Abstract
To study the efficacy of local application of oral probiotics in improving oral health in recurrent aphthous ulcer and oral candidiasis. Eighty patients with diagnosis of recurrent aphthous ulcer and oral candidiasis were included in the study. They were divided into group A = 40 patients (patients treated with oral application of probiotic as an adjuvant) and group B = 40 patients (patients treated without probiotic). Both the groups were divided into two subgroups, group AU and group BU for recurrent aphthous ulcer and group AC and BC for oral candidiasis. Clinical signs and symptoms were assessed at the beginning of the study and at the end of the study. Pregnant or lactating women, patients with localised or systemic diseases such as Steven Johnson syndrome, ulcerative colitis, Behcet’s syndrome and patients on chemotherapy or radiotherapy were excluded from the study. Bacillus Clausii, was used as a probiotic in our study. Patients in group A showed significant improvement in erythema (p = 0.001), pain reduction (p = 0.0001), decreased oral thrush (p = 0.006) and burning sensation in the mouth (p = 0.005) on day 5, whereas there was no significant difference on day 10 follow up. The study demonstrated the efficacy and rapidity of response to oral probiotic as an adjuvant in treating aphthous ulcer and oral candidiasis. Hence, oral application of probiotics can be used as an adjuvant in treating various oral pathology.
Keywords: Bacillus Clausii, Oral candidiasis, Probiotic, Recurrent aphthous ulcer
Introduction
The term “probiotics” was first introduced in 1965 by Lilly and Stillwell. In contrast to antibiotics, probiotics were defined as microbially derived factors that stimulate the growth of other useful organisms [1]. First probiotic species to be introduced in research was Lactobacillus acidophilus by Hull et al. in 1984; followed by Bifidobacteriumbifidum by Holcombh et al. in 1991 [2]. Probiotic is used to treat various medical conditions such as antibiotic associated diarrhoea, cancer and immunosuppressive states, short bowel syndrome, inflammatory bowel disease and lactose intolerance and have shown improved results after using probiotics [3].
In oral cavity, probiotics can create a biofilm which competes with carcinogenic bacteria and periodontal pathogens [3]. They also modulate host immune response and strengthen the immune system and help to prevent diseases [4]. A literature review shows that oral probiotic use is associated with a significant improvement in oral health. Currently, the role of probiotic on oral health and oral cavity disorders such as aphthous ulcers, halitosis and oral candidiasis is limited.
Neverthless, further studies would be needed to show probiotics efficacy to improve oral health. Hence, the aim of our present study is to establish the efficacy of local application of oral probiotics in improving oral health in recurrent aphthous ulcer and oral candidiasis.
Materials and Methodology
80 patients attending to the department of otolaryngology, Kempegowda Institute of medical sciences, Bangalore were taken into the study of which 40 patients were diagnosed with recurrent aphthous ulcers and 40 patients were diagnosed with oral candidiasis and were randomly divided into two groups based on those receiving oral application of probiotics as an adjuvant (GROUP = A) and those without probiotics (GROUP = B). Further, they were divided into two subgroups based on the clinical diagnosis of recurrent aphthous ulcer (group AU and BU) and oral candidiasis (group AC and BC) respectively. The aim of the study was to assess the efficacy of local application of oral probiotics in treating various oral pathology. Study period was for 10 days. Patients were assessed on day 1(visit 1), day 5 (visit 2) and day 10(visit 3). Pregnant or lactating women, patients with localised or systemic diseases such as Steven Johnson syndrome, ulcerative colitis, Behcet’s syndrome and patients on chemotherapy or radiotherapy were excluded from the study.
In our study we used Bacillus Clausii probiotic for local application twice daily for 1 week as an adjuvant in Group A. In Group B, subgroup BU had triamicinolone paste for local application twice daily, and subgroup BC had clotimazole mouth paint application twice daily.
Clinical signs and symptoms were assessed at the beginning of the study and at the end of the study. The intensity of the signs and symptoms were expressed as 0: absent, 1: mild, 2: moderate and 3: severe.
The clinical parameters that were assessed include: number of ulcers present, size of the ulcer [expressed in millimetres ranging from size1 (1 mm), size 2 (2 mm), size 3 (3 mm), size 4 (4 mm)], severity of pain, erythema, oral thrush, burning sensation of mouth and difficulty in swallowing. Their corresponding scores were given as no symptoms (0), mild (1), moderate (2), severe or unbearable (3).
Results were analysed statistically using Chi square and student’s t test.
Results
There were 49 males and 31 females. The mean age was 33.7 ± 1.74 years, with a range of 20–60 years. 37% were in the age group of 20–30 years, 23% in the age group of 30–40 years, while 24% were aged between 40 and 50 years and 16% between 50 and 60 years.
In patients with recurrent aphthous ulcer, 18 of 20 patients in group AU showed complete remission of the clinical symptoms and 14 patients in group BU showed complete healing at the end of treatment.
The mean value of reduction in number and size of ulcer in both groups were equal in both groups of recurrent aphthous ulcers (Table 1).
Table 1.
Mean value of sub group aphthous ulcer
| Visit | Sub group (aphthous ulcers) | Independent Samples t test | |||||
|---|---|---|---|---|---|---|---|
| AU | BU | ||||||
| Mean | SD | Mean | SD | T | Sig | ||
| 1 | No. of ulcer | 1.6 | 0.68 | 1.65 | 0.67 | 0.234 | 0.816 |
| Size of ulcer | 1.7 | 0.86 | 1.7 | 0.80 | 0.000 | 1.000 | |
| Erythema | 1.25 | 0.44 | 1.3 | 0.80 | 0.24 | 0.808 | |
| Degree of pain | 2.95 | 0.82 | 2.65 | 0.81 | 1.158 | 0.253 | |
| 5 | No. of ulcer | 1.05 | 0.88 | 1.05 | 0.94 | 0.000 | 1.000 |
| Size of ulcer | 1.10 | 0.71 | 1.30 | 0.80 | 0.831 | 0.410 | |
| Erythema | 0.25 | 0.44 | 0.75 | 0.44 | 3.561 | 0.001 | |
| Degree of pain | 0.95 | 0.75 | 2.00 | 0.64 | 4.705 | 0.0001 | |
| 10 | No. of ulcer | 0.15 | 0.37 | 0.25 | 0.44 | 0.776 | 0.4422 |
| Size of ulcer | 0.15 | 0.37 | 0.35 | 0.59 | 1.292 | 0.2040 | |
| Erythema | 0.00 | 0.00 | 0.15 | 0.37 | 1.831 | 0.0749 | |
| Degree of pain | 0.00 | 0.00 | 0.05 | 0.22 | 1.000 | 0.3236 | |
Patients in subgroup AU had significant reduction in erythema (p = 0.001) with mean value reduction from 1.25 to 0.25 on day 5 and 0.00 on day 10 as compared to subgroup BU, where the mean value reduced from 1.3 to 0.75 on day 5 and 0.15 on day 10 (Table 1).
At enrolment, group AU had severe pain in 9 patients, moderate in 7, and mild in 4 patients. At the end of therapy, it persisted mildly only in 4 patients. In group BU, pain was severe in 8 patients, moderate in 9 and mild in 3 at enrolment. At end of treatment, pain persisted moderately in 1 patient and mildly in 5 patients. There was significant difference (p = 0.0001) in degree of pain in subgroup AU, where the mean value reduced from 2.95 to 0.95 on day 5 and to 0 on day 10 follow up. In subgroup BU, the mean value reduced from 2.65 to 2.00 on day 5 and to 0.05 on day 10 (Fig. 1).
Fig. 1.
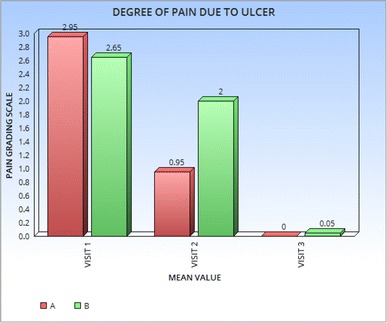
Mean value of degree of pain due to ulcer in subgroup aphthous ulcer
In patients with oral candidiasis, 19 of 20 patients in group AC showed remarkable improvement as compared to patients in group BC, where only 15 had complete remission at the end of treatment.
At the beginning of treatment patients in both group had oral thrush. There was statistically significant reduction in group AC on day 5, while on day 10 both groups achieved similar results (Fig. 2).
Fig. 2.
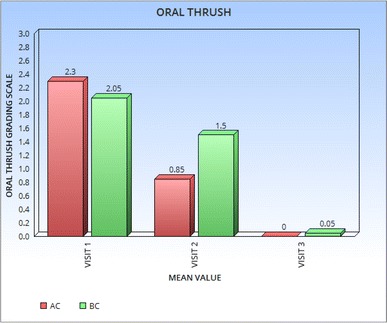
Mean value of oral thrush in subgroup oral candidiasis
In group AC, at enrolment, perilesional erythema was severe in 13 patients, moderate in 5 and mild in 2. At the end of therapy erythema persisted as mild only in 3 patients showing a statistically significant reduction in intensity of symptom on day 5 (Table 2). In group BC, 11 had severe erythema, moderate in 5 and mild in 4; of which 13 patients had complete regression of symptoms on day 10, while erythema persisted in other 7 patients. However, on day 10, the response to the treatment was same.
Table 2.
Mean Value Of Subgroup Oral Candidiasis
| Visit | Sub group (oral candidiasis) | Independent Samples t test | |||||
|---|---|---|---|---|---|---|---|
| AC | BC | ||||||
| Mean | SD | Mean | SD | T | Sig (p value) | ||
| 1 | Oral thrush | 2.3 | 0.73 | 2.05 | 0.75 | 1.068 | 0.292 |
| Burning sensation in mouth | 1.55 | 0.60 | 1.60 | 0.59 | 0.264 | 0.790 | |
| Erythema | 1.45 | 0.68 | 1.35 | 0.74 | 3.574 | 0.001 | |
| Difficulty in swallowing | 1.9 | 0.78 | 1.8 | 0.69 | 0.420 | 0.670 | |
| 5 | Oral thrush | 0.85 | 0.81 | 1.50 | 0.60 | 2.867 | 0.006 |
| Burning sensation in mouth | 0.30 | 0.65 | 0.90 | 0.64 | 2.940 | 0.005 | |
| Erythema | 0.40 | 0.50 | 1.00 | 0.56 | 3.574 | 0.001 | |
| Difficulty in swallowing | 0.70 | 0.73 | 1.00 | 0.632 | 1.391 | 0.170 | |
| 10 | Oral thrush | 0.00 | 0.00 | 0.05 | 0.22 | 1.000 | 0.323 |
| Burning sensation in mouth | 0.00 | 0.00 | 0.20 | 0.41 | 2.179 | 0.356 | |
| Erythema | 0.00 | 0.00 | 0.05 | 0.22 | 1.000 | 0.323 | |
| Difficulty in swallowing | 0.25 | 0.55 | 0.15 | 0.37 | 0.676 | 0.502 | |
There was significant reduction in local burning sensation in patients in group AC, with mean value reduction from 1.55 to 0.3 on day 5 and 0 on day 10 as compared to subgroup BU, where the mean value reduced from 1.6 to 0.9 on day 5 and 0.2 on day 10 (Table 2).
Group AC, had 17 patients with difficulty in swallowing on enrolment. At the end of treatment, only 3 had difficulty in swallowing. No significant difference was found in group BC, where 18 patients had difficulty in swallowing pretreatment, with only 2 persisted to have difficulty in swallowing at the end of treatment (Fig. 3).
Fig. 3.
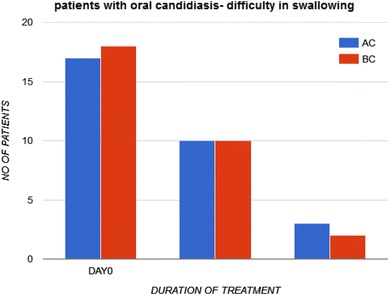
To assess difficulty in swallowing in patients with oral candidiasis
Discussion
A study by Sookhee et al. with 3790 lactic acid bacterial strains showed inhibitory effect against other microorganisms, including candida.
In oral cavity, probiotics can create a biofilm, acting as a protective lining for oral tissues against oral diseases [3]. Minor recurrent aphthous ulcers of size less than 5 mm is more common than major ulcers. These lesions usually heal within 1–2 weeks without scarring [5]. In our study, patients with aphthous ulcer, the mean numbers of ulcer and size of ulcer reduced equally in both, whereas erythema and degree of pain significantly reduced in group A patients compared to group B (p = 0.001 and 0.0001 respectively) on day 5 of treatment. However, Nalini et al. [6] found significant reduction in size of the ulcer in patients using probiotic lozenges. In their study, on day 10 follow up, patients in both group had symptomatic improvement, with no significant difference in both groups.
Candida albicans is the most common isolated candida species in the oral cavity and constitute up to 80% of the clinical isolates [7]. However some factors, such as immunosuppression and use of broadspectrum antimicrobial drugs, can favour their overgrowth and the development of candidiasis [8]. According to Boirivant and Strober [9], probiotics can improve the defence function of epithelial cells by induction of cytokine secretion and the production of immunoglobulins and antimicrobial substances. Similar findings were found by Hatakka et al. [10] and Daniel et al. [11], showing 75% reduction in yeast count and decreasing the prevalence from 30 to 21%. In our study, signs of oral thrush was absent or minimal in the oral cavity after probiotics use.
In our study, on day 5 follow up there was significant reduction in oral thrush, burning sensation in mouth and erythema in group A as compared to group B (p = 0.006, 0.005 and 0.001 respectively). Symptom of difficulty in swallowing reduced equally in both groups.
Overall probiotic (Bacillus Clausii) as a local adjuvant, significantly reduces the symptoms and signs of recurrent aphthous ulcer and oral candidiasis.
Conclusion
The study demonstrated the efficacy and rapidity of response to oral probiotic as an adjuvant in treating aphthous ulcer and oral candidiasis. Hence, oral application of probiotics can be used as an adjuvant in treating various oral pathology.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no competing interests.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Contributor Information
M. Nirmala, Email: nimmi.santy@gmail.com
Ganga J. Kamath, Email: ganga.veena@gmail.com
References
- 1.Koybasi Serap, et al. Recurrent aphthous stomatitis: investigation of possible etiologic factors. Am J Otolaryngol. 2006;27(4):229–232. doi: 10.1016/j.amjoto.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 2.Tanboga I, Caglar E, Kargul B. Campaign of probiotic food consumption in Turkish children, oral perspectives ‘Probiotics for your child’. Int J Pediatr Dent. 2003;13(Suppl 1):59. [Google Scholar]
- 3.Caglar E, Kargul B, Tanboga I. Bacteriotherapy and probiotics’ role on oral health. Oral Dis. 2005;11(3):131–137. doi: 10.1111/j.1601-0825.2005.01109.x. [DOI] [PubMed] [Google Scholar]
- 4.Deepa D, Mehta DS. Is the role of probiotics friendly in the treatment of periodontal diseases!! J Indian Soc Periodontol. 2009;13(1):30. doi: 10.4103/0972-124X.51892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guarner Francisco, et al. World Gastroenterology Organisation Practice Guideline: probiotics and Prebiotics-May 2008: guideline. South Afr Gastroenterol Rev. 2008;6(2):14–25. [Google Scholar]
- 6.Nalini A, Praveen K et al. (2014) A randomized, open label, clinical study of synbiotics in patients with recurrent minor aphthous ulcers. Res J Pharm Biol Chem Sci 5(2):1900–1905
- 7.Marsh PD, et al. Oral microbiology. Amsterdam: Elsevier; 2009. [Google Scholar]
- 8.Vecchiarelli Anna, et al. New approaches in the development of a vaccine for mucosal candidiasis: progress and challenges. Front Microbiol. 2012;3:13. doi: 10.3389/fmicb.2012.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boirivant Monica, Strober Warren. The mechanism of action of probiotics. Curr Opin Gastroenterol. 2007;23(6):679–692. doi: 10.1097/MOG.0b013e3282f0cffc. [DOI] [PubMed] [Google Scholar]
- 10.Hatakka K, et al. Probiotics reduce the prevalence of oral Candida in the elderly—a randomized controlled trial. J Dent Res. 2007;86(2):125–130. doi: 10.1177/154405910708600204. [DOI] [PubMed] [Google Scholar]
- 11.Rönnqvist Daniel, et al. Lactobacillus fermentum Ess-1 with unique growth inhibition of vulvo-vaginal candidiasis pathogens. J Med Microbiol. 2007;56(11):1500–1504. doi: 10.1099/jmm.0.47226-0. [DOI] [PubMed] [Google Scholar]


