Abstract
In patients with asymptomatic cervical lymphadenopathy, the physician often has to choose between evaluation via follow-up or open biopsy. Follow-up evaluation may lead to a delayed diagnosis of lymphoma, while an open biopsy is associated with surgical risks and costs. This dilemma can be avoided using predictive parameters. In the present study, we aimed to investigate whether neutrophil-to-lymphocyte ratio (NLR), a parameter which can be assessed quickly with ease and at low cost, has predictive value for Hodgkin’s lymphoma diagnosis in patients with asymptomatic cervical lymphadenopathy. A total of 46 patients with asymptomatic cervical lymphadenopathy who underwent open biopsy were included in the study. Based on the biopsy results, the patients were divided into two groups, Hodgkin lymphoma (26 patients) and reactive lymphadenopathy (20 patients). The mean NLR in the groups was calculated and compared based on the results of complete blood count performed before biopsy. We found that mean NLR (P = 0.022) and mean neutrophil count (P = 0.046) were higher and mean lymphocyte count was lower (P = 0.054) in patients with Hodgkin’s lymphoma compared to those in patients with reactive lymphadenopathy. Our results indicate that a high NLR may have predictive value for Hodgkin’s lymphoma diagnosis in patients with asymptomatic cervical lymphadenopathy.
Keywords: Neutrophil-to-lymphocyte ratio, Hodgkin lymphoma, Lymphadenopathy, Biopsy, Predictive value
Introduction
Lymphadenopathy is a clinical term describing an increase in lymph node size, and it also refers to lymph nodes that are abnormal in size, consistency, or number. The possible causes are infectious, immunological, neoplastic, or metabolic disorders [1, 2]. The head and neck region has a rich lymphatic system with approximately one-third of all lymph nodes in the body, and, therefore, it is not surprising that lymphoma is often found in the neck [3]. The presence of lymph nodes larger than 1 cm in this region, persisting for more than 4 weeks, requires diagnostic evaluation [1, 4].
Lymphoma is the most common diagnosis for a persistent enlarging unilateral neck mass in young patients aged 20–40 years. The two main types of lymphoma are Hodgkin’s lymphoma (HL) and non-Hodgkin’s lymphoma (NHL). HL often presents as painless cervical lymphadenopathy. ‘B symptoms’ can be seen in both HL and NHL with fever, weight loss, and night sweats, while some patients do not have any symptoms and may present with asymptomatic cervical lymphadenopathy [3, 5]. Approximately 25% of patients with HL are known to have B symptoms, while a study by Storck et al. [6, 7] found this rate to be 11%. Ultrasonography-guided fine-needle aspiration biopsy is used to diagnose cervical metastatic carcinoma and the primary focus can be detected via upper aerodigestive endoscopy. However, in lymphomas, cytological evaluation is usually not sufficient and open biopsy is often necessary [8]. In patients without any other symptoms other than cervical lymphadenopathy, both clinicians and surgeons are faced with a decision between choosing follow-up evaluation or open biopsy. The decision of surgery is associated with possible risks (bleeding, nerve injury) and costs to the patient, while the decision to follow-up may lead to a delayed diagnosis of lymphoma. This high lights the need for predictive parameters to prevent false negative diagnoses in patients undergoing biopsy.
In the present study, we aimed to determine whether neutrophil-to-lymphocyte ratio (NLR) has predictive value for HL diagnosis in patients with asymptomatic cervical lymphadenopathy.
Materials and Methods
A retrospective case–control study was conducted at the Ankara Numune Training and Research Hospital. The study was conducted in accordance with the principles of the Helsinki Declaration and was approved with the 12/13/2018 dated and 18-2357 numbered permission by the Ankara Numune Training and Research Hospital Corporate Ethics Committee.
Records of patients with asymptomatic cervical lymphadenopathy who underwent a diagnostic excisional lymph node biopsy between 2012 and 2017 were retrospectively reviewed and 71 patients were selected. One patient with thyroid papillary carcinoma metastasis, five patients with malignant epithelial tumors, four patients with lipoma, three patients with nasopharyngeal carcinoma metastasis, four patients with granulomatous lymphadenitis, and eight patients with NHL were excluded from the study. A total of 46 patients were finally included in the study. These patients were divided into two groups, reactive lymphadenopathy (RAL), and HL. All patients underwent upper aerodigestive endoscopy and no mucosal pathology was found before biopsy. Prophylactic antibiotics (amoxicillin + clavulanic acid, 1000 mg, twice a day) were given to all patients for 2 weeks. In cases showing no change in neck swelling, a biopsy decision was made with the suspicion of lymphoma. Blood samples taken from a peripheral vein before biopsy were tested using a Sysmex WE-2100 (Sysmex Corporation, Kobe, Kansai, Japan) and hemoglobin levels, erythrocyte count, leukocyte count, neutrophil count, lymphocyte count, and platelet count were determined. The NLR was calculated by dividing the neutrophil count with the lymphocyte count. Data of age, gender, lymph node size, complete blood count, and NLR in the HL and RAL groups were added into a data base and compared via statistical analysis.
The Shapiro–Wilk test was used to assess the distribution of the data. Continuous data were expressed as mean with standard deviation. Intergroup comparisons of independent variables were performed using Student’s t test and Mann–Whitney U tests for normally and non-normally distributed variables, respectively. A value of P < 0.05 was deemed to represent statistical significance. The software SPSS (SPSS for Windows version 21.0; SPSS Inc, Chicago, IL, USA) was used for all statistical analyses.
Results
The mean age of the 20 patients (12 males, 8 females) in the RAL group was 35.15 ± 14.5 years; the mean age of the 26 patients (16 males, 10 females) in the HL group was 40.15 ± 13.58 years, with no significant difference between the two groups (P = 0.307). The mean lymph node size was 16.15 ± 13.79 mm in the RAL group and 16.95 ± 8.10 mm in the HL group, with a statistically significant difference between the groups (P = 0.029). Hemoglobin (Hb) levels were also significantly different between the groups (P = 0.041); the mean Hb in the RAL group was 14.51 ± 1.60 g/dL and that in the HL group was 13.08 ± 2.91 g/dL.
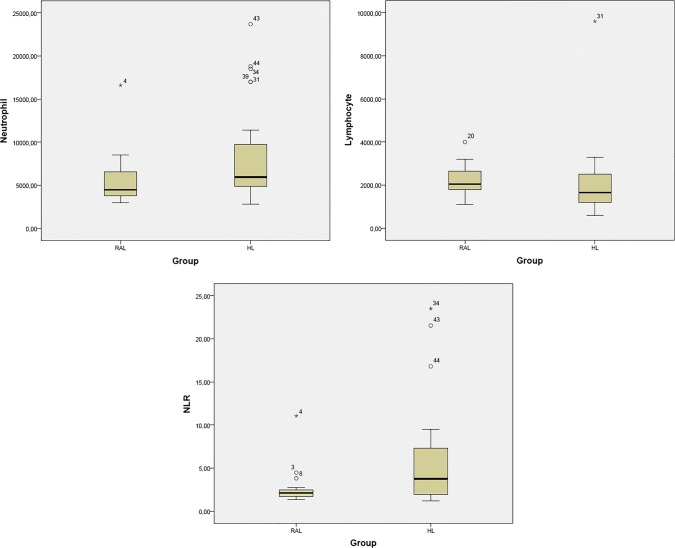
The mean leukocyte count in the RAL group was 8.6 ± 3.3 × 103/µL, while it was 11.2 ± 5.6 × 103/µL in the HL group; this difference was not statistically significant (P = 0.116). There was also no significant difference in mean platelet count between the groups (P = 0.219); the RAL group had a count of 275.7 ± 82.6 × 103/µL, and the HL group had a count of 321.7 ± 129.7 × 103/µL. However, the mean neutrophil count was 5.5 ± 3.0 × 103/µL in the RAL group and 8.4 ± 5.7 × 103/µL in the HL group, and this difference was statistically significant (P = 0.046). No significant difference was found in the mean lymphocyte counts (P = 0.054) between the groups, which was 2.2 ± 0.6 × 103/µL in the RAL group and 2.0 ± 1.6 × 103/µL in the HL group. The NLR was significantly higher in the HL group than in the RAL group (P = 0.022), being 2.6 ± 2.1 in the RAL group and 5.8 ± 6.0 in the HL group (Table 1, Fig. 1).
Table 1.
Comparison between the reactive lymphadenopathy group and the Hodgkin’s lymphoma group in terms of lymph node size and complete blood count
| Mean ± standard deviation | P | ||
|---|---|---|---|
| RAL | HL | ||
| Lymph node size (mm) | 16,95 ± 8,10 | 25.15 ± 13.79 | 0.029 |
| Hemoglobin (g/dL) | 14.51 ± 1.60 | 13.08 ± 2.91 | 0.041 |
| Leukocyte (× 103/µL) | 8.6 ± 3.3 | 11.2 ± 5.6 | 0.116 |
| Platelet (× 103/µL) | 275.7 ± 82.6 | 321.7 ± 129.7 | 0.219 |
| Neutrophil (× 103/µL) | 5.5 ± 3.0 | 8.4 ± 5.7 | 0.046 |
| Lymphocyte (× 103/µL) | 2.2 ± 0.6 | 2.0 ± 1.6 | 0.054 |
| NLR | 2.6 ± 2.1 | 5.8 ± 6.0 | 0.022 |
RAL reactive lymphadenopathy, HL Hodgkin lymphoma, NLR neutrophil lymphocyte ratio
P values in bold represent statistical significance
Fig. 1.
Comparison of neutrophil count, lymphocyte count, and NLR between the reactive lymphadenopathy group and the Hodgkin’s lymphoma group
Discussion
A number of factors are involved in the etiology of cervical lymphadenopathy, including malignancies, infections, autoimmune diseases, miscellaneous and unusual conditions, and iatrogenic (MIAMI) causes being the major ones. The most common cause among the above is infections [9]. It is often challenging for physicians to reach a decision to perform biopsy in patients with asymptomatic cervical lymphadenopathy. If the underlying cause is infection, this can be easily determined via a detailed medical history and thorough physical examination. Lymphadenopathies related to epithelial carcinoma metastases can be diagnosed via upper aerodigestive tract endoscopy and fine-needle aspiration biopsy (FNAB) with ultrasound guidance (USG). However, lymphomas often require open biopsy for diagnosis. FNAB is often insufficient to diagnose lymphoma and to type the disease [10, 11]. Every patient who undergoes open biopsy based on a suspicion of lymphoma is not diagnosed with lymphoma; thus, patients with reactive lymphadenopathy undergo unnecessary surgery. To prevent such false negative situations when making decisions regarding biopsy, predictivity studies are conducted using pre-biopsy blood parameters. In a study comparing blood parameters of malignant lymphoma patients with those of benign lymphadenopathy patients, Matsumoto et al. determined that serum lactate dehydrase (LDH) and soluble interleukin-2 receptor (sIL-2r) levels were significantly higher in malignant lymphoma patients. In the same study, they found that the age of patients in the lymphoma group was significantly higher and their lymph node sizes were significantly larger [12]. Similarly, Orita et al. [8] found that serum LDH and sIL-2r levels were higher in patients with malignant lymphoma than in patients with reactive lymphadenopathy. Tsuji et al. [13] found in their study that serum thymidine kinase and sIL-2r levels were higher in lymphoma patients than in patients with benign lymphadenopathy. Although these three studies showed strong predictive values supporting pre-biopsy lymphoma suspicion, the high cost of detecting the levels of sIL-2r and serum thymidine kinase makes it challenging to use these two blood values in routine practice.
It is now well-recognized that inflammation plays an important role in cancer development and may affect the prognosis of cancer patients. Inflammatory conditions may facilitate tumor growth, invasion, and metastasis, via neutrophils and lymphocytes infiltrating into the tumor microenvironment. Keratinocyte-derived chemokines, macrophage inflammatory protein 2, and IL-8 secreted from tumor cells are known to cause neutrophil migration to the tumor site. During inflammation, neutrophils and monocytes produce angiogenic factors and cause lymphangiogenesis, leading to tumor invasion and progression [14–16]. Lymphocytes are important elements of a healthy immune system. They are absolutely necessary for an effective anti-tumoral cellular immune response. In contrast to neutrophils, lymphocytes play a role in host defense against tumor cells, and, therefore, lymphocyte infiltration is associated with a good prognosis [17]. It has been shown that neutrophils can inhibit tumor suppressor properties of lymphocytes and natural killer cells, and this is closely linked to neutrophil counts [18]. The present study found that the neutrophil count was significantly higher in the HL group compared to that in the RAL group.
Damar et al. compared lymphocyte counts in patients with benign salivary gland tumors and those in patients with malignant salivary gland tumors before treatment, and found that the counts were significantly lower in patients with malignant tumors than in patients with benign tumors [19]. Similarly, Kum et al. [20] compared lymphocyte counts in patients with benign or malignant laryngeal lesions and found that lymphocyte counts were significantly lower in patients with malignant lesions. The present study found that the lymphocyte count was lower in patients with HL than in patients with RAL; however, the difference was not statistically significant.
NLR is a low-cost and easily available indicator of inflammation and high NLR has been shown to adversely affect survival, especially when used as a prognostic indicator in hematologic malignancies [21, 22]. NLR is an indicator of the balance between a pro-tumoral inflammatory state and an anti-tumor immunity state. NLR increases due to an increase in neutrophil numbers or a decrease in lymphocyte numbers, shifting the equilibrium in favor of the pro-tumoral inflammatory state, while an increase in lymphocyte numbers or decrease in neutrophil numbers shifts this equilibrium in favor of an anti-tumor immunity status. Therefore, while any increase in NLR is an indicator of poor prognosis, a decrease in NLR is an indicator of good prognosis [23]. Although NLR has been used in many studies as a prognostic and predictive indicator, no studies showing its usability as a parameter supporting a suspicion of lymphoma in patients with undiagnosed adult cervical lymphadenopathy have been reported.
Marcheselli et al., in a study of 990 patients with nodular sclerosing type HL, showed that high NLR (≥ 6) affected both progression-free survival and overall survival negatively [24]. Seretis et al. [25] found that mean NLR was higher in patients with papillary thyroid carcinoma than in patients with benign thyroid pathology and suggested that high NLR could be used to detect incidental papillary carcinoma in patients with benign thyroid pathology. In another study, Jiang et al. compared the NLR in patients with gastric polyps or benign gastric stromal tumors with the NLR in newly diagnosed gastric cancer patients, and found the NLR to be significantly higher in the malignancy group. Studies have also reported that high NLR may be associated with malignancy in patients with undiagnosed gastric tumors [26]. Kilincalp et al. [27] have compared NLR in patients with diagnosed cancer with NLR in patients with benign pathology suspected of colorectal cancer undergoing endoscopic biopsy, and found the NLR to be higher in the malignancy group.
Many studies so far have addressed the cut-off value of NLR [28–30]. Forget et al. found normal NLR values in non-geriatric healthy adults, ranging from 0.78 to 3.53 [31]. Cho et al. [32] described high NLR as ≥ 2.7 and showed that patients with head and neck cancer with high NLR had a worse prognosis. In the present study, we found the NLR to be significantly higher in patients with HL than in those without HL; the mean NLR was 2.6 ± 2.1 in the RAL group and 5.8 ± 6.0 in the HL group.
The limitations of the present study include its retrospective nature and the low number of patients. Further prospective studies with more patients are needed to validate our findings.
Conclusion
Our results that high NLR, a parameter that can be evaluated at low cost with relative ease and speed, may have predictive value for HL diagnosis in patients with asymptomatic cervical lymphadenopathy.
Compliance with Ethical Standards
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical Rules
The study was conducted in accordance with the principles of the Helsinki Declaration and was approved with the 12/13/2018 dated and 18-2357 numbered permission by the Ankara Numune Training and Research Hospital Corporate Ethics Committee.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Al Kadah B, Popov HH, Schick B, Knöbber D. Cervical lymphadenopathy: study of 251 patients. Eur Arch Otorhinolaryngol. 2015;272:745–752. doi: 10.1007/s00405-014-3315-9. [DOI] [PubMed] [Google Scholar]
- 2.Abba AA, Khalil MZ. Clinical approach to lymphadenopathy. Ann Niger Med. 2012;6:11–17. doi: 10.4103/0331-3131.100201. [DOI] [Google Scholar]
- 3.Herd MK, Woods M, Anand R, Habib A, Brennan PA. Lymphoma presenting in the neck: current concepts in diagnosis. Br J Oral Maxillofac Surg. 2012;50:309–313. doi: 10.1016/j.bjoms.2011.03.263. [DOI] [PubMed] [Google Scholar]
- 4.Nolder AR. Paediatric cervical lymphadenopathy: when to biopsy? Curr Opin Otolaryngol Head Neck Surg. 2013;21:567–570. doi: 10.1097/MOO.0000000000000003. [DOI] [PubMed] [Google Scholar]
- 5.Urquhart A, Berg R. Hodgkin’s and non-Hodgkin’s’lymphoma of the head and neck. Laryngoscope. 2001;111:1565–1569. doi: 10.1097/00005537-200109000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Connors JM. Hodgkin’s lymphoma. In: Goldman L, Schafer AI, editors. Goldman’s cecil medicine. 24. New York: Elsevier Saunder; 2012. pp. 1228–1233. [Google Scholar]
- 7.Storck K, Brandstetter M, Keller U, Knopf A. Clinical presentation and characteristics of lymphoma in the head and neck region. Head Face Med. 2019;15:1. doi: 10.1186/s13005-018-0186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orita Y, Nose S, Sato Y, Miki K, Domae S, Hirai M, et al. Cervical lymph node extirpation for the diagnosis of malignant lymphoma. Surg Today. 2013;43:67–72. doi: 10.1007/s00595-012-0149-1. [DOI] [PubMed] [Google Scholar]
- 9.Gaddey HL, Riegel AM. Evaluation of unexplained lymphadenopathy. Am Fam Phys. 2016;94:896–903. [PubMed] [Google Scholar]
- 10.Park YM, Oh KH, Cho JG, Baek SK, Kwon SY, Jung KY, et al. Analysis of efficacy and safety of core-needle biopsy versus fine-needle aspiration cytology in patients with cervical lymphadenopathy and salivary gland tumour. Int J Oral Maxillofac Surg. 2018;47:1229–1235. doi: 10.1016/j.ijom.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Kwon M, Yim C, Baek HJ, Lee JS, Seo JH, Kim JP, et al. Ultrasonography-guided core needle biopsy of cervical lymph nodes for diagnosing head and neck lymphoma compared with open surgical biopsy: exploration for factors that shape diagnostic yield. Am J Otolaryngol. 2018;39:679–684. doi: 10.1016/j.amjoto.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto F, Itoh S, Ohba S, Yokoi H, Furukawa M, Ikeda K. Biopsy of cervical lymph node. Auris Nasus Larynx. 2009;36:71–74. doi: 10.1016/j.anl.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Tsuji T, Satoh K, Nakano H, Nishide Y, Uemura Y, Tanaka S, et al. Predictors of the necessity for lymph node biopsy of cervical lymphadenopathy. J Craniomaxillofac Surg. 2015;43:2200–2204. doi: 10.1258/ce.2008.008001. [DOI] [PubMed] [Google Scholar]
- 14.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji H, Houghton AM, Mariani TJ, Perera S, Kim CB, Padera R, et al. K-ras activation generates an inflammatory response in lung tumors. Oncogene. 2006;25:2105–2112. doi: 10.1038/sj.onc.1209237. [DOI] [PubMed] [Google Scholar]
- 16.Cassatella MA. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–26. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 17.Rabinowich H, Cohen R, Bruderman I, Steiner Z, Klajman A. Functional analysis of mononuclear cells infiltrating into tumors: lysis of autologous human tumor cells by cultured infiltrating lymphocytes. Cancer Res. 1987;47:173–177. [PubMed] [Google Scholar]
- 18.Shau HY, Kim A. Suppression of lymphokine-activated killer induction by neutrophils. J Immunol. 1988;141:4395–4402. [PubMed] [Google Scholar]
- 19.Damar M, Dinc AE, Erdem D, Aydil U, Kizil Y, Eravcı FC, et al. Pretreatment neutrophil–lymphocyte ratio in salivary gland tumors is associated with malignancy. Otolaryngol Head Neck Surg. 2016;155:988–996. doi: 10.1177/0194599816659257. [DOI] [PubMed] [Google Scholar]
- 20.Kum RO, Ozcan M, Baklaci D, Kum NY, Yilmaz YF, Gungor V, et al. Elevated neutrophil-to-lymphocyte ratio in squamous cell carcinoma of larynx compared to benign and precancerous laryngeal lesions. Asian Pac J Cancer Prev. 2014;15:7351–7355. doi: 10.7314/APJCP.2014.15.17.7351. [DOI] [PubMed] [Google Scholar]
- 21.Wang S, Ma Y, Sun L, Shi Y, Jiang S, Yu K, et al. Prognostic significance of pretreatment neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in patients with diffuse large B-cell lymphoma. Biomed Res Int. 2018;2018:1–8. doi: 10.1155/2018/9651254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mu S, Ai L, Fan F, Qin Y, Sun C, Hu Y. Prognostic role of neutrophil-to-lymphocyte ratio in diffuse large B cell lymphoma patients: an updated dose-response meta-analysis. Cancer Cell Int. 2018;18:1–9. doi: 10.1186/s12935-018-0609-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mallappa S, Sinha A, Gupta S, Chadwick SJD. Preoperative neutrophil to lymphocyte ratio > 5 is a prognostic factor for recurrent colorectal cancer. Colorectal Dis. 2013;15:323–328. doi: 10.1111/codi.12008. [DOI] [PubMed] [Google Scholar]
- 24.Marcheselli R, Bari A, Tadmor T, Marcheselli L, Cox MC, Pozzi S, et al. Neutrophil-lymphocyte ratio at diagnosis is an independent prognostic factor in patients with nodular sclerosis Hodgkin lymphoma: results of a large multicenter study involving 990 patients. Hematol Oncol. 2017;35:561–566. doi: 10.1002/hon.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seretis C, Gourgiotis S, Gemenetzis G, Seretis F, Lagoudianakis E, Dimitrakopoulos G. The significance of neutrophil/lymphocyte ratio as a possible marker of underlying papillary microcarcinomas in thyroidal goiters: a pilot study. Am J Surg. 2013;205:691–696. doi: 10.1016/j.amjsurg.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Jiang Y, Xu H, Jiang H, Ding S, Zheng T. Pretreatment neutrophil–lymphocyte count ratio may associate with gastric cancer presence. Cancer Biomark. 2016;16:523–528. doi: 10.3233/CBM-160593. [DOI] [PubMed] [Google Scholar]
- 27.Kilincalp S, Çoban Ş, Akinci H, Hamamcı M, Karaahmet F, Coşkun Y, et al. Neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, and mean platelet volume as potential biomarkers for early detection and monitoring of colorectal adenocarcinoma. Eur J Cancer Prev. 2015;24:328–333. doi: 10.1097/CEJ.0000000000000092. [DOI] [PubMed] [Google Scholar]
- 28.Cho J-K, Kim MW, Choi IS, Moon UY, Kim MJ, Sohn I, et al. Optimal cutoff of pretreatment neutrophil-to-lymphocyte ratio in head and neck cancer patients: a meta-analysis and validation study. BMC Cancer. 2018;18:969. doi: 10.1186/s12885-018-4876-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vano YA, Oudard S, By MA, Tetu P, Thibault C, Aboudagga H, et al. Optimal cut-off for neutrophil-to-lymphocyte ratio: fact or fantasy? A prospective cohort study in metastatic cancer patients. PLoS ONE. 2018;13:1–13. doi: 10.1371/journal.pone.0195042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abbate V, Dell’Aversana Orabona G, Salzano G, Bonavolonta P, Maglitto F, Romano A, et al. Pre-treatment neutrophil-to-lymphocyte ratio as a predictor for occult cervical metastasis in early stage (T1–T2 cN0) squamous cell carcinoma of the oral tongue. Surg Oncol. 2018;27:503–507. doi: 10.1016/j.suronc.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Forget P, Khalifa C, Defour JP, Latinne D, Van Pel MC, De Kock M. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res Notes. 2017;10:1–4. doi: 10.1186/s13104-016-2335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho Y, Kim J, Yoon H, Lee C, Keum K, Lee I. The prognostic significance of neutrophil-to-lymphocyte ratio in head and neck cancer patients treated with radiotherapy. J Clin Med. 2018;7:512. doi: 10.3390/jcm7120512. [DOI] [PMC free article] [PubMed] [Google Scholar]