Abstract
The market of probiotics is growing dynamically for the food and supplements, which provides better health to an individual. Probiotics are used as dietary management for diseases, but it varies between regions and persons. Systems biology can help in resolving the strain specificity of probiotics by studying their genome level organization. In this review, we have compiled facets of systems biology and next-generation omics methods such as metagenomics, proteomics and metabolomics. These tools are crucial for the optimization of the metabolic processes in probiotics and hence, their use for human health. The limitations and challenges associated with the development of probiotics involve their stability and function in different individuals. Systems biology facilitates emerging metabolic engineering approaches to improve probiotics strain for their broader application. This review provides comprehensive and updated knowledge of engineered probiotics as therapeutics and various challenges in the development of engineered probiotics.
Keywords: Probiotics, Systems biology, Proteomics, Metabolomics, Metagenomics
Introduction
The human gastrointestinal tract (GIT) addresses a robust microbial ecosystem known as gut microbiota. Maintenance of human health is moderately dependent on the intestinal ecosystem and changes in this might contribute towards the advancement of disease-causing microorganisms (Terpou et al. 2019). Systems biology is an advanced approach to study the cellular activity of the cells. This approach explores the gut microbiota and their genomes having biotherapeutic targets of disease control (Yadav et al. 2018a, b). Probiotic bacteria act as live biotherapeutic product which can be used in elevating individual’s health as well as reduce the risk of other metabolic disorders. According to Hill et al. 2014, probiotics are “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” (Hill et al. 2014). This also modulates antibody or cell-mediated response against the pathogenic bacteria (Singh et al. 2019). Lactobacillus, Bifidobacteria, Weissella spp., Escherichia coli Strain Nissle 1917, Saccharomyces boulardi and Bacillus spp. have been identified with the help of these tools as potential candidates for biotherapeutic interventions (Kota et al. 2018; Gronbach et al. 2010). Food and supplement industries are stressed to provide health beneficial product. They need to develop functionally superior and nutritional products. Probiotics, prebiotics and synbiotics (a combination of probiotics and prebiotics) comprise a huge segment of the functional food market (Khangwal and Shukla 2019a). Multiple omics studies are providing a better platform for optimization of probiotic strains, which are beneficial for food and supplement industries in terms of specificity, accuracy, and sensitivity (Isaac et al. 2019). A potential probiotic is selected on the basis of bile salt hydrolase action, acid forbearance, cell facade hydrophobicity, auto-aggregation, co-aggregation, antimicrobial activity and their production depends on carbon source uptake (Kumar et al. 2019; Chlebowska-Śmigiel et al. 2019). Apart from all these factors time, temperature and pH are also essential for the growth and selection of probiotics. The most favorable temperature for their growth ranges 28–32 °C. The most familiar genera used as probiotics for human consumption are Lactobacillus and Bifidobacterium (Khangwal and Shukla 2019b). Probiotic bacteria are used in the production of food products as they play a crucial role in subsidizing ailments related to diseases such as inflammatory bowel disease (IBD), infectious diseases, allergies, etc. (Sireswar et al. 2019). These types of foods are rich in nutrition with live beneficial microbes, which shows high antioxidant activity, hydrophobicity, enzyme production for nutrients availability (Menezes et al. 2019). In 1995, Gibson and Roberfroid recommended the use of non-digestible food components that can stimulate the growth of gut microbiota know as prebiotics. Later they modified the definition “A prebiotic is a selectively fermented component which permits specific transformations in activity and/or composition in the gastrointestinal microflora and provides health profit to host” (Gibson and Roberfroid 1995). The field of nutrition supplementation and probiotics production is revolutionized by microbial engineering (Jin Song et al. 2019). In this revolutionary time, systems biology plays a great role in winding the enormous data provided by different omics experiments such as proteomics, metabolomics, transcriptomics and genomics with the help of various mathematical and computational tools for microbial engineering and their use in probiotics.
The term probiogenomics define insight into the variety and development of probiotic bacteria. This tool is helpful in revealing the molecular base for their health improving effects. These studies are essential to understand the properties of cellular metabolite, interlink metabolite, and their production as one integrated system rather than using information about the relationship between different molecules (Ventura et al. 2009). Advancement of systems biology provides knowledge about various biomarkers to diagnose and in the treatment of various diseases or metabolic disorders. For example, gene expression profiling is an influential diagnosis method to predict HER cancer in a juvenile patient, and it is found to be more precise in contrast to typical histological tests (Yadav et al. 2018a, b; Van De Vijver et al. 2002). The human gut includes a consortium of the various microorganisms (especially bacteria), which are unique to every person and change with age, diet, drug input, and medical or surgical interference. Gut microbiota is different as analyzed from upper GIT (gastrointestinal tract) to lower GIT (Bäckhed et al. 2005). Lower GIT (ileum and colon) is more crowded as most of the nutrient absorption takes place here only. This microbiota is involved in nutrient absorption and detoxification of xenobiotic compounds. They provide immunity against various pathological microorganisms and perform various enzymatic reactions. These reactions involve enzymes that are not coded by the human genome but are vital for human development (He et al. 2013). For example, in CVD (Cardiovascular disease) conditions; the continuous use of engineered probiotics can reduce the plaque formation of infectious agents because microbes have direct associations with atherosclerosis and infectious agents. Previously, the first-line treatment for CVD prevention is statins; their special effects on gut microbiota functions and composition are yet unidentified. To resolve these circumstances, genome analysis is necessary and the direct procedure of genome analysis is known as metagenomics (Chiu and Miller 2019). The metagenomic analysis of patient’s fecal microbiome of symptomatic atherosclerosis with healthy controls demonstrates the functional and compositional alterations in the gut metagenome. There was enrichment of genes encoding peptidoglycan synthesis, depletion in phytoene dehydrogenase and decreased production of β-carotene in serum. This study revealed the association of microbiota with healthy and diseased person. A diseased person had enriched Collinsella genus while the healthy persons had proliferating Roseburia and Eubacterium genus (Karlsson et al. 2012). It is anti-inflammatory, and also augmented the levels of genes for the combination of peptidoglycan, which is proinflammatory, in the gut microbiome of atherosclerosis patients. The active depiction of the ecosystem and the classification of bacteria, which is lively in human diseases, can be studied by the shotgun metagenome technique (Korem et al. 2015). The metagenomics focussed on whole genome shotgun sequencing, which provides the knowledge about the community of microbes, capabilities of microbes, data overlapped by metagenomics and meta-transcriptomics is about 41% (Franzosa et al. 2014). The use of systems biology to study the gut microbiota has many challenges such as wide variety and specificity to every individual complicates the use of systems biology approaches. It is tough to conclude the normal microbiota in the diverse individuals as microbiota is specified to an individual (Ehrlich 2010). This review is emphasized on recent omics approaches used for improvement and optimization of health beneficial probiotics.
Metagenomics
This technique allows the analysis of genetic structure, identification of genetic changes by whole genome sequencing and also helps in the characterization of microbiota establishment process in new borns (De Filippo et al. 2010). This in silico study provides information to understand the adaptation of bacterial genomes in its ecological niche, which is in the gut. The limitation of this study is not able to supply information about taxa phylogeny and variety. To resolve this problem, a comparative genomic hybridization (CGH) approach is used to establish phylogenetic relationships among the reference genome and new isolates. The recent studies depict metagenomic approaches to determine compositions of supplements with the help of next generation sequencing. These analyses are based on rRNA-associated sequences (Lugli et al. 2019). This technique is culture free and sequence based for the whole microbial genome and it comprises a set of metagenomes which assist in the characterization of functional and compositional distinctiveness (Jovel et al. 2016). The initiative facet of this tool is that they do not need sequencing or cloning of meticulous genes, but main drawback of this tool is not able to determine the key microbial actors contributing to gut metabolic functions (Mayers et al. 2017). A metagenomics approach depends on the recovery and sequencing of microbial mRNAs of the gut (Turroni et al. 2016). This study results in the appearance of meticulous genes of the population. Moreover, structural data can be created to identify the special utility of genes (Graham et al. 2016). This analysis is also beneficial for decision of the ideal treatments for diverse disorders. For instance, the study on breast cancer patients is performed by compressive shotgun metagenomic analysis to prove that the density of microbes amplifies steadily in cancer pateints in contrast to normal individuals. Metagenomics also discovered the significance of green tea polyphenols (GTP) on human intestinal microbiota, and this demonstrated a considerable reduction in the Firmicutes/Bacteroidetes and also help in the enhancement of gut dysbiosis provoked by fatness (Zhang 2018). Moreover, this is also good to know about the biosynthesis of secondary metabolites, upregulation of pathways involved in the metabolism of amino acids with downregulation of pathways related to the pathogenic bacteria virulence and its cell signaling. This approach is helpful in improving the health of humans and animals through the regulation of gut microbiota (Xu et al. 2019). The cost of metagenomics analysis is around 100 USD.
Proteomics
Proteomics helps out to understand the biological functioning of a cell; it provides us a brief knowledge of protein expression and the function of post-translational modifications. Proteomics of LAB offers the proteome map of bacteria, which confers the general idea of protein content for bacteria. This will help to understand the adaptation of microbial gut under harsh conditions such as low pH and presence of bile acids, and to study the localization of proteins on the surface or inside the cell (Vinusha et al. 2018). Initially, proteomics studies are dependent on one-dimensional (1D) electrophoresis for generating the protein fingerprints, and the drawback of this technique is not able to recognize the individual proteins (Aires and Butel 2011). After that, the two-dimensional (2D) gel electrophoresis-based proteomic technology is used; in this, according to isoelectric point proteins are separated. Proteins mapping on the 2-D gel is the initiation of proteome studies, and this will help in furthermore studies of proteomics. General 2-D proteome mapping is carried out for the Lactobacillus casei Zhang, L. reuteri, L. rhamnosus GG, L. plantarum 299 V strains of probiotics (Wu et al. 2009; Lee et al. 2008; Hamon et al. 2011). The single strain interactions in co-culture have been depicted by proteomics and also describe changes in surface associated proteins. Proteome study of Lactobacillus casei Zhang to depict low pH response can be done by DIGE (differential in gel electrophoresis). DIGE is used to study organic acidemias and mainly methylmalonic acidemia (MMA) (Imperlini et al. 2016). This technique also allocates to detect differences of protein abundance in probiotic strain Lactobacillus rhamnosus GG grown in industrial or laboratory type media at variable stages of the growth phase. On the next level of proteomics study, the procedures used are gel-free, or mass profiling such as separation on liquid chromatography (LC) and identification of protein is done using mass spectrometry (MS) or by matrix-assisted laser desorption/ionization-time-of-flight (MALDI-TOF) (Choi et al. 2019). Proteome map of probiotic bacteria facilitates the study of their stress responses, stress tolerance, strains comparision and growth phases. This is also used for the differentiation and characterization between congenital disorders of glycosylation (CDG) using surface enhanced laser desorption/ionization (SELDI-TOF–MS) (Mills et al. 2006). We can also use a Label-free shotgun proteomic approach, which is beneficial to detect quantitative changes in the proteome with different temperature variations and availability of oxygen. After using different methods of proteomics, we can evaluate with in silico data of proteome. The in silico tools such as SurfG tool used to group the identified protein according to the membrane (MEM), potentially surface-exposed (PSE), cytoplasmic (CYT) and secreted (SEC) protein. Further, we can use KEGG enrichment analysis of expressed proteins (Marques Da Silva et al. 2019). The cost of proteomic analysis is approximately 60–90 USD.
Metabolomics
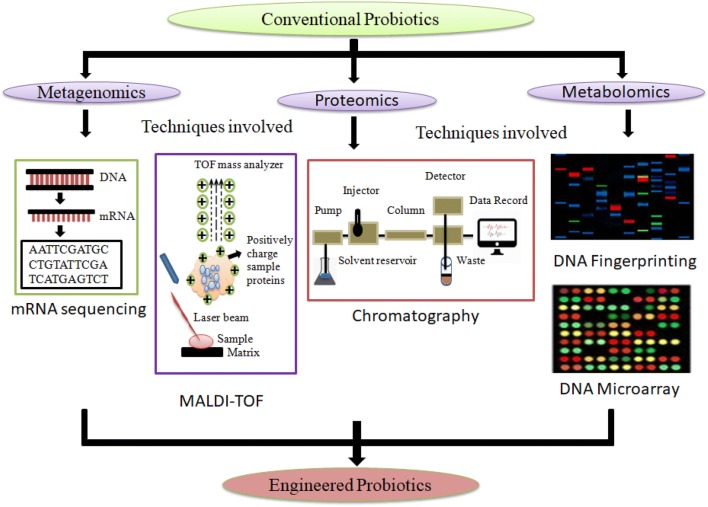
Metabolomics helps us in determining different metabolic changes in host depending upon metabolic engineering of probiotics and also reveal about rate limiting reactions for the production of useful chemicals (Ohtake et al. 2017). Metabolomics is also known as metabolic profiling, which deals with the quantitative analysis and determines the molecules of different molecular mass. This technique is very crucial to study the levels of metabolites under meticulous conditions and also had been used for the optimization of strains (Khangwal and Shukla, 2019c). The development of mass spectrometry (MS) and nuclear magnetic resonance (NMR) facilitates in the field of metabolomics successfully function in vast areas of food science (Kumar et al. 2018). This also represents the pathological or physiological status of an organism, which is helpful in the study of toxicology, molecular pathology, and disease diagnosis. Metaomics analysis of gut microbiota helps us to disclose the targets of probiotic actions. The potential of metabolomics was studied in gnotobiotic mice. The mice were colonized with a 15-model species of human gut microbiota, which includes Bacteroides uniformis, B. ovatus, B. thetaiotaomicron, B. caccae, B. WH2, B. vulgatus, Clostridium scindens, C. spiroforme, Dorea longicatena, Collinsella aerofaciens, Eubacterium rectal, Parabacteroides distasonis, Faecalibacterium prausnitzii, Ruminococcus obeum, and R. torques. Following the introduction of fermented milk product, there were no considerable changes observed in metagenome but, metatranscriptomics of fecal samples indicated the consequences of milk product on the expression of microbial enzymes which were involved in the metabolism of carbohydrates (McNulty et al. 2011). Among all the omics studies, a fluxomic analysis provides the closest description of a cell and it is widely used for developing various strains (Kambouris et al. 2018). Using all these approaches, plenty of knowledge has been extracted, such as the sequencing of 16S rDNA clones, DNA microarrays, fingerprinting techniques of 16S rDNA amplicons, high-throughput sequencing and fluorescent in situ hybridization. Less number of studies has been conducted on metabolomic analysis for stress-induced responses of bacteria and thus integrated approaches are used particularly in probiotic bacterial systems (Zhu et al. 2015). The cost of metabolomic analysis per sample is approximately 150–280 USD and differs according to UC-based and non UC-based clients. Insights of probiotic engineering techniques from various omics tools are provided in Fig. 1.
Fig. 1.
A snapshot of probiotic engineering by means of various omics tools
Role of engineered probiotics in disease
When probiotic bacteria are modified by traditional, genetic engineering or with the help of computional biology to produce novel varities is known engineered probiotics. The bioengineered microorganism is first and foremost aimed to augment nutrient utilization, lessen the enteric methanogenesis, present defense against harmful microbes, and restrain infectious agents (Singh et al. 2019). Appliance of computational engineering, synthetic biology, rDNA technology have presented colossal possibilities to employ microorganisms for producing a range of therapeutics and nutraceuticals (Sola-Oladokun et al. 2017; Yadav and Shukla 2019). These are used in the treatment of various human diseases; they can be immunomodulatory, metabolic or related to normal gut microbiota distortion. Pediatric intestinal diseases include different clinical conditions like acute diarrhea, Helicobacter pylori infection, necrotizing enterocolitis (NEC), inflammatory bowel diseases (IBD), Antibiotic-associated Diarrhea (AAD) cystic fibrosis, etc. The first commercialized genetically modified probiotic is Zbiotics.
Infectious disease
Lactobacillus rhamnosus HN001 is used for inflammatory disease treatment because of its probiotics action, but the specific mode of action of this probiotic is not yet fully resolved. Recent reports show their mode of action in which the microbial DNA receptor known as toll-like receptor-9 (TLR-9) is activated by L. rhamnosus HN001, which acts as a therapeutic target. Therefore L. rhamnosus HN001 can attenuate NEC through microbial DNA in in vitro studies, and the activation of this type of protection is advantageous as they don’t have any toxic effect (Plaza-Diaz et al. 2019; Copeland et al. 2009). LAB is also helpful in the treatment of inflammatory bowel diseases using different modes of action. They can treat IBD by modulation of intestinal microbiota, as reported by Madsen et al. for curing colitis in IL-10 knockout mice (Madsen et al. 2000). Other modes of action include the improvement in intestinal barrier functions and the diminution of oxidative stress (LeBlanc et al. 2017). Probiotic bacterium Lactobacillus reuteri was studied to prevent NEC. It controls the immune response and inhibiting the enteric infection. The bacterium is producing an effective antimicrobial compound, which helps to inhibit the growth of harmful microorganisms. It also modulates tumor necrosis factor alpha (TNF-α) synthesized from activated monocytoid cells of bacterial lipopolysaccharides. The strains of L. reuteri derived from a human, which help in reducing the lipopolysaccharides induced-inflammation in epithelial cells of the small intestine are ATCC PTA4659, ATCC PTA 6475, DSM17938 (Yadav et al. 2018b).
Peptic ulcers
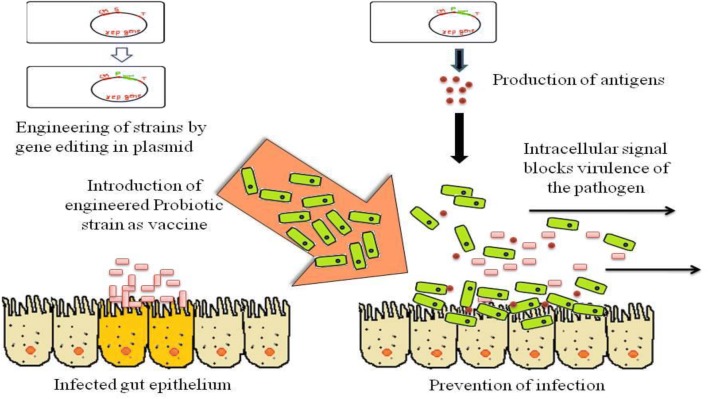
The probiotic bacteria used in the treatment of peptic ulcer and gastritis are L. acidophilus, L. rhamnosus, L. casei, and L. bulgaricus (Ghoshal et al. 2018). A vaccine against H. pylori has been developed by engineering lpp20 gene from H. pylori, and the construct elicits immune response during in vivo testing by delivery of antigens (Zhang et al. 2016). Helicobacter pylori cause the pathogenesis of peptic ulcer and gastritis. The pictorial representation for the treatment of peptic ulcers with the help of engineered probiotics is given in Fig. 2.
Fig. 2.
The schematic diagram showing possible use of engineered probiotics in the gut epithelium
Hypertension
A recent observation shows that hypertension is becoming a significant health issue, and it is a critical requirement in the present scenario to deal with this health problem. It has been reported that angiotensin-converting enzyme inhibitory peptides (ACEIPs) helps in the relaxation of blood vessels and diminishes the water reabsorption by kidneys. Hence it results in lowering the blood pressure, and it can be used as a possible alternative to cure hypertension. Lactobacillus plantarum NC8 strain is modified to deliver ACEIPs enzyme. Its coding sequence from yellowfin sole frame protein (YFP) and tuna frame protein (TFP) connected via arginine linker was observed for the effective reduction of systolic blood pressure in hypertensive rats (Aguilar-Toalá et al. 2018).
Diabetes mellitus
Diabetes mellitus type 1 (DM1) is the type of inherited disease. In this disease, autoreactive T cells destroy the pancreatic B cells and lead to lower the production of insulin. In this condition, the patient depends on the insulin injection. A successful observation is done by fusion protein HSP65-6P277 expression by engineered Lactococcus lactis NZ9000, which obstructs the DM1 in non-obese diabetic (NOD) mice with better tolerance of glucose and reduced insulitis (Ma et al. 2014). Engineered probiotic bacteria also help in managing the DM2 (Diabetes mellitus type 2). An incretin-derived hormone, glucagon-like peptide-1 (GLP-1), assists in the improvement of pancreatic functions near its short half-life. The therapeutic application can be attained by engineering commensal bacteria like Bifidobacterium longum to express and secrete GLP1 directly into the colon, which is biologically active in the form of the penetratin-GLP-1 fusion protein (Wei et al. 2015; Chua et al. 2017). Genetically modified lactic acid bacteria are designed for the delivery of therapeutic heterologous proteins.
Allergy
House dust mites (HDM) are asthmatic and allergic. The patients have a positive reaction towards the Dermatophagoides pteronyssinusords Der p2 (Thomas et al. 2002; Chua et al. 2017). When recombinant Lactobacillus lactis is fed to the BALB/c mouse, it overexpresses the Der p2 (DM) and recognized by a polyclonal anti-Der p2 antibody (Ai et al. 2014). The prevention of inflammation in Der p2-sensitized mice is done by reducing the production of IgE antibodies and reactivity of T-cells upon HDM exposure. It is observed that the genetically modified L. lactis can reduce approximately 50% of IgE reactivity along with up-regulating the IgG, which diminishes the interleukin-4 (IL-4) level in the spleen (Ai et al. 2015). In the case of Der p2-derived, peptides (DPs) over-expresses the T cell epitopes of Der p2 in L. lactis.
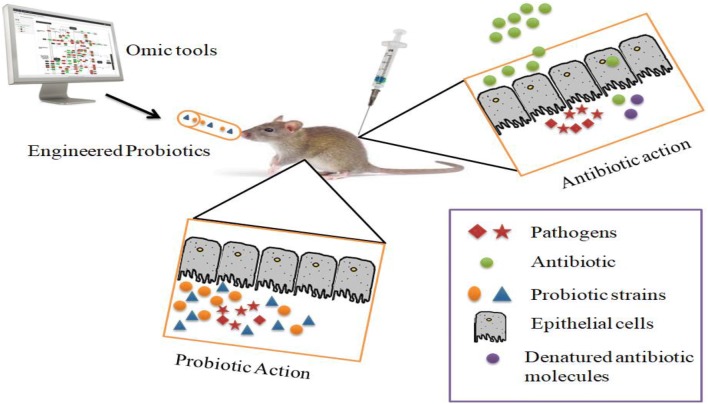
In comparison to DM, DPs were not eliminated IgE-binding capacity, but they can reduce the T cell reactivity and successfully hinder the Der p2-induced allergic responses in Der p2-sensitized mice. An engineered food grade L. lactis can be programmed to produce IL-10, which is therapeutically effective when intragastrically administrated. It means that the anti-inflammatory cytokines will be delivered to a specific organ without any trouble (Ai et al. 2016). When antibiotics are directly administered or injected; antibiotic molecules denatured or show less activity at the targeted site while engineered probiotics have target specificity and reach the target site and produce therapeutic molecules in the response of infection, as revealed in Fig. 3.
Fig. 3.
A proposed snapshot of probiotics based drug delivery
Cancer
Cancer biology has significantly benefited from systems biology approaches, especially in identifying markers for specific cancers and using these to forecast treatment stratagems for individual patients (Peñalver Bernabé et al. 2018). Colorectal cancer (CRC) is the most common cancer worldwide and chemotherapy is the universal treatment for cancer, but this leads to side effects. According to in vivo study, Lactobacillus paracasei subsp. paracasei NTU 101, in combination with chemotherapy on CRC, is effective in overcoming the chemotherapeutic side effects. This shows considerable changes in tumor growth and metastasis in comparison to chemotherapy individually. NTU 101FM is also able to control oxidative stress in the tumor, intestine and pro-inflammatory cytokines (Chang et al. 2019).
The ability of colonization in solid tumors is shown by Bifidobacterium longum, and it provides a robust immune response. Engineered B. longum can express tumstatin to proliferate in the hypoxic regions and produce angiostatins. They hinder the proliferation and stimulate apoptosis in tumors (Wei et al. 2016). The rate of the endurance of the engineered B. longum treated mice is increased in comparison to the control and shows a significant decrease in tumor metastasis. According to recent studies, it is pragmatic that successfully engineered B. longum expresses the herpes simplex virus thymidine kinase (HSV-TK). When HSV-TK is used with ganciclovir (GCV), it helps in suicide gene therapies (Zhou et al. 2016). Engineered Salmonella spp. has anti-cancer therapeutic properties. Engineered attenuated Salmonella is multifaceted, and it is used as a drug delivery vehicle. The cytokines produced as interferon-gamma (IFN-g) is helpful in destroying tumor cells and also boost immune response besides tumor cell destruction (Zheng et al. 2017). Engineered Salmonella spp. can also be a used as tumor detection tool because it’s able to detect tumors having a poor prognosis, like gliomas and neuroblastomas (Guo et al. 2015; Chen et al. 2015). Some other engineered probiotics with their significance in therapeutics are enlisted in Table 1. According to present studies, we can say that the engineering approaches applied in probiotics have a good impact on human health.
Table 1.
Engineered probiotics and their significance in therapeutics
| Engineered probiotics | Disease | Models | Significances | References |
|---|---|---|---|---|
| Lactobacillus plantarum | Diarrhea | Piglets | Produce porcine lactoferrin (pLF) which used as feed additive | Xu et al. (2016) |
| Lactobacillus plantarum | Hyperoxaluria | Rats | Express oxalate-degrading gene (OxdC) for recurring calcium oxalate (CaOx) stone disease | Paul et al. (2018) |
| Lactobacillus casei | Diarrhea | Hela cell lines | Produce high amount of bioactive compounds including conjugated linoleic acid (CLA) which inhibits enteric bacterial pathogens and also upgrade host immune system | Tabashsum et al. (2018) |
| Lactobacillus gasseri | Type I diabetes | Rats | Increase insulin concentration as well as rise glucose tolerance than control | Duan et al. (2015) |
| Lactococcus lactis | Inflammatory bowel disease | Mice, Murine |
Expression of anti-inflammatory cytokines (IL-10) |
Del Carmen et al. (2014), Martín et al. (2014) |
| Lactobacillus plantarum | Hypertension | Rats |
Decreased systolic blood pressure, endothelin and angiotensin II in plasma, kidney and heart |
Yang et al. (2015) |
| Lactococcus lactis + Lactoferrin | Sepsis | Animal models and humans | Produce recombinant bovine lactoferrin on the basis of green fluorescent protein fused expression system | Shigemori et al. (2017) |
Conclusion and future perspectives
Systems biology is an emerging approach having the colossal potential for advance studies of probiotic bacteria. This plays an essential role in the diagnosis and treatment of various types of human diseases and disorders. This study is beneficial for the production of effective and efficient biomedical products. Systems biology creates a new era of designed probiotics having therapeutic applications to enhance the immune system and metabolism. It is also necessary to authenticate predictive models on significant data, but current validation methods rely on simulated data to verify the precision of their methods. Simulation of the data is the initial approach for method optimization; this rarely resembles with the reality. Moreover, in the future perspective view, the concept of probiotics evolves parallel with the knowledge on functional gut microbiome, a research area that has been developed enormously with the use of metagenomics, proteomics metabolomics and other omic tools. All these tools and techniques are necessary to know about the metabolic and biochemical network studies of an industrially important strain.
Acknowledgements
The authors acknowledge Maharshi Dayanand University, Rohtak, India for providing infrastructure facility. PS acknowledges the infrastructural support from Department of Science and Technology, New Delhi, Govt. of India, through FIST grants (Grant No. 1196 SR/FST/LS-I/2017/4) and Department of Biotechnology, Government of India (Grant no. BT/PR27437/BCE/8/1433/2018).
Author contribution
Both authors have equal contribution.
Compliance with ethical standards
Conflict of interest
The authors don’t have any conflict of interest.
Ethical statement
This review article does not contain any studies with human participants or animals performed by any of the authors.
References
- Aguilar-Toalá JE, Garcia-Varela R, Garcia HS, Mata-Haro V, González-Córdova AF, Vallejo-Cordoba B. Postbiotics: an evolving term within the functional foods field. Trends Food Sci Technol. 2018;75:105–114. doi: 10.1016/j.tifs.2018.03.009. [DOI] [Google Scholar]
- Ai C, Zhang Q, Ren C, Wang G, Liu X, Tian F, et al. Genetically engineered Lactococcus lactis protect against house dust mite allergy in a BALB/c mouse model. PLoS One. 2014;9:109461. doi: 10.1371/journal.pone.0109461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai C, Zhang Q, Ding J, Ren C, Wang G, Liu X. Suppression of dust mite allergy by mucosal delivery of a hypoallergenic derivative in a mouse model. Appl Microbiol Biotechnol. 2015;99:4309–4319. doi: 10.1007/s00253-015-6407-6. [DOI] [PubMed] [Google Scholar]
- Ai C, Zhang Q, Ding J, Wang G, Liu X, Tian F. Mucosal delivery of allergen peptides expressed by Lactococcus lactis inhibit allergic responses in a BALB/c mouse model. Appl Microbiol Biotechnol. 2016;100:1915–1924. doi: 10.1007/s00253-015-7187-8. [DOI] [PubMed] [Google Scholar]
- Aires J, Butel MJ. Proteomics, human gut microbiota and probiotics. Expert Rev Proteom. 2011;8:279–288. doi: 10.1586/epr.11.5. [DOI] [PubMed] [Google Scholar]
- Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- Chang CY, Ho BY, Pan TM. Lactobacillus paracasei subsp. paracasei NTU 101-fermented skim milk as an adjuvant to uracil-tegafur reduces tumor growth and improves chemotherapy side effects in an orthotopic mouse model of colorectal cancer. J Funct Foods. 2019;55:36–47. doi: 10.1016/j.jff.2019.02.025. [DOI] [Google Scholar]
- Chen JQ, Zhan YF, Wang W, Jiang SN, Li XY. The engineered Salmonella typhimurium inhibits tumorigenesis in advanced glioma. OncoTargets Ther. 2015;8:2555. doi: 10.2147/OTT.S86899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CY, Miller SA. Clinical metagenomics. Nat Rev Genet. 2019;20:341–355. doi: 10.1038/s41576-019-0113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowska-Śmigiel A, Kycia K, Neffe-Skocińska K, Kieliszek M, Gniewosz M, Kołożyn-Krajewska D. Effect of pullulan on physicochemical, microbiological, and sensory quality of yogurts. Curr Pharm Biotechnol. 2019;20(6):489–496. doi: 10.2174/1389201020666190416151129. [DOI] [PubMed] [Google Scholar]
- Choi KR, Jang WD, Yang D, Cho JS, Park D, Lee SY. Systems metabolic engineering strategies: integrating systems and synthetic biology with metabolic engineering. Trends Biotechnol. 2019 doi: 10.1016/j.tibtech.2019.01.003. [DOI] [PubMed] [Google Scholar]
- Chua KJ, Kwok WC, Aggarwal N, Sun T, Chang MW. Designer probiotics for the prevention and treatment of human diseases. Curr Opin Chem Biol. 2017;40:8–16. doi: 10.1016/j.cbpa.2017.04.011. [DOI] [PubMed] [Google Scholar]
- Copeland DR, McVay MR, Dassinger MS, Jackson RJ, Smith SD. Probiotic fortified diet reduces bacterial colonization and translocation in a long-term neonatal rabbit model. J Pediatr Surg. 2009;44(6):1061–1064. doi: 10.1016/j.jpedsurg.2009.02.014. [DOI] [PubMed] [Google Scholar]
- De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci. 2010;107(33):14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Carmen S, Rosique RM, Saraiva T, Zurita-Turk M, Miyoshi A, Azevedo V, et al. Protective effects of lactococci strains delivering either IL-10 protein or cDNA in a TNBS-induced chronic colitis model. J Clin Gastroenterol. 2014;48:S12–S17. doi: 10.1097/MCG.0000000000000235. [DOI] [PubMed] [Google Scholar]
- Duan FF, Liu JH, March JC. Engineered commensal bacteria reprogram intestinal cells into glucose-responsive insulin-secreting cells for the treatment of diabetes. Diabetes. 2015;64:1794–1803. doi: 10.2337/db14-0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich SD. Metagenomics of the intestinal microbiota: potential applications. Gastroenterol Clin Biol. 2010;34:S23–S28. doi: 10.1016/S0399-8320(10)70017-8. [DOI] [PubMed] [Google Scholar]
- Franzosa EA, Morgan XC, Segata N, Waldron L, Reyes J, Earl AM, et al. Relating the metatranscriptome and metagenome of the human gut. Proc Natl Acad Sci. 2014 doi: 10.1073/pnas.1319284111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoshal UC, Gwee KA, Holtmann G, Li Y, Park SJ, Simadibrata M, et al. The role of the microbiome and the use of probiotics in gastrointestinal disorders in adults in the Asia–Pacific region-background and recommendations of a regional consensus meeting. J Gastroenterol Hepatol. 2018;33:57–69. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- Graham EB, Knelman JE, Schindlbacher A, Siciliano S, Breulmann M, Yannarell A, et al. Microbes as engines of ecosystem function: when does community structure enhance predictions of ecosystem processes. Front Microbiol. 2016;7:214. doi: 10.3389/fmicb.2016.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronbach K, Eberle U, Müller M, Ölschläger TA, Dobrindt U, Leithäuser F, Niess JH, Döring G, Reimann J, Autenrieth IB, Frick JS. Safety of probiotic Escherichia coli strain Nissle 1917 depends on intestinal microbiota and adaptive immunity of the host. Infect Immun. 2010;78(7):3036–3046. doi: 10.1128/IAI.00218-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo ZL, Yu B, Ning BT, Chan S, Lin QB, Li JCB, et al. Genetically modified” obligate” anaerobic Salmonella typhimurium as a therapeutic strategy for neuroblastoma. J Hematol Oncol. 2015;8(1):99. doi: 10.1186/s13045-015-0196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon E, Horvatovich P, Izquierdo E, Bringel F, Marchioni E, Aoude-Werner D, et al. Comparative proteomic analysis of Lactobacillus plantarum for the identification of key proteins in bile tolerance. BMC Microbiol. 2011;11:63. doi: 10.1186/1471-2180-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Marco ML, Slupsky CM. Emerging aspects of food and nutrition on gut microbiota. J Agric Food Chem. 2013;61:9559–9574. doi: 10.1021/jf4029046. [DOI] [PubMed] [Google Scholar]
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- Imperlini E, Santorelli L, Orrù S, Scolamiero E, Ruoppolo M, Caterino M. Mass spectrometry-based metabolomic and proteomic strategies in organic acidemias. Biomed Res Int. 2016 doi: 10.1155/2016/9210408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac NI, Philippe D, Nicholas A, Raoult D, Eric C. Metaproteomics of the human gut microbiota: challenges and contributions to other OMICS. Clin Mass Spectrom. 2019 doi: 10.1016/j.clinms.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Song S, Woodhams DC, Martino C, Allaband C, Mu A, Javorschi-Miller-Montgomery S, Suchodolski JS, Knight R. Engineering the microbiome for animal health and conservation. Exp Biol Med. 2019;244(6):494–504. doi: 10.1177/1535370219830075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovel J, Patterson J, Wang W, Hotte N, O’Keefe S, Mitchel T, et al. Characterization of the gut microbiome using 16S or shotgun metagenomics. Front Microbiol. 2016;7:459. doi: 10.3389/fmicb.2016.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambouris ME, Pavlidis C, Skoufas E, Arabatzis M, Kantzanou M, Velegraki A, Patrinos GP. Culturomics: a new kid on the block of OMICS to enable personalized medicine. Omics. 2018;22(2):108–118. doi: 10.1089/omi.2017.0017. [DOI] [PubMed] [Google Scholar]
- Karlsson FH, Fåk F, Nookaew I, Tremaroli V, Fagerberg B, Petranovic D, et al. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun. 2012;3:1245. doi: 10.1038/ncomms2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khangwal I, Shukla P. Prospecting prebiotics, innovative evaluation methods, and their health applications: a review. 3 Biotech. 2019;9(5):187. doi: 10.1007/s13205-019-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khangwal I, Shukla P. Potential prebiotics and their transmission mechanisms: recent approaches. J Food Drug Anal. 2019 doi: 10.1016/j.jfda.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khangwal I, Shukla P. Combinatory biotechnological intervention for gut microbiota. Appl Microbiol Biotechnol. 2019;103(9):3615–3625. doi: 10.1007/s00253-019-09727. [DOI] [PubMed] [Google Scholar]
- Korem T, Zeevi D, Suez J, Weinberger A, Avnit-Sagi T, Pompan-Lotan M, et al. Growth dynamics of gut microbiota in health and disease inferred from single metagenomic samples. Science. 2015 doi: 10.1126/science.aac4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota RK, Ambati RR, AK YVV, Srirama K, Reddy PN. Recent advances in probiotics as live biotherapeutics against gastrointestinal diseases. Curr Pharm Des. 2018;24(27):3162–3171. doi: 10.2174/1381612824666180717105128. [DOI] [PubMed] [Google Scholar]
- Kumar A, Mosa KA, Ji L, Kage U, Dhokane D, Karre S, et al. Metabolomics-assisted biotechnological interventions for developing plant-based functional foods and nutraceuticals. Crit Rev Food Sci Nutr. 2018;58:1791–1807. doi: 10.1080/10408398.2017.1285752. [DOI] [PubMed] [Google Scholar]
- Kumar R, Sood U, Gupta V, Singh M, Scaria J, Lal R. Recent advancements in the development of modern probiotics for restoring human gut microbiome dysbiosis. J Microbiol. 2019 doi: 10.1007/s12088-019-00808-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc JG, Chain F, Martín R, Bermúdez-Humarán LG, Courau S, Langella P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb Cell Fact. 2017;16(1):79. doi: 10.1186/s12934-017-0691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Lee HG, Choi YJ. Proteomic analysis of the effect of bile salts on the intestinal and probiotic bacterium Lactobacillus reuteri. J Biotechnol. 2008;137:14–19. doi: 10.1016/j.jbiotec.2008.07.1788. [DOI] [PubMed] [Google Scholar]
- Lugli GA, Mangifesta M, Mancabelli L, Milani C, Turroni F, Viappiani A, et al. Compositional assessment of bacterial communities in probiotic supplements by means of metagenomic techniques. Int J Food Microbiol. 2019;294:1–9. doi: 10.1016/j.ijfoodmicro.2019.01.011. [DOI] [PubMed] [Google Scholar]
- Ma Y, Liu J, Hou J, Dong Y, Lu Y, Jin L, et al. Oral administration of recombinant Lactococcus lactis expressing HSP65 and tandemly repeated P277 reduces the incidence of type I diabetes in non-obese diabetic mice. PLoS One. 2014;9:105701. doi: 10.1371/journal.pone.0105701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen KL, Doyle JS, Tavernini MM, Jewell LD, Rennie RP, Fedorak RN. Antibiotic therapy attenuates colitis in interleukin 10 gene-deficient mice. Gastroenterol. 2000;118(6):1094–1105. doi: 10.1016/S0016-5085(00)70362-3. [DOI] [PubMed] [Google Scholar]
- Marques Da Silva W, Oliveira LC, Soares SC, Sousa CS, Tavares GC, Resende CP, Pereira FL, Ghosh P, Figueiredo HC, Azevedo VA. Quantitative proteomic analysis of the response of probiotic putative Lactocuccus lactis NCDO 2118 strain to different oxygen availability under temperature variation. Front Microbiol. 2019;10:759. doi: 10.3389/fmicb.2019.00759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín R, Martín R, Chain F, Chain F, Miquel S, Miquel S, et al. Effects in the use of a genetically engineered strain of Lactococcus lactis delivering in situ IL-10 as a therapy to treat low-grade colon inflammation. Hum Vaccin Immunother. 2014;10(6):1611–1621. doi: 10.4161/hv.28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayers MD, Moon C, Stupp GS, Su AI, Wolan DW. Quantitative metaproteomics and activity-based probe enrichment reveals significant alterations in protein expression from a mouse model of inflammatory bowel disease. J Proteome Res. 2017;16(2):1014–1026. doi: 10.1021/acs.jproteome.6b00938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty NP, Yatsunenko T, Hsiao A, Faith JJ, Muegge BD, Goodman AL, et al. The impact of a consortium of fermented milk strains on the gut microbiome of gnotobiotic mice and monozygotic twins. Sci Transl Med. 2011;3(106):106ra106. doi: 10.1126/scitranslmed.3002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes AG, Ramos CL, Cenzi G, Melo DS, Dias DR, Schwan RF. Probiotic potential, antioxidant activity, and phytase production of indigenous yeasts isolated from indigenous fermented foods. Probiotics Antimicro. 2019;26:1–9. doi: 10.1007/s12602-019-9518-z. [DOI] [PubMed] [Google Scholar]
- Mills K, Mills P, Jackson M, Worthington V, Beesley C, Mann A, et al. Diagnosis of congenital disorders of glycosylation type-I using protein chip technology. Proteomics. 2006;6(7):2295–2304. doi: 10.1002/pmic.200500682. [DOI] [PubMed] [Google Scholar]
- Ohtake T, et al. Metabolomics-driven approach to solving a CoA imbalance for improved 1-butanol production in Escherichia coli. Metab Eng. 2017;41:135–143. doi: 10.1016/j.ymben.2017.04.003. [DOI] [PubMed] [Google Scholar]
- Paul E, Albert A, Ponnusamy S, Mishra SR, Vignesh AG, Sivakumar SM, et al. Designer probiotic Lactobacillus plantarum expressing oxalate decarboxylase developed using group II intron degrades intestinal oxalate in hyperoxaluric rats. Microbiol Res. 2018;15:65–75. doi: 10.1016/j.micres.2018.06.009. [DOI] [PubMed] [Google Scholar]
- Peñalver Bernabé B, Cralle L, Gilbert JA. Systems biology of the human microbiome. Curr Opin Biotechnol. 2018;51:146–153. doi: 10.1016/j.copbio.2018.01.018. [DOI] [PubMed] [Google Scholar]
- Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A. Mechanisms of action of probiotics. Adv Nutr. 2019 doi: 10.1093/advances/nmy063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemori S, Namai F, Yamamoto Y, Nigar S, Sato T, Ogita T, Shimosato T. Genetically modified Lactococcus lactis producing a green fluorescent protein–bovine lactoferrin fusion protein suppresses proinflammatory cytokine expression in lipopolysaccharide-stimulated RAW 264.7 cells. J Dairy Sci. 2017;100(9):7007–7015. doi: 10.3168/jds.2017-12872. [DOI] [PubMed] [Google Scholar]
- Singh B, Mal G, Gautam SK, Mukesh M. Designer probiotics: the next-gen high efficiency biotherapeutics. Adv Anim Biotechnol. 2019 doi: 10.1007/978-3-030-21309-1_7. [DOI] [Google Scholar]
- Sireswar S, Ghosh I, Dey G. First and second generation probiotic therapeutics for inflammatory bowel disease. PharmaNutrition. 2019 doi: 10.1016/j.phanu.2019.100159. [DOI] [Google Scholar]
- Sola-Oladokun B, Culligan EP, Sleator RD. Engineered probiotics: applications and biological containment. Annu Rev Food Sci T. 2017;8:353–370. doi: 10.1146/annurev-food-030216-030256. [DOI] [PubMed] [Google Scholar]
- Tabashsum Z, Peng M, Salaheen S, Comis C, Biswas D. Competitive elimination and virulence property alteration of Campylobacter jejuni by genetically engineered Lactobacillus casei. Food Control. 2018;85:283–291. doi: 10.1016/j.foodcont.2017.10.010. [DOI] [Google Scholar]
- Terpou A, Papadaki A, Lappa IK, Kachrimanidou V, Bosnea LA, Kopsahelis N. Probiotics in food systems: significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients. 2019;11(7):1591. doi: 10.3390/nu11071591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas WR, Smith WA, Hales BJ, Mills KL, O’Brien RM. Characterization and immunobiology of house dust mite allergens. Int Arch Allergy Immunol. 2002;129:1–18. doi: 10.1159/000065179. [DOI] [PubMed] [Google Scholar]
- Turroni F, Milani C, Duranti S, Mancabelli L, Mangifesta M, Viappiani A, et al. Deciphering bifidobacterial-mediated metabolic interactions and their impact on gut microbiota by a multi-omics approach. ISME J. 2016;10(7):1656. doi: 10.1038/ismej.2015.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Vijver MJ, He YD, Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, Parrish M. A gene-expressionsignature as a predictor of survival in breast cancer. N Engl J Med. 2002;347(25):1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- Ventura M, O’flaherty S, Claesson MJ, Turroni F, Klaenhammer TR, Van Sinderen D, O’toole PW. Genome-scale analyses of health-promoting bacteria: probiogenomics. Nat Rev Microbiol. 2009;7(1):61. doi: 10.1038/nrmicro2047. [DOI] [PubMed] [Google Scholar]
- Vinusha KS, Deepika K, Johnson TS, Agrawal GK, Rakwal R. Proteomic studies on lactic acid bacteria: a review. Biochem Biophys Rep. 2018;14:140–148. doi: 10.1016/j.bbrep.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wei SH, Chen YP, Chen MJ. Chen MJ (2015) Selecting probiotics with the abilities of enhancing GLP-1 to mitigate the progression of type 1 diabetes in vitro and in vivo. J Funct Foods. 2015;8:473–486. doi: 10.1016/j.jff.2015.08.016. [DOI] [Google Scholar]
- Wei C, Xun AY, Wei XX, Yao J, Wang JY, Shi RY. Bifidobacteria expressing tumstatin protein for antitumor therapy in tumor-bearing mice. Technol Cancer Res T J. 2016;15:498–508. doi: 10.1177/1533034615581977. [DOI] [PubMed] [Google Scholar]
- Wu R, Wang W, Yu D, Zhang W, Li Y, Sun Z, et al. Proteomics analysis of Lactobacillus casei Zhang, a new probiotic bacterium isolated from traditional home-made koumiss in Inner Mongolia of China. Mol Cell Proteom. 2009;8:2321–2338. doi: 10.1074/mcp.M800483-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YG, Yu H, Zhang L, Liu M, Qiao XY, Cui W, et al. Probiotic properties of genetically engineered Lactobacillus plantarum producing porcine lactoferrin used as feed additive for piglets. Process Biochem. 2016;51:719–724. doi: 10.1016/j.procbio.2016.03.007. [DOI] [Google Scholar]
- Xu H, Zhao F, Hou Q, Huang W, Liu Y, Zhang H, Sun Z. Metagenomic analysis revealed beneficial effects of probiotics in improving the composition and function of the gut microbiota in dogs with diarrhoea. Food Funct. 2019;10:2618–2629. doi: 10.1039/C9FO00087A. [DOI] [PubMed] [Google Scholar]
- Yadav M, Shukla P. Efficient engineered probiotics using synthetic biology approaches: a review. Biotechnol Appl Biochem. 2019 doi: 10.1002/bab.1822. [DOI] [PubMed] [Google Scholar]
- Yadav R, Kumar V, Baweja M, Shukla P. Gene editing and genetic engineering approaches for advanced probiotics: a review. Crit Rev Food Sci Nutr. 2018;58(10):1735–1746. doi: 10.1080/10408398.2016.1274877. [DOI] [PubMed] [Google Scholar]
- Yadav R, Singh KP, Shukla P. Metabolic engineering for probiotics and their genome-wide expression profiling. Curr Protein Pept Sc. 2018;19(1):68–74. doi: 10.2174/1389203718666161111130157. [DOI] [PubMed] [Google Scholar]
- Yang G, Jiang Y, Yang W, Du F, Yao Y, Shi C, Wang C. Effective treatment of hypertension by recombinant Lactobacillus plantarum expressing angiotensin converting enzyme inhibitory peptide. Microb Cell Fact. 2015;14:202. doi: 10.1186/s12934-015-0394-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Probiotic effects of Lactobacillus casei Zhang: from single strain omics to metagenomics. Chinese Sci Bull. 2018;64(3):307–314. doi: 10.1360/N972018-00591. [DOI] [Google Scholar]
- Zhang R, Peng X, Duan G, Shi Q, Chen S, Wang C, et al. An engineered Lactococcus lactis strain exerts significant immune responses through efficient expression and delivery of Helicobacter pylori Lpp20 antigen. Biotechnol Lett. 2016;38(12):2169–2175. doi: 10.1007/s10529-016-2209-x. [DOI] [PubMed] [Google Scholar]
- Zheng JH, Nguyen VH, Jiang SN, Park SH, Tan W, Hong SH, et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci Transl Med. 2017 doi: 10.1126/scitranslmed.aak9537. [DOI] [PubMed] [Google Scholar]
- Zhou H, He Z, Wang C, Xie T, Liu L, Liu C. Intravenous administration is an effective and safe route for cancer gene therapy using the bifidobacterium-mediated recombinant HSV-1 thymidine kinase and ganciclovir. Int J Mol Sci. 2016;17:891. doi: 10.3390/ijms17060891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Pei G, Niu X, Shi M, Zhang M, Chen L, Zhang W. Metabolomic analysis reveals functional overlapping of three signal transduction proteins in regulating ethanol tolerance in cyanobacterium Synechocystis sp. PCC 6803. Mol BioSyst. 2015;11(3):770–782. doi: 10.1039/C4MB00651H. [DOI] [PubMed] [Google Scholar]