ABSTRACT
Background
High-fat meal (HFM) consumption may induce transient postprandial atherogenic responses, including impairment of vascular endothelial function, in individuals with overweight/obesity. Red beetroot juice (RBJ) may modulate endothelial function and other measures of cardiometabolic health.
Objective
This study investigated the impact of acute and chronic RBJ consumption, including nitrate-dependent and -independent effects, on postprandial endothelial function and other cardiometabolic responses to a HFM.
Methods
Fifteen men and postmenopausal women with overweight/obesity were enrolled in this randomized, double-blind, placebo-controlled, 4-period, crossover clinical trial. Following an overnight fast, participants underwent baseline assessment of endothelial function (reactive hyperemia index; RHI) and hemodynamics, and biological sample collection. In random order, participants consumed 70 mL (acute visit) of: 1) RBJ, 2) nitrate-free RBJ (NF-RBJ), 3) placebo + nitrate (PBO + NIT), or 4) placebo (PBO), followed by a HFM. RHI was remeasured 4 h post-HFM, and hemodynamic assessment and biological sample collection were performed 1, 2, and 4 h post-HFM consumption. Participants consumed treatments daily for 4 wk (chronic visit), and assessments were repeated before/after the HFM (without consuming treatments).
Results
HFM consumption did not induce significant impairment of postprandial RHI. No significant differences in RHI were detected across treatment groups following acute or chronic exposure, despite increases in circulating nitrate/nitrite (NOx) concentrations in the RBJ and PBO + NIT groups compared with PBO and NF-RBJ (P < 0.0001 for all time points at the acute visit; P < 0.05 for all time points at the chronic visit). Although the HFM led to significant alterations in several secondary outcomes, there were no consistent treatment effects on postprandial cardiometabolic responses.
Conclusions
HFM consumption did not impair postprandial endothelial function in this population, and RBJ exposure did not alter postprandial endothelial function or other outcomes despite increasing NOx concentrations. This trial is registered at clinicaltrials.gov as NCT02949115.
Keywords: betalains, cardiovascular disease, dietary nitrate, inflammation, oxidative stress, nitric oxide, postprandial dysmetabolism, vascular function, vasodilation
Introduction
Advancing age is the primary risk factor for atherosclerotic cardiovascular disease (CVD), largely due to adverse effects on the arteries (1, 2), including vascular endothelial dysfunction, which is characterized by impaired endothelium-dependent vasodilation. Nitric oxide (NO) is critical to cardiovascular health as it promotes vasodilation, blood flow, antithrombotic, and anti-inflammatory effects. A central driver of vascular endothelial dysfunction is reduced NO bioavailability secondary to oxidative stress (3). Inflammation promotes vascular endothelial dysfunction through a bidirectional relationship with oxidative stress (2, 3). The progression of atherosclerosis is characterized by chronic oxidative stress, activation of proinflammatory pathways, and recruitment and adhesion of immune cells to the endothelium (4). Previous research suggests that consumption of a single high-fat meal (HFM) may induce transient postprandial atherogenic responses including impairment of vascular endothelial function, hypertriglyceridemia, hyperglycemia, inflammation, and oxidative stress (5–10) that are exacerbated in individuals with overweight and obesity (8). Indeed, even mildly elevated postprandial glucose and triglyceride concentrations have been linked to the development of atherosclerosis and other CVDs in the general population (5, 11). As such, repeated HFM consumption may accelerate atherogenesis in aging individuals with overweight and obesity, and dietary interventions that attenuate these responses may contribute to the preservation of cardiovascular health. Nonetheless, previous research has also demonstrated that consumption of a HFM did not induce postprandial vascular endothelial dysfunction in obese or normal weight individuals (12). These discrepancies in the evidence warrant further investigation to better understand the impact of a HFM on postprandial vascular endothelial function.
Consumption of red beetroot (Beta vulgaris L.) juice (RBJ) has emerged as a potential therapeutic approach for reducing CVD risk. Research has demonstrated antioxidant (13–15), anti-inflammatory (16, 17), antihypertensive (18–20), and cardiometabolic-protective (18, 20–23) effects of RBJ and its bioactive components in animals and humans, though results have been equivocal with respect to cardiovascular health. For instance, a recent systematic review found that although there is evidence that acute and chronic RBJ intake (and other dietary sources of inorganic nitrate) is an effective means for increasing NO bioavailability and improving cardiovascular health (e.g. blood pressure, endothelial function, arterial stiffness), others have not observed beneficial effects. Favorable effects were primarily observed in healthy populations, with research being limited and more discrepant in populations with an increased CVD risk (24). Observed cardiometabolic-protective effects of RBJ have been primarily attributed to the high inorganic nitrate content of RBJ, as inorganic nitrate is reduced via the enterosalivary nitrate-nitrite-NO pathway to NO in an endothelium-independent manner (18, 25). Hence, dietary inorganic nitrate may be an effective approach for improving vascular health in individuals or situations in which vascular endothelial dysfunction is present. Underappreciated is the fact that RBJ is also rich in other bioactive compounds including betalains, flavonoids, carotenoids, and ascorbic acid, which also have antioxidant, anti-inflammatory, and cardiometabolic-protective effects (26). In fact, a previous meta-analysis observed similar blood pressure outcomes when comparing nitrate-rich RBJ with nitrate-depleted RBJ, suggesting that RBJ may also have nitrate-independent effects (27). To our knowledge, the nitrate-independent effects of RBJ on health outcomes have not been investigated with respect to other health outcomes, with the exception of blood pressure. In addition, polyphenols and ascorbic acid can enhance the reduction of nitrate to nitrite and to NO (28, 29). The purpose of this randomized, double-blind, placebo-controlled, 4-period crossover clinical trial was to investigate the impact of both acute and chronic RBJ consumption on vascular endothelial function and other cardiometabolic responses to HFM consumption. This study also aimed to investigate underlying mechanisms contributing to clinical responses, including nitrate-dependent and -independent effects of RBJ. To achieve the latter, we used 1) a placebo (PBO) concentrate devoid of nitrate or polyphenols, 2) RBJ concentrate, 3) nitrate-depleted RBJ concentrate, and 4) a PBO concentrate with an equivalent dose of nitrate to that of the RBJ. To our knowledge, this is the first clinical trial designed to isolate the effects of inorganic nitrate in RBJ on cardiometabolic health, with the exception of blood pressure which was previously investigated in a meta-analysis (27). We hypothesized that acute and chronic RBJ consumption would attenuate postprandial impairment of vascular endothelial function, through nitrate-dependent and -independent mechanisms.
Methods
Study population
Men and postmenopausal women (≥1 y absence of menses) aged 40–65 y and with a BMI (kg/m2) between 25 and 39.9 were recruited to participate in this trial. Exclusion criteria included taking nitrate, antihypertensive, lipid-lowering, acid reflux, hypoglycemic, phosphodiesterase 5 inhibitor, or hormone replacement medications, triglyceride concentrations ≥250 mg/dL, hemoglobin A1c ≥6.5%, diagnosed hypertension or a blood pressure greater than 139/89 mmHg, CVD, diabetes, cancer, kidney, liver, or pancreatic disease, participating in a weight loss program or actively trying to lose weight, smokers, heavy drinkers (>3 drinks on any given occasion and/or >7 drinks/wk for women, or >4 drinks on any given occasion and/or >14 drinks/wk for men), allergy to test meals or treatments, or consuming >2 servings of RBJ/wk.
Participant recruitment
Participants were recruited from the greater Fort Collins, Colorado area through advertisements in local newspapers, Colorado State University webpages and e-mail, flyer distribution, direct mailers, and via clinicaltrials.gov between November 2016 and December 2017. Individuals sent an email or called to indicate interest and were then asked a series of questions regarding their health history to determine eligibility through a phone prescreening. Qualified individuals were invited for an onsite screening visit where they provided written informed consent, and inclusion and exclusion criteria were confirmed. Specifically, a detailed health history was obtained from the participant, followed by seated rest in a quiet room for 10 min prior to blood pressure assessment. Seated brachial blood pressure was measured in triplicate, with each measurement separated by 1 min, using an automatic device (IntelliSense® Blood Pressure Monitor HEM-907XL, Omron Healthcare, Inc.). A finger stick blood draw was performed to assess lipid profiles (Alere Cholestech LDX® Analyzer, Abbott) and hemoglobin A1c (Alere Afinion Analyzer System, Abbott). Anthropometric measurements (i.e. height, weight, and waist and hip circumferences) were performed.
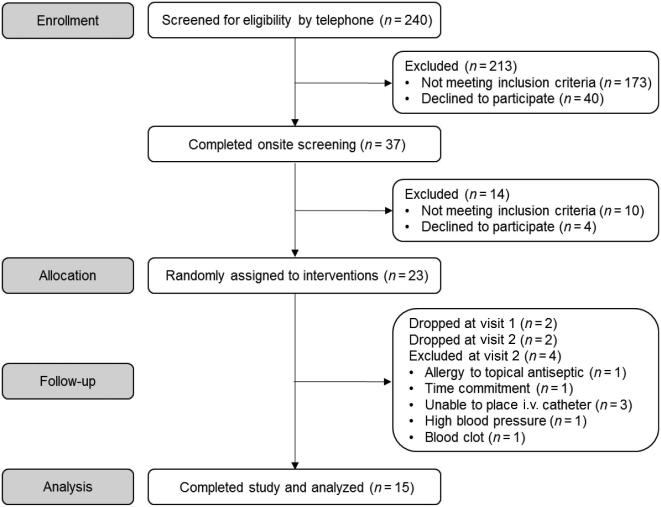
A detailed schematic of participant recruitment and enrollment for the study is provided in Figure 1. A total of 240 individuals responded to advertisements, 37 of which met inclusion criteria through the phone prescreening and completed the onsite screening visit. Of those, 23 met inclusion criteria, agreed to partake in the study, and were randomly assigned. Eight participants withdrew or were excluded from the study, and therefore data are reported for 15 participants who completed all protocol-specified procedures. This trial was conducted in accordance with the Declaration of Helsinki, was approved by the Colorado State University Institutional Review Board (16-6495HH), and is registered at clinicaltrials.gov as NCT02949115.
FIGURE 1.
CONSORT flow diagram of participants through the trial.
Study design and intervention
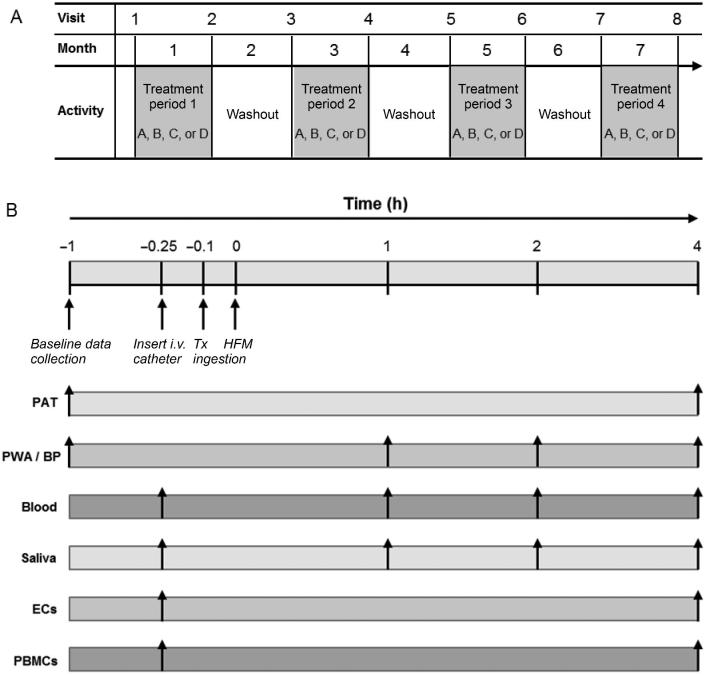
This was a randomized, double-blind, placebo-controlled, 4-period crossover trial in which participants completed 2 postprandial challenges during each treatment period, where each testing period lasted ∼5–6 h (times varied for intravenous catheter placement and blood sample collection). Overall study design, schedule of study visits, and a schematic of the test day timeline for data collection and measurements are presented in Figure 2. Study treatments included 70 mL of: 1) RBJ; 2) nitrate-free RBJ concentrate (NF-RBJ); 3) PBO concentrate; and 4) PBO concentrate + potassium nitrate (PBO + NIT). Nutrient composition of the treatments are presented in Supplemental Table 1. The PBO was devoid of polyphenols and nitrate, and had similar degrees of sweetness, flavor, and color to the RBJ. Prepackaged RBJ and NF-RBJ concentrates were purchased from James White Drinks, Ltd., pharmaceutical grade potassium nitrate was purchased from Spectrum Pharmacy Products, and PBO powder was purchased from Flavor Dynamics, Inc. PBO and PBO + NIT concentrates were prepared, packaged, and labelled by staff in the Kendall Reagan Nutrition Center in the Department of Food Science and Human Nutrition at Colorado State University. All treatments were packaged in individual bottles with the same packaging (from James White Drinks, Ltd.) to ensure blinding. Randomization permutations of treatments were created using the second generator at Randomization.com (www.randomization.com). Participants were assigned to randomization sequences in order of qualification and enrollment into the study. Bottles were labeled with participant ID and according to randomization sequences; however, study participants were not aware of their randomization sequences or treatments.
FIGURE 2.
(A) Overall study design and schedule of participant study visits. After enrollment, participants were randomized to receive four 70 mL treatments in random order: 1) placebo (PBO), 2) red beetroot juice (RBJ), 3) placebo + nitrate (PBO + NIT), and 4) nitrate-free RBJ (NF-RBJ). Each treatment period consisted of 2 postprandial challenges (i.e. first and last day of each 4-wk treatment period), followed by 4 wk of daily treatment consumption. Each treatment period was separated by a 4-wk washout period. Participants were enrolled in the trial for an 8-mo period. (B) Schematic of the test day timeline for data collection and measurements. Participants were randomly assigned to treatments A, B, C, or D for each treatment period in random order. BP, blood pressure; EC, endothelial cell; HFM, high-fat meal; i.v., intravenous; PAT, peripheral arterial tonometry; PBMC, peripheral blood mononuclear cell; PWA, pulse wave analysis; Tx, treatment.
The 2 postprandial challenges occurred on the first and last day of each treatment period, with 4 wk of daily treatment consumption in between. Each treatment period was separated by a 4-wk washout. During the first test day of each treatment period (acute test visit), preprandial assessments were performed followed by consumption of respective treatments 10 min prior to consuming a HFM to assess the acute impact of treatments on postprandial responses. The HFM was a breakfast meal consisting of 1 bagel, 1 tablespoon of butter (14.18 g), 2 tablespoons of cream cheese (29 g), 1 tablespoon of apple or peach jelly (20 g), 2 boiled eggs, and 1 cup of whole milk (240 mL). The meal composition was modeled after previous research demonstrating that HFMs containing ∼50 g fat or more impaired endothelial function 3–4 h following meal consumption, corresponding with peak triglyceride concentrations (30, 31). Nutrient composition of the test meal is presented in Supplemental Table 2. Following the first test day of each treatment period, participants consumed respective treatments daily until returning to the clinical research facility 4 wk later for a follow-up test visit (chronic test visit). During the follow-up test visit, preprandial assessments were performed and subjects did not consume their respective treatment prior to consuming a HFM in order to assess the chronic effects of the treatment on postprandial responses.
Enrolled participants were provided a 2-wk supply of treatments at a time and asked to consume one 70 mL bottle in the morning daily for 4 wk. Treatment compliance was assessed by asking study participants to 1) return empty and/or unused treatment bottles at their next visit, and 2) record the date and time their treatment was consumed each day, and to document missing doses and the reason for missing the dose (e.g. sick, fell asleep, forgot) in a daily dosing diary. Noncompliance was defined as missing ≥1 dose per wk.
Participants agreed to adhere to their usual dietary habits, to avoid use of antibacterial mouthwash, and to maintain their physical activity level during the course of the study. They also agreed to abstain from caffeine, alcohol, prescription and over-the-counter medications, dietary supplements, brushing their teeth during the 12 h before the start of all test visits, and to avoid intense physical activity for 24 h prior to their test visit. All vascular and hemodynamic measurements were performed in a quiet, dimly-lit, temperature-controlled room (20–25°C).
The primary outcome measure for this trial was postprandial reactive hyperemia index (RHI), relative to preprandial RHI and PBO, at the acute and chronic test visits. RHI is a validated measure of microvascular endothelial function that is predictive of atherosclerosis and future cardiovascular events (32–34). Secondary outcome measures included arterial stiffness (augmentation index [AIx] and augmentation index corrected for heart rate at 75 beats per minute [AIx@75]), hemodynamics (brachial and aortic systolic blood pressure, diastolic blood pressure, pulse pressure, heart rate, mean arterial pressure, and augmented pressure), biochemical markers of cardiovascular health, metabolism, inflammation, oxidative stress, and endoplasmic reticulum (ER) stress (blood triglycerides, glucose, insulin, and plasma and saliva nitrate/nitrite [NOx]), peripheral blood mononuclear cell (PBMC) gene expression (NADPH oxidase, NF-κB, TLR-4, TNF-α, GADD34, and XBP1s), and endothelial cell protein expression (NADPH oxidase).
Anthropometrics
Height without shoes was measured using a scale-mounted stadiometer to the nearest 0.5 cm and weight was assessed using a digital scale (Health o Meter® Professional 500 KL, Sunbeam Products, Inc.). BMI was calculated as weight in kilograms divided by height in meters squared. Midabdominal waist circumference and hip circumference were measured using a Gulick fiberglass measuring tape with a tension handle (Creative Health Products, Inc.).
Vascular endothelial function
Digital artery endothelium-dependent vasodilation was assessed using a noninvasive, reproducible plethysmographic method (EndoPAT2000, Itamar Medical, Ltd.) as previously described (34, 35) and in accordance with the conditions specified by the manufacturer. After 10 min of supine rest, pneumatic finger-tip probes were placed on each index finger and a blood pressure cuff was placed on the experimental (nondominant) upper arm, whereas the other arm served as the contralateral control, with both arms at rest on arm supports. After an equilibration period (i.e. baseline recording of pulse amplitude for 5 min on each arm), the cuff on the experimental arm was inflated to 200 mmHg or 60 mmHg higher than the participant’s systolic blood pressure (whichever was higher) for 5 min to occlude the brachial artery. The cuff was then deflated to induce reactive hyperemia and postocclusion peripheral arterial tonometry (PAT)-signals were recorded for an additional 5 min in both arms. RHI, an index of flow-mediated dilation, was derived as the ratio of the average pulse wave amplitude during hyperemia (60 to 120 s of the postocclusion period) to the average pulse wave amplitude during baseline in the occluded hand, divided by the same value in the control hand and then multiplied by a baseline correction factor. Framingham RHI (F-RHI), which uses a different postocclusion period (90–120 s) without baseline correction and has a natural logarithmic transformation applied to the resulting ratio, is also reported. RHI and F-RHI have been shown to correlate with CVD risk (32, 36).
Hemodynamics and arterial stiffness
Brachial pulse pressure was calculated as the difference between mean systolic blood pressure and diastolic blood pressure. Central aortic blood pressure and related hemodynamic parameters (e.g. aortic mean arterial pressure, aortic pulse pressure) were derived from brachial pressure waveforms using a validated transfer function and automatically recorded. Aortic AIx, a measure of pulse wave reflection and arterial stiffness, was automatically calculated as the ratio between augmented aortic pressure (i.e. difference between the first and second derived aortic systolic peaks) and aortic pulse pressure. Both AIx and AIx normalized to a heart rate of 75 beats per min (AIx@75) are reported, as AIx can be influenced by heart rate, though this approach may not be generalizable to all populations (37, 38).
At the screening visit, aortic stiffness was assessed by measuring carotid-femoral pulse wave velocity (aPWV) in the supine position (SphygmoCor XCEL, AtCor Medical, Inc.) (39). Carotid and femoral waveforms were simultaneously captured using applanation tonometry above the carotid artery and a femoral blood pressure cuff. Distance between the sternal notch and the carotid artery, the sternal notch to the top of the femoral cuff, and the femoral artery to the top of the femoral cuff was measured with a nonelastic measuring tape. The distance traveled and transit time were automatically determined by the SphygmoCor system and used to calculate aPWV, which is expressed as distance over transit time (i.e. meters per second). Three measurements were obtained and averaged for analysis. At the beginning of each testing visit and following 10 min of supine rest, brachial and aortic blood pressure, and AIx were measured in the nondominant arm (SphygmoCor XCEL, AtCor Medical, Inc.) and the mean value of 3 measurements was used in analyses.
Blood and saliva collection and biochemical analyses
Following baseline vascular and hemodynamic assessments, an intravenous catheter was placed into an antecubital vein. Blood was collected in vacutainers with EDTA (BD) for plasma separation, centrifuged according to the manufacturers’ instructions, aliquoted, and stored at –80°C until analysis. A slow saline drip was then initiated to keep the line patent for serial blood draws. Saliva was collected directly into cryovials using a saliva collection aid (SalivaBio, Inc.) and stored at –80°C until analysis.
Plasma triglyceride, glucose, and insulin concentrations were analyzed using an AU480 Automated Chemistry Analyzer (Beckman Coulter) at the University of Colorado-Denver Colorado Clinical and Translational Sciences Institute. HOMA-IR was calculated using the HOMA2 Calculator v2.2.3 (Radcliffe Department of Medicine, Diabetes Trial Unit, University of Oxford) based on initial fasting baseline values insulin and glucose measurements (40). Plasma samples were filtered using 30 kDa molecular weight cut-off filters (Millipore Sigma) to reduce the presence of hemoglobin prior to NOx analysis. Plasma and saliva NOx concentrations were measured using commercially available colorimetric assay kits according to the manufacturers’ instructions (Cayman Chemical).
PBMC isolation and gene expression analyses
A portion of venous blood (17 mL) collected in EDTA plasma vacutainers was used for PBMC isolation. Whole blood was transferred into 50 mL conical tubes and diluted with an equal amount of PBS containing 2% FBS. Diluted blood was added to a SepMate 50 mL tube containing a density gradient medium (Lymphoprep, STEMCELL Technologies), and PBMCs were isolated per the manufacturer's protocol. Briefly, after centrifugation at 1200 × g for 10 min with the brake on, the top layer containing the enriched PBMCs was poured into a fresh conical tube and washed twice with PBS containing 2% FBS at room temperature. The cell pellet was resuspended with PBS + 2% FBS at room temperature. After cell counting, PBMCs were cryopreserved in a cryopreservation medium (CryoStor® CS10, STEMCELL Technologies) at 5 × 106 cells per 1 mL and placed inside a Nalgene® Mr. Frosty® Cryo 1°C Freezing Container (Thermo Fisher Scientific) at −80°C for 24 h. Cells were then transferred into liquid nitrogen where they were stored until analysis.
Total RNA was extracted with Trizol reagent according to the manufacturer's protocol (Invitrogen). For real-time PCR, reverse transcription was performed using 0.5 μg of DNase-treated RNA, Superscript II RnaseH- and random hexamers. PCR reactions were performed in 96-well plates using transcribed cDNA and IQ-SYBR green master mix (Bio Rad Laboratories). Primer sets are provided in Supplemental Table 3. PCR efficiency was between 90% and 105% for all primer and probe sets and linear over 5 orders of magnitude. The specificity of products generated for each set of primers was examined for each amplicon using a melting curve and gel electrophoresis. Reactions were run in triplicate and data were calculated as the change in cycle threshold (∆CT) for the target gene relative to the ∆CT for β2-microglobulin (control/reference gene) according to the procedures of Muller et al. (41).
Endothelial cell biopsy and protein expression analyses
Endothelial cell collection and protein expression analyses were performed as previously described (42, 43). Endothelial cells were biopsied from the antecubital vein as venous endothelial cell protein expression correlates with arterial endothelial cell protein expression (44). Briefly, endothelial cells were biopsied using sterile 0.025 inch (1 inch = 25.4 mm) J-wires (GuideRightTM, St. Jude Medical) advanced through an intravenous catheter ∼4 cm beyond the tip of the catheter and withdrawn. The distal portion of the wire was transferred to a 50 mL conical tube containing a buffer solution, and cells were recovered by centrifugation, fixed with formaldehyde, plated onto microscope slides, and stored at –80°C until analysis.
Slides were stained for NADPH oxidase/p47 subunit (Sigma-Aldrich), and a complementary fluorescent secondary Alexafluor 555 antibody (Invitrogen). Slides were also stained for vascular endothelial-cadherin (Abcam) for positive identification of endothelial phenotype and DAPI (4′, 6′-diamidino-2-phenylindole hydrochloride; Vector Laboratories) for nuclear integrity. Images were digitally captured and analyzed using cellSens Software (Olympus Corporation). Values are reported as ratios of subject endothelial cell protein expression to human umbilical vein endothelial cell (HUVEC; control cells) protein expression. This ratio is reported to minimize the possible confound of differences in staining intensity among different staining sessions.
Sample size estimation and statistical analyses
Sample size was estimated with a minimum anticipated difference between the RBJ and PBO groups of 0.3 with an SD of 0.392 based on previous work (34). A crossover design was considered and hence a moderate intraclass correlation of 0.3 was used for calculation. A final sample size of 15 subjects in the study was estimated to provide a statistical power >90% and a confidence of 99% with a 2-tailed hypothesis. Collected data were stored electronically using Research Electronic Data Capture (REDCap) for secure data management (45, 46). As a measure of quality control, data were double-entered by 2 individuals and evaluated for consistency by a third person. Subject characteristics were analyzed using descriptive statistics from data collected at the screening visit. For each treatment and exposure (acute compared with chronic) arm, data were tested for normality using Shapiro–Wilks tests (PROC UNIVARIATE, SAS, version 9.4; SAS Institute) and confirmed using QQ-plot observations. Data not conforming to normal distribution were natural log-transformed before statistical analysis to accommodate assumptions of normality. Outlier removal may have resulted in fewer evaluable subjects for primary and secondary outcomes. Thus, no data were removed from the models. For the primary and secondary outcomes (e.g. RHI, hemodynamics, blood and saliva biomarkers), linear mixed models (PROC MIXED, SAS) were used to assess main and interaction effects of treatment (PBO, RBJ, PBO + NIT, NF-RBJ) and time (0 h and 1, 2, 4 h postprandial) at each respective visit. In these models, time, treatment, and time*treatment interaction were set as fixed effects, and subject and treatment order were set as random effects. Age, sex, and BMI were included in the models as covariates. When assessing the difference from the acute visit to the chronic visit (week 4 compared with week 0), a linear mixed model, with the same random and fixed effects as well as covariates, was used. AUC was calculated for postprandial glucose, insulin, triglyceride, and NOx concentrations using the trapezoidal rule, and incremental AUC (iAUC) was calculated for glucose, insulin, and triglycerides in the same way after controlling for baseline. Differences in AUC between treatment groups for each postprandial value were assessed using a generalized linear mixed model (PROC GLM, SAS) with Tukey's test for multiple comparisons for repeated measures. For AUC, time, treatment, and time*treatment interaction were set as fixed effects, subject and treatment order were set as random effects. For gene expression analysis, differences in relative expression (expressed as fold-change) within each treatment group were analyzed with a mixed model (PROC MIXED, SAS) to assess the magnitude of change over time. The same fixed and random effects and covariates previously stated were used. The 0-h time point at the acute visit was used as a reference time point for fold-change comparisons within each treatment group. For endothelial cell protein expression analyses, baseline/preprandial (0 h) differences between the acute and chronic test visits were assessed by a linear mixed model (PROC MIXED, SAS). The linear mixed model used for endothelial cell protein expression analyses included time (acute baseline compared with chronic baseline), treatment, and time*treatment as fixed effects and subject and treatment order as random effects. All results are presented as least squares mean ± SEMs, and 95% CIs are reported for fold-changes in gene expression. Statistical significance was set at a 2-sided α level of 0.05.
Results
Baseline characteristics, anthropometrics, and treatment compliance
Screening and baseline (RHI and HOMA-IR only) characteristics of participants those who completed the study, as well as those lost to follow-up are presented in Table 1. There were no significant changes in anthropometric measurements over the course of the study (data not shown). All study participants that completed the study were compliant with their treatments (missing ≤one 70 mL treatment per week on average, or >85% compliant). Treatment compliance was 93.7% across all treatment groups (overall compliance), and 92.3%, 95.9%, 95.5%, and 92.9% for the PBO, RBJ, PBO + NIT, and NF-RBJ treatment periods, respectively.
TABLE 1.
Screening and select baseline characteristics of participants who completed the study and those lost to follow-up
| Completed (n = 15) | Lost to follow-up (n = 8) | |
|---|---|---|
| Age, y | 53 ± 2 (42–65) | 51 ± 2 (43–64) |
| Years postmenopausal (women only) | 5 ± 1 (1–8) | 10 ± 2 (1–16) |
| Sex, M:F (n) | 7:8 | 2:6 |
| BMI, kg/m2 | 29.8 ± 0.9 (26.2–36.4) | 30.0 ± 1.5 (25.2–36.9) |
| Waist-to-hip ratio | 0.86 ± 0.02 (0.76–1.00) | 0.86 ± 0.02 (0.78–0.93) |
| Total cholesterol, mg/dL | 202 ± 11 (100–278) | 219 ± 9 (190–271) |
| HDL, mg/dL | 59 ± 4 (17–85) | 55 ± 3 (42–69) |
| LDL, mg/dL | 131 ± 0 (86–181) | 144 ± 10 (116–190) |
| Triglycerides, mg/dL | 112 ± 16 (45–249) | 121 ± 21 (45–222) |
| HDL:LDL ratio | 0.48 ± 0.05 (0.27–0.86) | 0.38 ± 0.04 (0.24–0.58) |
| Hemoglobin A1c, % | 5.3 ± 0.1 (5.0–6.0) | 5.4 ± 0.1 (5.0–5.8) |
| HOMA-IR | 0.79 ± 0.11 (0.21–1.52) | — |
| SBP, mmHg | 121 ± 3 (97–136) | 115 ± 3 (103–123) |
| DBP, mmHg | 78 ± 2 (62–89) | 80 ± 3 (66–88) |
| RHI* | 1.74 ± 0.09 (1.26–2.38) | 2.17 ± 0.26 (1.27–3.79) |
| PWV, m/s | 7.2 ± 0.4 (4.6–10.0) | 6.9 ± 0.25 (5.4–7.6) |
Values are mean ± SEM (ranges).
RHI and HOMA-IR were measured at the baseline visit (HOMA-IR not calculated for those who dropped the study due to not analyzing blood biomarkers at baseline visit). DBP, diastolic blood pressure; PWV, pulse wave velocity; RHI, reactive hyperemia index; SBP, systolic blood pressure; WC, waist circumference.
Acute treatment effects on postprandial vascular endothelial function
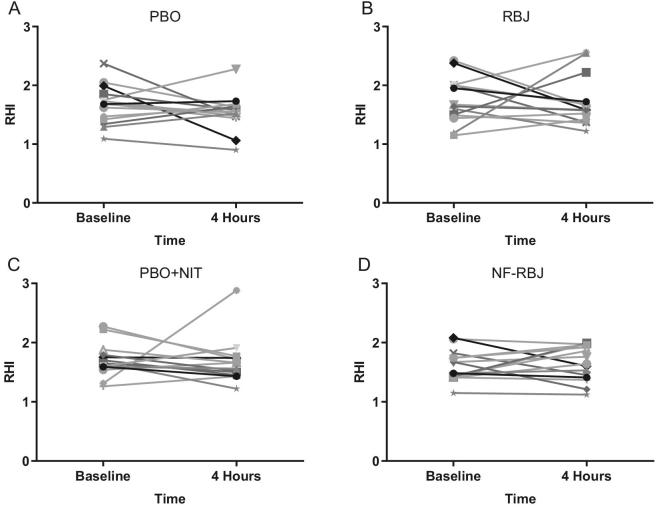
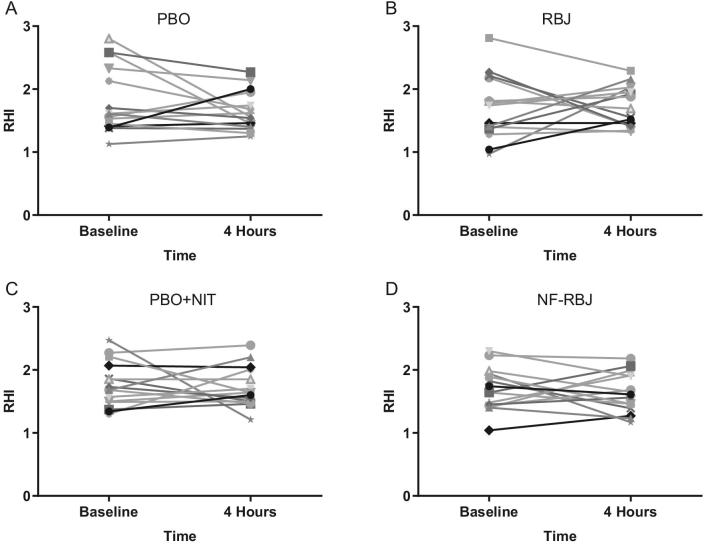
There were no significant time*treatment interaction effects for RHI or F-RHI across treatment groups, nor were there significant effects of time at the acute test visit (Figure 3). Individual RHI responses are shown in Figure 4.
FIGURE 3.
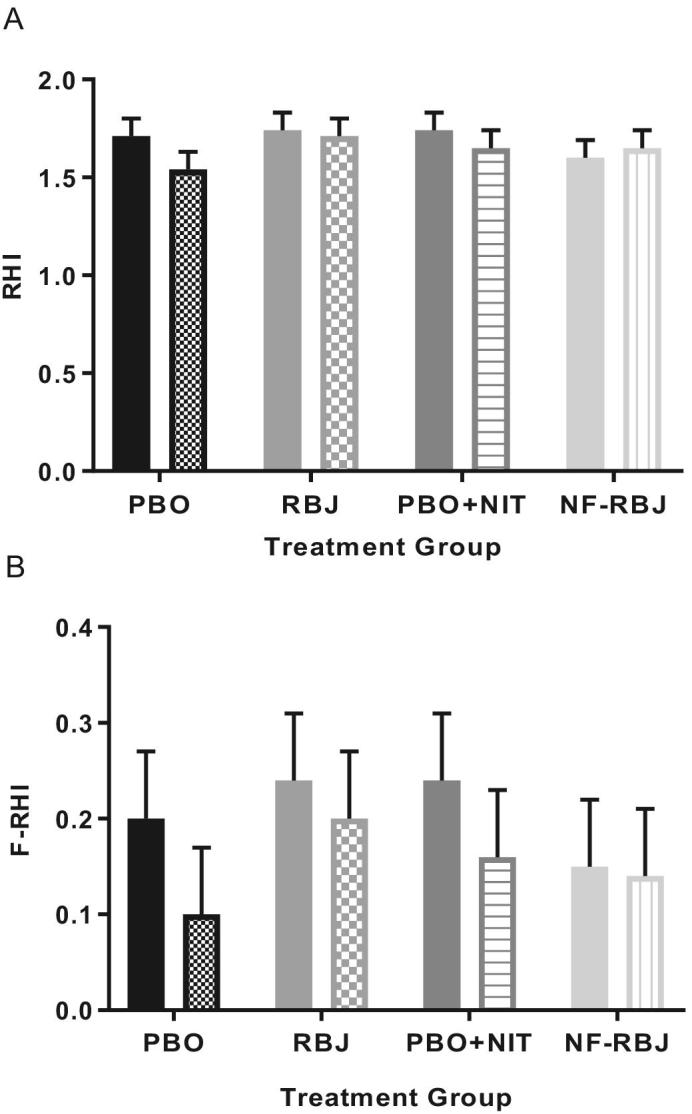
Effects of PBO, RBJ, PBO + NIT, and NF-RBJ on RHI (A) and F-RHI (B) before (0 h) and 4 h after consuming a high-fat meal at the acute visit. Data are least square means ± SEM, n = 15. Means were compared with the use of the PROC MIXED procedure in SAS version 9.4. There were no significant effects of time, treatment, or interaction effect of time*treatment of RHI or F-RHI in the model. Solid color = before meal (T0), and patterned color = 4 h after meal and treatment consumption (T4). RHI, reactive hyperemia index; F-RHI, Framingham-RHI; PBO, placebo; RBJ, red beetroot juice; PBO + NIT, PBO + nitrate; NF-RBJ, nitrate-free RBJ.
FIGURE 4.
Individual effects of PBO (A), RBJ (B), PBO + NIT (C), and NF-RBJ (D) on RHI before (0 h) and 4 h after consuming a high-fat meal at the acute visit. Data are least square means ± SEM, n = 15. Values were obtained with the use of the PROC MIXED procedure in SAS version 9.4. RHI, reactive hyperemia index; PBO, placebo; RBJ, red beetroot juice; PBO + NIT, PBO + nitrate; NF-RBJ, nitrate-free RBJ.
Acute treatment effects on postprandial hemodynamics
Hemodynamic parameters at the acute test visit are presented in Supplemental Table 4. There were significant time*treatment interaction effects for several of the parameters including brachial pulse pressure, aortic systolic blood pressure, aortic diastolic blood pressure, aortic mean arterial pressure (all P < 0.05) as shown in Supplemental Table 4. Specifically, brachial systolic blood pressure was significantly higher in the PBO + NIT group than the NF-RBJ group at the 1-h time point (time*treatment P < 0.05). For brachial pulse pressure, the NF-RBJ group was significantly lower than all other groups at the 1-h time point (time*treatment P < 0.05). Aortic pulse pressure was found to be significantly lower in the PBO + NIT group than all other groups (time*treatment P < 0.05) at the 4-h time point. As expected, significant effects of time, and thus the HFM, were noted for several parameters including aortic systolic blood pressure, aortic diastolic blood pressure, aortic heart rate, aortic mean arterial pressure, and augmented pressure; however, no time*treatment interaction effects were observed for those parameters.
Preprandial and postprandial AIx and AIx@75 are presented in Figure 5. At the 4-h time point, the RBJ and PBO + NIT groups had a significantly lower AIx than the PBO group (time*treatment P = 0.0039 and P = 0.0174, respectively). No other significant differences were observed for AIx or AIx@75 for any time point or treatment group.
FIGURE 5.
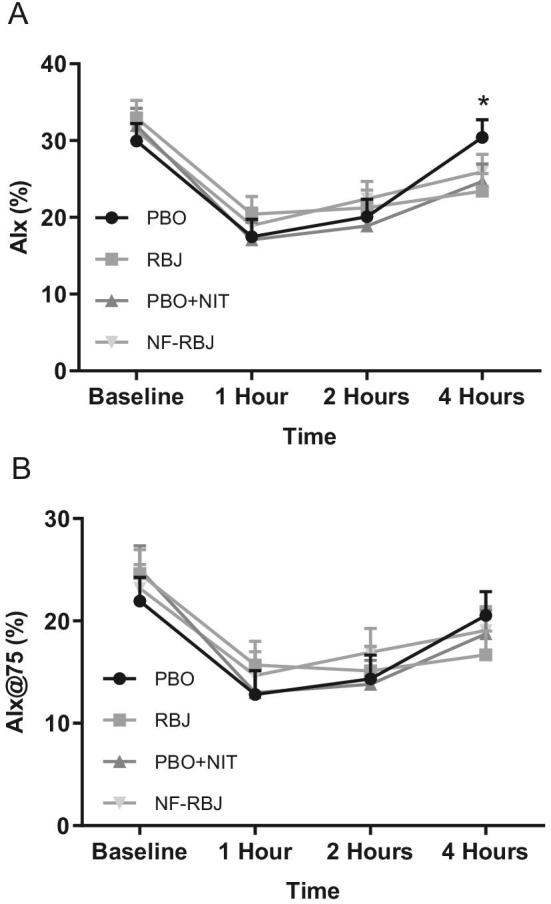
Effects of PBO, RBJ, PBO + NIT, and NF-RBJ on AIx (A) and Aix@75 (B) before (0 h) and 1, 2, and 4 h after consuming a high-fat meal at the acute visit. Data are least square means ± SEM, n = 15. Values were compared with the use of the PROC MIXED procedure in SAS version 9.4. Time points annotated with symbols represent a significant time*treatment interaction between treatment groups. *PBO significantly different than RBJ and PBO + NIT (both P < 0.05). No other significant effects for AIx or AIx@75 were observed. AIx, augmentation index; AIx@75, augmentation index at 75 beats per min; PBO, placebo; RBJ, red beetroot juice; PBO + NIT, PBO + nitrate; NF-RBJ, nitrate-free RBJ.
Acute treatment effects on postprandial blood and saliva biomarkers
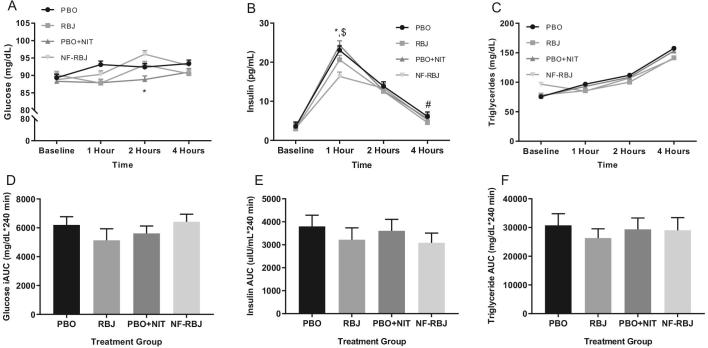
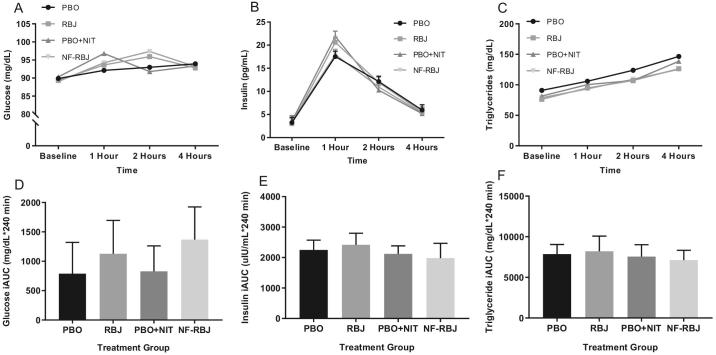
Plasma glucose concentrations were significantly lower in the PBO + NIT group than the NF-RBJ group at the 2-h time point (time*treatment P = 0.0269, Figure 6A). No other significant differences in plasma glucose concentrations were observed among treatment groups. Plasma insulin peaked at the 1-h time point in all treatment groups (all P < 0.0001), with the NF-RBJ group having significantly lower insulin concentrations than the PBO and PBO + NIT groups (time*treatment P = 0.0193 and 0.0068, respectively; Figure 6B). No significant differences across treatment groups were observed at any time point for postprandial triglyceride concentrations (Figure 6C). There were no significant differences in glucose, insulin, or triglyceride iAUC across treatment groups (Figure 6D and E).
FIGURE 6.
Plasma concentrations of glucose (A), insulin (B), and triglycerides (C) at baseline (0 h), and 1, 2, and 4 h after a high-fat meal and PBO, RBJ, PBO + NIT, and NF-RBJ treatment ingestion, and postprandial (0–240 min) glucose (D), insulin (E), and triglyceride (F) incremental AUC (iAUC) at the acute visit. Data in A–C are presented as log-transformed least square means ± SEM, n = 15. Data in D–F are presented as untransformed mean ± SEM. Values in A–C were compared with the use of the PROC MIXED procedure, whereas iAUC values in D–F were compared by use of the PROC GLM procedure with Tukey's multiple comparison test in SAS version 9.4. All time points were significantly different from baseline for plasma insulin and triglyceride concentrations among all treatment groups. Time points annotated with symbols represent significant time*treatment interaction between treatment groups. *PBO + NIT significantly different than NF-RBJ; $PBO + NIT significantly different than PBO; #RBJ significantly different than PBO, all P < 0.05. No significant differences between treatment groups at any time point for postprandial triglyceride concentrations were observed. There were no significant differences in postprandial glucose, insulin, or triglyceride AUC between treatment groups. PBO, placebo; RBJ, red beetroot juice; PBO + NIT, PBO + nitrate; NF-RBJ, nitrate-free RBJ.
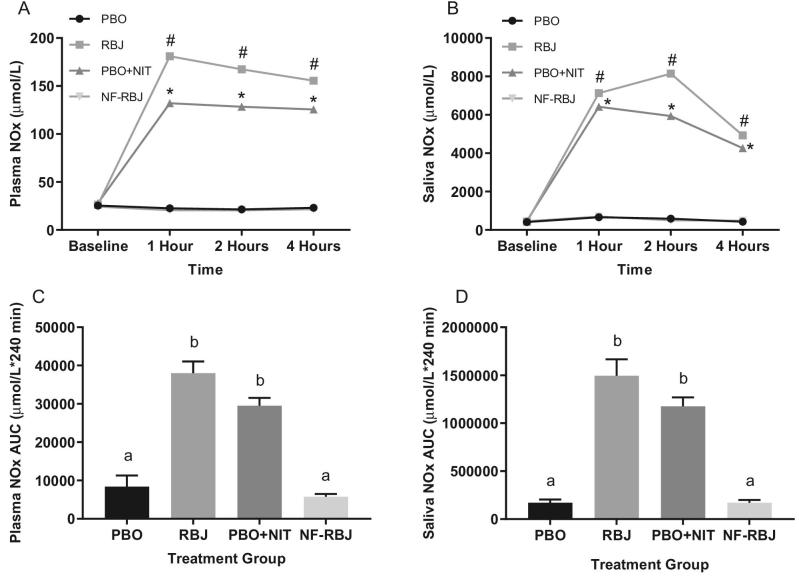
At the 1-, 2-, and 4-h time points, plasma and saliva NOx concentrations were significantly higher in the RBJ and PBO + NIT groups than the PBO and NF-RBJ groups (time*treatment P < 0.0001 for all time points), as well as compared with baseline (all P < 0.0001), whereas the PBO and NF-RBJ values remained unchanged throughout the 4-h testing period (Figure 7A and B). Similarly, plasma and saliva NOx AUC for the RBJ and PBO + NIT groups were significantly higher than PBO and NF-RBJ groups (P < 0.05, Figure 7C and D).
FIGURE 7.
Plasma NOx concentrations (A) and saliva NOx concentrations (B) at baseline (0 h) and 1, 2, and 4 h after a high-fat meal and PBO, RBJ, PBO + NIT, and NF-RBJ treatment ingestion, and postprandial (0–240 min) plasma NOx (C) and saliva NOx (D) AUC at the acute visit. Data in A–C are presented as log-transformed least square means ± SEM, n = 15. Data in D–F are presented as untransformed mean ± SEM. Values in A–C were compared with the use of the PROC MIXED procedure, whereas AUC values in D–F were compared by use of the PROC GLM procedure with Tukey's multiple comparison test in SAS version 9.4. All time points were significantly different than baseline for plasma and saliva NOx concentrations in the RBJ and PBO + NIT groups. Time points annotated with symbols represent significant time*treatment interactions between treatment groups. #RBJ significantly different from PBO and NF-RBJ, P < 0.0001. *PBO + NIT significantly different from PBO and NF-RBJ, P < 0.0001. Treatment groups annotated with different letters were significantly different from one another, P < 0.05. NOx, nitrate/nitrite; PBO, placebo; RBJ, red beetroot juice; PBO + NIT, PBO + nitrate; NF-RBJ, nitrate-free-RBJ.
Acute treatment effects on PBMC gene expression
Gene expression results at the acute test visit are presented in Supplemental Table 6. In the PBO group, there was a 1.9-fold increase in TLR-4 at the 4-h time point relative to baseline (95% CI: 1.4, 2.4; P = 0.0096). In the RBJ group, there was a 1.5-fold increase of p47phox (95% CI: 1.1, 1.9; P = 0.0469), a 1.7-fold increase of TLR-4 (95% CI: 1.3, 2.1; P = 0.0214), and a 0.6-fold decrease of GADD34 (95% CI: 0.4, 0.8; P = 0.01) at the 4-h time point relative to baseline. In the NF-RBJ group, there was a 0.6-fold decrease in TNF-α (95% CI: 0.3, 0.8; P = 0.0128) and GADD34 (95% CI: 0.3, 0.9; P = 0.0282) at the 4-h time point relative to baseline. No other within-treatment group differences were observed.
Chronic treatment effects on preprandial and postprandial vascular endothelial function
There were no significant time*treatment interaction effects for RHI or F-RHI following 4 wk of daily treatment consumption, nor were there significant effects of time (Figure 8). Individual RHI responses are shown in Figure 9. There were no significant preprandial or postprandial differences within treatment groups at 4 wk compared with 0 wk (data not shown).
FIGURE 8.
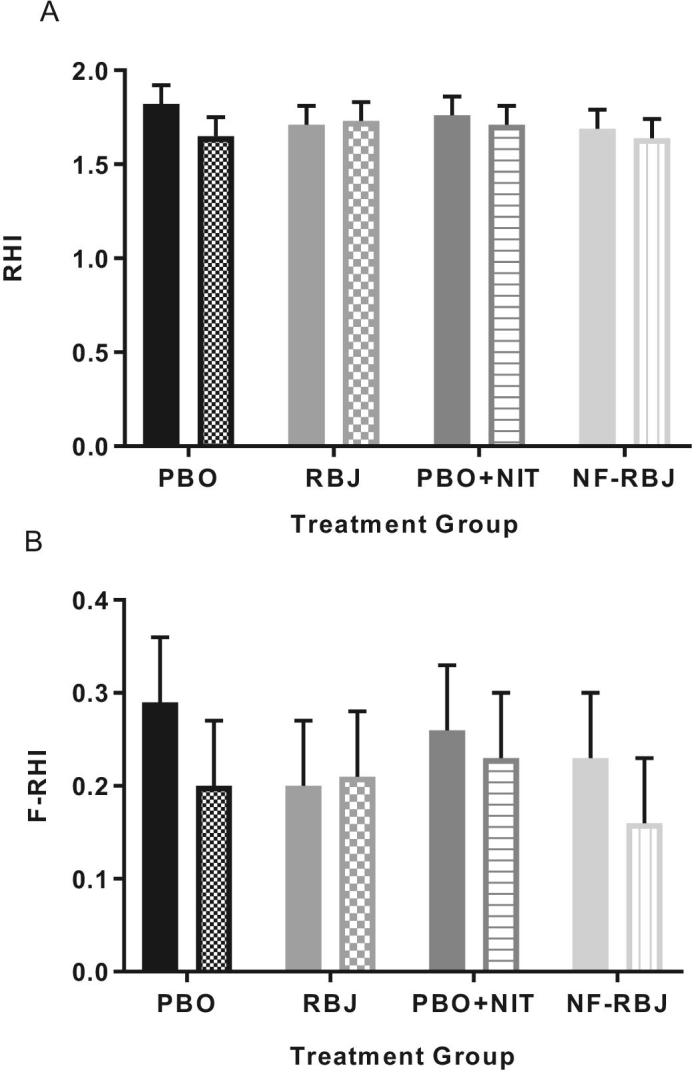
Effects of PBO, RBJ, PBO + NIT, and NF-RBJ on RHI (A) and F-RHI (B) after 4 wk treatment ingestion at baseline (0 h) and 4 h after consuming a high-fat meal at the chronic visit. Data are least square means ± SEM, n = 15. Means were compared with the use of the PROC MIXED procedure in SAS version 9.4. There were no significant effects of time, treatment, or interaction effect of time*treatment of RHI or F-RHI in the model. Solid color = before meal (T0), and patterned color = 4 h after meal and treatment consumption (T4). RHI, reactive hyperemia index, F-RHI, Framingham-RHI; PBO, placebo; RBJ, red beetroot juice; PBO + NIT, PBO + nitrate; NF-RBJ, nitrate-free RBJ.
FIGURE 9.
Individual effects of PBO (A), RBJ (B), PBO + NIT (C), and NF-RBJ (D) on RHI after 4-wk treatment ingestion at baseline (0 h) and 4 h after consuming a high-fat meal at the chronic visit. Data are least square means ± SEM, n = 15. Values were obtained with the use of the PROC MIXED procedure in SAS version 9.4. RHI, reactive hyperemia index; PBO, placebo; RBJ, red beetroot juice; PBO + NIT, PBO + nitrate; NF-RBJ, nitrate-free RBJ.
Chronic treatment effects on preprandial and postprandial hemodynamics
Preprandial and postprandial hemodynamic parameters at the chronic test visit are presented in Supplemental Table 5. After 4 wk of daily RBJ consumption, preprandial augmented pressure was significantly lower than baseline (P = 0.0125), whereas preprandial AIx@75 was significantly lower at 4 wk than baseline (0 wk) following PBO + NIT consumption (P = 0.0363). There were no significant preprandial differences within treatment groups at 4 wk compared with 0 wk for the remaining parameters (data not shown).
With respect to postprandial hemodynamic parameters, brachial systolic blood pressure was significantly lower in the NF-RBJ group than all other groups at the 1-h time point, whereas PBO + NIT was significantly higher than PBO, RBJ, and NF-RBJ at the 4-h time point (both time*treatment P < 0.05). For brachial diastolic blood pressure, the NF-RBJ group was significantly lower than all other groups at the 2-h time point (time*treatment P < 0.05). At the 4-h time point, the PBO + NIT group was significantly higher than all other treatment groups whereas NF-RBJ was significantly lower than all other groups (time*treatment P < 0.05). For brachial pulse pressure, the NF-RBJ group was significantly lower than the PBO + NIT group at the 4-h time point (time*treatment P < 0.05). For aortic systolic blood pressure, the NF-RBJ group was significantly lower than the PBO + NIT group at the 2-h time point, whereas the PBO, RBJ, and NF-RBJ groups were significantly lower than the PBO + NIT group at the 4-h time point (all time*treatment P < 0.05). For aortic diastolic blood pressure, the NF-RBJ was significantly lower than all other treatment groups at the 2-h time point, whereas the PBO, RBJ, and NF-RBJ groups were significantly lower than the PBO + NIT group at the 4-h time point (all time*treatment P < 0.05). For aortic pulse pressure, the NF-RBJ group was significantly lower than the PBO + NIT group at the 4-h time point (time*treatment P < 0.05). For aortic heart rate, the PBO + NIT group was significantly lower than the NF-RBJ group (time*treatment P < 0.05). For aortic mean arterial pressure, the NF-RBJ group was significantly lower than the RBJ and PBO + NIT groups at the 2-h time point, whereas all groups were significantly lower than the PBO + NIT group at the 4-h time point (all time*treatment P < 0.05). For augmented pressure, the PBO + NIT group was significantly lower than the NF-RBJ group at the 1-h time point (time*treatment P < 0.05).
When comparing postprandial responses at the chronic visit versus the acute visit, there was a significant postprandial increase in aortic pulse pressure at the 4-h time point within the PBO + NIT group at 4 wk compared to 0 wk (32 ± 1 mmHg compared with 39 ± 1 mmHg, respectively, time*treatment P = 0.02), whereas there was a significant postprandial decrease in AIx within the PBO group at 4 wk compared with 0 wk at the 4-h time point (30 ± 2% compared with 26 ± 2%, time*treatment P = 0.0426). There were no significant postprandial differences within treatment groups at 4 wk compared with 0 wk for the remaining parameters (data not shown).
Chronic treatment effects on preprandial and postprandial blood and saliva biomarkers
Preprandial and postprandial AIx and AIx@75 are presented in Figure Figure 10. There were no significant preprandial or postprandial differences within treatment groups at 4 wk compared with 0 wk for plasma glucose, insulin, or triglycerides (data not shown) except for the PBO group which had a significant preprandial increase at 4 wk compared with 0 wk for triglycerides (91 ± 1 mg/dL compared with 79 ± 1 mg/dL, time*treatment P = 0.0361). Plasma insulin peaked at the 1-h time point in all treatment groups (all P < 0.0001), however, postprandial glucose, insulin, and triglyceride concentrations and iAUC did not differ across treatment groups following 4 wk of chronic treatment consumption (Figure 11A–F).
FIGURE 10.
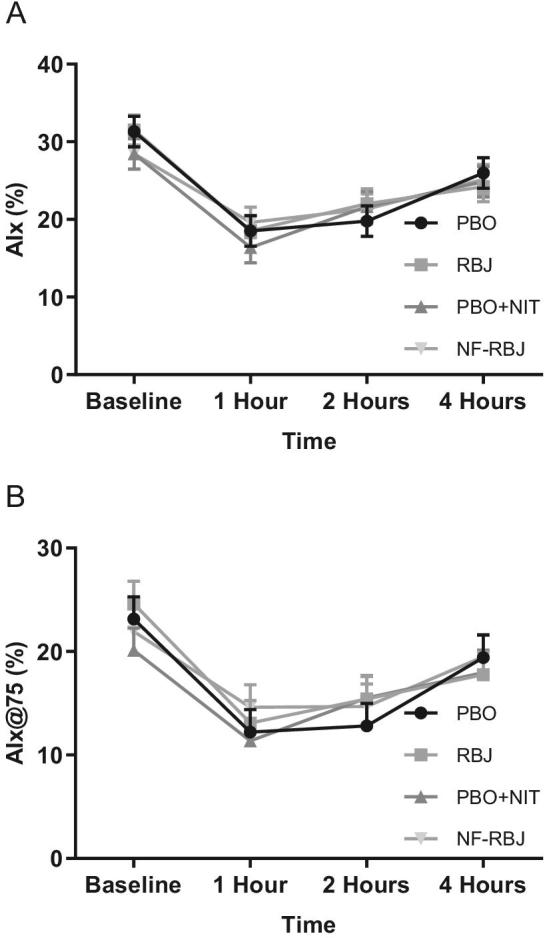
AIx (A) and Aix@75 (B) after 4 wk PBO, RBJ, PBO + NIT, and NF-RBJ treatment ingestion at baseline (0 h) and 1, 2, and 4 h after consuming a high-fat meal at the chronic visit. Data are least square means ± SEM, n = 15. Values were compared with the use of the PROC MIXED procedure in SAS version 9.4. For both outcomes, there were no significant effects of time, treatment, or main interaction effect of time*treatment in the models. AIx, augmentation index; AIx@75, augmentation index at 75 beats per min; PBO, placebo; RBJ, red beetroot juice; PBO + NIT, PBO + nitrate; NF-RBJ, nitrate-free RBJ.
FIGURE 11.
Plasma concentrations of glucose (A), insulin (B), and triglycerides (C) after 4 wk PBO, RBJ, PBO + NIT, and NF-RBJ treatment ingestion at baseline (0 h) and 1, 2, and 4 h after consuming a high-fat meal, and postprandial (0–240 min) glucose (D), insulin (E), and triglyceride (F) incremental AUC (iAUC) at the chronic visit. Data in A–C are presented as log-transformed least square means ± SEM, n = 15. Data in D–F are presented as untransformed mean ± SEM. Values in A–C were compared with the use of the PROC MIXED procedure, whereas iAUC values in D–F were compared by use of the PROC GLM procedure with Tukey's multiple comparison test in SAS version 9.4. All time points were significantly different from baseline for plasma insulin and triglyceride concentrations among all treatment groups. For plasma glucose, insulin, and triglyceride responses, there were no significant effects of time, treatment, or interaction effect of time*treatment. PBO, placebo; RBJ, red beetroot juice; PBO + NIT, PBO + nitrate; NF-RBJ, nitrate-free RBJ.
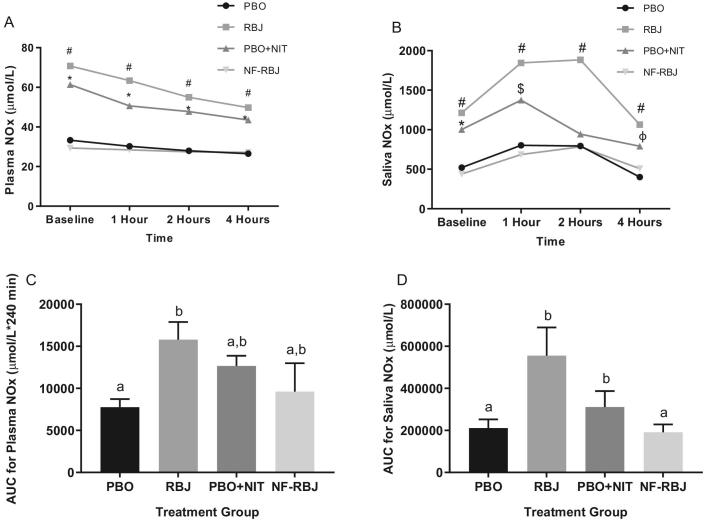
There were significant preprandial increases in the RBJ and PBO + NIT groups at 4 wk compared with 0 wk for plasma NOx (P < 0.001) and saliva NOx (P < 0.05). At the 1-, 2-, and 4-h time points, plasma NOx concentrations in the RBJ and PBO + NIT groups were significantly higher (time*treatment P < 0.01 for all time points) than the PBO and NF-RBJ groups with these concentrations remaining unchanged throughout the 4-h testing period (Figure 12A). Saliva NOx concentrations were significantly higher in the RBJ group than the PBO and NF-RBJ groups at the 1-, 2-, and 4-h time points (all time points, time*treatment P < 0.001), whereas saliva NOx concentrations in the RBJ and PBO + NIT groups were significantly higher than both PBO and NF-RBJ groups only at the 1-h time point (time*treatment P < 0.001) (Figure 12B). Plasma NOx AUC was significantly higher in the RBJ group compared with the PBO group, and saliva NOx AUC was significantly higher in the RBJ group compared with the PBO and NF-RBJ groups (time*treatment P < 0.05, Figure 12C and D).
FIGURE 12.
Plasma NOx concentrations (A) and saliva NOx concentrations (B) after 4 wk PBO, RBJ, PBO + NIT, and NF-RBJ treatment ingestion at baseline (0 h) and 1, 2, and 4 h after consuming a high-fat meal, and postprandial (0–240 min) plasma NOx (C) and saliva NOx (D) AUC at the chronic visit. Data in A–C are presented as log-transformed least square means ± SEM, n = 15. Data in D–F are presented as untransformed mean ± SEM. Values in A–C were compared with the use of the PROC MIXED procedure, whereas AUC values in D–F were compared by use of the PROC GLM procedure with Tukey's multiple comparison test in SAS version 9.4. All time points were significantly different than baseline for plasma NOx concentrations in the RBJ and PBO + NIT groups. Time points annotated with symbols represent significant time*treatment interactions between treatment groups. #RBJ significantly different from PBO and NF-RBJ; *PBO + NIT significantly different from PBO and NF-RBJ; $PBO + NIT significantly different than NF-RBJ; all P < 0.01. Treatment groups annotated with different letters were significantly different from one another, P < 0.05. NOx, nitrate/nitrite; PBO, placebo; RBJ, red beetroot juice; PBO + NIT, PBO + nitrate; NF-RBJ, nitrate-free RBJ.
Chronic treatment effects on preprandial and postprandial PBMC gene expression
Preprandial and postprandial PBMC gene expression at the chronic test visit are presented in Supplemental Table 6. In the PBO group, there was a significant reduction in relative expression of TNF-α and GADD34 between 0 and 4-h time points at the chronic visit (P = 0.0357 and P = 0.058, respectively). In the RBJ group, there was a significant increase and decrease in relative expression of TLR-4 and GADD34, respectively, between 0 and 4-h time points at the chronic visit (P = 0.0037 and P = 0.0102, respectively). In the PBO + NIT group, there was a significant reduction in relative expression of GADD34 between 0 and 4-h time points (P = 0.0218). In the NF-RBJ group, there was a significant increase and decrease in relative expression of TLR-4 and GADD34, respectively, between the 0 and 4-h time points (P = 0.0311 and P = 0.0375). No other significant differences between 0 and 4-h time points within treatment groups were observed at the chronic visit. There were few significant differences in relative expression at the 4-h time point when compared with baseline, such as a 0.5-fold decrease of XBP1s in the PBO group (95% CI: 0.3, 0.8; P = 0.0105), a 1.9-fold increase of TLR-4 (95% CI: 1.5, 2.3; P = 0.004), and a 0.4-fold decrease of GADD34 (95% CI: 0.2, 0.6; P < 0.001) in the RBJ group, a 0.6-fold decrease of TNF-α in the PBO + NIT group (95% CI: 0.3, 0.9; P = 0.0391), and a 2.4-fold increase in TLR-4 (95% CI: 1.5, 3.3; P = 0.0285) in the NF-RBJ group.
Chronic treatment effects on preprandial endothelial cell protein expression
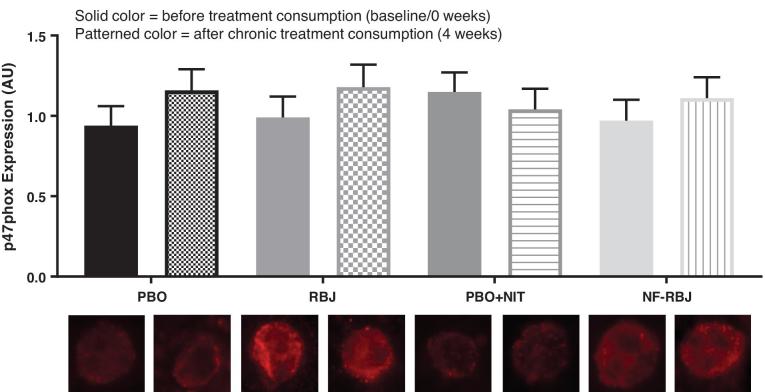
No significant differences were observed for endothelial cell NADPH oxidase/phox47 subunit protein expression within or between groups at 4 wk compared with 0 wk (Figure 13).
FIGURE 13.
Mean endothelial cell protein expression at baseline and after 4 weeks of PBO, RBJ, PBO+NIT, and NF-RBJ consumption. Data are normalized to human umbilical vein endothelial cell protein expression via immunofluorescence. Values represent least square mean AU ± SEM, n = 15. Values were compared with the use of the PROC MIXED procedure in SAS version 9.4. There were no significant main effects of time, treatment, or main interaction effect of time*treatment in the model. There were no significant differences in endothelial protein expression of p47phox within or between treatment groups at any time point. Abbreviations: AU, arbitrary units; PBO, placebo; RBJ, red beetroot juice; PBO+NIT, PBO+nitrate; NF-RBJ, nitrate-free-RBJ.
Discussion
To our knowledge, this is the first randomized controlled trial to investigate the acute and chronic effects of RBJ supplementation, including nitrate-dependent and -independent effects, on vascular endothelial function and other cardiometabolic responses to HFM consumption in middle-aged/older adults with overweight and obesity. We found that acute and chronic RBJ and PBO + NIT supplementation increased saliva and plasma NOx concentrations compared with PBO and NF-RBJ, but these increases were not paralleled by significant differences in postprandial vascular endothelial function across treatment groups. Postprandial vascular endothelial function was not altered by the PBO + NIT and NF-RBJ treatments, ruling out any sole contribution of inorganic nitrate or other bioactive compounds in RBJ on this outcome in the present study. There were no significant within-group declines in RHI following HFM and PBO consumption, suggesting that the HFM did not significantly impair postprandial vascular endothelial function even though it led to significant changes in several hemodynamic parameters, and in plasma insulin and triglyceride concentrations.
Although some previous research suggests that consumption of a HFM leads to exacerbated postprandial impairment of vascular endothelial function in individuals with overweight and obesity, this premise is not supported by our current findings, which are in agreement with others. Ayer et al. (12) did not observe significant within- or between-group differences in measures of vascular endothelial function including RHI, brachial artery flow-mediated dilation (FMD), or hyperemic forearm blood flow (FBF) at 1 and 3 h following consumption of a HFM (1000 kcal, 60 g fat) in young adults with obesity compared with a normal body weight. In a separate study by Raitakari et al. (47), a saturated fatty acid-rich HFM (1030 kcal, 61 g fat) was actually found to increase brachial artery basal diameter, FBF, and post-ischemic hyperemia with no change in FMD. Other studies with healthy adults have shown that transient impairment of FMD at 2, 3, and 4 h following HFM consumption were strongly associated with the magnitude of postprandial triglyceride concentrations, as well as leukocyte superoxide production (9, 10). In a dose-response study, Schwander et al. (48) observed significant increases in plasma triglyceride iAUC over a 6-h period in men with obesity following consumption of HFMs containing 1000 kcal (68 g fat) or 1500 kcal (102 g fat), and in serum IL-6 concentrations but only following consumption of the 1500 kcal HFM. They did not observe significant increases in these parameters following consumption of a 500 kcal HFM (34 g fat) in men with obesity, or following consumption of any of the meals in normal weight men. Vascular endothelial function was not assessed in that study; however, their data suggest that a higher caloric and fat challenge may be needed to induce postprandial inflammation, and thus likely oxidative stress and impairment of vascular endothelial function. Indeed, although we did observe a moderate increase in postprandial triglycerides, consumption of the HFM did not provoke postprandial hyperglycemia, inflammation, oxidative stress, or ER stress. As those processes are believed to be major contributors to postprandial impairment of vascular endothelial function, the lack of an effect on postprandial RHI may be explained by the neutral glycemic, proinflammatory, oxidative stress, and ER stress responses to the HFM in our study. Similarly, Berry et al. (49) showed that a stearic acid-rich HFM attenuated the postprandial impairment of vascular endothelial function, likely due to a blunted effect on postprandial triglyceride and subsequently oxidative stress responses when compared with an oleic acid-rich HFM. In general, the effect of a HFM on vascular endothelial function (and other cardiometabolic responses) is uncertain due to significant interindividual and group variability, as well as several other factors including the type, source, and amount of fats used.
Major discrepancies among studies in this area, including the present study, are the different techniques used to assess vascular endothelial function (e.g. EndoPat assessed RHI compared with ultrasound assessed FMD, FBF, etc.), as well as different time points chosen to assess cardiometabolic parameters and the duration of the postprandial testing period. We therefore cannot rule out the fact that the techniques chosen to measure vascular endothelial function, the times of performing measurements, or the postprandial testing period duration may be factors contributing to our findings. However, considering that adverse, neutral, and even positive effects on postprandial vascular endothelial function have been observed using all of the aforementioned techniques, the technique utilized for assessing vascular endothelial function does not appear to be a driver of the discrepant findings. Nonetheless, considering the discrepant findings among published studies in this area, robust human studies designed specifically to evaluate causes of interindividual and group variability in men and women are needed to better understand the impact of HFM consumption on CVD risk.
Several studies investigating the efficacy of RBJ, primarily as a rich source of inorganic nitrate, have shown promising but mixed results on measures of cardiovascular health (27, 50, 51). Inorganic nitrate supplementation may compensate for disrupted endothelium-dependent pathways for NO production and bioavailability that contribute to vascular endothelial dysfunction, hypertension, and other CVD risk factors (52). RBJ increases NO bioavailability via the enterosalivary nitrate-nitrite-NO endothelium-independent pathway which is complementary to the L-arginine-NO synthase endothelium-dependent pathway (53). Since transient impairment of vascular endothelial function has been observed following HFM consumption in several previous studies, we hypothesized that acute and chronic supplementation of RBJ would increase NO bioavailability, in part, through the enterosalivary nitrate-nitrite-NO pathway, and therefore increase postprandial RHI compared with preprandial RHI and PBO. After acute and chronic ingestion of RBJ or PBO + NIT in the present study, plasma and saliva NOx concentrations increased significantly, but were not paralleled by significant effects on postprandial RHI values. To our knowledge, only 1 previous study has evaluated the impact of acute RBJ consumption on postprandial vascular endothelial function following the consumption of a HFM (1122 kcal, 57 g fat) (23). Contrary to our study, Joris and Mensink showed attenuated impairment of postprandial FMD following the consumption of 140 mL of concentrated RBJ in men with overweight and obesity. That study differed from ours in that they measured brachial artery FMD compared with RHI, vascular endothelial function was assessed 2 h postprandially compared with 4 h, the study included only men, and the RBJ dose provided was double that provided in our study. It is possible the timing of endothelial function assessment could have influenced the present findings. Although impairment of endothelial function has been observed at 2- and 4-h post-HFM consumption (9, 10), the 4-h time point was chosen for our study due to the fact that postprandial triglycerides and maximal impairment of FMD have been shown to occur 3–4 h following HFM consumption (6). Nonetheless, impaired FMD has been observed 2 h following HFM consumption due to enhanced oxidative stress (9) and we therefore cannot rule out the possibility that RHI may have been impaired at the 2-h time point. Importantly, the neutral effect of RBJ on postprandial RHI in the present study may be due, in part, to the lack of a major effect of the HFM on RHI. Another possible contributor to our findings is the dose of RBJ provided. Most studies that have evaluated the impact of RBJ (and inorganic nitrate) on endothelial function or other measures of cardiometabolic health have used at least double that used in the present study (≥two 70 mL bottles). However, we chose to use a dose likely to be more realistic for the typical consumer and less of a burden. Nonetheless, studies using doses of inorganic nitrate above (>300 mg) and below (≤300 mg) the concentration provided in our study have shown beneficial effects or no effects. Therefore, dose may be a contributing factor but is likely not the only factor responsible for the lack of an observed effect. It is important to note that the cardiovascular effects of inorganic nitrate can vary due to individual differences in oral hygiene and the oral microbiota, including the abundance of nitrate-reducing oral bacteria (54). Exploration of the impact of our treatments on the oral microbiota is underway. Finally, a recent meta-analysis suggests that the effects of inorganic nitrate and RBJ supplementation on vascular endothelial function and blood pressure are reduced in older subjects, as well as those with overweight and obesity, and with an increased cardiometabolic risk in general (21, 27). Thus, the study population included in our investigation may be less responsive to RBJ and/or inorganic nitrate intake.
Several studies have investigated the acute vasodilatory effects of a single dose of RBJ (in the absence of a HFM), and have found increased FMD to be associated with increased circulating NOx concentrations (18, 19, 55, 56). There are many differences among those studies and the present study, including experimental design, volume of RBJ (i.e. 140–500 mL), RBJ type (i.e. concentrate compared with juice), dose of inorganic nitrate provided (i.e. 1.1–45 mmol/L), placebo used (e.g. equivalent volumes of water, potassium nitrate in water, NF-RBJ, and juice), and study population (i.e. healthy young lean men, hypertensive men, overweight and obese men, healthy men and women). It is possible that any potential for RBJ to increase RHI following acute consumption was negated by consumption of the HFM in the present study, or any of the aforementioned variables.
Hemodynamic parameters and AIx are reduced following meal consumption, potentially due to the vasodilatory effect of insulin or other direct effects on the vascular tissue (57, 58). As expected, insulin peaked 1 h postprandially which coincided with reductions in several hemodynamic parameters and AIx. Overall, there were no clear or consistent effects of any one treatment on hemodynamic parameters following acute or chronic consumption. Considering blood pressure values were relatively normal at baseline, we did not expect to see major changes in these values. In fact, these data suggest that consumption of RBJ or other nitrate-containing supplements (at the dose provided in this study) is safe for normotensive individuals to ingest without exerting major hypotensive effects. On the other hand, AIx is a measure of pulse wave reflection and arterial stiffness. A postprandial decrease in AIx is suggested to be a protective hemodynamic response reflective of lower systolic loading (59). We observed significant decreases in AIx 4 h post-HFM consumption in the RBJ and PBO + NIT groups compared with PBO following acute treatment exposure. It is possible that this represents reversible changes in arterial stiffness, presumably from smooth muscle relaxation (57). There were small but significant differences between RBJ and PBO 4 h after acute treatment ingestion suggesting that RBJ may exert mild effects on insulin sensitivity. After adjusting for heart rate, these effects were no longer significant. Heart rate has been shown to have a linear relation with AIx which may be related to alterations in timing of the reflected pressure wave (60). Thus, AIx is often standardized at 75 beats per minute; however, this has been argued to be a physiologically inappropriate approach as it may not apply to all populations (38) and thus both values should be considered.
Based on previous work (7, 61, 62), we expected a response to HFM consumption reflective of proinflammation, oxidative stress, and ER stress that would be detected in PBMCs. In line with the overall results of the present study, we did not observe substantial or consistent responses in PBMC gene expression to the HFM or treatments. Postprandial proinflammatory responses are believed to be caused in part by the absorption of lipopolysaccharide by enterocytes during triglyceride-rich chylomicron secretion, and circulating triglyceride and glucose concentrations following HFM consumption (63, 64). Given that the HFM did not provoke statistically significant impairment of RHI or robust metabolic derangements, these findings are not surprising. Baseline health status (e.g. composition of the gut microbiota, degree of insulin resistance) may be a contributing factor to variability in postprandial responses to a meal (65). RBJ also had no influence on endothelial cell NADPH oxidase protein expression, suggesting that RBJ did not reduce vascular superoxide production following chronic treatment consumption.
There are several strengths of the present study including the randomized, double-blind, placebo-controlled crossover design, the exploration of nitrate-dependent and -independent effects, and potential synergy of the bioactive compounds in RBJ, as well as the acute and chronic effects of treatment consumption on postprandial responses to a HFM. In addition, our study included an aging population of both men and postmenopausal women at risk of developing CVD. We used a dose of RBJ that would be practical for daily consumption, whereas most other research has used ≥140 mL (the equivalent of 2 doses of RBJ concentrate). Lastly, we utilized a HFM challenge representative of the typical meal composition consumed in the Western diet.
There are several limitations of the present study. Although inclusion of both men and women should be considered a strength, it is possible that there were sex differences in meal and treatment responses and our study was not powered to evaluate that. With respect to attrition, there was a high level of dropout (n = 8, 35% attrition); however, these individuals dropped out or were excluded within the first 4 wk of participating in the study due to nontreatment related issues such as an inability to place an intravenous catheter, allergy to topical antiseptic, etc. Although a high rate of loss to follow-up can lead to attrition bias, comparison of screening and baseline characteristics between those who completed the trial and those who were lost to follow-up suggests no major differences, and thus attrition bias is unlikely. The study population included a metabolically healthy overweight/obese study population (i.e. without the presence of hypertension, diabetes, etc.). The multiple exclusion criteria, which were warranted to minimize confounding variables, may have resulted in a sample of relatively metabolically healthy, middle-aged/older adults with overweight or obesity, whose metabolic flexibility minimized or preserved the effects of the HFM. The challenge meal used may have lacked adequate calories and fat to induce postprandial vascular endothelial dysfunction in this population. Previous research has adjusted the caloric and fat content of a HFM challenge to individual body weight, body surface area, or resting metabolic rate, whereas we chose to standardize the caloric and fat content of the meal to represent typical Western intake. In addition, the postprandial duration and timing of the measurements may not have been sufficient to capture responses to the meal and treatments. It is also possible that the volume of RBJ may not have provided a large enough dose of inorganic nitrate or other bioactive compounds to modulate the outcomes studied. Lastly, measurement of NOx does not distinguish between the amount of nitrate and nitrite, and thus any influence on nitrate reduction to nitrite cannot be determined. Future research studies are planned to examine differences in the oral and gut microbiota among the study participants in the present study and their relation with treatment responses and nonresponses.
In summary, this study confirms that the nitrate-nitrite-NO pathway is intact in healthy, middle-aged/older adults with overweight and obesity following acute and chronic consumption of concentrated RBJ. Although consumption of the HFM led to postprandial alterations in several cardiometabolic parameters, we were unable to detect HFM-induced impairment of vascular endothelial function. Acute nor chronic RBJ or other treatment supplementation did not consistently modulate postprandial cardiometabolic responses to a HFM. Additional research in this area is needed, particularly with respect to interindividual and group variability in response to HFM and RBJ consumption.
Supplementary Material
Acknowledgements
The authors’ contributions were as follows—NSL, CLM, TLW, and SAJ: designed the research; NSL, HJV, SCH, KAM, ARV, EKF, CLM, and SAJ: collected the data; KLH: provided medical oversight; NSL, HJV, SCH, KAM, ARV, and EKF: performed data entry; NSL, HJV, YW, and MJP: performed biological sample analyses; NSL and SR: performed statistical analysis; NSL, CLM, TLW, KLH, DRS, MJP, and SAJ: interpreted the data; NSL and SAJ: wrote the initial manuscript; all authors reviewed, edited, and commented on subsequent drafts of the manuscript; and all authors read and approved the final version of the manuscript. SAJ: had primary responsibility for final content.
Notes
Supported by the National Institute of Food and Agriculture, USDA, Hatch under 1009727 (SAJ, CLM, TLW). Also supported by a mini grant from the College of Health and Human Sciences at Colorado State University (SAJ). Blood biochemical analyses performed at the Colorado Clinical and Translational Sciences Institute (University of Colorado-Denver) was supported by NIH/National Center for Advancing Translational Sciences (NCATS) Colorado Clinical and Translational Science Awards (CTSA) Grant Number UL1 TR001082-04. The content is the authors’ sole responsibility and does not necessarily represent official NIH views. Funding entities had no role in the design, implementation, analysis, and/or interpretation of this study and its results.
Author disclosures: the authors report no conflicts of interest.
Supplemental Tables 1–6 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used: AIx, augmentation index; AIx@75, augmentation index corrected for heart rate at 75 beats per minute; aPWV, aortic pulse wave velocity; CVD, cardiovascular disease; ER, endoplasmic reticulum; FBF, forearm blood flow; FMD, flow-mediated dilation; F-RHI, Framingham-reactive hyperemia index; HFM, high-fat meal; NF-RBJ, nitrate-free red beetroot juice; NOx, nitrate/nitrite; PBMC, peripheral blood mononuclear cell; PBO, placebo; PBO + NIT, placebo plus potassium nitrate; RBJ, red beetroot juice; RHI, reactive hyperemia index.
References
- 1. Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part III: cellular and molecular clues to heart and arterial aging. Circulation 2003;107(3):490–7. [DOI] [PubMed] [Google Scholar]
- 2. Rossman MJ, LaRocca TJ, Martens CR, Seals DR. Healthy lifestyle-based approaches for successful vascular aging. J Appl Physiol (1985) 2018;125(6):1888–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seals DR, Kaplon RE, Gioscia-Ryan RA, LaRocca TJ. You're only as old as your arteries: translational strategies for preserving vascular endothelial function with aging. Physiology (Bethesda) 2014;29(4):250–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Botham KM, Wheeler-Jones CP. Postprandial lipoproteins and the molecular regulation of vascular homeostasis. Prog Lipid Res 2013;52(4):446–64. [DOI] [PubMed] [Google Scholar]
- 5. O'Keefe JH, Bell DS. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am J Cardiol 2007;100(5):899–904. [DOI] [PubMed] [Google Scholar]
- 6. de Koning EJ, Rabelink TJ. Endothelial function in the post-prandial state. Atheroscler Suppl 2002;3(1):11–6. [DOI] [PubMed] [Google Scholar]
- 7. Herieka M, Erridge C. High-fat meal induced postprandial inflammation. Mol Nutr Food Res 2014;58(1):136–46. [DOI] [PubMed] [Google Scholar]
- 8. Jonk AM, Houben AJ, Schaper NC, de Leeuw PW, Serne EH, Smulders YM, Stehouwer CD. Obesity is associated with impaired endothelial function in the postprandial state. Microvasc Res 2011;82(3):423–9. [DOI] [PubMed] [Google Scholar]
- 9. Bae JH, Bassenge E, Kim KB, Kim YN, Kim KS, Lee HJ, Moon KC, Lee MS, Park KY, Schwemmer M. Postprandial hypertriglyceridemia impairs endothelial function by enhanced oxidant stress. Atherosclerosis 2001;155(2):517–23. [DOI] [PubMed] [Google Scholar]
- 10. Marchesi S, Lupattelli G, Schillaci G, Pirro M, Siepi D, Roscini AR, Pasqualini L, Mannarino E. Impaired flow-mediated vasoactivity during post-prandial phase in young healthy men. Atherosclerosis 2000;153(2):397–402. [DOI] [PubMed] [Google Scholar]
- 11. Ceriello A, Genovese S.. Atherogenicity of postprandial hyperglycemia and lipotoxicity. Reviews in Endocrine and Metabolic Disorders 2016;17(1):111–6. [DOI] [PubMed] [Google Scholar]
- 12. Ayer JG, Harmer JA, Steinbeck K, Celermajer DS. Postprandial vascular reactivity in obese and normal weight young adults. Obesity (Silver Spring) 2010;18(5):945–51. [DOI] [PubMed] [Google Scholar]
- 13. Kanner J, Harel S, Granit R. Betalains–a new class of dietary cationized antioxidants. J Agric Food Chem 2001;49(11):5178–85. [DOI] [PubMed] [Google Scholar]
- 14. Kujawska M, Ignatowicz E, Murias M, Ewertowska M, Mikolajczyk K, Jodynis-Liebert J. Protective effect of red beetroot against carbon tetrachloride- and N-nitrosodiethylamine-induced oxidative stress in rats. J Agric Food Chem 2009;57(6):2570–5. [DOI] [PubMed] [Google Scholar]
- 15. Sakihama Y, Maeda M, Hashimoto M, Tahara S, Hashidoko Y. Beetroot betalain inhibits peroxynitrite-mediated tyrosine nitration and DNA strand cleavage. Free Radic Res 2012;46(1):93–9. [DOI] [PubMed] [Google Scholar]
- 16. Zielinska-Przyjemska M, Olejnik A, Dobrowolska-Zachwieja A, Grajek W. In vitro effects of beetroot juice and chips on oxidative metabolism and apoptosis in neutrophils from obese individuals. Phytother Res 2009;23(1):49–55. [DOI] [PubMed] [Google Scholar]
- 17. Martinez RM, Longhi-Balbinot DT, Zarpelon AC, Staurengo-Ferrari L, Baracat MM, Georgetti SR, Sassonia RC, Verri WA Jr., Casagrande R. Anti-inflammatory activity of betalain-rich dye of Beta vulgaris: effect on edema, leukocyte recruitment, superoxide anion and cytokine production. Arch Pharm Res 2015;38(4):494–504. [DOI] [PubMed] [Google Scholar]
- 18. Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N et al.. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 2008;51(3):784–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kapil V, Khambata RS, Robertson A, Caulfield MJ, Ahluwalia A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension 2015;65(2):320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hobbs DA, Goulding MG, Nguyen A, Malaver T, Walker CF, George TW, Methven L, Lovegrove JA. Acute ingestion of beetroot bread increases endothelium-independent vasodilation and lowers diastolic blood pressure in healthy men: a randomized controlled trial. J Nutr 2013;143(9):1399–405. [DOI] [PubMed] [Google Scholar]
- 21. Lara J, Ashor AW, Oggioni C, Ahluwalia A, Mathers JC, Siervo M. Effects of inorganic nitrate and beetroot supplementation on endothelial function: a systematic review and meta-analysis. Eur J Nutr 2016;55:451–9. [DOI] [PubMed] [Google Scholar]
- 22. Velmurugan S, Gan JM, Rathod KS, Khambata RS, Ghosh SM, Hartley A, Van Eijl S, Sagi-Kiss V, Chowdhury TA, Curtis M et al.. Dietary nitrate improves vascular function in patients with hypercholesterolemia: a randomized, double-blind, placebo-controlled study. Am J Clin Nutr 2016;103(1):25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Joris PJ, Mensink RP. Beetroot juice improves in overweight and slightly obese men postprandial endothelial function after consumption of a mixed meal. Atherosclerosis 2013;231(1):78–83. [DOI] [PubMed] [Google Scholar]
- 24. Blekkenhorst LC, Bondonno NP, Liu AH, Ward NC, Prince RL, Lewis JR, Devine A, Croft KD, Hodgson JM, Bondonno CP. Nitrate, the oral microbiome, and cardiovascular health: a systematic literature review of human and animal studies. Am J Clin Nutr 2018;107(4):504–22. [DOI] [PubMed] [Google Scholar]
- 25. Rathod KS, Velmurugan S, Ahluwalia A. A ‘green’ diet-based approach to cardiovascular health? Is inorganic nitrate the answer? Mol Nutr Food Res 2016;60(1):185–202. [DOI] [PubMed] [Google Scholar]
- 26. Clifford T, Howatson G, West DJ, Stevenson EJ. The potential benefits of red beetroot supplementation in health and disease. Nutrients 2015;7(4):2801–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bahadoran Z, Mirmiran P, Kabir A, Azizi F, Ghasemi A. The nitrate-independent blood pressure-lowering effect of beetroot juice: a systematic review and meta-analysis. Adv Nutr 2017;8(6):830–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lundberg JO, Gladwin MT, Weitzberg E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat Rev Drug Discov 2015;14(9):623–41. [DOI] [PubMed] [Google Scholar]
- 29. Lundberg JO, Gladwin MT, Ahluwalia A, Benjamin N, Bryan NS, Butler A, Cabrales P, Fago A, Feelisch M, Ford PC et al.. Nitrate and nitrite in biology, nutrition and therapeutics. Nat Chem Biol 2009;5(12):865–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wallace J, Johnson B, Padilla J, Mather K. Postprandial lipaemia, oxidative stress and endothelial function: a review. Int J Clin Pract 2010;64(3):389–403. [DOI] [PubMed] [Google Scholar]
- 31. Jackson KG, Armah CK, Minihane AM. Meal fatty acids and postprandial vascular reactivity. Biochem Soc Trans 2007;35(Pt 3):451–3. [DOI] [PubMed] [Google Scholar]
- 32. Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, Pumper GM, Lerman LO, Lerman A. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J 2010;31(9):1142–8. [DOI] [PubMed] [Google Scholar]
- 33. Bonetti PO, Pumper GM, Higano ST, Holmes DR Jr., Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol 2004;44(11):2137–41. [DOI] [PubMed] [Google Scholar]
- 34. McCrea CE, Skulas-Ray AC, Chow M, West SG. Test-retest reliability of pulse amplitude tonometry measures of vascular endothelial function: implications for clinical trial design. Vasc Med 2012;17(1):29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brant LC, Barreto SM, Passos VM, Ribeiro AL. Reproducibility of peripheral arterial tonometry for the assessment of endothelial function in adults. J Hypertens 2013;31(10):1984–90. [DOI] [PubMed] [Google Scholar]
- 36. Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, Mitchell GF, Sheffy J, Vita JA, Benjamin EJ. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation 2008;117(19):2467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol 2000;525 (Pt 1):263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stoner L, Faulkner J, Lowe A, Lambrick DM, Young JM, Love R, Rowlands DS. Should the augmentation index be normalized to heart rate? J Atheroscler Thromb 2014;21(1):11–6. [DOI] [PubMed] [Google Scholar]
- 39. Hwang MH, Yoo JK, Kim HK, Hwang CL, Mackay K, Hemstreet O, Nichols WW, Christou DD. Validity and reliability of aortic pulse wave velocity and augmentation index determined by the new cuff-based SphygmoCor Xcel. J Hum Hypertens 2014;28(8):475–81. [DOI] [PubMed] [Google Scholar]
- 40. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27(6):1487–95. [DOI] [PubMed] [Google Scholar]
- 41. Muller PY, Janovjak H, Miserez AR, Dobbie Z. Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques 2002;32(6):1372–4, 1376, 1378–9. [PubMed] [Google Scholar]
- 42. Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear NF kappa B, reduced I kappa B alpha, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell 2008;7(6):805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, Seals DR. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol 2009;297(1):H425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Silver AE, Christou DD, Donato AJ, Beske SD, Moreau KL, Magerko KA, Seals DR. Protein expression in vascular endothelial cells obtained from human peripheral arteries and veins. J Vasc Res 2010;47(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Obeid JS, McGraw CA, Minor BL, Conde JG, Pawluk R, Lin M, Wang J, Banks SR, Hemphill SA, Taylor R et al.. Procurement of shared data instruments for Research Electronic Data Capture (REDCap). J Biomed Inform 2013;46(2):259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Raitakari OT, Lai N, Griffiths K, McCredie R, Sullivan D, Celermajer DS. Enhanced peripheral vasodilation in humans after a fatty meal. J Am Coll Cardiol 2000;36(2):417–22. [DOI] [PubMed] [Google Scholar]
- 48. Schwander F, Kopf-Bolanz KA, Buri C, Portmann R, Egger L, Chollet M, McTernan PG, Piya MK, Gijs MA, Vionnet N et al.. A dose-response strategy reveals differences between normal-weight and obese men in their metabolic and inflammatory responses to a high-fat meal. J Nutr 2014;144(10):1517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Berry SE, Tucker S, Banerji R, Jiang B, Chowienczyk PJ, Charles SM, Sanders TA. Impaired postprandial endothelial function depends on the type of fat consumed by healthy men. J Nutr 2008;138(10):1910–4. [DOI] [PubMed] [Google Scholar]
- 50. Lara J, Ashor AW, Oggioni C, Ahluwalia A, Mathers JC, Siervo M. Effects of inorganic nitrate and beetroot supplementation on endothelial function: a systematic review and meta-analysis. Eur J Nutr 2016;55(2):451–9. [DOI] [PubMed] [Google Scholar]
- 51. Bonilla Ocampo DA, Paipilla AF, Marin E, Vargas-Molina S, Petro JL, Perez-Idarraga A. Dietary nitrate from beetroot juice for hypertension: a systematic review. Biomolecules 2018;8(4):E134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Munzel T, Daiber A. Inorganic nitrite and nitrate in cardiovascular therapy: a better alternative to organic nitrates as nitric oxide donors? Vascul Pharmacol 2018;102:1–10. [DOI] [PubMed] [Google Scholar]
- 53. Lidder S, Webb AJ. Vascular effects of dietary nitrate (as found in green leafy vegetables and beetroot) via the nitrate‐nitrite‐nitric oxide pathway. Br J Clin Pharmacol 2013;75(3):677–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vanhatalo A, Blackwell JR, L'Heureux JE, Williams DW, Smith A, van der Giezen M, Winyard PG, Kelly J, Jones AM. Nitrate-responsive oral microbiome modulates nitric oxide homeostasis and blood pressure in humans. Free Radic Biol Med 2018;124:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kapil V, Milsom AB, Okorie M, Maleki-Toyserkani S, Akram F, Rehman F, Arghandawi S, Pearl V, Benjamin N, Loukogeorgakis S et al.. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension 2010;56(2):274–81. [DOI] [PubMed] [Google Scholar]
- 56. Kenjale AA, Ham KL, Stabler T, Robbins JL, Johnson JL, Vanbruggen M, Privette G, Yim E, Kraus WE, Allen JD. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. J Appl Physiol (1985) 2011;110(6):1582–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ahuja KD, Robertson IK, Ball MJ. Acute effects of food on postprandial blood pressure and measures of arterial stiffness in healthy humans. Am J Clin Nutr 2009;90(2):298–303. [DOI] [PubMed] [Google Scholar]
- 58. Greenfield JR, Samaras K, Chisholm DJ, Campbell LV. Effect of postprandial insulinemia and insulin resistance on measurement of arterial stiffness (augmentation index). Int J Cardiol 2007;114(1):50–6. [DOI] [PubMed] [Google Scholar]
- 59. Phillips LK, Peake JM, Zhang X, Hickman IJ, Kolade O, Sacre JW, Huang BE, Simpson P, Li SH, Whitehead JP et al.. The effect of a high-fat meal on postprandial arterial stiffness in men with obesity and type 2 diabetes. J Clin Endocrinol Metab 2010;95(9):4455–9. [DOI] [PubMed] [Google Scholar]
- 60. Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol 2000;525(1):263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sies H, Stahl W, Sevanian A. Nutritional, dietary and postprandial oxidative stress. J Nutr 2005;135(5):969–72. [DOI] [PubMed] [Google Scholar]
- 62. Rangel-Zuniga OA, Haro C, Perez-Martinez P, Delgado-Lista J, Marin C, Quintana-Navarro GM, Tinahones FJ, Malagon MM, Lopez-Segura F, Lopez-Miranda J et al.. Effect of frying oils on the postprandial endoplasmic reticulum stress in obese people. Mol Nutr Food Res 2014;58(11):2239–42. [DOI] [PubMed] [Google Scholar]
- 63. Vors C, Pineau G, Drai J, Meugnier E, Pesenti S, Laville M, Laugerette F, Malpuech-Brugere C, Vidal H, Michalski MC. Postprandial endotoxemia linked with chylomicrons and lipopolysaccharides handling in obese versus lean men: a lipid dose-effect trial. J Clin Endocrinol Metab 2015;100(9):3427–35. [DOI] [PubMed] [Google Scholar]
- 64. Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation 2002;106(16):2067–72. [DOI] [PubMed] [Google Scholar]
- 65. Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan M. Personalized nutrition by prediction of glycemic responses. Cell 2015;163(5):1079–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.