Abstract
The aim of this study is to highlight involvement of facial nerve as a presenting symptom in rhino cerebral mucormycosis. A retrospective longitudinal study was carried out for a period of 1 year from May 2017 to May 2018 in Department of Otorhinolaryngology of Dr. D. Y. Patil Medical College and Hospital, Kolhapur. The usual presentation include nasal stuffiness, headache, eye pain and orbital swelling, ophthalmoplegia and visual loss. However we had four patients who presented to our OPD with facial nerve palsy and rhino cerebral mucormycosis. All four patients were diabetic. The available clinical and laboratory data was retrospectively collected and analyzed. Facial nerve palsy is an unusual but significant sign in presentation of mucormycosis. It could be misdiagnosed as CVA with subsequent delay in the treatment. A high index of suspicion for mucormycosis in diabetic patients presenting with facial palsy will be helpful in achieving early and accurate diagnosis with prompt management and better outcome.
Keywords: Rhino cerebral mucormycosis, Mucormycosis, Facial nerve palsy, Complications of diabetes
Introduction
The term ‘mucormycosis’ was coined by an American pathologist R. D. Baker. The incidence of mucormycosis reported in literature is approximately 1.7 cases per 1,000,000 populations per year [1]. It is an opportunistic, fulminant fungal infection caused by Rhizopus species of the order—mucorales. It affects mainly the immunocompromised patients, especially those with uncontrolled diabetes mellitus (DM). In addition, patients with organ transplant or hematopoietic stem cell transplant, neutropenia or malignancy are at high risk [2]. Depending on the site affected, the disease may manifest in six different forms, rhino cerebral, pulmonary, cutaneous, gastro-intestinal, orbital and disseminated. Rhino cerebral mucormycosis (RCM) is the commonest type accounting for about 30–50% of cases [3]. With the increasing incidence of diabetes, the complications are bound to increase. Rhino cerebral Mucormycosis is one of the complications seen on the rise recently. Early diagnosis is very important factor not only to cure the disease completely but also to improve the outcome by reducing the need for extensive surgical resection. Typical presentation of RCM includes nasal stuffiness, headache, retro-orbital pain, orbital swelling, ophthalmoplegia and visual loss in diabetic patients. However we had four patients who presented with unilateral lower motor neuron facial palsy. On further evaluation they were diagnosed as rhino cerebral mucormycosis. The purpose of this article is to make the ENT surgeon aware of this uncommon presentation of RCM.
Present article is a retrospective longitudinal study carried out for a period of one year from May 2017 to May 2018 in Department of Otorhinolaryngology, Dr. D. Y. Patil Medical College and Hospital, Kolhapur, Maharashtra. The available clinical and laboratory data was analyzed. As it is a retrospective study, informed consent from patients was not possible but ethical approval was taken from Institutional Ethical Committee.
Case 1
A 52 year old, male patient presented to ENT OPD with right sided lower motor neuron (LMN) facial palsy of 2 days duration. On examination, he had a necrotic ulcer on right side of hard palate not crossing the midline. Nose examination was normal. Biopsy taken from the palate ulcer was reported as mucormycosis and on investigating he was found to be in diabetic ketoacidosis. Patient was immediately admitted and treated with Inj. Amphotericin B. But he did not respond to the treatment and expired on the 5th day.
Case 2
A 67 year old diabetic male had presented with right sided facial paresis for 1 month and right sided orbito-maxillary facial swelling for 4 days. On examination he had blackish necrotic crusts—eschar in both nasal cavities with grade III right sided LMN facial palsy. He also had oroantral fistula on right side. Diagnostic nasal endoscopy was done and tissue sent for HPR, was positive for Mucormycosis. His CT scan showed a well defined soft tissue density in right retro bulbar region. Repeated surgical debridement with iv Amphotericin B was the line of management. Optimal dose of 1600 mg was administered until the biopsy was reported as negative for mucormycosis. The Facial paresis improved as seen in Fig. 1.
Fig. 1.
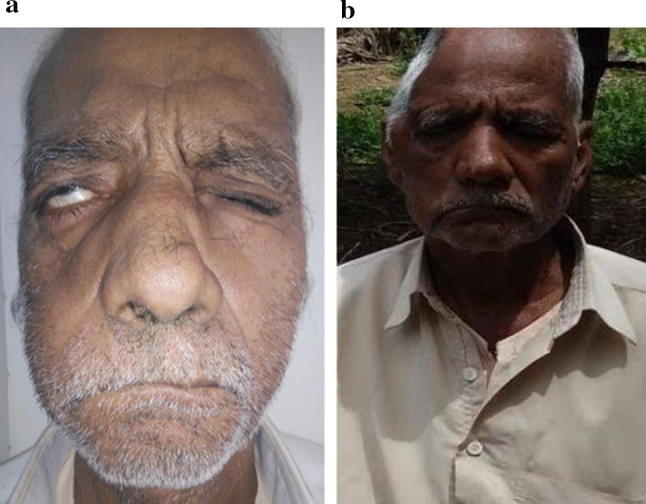
a On presentation—right LMN facial palsy, b improvement of facial palsy on completion of treatment
Case 3
Third patient was a 57 years old diabetic male. He was referred to us from medicine OPD with complaints of headache for 15 days and left facial nerve palsy for 3 days. On ENT examination, he had eschar in both the nasal cavities with anterior septal perforation, ischemic necrotic patch over left side of hard palate and left LMN grade III facial nerve palsy. Right eye examination revealed ptosis and ophthalmoplegia (Fig. 2). CT scan showed bilateral fronto-maxillary and right orbital soft tissue densities. Histopathology report was suggestive of Mucormycosis. Wide surgical debridement was done and injection Amphotericin B was administered. In spite of this, the patient worsened clinically and expired on 6th day.
Fig. 2.
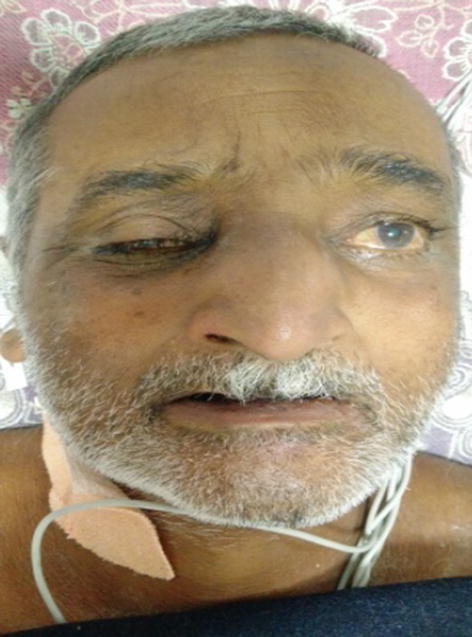
Image showing right eye ptosis with left facial palsy
Case 4
Fourth patient was a 27 year old diabetic and chronic alcoholic male with headache and left facial palsy for 8 days (Fig. 3). On ENT examination, he had blackish eschar in both nasal cavities with left LMN facial palsy grade III. His CT PNS showed pansinusitis. Left side had more extensive involvement than right side. Tissue for HPR was reported as broad, aseptate hyphae right angle to each other, suggestive of Mucormycosis. Amphotericin B was given intravenously and surgical debridement done till HPR was negative for mucormycosis. Optimal dose of 1450 mg of Amphotericin B was given. Patient was followed up regularly. Facial palsy improved and patient had complete eye closure.
Fig. 3 .
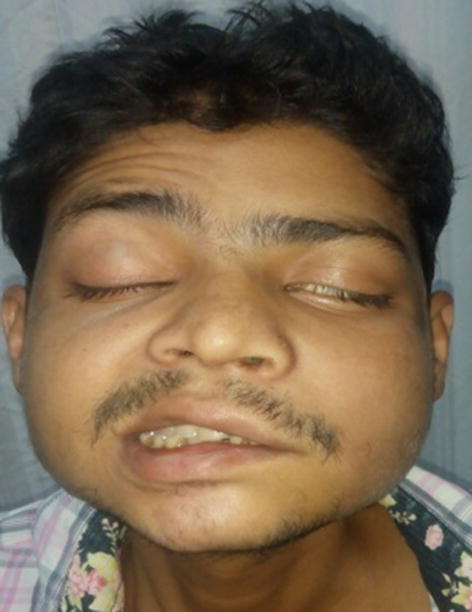
Patient having left LMN facial palsy
Discussion
Rhino cerebral mucormycosis begins in the nasal cavity and spreads to paranasal sinuses via angular, ethmoidal and lacrimal vessels. From paranasal sinuses, it invades into the retro-orbital region. Usually, it presents with facial or orbital swelling and pain, diabetes being the most common predisposing factor. In diabetes, the elevated blood sugar levels and acidemia produces a favorable environment for the spores to germinate and hyphae production. The pathogenesis is due to angio-invasion by hyphae leading to thrombosis which eventually occludes the vessels and causes ischemia. This produces the characteristic black necrotic eschar on which the fungus thrives and causes rapid spread of infection into surrounding area [3].
Presentation of mucormycosis as facial nerve palsy has been reported in few isolated cases. The involvement of various tissues in rhino-cerebral area can lead to clinical presentation mimicking other conditions like cerebro vascular accidents (CVA) [4]. The aim of this study is to highlight involvement of facial nerve paresis as a presenting feature in rhino cerebral mucormycosis. We had four patients who presented with facial nerve paresis with rhino cerebral mucormycosis. Three of them were known diabetics whereas one was diagnosed on admission (Table 1). All of them underwent repeated surgical debridement followed by intravenous Amphotericin B infusion. Two of the patients survived and the other two expired. The two expired patients could complete only 300 mg and 700 mg of Amphotericin B infusion (Table 2). Facial nerve paresis improved in the two patients (who survived) after completion of treatment. However none of these patients had any ear complaints and ear examination was normal in all four patients and no other cranial nerve was involved. Frequency of facial nerve paralysis in RCM is 11%. The exact mechanism of involvement of facial nerve is unknown, and no definite pathology of the facial nerve has been identified.
Table 1.
Clinical features of patients having mucormycosis with facial palsy
| Clinical features | Case 1 | Case 2 | Case 3 | Case 4 |
|---|---|---|---|---|
| Facial palsy | + | + | + | + |
| Nasal eschar | + | + | + | + |
| Nasal discharge | − | − | + | + |
| Hard palate—necrotic patch | + | + | + | + |
| KOH mount | Fungal elements seen | Fungal elements seen | Fungal elements seen | Fungal elements seen |
| HPR | Broad, aseptate hyphae at right angle to each other | Broad, aseptate hyphae at right angle to each other | Broad, aseptate hyphae at right angle to each other | Broad, aseptate hyphae at right angle to each other |
| Diabetics | + | + | + | + |
| Ketoacidosis | + | − | + | + |
| Duration of illness | 2 days | 1 month | 15 days | 8 days |
Table 2.
Endoscopic findings, CT scan findings and treatment of the patients
| Sr. No | Age, sex | Endoscopy findings | CT scan findings | Treatment |
|---|---|---|---|---|
| 1 | 52,M | Blackish crusts—eschar in both nostrils | Soft tissue shadow in bilateral maxillary sinus, sphenoid, ethmoid and frontal sinuses predominantly on right side | iv Amphotericin B (300 mg) with repeated surgical debridement. Patient expired and could not complete the treatment |
| 2 | 70, M | Blackish crusts—eschar in both nostrils | Soft tissue density noted in retrobulbar region at the floor of right orbit. Chronic sinusitis of right maxillary sinus | iv Amphotericin B (1600 mg) with repeated surgical debridement |
| 3 | 57, M | Blackish crusts—eschar in both nostrils | Bilateral frontomaxiallary soft tissue swelling and right orbital swelling | iv Amphotericin B (700 mg) with repeated surgical debridement. Patient expired and could not complete the treatment |
| 4 | 27, M | Blackish crusts—eschar in both nostrils | Soft tissue shadow in bilateral maxillary sinus, sphenoid, ethmoid and frontal sinuses predominantly on left side | iv Amphotericin B (1450 mg) with repeated surgical debridement |
Involvement of pterygopalatine fossa has been reported by some authors as a route of spread of mucormycosis to the facial nerve. Pterygopalatine fossa is also considered to be a reservoir of mucor from where it spreads to retro global space of orbit and infra temporal space [5]. So, the infection reaching the pterygopalatine fossa can spread to the inferior orbital fissure, orbital apex, and infra temporal fossa.
Recent studies have demonstrated spread of Mucorales species along peripheral nerves [4]. The juxtaposition of the Pterygopalatine fossa and presence of many connections of vascular and neural tissue make it a likely route of perineural invasion to the cranial tissues [4]. Perineural extension through Vidian nerve is also one of the possibilities [4]. The Vidian nerve is a continuation of greater superficial petrosal nerve. An isolated involvement of the intracanalicular facial nerve within the temporal bone can occur along the nerve sheath of this nerve [6]. This explains facial nerve affection without involvement of any other cranial nerves as seen in our study.
Facial nerve may also be affected by direct spread through the Eustachian tube or through the vascular channels into middle ear [3]. This fungus is a normal commensal in the nose and nasopharynx of healthy individuals and can get opportunistic in immunocompromised diabetic state.
Another reason of involvement of facial nerve palsy can be the pathology of resistance arteries in diabetic patients which may cause oedema and localize facial nerve ischemia. This would compromise the blood supply to the nerve leading to palsy [7]. In most of the diabetic cases with facial palsy, the chorda tympani is not affected and hence the taste sensation remains unaffected. This occurs because the bifurcation of chorda tympani nerve is proximal to the site of lesion [8]. Unlike in cases of facial nerve palsy with viral or any other aetiology where the entire facial nerve is involved.
Facial nerve palsy can also be incidental and may be idiopathic as in Bell’s palsy [3].
Spread of mucormycosis can be identified using contrast enhanced MRI. Facial nerve involvement may be diagnosed by widening of pterygopalatine fossa, seen on MRI. Perineural invasion has been documented using contrast enhanced MRI [9]. But imaging studies carry their own limitations and tissue culture is the gold standard for diagnosis of mucormycosis. Diagnosis is confirmed histologically by detection of aseptate hyphae with right angled branching which is pathognomonic of mucormycosis [10]. The impact of facial nerve palsy on the overall prognosis of patients with mucormycosis is yet to be determined and cannot be derived from our case series.
Conclusion
Mucormycosis is a rapidly progressive and fatal fungal infection. Early diagnosis and treatment is the key for survival of the patient. Facial nerve palsy is a significant presenting feature of mucormycosis. Such patients may be misdiagnosed as CVA with subsequent delay in treatment. A high index of suspicion for mucormycosis in diabetic patients presenting with facial palsy will be helpful in achieving early and accurate diagnosis with prompt management and better outcome.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
The study was approved by the Institutional Ethics Committee.
Contributor Information
Rajashri Mane, Email: manerajashri45@gmail.com.
Balasaheb Patil, Email: drbalasaheb6@gmail.com.
Anjana Mohite, Email: dranjanamohite@gmail.com.
Roshni Mohanty, Email: roshni040493@gmail.com.
References
- 1.Bouza E, Munoz P, Guinea J. Mucormycosis: an emerging disease. Clin Microbiol Infect. 2006;12:7–23. doi: 10.1111/j.1469-0691.2006.01604.x. [DOI] [Google Scholar]
- 2.Mane RS, Watve JK, Mohite AA, et al. Rhinocerebral mucormycosis: a deadly disease on the rise. Indian J Otolaryngol Head Neck Surg. 2007;59:112. doi: 10.1007/s12070-007-0034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakshi SS. An unusual cause for facial nerve palsy: mucormycosis. Int J Diabetes Dev Ctries. 2016;36:385. doi: 10.1007/s13410-016-0468-7. [DOI] [Google Scholar]
- 4.Aloosi SN. Facial nerve palsy as frequent presentation in patient with rhinocerebral mucormycosis. J Oral Dental Res. 2018;5(1):55–69. [Google Scholar]
- 5.Hosseini SM, Borghei P. Rhinocerebral mucormycosis: pathways of spread. Eur Arch Otorhinolaryngol. 2005;262:932–938. doi: 10.1007/s00405-005-0919-0. [DOI] [PubMed] [Google Scholar]
- 6.Swift AC, Denning DW. Skull base osteitis following fungal sinusitis. J Laryngol Otol. 1998;112(1):92–97. doi: 10.1017/S0022215100140009. [DOI] [PubMed] [Google Scholar]
- 7.Shekar V, Sikander J, Rangdhol V, Naidu M. Facial nerve paralysis: a case report of rare complication in uncontrolled diabetic patient with mucormycosis. J Nat Sci Biol Med. 2015;6:226–228. doi: 10.4103/0976-9668.149195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pecket P, Schattner A. Concurrent Bell’s palsy and diabetes mellitus: a diabetic mononeuropathy? J Neurol Neurosurg Psychiatry. 1982;45:652–655. doi: 10.1136/jnnp.45.7.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Safdar A, Jr DMP, Talwani R, Thompson CR. Intracranial perineural extension of invasive mycosis: a novel mechanism of disease propagation by Aspergillus fumigatus. Clin Infect Dis. 2002;35(5):e50–e53. doi: 10.1086/341972. [DOI] [PubMed] [Google Scholar]
- 10.Ferry AP, Abedi S. Diagnosis and management of rhinoorbitocerebral mucormycosis (phycomycosis). A report of 16 personally observed cases. Ophthalmology. 1983;90:1096–1104. doi: 10.1016/S0161-6420(83)80052-9. [DOI] [PubMed] [Google Scholar]


