In this series a clinician extemporaneously discusses the diagnostic approach (regular text) to sequentially presented clinical information (bold). Additional commentary on the diagnostic reasoning process (italic) is interspersed throughout the discussion.
A 53-year-old policeman with a history of gastroesophageal reflux disease (GERD) presented to his primary care physician for routine follow-up. On review of systems, he reported multiple episodes of new-onset chest discomfort over the previous month. He described it as non-radiating, left parasternal burning or pressure, which differed from his usual GERD symptoms. The discomfort lasted up to 30 minutes, occurred at rest or with exertion, and always resolved spontaneously.
Causes of chest pain include cardiac, pulmonary, gastrointestinal, and musculoskeletal processes. The patient’s age and sex trigger consideration of angina because it is a “cannot miss” diagnosis. But the characteristics of his chest discomfort define it as non-anginal (i.e., lacking the classic features of substernal chest pain precipitated by exertion and relieved by rest), which significantly lowers the pretest probability of coronary artery disease (CAD). The substernal, burning nature of the pain and prior history of GERD increase the probability of a gastrointestinal etiology; however, the discomfort differs from his usual GERD symptoms, making that diagnosis less likely. Esophageal spasm, a mimicker of angina, also deserves consideration. It presents as visceral, pressure-like squeezing pain, occurring at rest or with exertion, and may be exacerbated by GERD. Further complicating the differentiation between angina and esophageal spasm is the fact that nitroglycerin, a smooth muscle relaxant, may relieve symptoms in both conditions. Musculoskeletal diseases, such as costochondritis or muscle strain, may also cause chest pain, but the fact that it occurs without movement makes these causes unlikely.
The discussion highlights two analytic reasoning approaches: worst-case scenario versus Bayesian reasoning. The discussant immediately focuses on CAD given its potentially life-threatening sequelae and employs a Bayesian analytic approach by focusing on pretest probability. He notes that the patient’s chest discomfort is non-anginal, which significantly reduces the pretest probability of CAD. This patient’s age, sex, and non-anginal or non-specific chest pain predict a 34% probability of CAD (Table 1).1
Table 1.
CAD Pretest Probability Prediction Model
| Age | Men | Women | ||||
|---|---|---|---|---|---|---|
| Non-specific chest pain | Atypical chest pain | Typical chest pain | Non-specific chest pain | Atypical chest pain | Typical chest pain | |
| 30–39 | 18 | 30 | 59 | 5 | 10 | 28 |
| 40–49 | 25 | 38 | 69 | 8 | 14 | 37 |
| 50–59 | 34 | 50 | 77 | 12 | 20 | 47 |
| 60–69 | 44 | 60 | 84 | 17 | 28 | 58 |
Modified from: Genders TSS, et al. A clinical prediction rule for the diagnosis of coronary artery disease: validation, updating and extension. Eur Heart J. 2011, 32:1316–1330
He had a history of hypercholesterolemia treated with statins for more than 10 years. His low-density lipoprotein (LDL) level was consistently below 100 mg/dl. He never smoked. He had a negative exercise stress test for atypical chest pain performed more than 10 years ago. He had sleep apnea and regularly used continuous positive airway pressure (CPAP) therapy. He had a remote history of a postoperative deep venous thrombosis (DVT) after meniscal knee surgery.
The absence of a textbook pattern for his symptoms and relatively low pretest probability for CAD make me wonder about other life-threatening conditions like aortic dissection or pericardial tamponade. However, neither the quality of the chest discomfort nor the prolonged time course fits. Pulmonary embolism (PE) merits consideration given the patient’s history of provoked DVT. However, lack of shortness of breath or pleuritic chest pain would be unusual.
The discussant continues applying a Bayesian lens by considering how contextual risk factors impact pretest probability. The literature suggests doctors poorly estimate pretest probability 2because of the availability heuristic, a cognitive bias wherein the probability of memorable (e.g., recent, dramatic, or highly publicized) diseases is overestimated while the probability of other conditions is underestimated. 3In addition, even when provided accurate pretest probability estimates, clinicians do not fully adjust their pretest probability estimates due to a cognitive attachment (“anchor”) to the initial pretest probability, a phenomenon known as anchoring. 3In practice, clinicians typically use pattern recognition to develop a gestalt or “quick and dirty” initial estimation of pretest probability.
He has a significant family history including a father and four paternal uncles with CAD in their early fifties. All had high cholesterol and smoked cigarettes. A brother who did not smoke had a myocardial infarction at age 38. Two sisters and 3 other brothers were in good health. His medications included low-dose aspirin, omeprazole, simvastatin, and ezetimibe. He did not drink alcohol. He exercised regularly.
Although the strong family history increases the concern for CAD, it is difficult to estimate the magnitude of risk due to genetic predisposition. I do wonder about the possibility of familial hypercholesterolemia or rarer, genetic causes of CAD.
The lack of a definite diagnostic pattern match and a potentially life-threatening symptom (chest pain) lead to a cautious analytical reasoning approach, in which the discussant highlights the similar clinical presentations of angina and esophageal spasm. The physician has difficulty determining how much the patient’s family history of premature CAD should impact disease probability. Physicians may vacillate between analytical and intuitive diagnostic reasoning (e.g., pattern recognition) depending on their knowledge and available evidence.
On examination, the patient was afebrile with a blood pressure of 125/71, heart rate of 82, and respiratory rate of 12. His cardiac examination demonstrated a regular rhythm and rate with no murmurs, rubs, or gallops. He had a normal jugular venous pressure, normal peripheral pulses, and no edema. His lungs were clear. The remainder of his examination was normal.
In patients with chest pain, there are few highly sensitive or specific findings other than asymmetric blood pressure readings or pulse deficits in aortic dissection and pericardial friction rub in pericarditis. A resting electrocardiogram (ECG) may show abnormalities suggestive of CAD (e.g., Q waves), but is not sensitive enough to exclude that diagnosis. A lipid profile (to confirm statin adherence) and screening for diabetes should be considered, as well as a complete blood count to rule out anemia as an exacerbating factor for angina.
The discussant demonstrates Bayesian reasoning in thinking about how the sensitivity and specificity of a given test impact the posttest probability of CAD. Useful mnemonics include “Positive SpIn” and “Negative SnOut”: a positive high specificity test effectively rules in the diagnosis, whereas a negative high sensitivity test effectively rules it out. Likelihood ratios (LRs) operationalize these test characteristics to estimate an individual patient’s posttest probability using Bayes’ rule (see below). The discussant highlights the lack of sensitivity of several non-invasive tests for diagnosis of obstructive CAD.
The ECG was normal. His electrolytes, BUN, creatinine, and CBC were normal.
The normal resting ECG is not surprising, but it is a low sensitivity test. Using a negative LR of 0.73 for a resting ECG,4 the likelihood ratio nomogram (Fig. 1) produces a posttest CAD probability of 27% (i.e., line connecting pretest probability of 34% from the Diamond-Forrester table and LR− of 0.73). The next step is to consider an exercise stress test, which has a greater sensitivity. Given the potentially fatal nature of untreated CAD and relatively benign nature of stress testing, I have a low threshold for ordering this non-invasive test. A CAD probability of 27% exceeds this threshold.
Figure 1.
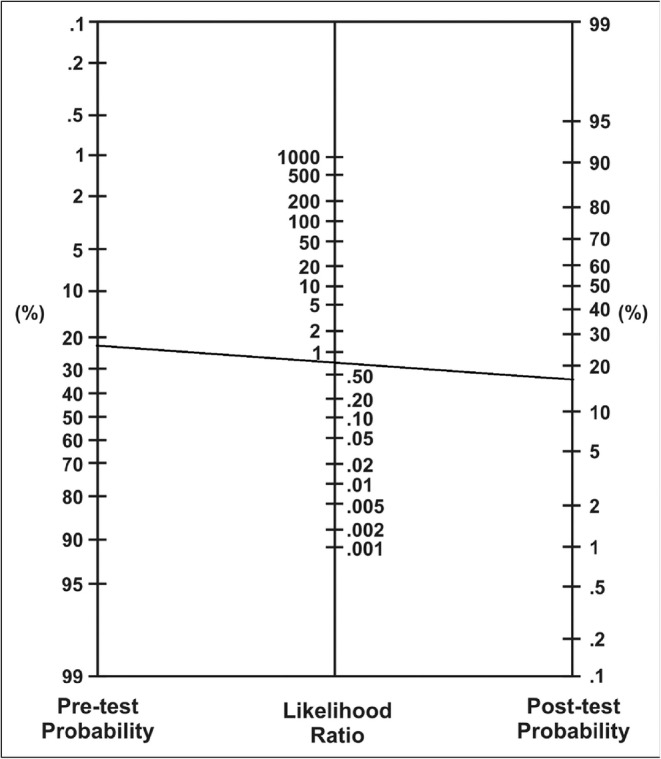
The impact of a negative resting ECG on the posttest probability of CAD.
Clinicians implicitly make decisions using their estimated probability of disease based on history, physical examination, and results of diagnostic testing, as well as the harms and benefits of tests and treatments. A critical question to consider before ordering a test is “Will the test results change my management?” In contrast, a more prescriptive or normative approach involves using threshold probabilities (of disease) to provide guidance for when to test or treat (Fig. 2).5For probabilities of disease below the “testing threshold,” neither testing nor treatment should be pursued. Conversely, with probabilities of disease above the “treatment threshold,” treatment should be initiated without further testing. For intermediate probabilities between these two thresholds, additional testing should be performed to clarify the probability estimate.
Figure 2.
Adapted from Pauker and Kassirer. NEJM. 1980; 302:1109–1117.
An exercise radionuclide myocardial perfusion imaging (rMPI) test was performed. The patient exercised for 13 min and stopped due to fatigue and knee pain, reaching 105% of his target heart rate. There were no ECG changes. The calculated Duke treadmill score was 13, a low-risk result. However, the rMPI showed a large, reversible, inferior defect consistent with ischemia. Left ventricular ejection fraction was normal.
Understanding test characteristics is essential to high-value clinical decision-making. As discussed above, the probability of CAD based on history, physical exam, and resting ECG was approximately 27%.1 We can more efficiently determine posttest probability by multiplying the LRs of the negative exercise ECG test (LR− = 0.4)6 times the positive rMPI (LR+ = 3.6)7 to obtain a combined LR of 1.44. Using the nomogram (Fig. 1), this LR combined with a pretest probability of 27% yields a posttest CAD probability of 35%.
At this point, my working diagnosis is CAD, although esophageal spasm is still possible. With a CAD probability of 35% and large perfusion imaging defect, some clinicians would pursue coronary angiography. A coronary intervention may improve survival in patients with moderate-to-large ischemia, but carries risks of complications. On the other hand, labeling the patient with CAD can also have negative personal and social consequences. I would discuss the risks of cardiac catheterization with the patient and explore his preferences.
The discussant articulates an explicit Bayesian estimation of CAD likelihood. Some clinicians might view a large defect on stress imaging as definitive evidence of CAD. However, the relatively low pretest probability and normal exercise ECG suggest the patient has a 65% chance of not having CAD. Moreover, the Duke treadmill score of 13 (maximum exercise time in minutes − [5 × ST segment deviation in mm] − [4 × angina index]; angina index = 0 [no angina], 1 [non-limiting angina], or 2 [exercise limiting angina]) suggests an average annual mortality between 0.3 and 0.9% (low-risk score ≥ 5). However, up to 16% of low-risk patients may have obstructive single-vessel CAD, which would be consistent with the patient’s imaging results. 8
The discussant emphasizes the importance of shared decision-making, particularly in diagnostically ambiguous cases. According to the Institute of Medicine’s Crossing the Quality Chasm report, patient-centered care is “respectful of and responsive to individual patient preferences, needs, and values and ensures that patient values guide all clinical decisions.” The use of decision aids to facilitate the discussion of benefits and harms has been shown to improve patients’ knowledge, accuracy of risk perception, and likelihood of choosing options consistent with their values. 9However, integrating shared decision-making into routine clinical practice remains quite challenging. 10, 11
The PCP discussed the findings, specifically that the imaging suggested single-vessel CAD, but that his exercise performance placed him in a low-risk category. The PCP outlined 2 possible strategies: “labeling” the patient with mild CAD and treating medically, or determining the extent of disease with coronary angiography. The PCP was concerned that catheterization might lead to a stent placement with its incumbent risks and uncertain benefits. The patient and his family were anxious about the diagnostic uncertainty, particularly the risk of myocardial infarction, given his stressful job as a policeman. After further consultation, he decided to undergo a cardiac catheterization. In the interim, the patient was prescribed sublingual nitroglycerin and a beta-blocker.
Given his strong family history and 35% probability of CAD, the patient is appropriately worried. A normal catheterization would rule out obstructive CAD; plus testing for coronary reactivity with injection of intracoronary ergonovine might provide evidence for coronary vasospasm, an alternative diagnostic hypothesis.
A confounding aspect of chest pain management is that cardiac catheterization is both a diagnostic and therapeutic procedure. If an obstructive lesion is found, treatment (e.g., coronary intervention) may occur despite unclear or uncertain benefits. This discussion highlights the impact of uncertainty on clinical decision-making. Although the patient’s prognosis is excellent, the desire to achieve greater diagnostic certainty leads to additional testing. While the evidence for sequential testing is sparse, appropriate use criteria have been developed to promote rational use of imaging services. For patients with low pretest probability, coronary angiography may be appropriate, because the risk of missing the diagnosis of CAD outweighs the risk of the procedure. Nevertheless, physician judgment, patient preferences, and insurance coverage also influence these decisions. 11
One week before the scheduled catheterization, the patient was in a meeting at work and suddenly developed chest pain, initially described as a burning, which radiated down his left arm. He became pale and diaphoretic and took a sublingual nitroglycerin, which relieved the pain in a few minutes. He was brought to the emergency room, where an ECG and troponins were both normal. After being admitted to the hospital, he had no further episodes of chest pain. A cardiac catheterization revealed normal coronary arteries. While the exact cause of his chest pain remained uncertain, a presumptive diagnosis of GERD was made and additional acid suppression was recommended for symptom relief. Additional evaluation was not deemed necessary since there were no other worrisome clinical features and the symptoms did not recur.
The normal catheterization is surprising, especially since the stress rMPI testing suggested CAD. However, his symptoms were atypical and relief with nitroglycerin is not entirely specific for angina. Even after a negative ECG stress with positive rMPI, the calculated probability of CAD was only 35%. Furthermore, his exercise tolerance suggested an excellent prognosis. In this case, the “subjective” clinical assessment was more accurate than the “objective” rMPI defect, which turned out to be a false positive test result.
The discussant reemphasizes Bayesian pretest probability estimation. There are very few tests with sufficiently high sensitivity and specificity to rule in or rule out a disease. In addition, every test result requires interpretation or clinical judgment. In this case, the clinician and patient remained “anchored” on the impressively abnormal imaging defect, a common cognitive trap. 12
DISCUSSION
In busy clinical settings, clinical decisions are often made using gestalt or intuitive judgments. However, slower analytic approaches may enhance clinical decision-making, including use of likelihood ratios and on-line clinical prediction rules to more accurately estimate disease probability. These two, complementary approaches (‘fast’ and ‘slow’ thinking) are known as dual process theory.
This case highlights the Bayesian clinical reasoning approach and influential effect of “anchoring” on abnormal imaging tests. Bayesian reasoning can be used to explain diagnostic and therapeutic choices and ideally help patients make informed decisions. Conflicting test results (e.g., positive exercise rMPI testing but low-risk Duke treadmill score) can make the application of Bayesian reasoning challenging in practice. Furthermore, diagnostic uncertainty remains a powerful driver of testing even when test results are not likely to improve care.13 Patients’ psychological needs and personal preferences sometimes conflict with high-value care principles, requiring a delicate balancing act for clinicians. Explicit shared decision-making discussions about uncertainty between physicians and patients may help both to deal with it more effectively.
CLINICAL PEARLS
The CAD consortium table for estimated CAD risk 1(Table 1) enables clinicians to estimate pretest probabilities of CAD based on age, sex, and presenting symptoms.
A low-risk Duke treadmill score of ≥ 5 predicts a low annual mortality of 0.25% per year. 14However, annual mortality may be as high as 1.1% for individuals with high-risk clinical features including male sex, history of myocardial infarction, and diabetes. 15Nuclear rMPI testing has a limited impact on risk stratification in patients with low-risk clinical features. 1The combination of the patient’s low-risk treadmill score and lack of high-risk clinical features suggested a 7-year cardiac survival of 99% and non-fatal MI-free survival of 96%. 16
Likelihood ratios provide a sense of the value of a test. Their impact on disease probability can be determined through nomograms or online posttest probability calculators. Likelihood ratios can be calculated with sensitivity and specificity data, which are available in the JAMA®rational clinical exam series and McGee’sEvidence-based Physical Diagnosis.17
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Genders TSS, Steyerberg EW, Alkadhi H, Leschka S, Desbiolles L, Nieman K, et al. The CAD Consortium. A clinical prediction rule for the diagnosis of coronary artery disease: validation, updating and extension. Eur Heart J. 2011;32:1316–1330. doi: 10.1093/eurheartj/ehr014. [DOI] [PubMed] [Google Scholar]
- 2.Poses RM, et al. The accuracy of experienced physicians’ probability estimates for patients with sore throats: implications for decision making. JAMA. 1985;254(7):925–29. doi: 10.1001/jama.1985.03360070063024. [DOI] [PubMed] [Google Scholar]
- 3.Redelmeier DA. Improving patient care. The cognitive psychology of missed diagnoses. Ann Intern Med. 2005;142(2):115–20. doi: 10.7326/0003-4819-142-2-200501180-00010. [DOI] [PubMed] [Google Scholar]
- 4.Mahmoodzadeh S, Moazenzadeh M, Rashidinejad H, Sheikhvatan M. Diagnostic performance of electrocardiography in the assessment of significant coronary artery disease and its anatomical size in comparison with coronary angiography. J Res Med Sci. 2011;16(6):750–755. [PMC free article] [PubMed] [Google Scholar]
- 5.Pauker SG, Kassirer JP. The threshold approach to clinical decision making. N Engl J Med. 1980;302(20):1109–17. doi: 10.1056/NEJM198005153022003. [DOI] [PubMed] [Google Scholar]
- 6.Garber AM, Solomon NA. Cost-effectiveness of alternative test strategies for the diagnosis of coronary artery disease. Ann Intern Med. 1999;130(9):719–28. doi: 10.7326/0003-4819-130-9-199905040-00003. [DOI] [PubMed] [Google Scholar]
- 7.Underwood SR, Anagnostopoulos C, Cerqueira M, et al. Myocardial perfusion scintigraphy: the evidence. Eur J Nucl Med Mol Imaging. 2004;31:261–91. doi: 10.1007/s00259-003-1344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw LJ, Peterson ED, Shaw LK, Kesler KL, DeLong ER, Harrell FE, Muhlbaier LH, Mark DB. Use of a prognostic treadmill score in identifying diagnostic coronary disease subgroups. Circulation. 1998;98(16):1622–30. doi: 10.1161/01.CIR.98.16.1622. [DOI] [PubMed] [Google Scholar]
- 9.Stacey D, Légaré F, Col NF, Bennett CL, Barry MJ, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2014;(1):CD001431. 10.1002/14651858.CD001431.pub4. [DOI] [PubMed]
- 10.Barry MJ, Edgman-Levitan S. Shared Decision Making — The Pinnacle of Patient-Centered Care. New Engl J Med. 2012;366(9):780–1. doi: 10.1056/NEJMp1109283. [DOI] [PubMed] [Google Scholar]
- 11.Walsh MN, Bove AA, Cross RR, Ferdinand KC, Forman DE, Freeman AM, et al. ACCF 2012 Health policy statement on patient-centered care in cardiovascular medicine: a report of the American College of Cardiology Foundation Clinical Quality Committee. J Am Coll Cardiol. 2012;59(23):2125–43. doi: 10.1016/j.jacc.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Botros J, Rencic J, Centor RM, Henderson MC. Anchors away. J Gen Intern Med. 2014;29(10):1414–8. doi: 10.1007/s11606-014-2879-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kassirer JP. Our stubborn quest for diagnostic certainty: a cause of excessive testing. NEJM. 2010;320(22):14890–1491. doi: 10.1056/NEJM198906013202211. [DOI] [PubMed] [Google Scholar]
- 14.Cannon CP, Weintraub WS, Demopoulos LA, Vicari R, Frey MJ, Lakkis N, Neumann FJ, Robertson DH, DeLucca PT, DiBattiste PM, Gibson CM. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. New Engl J Med. 2001;344(25):1879–87. doi: 10.1056/NEJM200106213442501. [DOI] [PubMed] [Google Scholar]
- 15.Mark DB, Shaw L, Harrell F, Hlatky M, Lee K, Bengston JR, et al. Prognostic value of a treadmill exercise score in outpatients with suspected coronary artery disease. N Engl J Med. 1991;325(12):849–53. doi: 10.1056/NEJM199109193251204. [DOI] [PubMed] [Google Scholar]
- 16.Poornima IG, Miller T, Christian T, Hodge D, Bailey KR, Gibbons RJ. Utility of myocardial perfusion imaging in patients with low-risk treadmill scores. J Am Coll Card. 2004;43(2):194–199. doi: 10.1016/j.jacc.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 17.McGee SR. Evidence-based physical diagnosis. 3. Philadelphia: Elsevier Health Sciences; 2012. [Google Scholar]



