Abstract
Malignant tumors consist of both carcinoma cells and tumor associated host cells. Host cells has started to receive more attention regarding their role in tumor progression such as invasion and metastasis. Fibroblasts that are incorporated in the tumoral stroma are called as peritumoral fibroblasts, reactive stroma, cancer-related fibroblasts or myofibroblasts. In general fibroblasts next to nich of cancer cells express alpha–smooth muscle actin (α-SMA) which is an important marker for differentiated myofibroblasts. Our aim is to investigate the role of α-SMA positive myofibroblasts in the development and progression of laryngeal carcinoma. The proportion of α-SMA positive myofibroblasts are scored from (1 +) to 3( +) in laryngeal dysplasia (n = 17) and microinvasive and invasive squamous cell carcinoma (n = 66). α-SMA expression in invasive carcinoma and dysplasia was analyzed statistically. No stromal myofibroblast was detected in mild dysplasia (score 0). Among 12 cases of moderate-severe dysplasia, in only 3 cases low α-SMA expression (score 1) was observed and in 9 cases there were no stromal myofibroblasts (score 0) were. In most cases of invasive carcinoma, high α-SMA expression (score 2, 3) was seen. α-SMA positive stromal myofibroblasts were significantly higher in invasive laryngeal squamous cell carcinoma compared to dysplasia (p < 0.05). Results of our study suggested that α-SMA positive stromal myofibroblasts play an important role in creating the permissive environment for tumor invasion in laryngeal squamous cell carcinoma. According to this data, we think that the presence of stromal myofibroblasts might be used as a helpful criterion in the differential diagnosis of true invasion and pseudoinvasion.
Keywords: Myofibroblast, Larynx, Squamous cell carcinoma, Laryngeal dysplasia, Actin
Introduction
Laryngeal cancer is the most common neoplasm of the head and neck region, accounting for 2% of all cancers in adults [1]. The incidence of the disease is 1% in younger patients [2, 3]. Laryngeal cancer is the second leading cancer in men in Turkey, which accounts for 7% of all deaths among men [4]. Laryngeal carcinoma is the most manageable malignancy of head and neck with appropriate diagnostic and therapeutic modalities.
Malignant tumors contain cancer cells and tumor-associated host cells. In recent years, host cells have become more interesting than ever by researchers, as they may play a role in tumor formation including invasion and metastasis, and thanks to the high rate of treatment response. Tumor-associated host cells include endothelial cells, inflammatory cells, immunocytes, macrophages, and fibroblasts [5, 6]. Fibroblasts, which are located within the tumor stroma, are defined as peritumoral fibroblasts, reactive stroma, cancer-associated fibroblasts, and myofibroblasts [7]. Fibroblastic cells which are adjacent to cancer cells typically express α-SMA, a critical probe for myofibroblastic differentiation. Myofibroblasts physiologically and pathologically modulate stroma through direct cell-to-cell contact as well as matrix metalloproteinase, tissue inhibitor of metalloproteinase, extracellular matrix components, growth factors, cytokines, chemokines, and expression of specific receptors of secreted lipid products [8].
Fibroblasts are the most crucial stromal cells in the development of carcinoma. Recent studies have demonstrated that stromal fibroblasts have a profound role in the proliferation and progression of tumor [9–13]. α-SMA positive stromal fibroblasts, in particular, stimulate neoangiogenesis and thereby activate tumor cell proliferation, by releasing angiogenic chemokine SDF-1 [14, 15].
In this study, we aimed to investigate the importance of presence of α-SMA-positive stromal fibroblasts in laryngeal squamous cell carcinoma, comparing the results with those of patients with dysplasia, and to assess the possible role of these cells in the development and progression of laryngeal carcinoma.
Materials and Methods
This retrospective study included a total of 83 patient including 17 with laryngeal dysplasia and 66 with micro-invasive and invasive squamous cell carcinoma who were admitted to our hospital between June 2011–June 2013 based on the paraffin blocks in the pathological records. Clinical data regarding age, sex and localization of the lesion were extracted from hospital records. Patients whose paraffin blocks were unavailable (n = 5) were excluded from the study. Five samples of normal mucosa were used as the control group.
Squamous cell carcinoma is classified as well differentiated, moderately differentiated and poorly differentiated according to the study of Neville et al. [16]. Well differentiated tumors are defined as slowly growing tumors with low cellularity and minimum nuclear pleomorphism showing high amount of keratinization. Poorly differentiated tumors have a rapid growth pattern, high cellularity and severe nuclear pleomorphism with minimal or no keratinization. Moderately differentiated tumors show transient features between these two types.
Three to four µm sections were obtained from selected paraffin blocks and fully automated immunohistochemical staining procedure was done using the Leica Bond-Max with the Bond Polymer Refine Detection kit and heat-induced retrieval pH 8.0 Bond max ER2 (EDTA) solution in the medical pathology laboratory. The tissue sections were immunohistochemically stained with α-SMA (Leica®) using biotin–streptavidin–peroxidase technique. Ductal carcinoma of the breast was used as positive control. α-SMA positive blood vessel endothelial cells were considered as internal positive control. The slides were examined under 400× magnification of light microscope (Olympus, BX51). α-SMA positive myofibroblasts within the squamous cell carcinoma and near the tumoral islands as well as the myofibroblasts within the stroma under the dysplastic epithelium were counted and noted. α-SMA positive blood vessel endothelial cells were excluded from the analysis.
To quantify α-SMA positive myofibroblasts, the cells were counted at the highest numbered ten high-power fields (HPF). The area of percentage of positive staining was scored as 0 (0%), 1 (1–33%), 2 (34–66%), and 3 (67–100%). Low α-SMA expression was considered as score 1. High α-SMA expression was considered as score 2 and 3. The intensity of staining with α-SMA was also evaluated. Scores 1, 2 and 3 were graded as mild, moderate, and intense expression of α-SMA, respectively [17].
The degree of dysplasia in noninvasive lesions was revised and noted for further grouping. The scores of stromal myofibroblasts for every degree of dysplasia and invasive carcinoma were recorded. The scores were then compared according to the level of dysplasia. In addition, α-SMA percentage scores were compared between noninvasive and invasive laryngeal tumoral lesions.
Statistical analysis was performed using SPSS statistical package (version 13.0; SPSS Inc, Chicago, Ill) along with χ2 and Kruskal–Wallis tests. A p value of 0.05 was considered as statistically significant.
Results
The mean age was 60.4 years (range, 40–86 years). Among 83 patients, 82 were male and 1 patient was female. Among 17 dysplasia, 5 were mild, 12 were moderate to severe, and among 66 invasive tumors; 2 were microinvasive and 64 were invasive. The mean size of the invasive tumors was 2.85 cm (minimum 1 cm, maximum 5.5 cm).
According to the pathological stage; 9 of the invasive carcinomas were T1 (14.4%), 21 were T2 (32.8%), 20 were T3 (31.2%) and 14 were T4 (21.6%). Forty-three of the invasive carcinomas had no lymph node involvement (N0) (67.18%), whilst 12 cases were N1 (18.75%), 1 case was N2a (1.56%), 2 cases were N2b (3.13%), and 6 cases were N2c (9.38%). Among invasive carcinomas, 7 were well differentiated (11%), 40 were moderately differentiated (62.5%) and 17 were poorly differentiated tumors (26.5%) (Table 1).
Table 1.
General features of the tumors
| Gender (all patients) | |
| Male | 82 (98.8) |
| Female | 1 (1.2%) |
| Dysplasia (total) | 17 |
| Mild dysplasia | 5 (29.41%) |
| Moderate to severe dysplasia | 12 (70.59%) |
| Invasive tumor (total) | 66 |
| Invasive carcinoma | 64 (96.9%) |
| Microinvasive carcinoma | 2 (3.1%) |
| Stage (invasive carcinoma) | |
| Tl | 9 (14.4%) |
| T2 | 21 (32.8%) |
| T3 | 20 (31.2%) |
| T4 | 14 (21.6%) |
| Lymph node stage (invasive carcinoma) | |
| N0 | 43 (67.18%) |
| N1 | 12 (18.75%) |
| N2a | 1 (1.56%) |
| N2b | 2 (3.13%) |
| N2c | 6 (9.38%) |
| Differentiation (invasive carcinoma) | |
| Well differentiated | 7 (10.93%) |
| Moderately differentiated | 40 (62.50%) |
| Poorly differentiated | 17 (26.57%) |
Clinicopathological parameters such as age, sex, size of the tumor, lymph node status, pathological stage, involvement of the thyroid cartilage and distant metastasis showed no significant correlation with α-SMA percentage scores (p = 0.22, p = 0.09, p = 0.44, p = 0.17, p = 0.69, p = 0.76, p = 0.79, respectively).
No stromal myofibroblasts were found in patients with mild laryngeal dysplasia [score 0]. Nine of 12 patients with moderate-to-severe dysplasia had no stromal myofibroblasts (Fig. 1), while 3 had low α-SMA expression. On the other hand, patients with invasive carcinoma had high α-SMA expression (Fig. 2). In addition, the α-SMA percentage scores were statistically significantly higher in patients with invasive laryngeal carcinoma (p = 0.000) as well as in poorly differentiated carcinomas (p = 0.000) (Table 2). All cases in this study showed intense staining with α-SMA. α-SMA positive stromal myofibroblasts had significantly more numerous stromal bands at the invasive front of the tumor (Fig. 3).
Fig. 1.
Sections of severe dysplasia lack α-SMA in myofibroblasts. (Original magnification, ×100 and ×200)
Fig. 2.
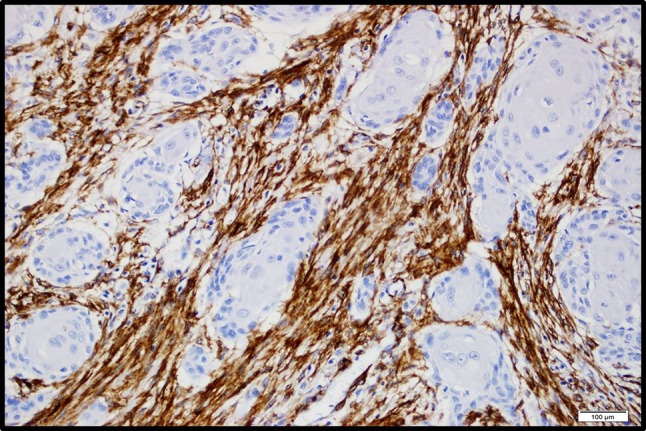
Images of representative high α-SMA expression scores in laryngeal squamous cell carcinoma. α-SMA positive (brown) cells are located within stroma surrounding tumor cells. (Original magnification, ×100)
Table 2.
α-SMA percentage scores in laryngeal dysplasia/carcinoma cases
| Laryngeal dysplasia/carcinoma | Myofibroblast Score | p value | |||
|---|---|---|---|---|---|
| Score 0 | Score 1 | Score 2 | Score 3 | ||
| Invasive carcinoma | |||||
| Mild dysplasia | 5 | 0 | 0 | 0 | p < 0.05 |
| Moderate to severe dysplasia | 9 | 3 | 0 | 0 | |
| Microinvasive carcinoma | 1 | 1 | 0 | 0 | |
| Differentiation (invasive carcinoma) | |||||
| Well differentiated | 1 | 2 | 4 | 0 | p < 0.05 |
| Moderately differentiated | 1 | 6 | 23 | 10 | |
| Poorly differentiated | 0 | 4 | 9 | 4 | |
Fig. 3.

Myofibroblasts were present in band-like stromal structures at the invasive front of the tumor. (Original magnification, ×200)
Discussion
Tumor microenvironment is one of the most popular foci among many studies worldwide. In epithelial malignant tumors such as squamous cell carcinoma, stromal cells including fibroblasts, endothelial cells, and inflammatory cells also collaborate with epithelial cells in tumor progression, angiogenesis, local invasion, and metastasis [18]. Recent findings have increasingly suggested that all stromal cells and fibroblasts, in particular, are crucial regulators of tumor progression [19, 20].
There is growing evidence that active fibroblasts, a subtype of fibroblasts, may play a role in the proliferation of tumor cells and progression of tumor [18, 21]. In the early proliferation stage of epithelial cells, the neoplasm has been found to be embedded within the tissue, which is transformed into the reactive stroma with increased fibroblasts and capillary veins due to the effects of growth factors released by cancer cells [12, 22]. Under these circumstances, original stromal fibroblasts are activated and some produce modified phenotype expressing α-SMA, like fibroblasts which are associated with wound healing. This phenotype is consistent with myofibroblasts [23]. Signaling pathways for fibroblast transformation into stromal myofibroblasts are still debated.
Stromal myofibroblasts also have been suggested to originate from cells other than stromal fibroblast. Recent studies have indicated that bone marrow and periadventitial cells (pericytes and vascular smooth muscle cells) may be an origin for stromal myofibroblasts [15]. Malignant epithelial cells are also suggested to be a critical origin for stromal myofibroblasts. This phenomenon is termed as epithelial-mesenchymal transformation, in which epithelial cells loss their epithelial characteristics and acquire more motile characteristics of mesenchymal cells. Epithelial-mesenchymal transformation was first defined during embryogenesis and is believed to play a role in the development and progression of tumor cells [24, 25].
In the study of Ochicha et al., myofibroblasts were found more prominent in dysplastic Barrett metaplasia when compared with the non-dysplastic foci. They suggested that myofibroblasts may have a role in the development and malignant transformation of Barrett’s esophagus [26]. Similar to our study, Chaudhary et al. [17] found that myofibroblastic proliferation is undetectable in normal oral mucosa and low risk dysplasia but increased in malignant disorders such as oral squamous cell carcinoma and verrucous carcinoma. They suggested that myofibroblastic proliferation highlighted by α-SMA can be used as a stromal marker of oral malignant lesions [17]. This finding is concordant with other various studies such as Kellerman et al., Seifi et al. and Etemad Moghadam et al. [27–29]. In addition, Etemad Moghadam et al. and Chaudary et al. found no myofibroblastic expression in normal oral mucosa [17, 27]. Similarly, we observed higher α-SMA expression in invasive carcinomas [29].
Chaudary et al. [17] found that loss of tumor differentiation affected percentage of α-SMA positive cells and suggested that α-SMA expression may have played a role in predicting prognosis. Accordingly, we found that poorly differentiated carcinomas had higher α-SMA expression scores (p = 0.000).
In conclusion, our study results show that the percentage of α-SMA positive stromal myofibroblasts is higher in the stroma of the laryngeal squamous cell carcinoma, compared to epithelial dysplasia. Furthermore, these findings suggest that α-SMA positive stromal myofibroblasts may play a critical role in the designation of a suitable stromal environment for tumor invasion in invasive laryngeal carcinoma. Therefore, we believe that the presence of stromal myofibroblasts may be an additional criterion for the differential diagnosis of invasion and pseudoinvasion. However, further large-scale studies are required to establish the possible role of tumor-associated stromal myofibroblasts in laryngeal dysplasia and invasive carcinoma.
Author’s Contribution
AOC: planning the study, revaluation of histopathologic diagnosis of all the cases; evaluation of the results, writing the manuscript and financial support of the study. YD: review and editing of the manuscript, evaluation of the results. AS: planning the study, evaluation of the results and financial support of the study. FD: evaluation of the results. IO: evaluation of the results. KI: providing the clinical data and providing the specimens for control group.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Aylin Orgen Calli, Phone: +90 5304151073, Email: orgencalli@gmail.com.
Yelda Dere, Email: yeldamorgul@gmail.com.
Aysegül Sari, Email: aysegulakder@gmail.com.
Fikret Dirilenoglu, Email: fikretdirilenoglu@gmail.com.
Irem Onur, Email: iremonur@yahoo.com.
Kadir İmre, Email: kadir_imre@yahoo.com.
References
- 1.Kirchner JA, Carter D. Pathology of the larynx. In: Mills SE, editor. Sternberg’s diagnostic surgical pathology. 4. Noida: Gopson Papers Ltd; 2004. pp. 1007–1032. [Google Scholar]
- 2.Lingen MW. Head and neck. In: Kumar V, Abbas AK, Aster JC, editors. Robbins and cotran pathologic basis of disease. 9. Philadelphia: Elsevier Saunders; 2015. pp. 738–740. [Google Scholar]
- 3.Lipkin A, Miller RH, Woodson GE. Squamous cell carcinoma of the oral cavity, pharynx, and larynx in young adults. Laryngoscope. 1985;95:790–793. doi: 10.1288/00005537-198507000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Elci OA, Elci MA, Blair A, Dosemeci M. Risk of laryngeal cancer by ocupational chemical exposure in Turkey. J Occup Environ Med. 2003;45:1100–1106. doi: 10.1097/01.jom.0000085890.50021.6f. [DOI] [PubMed] [Google Scholar]
- 5.Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer. 2007;7:139–147. doi: 10.1038/nrc2067. [DOI] [PubMed] [Google Scholar]
- 6.Barth PJ, Westhoff CC. CD341 fibrocytes: morphology, histogenesis and function. Curr Stem Cell Res T. 2007;2:221–227. doi: 10.2174/157488807781696249. [DOI] [PubMed] [Google Scholar]
- 7.Desmouliere A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int J Dev Biol. 2004;48:509–517. doi: 10.1387/ijdb.041802ad. [DOI] [PubMed] [Google Scholar]
- 8.Powell DW, Adegboyega PA, DiMari JF, Mifflin RC. Epithelial cells and their neighbors. I. Role of intestinal myofibroblasts in development, repair, and cancer. Am J Physiol-Gastr L. 2005;289:G2–G7. doi: 10.1152/ajpgi.00075.2005. [DOI] [PubMed] [Google Scholar]
- 9.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 10.Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS ONE. 2009;4:e4992. doi: 10.1371/journal.pone.0004992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sridhara SU, Choudaha N, Kasetty S, Joshi PS, Kallianpur S, Tijare M. Stromal myofibroblasts in nonmetastatic and metastatic oral squamous cell carcinoma: an immunohistochemical study. J Oral Maxillofac Pathol. 2013;17:190–194. doi: 10.4103/0973-029X.119758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bello IO, Vered M, Dayan D, Dobriyan A, Yahalom R, Alanen K, et al. Cancer-associated fibroblasts, a parameter of the tumour microenvironment, overcomes carcinoma-associated parameters in the prognosis of patients with mobile tongue cancer. Oral Oncol. 2011;47:33–38. doi: 10.1016/j.oraloncology.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Routray S, Sunkavali A, Bari KA. Carcinoma-associated fibroblasts, its implication in head and neck squamous cell carcinoma: a mini review. Oral Dis. 2014;20:246–253. doi: 10.1111/odi.12107. [DOI] [PubMed] [Google Scholar]
- 14.Astekar M, Metgud R, Sharma A, Soni A. Hidden keys in stroma: unlocking the tumor progression. J Oral Maxillofac Pathol. 2013;17:82–88. doi: 10.4103/0973-029X.110742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Neville BW, Day TA. Oral cancer and precancerous lesions. CA-Cancer J Clin. 2002;52:195–215. doi: 10.3322/canjclin.52.4.195. [DOI] [PubMed] [Google Scholar]
- 17.Chaudhary M, Gadbail AR, Vidhale G, Mankar MP, Gondivkar SM, Gawande M, et al. Comparison of myofibroblasts expression in oral squamous cell carcinoma, verrucous carcinoma, high risk epithelial dysplasia, low risk epithelial dysplasia and normal oral mucosa. Head Neck Pathol. 2012;6:305–313. doi: 10.1007/s12105-012-0335-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 19.Tlsty TD, Hein PW. Know thy neighbor: stromal cells can contribute oncogenic signals. Curr Opin Genet Dev. 2001;11:54–59. doi: 10.1016/S0959-437X(00)00156-8. [DOI] [PubMed] [Google Scholar]
- 20.Elenbaas B, Weinberg RA. Heterotopic signaling between epithelial tumor cells and fibroblasts in carcinoma formation. Exp Cell Res. 2001;264:169–184. doi: 10.1006/excr.2000.5133. [DOI] [PubMed] [Google Scholar]
- 21.Mueller MM, Fusening NE. Friends or foes—bipolar effects of the tumor stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 22.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123–10128. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 23.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodeling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 24.Nawshad A, LaGamba D, Polad A, Hay ED. Transforming growth factor-β signaling during epithelial-mesenchymal transformation: implications for embryogenesis and tumor metastasis. Cells Tissues Organs. 2005;179:11–23. doi: 10.1159/000084505. [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Ochicha O, Pringle JH, Mohammed AZ. Immunohistochemical study of epithelial-myofibroblast interaction in Barrett metaplasia. Indian J Pathol Microbiol. 2010;53:262–266. doi: 10.4103/0377-4929.64341. [DOI] [PubMed] [Google Scholar]
- 27.Seifi S, Shafaei S, Shafigh E, Sahabi SM, Ghasemi H. Myofibroblast stromal presence and distribution in squamous epithelial carcinomas, oral dysplasia and hyperkeratosis. Asian Pac J Cancer Prev. 2010;11:359–364. [PubMed] [Google Scholar]
- 28.Kellermann MG, Sobral LM, da Silva SD, Zecchin KG, Graner E, Lopes MA, et al. Mutual paracrine effects of oral squamous cell carcinoma cells and normal oral fibroblasts: induction of fibroblast to myofibroblast transdifferentiation and modulation of tumor cell proliferation. Oral Oncol. 2008;44:509–517. doi: 10.1016/j.oraloncology.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Etemad-Moghadam S, Khalili M, Tirgary F, Alaeddini M. Evaluation of myofibroblasts in oral epithelial dysplasia and squamous cell carcinoma. J Oral Pathol Med. 2009;38:639–643. doi: 10.1111/j.1600-0714.2009.00768.x. [DOI] [PubMed] [Google Scholar]



