Abstract
The aim of this study was to evaluate the efficacy and safety of proton beam therapy for patients with locally recurrent parotid cancer. Between 2009 and 2012, ten patients with locally recurrent parotid gland cancer were treated with proton beam therapy (70.2 Gy equivalents in 32 fractions) with or without intra-arterial infusion chemotherapy of cisplatin (50 mg/body/week, for a total of 5–8 weeks). The median follow-up was 24 months (range 10–49 months). The 1-year overall survival and local control rates were 80 %, and the 3-year overall survival and local control rates were 60 %. None of the patients experienced grade 3–5 toxicities in the treatment or the follow-up periods. These findings suggest that proton beam therapy could be applied effectively and safely for patients with locally recurrent parotid gland cancer.
Keywords: Intra-arterial chemotherapy, Local recurrence, Parotid gland cancer, Proton beam therapy, Re-irradiation
Introduction
Of all the salivary gland tumors, 85 % are benign and 15 % are malignant. Salivary gland cancers are uncommon and comprise 3–6 % of all head and neck cancers [1]. Their site of origin can be the major (parotid, submandibular, and sublingual) or the minor salivary glands (in the palate, the nasal cavity, the paranasal sinuses, the trachea, and the lacrimal glands). Most salivary gland cancers arise from the parotid glands, and 20–25 % of parotid gland tumors are malignant. These cancers have varying histology and diverse biologic behaviors. Histologically, mucoepidermoid carcinoma is the most common malignant salivary gland tumor of the parotid glands. Surgery with adjuvant radiotherapy is the standard and curative treatment modality [1, 2]. Chemotherapy is not routinely performed except in the palliative or the recurrent disease setting. However, the clinical response rate to chemotherapy and the prognosis are poor [3]. We treated recurrent parotid gland cancers with proton beam therapy (PBT), which can be used to deliver high-dose radiation to the tumor, while minimizing the doses delivered to the surrounding normal tissues.
The purpose of this study was to clarify the efficacy and safety of PBT for recurrent parotid gland cancer. The study was approved by the Ethics Committee of the Southern Tohoku Proton Therapy Center.
Methods
Eligibility
Patients with histopathologically confirmed, recurrent parotid gland cancers, including adenoid cystic carcinoma (ACC), epithelial myoepithelial carcinoma (EMC), mucoepidermoid carcinoma (MC), sarcoma, acinic cell carcinoma (acinic cell Ca), and squamous cell carcinoma (SCC), were enrolled in this study. All patients were diagnosed as having recurrence at the primary tumor site after initial treatment. Patients who had recurrent lymph node metastases or distant metastases were excluded. The specific eligibility criteria included an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2; adequate hematological, hepatic, and renal functions (white blood cells >3500 µL; neutrophils >2000 µL; platelet count >100,000 µL; hemoglobin >8 g/dL; AST and ALT < 3 times the upper limit of normal; total bilirubin <1.5 mg/dL; creatinine clearance >30 mL/min); and a life expectancy >3 months.
Proton Beam Therapy
The irradiation field of the PBT was focused on the primary tumor only. The gross tumor volume (GTV) was defined as any visible evidence of disease on physical examination or on any imaging modality, including CT, MRI, and/or PET/CT. The clinical target volume (CTV) was defined as the GTV with a 3–5 mm margin in all directions to cover microscopic disease. The CTV was expanded by 3–5 mm in all directions to allow for setup uncertainty, to create the planning target volume (PTV), but it did not extend to the critical organs at risk, such as the brainstem and spinal cord. The photon beam energy and the spread-out Bragg peak were fine-tuned so that the PTV was covered by at least a 90 % isodose volume of the prescribed dose. A fraction size of 2 or 2.2 Gy equivalents (GyE) was delivered daily, 5 days per week. The total dose of PBT was approximately 70.2 GyE in 32 fractions over 7 weeks.
Intra-Arterial Infusion Chemotherapy Via the Superficial Temporal Artery
Before treatment, three-dimensional CT angiography of the carotid artery was necessary to identify the main tumor feeding arteries and determine the morphology and the course of the tumor feeding artery from the external carotid artery (Fig. 1a) [4]. Catheterization from the superficial temporal artery (the HFT method) was performed, as previously reported [4]. The tip of the catheter was placed in the posterior auricular artery branch of the external carotid artery (Fig. 1b).
Fig. 1.
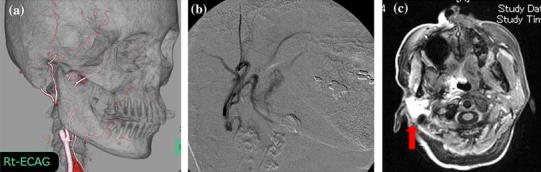
a Three-dimensional CT angiography of the right external carotid artery. b Digital subtraction angiogram (DSA) of retrograde intra-arterial infusion. c Axial view of the MR image after infusion of a small amount of contrast medium through the catheter for arterial infusion chemotherapy. This image shows tumor staining of the right parotid gland (arrow)
The extent of arterial injection was confirmed using angiography or MRI with an extremely low dose of contrast medium slowly infused by way of the catheter (Fig. 1c) [5].
Cisplatin (CDDP) was administered as an intra-arterial infusion at doses of 50 mg/body for 5 h once a week, with a total of 5–8 courses. During the arterial infusion of CDDP, sodium thiosulfate (STS), a CDDP-neutralizing agent, was also administered intravenously at 10 g/body for 8 h, starting 1 h before the CDDP infusion.
Evaluation and Follow-Up
The clinical response was judged 3 months after PBT, according to the Response Evaluation Criteria in Solid Tumor guidelines. The overall survival (OS) and local control (LC) rates were calculated using the Kaplan–Meier method. Events were measured from the start of treatment. Acute and late toxicities were evaluated in accordance with the Common Terminology Criteria for Adverse Events (CTCAE), v4.0. The evaluation categories were blood cell counts, renal function, oral mucositis, dermatitis, alopecia, nausea, fever, and brain necrosis.
Results
Patient Characteristics and Treatment Results
The patient characteristics and treatment results are shown in Table 1. Between June 2009 and June 2012, ten patients with local recurrence of parotid carcinoma were enrolled in the present study. There were eight males and two females, with a median age of 62 years (range 43–83 years). Histologically, the tumors were diagnosed as ACC (n = 4), EMC (n = 2), sarcoma (n = 1), SCC (n = 1), MC (n = 1), and acinic cell Ca (n = 1). The TNM status of the locally recurrent tumors was rT4a (n = 6), rT3 (n = 2), rT2 (n = 1), and rT4b (n = 1). None of the patients had lymph node or distant metastases. The initial treatments consisted of surgery (n = 4); surgery and postoperative radiotherapy (n = 4); and chemoradiotherapy (n = 2). For those treated with radiotherapy, the median total irradiation dose was 57.5 Gy. In the present study, all patients were treated with PBT, with a median total dose of 66 GyE (range, 56–77 GyE per 28–30 fractions). Five patients were treated with intra-arterial infusion chemotherapy of CDDP combined with PBT, with a median total CDDP dose of 400 mg/body (range 250–500 mg).
Table 1.
Patient characteristics and treatment results
| Patient | Age (years) | Histology | TNM-status | Initial treatment | PBT (GyE) | IAC | Types of recurrence | Survival (months) | Status |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 65 | ACC | rT4aN0M0 | Surgery, RT (50 Gy) | 66 | No | Local | 45 | DOD |
| 2 | 83 | Sarcoma | rT3N0M0 | Surgery | 68.2 | No | No | 49 | NED |
| 3 | 67 | EMC | rT4bN0M0 | Surgery, RT (40 Gy) | 60 | No | Local | 39 | DOD |
| 4 | 55 | SCC | rT4aN0M0 | Chemo, RT (70 Gy) | 56 | CDDP 500 mg | Local | 10 | DOD |
| 5 | 59 | ACC | rT4aN0M0 | Surgery, RT (50 Gy) | 61.6 | CDDP 400 mg | Skin metastasis | 14 | DOD |
| 6 | 75 | ACC | rT3N0M0 | Surgery | 77 | No | Local | 15 | DOD |
| 7 | 69 | EMC | rT4aN0M0 | Chemo, RT (70 Gy) | 74 | CDDP 500 mg | Lung metastasis | 12 | DOD |
| 8 | 54 | ACC | rT4aN0M0 | Surgery | 74.8 | CDDP 250 mg | No | 25 | NED |
| 9 | 53 | MC | rT4aN0M0 | Surgery, RT (64.9 Gy) | 60 | CDDP 300 mg | No | 23 | NED |
| 10 | 43 | Acinic cell Ca | rT2N0M0 | Surgery | 74.8 | No | No | 49 | NED |
ACC adenoid cystic carcinoma, EMC epicelial myoepithelial carcinoma, SCC squamous cell carcinoma, MC mucoepidermoid carcinoma, Acinic cell Ca acinic cell carcinoma, RT radiotherapy, IAC intra-arterial infusion chemotherapy, CDDP cisplatin, OS overall survival, LC local control, DOD dead of disease, NED no evidence of disease
Tumor Control and Survival Rates
The median follow-up was 24 months (range 10–49 months). Eight patients achieved a complete response (CR), one patient achieved a partial response (PR), and one patient achieved stable disease (SD). The response rate to this treatment was 90 % (Fig. 2c). MR images of the right parotid gland for the patient with a CR are shown in Fig. 2a. The 1-year OS and LC rates were 80 %, and the 3-year OS and LC rates were 60 % (Fig. 3). During the follow-up period, four patients died of local recurrence at the primary tumor site, and two patients died of multiple metastases (Table 1).
Fig. 2.
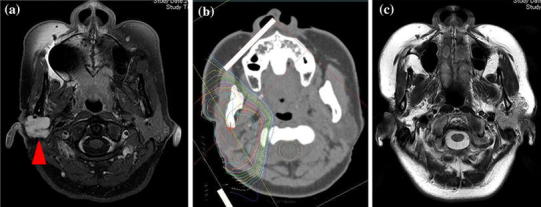
a MR image of the right parotid gland of a patient with recurrent adenoid cystic carcinoma (rT4aN0M0) before treatment (arrowhead). There was recurrence at the primary tumor site 36 months after the initial surgical treatment. Patients received PBT combined with intra-arterial chemotherapy at our institution (PBT total 74.8 GyE; CDDP total 250 mg/body). b Dose distribution map of proton beam therapy (the outside line shows <10 % of the prescription dose). c MR image of the same patient 38 months after treatment. The primary tumor lesion has disappeared, and this patient has achieved a complete response
Fig. 3.
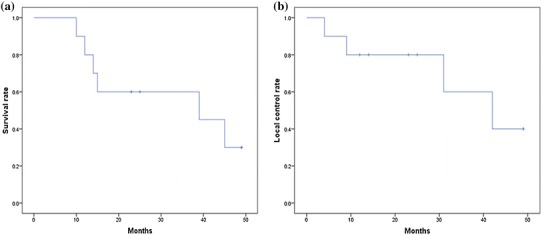
Overall survival (OS) rates (a) and local control (LC) rates (b) using the Kaplan–Meier method. The 1-year OS and LC rates are 80 %, and the 3-year OS and LC rates are 60 %
Toxicity
Table 2 shows the therapy-related toxicities. All patients experienced grade 2 dermatitis, and one patient had grade 1 brain necrosis. During the treatment and follow-up periods, no severe complications, such as neurological complications and grade 3–5 toxicities, were encountered.
Table 2.
Toxities
| Toxicities | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|
| Hematological | |||||
| Leukopenia | 0 | 1 | 0 | 0 | 0 |
| Neutropenia | 0 | 1 | 0 | 0 | 0 |
| Thrombocytopenia | 0 | 1 | 0 | 0 | 0 |
| Anemia | 0 | 2 | 0 | 0 | 0 |
| Non-hematological | |||||
| Mucositis | 4 | 3 | 0 | 0 | 0 |
| Dermatitis | 0 | 10 | 0 | 0 | 0 |
| Alopecia | 3 | 0 | 0 | 0 | 0 |
| Nausea | 2 | 1 | 0 | 0 | 0 |
| Fever | 2 | 0 | 0 | 0 | 0 |
| Brain necrosis | 1 | 0 | 0 | 0 | 0 |
Discussion
Combined-modality treatment with surgery followed by postoperative radiotherapy is the standard treatment for salivary gland cancers. Various studies have demonstrated the benefit of surgery combined with postoperative radiotherapy in improving local control and overall survival. Therasa et al. have reported a local control rate of 94 % at 5 years and 73 % at 10 years. Metastatic control was 82 % at 5 years and 63 % at 10 years [6]. However, local tumor recurrence can develop after incomplete tumor removal, and surgical treatment for recurrent cancer often requires a larger resection than in primary treatment. This is due to the difficulty of radically resecting a recurrent tumor lesion with a sufficient safety margin, because of its anatomical and pathological features [7]. Therefore, some cases are inoperable, and patients who undergo these surgical treatments often experience a deterioration in their quality of life (QOL) caused by dysfunction (such as dysphagia and dysarthria) and poor cosmetic impairment.
In a report by Pederson et al. [8] on chemotherapy combined with re-irradiation for salivary gland malignancies, the 3-year OS rate was 35.7 %. Nakamura et al. [9] reported that severe late toxicity was seen with total radiation doses (the sum of the initial and the re-treatment radiation doses) of more than 110 Gy. This may be the upper limit dose that can be given, and it is thought to be the optimal dose for recurrent tumors. Furthermore, the lifetime total doses for the spinal cord are restricted to 50 Gy. Therefore, it is difficult to administer high-dose radiation only to the tumor using traditional photon therapy. PBT has unique physical properties (e.g., the Bragg peak) and an excellent dose distribution for head and neck carcinomas, which limit the amount of normal tissue irradiated in the head and neck, while maximizing the radiation delivered to the tumor (Fig. 2b) [10].
As previously mentioned, the clinical response rate in the present study was 90 %, and the 3-year OS and LC rates were 60 %. Furthermore, the six patients who received radiotherapy in the initial treatment had no severe toxicities during the treatment and follow-up period in the present study. Limiting unnecessary radiation to normal tissues in the head and neck region can therefore result in a profound improvement in QOL, both during and after treatment [11]. In the present study, using PBT completely spared the critical organs, such as the brain, the brainstem, the spinal cord, and others. Selective injection of cytostatic agents into the tumor-feeding artery is an effective method to achieve higher doses of these agents in the tumors with less systemic toxicity than with intravenous chemotherapy [12]. Mitsudo et al. [13, 14] reported that a combination of radiotherapy with concurrent intra-arterial infusion chemotherapy is a useful and effective approach in the treatment of advanced head and neck carcinomas.
In the present study, of five patients treated with intra-arterial CDDP infusion chemotherapy combined with PBT, only one patient experienced local failure. This result suggests that intra-arterial infusion chemotherapy can improve local control. A report by Teo et al. [15] concluded that optimal locoregional treatment can reduce the possibility of distant metastases and improve overall survival. Therefore, we believe that good local control can lead to good overall survival.
In conclusion, PBT with or without combined intra-arterial infusion chemotherapy could become a new treatment modality for patients with recurrence of parotid gland cancer, especially in cases that are inoperable or require re-irradiation. Further studies of this treatment are required to better determine the long-term prognosis and the possibility of late toxicities.
Compliance with Ethical Standards
Conflict of interest
All authors have no conflicts of interest to declare.
Informed Consent
This study is a retrospective study. Therefore, this type of study formal consent is not required.
Human and Animal Rights
This article does not contain any studies with animals performed by any of the authors.
References
- 1.Armstrong JG, Harrison LB, Spiro RH, Fass DE, Strong EW, Fuks ZY. Malignant tumors of major salivary gland origin. A matched-pair analysis of the role of combined surgery and postoperative radiotherapy. Arch Otolaryngol Head Neck Surg. 1990;116:290–293. doi: 10.1001/archotol.1990.01870030054008. [DOI] [PubMed] [Google Scholar]
- 2.Bell RB, Dierks EJ, Homer L, Potter BE. Management and outcome of patients with malignant salivary gland tumors. J Oral Maxillofac Surg. 2005;63:917–928. doi: 10.1016/j.joms.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Surakanti SG, Agulnik M. Salivary gland malignancies: the role for chemotherapy and molecular targeted agents. Semin Oncol. 2008;35:309–319. doi: 10.1053/j.seminoncol.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Fuwa N, Ito Y, Matsumoto A, et al. A combination therapy of continuous superselective intraarterial carboplatin infusion and radiation therapy for locally advanced head and neck carcinoma. Phase 1 study. Cancer. 2000;89:2099–2105. doi: 10.1002/1097-0142(20001115)89:10<2099::AID-CNCR10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura T, Fuwa N, Takayama K, et al. A new method using MRI to delineate areas of head and neck cancer targeted by intra-arterial infusion via a superficial temporal artery. Oral Oncol. 2011;47:387–390. doi: 10.1016/j.oraloncology.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Theresa AG, David WE, Vivian W. Adenoid cystic carcinoma of the major salivary glands treated with surgery and radiation. The Laryngoscope. 2005;115:1278–1282. doi: 10.1097/01.MLG.0000165381.64157.AD. [DOI] [PubMed] [Google Scholar]
- 7.Garden AS, Weber RS, Morrison WH, Ang KK, Peters LJ. The influence of positive margins and nerve invasion in adenoid cystic carcinoma of the head and neck treated with surgery and radiation. Int J Radiat Oncol Biol Phys. 1995;32(3):619–626. doi: 10.1016/0360-3016(95)00122-F. [DOI] [PubMed] [Google Scholar]
- 8.Pederson A, Salama J, Haraf D, Witt M, Stenson K, Vokes E, et al (2008) Chemoradiotherapy for locoregionally advanced and high risk salivary gland malignancies. In: Wolf GT, Weymuller EA, Myers J (eds) Poster session presented at: 7th international conference on head and neck cancer. 2008 Jul 19–23, The American Head and Neck Society, San Francisco
- 9.Nakamura T, Kodaira T, Tachibana H, et al. Chemoradiotherapy for locally recurrent nasopharyngeal carcinoma: treatment outcome and prognostic factors. Jpn J Clin Oncol. 2008;38:803–809. doi: 10.1093/jjco/hyn104. [DOI] [PubMed] [Google Scholar]
- 10.Frank SJ, Selek U. Proton beam therapy for head and neck malignancies. Curr Oncol Rep. 2010;12:202–207. doi: 10.1007/s11912-010-0089-0. [DOI] [PubMed] [Google Scholar]
- 11.Fokas E, Kraft G, An H, et al. Ion beam radiobiology and cancer: time to update ourselves. Biochim Biophys Acta. 2009;1796(2):216–229. doi: 10.1016/j.bbcan.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Tohnai I, Fuwa N, Hayashi Y, et al. New superselective intra-arterial infusion via superficial temporal artery for cancer of the tongue and tumor tissue platinum concentration after carboplatin (CBDCA) infusion. Oral Oncol. 1998;34:387–390. doi: 10.1016/S1368-8375(98)00018-9. [DOI] [PubMed] [Google Scholar]
- 13.Mitsudo K, Koizumi T, Iida M, et al. Retrograde superselective intra-arterial chemotherapy and daily concurrent radiotherapy for stage III and IV oral cancer: analysis of therapeutic results in 112 cases. Radiother Oncol. 2014;111:306–310. doi: 10.1016/j.radonc.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Mitsudo K, Shigetomi T, Fujimoto Y, et al. Organ preservation with daily concurrent chemoradiotherapy using superselective intra-arterial infusion via a superficial temporal artery for T3 and T4 head and neck cancer. Int J Radiat Oncol Biol Phys. 2011;79:1428–1435. doi: 10.1016/j.ijrobp.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Teo PM, Chan AT, Lee WY, et al. Failure patterns and factors affecting prognosis of salivary gland carcinoma: retrospective study. Hong Kong Med J. 2000;6:29–36. [PubMed] [Google Scholar]


