bondi et al., 1999; Bunce et al., 2004). However, the effect of APOE ε4 in the preclinical phase may be contingent on other factors such as the level of amyloid aggregation (Mormino et al., 2014) and homozygotic vs. heterozygotic status (Caselli et al., 1999). Clinically normal carriers of APOE ε4 with high levels of amyloid aggregation experienced the highest levels of cognitive decline as compared to ε4 non-carriers and those with lower Aβ aggregation (Mormino et al., 2014). Cognitively healthy APOE ε4 homozygotic carriers also experienced memory decline earlier than heterozygotic carriers (Caselli et al., 1999).
ABCA7
Outside of APOE, ABCA7 is the strongest genetic risk factor for AD in African Americans (Reitz et al., 2013). As a member of the super-family of adenosine triphosphate (ATP)-binding cassette (ABC) transporters, ABCA7 is another gene that regulates the homeostasis of phospholipids and cholesterol in the central nervous system and peripheral tissues. ABCA7 gene expression has been linked to AD via the dysregulation of lipid metabolism (Zhao et al., 2015; Aikawa et al., 2018).
ABCA7 single nucleotide polymorphism (SNP) rs115550680 is associated with the development of LOAD in African Americans with an effect size (OR = 1.79; 70%–80% increase in risk) that is comparable to that of APOE ε4 (Reitz et al., 2013). ABCA7 rs115550680 is hypothesized to contribute to AD in African Americans through amyloid precursor protein (APP) processing and the suppression of Aβ clearance (Cukier et al., 2016).
In cognitively healthy elderly subjects and MCI patients, cortical Aβ load is associated with disrupted functional connectivity within the MTL and impaired memory performance (Song et al., 2015). As such, Aβ plaques may play a key role in facilitating tauopathy in the MTL, and therefore lead to disrupted functional connectivity in the MTL circuitry. Hardy and Selkoe (2002) suggest that one of the functions of ABCA7 in AD may be Aβ facilitated tauopathy: as Aβ deposition accumulates in cortical regions within the default mode network (DMN), it may lead to concurrent accumulation of tau tangles in the MTL via reciprocal connections through the entorhinal cortex (EC) (Pooler et al., 2015). Hence, the cortico-MTL circuit may be the neural network underlying ABCA7 rs115550680-related AD pathology.
A recently published study examining the impact of ABCA7 rs115550680 genotype on the cortico-MTL network function in a group of cognitively healthy older African Americans found ABCA7-related dissociation in EC resting state functional connectivity (Sinha et al., 2019). Specifically, the risk variant was associated with increased functional connectivity between the EC and other MTL regions, including hippocampal subfields, coincident with decreased connectivity between the EC and medial prefrontal cortex (mPFC; Sinha et al., 2019). These findings suggest that for individuals with the risk ABCA7 rs115550680 genotype, impaired cortical connectivity leads to a more functionally isolated EC at rest, which translates into aberrant EC-MTL hyper-synchronization (Sinha et al., 2019).
While direct claims cannot be made about the exact mechanism underlying the aforementioned alterations in cortico-MTL network function, when considering the relevance of Aβ in ABCA7-related AD pathogenesis, these results may reflect the combined reinforcement between amyloid and tau pathology in the EC (Sinha et al., 2019). Thus, anomalous MTL functional connectivity may be an additional neural correlate of future cognitive decline in African Americans. This ABCA7 variant is monomorphic in European Americans (Reitz et al., 2013; Machiela and Chanock, 2015), and consequently, it does not confer any increased risk for AD in this group. However, recent studies of functional connectivity in MCI and AD patients have reported a similar disconnection of the MTL from other nodes of the DMN, particularly mPFC, but increased connectivity locally within the MTL, between EC and other subregions of the MTL (Das et al., 2013; Pasquini et al., 2015). As such, MTL network dysfunction may be a ubiquitously applicable AD biomarker for preclinical AD detection.
Indirect Effects of Genetics
The Interaction With Aerobic Fitness
Modifiable lifestyle factors, such as diet, exercise, and aerobic fitness, contribute to AD risk. In particular, aerobic fitness is one cardiovascular disease management method that has been associated with decreased levels of cognitive decline and reduced risk of AD in several previous studies (Colcombe and Kramer, 2003; Kramer et al., 2005, 2006). Aerobic activity has been found to aid in brain lipid homeostasis and in the reduction of Aβ deposit accumulation (Maesako et al., 2012; He et al., 2017; Houdebine et al., 2017). Recent work has also argued that increased levels of aerobic fitness can attenuate the adverse influence of AD-related polygenic vulnerability derived from genes implicated in lipid homeostasis, including APOE and ABCA7 (Schultz et al., 2017).
In addition to ABCA7 rs115550680 (reviewed under Direct Effects of Genetics), which has been identified as a genetic risk factor for AD in African Americans, another ABCA7 SNP (rs3764650) has been identified as a susceptibility locus for AD in European Americans (Hollingworth et al., 2011; Naj et al., 2011). ABCA7 rs3764650 has a lower effect size in African Americans (OR = 1.23), increasing AD risk by about 10%–20% (Reitz et al., 2013). However, this SNP has been found to influence overall ABCA7 expression (the conversion of DNA instructions into functional products and proteins), and, dysfunctional ABCA7 expression levels are associated with AD risk (Vasquez et al., 2013; Aikawa et al., 2018).
While the overall effects of ABCA7 rs3764650 on cognition seem to be minimal (Vivot et al., 2015; Andrews et al., 2016, 2017), it has been found to alter cognition in subgroups stratified on factors such as gender and disease progression. In healthy elderly, an association between rs3764650 and cognitive decline was found selectively in females (Nettiksimmons et al., 2016), and, in individuals with a final diagnosis of MCI or AD, this SNP was associated with increased rates of memory decline (Karch et al., 2012; Carrasquillo et al., 2015).
A study of healthy older African Americans found that ABCA7 rs3764650 modulates the association between aerobic fitness level (as measured by maximal oxygen consumption, VO2 max) and mnemonic flexibility—the ability to flexibly apply and recombine information from past learning—as measured by generalization following rule learning (Berg et al., 2019). In particular, for carriers of the non-risk genotype, higher levels of aerobic fitness were significantly associated with fewer generalization errors. Conversely, carriers of the risk genotype did not show any relationship between aerobic fitness and generalization. Successful mnemonic flexibility is known to depend on the integrity of the MTL (Myers et al., 2002, 2008), a major site of neuroplasticity that is sensitive to the effects of exercise and aerobic fitness (Cotman et al., 2007). The results of Berg et al. (2019) therefore imply that the ABCA7 risk genotype may attenuate the neuro-protective value of aerobic fitness in cognitively healthy older African Americans.
Analogous to this study, others have found that in European Americans, APOE ε4+ individuals did not receive the same benefits as APOE ε4− individuals from higher levels of aerobic fitness or following an exercise intervention, with fitness only reducing the risk for dementia in non-carriers (Podewils et al., 2005; Lautenschlager et al., 2008). On the contrary, some self-reported studies of physical activity found that the neuro-protective effects of fitness were exclusive to APOE ε4 carriers (Schuit et al., 2001; Rovio et al., 2005; Smith et al., 2011). Additionally, in African Americans the APOE ε4 genotype has been found to influence exercise-related upregulation of BDNF (brain-derived neurotrophic factor), a gene associated with neuroplasticity and hippocampal volume (Erickson et al., 2011); non-carriers of the ε4 allele exclusively experienced a significant increase in BDNF levels after 6 months of exercise, while carriers did not (Allard et al., 2017).
Research on the interactive effects of aerobic fitness and genetic risk for AD is still in the early stages, with the various studies containing methodological and racial differences in subject populations. Albeit equivocal, these results do provide evidence of the modulating effect of genetic variation on modifiable AD risk factors.
Discussion
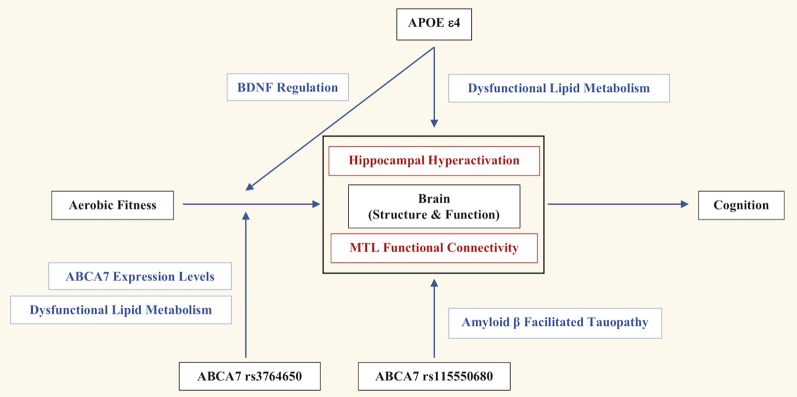
Here, we reviewed research outlining the influence of genetic risk on MTL neural and cognitive function. We present a novel comprehensive outline of how genotypic variation may contribute to AD and impaired cognitive function (Figure 1). Overall, aerobic fitness influences neural structure and function, which then affects cognition. APOE ε4 directly impacts the brain via dysfunctional lipid metabolism, leading to aberrant hippocampal hyperactivation, and therefore, impaired mnemonic discrimination of episodic memories (Sinha et al., 2018). The indirect effects of APOE ε4 via fitness remain somewhat ambiguous, with some studies reporting aerobic fitness-related benefits only in APOE ε4− individuals (Podewils et al., 2005; Lautenschlager et al., 2008; Allard et al., 2017), while other studies report those benefits only in APOE ε4+ individuals (Schuit et al., 2001; Rovio et al., 2005; Smith et al., 2011). However, exercise-induced upregulation of BDNF, and its influence on hippocampal plasticity, may serve as a possible mechanism for the indirect influence of APOE ε4 (Allard et al., 2017).
Figure 1.
The genetic and lifestyle factors that contribute to Alzheimer’s disease (AD) risk and impaired cognitive function in African Americans. Overall, aerobic fitness influences brain structure and function, which then affects cognition. Apolipoprotein E (APOE) ε4 directly impacts brain structure and function via dysfunctional lipid metabolism, leading to aberrant hippocampal hyperactivation and therefore, impaired mnemonic discrimination of episodic memories. APOE ε4 indirectly influences the effects of aerobic exercise on hippocampal plasticity and volume through the regulation of BDNF. ABCA7 rs115550680 directly impacts the brain through amyloid-β (Aβ) facilitated tauopathy, which negatively influences medial temporal lobe (MTL) functional connectivity, and consequently, behavioral generalization. ABCA7 rs3764650 moderates the effects of aerobic fitness through dysfunctional lipid metabolism and ABCA7 expression, which indirectly impairs behavioral generalization.
Meanwhile, ABCA7 rs115550680 directly impacts the brain through Aβ facilitated tauopathy, which negatively influences MTL functional connectivity, and consequently, behavioral generalization (Sinha et al., 2019). Although ABCA7 rs3764650 is not a causative variant for AD in African Americans, and does not directly impact brain structure and function, it appears to confer indirect consequences on cognition and AD risk by moderating the effects of aerobic fitness through dysfunctional lipid metabolism and ABCA7 expression (Berg et al., 2019).
While the current schematic (Figure 1) of genetic influences on AD risk in African Americans is a first step, additional studies are needed to verify the molecular mechanisms underlying the link between genetic risk and pathogenic pathways; the potential contribution of brain lipid homeostasis in the MTL should be a focal point. It is also important to determine if ABCA7 and APOE have any common pathways mediating the effect on MTL structure and function. Furthermore, comprehensive single-cell type transcriptome analyses in human and mouse brains may be necessary to determine cell-specific contributions of ABCA7 risk variants to AD pathogenesis. For instance, ABCA7 rs115550680-related dysregulation of lipid metabolism may specifically target the neurons accelerating APP processing and Aβ production, while, ABCA7 rs3764650 may impact Aβ clearance by the microglia, known to play a pivotal role in mediating exercise-dependent enhancement of hippocampal neurogenesis (Vukovic et al., 2012).
Several studies have shown qualitative and quantitative differences in AD between African Americans and European Americans. One such study found racial differences in cerebrospinal fluid (CSF) and structural MRI biomarkers of AD in an elderly cohort; despite comparable CSF Aβ42 levels, white matter hyperintensity (WMH) volume, and hippocampal volume, the same degree of WMH had a greater influence on cognition in African Americans as compared to European Americans (Howell et al., 2017). Since WMH is a marker of vascular dysfunction, which African Americans experience at a higher rate than European Americans (Obisesan et al., 2012), these results may indicate that genes such as APOE and ABCA7, which regulate lipid metabolism, differentially affect African Americans. For example, the direct and indirect effects of ABCA7 have not been validated in other racial groups. ABCA7 rs115550680 is monomorphic on the non-risk minor “A” allele in European Americans (Reitz et al., 2013; Machiela and Chanock, 2015). As such, ABCA7 rs115550680 may confer AD risk selectively in African Americans, and, in conjunction with the indirect effects of ABCA7 rs3764650, may contribute to the higher incidence rate of dementia and AD in this population.
It is imperative that the studies presented here be replicated across diverse subject populations for a more representative and comprehensive understanding of AD progression and outcomes. At the same time, it will be crucial for future studies to examine race-specific AD biomarkers and consequences. Finally, researchers should explore the interplay between genetic variation and other modifiable lifestyle factors, such as diet and sleep patterns, to understand whether the benefits of potential interventions are similar for those with and without a genetic risk for dementia and AD.
Author Contributions
CB and NS conducted background research. CB drafted the manuscript. NS and MG provided critical review of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. Grants to MG from the NIH’s National Institute on Aging (1R01AG053961) and the New Jersey Department of Health’s Office of Minority and Multicultural Health (MH-STT-15-001).
References
- Aikawa T., Holm M.-L., Kanekiyo T. (2018). ABCA7 and pathogenic pathways of Alzheimer’s disease. Brain Sci. 8:27. 10.3390/brainsci8020027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard J. S., Ntekim O., Johnson S. P., Ngwa J. S., Bond V., Pinder D., et al. (2017). APOEε4 impacts up-regulation of brain-derived neurotrophic factor after a six-month stretch and aerobic exercise intervention in mild cognitively impaired elderly African Americans: a pilot study. Exp. Gerontol. 87, 129–136. 10.1016/j.exger.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association (2019). 2019 Alzheimer’s disease facts and figures. Alzheimers Dement. 15, 321–387. 10.1016/j.jalz.2019.01.010 [DOI] [Google Scholar]
- Andrews S. J., Das D., Anstey K. J., Easteal S. (2017). Late onset alzheimer’s disease risk variants in cognitive decline: the PATH through life study. J. Alzheimers Dis. 57, 423–436. 10.3233/JAD-160774 [DOI] [PubMed] [Google Scholar]
- Andrews S. J., Das D., Cherbuin N., Anstey K. J., Easteal S. (2016). Association of genetic risk factors with cognitive decline: the PATH through life project. Neurobiol. Aging 41, 150–158. 10.1016/j.neurobiolaging.2016.02.016 [DOI] [PubMed] [Google Scholar]
- Bakker A., Albert M. S., Krauss G., Speck C. L., Gallagher M. (2015). Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by fMRI and memory task performance. NeuroImage Clin. 7, 688–698. 10.1016/j.nicl.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A., Krauss G. L., Albert M. S., Speck C. L., Jones L. R., Stark C. E., et al. (2012). Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron 74, 467–474. 10.1016/j.neuron.2012.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes L. L., Bennett D. A. (2014). Alzheimer’s disease in African Americans: risk factors and challenges for the future. Health Aff. 33, 580–586. 10.1377/hlthaff.2013.1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart M., Snyder H. M., Carrillo M. C., Fazio S., Kim H., Johns H. (2015). Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. 11, 718–726. 10.1016/j.jalz.2015.05.016 [DOI] [PubMed] [Google Scholar]
- Benjamin E. J., Muntner P., Bittencourt M. S. (2019). Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 139, e56–e528. 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- Berg C. N., Sinha N., Gluck M. A. (2019). ABCA7 risk genotype diminishes the neuroprotective value of aerobic fitness in healthy older African Americans. Front. Aging Neurosci. 11:73. 10.3389/fnagi.2019.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi M. W., Salmon D. P., Galasko D., Thomas R. G., Thal L. J. (1999). Neuropsychological function and apolipoprotein E genotype in the preclinical detection of Alzheimer’s disease. Psychol. Aging 14, 295–303. 10.1037//0882-7974.14.2.295 [DOI] [PubMed] [Google Scholar]
- Bookheimer S. Y., Strojwas M. H., Cohen M. S., Saunders A. M., Pericak-Vance M. A., Mazziotta J. C., et al. (2000). Patterns of brain activation in people at risk for Alzheimer’s disease. N. Engl. J. Med. 343, 450–456. 10.1056/NEJM200008173430701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunce D., Fratiglioni L., Small B. J., Winblad B., Bäckman L. (2004). APOE and cognitive decline in preclinical Alzheimer disease and non-demented aging. Neurology 63, 816–821. 10.1212/01.wnl.0000137041.86153.42 [DOI] [PubMed] [Google Scholar]
- Carrasquillo M. M., Crook J. E., Pedraza O., Thomas C. S., Pankratz V. S., Allen M., et al. (2015). Late-onset Alzheimer’s risk variants in memory decline, incident mild cognitive impairment and Alzheimer’s disease. Neurobiology of Aging 36, 60–67. 10.1016/j.neurobiolaging.2014.07.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli R. J., Graff-Radford N. R., Reiman E. M., Weaver A., Osborne D., Lucas J., et al. (1999). Preclinical memory decline in cognitively normal apolipoprotein E-ε4 homozygotes. Neurology 53, 201–207. 10.1212/wnl.53.1.201 [DOI] [PubMed] [Google Scholar]
- Cass S. P. (2017). Alzheimer’s disease and exercise: a literature review. Curr. Sports Med. Rep. 16, 19–22. 10.1249/JSR.0000000000000332 [DOI] [PubMed] [Google Scholar]
- Colcombe S. J., Kramer A. F. (2003). Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol. Sci. 14, 125–130. 10.1111/1467-9280.t01-1-01430 [DOI] [PubMed] [Google Scholar]
- Cotman C. W., Berchtold N. C., Christie L.-A. (2007). Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 30, 464–472. 10.1016/j.tins.2007.06.011 [DOI] [PubMed] [Google Scholar]
- Cukier H. N., Kunkle B. W., Vardarajan B. N., Rolati S., Hamilton-Nelson K. L., Kohli M. A., et al. (2016). ABCA7 frameshift deletion associated with Alzheimer disease in African Americans. Neurol. Genet. 2:e79. 10.1212/NXG.0000000000000079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S. R., Pluta J., Mancuso L., Kliot D., Orozco S., Dickerson B. C., et al. (2013). Increased functional connectivity within medial temporal lobe in mild cognitive impairment. Hippocampus 23, 1–6. 10.1002/hipo.22051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis N. A., Browndyke J. N., Stokes J., Need A., Burke J. R., Welsh-Bohmer K. A., et al. (2010). Temporal lobe functional activity and connectivity in young adult APOE ε4 carriers. Alzheimers Dement. 6, 303–311. 10.1016/j.jalz.2009.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson B. C., Salat D. H., Greve D. N., Chua E. F., Rand-Giovannetti E., Rentz D. M., et al. (2005). Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology 65, 404–411. 10.1212/01.wnl.0000171450.97464.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G., Kim T.-W. (2011). Linking lipids to Alzheimer’s disease: cholesterol and beyond. Nat. Rev. Neurosci. 12:484 10.1038/nrn3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias-Sonnenschein L. S., Viechtbauer W., Ramakers I. H. G. B., Verhey F. R. J., Visser P. J. (2011). Predictive value of APOE-ε4 allele for progression from MCI to AD-type dementia: a meta-analysis. J. Neurol. Neurosurg. Psychiatry 82, 1149–1156. 10.1136/jnnp.2010.231555 [DOI] [PubMed] [Google Scholar]
- Erickson K. I., Voss M. W., Prakash R. S., Basak C., Szabo A., Chaddock L., et al. (2011). Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. U S A 108, 3017–3022. 10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer L. A., Cupples L. A., Haines J. L., Hyman B., Kukull W. A., Mayeux R., et al. (1997). Effects of age, sex and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA 278, 1349–1356. 10.1001/jama.1997.03550160069041 [DOI] [PubMed] [Google Scholar]
- Filippini N., MacIntosh B. J., Hough M. G., Goodwin G. M., Frisoni G. B., Smith S. M., et al. (2009). Distinct patterns of brain activity in young carriers of the APOE-ε4 allele. Proc. Natl. Acad. Sci. U S A 106, 7209–7214. 10.1073/pnas.0811879106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothe N. P., Kendall B. J. (2016). Barriers, motivations and preferences for physical activity among female african american older adults. Gerontol. Geriatr. Med. 2:233372141667739. 10.1177/2333721416677399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J., Selkoe D. J. (2002). The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297, 353–356. 10.1126/science.1072994 [DOI] [PubMed] [Google Scholar]
- He X., Liu D., Zhang Q., Liang F., Dai G., Zeng J., et al. (2017). Voluntary exercise promotes glymphatic clearance of amyloid beta and reduces the activation of astrocytes and microglia in aged mice. Front. Mol. Neurosci. 10:144. 10.3389/fnmol.2017.00144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrie H. C., Murrell J., Baiyewu O., Lane K. A., Purnell C., Ogunniyi A., et al. (2014). APOE ε4 and the risk for Alzheimer disease and cognitive decline in African Americans and Yoruba. Int. Psychogeriatr. 26, 977–985. 10.1017/S1041610214000167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth P., Harold D., Sims R., Gerrish A., Lambert J.-C., Carrasquillo M. M., et al. (2011). Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat. Genet. 43:429. 10.1038/ng.803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houdebine L., Gallelli C. A., Rastelli M., Sampathkumar N. K., Grenier J. (2017). Effect of physical exercise on brain and lipid metabolism in mouse models of multiple sclerosis. Chem. Phys. Lipids 207, 127–134. 10.1016/j.chemphyslip.2017.06.002 [DOI] [PubMed] [Google Scholar]
- Howell J. C., Watts K. D., Parker M. W., Wu J., Kollhoff A., Wingo T. S., et al. (2017). Race modifies the relationship between cognition and Alzheimer’s disease cerebrospinal fluid biomarkers. Alzheimers Res. Ther. 9:88. 10.1186/s13195-017-0315-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo-Porrera A. M., Waldstein S. R. (2002). Cardiovascular risk factors and cognitive function in African Americans. J. Gerontol. B Psychol. Sci. Soc. Sci. 57, P377–P380. 10.1093/geronb/57.4.p377 [DOI] [PubMed] [Google Scholar]
- Karch C. M., Jeng A. T., Nowotny P., Cady J., Cruchaga C., Goate A. M. (2012). Expression of novel Alzheimer’s disease risk genes in control and Alzheimer’s disease brains. PLOS One 7:e50976. 10.1371/journal.pone.0050976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer A. F., Colcombe S. J., McAuley E., Scalf P. E., Erickson K. I. (2005). Fitness, aging and neurocognitive function. Neurobiol. Aging 26, 124–127. 10.1016/j.neurobiolaging.2005.09.009 [DOI] [PubMed] [Google Scholar]
- Kramer A. F., Erickson K. I., Colcombe S. J. (2006). Exercise, cognition and the aging brain. J. Appl. Physiol. 101, 1237–1242. 10.1152/japplphysiol.00500.2006 [DOI] [PubMed] [Google Scholar]
- Lautenschlager N. T., Cox K. L., Flicker L., Foster J. K., van Bockxmeer F. M., Xiao J., et al. (2008). Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA 300, 1027–1037. 10.1001/jama.300.9.1027 [DOI] [PubMed] [Google Scholar]
- Leal S. L., Yassa M. A. (2018). Integrating new findings and examining clinical applications of pattern separation. Nat. Neurosci. 21, 163–173. 10.1038/s41593-017-0065-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue M. W., Schu M., Vardarajan B. N., Buros J., Green R. C., Go R. C. P., et al. (2011). A comprehensive genetic association study of Alzheimer disease in african americans. Arch. Neurol. 68, 1569–1579. 10.1001/archneurol.2011.646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machiela M. J., Chanock S. J. (2015). LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics 31, 3555–3557. 10.1093/bioinformatics/btv402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maesako M., Uemura K., Kubota M., Kuzuya A., Sasaki K., Hayashida N., et al. (2012). Exercise is more effective than diet control in preventing high fat diet-induced β-amyloid deposition and memory deficit in amyloid precursor protein transgenic mice. J. Biol. Chem. 287, 23024–23033. 10.1074/jbc.M112.367011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson D. M. (2014). APOE ε4: the most prevalent yet understudied risk factor for Alzheimer’s disease. Alzheimers Dement. 10, 861–868. 10.1016/j.jalz.2014.06.015 [DOI] [PubMed] [Google Scholar]
- Mormino E. C., Betensky R. A., Hedden T., Schultz A. P., Ward A., Huijbers W., et al. (2014). Amyloid and APOE ε4 interact to influence short-term decline in preclinical Alzheimer disease. Neurology 82, 1760–1767. 10.1212/WNL.0000000000000431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers C. E., Kluger A., Golomb J., Ferris S., de Leon M. J., Schnirman G., et al. (2002). Hippocampal atrophy disrupts transfer generalization in non-demented elderly. J. Geriatr. Psychiatry Neurol. 15, 82–90. 10.1177/089198870201500206 [DOI] [PubMed] [Google Scholar]
- Myers C. E., Kluger A., Golomb J., Gluck M. A., Ferris S. (2008). Learning and generalization tasks predict short-term cognitive outcome in nondemented elderly. J. Geriatr. Psychiatry Neurol. 21, 93–103. 10.1177/0891988708316858 [DOI] [PubMed] [Google Scholar]
- Naj A. C., Jun G., Beecham G. W., Wang L.-S., Vardarajan B. N., Buros J., et al. (2011). Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat. Genet. 43, 436–441. 10.1038/ng.801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettiksimmons J., Tranah G., Evans D. S., Yokoyama J. S., Yaffe K. (2016). Gene-based aggregate SNP associations between candidate AD genes and cognitive decline. Age 38:41. 10.1007/s11357-016-9885-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton S., Matthews F. E., Barnes D. E., Yaffe K., Brayne C. (2014). Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 13, 788–794. 10.1016/S1474-4422(14)70136-X [DOI] [PubMed] [Google Scholar]
- Obisesan T. O., Gillum R. F., Johnson S., Umar N., Williams D., Bond V., et al. (2012). Neuroprotection and neurodegeneration in Alzheimer’s disease: role of cardiovascular disease risk factors, implications for dementia rates and prevention with aerobic exercise in african americans. Int. J. Alzheimers Dis. 2012:568382. 10.1155/2012/568382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer A., Good M. (2011). Hippocampal synaptic activity, pattern separation and episodic-like memory: implications for mouse models of Alzheimer’s disease pathology. Biochem. Soc. Trans. 39, 902–909. 10.1042/bst0390902 [DOI] [PubMed] [Google Scholar]
- Pasquini L., Scherr M., Tahmasian M., Meng C., Myers N. E., Ortner M., et al. (2015). Link between hippocampus’ raised local and eased global intrinsic connectivity in AD. Alzheimers Dement. 11, 475–484. 10.1016/j.jalz.2014.02.007 [DOI] [PubMed] [Google Scholar]
- Podewils L. J., Guallar E., Kuller L. H., Fried L. P., Lopez O. L., Carlson M., et al. (2005). Physical activity, APOE genotype and dementia risk: findings from the cardiovascular health cognition study. Am. J. Epidemiol. 161, 639–651. 10.1093/aje/kwi092 [DOI] [PubMed] [Google Scholar]
- Pooler A. M., Polydoro M., Maury E. A., Nicholls S. B., Reddy S. M., Wegmann S., et al. (2015). Amyloid accelerates tau propagation and toxicity in a model of early Alzheimer’s disease. Acta Neuropathol. Commun. 3:14. 10.1186/s40478-015-0199-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter H., Wisniewski T. (2012). Apolipoprotein E: essential catalyst of the Alzheimer amyloid cascade. Int. J. Alzheimers Dis. 2012:489428. 10.1155/2012/489428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajabli F., Feliciano B. E., Celis K., Hamilton-Nelson K. L., Whitehead P. L., Adams L. D., et al. (2018). Ancestral origin of APOE ε4 Alzheimer disease risk in Puerto Rican and African American populations. PLoS Genet. 14:e1007791. 10.1371/journal.pgen.1007791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagh Z. M., Noche J. A., Tustison N., Delisle D., Murray E. A., Yassa M. A. (2017). Anterolateral entorhinal-hippocampal imbalance in older adults disrupts object pattern separation. bioRxiv [Preprint]. 10.1101/162925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz C., Jun G., Naj A., Rajbhandary R., Vardarajan B. N., Wang L. S., et al. (2013). Variants in the atp-binding cassette transporter (abca7), Apolipoprotein Eε4 and the risk of late-onset alzheimer disease in african americans. JAMA 309, 1483–1492. 10.1001/jama.2013.2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovio S., Kåreholt I., Helkala E.-L., Viitanen M., Winblad B., Tuomilehto J., et al. (2005). Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 4, 705–711. 10.1016/S1474-4422(05)70198-8 [DOI] [PubMed] [Google Scholar]
- Schuit A. J., Feskens E. J. M., Launer L. J., Kromhout D. (2001). Physical activity and cognitive decline, the role of the Apolipoprotein e4 allele. Med. Sci. Sports Exerc. 33, 772–777. 10.1097/00005768-200105000-00015 [DOI] [PubMed] [Google Scholar]
- Schultz S. A., Boots E. A., Darst B. F., Zetterberg H., Blennow K., Edwards D. F., et al. (2017). Cardiorespiratory fitness alters the influence of a polygenic risk score on biomarkers of AD. Neurology 88, 1650–1658. 10.1212/wnl.0000000000003862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard D. P., Graves L. V., Holden H. M., Delano-Wood L., Bondi M. W., Gilbert P. E. (2016). Spatial pattern separation differences in older adult carriers and non-carriers for the apolipoprotein E epsilon 4 allele. Neurobiol. Learn. Mem. 129, 113–119. 10.1016/j.nlm.2015.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N., Berg C. N., Tustison N. J., Shaw A., Hill D., Yassa M. A., et al. (2018). APOE ε4 status in healthy older African Americans is associated with deficits in pattern separation and hippocampal hyperactivation. Neurobiol. Aging 69, 221–229. 10.1016/j.neurobiolaging.2018.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N., Reagh Z. M., Tustison N. J., Berg C. N., Shaw A., Myers C. E., et al. (2019). ABCA7 risk variant in healthy older African Americans is associated with a functionally isolated entorhinal cortex mediating deficient generalization of prior discrimination training. Hippocampus 29, 527–538. 10.1002/hipo.23042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. C., Nielson K. A., Woodard J. L., Seidenberg M., Durgerian S., Antuono P., et al. (2011). Interactive effects of physical activity and APOE-ε4 on BOLD semantic memory activation in healthy elders. Neuroimage 54, 635–644. 10.1016/j.neuroimage.2010.07.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z., Insel P. S., Buckley S., Yohannes S., Mezher A., Simonson A., et al. (2015). Brain amyloid-β burden is associated with disruption of intrinsic functional connectivity within the medial temporal lobe in cognitively normal elderly. J. Neurosci. 35, 3240–3247. 10.1523/JNEUROSCI.2092-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark S. M., Yassa M. A., Lacy J. W., Stark C. E. L. (2013). A task to assess behavioral pattern separation (BPS) in humans: data from healthy aging and mild cognitive impairment. Neuropsychologia 51, 2442–2449. 10.1016/j.neuropsychologia.2012.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M.-X., Cross P., Andrews H., Jacobs D. M., Small S., Bell K., et al. (2001). Incidence of AD in African Americans, Caribbean hispanics, and caucasians in northern Manhattan. Neurology 56, 49–56. 10.1212/wnl.56.1.49 [DOI] [PubMed] [Google Scholar]
- Toner C. K., Pirogovsky E., Kirwan C. B., Gilbert P. E. (2009). Visual object pattern separation deficits in nondemented older adults. Learn. Mem. 16, 338–342. 10.1101/lm.1315109 [DOI] [PubMed] [Google Scholar]
- Tran T. T., Speck C. L., Pisupati A., Gallagher M., Bakker A. (2017). Increased hippocampal activation in ApoE-4 carriers and non-carriers with amnestic mild cognitive impairment. Neuroimage Clin. 13, 237–245. 10.1016/j.nicl.2016.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez J. B., Fardo D. W., Estus S. (2013). ABCA7 expression is associated with Alzheimer’s disease polymorphism and disease status. Neurosci. Lett. 556, 58–62. 10.1016/j.neulet.2013.09.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivot A., Glymour M. M., Tzourio C., Amouyel P., Chêne G., Dufouil C. (2015). Association of Alzheimer’s related genotypes with cognitive decline in multiple domains: results from the Three-City Dijon study. Mol. Psychiatry 20, 1173–1178. 10.1038/mp.2015.62 [DOI] [PubMed] [Google Scholar]
- Vukovic J., Colditz M. J., Blackmore D. G., Ruitenberg M. J., Bartlett P. F. (2012). Microglia modulate hippocampal neural precursor activity in response to exercise and aging. J. Neurosci. 32, 6435–6443. 10.1523/jneurosci.5925-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesnes K. A., Annas P., Basun H., Edgar C., Blennow K. (2014). Performance on a pattern separation task by Alzheimer’s patients shows possible links between disrupted dentate gyrus activity and apolipoprotein E ε4 status and cerebrospinal fluid amyloid-β42 levels. Alzheimers Res. Ther. 6:20. 10.1186/alzrt250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa M. A., Lacy J. W., Stark S. M., Albert M. S., Gallagher M., Stark C. E. L. (2011a). Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus 21, 968–979. 10.1002/hipo.20808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa M. A., Mattfeld A. T., Stark S. M., Stark C. E. L. (2011b). Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proc. Natl. Acad. Sci. U S A 108, 8873–8878. 10.1073/pnas.1101567108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa M. A., Stark S. M., Bakker A., Albert M. S., Gallagher M., Stark C. E. L. (2010). High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic mild cognitive impairment. Neuroimage 51, 1242–1252. 10.1016/j.neuroimage.2010.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q.-F., Yu J.-T., Tan M.-S., Tan L. (2015). ABCA7 in Alzheimer’s disease. Mol. Neurobiol. 51, 1008–1016. 10.1007/s12035-014-8759-9 [DOI] [PubMed] [Google Scholar]