Abstract
Oral cancer; the sixth most common malignancy in the world has one of the lowest 5 year survival rates. This can be attributed mainly to the delay in diagnosis. The purpose of this study was to evaluate the efficacy of vital staining with toluidine blue dye as an adjunct to standard clinical examination to facilitate early detection of malignant lesions of oral cavity and oropharynx. A hospital based diagnostic test accuracy study was carried out on 55 subjects with oral mucosal disorders that included clinically suspicious premalignant or malignant lesions, in the Department of ENT, Academy of Medical Sciences, Pariyaram, Kannur, Kerala over a period of 2 years. All lesions were subjected to detailed clinical examination and toluidine blue staining; and dye retention was recorded with photographs. The results of staining were compared with findings on histopathological examination. The Sensitivity and specificity of toluidine blue test for the detection of malignancy was 92.6 and 67.9% respectively; and the overall diagnostic accuracy was 80%. The result was highly significant with a ‘p value’ <0.001. The results indicate that toluidine blue staining is a simple, non-invasive technique which can be a valuable adjunct in the diagnostic process of oral and oropharyngeal cancers.
Keywords: Toluidine blue, Vital staining, Oral and oropharyngeal cancers, Premalignant lesions, Early diagnosis
Introduction
Cancer is one of the most dreaded diseases human race is suffering nowadays. Oral cancer is the sixth most common cancer worldwide and third most common in Southeast Asia [1]. The incidence of oral cancer is very high in India accounting to about 30–40% of the cancer load [2] and this malignancy poses a significant challenge to health services, both preventive and diagnostic. Now the incidence of oral premalignant lesions in Indian population has increased manifold, especially among the young, may be due to the rising trend of pan masala and gutkha chewing [3].
Despite numerous advances in management, oral cancer has one of the lowest 5 year survival rates of about 50–60% [2]. This can be attributed to the advanced extent of the disease at the time of diagnosis, with over 60% of patients presenting in stages 3 and 4 [4]. If diagnosed earlier (stage 1) it is often curable with survival rates of up to 86% [5, 6].
Currently biopsy with histopathological examination is considered the gold standard for diagnosis of oral cancer. The major problem is when and where the biopsy should be taken and this depends on the clinical ability of the medical practitioner to differentiate premalignant and malignant lesions from reactive and inflammatory diseases [7]. There is no reliable method for early diagnosis in these cases and this is the most important factor responsible for the delay in diagnosis and thus poorer prognosis.
Various techniques have been developed to supplement clinical examination with the aim of improving early diagnosis of oral and oropharyngeal cancer such as toluidine blue staining [8–10, 12, 14, 16–23], autofluorescence [9], veloscope [10], acetic acid staining, chemiluminescence [11] and oral exfoliative cytology [10, 12].
Among these, most commonly used and widely studied technique is invivo staining with toluidine blue. However studies published over the last 3 decades using toluidine blue to identify dysplastic and malignant lesions of oral mucosa have reported very disparate data resulting in controversies for its use [8, 9, 12, 14, 16–23]. Also there is a dearth of published studies on toluidine blue staining of oral cancer in our state where the prevalence of oral cancer is on the rise representing about 14% of all cancer cases at Regional Cancer Centre, Kerala [13]. Hence the need for this study, in which we assessed the diagnostic efficacy of 1% solution of modified toluidine blue to identify oral premalignant and malignant lesions.
Materials and Methods
This hospital based diagnostic test accuracy study was conducted over a period of 2 years after approval from the institutional ethics committee. 55 subjects presenting to ENT OPD with suspicious oral lesions were enrolled after obtaining full informed written consent. From the selected subjects with oral and oropharyngeal lesions, detailed history and epidemiological data, with special emphasis on age, duration of disease and exposure to possible carcinogenic substances was recorded with a questionnaire, after obtaining informed consent. All underwent detailed head and neck examination and were subjected to staining with 1% solution of Modified toluidine blue. All the lesions were biopsied under local anaesthesia by punch biopsy/wedge biopsy and sent for histopathological evaluation.
Preparation of Mouth Rinses
As described by Mashberg [14], a 100 ml of 1% toluidine blue solution was prepared by mixing 1 g toluidine blue powder, 10 ml 1% acetic acid, 4.19 ml absolute alcohol and 86 ml distilled water with pH regulated at 4.5.
1% acetic acid solution was prepared by diluting 5% acetic acid. It was done by mixing 1 part of acetic acid with 4 parts of distilled water.
Staining Protocol [14]
The technique of application involved rinsing of the mouth with water for 20 s to remove debris. Followed by application of 1% acetic acid for 20 s to remove ropey saliva. Then 1% toluidine blue oral rinse was given for 20 s. Again 1% acetic acid rinse was performed to remove mechanically retained stain. Finally the mouth was rinsed with water.
Interpretation
The interpretation was based on colour; dark blue (royal/navy) stain was considered positive (Figs. 1, 2). Light blue staining or when no colour was observed it was interpreted as negative.
Fig. 1.
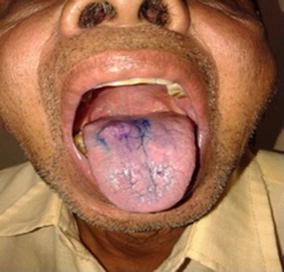
Benign lesion after toluidine blue staining
Fig. 2.
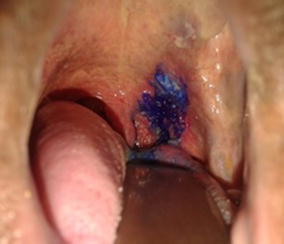
Malignant lesion after toluidine blue staining
For statistical analysis, we used histopathologic assessment as the gold standard with which toluidine blue stain retention was compared. We computed sensitivity, specificity, positive predictive value and negative predictive value from true positive, true negative, false positive and false negative results to get the diagnostic accuracy of the test.
Data analysis was performed using SPSS version 17.0 for qualitative data. Chi-square test and Fischer’s exact t test was used to compare the groups and assess the association. ‘p value’ of less than 0.05 was considered significant.
Results and Analysis
Most of the subjects in the study were above 50 years of age (78.2%) with mean age being 60.87 of which 60% were males. Of the 55 subjects, 76.4% (42/55) had lesions of oral cavity and 23.6% (13/55) had lesions of oropharynx. In the oral cavity, buccal mucosa was the commonest site; accounting for 38.2% (21/55). Next common site was anterior 2/3rd of tongue with 36.4%.
61.8% (34/55) of the lesions were positively stained with toluidine blue and 38.2% (21/55) were stained negative. Following histological examination of the biopsy samples, 27 (49.1%) were diagnosed as malignant i.e. Oral squamous cell carcinoma; of which 29.1% (16/55) were moderately differentiated; 18.2% (10/55) well differentiated; and 1.8% (1/55) were poorly differentiated. 28 (50.9%) were diagnosed as benign (with no dysplasia) (Fig. 3).
Fig. 3.
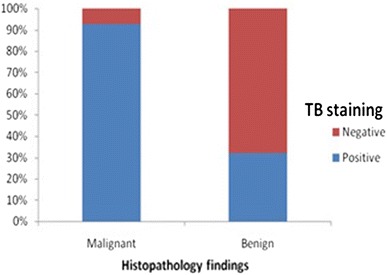
Diagnostic validity of toluidine blue
88.9% of subjects with malignancy (24/27) had one or more addictions (smoking, alcoholism, pan chewing) with a significant association, “p value” of 0.002; while only 50% (14/28) of subjects with benign lesions had addiction.
Of the 27 cases of malignant lesions, 25 (92.6%) retained toluidine blue stain i.e. True Positive (TP) while 2 (7.4%) lesions failed to retain the dye i.e. False Negative (FN). 9 Out of 34 toluidine blue positive lesions were diagnosed as benign/inflammatory lesions i.e. False Positive (FP). Out of the 21 toluidine blue negative lesions; 19 were histologically proved to be benign i.e. True Negative (TN) (Table 1).
Table 1.
Diagnostic validity of toluidine blue (sensitivity = TP/(TP + FN) = 92.6% specificity = TN/(TN + FP) = 67.9% positive)
| Toluidine blue staining | Histopathology | Total | |
|---|---|---|---|
| Malignant | Benign | ||
| Positive | 25 | 9 | 34 |
| Negative | 2 | 19 | 21 |
| Total | 27 | 28 | 55 |
The study showed a significant association between toluidine blue staining and malignancy, with a “p value” of <0.001.
Discussion
Prevention, early detection and prompt referral for diagnosis offer the best hope to a patient with cancer, providing the best chance to cure. Vital dyes or stains such as Crystal violet, Lugol’s iodine, toluidine blue [15] etc. have emerged in the recent years as useful diagnostic tools in detecting early potentially malignant and malignant lesions. It is a simple, easy to perform, non-invasive, inexpensive method which can be done as an outpatient procedure without time delay.
One of the first published reports of toluidine blue was cited in 1949 in the Journal of American Medical Association (JAMA) [16]. It selectively stains acidic tissue components (carboxylates, sulphates and phosphate radicals) such as DNA and RNA [8] and can detect early nuclear changes of malignancy.
In our study, we found a sensitivity of 92.6% and specificity of 67.9%. These values were comparable to those reported by Hegde et al. [17] (97.29 and 62.5% respectively) and were considerably higher than those reported by Ram and Siar in their study [18] (70.3 and 25% respectively).
Our sensitivity was comparable to the study of Shedd et al. [19], meta-analysis done by Rosenberg and Cretin [6] (92.6 vs 93% and 93.5–97.8% respectively) and the study by Allegra et al. [20] (92.6 vs 96.2% respectively). But our specificity being lower than all three of them (67.9 vs 100%, 92.9–73.3% and 77.7% respectively).
Our sensitivity was slightly lower than that reported by Nagaraju et al. [21] (92.6 vs 100% respectively) with a higher specificity (67.9 vs 40% respectively).
The variations in results may be due to the fact that inflammatory and ulcerative lesions tend to retain the dye and therefore yield a higher number of false positives. Also in hyperkeratotic lesions dye penetration to the deeper epithelial layers is hampered there by producing false negative results. Moreover staining results may vary subjected to interobserver variation, staining protocol and procedure and type of lesions.
Our positive predictive value was 73.5% which was higher than that reported by Onofre et al. [22] (43.5%) and Rodriguez et al. [23] (35.2%) but lower than that reported by Nagaraju et al. [21] (94.8%) and Allegra et al. [20] (86.6%). This may be due to the fact that prevalence of dysplastic/malignant lesions was higher in our study when compared to the first two studies (49.09 vs 26% and 18.1% respectively), and lower when compared to the last two (49.09 vs 91.67% and 60% respectively). Negative predictive value of our study was 90.4% which was comparable to studies of Onofre et al. [22] (88.9%), Rodriguez et al. [23] (90.2%) and Allegra et al. [20] (93.3%) and slightly lower to that of Nagaraju et al. [21] (100%).
This suggests that if prevalence is high, positive results will confirm the presence of the disease whereas if prevalence is low, a positive result does not permit its confirmation. Hence if toluidine blue dye test is used in high risk population, diagnostic validity of the test increases. On the other hand, when prevalence is low, a negative test result permits to rule out the disease safely.
The limitation of our study was that it being a hospital based study, does not discuss the utility of toluidine blue dye test in primary care settings.
Conclusion
The diagnostic efficacy of toluidine blue in detecting oral premalignant and malignant lesions was assessed in our study and we got a high sensitivity of 92.6% and a moderate specificity of 67.9% and a diagnostic accuracy of 80%.
To conclude, toluidine blue staining can be a valuable adjunctive method to clinical examination to decide the biopsy site and whether a biopsy should be taken when the clinical examination shows dubious lesions and this can contribute to the early diagnosis of oral and oropharyngeal cancer. Also it can be used in the follow up of oral premalignant lesions. The simplicity of the test procedure advocates for its role in routine practice. But as a definite consensus has not been achieved so far regarding the routine use of toluidine blue; further studies are needed.
Compliance with Ethical Standards
Conflict of interest
The authors declare that there are no conflicts of interests.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45(4–5):309–316. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Sankaranarayanan R, Nair MK, Mathew B, Balaram P, Sebastian P, Dutt SC. Recent results of oral cancer research in Kerala, India. Head Neck. 1992;14:107–112. doi: 10.1002/hed.2880140206. [DOI] [PubMed] [Google Scholar]
- 3.Mathew AL, Pai KM, Sholapurkar AA, Vengal M. The prevalence of oral mucosal lesions in patients visiting a dental school in Southern India. Indian J Dent Res. 2008;19:99–103. doi: 10.4103/0970-9290.40461. [DOI] [PubMed] [Google Scholar]
- 4.Gomez I, Warnakulasuriya S, Varela-Centelles PI, et al. Is early diagnosis of oral cancer a feasible objective? Who is to blame for diagnostic delay. Oral Dis. 2010;16:333–342. doi: 10.1111/j.1601-0825.2009.01642.x. [DOI] [PubMed] [Google Scholar]
- 5.Sciubba JJ. Oral cancer. The importance of early diagnosis and treatment. Am J Clin Dermatol. 2001;2:239–251. doi: 10.2165/00128071-200102040-00005. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg D, Cretin S. Use of meta-analysis to evaluate tolonium chloride in oral cancer screening. Oral Surg Oral Med Oral Pathol. 1989;67(5):621–627. doi: 10.1016/0030-4220(89)90286-7. [DOI] [PubMed] [Google Scholar]
- 7.López-Jornet P, Camacho-Alonso F, Martinez-Beneyto Y, Seoane-Les-ton J. Influence of years of professional experience in relation to the diagnostic skill of general dental practitioners (GDPs) in identifying oral cancer and precancerous lesions. Int Dent J. 2008;58:127–133. doi: 10.1111/j.1875-595X.2008.tb00187.x. [DOI] [PubMed] [Google Scholar]
- 8.Martin IC, Kerawala CJ, Reed M. The application of toluidine blue as a diagnostic adjunct in the detection of epithelial dysplasia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:444–446. doi: 10.1016/S1079-2104(98)90071-3. [DOI] [PubMed] [Google Scholar]
- 9.Lingen MW, Kalmar JR, Karrison T, Speight PM. Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol. 2008;44:10–22. doi: 10.1016/j.oraloncology.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patton LL, Epstein JB, Kerr AR. Adjunctive techniques for oral cancer examination and lesion diagnosis: a systematic review of the literature. J Am Dent Assoc. 2008;139(7):896–905. doi: 10.14219/jada.archive.2008.0276. [DOI] [PubMed] [Google Scholar]
- 11.Huber MA, Bsoul SA, Terezhalmy GT. Acetic acid wash and chemiluminescent illumination as an adjunct to conventional oral soft tissue examination for the detection of dysplasia: a pilot study. Quintessence Int. 2004;35:378–384. [PubMed] [Google Scholar]
- 12.Rajmohan M, Rao UK, Joshua E, Rajasekaran ST, Kannan R. Assessment of oral mucosa in normal, precancer and cancer using chemiluminescent illumination, toluidine blue supravital staining and oral exfoliative cytology. J Oral Maxillofac Pathol. 2012;16(3):325–329. doi: 10.4103/0973-029X.102476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sankaranarayanan R. Oral cancer in India. An epidemiologic and clinical review. Oral Surg Oral Med Oral Pathol. 1990;69:325–330. doi: 10.1016/0030-4220(90)90294-3. [DOI] [PubMed] [Google Scholar]
- 14.Mashberg A. Re-evaluation of toluidine blue application as a diagnostic adjunct in the detection of asymptomatic oral squamous cell carcinoma: a continuing prospective study of oral cancer. Cancer. 1980;46:758–763. doi: 10.1002/1097-0142(19800815)46:4<758::AID-CNCR2820460420>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Scully C, Malamos D, Levers BG, Porter SR, Prime SS. Sources and patterns of referrals of oral cancer: role of general practitioners. Br Med J (Clin Res Ed) 1986;293:599–601. doi: 10.1136/bmj.293.6547.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porter SR, Scully C. Early detection of oral cancer in the practice. Br Dent J. 1998;185(2):72–74. doi: 10.1038/sj.bdj.4809732. [DOI] [PubMed] [Google Scholar]
- 17.Hegde MC, Kamath PM, Sreedharan S, Dannana NK, Raju RM. Supravital staining: it’s role in detecting early malignancies. Indian J Otolaryngol Head Neck Surg. 2006;58(1):31–34. doi: 10.1007/BF02907735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ram S, Siar CH. Chemiluminescence as a diagnostic aid in the detection of oral cancer and potentially malignant epithelial lesions. Int J Oral Maxillofacial Surg. 2005;34:521–527. doi: 10.1016/j.ijom.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Shedd DP, Hukill P, Bahn S. In vivo staining properties of oral cancer. Am J Surg. 1965;110:631–634. doi: 10.1016/0002-9610(65)90052-8. [DOI] [PubMed] [Google Scholar]
- 20.Allegra E, Lombardo N, Puzzo L, Garozzo A. The usefulness of toluidine staining as a diagnostic tool for precancerous and cancerous oropharyngeal and oral cavity lesions. Acta Otorhinolaryngol Ital. 2009;29:187–190. [PMC free article] [PubMed] [Google Scholar]
- 21.Nagaraju K, Prasad S, Ashok L. Diagnostic efficiency of toluidine blue with Lugol’s iodine in oral premalignant and malignant lesions. Indian J Dent Res. 2010;21(2):218–223. doi: 10.4103/0970-9290.66633. [DOI] [PubMed] [Google Scholar]
- 22.Onofre MA, Sposto MR, Navarro CM. Reliability of toluidine blue application in the detection of oral epithelial dysplasia and in situ and invasive squamous cell carcinomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:535–540. doi: 10.1067/moe.2001.112949. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez PC, Lapiedra RC, Gomez GE, Martinez SL, Warnakulasuriya S. The use of toluidine blue in the detection of premalignant and malignant oral lesions. J Oral Pathol Med. 2011;40:300–304. doi: 10.1111/j.1600-0714.2010.00985.x. [DOI] [PubMed] [Google Scholar]


