Abstract
Xanthelasma palpebrarum (XP) is the most common form of cutaneous xanthomata, and is important aesthetically, because of its close relation to the eyes, as well as medically for its association with cardiovascular disease (CVD). To provide avant-garde review discussing the various aspects of XP, including its aetio-pathogenesis and various treatment modalities. A structured Pubmed and Medline were searched for relevant articles. The finding of recent research has strongly espoused the link between XP and CVD, and mechanisms have been suggested for its formation. The new technologies have led to a multitude of treatment options for XP. XP is a multi-faceted entity; other than simple treatment of the cosmetic aspect of the disease, one must be cognizant of its cardiovascular implications.
Keywords: Xanthelasma palpebrarum, Lipid, Cardiovascular disease, Tri-chloroacetic acid, Laser, Blepharoplasty
Introduction
Xanthelasma palpebrarum (XP) is the most common form of cutaneous xanthomata [1, 2], so called because of its location close to the eye. It is derived from the Greek words, xanthos which means yellow and elasma which is a beaten metal plate. It is more commonly seen in middle-aged to elderly adults, with an incidence of 0.3% in males and 1.1% in females [3]. It is not a disease of modern times; the painting of Mona Lisa by Leonardo da Vinci (1503–1506) represents possibly the first recorded evidence of XP, as suggested by Dequeker et al. [4].
Patients often present to their doctor because of cosmetic concerns and request treatment of these aesthetically undesirable lesions in a prominent part of their face. Lesions are typically raised, soft, yellowish, irregularly shaped plaques that are slow growing, and are usually found over the medial aspect of the eye [1, 2]. They can also be found over the upper and lower eyelids, and circumferential lesions have been described [5]. The doctor not only needs to address the aesthetic concerns of the patient, but also needs to be cognizant of the aetiopathogenesis of this condition and its association with atherosclerotic cardiovascular disease (CVD).
Histopathology
Xanthelasma comprises mainly of perivascular and periadnexal foamy histiocytes and occasional Touton giant cells in the upper and middle dermis [6], giving a macroscopic appearance of an unsightly soft, yellowish plaque. The intracellular vacuoles contain esterified cholesterol, and there may be surrounding inflammation and fibrosis. Lymphocytes and mast cells [7] are common components of the inflammatory matrix. There appear to be similarities in the ultra-structural makeup of xanthomata and evolving atheromas, for instance between the intimal smooth muscle cells of arteries and the perithelial cells of dermal capillaries, hence suggesting that analogous mechanisms may be involved [8, 9]. There is variation in terms of the extensiveness of involvement, with the upper eyelid and medial canthus frequently affected. The fact that the periocular region is preferentially affected implies that local factors, such as constant movement and friction, may play a role in its pathogenesis, though its mechanism is not well elucidated.
Aetiopathogenesis and Prevalence
While XP is usually diagnosed by clinical appearance alone, it may be an important marker of underlying disease. It is present in a fair proportion of patients with familial hyperlipoproteinemia, most frequently the type-IIa Fredrickson phenotype [10–12]. It is classically associated with an increased risk of atherosclerotic CVD. Hence it is generally recommended that patients with XP should at least have a basic lipid screen, including low and high-density lipoprotein (LDL and HDL) levels. Reported prevalence of atherosclerotic CVD amongst XP patients have been varied, with numbers ranging from 15 to 70% [11, 13–16]. Conversely other studies have found little or no increase in the risk of atherosclerosis [12]. This wide range may be attributed to limitations and differences in methodology over the years [1, 17].
Association Between Xanthelasmata, Hyperlipidemia and Cardiovascular Disease
Although many patients with XP have raised total cholesterol levels, up to half of these may have normal lipid levels. While it is generally accepted that xanthelasmata in hyperlipidemic patients are associated with higher LDL and lower HDL levels, both of which are established atherogenic risk factors, the reasons behind the evolution of xanthelasma in normolipidemic patients remain more obscure. Possible implicating factors other than hyperlipoproteinemia include increased vascular lipid permeability, lipid synthesis and macrophagic uptake [18]. Other apolipoproteins have been shown to be associated with XP formation, though not conclusively. For instance, apolipoprotein B (Apo-B), a marker of atherosclerotic CVD, was reported to be elevated in normolipidemic XP patients by Douste-Blazy et al. [19], but not so by Gomez et al. [12] Likewise, Douste-Blazy et al. [19] too found increased prevalence of apolipoprotein E2 and E3 phenotypes in normolipidemic XP patients, but the results were not replicated by Gomez et al. [12].
More recently carotid intima media thickness measurements (CIMT) are accepted as a surrogate marker for generalised atherosclerosis and risk of CVD [20]. Noel [21] was one of the first to evaluate CIMT in patients with XP, and found an increase in mean CIMT values in patients with XP compared to controls. Pandhi et al. [22] found the same results in his study involving BMI matched controls, and went on to conclude that XP may be a risk factor for atherosclerosis regardless of the patient’s lipid levels. There was, however, no correlation with the duration or extent of the lesion. Another index of arterial stiffness is cardio-ankle vascular index (CAVI) which is significant marker for sub-clinical atherosclerosis. Akyuz A R et al. investigated the association between CAVI and XP, and concluded that XP was an independent predictor for abnormal CAVI [23].
Other Associations of Xantholesma
Besides lipid abnormalities, XP has also been associated with allergic contact dermatitis, periorbital hyperpigmentation, smoking and obesity [24, 25]. XP can also develop following surgical procedures such as injection fillers [26], and septorhinoplasty [27] (See Table 1). An inflammatory mechanism with resultant vascular permeability and oedema has been proposed [28]. Local trauma has been shown to increase capillary leakage of LDLs, and xanthomas are a result of subsequent inflammatory reactions. For instance, hyaluronic acid from injections in the extracellular matrix can bind extravasated LDL, and this LDL-glycosaminoglycan complex is taken up more easily by macrophages than native LDL [29]. Furthermore, glycosaminoglycans promote the oxidation of LDLs, which in turn leads to foam cell formation. Nilotinib, a tyrosine kinase inhibitor used in the treatment of chronic myloid leukemia is also believed to cause both lipid panel abnormalities and cutaneous lesions [30].
Table 1.
|
Clinico-pathological conditions Familial hyperlipoproteinemia Macrophage abnormalities Allergic contact dermatitis Periorbital hyperpigmentation Smoking Obesity Injection of intradermal hyaluronic acid fillers Post septorhinoplasty A new side effect of Nilotinib |
Treatment Options
Until about 30 years ago, XPs were only effectively removed by surgery with cold steel instruments [31]. Although, meticulous percutaneous resection of the lesions followed by direct suture has remained one of the more popular techniques, the high risk of recurrence and other complications like ectropion are among the reasons which have drawn surgeons to seek alternative methods of removal. The relative paucity of skin at the lower lid means that repeated resections of recurrences may not be possible if cosmesis is to be preserved [32], hence the search for skin sparing techniques. With the advent of medical technology and research, a plethora of options has surfaced (See Table 2).
Table 2.
Different modalities of treatment for XPs
| Modality of treatment | Principle | No. of session | Advantage | Side effects |
|---|---|---|---|---|
| Surgical excision [33, 34] | Meticulous percutaneous resection of the lesions followed by direct suture with or without flap | One or revision surgery |
Simple Best for lesion involving deep dermis and muscle Combined with blepharoplasty |
Recurrence, Ectropion Scar contracture Revision surgery |
| TCA peeling (concentration 35–100%) [35–39] | Dissolving lipids and coagulating proteins | 1–12 |
Simple Low-cost Out-patient clinic procedure |
Hypo- and Hyper-pigmentation Scarring Accidental application to the conjunctiva or cornea—atrophy, Koebner-like phenomenon |
| Low/Mid Voltage Radiofrequency [40] | Thermal energy induces vaporization at the cellular level in tissues | Single |
Quick, Inexpensive Relatively safe |
Hypo- and Hyper-pigmentation Pain |
| Cryo-surgery (nitrous oxide) [44, 45] | Ice crystal formation, Cellular dehydration and electrolyte disruption, Thermal shock, Enzyme inhibition or Effect on proteins | 1–21 Cycle |
Easy for application Low risk of adverse effects |
Recurrence, Hypopigmentation Healing is slow Extensive lesions are difficult to treat |
| CO2 laser (ultra pulse) (10,600 nm) [47–50] | Absorbed by the extracellular fluid of tissue cells, leading to non-specific vaporisation | 1–5 Sessions |
Less downtime, High patient satisfaction |
Hyperpigmentation, scaring, recurrence |
| Erbium:YAG laser (2940 nm) [51–55] | Absorption by water-containing tissues | Single | Suitable for early lesion |
Mild erythema. Hypo- and Hyper-pigmentation |
| Nd:YAG laser (1064 nm) [56–59] | Q-switched—cellular fragmentation via shock waves | Single | Large lesions | Erythema |
| KPT laser (532 nm) [60, 61] | Continuous wave | Single | Safe |
Hypopigmentation, Recurrences |
| Diode laser (1450 nm) [66, 67] | Preferentially targets water | Single | Satisfactory result | Transient focal hyperpigmentation |
| Pulsed dye laser (585 nm) [31, 62] | Vascular specific, targeting small vessels | 5 Sessions | High patient satisfaction | Blisters, crusting and ectropion |
| Intra-lesional Pingyangmycin [73] | A broad-spectrum antitumor antibiotic | 1 Session | Cheap, effective, and safe | No complications |
Surgical Excision
Hoon Young Lee et al. graded XP into four types, on basis of location and extent of lesion. Grade I lesions involve only the upper eyelids; grade II extends to the medial canthal area; grade III involves medial side of both upper and lower eyelids, and grade IV ones have diffuse involvement of the medial and lateral side of both upper and lower eyelids. Hoon treated grade I and II lesions by simple excision while grade III and IV ones were managed by excision in combination with skin grafting, local flaps, blepharoplasty and medial epicanthoplasty [33]. Similarly, Kose R treated large XP defects with full thickness skin grafts harvested from the lateral aspect of the upper eyelid. Good patient satisfaction was achieved by performing blepharoplasty of the upper eyelid simultaneously with the excision of XPs [34]. Authors suggested that for lesions involving the deep dermis and/or muscle, cold steel dissection may be the most suitable treatment option (Fig. 1).
Fig. 1.
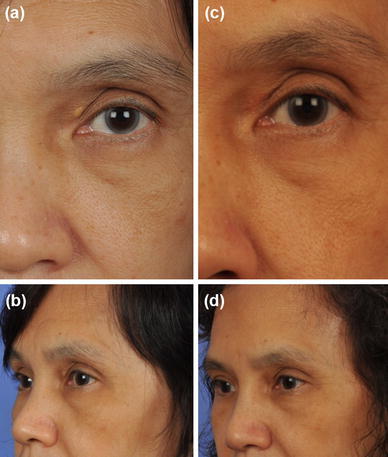
a, b (Pre-operative) and c, d (post operative) clinical photographs of a patient presenting with xanthelasma palpebrarum involving the left upper lid
Di- and Tri-Chloroacetic Acids (DCA and TCA)
These chemicals have been described in the treatment of XP. They are chlorinated acetic acids, and are used in different concentrations as tissue cauterants [35]. They work by dissolving lipids and coagulating proteins. A thin layer is applied over the lesion with a wooden applicator stick, with care taken to avoid the surrounding healthy skin. Blanching will subsequently be observed, and antibiotic creams are then applied. Side effects associated with this form of treatment include hypo- and hyperpigmentation, scarring and accidental application to the conjunctiva or cornea. Haygood et al. [36] treated 25 patients with 100% Bichloracetic acid (BCA), with 85% of them achieving initial complete clearance. The other 28% of the lesions required repeated application after an average period of 64 months, but these recurrences generally responded well to retreatment. TCA has increased in popularity recently, with different concentrations trialed. Cannon et al. [37] investigated the efficacy of 95% TCA in a retrospective review of 102 patients, of which 44 and 51 patients were examined clinically and interviewed respectively. They found a limited success rate of 61% at a mean follow up of 31.8 months. Hague and Ramesh [38] used varying concentrations (50, 70 and 100%) of TCA on 51 patients, and commented that higher concentrations worked better with papulo-nodular lesions and flat plaques, while 50% TCA was sufficient for macular lesions. However the duration of follow-up was not cited. In a study of 24 patients treated with 70% TCA, Nahas et al. [39] found that an average of 1.5 applications was required to treat till resolution and 25% of lesions had recurrence 6 months after treatment. Nonetheless, patient satisfaction was high, making this an attractive, simple and low-cost viable alternative for the treatment of XPs.
Low Voltage Radiofrequency
Radiofrequency works by causing vaporization at a cellular level in tissues. It initiates fibrosis followed by volume reduction during healing. This novel technique has been attempted by Dincer et al., on 15 patients. A dual-frequency radiofrequency machine was used at low power settings. Electrodes were applied superficially onto the lesions under local anaesthesia, following which creams were applied to reduce the risk of infection and hasten re-epithelialization. Pateints were followed up for 5 months, and success was graded according to a subjective 5 point scale (0 = no result, 0–25% Mild, 26–50% = moderate, 51–75% = good, and 76–100% = excellent). Preliminary results were promising, with 14 patients having at least a ‘good’ (second best) result. Of the 15 patients who were treated this way, 5 required a second session to achieve a satisfactory result. Main complications were pain, and hyper- or hypopigmentation. This technique is potentially a quick, inexpensive and relatively safe therapeutic modality. The authors recommended that it may be useful in cases where there are multiple lesions; lesions have indistinct borders or are situated close to the eyes [40].
Cryotherapy
Cryotherapy works by one or more of the following ways: ice crystal formation, thermal shock, enzyme inhibition, cellular dehydration and electrolyte disruption [41–43]. Dewan et al. [44] looked at 100 patients with a total of 237 lesions, and performed closed probe cryosurgery with nitrous oxide as the cryogen, without local anaesthesia. Freezing time was 15 s, and the number of freeze–thaw cycles ranged from 1 to 21 depending on the size and number of lesions. Follow up was set at 6 months, which showed that 68% of the cases had complete resolution of the lesions and 6% had incomplete resolution, with a tiny central area of hypopigmentation. The other 26% had either persistence or recurrence of the lesion. Labanderia et al. treated four cases of XPs with gentle liquid nitrogen spray cryotherapy. This treatment modality was advocated because of its easy applicability and minimal adverse effects [45].
Laser Therapy
Laser vaporisation is the latest technique in the treatment of XP. Within this category, the different lasers with their varying wavelengths and properties, coupled with a multitude of surgical techniques, give rise to an interesting range of treatment options [46].
Ablative Lasers
Carbon dioxide (CO2) lasers are one of most widely utilised lasers in the medical arena. Its 10,600 nm wavelength means that it is selectively absorbed by the extracellular fluid of tissue cells, leading to non-specific vaporisation, regardless of pigmentation. It is an ablative laser, hence the need for some form of anaesthesia during the procedure. It results in an open wound, therefore the need for wound care and dressing post-operatively. Its ablative effect, especially in the continuous mode, may result in collateral tissue damage leading to unpredictable scarring and asymmetrical skin retractions. With current technology, it has become possible to deliver high energy beams in extremely short impulses, beyond the skin thermal relaxation time. This ultrapulsed CO2 laser allows for more precise ablation of skin layers and less injury to the surrounding tissues. Raulin et al. [47] treated 23 patients (52 lesions) with the ultrapulsed CO2 laser with good effect, removing all lesions with a single treatment. Pigmentary changes were transient, seen in 17% of cases, without visible scarring. Three patients developed a recurrence, which the authors postulated may be due to an inadequate depth of treatment. Pathania et al. and Saif MYS et al. [48, 49] achieved similarly good results. A randomized control trial was conducted by Esmat et al. to compare the efficacy and safety of super pulsed (SP) and fractional CO2 laser in 20 adult patients with bilaterally symmetrical XPs. Randomly assigned XP lesions were treated with one session of super pulsed CO2 laser or 3–5 sessions of fractional CO2 laser. After removal, XP lesion on both sides showed remarkable improvement in size, colour and thickness of lesions. The lesions treated with SP CO2 laser showed significant improvement in colour and thickness of lesions. Nevertheless, better patient satisfaction and shorter time-out was observed with fractional CO2 laser [50].
The erbium:YAG laser is another form of ablative laser that has been tried. It has a wavelength of 2940 nm, with preferential absorption by water-containing tissues. It is less invasive compared to the conventional CO2 laser, making it possible to ablate thin layers of skin to a depth of a few nanometers, hence allowing for precise manipulation with less damage to surrounding healthy tissues. Post-treatment complications are also minimised because of the non-invasive nature of this laser, with erythema disappearing after 2 weeks for most patients. Borelli and Kaudewitz [51] treated 33 lesions in 15 patients and noted no recurrence in a follow-up period of 7–12 months. They concluded that this laser was a minimally invasive method suitable for treating early lesions before they become aesthetically problematic. In addition, he commented on the merits of the CO2 laser, for instance, better hemostasis and allowed for removal of deeper lesions which were also documented by Mannino et al. [52], Kaufman and Hibst [53], and Drnovsek-Olup and Vedlin [54] in 70 XPs, 9XPs and 32 XPs respectively. Levy and Trelles [55] described an inverted resurfacing technique with the erbium:YAG laser, whereby the eyelid skin is everted, exposing the lesional tissue and orbicularis oculi. The lesion is then precisely vaporised with the laser until normal tissue architecture is seen, following which the skin flaps are replaced and closed.
Non-ablative Lasers
Non-ablative lasers are gaining favour in recent years due to their non-invasive properties, safety profile, and possibility of using them in office based procedures. The 1064 nm Q-switched Nd:YAG laser has been in use since the 1990s for the treatment of tattoo and pigmented lesions. The mechanism of action is cellular fragmentation via shock waves generated from sudden energy transfer, confinement and release in a photoacoustic phenomenon [56]. Fusade [57] treated 38 lesions in 11 patients with this laser, and obtained good to excellent results (defined as 50% or more clearing) in 26 lesions. On the contrary, Karsai et al. [58] could not replicate Fusade’s results, getting instead a high dropout rate (due largely to apparent inefficacy) and low success rate (70–75% of treated lesions showed no clearance). However, later, Leonardo Marini treated 20 periocular XPs in twelve patients, all achieving at least good outcomes, defined as more than 50% clearance [59].
Another non-ablative laser is the KTP (Potassium Titanyl Phosphate) laser. It has a wavelength of 532 nm and was first time introduced by Berger C and Kopera D in the treatment of XP. They treated 33 lesions in 14 patients. After one to three sessions 86% patients showed convincing reduction of XPs without side effects. Moreover, 70% of patients tolerated procedure without any anaesthesia [60]. Recently a retrospective study was conducted by Greijimans et al. to evaluate the safety and efficacy of continuous wave KTP laser treatment for XPs. Overall, 97% (29/30) patient had excellent aesthetic results. However, recurrences were frequent (43%; 13/30) which warranted regular maintenance therapy [61].
The pulsed dye laser (PDL) was first described by Schönermark and Raulin [62], and had been used to some success by Karsai et al. [31]. In his prospective clinical trial of 38 cases (in 20 patients), a 585 nm wavelength laser was utilised. The laser is vascular specific, targeting small vessels to which the offending histiocytes are adherent to, thereby simultaneously causing the destruction of these cells. The 20 patients underwent five sessions with the laser, after which pre- and post-operative photographs were compared and assessed by independent examiners. Two-thirds of lesions showed clearance of more than 50%, while one-quarter had clearance of more than 75%. Patient satisfaction was high, and there were no major side effects like blisters, crusting and ectropion. Karsai et al. [31] suggested that a combination of ablative and non-ablative laser therapy holds promise in the removal of tuberous lesions.
Other kinds of lasers that have been utilised in recent years include the 1450 nm diode laser. It preferentially targets water, causing photothermal destruction of sebaceous lobules and hair follicles, up to 500 µm in depth [63]. It has been used for the number of years, safely and efficaciously, for a number of skin lesions including acne [63, 64], sebaceous hyperplasia [65] and scarring [66]. Its exact mechanism in the treatment of xanthelasma is not well elucidated, but it is believed that the foamy histiocytes are targeted by the 1450 nm wavelength energy emission. Park et al. [67] used it to good effect in 16 patients, with 75% achieving moderate to marked clearance (defined as more than 40%) after one to four sessions. Transient focal hyperpigmentation was the most common post-operative side effect, occurring in five patients.
Other Treatment Modalities
Non-surgical treatment of XP has limited applicability. A decade ago, Shields et al. published an observational case report on the disappearance of XP following oral simvastatin. They postulated that oral statins combined with a fat restricted diet might result in resolution of XP, and reduction in cardiac risk, especially in young patients [68]. A novel technique was introduced by Wang et al., making use of Pingyangmycin which is a broad spectrum antitumor antibiotic. Twenty-one lesions in 12 patients were treated by intralesional infiltration of this antibiotic. All patient except one achieved satisfactory result after 2 sessions without any complications like infection, atrophy, ulceration or scarring [69].
Discussion
Few studies have been published in literature comparing different modalities of treatment for XPs. Mourad B and colleagues assessed the clinical efficacy and tolerability of different concentration of topical TCA (35, 50, and 70%) against CO2 laser in 30 XPs. Both 70% TCA peeling and CO2 laser ablation demonstrated more significant clinical efficacy and patient satisfaction with minimum number of treatment sessions than 35 and 50% TCA peeling [70]. Similarly, a prospective study comparing ultra-pulsed CO2 laser and 30% TCU was conducted by Goel K et al. It suggested that both TCA and lasers are good for clinically small lesions but the laser works more effectively in severe lesions [71]. Another comparative study was conducted by Mona Abdelkader et al., this time comparing argon laser against the erbium: YAG laser. Thus concluded that former was good for small lesions while the later was better for larger nodular lesion [72]. On the other hand, Gungor S and co-workers documented no significant difference in the post treatment improvement scores as well as complication scores for both Erbium:YAG laser ablation and 70% TCA application [73].
Overall, some authors have suggested that surgical excision of deep dermal and/or muscle XPs may be the most suitable modality of treatment in experienced hands. Higher concentrated TCA in papulo-nodular XP lesions and 50% TCA in macular lesions showed promising results. XPs near the lid margin or close to the canthus can be managed safely with low voltage radiofrequency. Latterly, non-ablative lasers are well accepted more by patients as well as physicians because of their minimal side effects, desirable outcomes and may be performed as out-patient clinic procedures.
Conclusion
There is a myriad of options available for the treatment of XP, but thus far, a preferred modality has not emerged, as no one method has been shown to be irrefutably superior to the rest. Furthermore, only a limited number of comparison studies have been done, and they are relatively small with short follow-up durations [38, 68–71]. More research and development, as well as well-designed large prospective trials, will be needed to further our knowledge in this area of medicine. However, other than just treating the aesthetic aspect of this condition, one must be aware of its cardiovascular implications, thus allowing for holistic management of the patient.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Shailesh Khode, Phone: +65 97156564, Email: shaileshkhode@gmail.com.
Soon Heng Terry Tan, Email: tan.terry.sh@ktph.com.sg.
En-Pei Amanda Tan, Email: tan.amanda.ep@ktph.com.sg.
Sandeep Uppal, Email: uppal.sandeep@ktph.com.sg.
References
- 1.Bergman R. The pathogenesis and clinical significance of xanthelasma palpebrarum. J Am Acad Dermatol. 1994;30(2):236–242. doi: 10.1016/s0190-9622(94)70023-0. [DOI] [PubMed] [Google Scholar]
- 2.Domonkoa AN, Arnold HL, Jr, Odom RB. Andrews’ diseases of the skin: clinical dermatology. 7. Philadelphia: WB Saunders; 1982. pp. 658–660. [Google Scholar]
- 3.Jónsson A, Sigfŭsson N. Letter: significance of xanthelasma palpebrarum in the normal population. Lancet. 1976;1(7955):372. doi: 10.1016/s0140-6736(76)90140-9. [DOI] [PubMed] [Google Scholar]
- 4.Dequeker J, Muls E, Leenders K. Xanthelasma and lipoma in Leonardo da Vinci’s Mona Lisa. Isr Med Assoc J. 2004;6(8):505–506. [PubMed] [Google Scholar]
- 5.Kim J, Kim YJ, Lim H, Lee SI. Bilateral circular xanthelasma palpebrarum. Arch Plast Surg. 2012;39(4):435–437. doi: 10.5999/aps.2012.39.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarkany RPE, Breathnach SM, Seymour CA, et al. et al. Metabolic and nutritional diseases. In: Burns T, Breathnach S, Cox N, et al.et al., editors. Rook’s textbook of dermatology. Oxford: Blackwell; 2004. p. 57.1e57.124. [Google Scholar]
- 7.Matsumoto M, Kunimitsu S, Wada K, Ikeda M, Keyama A, Kodama H. Mast cell distribution, activation, and phenotype in xanthoma. J Am Acad Dermatol. 2007;56(6):1006–1012. doi: 10.1016/j.jaad.2006.05.071. [DOI] [PubMed] [Google Scholar]
- 8.Parker F, Odland GF. Experimental xanthoma. A correlative biochemical, histologic, histochemical, and electron microscopic study. Am J Pathol. 1968;53(4):537–565. [PMC free article] [PubMed] [Google Scholar]
- 9.Parker F, Peterson N, Odland GF. A comparison of cholesterol-ester fatty acid patterns in the blood and in evolving xanthoma and atheroma during cholesterol-feeding of rabbits. J Invest Dermatol. 1966;47(3):253–259. doi: 10.1038/jid.1966.138. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe A, Yoshimura A, Wakasugi T, et al. Serum lipids, lipoprotein lipids and coronary heart disease in patients with xanthelasma palpebrarum. Atherosclerosis. 1981;38:283–290. doi: 10.1016/0021-9150(81)90044-7. [DOI] [PubMed] [Google Scholar]
- 11.Ribera M, Pinto X, Argimon JM, et al. Lipid metabolism and apolipoprotein-E phenotypes in patients with xanthelasma. Am J Med. 1995;99:485–490. doi: 10.1016/s0002-9343(99)80224-1. [DOI] [PubMed] [Google Scholar]
- 12.Gómez JA, Gónzalez MJ, de Moragas JM, Serrat J, Gónzalez-Sastre F, Pérez M. Apolipoprotein E phenotypes, lipoprotein composition, and xanthelasmas. Arch Dermatol. 1988;124(8):1230–1234. doi: 10.1001/archderm.1988.01670080042015. [DOI] [PubMed] [Google Scholar]
- 13.Montgomery H, Osterberg A. Xanthomatosis: correlation of clinical, histopathologic, and chemical studies of cutaneous xanthoma. Arch Dermatol. 1938;37:373–401. [Google Scholar]
- 14.Vacca JB, Knight WA, Broun GO. Clinical observations regarding xanthelasma. Ann Intern Med. 1959;51:1019–1031. doi: 10.7326/0003-4819-51-5-1019. [DOI] [PubMed] [Google Scholar]
- 15.Epstein NN, Rosenmann RH, Gofman JW. Serum lipoproteins and cholesterol metabolism in xanthelasma. Arch Dermatol. 1952;65:70–81. doi: 10.1001/archderm.1952.01530200074011. [DOI] [PubMed] [Google Scholar]
- 16.Pedace JF, Winkelmann RK. Xanthelasma palpebrarum. J Am Med Assoc. 1965;193:893–894. doi: 10.1001/jama.1965.03090110031008. [DOI] [PubMed] [Google Scholar]
- 17.Bergman R. Xanthelasma palpebrarum and risk of atherosclerosis. Int J Dermatol. 1998;37(5):343–345. doi: 10.1046/j.1365-4362.1998.00362.x. [DOI] [PubMed] [Google Scholar]
- 18.Bergman R, Kasif Y, Aviram M, Maor I, Ullman Y, Gdal-On M, Friedman-Birnbaum R. Normolipidemic xanthelasma palpebrarum: lipid composition, cholesterol metabolism in monocyte-derived macrophages, and plasma lipid peroxidation. Acta Derm Venereol. 1996;76(2):107–110. doi: 10.2340/0001555576107110. [DOI] [PubMed] [Google Scholar]
- 19.Douste-Blazy P, Marcel YL, Cohen L, Giroux JM, Davignon J. Increased Frequency of Apo E-ND phenotype and hyperapobeta-lipoproteinemia in normolipidemic subjects with xanthelasmas of the eyelids. Ann Intern Med. 1982;96(2):164–169. doi: 10.7326/0003-4819-96-2-164. [DOI] [PubMed] [Google Scholar]
- 20.Greenland P, Abrams J, Aurigemma GP, et al. Prevention conference V: beyond secondary prevention: identifying the high-risk patient for primary prevention: non-invasive tests of atherosclerotic burden—Writing Group III. Circulation. 2000;101:E16e22. doi: 10.1161/01.cir.101.1.e16. [DOI] [PubMed] [Google Scholar]
- 21.Noël B. Premature atherosclerosis in patients with xanthelasma. J Eur Acad Dermatol Venereol. 2007;21(9):1244–1248. doi: 10.1111/j.1468-3083.2007.02252.x. [DOI] [PubMed] [Google Scholar]
- 22.Pandhi D, Gupta P, Singal A, Tondon A, Sharma S, Madhu SV. Xanthelasma palpebrarum: a marker of premature atherosclerosis (risk of atherosclerosis in xanthelasma) Postgrad Med J. 1038;2012(88):198–204. doi: 10.1136/postgradmedj-2011-130443. [DOI] [PubMed] [Google Scholar]
- 23.Akyuz AR, Turan T, Erkus ME, Gurbak KS, et al. Xanthelasma palpebrarum associated with increased cardio-ankle vascular index in asymptomatic subjects. Wien Klin Wochenschr. 2016;128(suppl 8):610–613. doi: 10.1007/s00508-016-0989-6. [DOI] [PubMed] [Google Scholar]
- 24.Bhat J, Smith AG. Xanthelasma palpebrarum following allergic contact dermatitis from para-phenylenediamine in a black eyelash-tinting product. Contact Dermat. 2003;49(6):311. doi: 10.1111/j.0105-1873.2003.0251g.x. [DOI] [PubMed] [Google Scholar]
- 25.Platsidaki E, Kouris A, Agiasofitou E, Antoniou C, Kontochristopoulos G. Periorbital hyperpigmentation in patients with xanthelasma palpebrarum :an interesting observation. J Clin Aesthet Dermatol. 2016;9(4):52–54. [PMC free article] [PubMed] [Google Scholar]
- 26.D’Acunto C, Pazzaglia M, Raone B, Misciali C, Badiali L, Neri I, Patrizi A. Xanthelasma palpebrarum: a new adverse reaction to intradermal fillers? Br J Dermatol. 2013;168(2):437–439. doi: 10.1111/j.1365-2133.2012.11152.x. [DOI] [PubMed] [Google Scholar]
- 27.Başterzi Y, Sari A. Xanthelasma palpebrarum after septorhinoplasty. Aesthetic Plast Surg. 2006;30(4):492–493. doi: 10.1007/s00266-006-0043-6. [DOI] [PubMed] [Google Scholar]
- 28.Scott PJ, Winterbourn CC. Low-density lipoprotein accumulation in actively growing xanthomas. J Atheroscler Res. 1967;7(2):207–223. doi: 10.1016/s0368-1319(67)80082-6. [DOI] [PubMed] [Google Scholar]
- 29.Seike M, Ikeda M, Matsumoto M, Hamada R, Takeya M, Kodama H. Hyaluronan forms complexes with low density lipoprotein while also inducing foam cell infiltration in the dermis. J Dermatol Sci. 2006;41(3):197–204. doi: 10.1016/j.jdermsci.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Sayin L, Ayli M, Oguz AK, Sevel GC. Xanthelasma palpebrarum: a new side effect of nilotinib. BMJ Case Rep. 2016 doi: 10.1136/bcr-2015-213511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karsai S, Czarnecka A, Raulin C. Treatment of xanthelasma palpebrarum using a pulsed dye laser: a prospective clinical trial in 38 cases. Dermatol Surg. 2010;36(5):610–617. doi: 10.1111/j.1524-4725.2010.01514.x. [DOI] [PubMed] [Google Scholar]
- 32.Parkes ML, Waller TS. Xanthelasma palpebrarum. Laryngoscope. 1984;94(9):1238–1240. doi: 10.1288/00005537-198409000-00019. [DOI] [PubMed] [Google Scholar]
- 33.Lee Hoon Young, Jin Ung Sik, Minn Kyung Won, Park Young-Oh. Outcomes of surgical management of xanthelasma palpebrarum. Arch Plast Surg. 2013;40:380–386. doi: 10.5999/aps.2013.40.4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kose R. Treatment of large xanthelasma palpebrarums with full-thickness skin grafts obtained by blepharoplasty. J Cutan Med Surg. 2013;17(3):197–200. doi: 10.2310/7750.2012.12065. [DOI] [PubMed] [Google Scholar]
- 35.Roberts HL. The Chloroacetic acids: a biochemical study. Br J Dermatol. 1926;38:323–391. [Google Scholar]
- 36.Haygood LJ, Bennett JD, Brodell RT. Treatment of xanthelasma palpebrarum with bichloracetic acid. Dermatol Surg. 1998;24(9):1027–1031. doi: 10.1111/j.1524-4725.1998.tb04297.x. [DOI] [PubMed] [Google Scholar]
- 37.Cannon PS, Ajit R, Leatherbarrow B. Efficacy of trichloroacetic acid (95%) in the management of xanthelasma palpebrarum. Clin Exp Dermatol. 2010;35(8):845–848. doi: 10.1111/j.1365-2230.2010.03818.x. [DOI] [PubMed] [Google Scholar]
- 38.Haque MU, Ramesh V. Evaluation of three different strengths of trichloroacetic acid in xanthelasma palpebrarum. J Dermatol Treat. 2006;17(1):48–50. doi: 10.1080/09546630500475708. [DOI] [PubMed] [Google Scholar]
- 39.Nahas TR, Marques JC, Nicoletti A, Cunha M, Nishiwaki-Dantas MC, Filho JV. Treatment of eyelid xanthelasma with 70% trichloroacetic acid. Ophthalmic Plast Reconstr Surg. 2009;25(4):280–283. doi: 10.1097/IOP.0b013e3181aa9a1f. [DOI] [PubMed] [Google Scholar]
- 40.Dincer D, Koc E, Erbil AH, Kose O. Effectiveness of low-voltage radiofrequency in the treatment of xanthelasma palpebrarum: a pilot study of 15 cases. Dermatol Surg. 2010;36(12):1973–1978. doi: 10.1111/j.1524-4725.2010.01770.x. [DOI] [PubMed] [Google Scholar]
- 41.Dawber R, Colver G, Jackson A, Pringle F. Cutaneous cryosurgery. Principles and Clinical practice. London: Martin Dunitz; 1992. [Google Scholar]
- 42.Kuflik EG, Gage AA. Cryosurgical treatment of skin cancer. Newyork: Igaku-Shoin; 1990. [Google Scholar]
- 43.Sheperd J, Dawber RP. The historical and scientific basis of cryosurgery. Clin Exp Dermatol. 1982;7:321–328. doi: 10.1111/j.1365-2230.1982.tb02434.x. [DOI] [PubMed] [Google Scholar]
- 44.Dewan SP, Kaur A, Gupta RK. Effectiveness of cryosurgery in xanthelasma palpebrarum. Indian J Dermatol Venereol Leprol. 1995;61(1):4–7. [PubMed] [Google Scholar]
- 45.Labandeira J, Vázquez-Osorio I, Figueroa-Silva O, Pereiro M, Jr, Toribio J. Tolerability and effectiveness of liquid nitrogen spray cryotherapy with very short freeze times in the treatment of xanthelasma palpebrarum. Dermatol Ther. 2015;28(6):346–350. doi: 10.1111/dth.12254. [DOI] [PubMed] [Google Scholar]
- 46.Delgado Navarro C, Lanuza García A, Llorca Cardeñosa A, Bañón-Navarro R, Corchero MG. Application of laser CO2 for the treatment of xanthelasma palpebrarum. Arch Soc Esp Oftalmol. 2013;88:320–322. doi: 10.1016/j.oftal.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 47.Raulin C, Schoenermark MP, Werner S, Greve B. Xanthelasma palpebrarum: treatment with the ultrapulsed CO2 laser. Lasers Surg Med. 1999;24(2):122–127. doi: 10.1002/(sici)1096-9101(1999)24:2<122::aid-lsm7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 48.Pathania V, Chatterjee M. Ultrapulse carbon dioxide laser ablation of xanthelasma palpebrarum: a case series. J Cutan Aesthet Surg. 2015;8(1):46–49. doi: 10.4103/0974-2077.155084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saif MYS. Xanthelasma palpebrarum treatment by CO2 laser. Bull Ophthalmol Soc Egypt. 2007;100:791–794. [Google Scholar]
- 50.Esmat SM, Elramly AZ, Abdel Halim DM, Gawdat HI, Taha HI. Fractional CO2 laser is an effective therapeutic modality of xanthelasma palpebrarum: a randomized clinical trial. Dermatol Surg. 2014;40(20):1349–1355. doi: 10.1097/DSS.0000000000000172. [DOI] [PubMed] [Google Scholar]
- 51.Borelli C, Kaudewitz P. Xanthelasma palpebrarum: treatment with the erbium:YAG laser. Lasers Surg Med. 2001;29(3):260–264. doi: 10.1002/lsm.1117. [DOI] [PubMed] [Google Scholar]
- 52.Mannino G, Papale A, De Bella F, Mollo R. Use of Erbium:YAG laser in the treatment of palpepral xanthelasmas. Opthalmic Surg Lasers. 2001;32:129–133. [PubMed] [Google Scholar]
- 53.Kaufmann R, Hibst R. Pulsed Erbium-YAG laser ablation in cutaneous surgery. Lasers Surg Med. 1996;19:324–330. doi: 10.1002/(SICI)1096-9101(1996)19:3<324::AID-LSM7>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 54.Drnovsek-Olup B, Vedlin B. Use of Erbium:YAG laser for benign skin disorders. Lasers Surg Ned. 1997;21:13–19. doi: 10.1002/(sici)1096-9101(1997)21:1<13::aid-lsm3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 55.Levy JL, Trelles MA. New operative technique for treatment of xanthelasma palpebrarum: laser-inverted resurfacing: preliminary report. Ann Plast Surg. 2003;50(4):339–343. doi: 10.1097/01.SAP.0000044249.10349.8D. [DOI] [PubMed] [Google Scholar]
- 56.Anderson RR, Margolis RJ, Watenabe S, Flotte T, Hruza GJ, Dover JS. Selective photothermolysis of cutaneous pigmentation by Q-switched Nd: YAG laser pulses at 1064, 532, and 355 nm. J Invest Dermatol. 1989;93(1):28–32. doi: 10.1111/1523-1747.ep12277339. [DOI] [PubMed] [Google Scholar]
- 57.Fusade T. Treatment of xanthelasma palpebrarum by 1064-nm Q-switched Nd: YAG laser: a study of 11 cases. Br J Dermatol. 2008;158(1):84–87. doi: 10.1111/j.1365-2133.2007.08194.x. [DOI] [PubMed] [Google Scholar]
- 58.Karsai S, Schmitt L, Raulin C. Is Q-switched neodymium-doped yttrium aluminium garnet laser an effective approach to treat xanthelasma palpebrarum? Results from a clinical study of 76 cases. Dermatol Surg. 2009;35(12):1962–1969. doi: 10.1111/j.1524-4725.2009.01314.x. [DOI] [PubMed] [Google Scholar]
- 59.Marini L. 1064 nm Q-switched photo-acoustic laser ablation of xanthelasma palpebrarum. J Laser Health Acad. 2013;1:48–51. [Google Scholar]
- 60.Berger C, Kopera D. KTP laser coagulation for xanthelasma palpebrarum. J Dtsch Dermatol. 2005;3(10):775–779. doi: 10.1111/j.1610-0387.2005.05746.x. [DOI] [PubMed] [Google Scholar]
- 61.Greijmans E, Luiting-Welkenhuyzen H, Luijks H, Bovenschen HJ. Continuous wave potassium titanyl phosphate laser treatment is safe and effective for xanthelasma palpebrarum. Dermatol Surg. 2016;42(7):860–866. doi: 10.1097/DSS.0000000000000772. [DOI] [PubMed] [Google Scholar]
- 62.Schönermark MP, Raulin C. Treatment of xanthelasma palpebrarum with the pulsed dye laser. Lasers Surg Med. 1996;19(3):336–339. doi: 10.1002/(SICI)1096-9101(1996)19:3<336::AID-LSM9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 63.Astner S. Clinical applicability of a 1 450 nm diode laser as adjunctive treatment for refractory acne. G Ital Dermatol Venereol. 2009;144(6):629–638. [PubMed] [Google Scholar]
- 64.Friedman PM, Jih MH, Kimyai-Asadi A, Goldberg LH. Treatment of inflammatory facial acne vulgaris with the 1450-nm diode laser: a pilot study. Dermatol Surg. 2004;30(2 Pt 1):147–151. doi: 10.1111/j.1524-4725.2004.30062.x. [DOI] [PubMed] [Google Scholar]
- 65.No D, McClaren M, Chotzen V, Kilmer SL. Sebaceous hyperplasia treated with a 1450-nm diode laser. Dermatol Surg. 2004;30(3):382–384. doi: 10.1111/j.1524-4725.2004.30105.x. [DOI] [PubMed] [Google Scholar]
- 66.Chua SH, Ang P, Khoo LS, Goh CL. Nonablative 1450-nm diode laser in the treatment of facial atrophic acne scars in type IV to V Asian skin: a prospective clinical study. Dermatol Surg. 2004;30(10):1287–1291. doi: 10.1111/j.1524-4725.2004.30402.x. [DOI] [PubMed] [Google Scholar]
- 67.Park EJ, Youn SH, Cho EB, Lee GS, Hann SK, Kim KH, Kim KJ. Xanthelasma palpebrarum treatment with a 1450-nm-diode laser. Dermatol Surg. 2011;37(6):791–796. doi: 10.1111/j.1524-4725.2011.01945..x. [DOI] [PubMed] [Google Scholar]
- 68.Shields CL, Mashayekhi A, Shields JA. Disappearance of eyelid xanthelasma following oral simvastatin (Zocor) Br J Ophthalmol. 2005;89:639–645. doi: 10.1136/bjo.2004.053058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang H, Shi Y, Guan H, Liu C, Zhang W, et al. Treatment of xanthelasma palpebrarum with intralesional pingyangmycin. Dermatol Surg. 2016;42(3):368–376. doi: 10.1097/DSS.0000000000000660. [DOI] [PubMed] [Google Scholar]
- 70.Mourad B, Elgarhy LH, Ellakkawy HA, Elmahdy N. Assessment of efficacy and tolerability of different concentrations of trichloroacetic acid vs. carbon dioxide laser in treatment of xanthelasma palpebrarum. J CosmetDermatol. 2015;14(3):209–215. doi: 10.1111/jocd.12148. [DOI] [PubMed] [Google Scholar]
- 71.Goel K, Sardana K, Garg VK. A prospective study comparing ultrapulse CO2 laser and trichloroacetic acid in treatment of Xanthelasmapalpebrarum. J Cosmet Dermatol. 2015;14(2):130–139. doi: 10.1111/jocd.12137. [DOI] [PubMed] [Google Scholar]
- 72.Mona Abdelkader and Shereen Ezzelregal Alashry Argon laser versus erbium:YAG laser in the treatment of xanthelasma palpebrarum. Saudi J Ophthalmol. 2015;29(2):116–120. doi: 10.1016/j.sjopt.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Güngör S, Canat D, Gökdemir G. Erbium:YAG laser ablation versus 70% trichloroacetıc acid application in the treatment of xanthelasma palpebrarum. J Dermatol Treat. 2014;25(4):290–293. doi: 10.3109/09546634.2013.777153. [DOI] [PubMed] [Google Scholar]


