Abstract
Parotid tumours are not uncommon. The management is surgical for benign and malignant parotid neoplasm. Due to the location of parotid gland and its intricate relationship with facial nerve, cosmetic and functional outcomes after parotid surgery are extremely important. Objectives of the study were to analyse facial nerve functions with emphasis on the quality of life of patients undergoing surgery for parotid neoplasm. A prospective study was conducted on patients presented with parotid neoplasm and undergone parotid surgery. Patient with malignant neoplasm were excluded. 30 patients with benign parotid neoplasm in final histopathology were included in the study. Post operative assessment of facial nerve was done using postparotidectomy facial nerve grading system. Symptom-specific QOL was assessed with the parotidectomy outcome inventory-8. Aesthetic outcome was evaluated with an ordinal scale. Posterior belly of digastric muscle and tragal pointer were the commonest landmark used for facial nerve identification. Temporary facial nerve dysfunction was present in six (20%) patients with marginal mandibular branch most commonly involved. 96% of the female patients and 91% of the male patients rated the cosmetic result as good or very good. A statistically significant difference is noted between superficial parotidectomy and total Parotidectomy for cosmetic outcome and sensory impairment. We noted that changed appearance due to resection of the parotid gland and scar and sensory impairment in the area affect the quality of life of patients and such affect are more after total conservative parotidectomy.
Keywords: Parotidectomy, Parotidectomy outcome inventory-8, POI-8, PPFNGS
Introduction
Parotid gland tumours are the most common salivary gland tumours. These tumours affect 1:100,000 people and represent 2–3% of tumours of the head and neck and 80% of salivary gland tumours [1, 2]. Majority of the lesions are benign with pleomorphic adenoma being the most common representing 60–70% of all parotid gland tumours, the management of the parotid gland neoplasm is surgical. Extracapsular dissection (ECD), superficial parotidectomy, total parotidectomy, radical and extended radical parotidectomy procedures are being performed for benign to malignant parotid tumours [3–6]. Superficial parotidectomy with facial nerve preservation is the most often indicated surgical procedure, mostly tumours are benign and confined to the glandular superficial lobe [5].
The most-feared postoperative complication after parotid surgery is facial nerve dysfunction [3–6]. The functional deficit may be total or partial, and may include all or a single branch of the nerve [6]. The post-parotidectomy facial nerve grading system (PPFNGS) is a new grading system designed for assessing the facial nerve function after parotidectomy in a quantitative and qualitative way [7]. Other complication includes infection, haemorrhage and haematoma, aesthetic problems, sensory changes, sialoceles and salivary fistulas, Frey’s syndrome (gustatory sweating and flushing) and tumour recurrence.
Functional outcome and complications after parotid surgery affect quality-of-life (QoL). Post parotidectomy QoL has been discussed in a limited number of studies so far and no uniform standard criteria or scale exist. Ciuman et al. [8] studied post-operative QoL using parotidectomy outcome inventory-8 (POI-8) [9].
The work was planned to study the surgical management of benign parotid neoplasm with emphasis on post-operative QoL of the patient. Post-operative facial nerve functions were evaluated using PPFNGS. POI-8 was used for assessment of QoL after surgery for benign neoplasm of parotid.
Material and Method
The study was conducted on the patients of parotid swelling, who attended IPD of Otorhinolaryngology and Head and Neck Surgery Department, J.L.N. Medical College and Associated Hospitals Ajmer, Rajasthan from August 2014 to July 2016.
The prospective cohort study included all cases of parotid neoplasm willing to undergo parotid surgery during the study period. Swellings of inflammatory and non inflammatory origin not proved to be neoplastic were excluded. Histopathologically proven malignant parotid tumours were excluded. The study protocol was reviewed and approved by the Ethical Committee of the Institute. A written fully explained consent stating the voluntary participation of subjects in the study was taken before the enrolment of the subjects. All cases selected for the study were evaluated using preformed proforma. Detailed history was taken as to age, sex, socioeconomic status, occupation, nature and duration of presenting symptoms, duration of the mass, associated pain, and intraoral drainage for all patients.
Physical examination of the parotid neoplasm included examination of parotid gland, palpation of neck for lymphadenopathy and evaluation of the facial nerve functions. Oral cavity examination was done which included inspection of Stenson’s duct opening for evidence of abnormal or purulent drainage. Oropharynx was examined for any bulge of lateral pharyngeal wall indicating parapharyngeal space involvement. Fine needle aspiration cytology, ultrasound, contrast enhanced computed tomography and magnetic resonance imaging were performed for all cases.
Patient and relatives were explained about the surgical procedure and possible complications especially possibility of facial nerve injury causing facial asymmetry, inability of closing the eye and a written consent for undergoing the surgery was obtained. Standard surgical procedure was followed in all cases. Modified Blair’s incision was used in almost all patients. Cartilaginous tragal pointer identified. The greater auricular nerve identified and its posterior branch is carefully preserved and posterior belly of digastrics were identified, the salivary tissue and fibrous tissue are separated step by step with careful use of a haemostat and the bipolar electrocautery, thus exposing the main trunk of the facial nerve at a point where the mastoid process, the cartilaginous portion of the auditory canal, and the superior border of the posterior belly of the digastric muscle meet. Once the main trunk of the facial nerve is identified, dissection is done in a plane superficial to the nerve toward the periphery of the gland. Dissection continues along the peripheral branches of the facial nerve from one direction to other and entire specimen is removed in one piece. Superficial musculoaponeurotic system (SMAS) or sternocleidomastoid (SCM) flaps were used to cover the facial nerve branches and fill the defect, Penrose drain is placed. The incision is closed in two layers, and pressure dressings applied. Dressing is reviewed on second post-operative day, drain removed. Oral antibiotics and analgesics were given for 5 days. Stitches were removed on 7th post-operative day.
Facial nerve functions were assessed on first post-operative day using PPFNGS suggested by Stodulski et al. [7]. This scale examines the function of four branches of the facial nerve. The activity was evaluated by giving to the each branch of the facial nerve from 0 to 4 points (“Appendix 1”).
Post-operative QoL assessment was done after 6 months. Instrument for the QoL assessment used were POI-8 and questionnaire for aesthetic satisfaction. POI-8 was given by Baumann in 2009, It consists of eight Likert-type scaled questions from 0 to 5. For comparability reasons the results were linearly transformed: X = (1[x/range] × 100) (“Appendix 2”).
For aesthetic satisfaction, patients were asked that “How would you rate your satisfaction with the aesthetic outcome of the operation?” Scoring was done from 0 to 10, zero being most satisfactory and 10 worse. 0 = normal or very good, 1–3 = good, 4–6 = average, 7–9 = poor, 10 = intolerable. The results were linearly transformed for comparability reasons using X = 1 − (x/range) × 100. Statistical analyses were done using open-epi software [10] (Fig. 1).
Fig. 1.
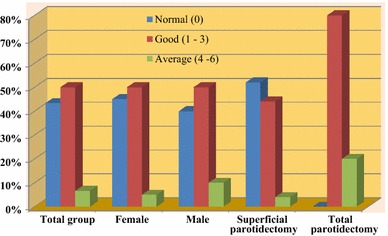
Patient satisfaction with the cosmetic result after parotid surgery for benign neoplasm (Score 0–10, zero being most satisfactory and 10 worse)
Results
30 patients with parotid swelling were included in the study with mean age of 36.4 ± 13.87 years (Table 1). According to final histopathological report, pleomorphic adenoma was the most common tumor seen in 24 cases (80.0%). Other benign neoplasms were Warthin’s tumour (6.67%), oncocytoma (3.33%), lipoma, and lymphoepithelial cyst. Superficial parotidectomy was performed in 25 cases (83.33%) and total conservative parotidectomy with facial nerve preservation in 5 cases (16.67%). Facial nerve was identified in all cases. Posterior belly of digastric muscle and tragal pointer were the commonest landmark used for facial nerve identification (Table 2).
Table 1.
Demographical characteristics of study population
| Total number of patients | 30 |
| Age (years) | |
| 11–20 | 4 (13.33%) |
| 21–30 | 10 (33.33%) |
| 31–40 | 6 (20%) |
| 41–50 | 5 (16.67%) |
| 51–60 | 3 (10%) |
| 61 above | 2 (6.67%) |
| Mean ± SD | 36.4 ± 13.87 years |
| Gender | |
| Male | 10 (33.3%) |
| Female | 20 (66.6%) |
Table 2.
Surgical management of benign parotid neoplasm
| n | Percentage | |
|---|---|---|
| Procedure performed (n = 30) | ||
| Superficial parotidectomy | 25 | 83.33 |
| Conservative total parotidectomy (with preservation of facial nerve) | 5 | 16.67 |
| Facial nerve landmarks used in identification | ||
| Posterior belly of digastric muscle | 30 | 100 |
| Tragal pointer | 30 | 100 |
| Tympanomastoid suture | 17 | 56.67 |
| Base of styloid process | 6 | 20.0 |
| Marginal mandibular branch | 3 | 10.0 |
| Flap used | ||
| SMAS | 16 | 53.33 |
| SCM | 6 | 20 |
| Final histo-pathological diagnosis | ||
| Pleomorphic adenoma | 24 | 80 |
| Warthins tumour | 2 | 6.67 |
| Oncocytoma | 1 | 3.33 |
| Lymphoepithelial cyst | 1 | 3.33 |
| Lipoma | 1 | 3.33 |
| Lymphoid tissue | 1 | 3.33 |
| Complications | ||
| Temporary dysfunction of the facial nerve | 6 | 20.0 |
| Wound dehiscence | 1 | 3.33 |
Post-operative facial nerve paresis was seen in 6 (20%) cases, which was recovered in due course in all patients. The marginal mandibular branch was involved in three patients, the temporal in one patient, temporal and zygomatic in one, and all branches in 1 patient. In the remaining 24 patients, the facial nerve functions were unaffected. None of the patient had permanent facial nerve dysfunction. However the transient facial nerve paresis was more commonly seen after total parotidectomy with facial nerve preservation as compared with superficial parotidectomy (p < 0.05). The average score for PPFNGS in the whole group was 15.56 (T3.9; Z3.9; B3.97; M3.80), while the mean score when assessing only cases with facial nerve dysfunction was 13.83 (T3.5; Z3.5; B3.83; M3) (Table 3). We noted that 13.33% patients reported sensory impairment such as hypoesthesia, dysesthesia, and temperature intolerance. Salivary fistula and Frey’s syndrome were not seen in any of the patients.
Table 3.
Post-operative facial nerve function assessment using post-parotidectomy facial nerve grading system (PPFNGS) as suggested by Stodulski et al. [7]
| 7th nerve function | n | PPFNGS |
|---|---|---|
| Normal | 24 | 16 (T4; Z4; B4; M4) |
| M branch deficit | 3 | 14.67 (T4;Z4;B4;M2.67) |
| T branch deficit | 1 | 15 (T3; Z4; B4; M4) |
| T and Z branches deficit | 1 | 14 (T3; Z3; B4; M4) |
| All branches deficit | 1 | 10 (T3; Z2; B3; M2) |
| Average paresis | 6 | 13.83 (T3.5; Z3.5; B3.83; M3) |
| Average in whole group | 30 | 15.56 (T3.9; Z3.9; B3.97; M3.8) |
After 6 months for aesthetic outcome, scoring was done from 0 to 10, zero being most satisfactory and 10 worse (0 = normal or very good, 1–3 = good, 4–6 = average, 7–9 = poor, 10 = intolerable) (Fig. 1). The mode was 0 (43.33%, very good) and the median was 1 (good) on the ordinal scale. The sex-based analysis showed that 96% of the female patients and 91% of the male patients rated the cosmetic result as good or very good. The mode was 0 (very good) and the median was 1 (good) for female patients and 1 (good) for male patients. After linear transformation to a 100-point scale, the value for the complete group was 91. Correlation analysis with extent of surgery showed statistically non-significant correlation between aesthetic outcome and extent of surgery.
Symptoms-specific QoL, using POI-8 assessed 6 months after surgery. The linearly transformed mean values of the symptom-specific QoL showed that most values are above 90, indicating a high symptom-specific QoL. Only the value for changed appearance due to resection of the parotid gland (tissue loss) after total parotidectomy with 72 was lower than 90 on the 100-point scale (Fig. 2). Statistical analysis showed statistically significant differences between superficial parotidectomy and total parotidectomy for changed appearance due to resection of the parotid gland (tissue loss) (p < 0.01), for abnormality of the scar (p < 0.01) and for sensory impairment in the area of operations and/or neck (p < 0.01).
Fig. 2.
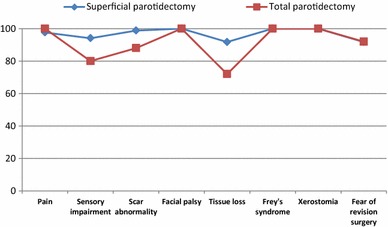
Symptom-specific quality of life using parotidectomy outcome inventory-8 (POI-8) after parotid surgery for benign parotid disease
Discussion
Surgery for benign parotid neoplasm could have significant impact on general QoL and global health status of the patient due to close proximity of the facial nerve and greater auricular nerve to the parotid gland and also due to anatomical position of the parotid gland. Ciuman et al. reported that outcome of parotid resection in benign disease has little impact on general and symptom-specific QoL. However, hypoesthesia or dysesthesia, Frey’s syndrome, and cosmetic discontent commonly exist and may affect symptom-specific QoL and general QoL [8]. Koch et al. [11] in their study reported no significant impact on general condition after parotid surgery, but found significantly positive correlations of the scores of facial nerve paresis, Frey’s syndrome, sensory deficit of the auricle, and cosmetic appearance with the general condition if only patients who sustained complications were considered. Gunsoy et al. [12] using EORTC QLQ-C30 and EORTC QLQ-H&N 35 questionnaires found a statistically significant correlation between the female gender and post-surgical pain, sleeplessness and the use of non-steroid anti-inflammatory drugs.
Facial nerve weakness is one of the most common post operative complications of the parotid surgery which affects QoL. In the study of Gaillard et al. [13] the incidence of postoperative facial nerve dysfunction was reported as 42.7% on the first postoperative day with normal facial nerve function 6 months after parotidectomy. Postoperative transient facial nerve dysfunction occurs up to 46.1% of cases, permanent damage is much less common, occurring 1.9–3.9% [14]. Sometimes the patient may have normal facial nerve function on recovery from anaesthesia facial nerve functions subsequently deteriorate may be due to the endoneural capillary endothelium injury by anoxia and trauma of surgery which leads to permeable capillary and oedema within the nerve. This may take some hours to develop and eventually resolve in few days [15]. The incidence of facial nerve paralysis is higher with total parotidectomy than with superficial parotidectomy, which may be related to stretch injury or as a result of surgical interference with the vasa nervosum [16].
The post-parotidectomy facial nerve grading system (PPFNGS) was suggested by Stodulski et al. [7] based on three grading systems (The regional House–Brackmann, Sydney and Yanagihara classification systems) for the assessment of the facial nerve function was used in the study. Mehle et al. reported that the marginal mandibular branch—64.1%, followed by buccal—20.5% are the most frequently affected branches. This may be because it is the longest of all facial nerve branches, comparatively more vigorous dissection of this branch in the tumours of the parotid tail, the paucity of anastomotic connections of this branch as compared with others, or an increased sensitivity to minimal trauma secondary to a smaller diameter or longer course [14].
Scar at the incision site and tissue loss after removal of the tumour affect on the cosmetic appearance the patient. Roh et al. [17] reported that patient scores regarding their scar and cosmetic appearance were significantly better after partial compared to superficial and total parotidectomy. Bianchi et al. [18] reported the use of the superficial musculoaponeurotic system, and the sternocleidomastoid muscle flap could further improve aesthetic outcome. Ciuman et al. [8] reported that over 87% of patients rated the cosmetic result as very good or good.
Sensory impairment after parotidectomy occurs mainly due to injury to greater auricular nerve. Ciuman et al. [8] reported that 54% of patients had some kind of sensory impairment, and 44.3% of them perceived it as disturbing. Patel et al. reported in a group of 53 patients, 30 patients (57%) with sensory impairment. Among patients experiencing symptoms, 23 (77%) reported only a little or no bother caused by the symptoms, and 27 (90%) reported no interference or almost none with their daily activities [19]. Klintworth et al. [20] reported in their series of 377 extracapsular dissections 10% of patients with hypoesthesia. In our study, sensory impairment was seen in only 13.33%. This was because of early identification and preservation of greater auricular nerve. We believe that careful preservation of greater auricular nerve is necessary and can easily be done. We experience that if the horizontal infra-auricular limb of modified Blair incision is deepened early it may result in transection of posterior branch of greater auricular nerve. So to preserve first the cervical incision made and the nerve is identified lower down, then the flap should be elevated along the greater auricular nerve and posterior branch is followed up to the lobule. By this means we can preserve posterior branch in most of the cases.
Frey’s syndrome, salivary fistula and sialocele have been reported as sequelae of parotidectomy. These sequelae were not shown by any of the patient in present study. We used SMAS and SCM flaps in 53.33 and 20% patients. Zhao et al. [21] showed that conserving the sub SMAS alone or together with a SCM flap decreases the incidence of Frey’s syndrome significantly. Ciuman et al. [8] reported that Frey’s syndrome occurs more often in superficial parotidectomy than in limited parotid surgery and shows a statistically significant impact on symptom-specific and general QoL.
We conclude that parotidectomy for benign lesions not have much effect on general QoL, however symptom specific outcome such as changed appearance due to resection of the parotid gland, and scar and sensory impairment in the area of operations and/or neck affects the patients and such affects are more after total conservative parotidectomy. We found PPFNGS and POI-8 are easy to use and suitable tools for QoL assessment after parotidectomy.
Acknowledgements
No financial funding received. Patients were treated free of cost under Chief Minister’s ‘MNDY (Mukhyamantri Nishulk Dava Yojana) scheme’ sponsored by Government of Rajasthan.
Appendix 1
The PPFGNS is used as per Creative Commons Attribution License with all credits to original authors and copyright holders from Eur Arch Otorhinolaryngol, Facial nerve grading after parotidectomy, 272(9), 2015, 2445–2450, Stodulski D, Skorek A, Mikaszewski B, Wiśniewski P, Stankiewicz C, Copyright © The Author(s) 2014 Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited (Tables 4, 5).
Table 4.
Assessment and recording of facial nerve function after parotidectomy (PPFNGS) [7]
| 7th nerve branch | Symmetry at rest and spontaneous movements | Assessed function | Points |
|---|---|---|---|
| Temporal (T) |
Forehead wrinkles Eyebrows level |
Forehead wrinkle Eyebrows raise |
0–4 |
| Zygomatic (Z) | Blinking | Eye closure | 0–4 |
| Buccal (B) | Nasolabial folds symmetry |
Cheeks raise Nose wrinkle |
0–4 |
| Marginal mandibular (M) |
Speecha Smilea Mouth corner symmetry |
Whistlea Showing teeth (grin)a |
0–4 |
| Whole nerve VII | 0–16 (Tx, Zx, Bx, Mx) |
aAlso for buccal branch
Table 5.
Scoring rules of the facial nerve function after parotidectomy (PPFNGS) [7]
| Degree | Description | Points |
|---|---|---|
| Complete function |
Symmetry at rest Symmetry at full range of movements |
4 |
| Slight paresis |
Symmetry at rest Slight asymmetry at full range of movements |
3 |
| Pronounced paresis |
Symmetry at rest Movement disorders with clear asymmetry |
2 |
| Profound paresis |
Asymmetry at rest Slight of the muscle movements |
1 |
| Paralysis |
Asymmetry at rest Lack of movements |
0 |
Appendix 2
The POI-8 is used from HNO, Development and validation of the Parotidectomy Outcome Inventory 8 (POI-8) Measurement of quality of life after parotidectomy in benign diseases, 57(9), 2009, 884–888, Baumann I, Cerman Z, Sertel S, Skevas T, Klingmann C, Plinkert PK, Springer Medizin Verlag 2009 “With permission of Springer” License No. 4103510708955 License date May 07, 2017.
| S. no. | No problem | Very small problem | Small problem | Moderate problem | Severe problem | it cannot be worse | |
|---|---|---|---|---|---|---|---|
| 1. | Pain in the area of operations and/or face | 0 | 1 | 2 | 3 | 4 | 5 |
| 2 | Sensory impairment in the area of operations and/or neck | 0 | 1 | 2 | 3 | 4 | 5 |
| 3 | Abnormality of the scar | 0 | 1 | 2 | 3 | 4 | 5 |
| 4 | Changed appearance due to facial nerve paralysis | 0 | 1 | 2 | 3 | 4 | 5 |
| 5 | Changed appearance due to resection of the parotid gland (tissue loss) | 0 | 1 | 2 | 3 | 4 | 5 |
| 6 | Perspiration in the area of operations (particularly at dinner) | 0 | 1 | 2 | 3 | 4 | 5 |
| 7 | Dryness of mouth as impact of the operation | 0 | 1 | 2 | 3 | 4 | 5 |
| 8 | I have fear of revision surgery | 0 | 1 | 2 | 3 | 4 | 5 |
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in presented study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Zhan KY, Khaja SF, Flack AB, Day TA. Benign parotid tumors. Otolaryngol Clin North Am. 2016;49(2):327–342. doi: 10.1016/j.otc.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Lin CC, Tsai MH, Huang CC, Hua CH, Tseng HC, Huang ST. Parotid tumours: a 10 year experience. Am J Otolaryngol. 2008;29(2):94–100. doi: 10.1016/j.amjoto.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2807 patients. Head Neck Surg. 1986;8(3):177–184. doi: 10.1002/hed.2890080309. [DOI] [PubMed] [Google Scholar]
- 4.Bradley PJ. Pleomorphic salivary adenoma of the parotid gland: which operation to perform? Curr Opin Otolaryngol Head Neck Surg. 2004;12(2):69–70. doi: 10.1097/00020840-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Larian B. Parotidectomy for benign parotid tumors. Otolaryngol Clin North Am. 2016;49(2):395–413. doi: 10.1016/j.otc.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Cracchiolo JR, Shaha AR. Parotidectomy for parotid cancer. Otolaryngol Clin North Am. 2016;49(2):415–424. doi: 10.1016/j.otc.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stodulski D, Skorek A, Mikaszewski B, Wiśniewski P, Stankiewicz C. Facial nerve grading after parotidectomy. Eur Arch Otorhinolaryngol. 2015;272(9):2445–2450. doi: 10.1007/s00405-014-3196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciuman RR, Oels W, Jaussi R, Dost P. Outcome, general, and symptom-specific quality of life after various types of parotid resection. Laryngoscope. 2012;122(6):1254–1261. doi: 10.1002/lary.23318. [DOI] [PubMed] [Google Scholar]
- 9.Baumann I, Cerman Z, Sertel S, Skevas T, Klingmann C, Plinkert PK. Development and validation of the parotidectomy outcome inventory 8 (POI-8). Measurement of quality of life after parotidectomy in benign diseases. HNO. 2009;57(9):884–888. doi: 10.1007/s00106-009-1991-3. [DOI] [PubMed] [Google Scholar]
- 10.Dean AG, Sullivan KM, Soe MM. OpenEpi: open source epidemiologic statistics for public health, version. www.OpenEpi.com. Accessed 06 Apr 2013
- 11.Koch M, Zenk J, Iro H. Long-term results of morbidity after parotid gland surgery in benign disease. Laryngoscope. 2010;120:724–730. doi: 10.1002/lary.20822. [DOI] [PubMed] [Google Scholar]
- 12.Gunsoy B, Vuralkan E, Sonbay ND, Simsek G, Tokgoz SA, Akin I. Quality of life following surgical treatment of benign parotid disease. Indian J Otolaryngol Head Neck Surg. 2013;65(Suppl 1):105–111. doi: 10.1007/s12070-012-0585-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaillard C, Périé S, Susini B, St Guily JL. Facial nerve dysfunction after parotidectomy: the role of local factors. Laryngoscope. 2005;115(2):287–291. doi: 10.1097/01.mlg.0000154735.61775.cd. [DOI] [PubMed] [Google Scholar]
- 14.Mehle ME, Kraus DH, Wood BG, Benninger MS, Eliachar I, Levine HL, Tucker HM, Lavertu P. Facial nerve morbidity following parotid surgery for benign disease: the Cleveland Clinic Foundation experience. Laryngoscope. 1993;103:386–388. doi: 10.1002/lary.5541030404. [DOI] [PubMed] [Google Scholar]
- 15.Ramadan MM. Facial nerve morbidity following parotid surgery. Suez Canal Univ Med J. 2003;6:29–34. [Google Scholar]
- 16.Bova R, Saylor A, Coman WB. Parotidectomy: review of treatment and outcomes. ANZ J Surg. 2004;74(7):563–568. doi: 10.1111/j.1445-2197.2004.02988.x. [DOI] [PubMed] [Google Scholar]
- 17.Roh JL, Kim HS, Park CI. Randomized clinical trial comparing partial parotidectomy versus superficial or total parotidectomy. Br J Surg. 2007;94(9):1081–1087. doi: 10.1002/bjs.5947. [DOI] [PubMed] [Google Scholar]
- 18.Bianchi B, Ferri A, Ferrari S, Copelli C, Sesenna E. Improving esthetic results in benign parotid surgery: statistical evaluation of facelift approach, sternocleidomastoid flap, and superficial musculoaponeurotic system flap application. J Oral Maxillofac Surg. 2011;69(4):1235–1241. doi: 10.1016/j.joms.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Patel N, Har-El G, Rosenfeld R. Quality of life after great auricular nerve sacrifice during parotidectomy. Arch Otolaryngol Head Neck Surg. 2001;127(7):884–888. [PubMed] [Google Scholar]
- 20.Klintworth N, Zenk J, Koch M, Iro H. Postoperative complications after extracapsular dissection of benign parotid lesions with particular reference to facial nerve function. Laryngoscope. 2010;120(3):484–490. doi: 10.1002/lary.20801. [DOI] [PubMed] [Google Scholar]
- 21.Zhao HW, Li LJ, Han B, Liu H, Pan J. Preventing post-surgical complications by modification of parotidectomy. Int J Oral Maxillofac Surg. 2008;37:345–349. doi: 10.1016/j.ijom.2007.11.022. [DOI] [PubMed] [Google Scholar]


