Abstract
Nasopharyngeal carcinoma (NPC) is a malignant disease derived from nasopharyngeal epithelial cells that have a higher tendency for invasion and metastasis to the cervical lymph nodes than other head–neck malignancies. NPC patients with the same stages often show different progressions and prognoses. This suggests that clinical stages are not sufficient to predict progressivity, so biomarkers are required to provide better progression predictions. Some literature shows that the development and progression of NPC is a complex mechanism involving various components of signal paths, it plays a role in regulating the process of proliferation, angiogenesis and apoptosis. One of the most studied is β-catenin protein that is a key component of the canonical Wnt signal pathway. The β-catenin protein is reported to have roles in increasing the proliferative pathway of c-Myc and cyclin D1, increasing the expression of IL-8 proangiogenesis factor, decreasing expression of RASSF1A tumor suppressor and inhibiting apoptosis through the barriers of caspace-9 activity. To determine the association of β-catenin expression and staging in nasopharyngeal carcinoma patients. The research design used was analytic observational research with cross sectional approach. Samples were enrolled using consecutive sampling. The β-catenin expression was examined from the NPC tissue paraffin block with the immunohistochemical cracking technique, using an anti-β-catenin rabbit polyclonal antibody from Boster Biotechnology, California, USA. The β-catenin expression was assessed visually using a binocular light microscope and a scoring method according to the Allred scale index by an Anatomical Pathology consultant. Statistical analysis was performed using Spearman’s test to determine the association between β-catenin expression and staging in NPC patients. The significance level was α = 0.05. The study was conducted from May to December 2015 at Otolaryngology Unit of Dr. Soetomo General Soetomo, Surabaya. There were 40 patients who fulfilled the inclusion criteria. Spearman test results obtained p value = 0.060. The correlation of β-catenin expression with staging in NPC patients was found to be non-significant (p > 0.05). There was no correlation between β-catenin expression and staging in NPC patients.
Keywords: Nasopharyngeal carcinoma, Progressivity, β-catenin expression, Staging
Introduction
Nasopharyngeal carcinoma (NPC) is a malignancy derived from the nasopharynx epithelium, has a higher tendency to invade surrounding tissue and metastasizes to regional lymph nodes than other neck head malignancies [1]. Clinical staging is a major factor in assessing the progression and prognosis of NPC. NPC patients with similar clinical stages often show different progressions and prognoses [2]. This suggests that clinical stages are not sufficient to predict progressivity or response against therapy (prognosis) in NPC. The molecular marker that can increase the prediction of progressivity is needed to develop a therapeutic strategy. Some literature shows that high expression of β-catenin is found in various malignancies such as prostate, breast and colorectal cancer and often associated with invasion and metastasis [3, 4]. Signal pathway analysis and gene expression profiles show that increased β-catenin expression resulting in an uncontrolled proliferation that promotes progression in NPC [5]. The association between β-catenin expression and staging in patients with NPC who visited in Dr. Soetomo General Hospital Surabaya is not yet known.
The current available therapeutic modalities resulted in increased survival of NPC patients, but high rates of recurrence and metastasis were still a major constraint. Most patients seek a treatment at an advanced stage. Some patients have showed detected metastases at the basic examination or not [6]. The progression of NPC was characterized by invasive and metastatic tendencies; patients with the same stage might exhibit different metastatic tendencies. Several studies have demonstrated the prevalence of different micro metastases in NPC patients with similar stages. Micro-metastases evaluation in peripheral circulation of NPC patients with RT-PCR method in a study by Notopuro et al. (2008) found 60% in stage III, 72% in stage IVA and 87.5% in stage IVB [7]. Whereas, Moussa et al. (2014) reported that micro metastases was obtained 0% at stage III, 20% at stage IVA and 44.44% at stage IVB [8].
The β-catenin expression in advanced-stage esophageal squamous cell carcinoma was significantly higher than in the early stages [9]. Research on oropharyngeal cancer showed that the high expression of β-catenin in the cytoplasm correlated with poor levels of histologic differentiation, advanced stage and success of therapy [9]. Wang et al. (2009) reported that β-catenin expression was positively correlated with clinical stage and inversely proportional to patient survival rate [4]. Luo et al. (2012) found a significant positive correlation between β-catenin cytoplasmic levels with tumor mass size, glandular metastases and clinical stage NPC [1]. While Xu et al. (2013) found that β-catenin expression with tumor stage T3 and T4 in NPC was significantly higher than T2 and T1 [10].
The β-catenin protein has a dual role as a major component of the canonical Wnt signaling pathway and regulates the intercellular adhesion between cells through the formation of the complex with E-cadherin in adherents junction [3]. Most NPC cells have high levels of β-catenin expression in the cytoplasm and nucleus [4, 11]. β-catenin interacts with transcription factors in NPC and results in increased gene expression that plays a role in accelerating cell cycle, facilitating cell proliferation and migration [10]. β-catenin in the nucleus has a role to activate the proliferative signals of cyclin D1 and c-Myc, increases IL-8 levels and inhibits RASSF1A expression. Cyclin D1 that binds to the CDK4/CDK6 complex plays a role in cell progression through the G1/S transition without passing through the genomic reparation process, whereas c-Myc activates the CDK2/cyclin E complex that promotes cell progression through G1 phase [11].
Interleukin-8 regulated the cytoskeleton to initiate and maintain endothelial migration in the angiogenesis process. IL-8 angiogenic response occurred through ERK cascade activation [12]. Interleukin-8 also played a role in improving NPC metastasis through activation of Akt signals [13]. Akt signal path activity in NPC caused a decreased E-cadherin expression and resulted in an increase in invasion [14]. The disorder of the E-cadherin/β-catenin complex management was positively correlated with metastasis in NPC [3].
Decreased RASSF1A expression significantly correlated with tumor mass size and lymph node metastasis in NPC [15]. Decreased RASSF expression was a negative effector of Ras resulted in an increase of Race activation that stimulated the transcription of MMPs; a type IV collagenase that played a role in invasion and cancer cell metastasis [16]. β-catenin that binds to the transcription factor within the nucleus also played roles in suppressing apoptosis through the cytochrome c release resistance resulting in decreased caspace-9 activity [17]. Therefore, the authors aimed to determine the association of β-catenin expression with staging in NPC patients who visited at Dr. Soetomo General Hospital Surabaya. The results of this study are expected to be used as an indicator to determine the progressiveness of NPC.
Methods
The study used an observational analytic with cross sectional approach. Subjects were NPC patients who visited at Otolaryngology Unit of Dr. Soetomo General Hospital Surabaya and fulfilled the inclusion and exclusion criteria. The inclusion criteria were NPC patients who had paraffin blocks of sufficient nasopharyngeal biopsy for immunohistochemical examination and β-catenin expression assessment, willing to participate in the study and sign the informed consent. Exclusion criteria were patients who had damaged paraffin blocks thus the immunohistochemical examination to assess β-catenin expression cannot be performed. The subjects of this study were NPC patients who have treated for the first time without a prior definitive history of therapy in Otolaryngology Unit of Dr. Soetomo General Hospital Surabaya with the results of biopsy examination/histopathology from PA Installation RSUD Dr. Soetomo and fulfilled the research criteria. Samples were enrolled using consecutive sampling.
The β-catenin expression was a brown image of the nucleus, membrane or cytoplasm. This expression was discovered by immunohistochemical examination of the NPC tissue paraffin block using rabbit polyclonal antibody anti β-catenin from Boster Biotechnology, California, USA. The assessment of β-catenin expression was assessed visually under a binocular light microscope with 400× enlargements by an anatomical pathology specialist independent consultant. The β-catenin expression was assessed using the Allred scale index [18]. The proportion score showed the percentage of positive colored cells (0 = no positive cells, 1 = < 1%, 2 = 1–10%, 2 = 11–33%, 3 = 11–33%, 4 = 34–66%, 5 = > 66%) [18]. The intensity score indicated the intensity of the staining (0 = no color reaction, 1 = weak color intensity, 2 = medium intensity, 3 = strong color intensity). The final score was the sum of positive cell presentation scores with color intensity scores on immunoreactive cells. The assessment of β-catenin expression was as follows: negative expression (−) if final score of 0, weak expression (+) if final score of 1–3, medium expression (++) if final score of 4–6 and strong expression (+++) if the final score of 7–12. The NPC stadium was determined by the NCCN guideline of 2011 [19].
The study protocol was approved by Dr. Soetomo Teaching Hospital Surabaya. Data obtained in the study were presented in the form of table and analyzed statistically. Spearman’s statistical test was used to determine the correlation between β-catenin protein expression with NPC stages. The expression of β-catenin protein and staging were an ordinal scale with significance level (α) = 0.05.
Results
Forty NPC patients who fulfilled the inclusion criteria were enrolled in this study. The largest age group was 50–59 years with 16 patients (40.00%) followed by age group of 40–49 years by 12 patients (30.00%). The youngest was 22 years old and the oldest was 68 years old. There were 26 male patients (65.00%) and 14 female (35.00%) with a ratio of 1.86:1. The largest ethnic group was the Javanese of 35 patients (87.50%), followed by 2 patients from the Madurese (5.00%). The most types of work were farmers of 17 patients (42.50%). It was obtained histopathology WHO type III of 37 patients (92.50%) and WHO type II of 3 patients (7.50%). The results of β-catenin expression examination based on stage in NPC patients were shown in Table 1.
Table 1.
The results of β-catenin expression examination at various stages of NPC
| β-catenin expression | Stages | Amount | % | |||
|---|---|---|---|---|---|---|
| I | II | III | IV | |||
| Negative (−) | 0 | 0 | 0 | 1 | 1 | 2.50 |
| Weak positive (+) | 0 | 0 | 1 | 0 | 1 | 2.50 |
| Moderate positive (++) | 1 | 2 | 14 | 13 | 30 | 75.00 |
| Strong positive (+++) | 0 | 0 | 1 | 7 | 8 | 20.00 |
| Total | 1 | 2 | 16 | 21 | 40 | 100.00 |
p = 0.060
Medium positive expression (++) was obtained in NPC stage I (1 patient). Stage II (2 patients) obtained a moderate positive expression (++). One patient with weak positive expression (+), 14 patients with moderate positive expression (++) and one patient with strong positive expression (+++) were found in stage III (16 patients). One patient with negative expression (−), 13 patients with moderate positive expression (++) and seven patients with strong positive expression (+++) were obtained in stage IV (21 patients). Spearman’s test was performed to determine the correlation of β-catenin expression with NPC stages. We obtained p value = 0.060. There was no significant correlation (p > 0.05) between β-catenin expression with stages (I, II, III and IV) in NPC patients. The result of β-catenin immunohistochemical staining was shown in Fig. 1.
Fig. 1.
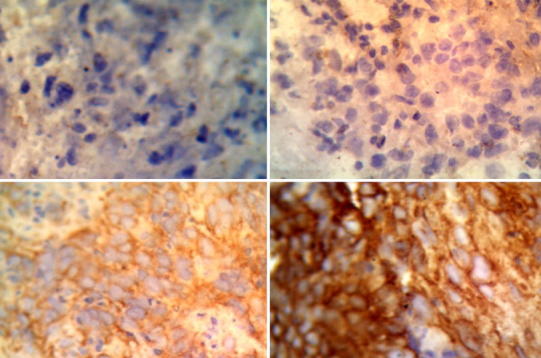
Result of β-catenin immunohistochemical staining with immunohistochemical technique. a Negative expression (−), b weak positive expression (+), c moderate positive expression (++), d strong positive expression (+++)
Discussion
Immunohistochemical results showed that an increase in β-catenin expression was not correlated to the increase in staging. We found one patient with moderate positive expression in stage I NPC whereas one patient with negative expression was obtained in stage IV. The result of statistical analysis obtained p value = 0.060, thus it means that the hypothesis of this study was not proven (p > 0.05). There was no correlation between β-catenin expression and NPC stage. The results of this study were not in accordance with some previous studies. Wang’s research et al. (2009) found that β-catenin expression was significantly higher in advanced stage than in the early stages (63.1% vs 40.7%, p = 0.041) [4]. Luo et al. (2012) reported that the high expression of β-catenin in the cytoplasm was significantly correlated with tumor size, lymph node metastasis and clinical stage [1]. Whereas, Xu et al. (2013) found that the β-catenin expression was significantly higher (p < 0.001) in the advanced stage of NPC (T4 + T3) than in the early stages (T1 + T2) [10].
The results of this study were similar to a study by Ruiz et al. (2011), which showed no significant correlation between β-catenin expression with tumor size, metastasis or therapeutic results in NPC [20]. This showed that β-catenin alone was not a major factor in the process of invasion and NPC metastasis moreover; other malignancies also obtained similar results. Osterheld et al. (2002) mentioned that there was no a significant correlation between β-catenin expression with tumor size and lymph node metastasis in adenocarcinoma of Barrett’s esophagus [21]. Single hospital series study in Norway of 722 colorectal carcinoma patients also showed no significant correlation between β-catenin expression levels with tumor stages and survival rates [19].
We obtained a number of signal paths that were interrupted in the NPC. Increased Wnt/β-catenin signal was an important cause of the accumulation of β-catenin in the cytoplasm thus it translocated into the nucleus. The role of β-catenin in NPC development occurred through several pathways. β-catenin formed a complex with T-CF/LEF in the nucleus to activate the target genes expression of the c-myc and cyclin D1 that regulated the proliferation [10]. β-catenin also bound to the promoter site of IL-8 which will increase the expression of IL-8 of an angiogenesis factor, it decreased the expression of RASSF1A of a TSG in NPC [3]. Activation of the Wnt/β-catenin signal pathway also plays a role in inhibiting apoptosis through cytochrome c release resistance resulting in the constraint of caspace-9 activity [17].
The unproven hypothesis of this study was probably due to the several factors that inhibit the activity of downstream signaling pathway of β-catenin, those who played a role in the development and progressivity of NPC. Several studies on NPC showed a constraint on the expression of c-Myc and cyclin D1 resulted in inhibited of G1/S transition, reduced IL-8 angiogenic response due to decreased expression of the receptor and increased release of cytochrome c through the role of VDAC1 protein in mitochondrial outer membranes; those caused an increase in caspace-9 activity [22–24].
The unproven hypothesis of this study was also probably due to the presence of other signaling pathways that could regulate the target gene of the β-catenin, those contributed to the development and progressivity of NPC. Several studies have shown that gene target expression of β-catenin in NPC contributed to the development and progression of NPC, it was also regulated by other signal pathways such as NF-κB, PI3K and AP-1. Expression of cyclin D1 and c-Myc was regulated by LMP1 through a signaling pathway of NF-κB [11, 25]. The NF-κB signal pathway was also reported to induce the expression of IL-8 [26] and suppressed the expression of RASSF1A through the stimulation of LMP1 [27]. The management of caspace-9 activation was reportedly arranged through the PI3K/Akt signal pathway [28].
The role of β-catenin increased the NPC proliferation that occurred through the activation of the proliferative signaling pathway of cyclin D1 and c-Myc. Cyclin D1 and c-Myc activated cdk4/cdk6 and cyclin E/cdk2 complexes that facilitated the G1/S transition resulted in the acceleration of cell cycle progression [3]. A study by Wu et al. (2014) showed that G1/S transition in cell cycle was inhibited by increased expression of miRNA-188 through direct resistance to the expression of a number of cyclin/cdk complexes, such as cdk2, cdk4, CCND1, CCND3, CCNE1, CCNE2 [22]. This showed that the effect of β-catenin on an increase of NPC proliferation could be inhibited by increased expression of miRNA-188. Increased expression of miR-188 was found in NPC cell cultures under hypoxic stress [29].
Cyclin D1 and c-Myc transcription were also regulated by signal paths other than β-catenin. Activation of the NF-κB signal path by LMP1 would improve the EGFR transcription. Furthermore, EGFR would bound to DNA in the cyclin promoter region cyclin D1 and cyclin E that resulted in an increase the transcription of both proteins [22]. c-Myc transcription induced LMP1 through activation of signal transducer, activator of transcription protein 3 (STAT 3) and transcription factor NF-κB [25].
Activation of the Wnt/β-catenin signal pathway inhibited apoptosis through cytochrome c release resistance resulted in the constraint of caspace-9 activity [17]. Other studies have shown that EBV infection in NPC cell culture resulted in increased cytochrome c release in the cytoplasm through the role of voltage-dependent anion channel protein 1 (VDAC1). The VDAC1 protein was found in the outer membrane of mitochondria and had a role to regulate pro-apoptotic signals in mammalian cells [24]. This showed that the apoptotic barrier effects by stimulation of the Wnt/β-catenin signal path could be reduced or eliminated by the role of VDAC1. Caspace-9 expression was also regulated by other pathways than β-catenin. A study by Jiang et al. (2010) on NPC xenographic mice showed that caspace-9 activation was regulated through the PI3K/Akt signal pathway [28].
Increased expression of β-catenin will elevate angiogenesis in NPC through increased IL-8 transcription [3]. Interleukin 8 played a role in the management of cytoskeleton to initiate and maintain endothelial migration in angiogenesis processes through activation of Gα12/Gα13 and PI3K. Activation of Gα12/Gα13 and PI3K might occur when IL-8 bonded to CXCR1 and CXCR2 receptors on endothelial [12]. Expressions of CXCR1 and CXCR2 might decrease through internalization mechanisms (endocytosis) and decreased response due to the desensitization process [23]. Decreased expression of CXCR1 and CXCR2 due to internalization process and desensitization resulted from increased IL-8 exposure; it was stimulated by β-catenin that resulted in a decreased angiogenic response of IL-8 to NPC. IL-8 expression was also regulated by the NF-κB signal path that induced by LMP-1 [26].
The role of β-catenin in increased NPC metastasis occurred through its resistance to RASSF1A, a negative effector of Ras. The RASSF1A suppression mechanism could also occur through mechanisms that did not involve β-catenin. Decreased RASSF1A expression could occur through LMP1 suppression mechanisms in RASSF1A promoter activity, it happened due to the activation of NFκB signaling that included C-terminal activating region (CTARs) in LMP1 [28]. Decreased RASSF1A expression resulted in an increase of Ras activity. Another mechanism that increased Ras activity was through direct stimulation by LMP1 [3]. Ras through its activation of Raf and MEK–ERK would stimulate the MMPs transcription of type IV collagenases that played a role in the invasion and metastasis of cancer cells. This showed that increased metastasis in NPC through the MMPs pathway may result from stimulation of β-catenin or direct stimulation of LMP1 against Ras [17].
E-cadherin proteins form complexes with β-catenin in adherents junction to maintain intercellular adhesion, mediate cell-to-cell communication and suppress metastasis. Several studies have shown that in NPC there was a decrease in E-cadherin expression which was positively correlated with an increase in stage [1, 10]. Decreased E-cadherin expression in NPC might result from hyper-methylation in the promoter region of the gene stimulated by the activity of the phosphatidylinositol 3-kinase (PI3K)/Akt signal pathway and resulted in increased invasion [14]. The PI3K/Akt signal pathway was included in a number of signal paths induced by LMP [11]. This showed that the role of β-catenin in suppressing metastasis through the formation of complexes with E-cadherin in adherents junction became insignificant because there was a decrease in E-cadherin expression in NPC.
Another factor causing the results of this study differs from previous studies was the correlation of β-catenin expression with clinical stages in NPC, differences in categorization of β-catenin expression and differences in NPC stage assessment system. A study by Wang’s et al. (2009), Luo et al. (2012) and Xu et al. (2013) classified the expression level of β-catenin into two categories (weak/strong or negative/positive expression) [1, 4, 10]. Meanwhile, this study has classified the level of β-catenin expression into four categories: negative, weak, moderate, and strong expression. The previous study by Wang et al. used the Fuzhou system (1992) to assess the staging, whereas, Luo et al. (2012) used AJCC in 2002. The components of staging that assessed in a study by Xu et al. were tumor size (T) and unmentioned staging assessment system [1, 4, 10]. This study the used AJCC 2011 in the staging of NPC [19].
Conclusion
There was no correlation between β-catenin expression and staging in patients with nasopharyngeal carcinoma.
References
- 1.Luo W, Fang W, Li S, Yao K. Aberrant expression of nuclear vimentin and related epithelial–mesenchymal transition markers in nasopharyngeal carcinoma. Int J Cancer. 2012;10:1863–1873. doi: 10.1002/ijc.27467. [DOI] [PubMed] [Google Scholar]
- 2.Wey WIKD. Current management strategy of nasopharyngeal carcinoma. Clin Exp Otorhinolaryngol. 2010;1:1–12. doi: 10.3342/ceo.2010.3.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chou J, Lin Y, Kim J, You L, Xu Z, He B, et al. Nasopharyngeal carcinoma—review of the molecular mechanism of tumorigenesis. Head Neck. 2008;30(7):946–963. doi: 10.1002/hed.20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang FL, Xiang G, Yuan TZ, Cao SM, Roo HL, Hou JH, et al. Expression and clinical significance of Wnt-1 and β-catenin in nasopharyngeal carcinoma. Chin J Cancer. 2009;8(1):72–79. [PubMed] [Google Scholar]
- 5.Shi W, Bastianutto C, Li A, Ordonez BP, Ng R, Chow KY. Multiple dysregulated pathways in nasopharyngeal carcinoma revealed by gene expression profilling. Int J Cancer. 2006;119:2467–2475. doi: 10.1002/ijc.22107. [DOI] [PubMed] [Google Scholar]
- 6.Chen C, Yi W, Gao J, Li XH, Shen LJ, Li BF, et al. Alternate endpoints to the 5-year overall survival and locoregional control for nasopharyngeal carcinoma: a retrospective analysis of 2,450 patients. Mol Clin Oncol. 2014;2:385–392. doi: 10.3892/mco.2014.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Notopuro H, Kentjono W, Handajani R, Notopuro F. Detection of cytokeratin 19 mRNA in blood as an early marker of micrometastatic tumor cells of nasopharyngeal carcinoma patients in surabaya. Folia Medica Indones. 2008;44(2):76–81. [Google Scholar]
- 8.Moussa SAB, Guemira F, Buisine MP. Detection of circulating tumor cells in the peripheral blood of nasopharyngeal carcinoma patients by nested reverse transcriptase polymerase assay for cytokeratin 19 mRNA. Afr J Biotechnol. 2014;13(3):378–384. [Google Scholar]
- 9.Santoro A, Pannone G, Papagerakis S, McGuff HS, Cafarelli B, Lepore S, et al. Beta-catenin and epithelial tumors: a study based on 374 oropharyngeal cancer. Biomed Res Int. 2014;64:1–13. doi: 10.1155/2014/948264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu L, Jiang Y, Zheng J, Xie G, Li J, Shi L, et al. Aberrant expression of β-catenin and E-cadherin is correlated with poor prognosis of nasopharyngeal cancer. Hum Pathol. 2013;44(7):1357–1364. doi: 10.1016/j.humpath.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 11.Zheng H, Li L, Hu D, Deng X, Cao Y. Role of Epstein–Barr virus encoded latent membrane protein 1 in the carcinogenesis of nasopharyngeal carcinoma. Cell Mol Immunol. 2007;4(3):185–196. [PubMed] [Google Scholar]
- 12.Brat DJ, Bellail AC, Van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol. 2005;7(2):122–133. doi: 10.1215/S1152851704001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li XJ, Peng L, Shan JY, Lu WH, Zhang JX, Chen S, et al. As an independent unfavorable prognostic factor, IL-8 promotes metastatic of nasopharyngeal carcinoma through induction of epithelial–mesenchimal transition and activation of AKT signalling. Carcinogenesis. 2012;33:1302–1309. doi: 10.1093/carcin/bgs181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yip WK, Seow H. Activation of phosphatidylinositol 3-kinase/Akt signalling bu EGF downregulates membranous E-cadherin and β-catenin and enhances invasion in nasopharygeal carcinoma cell. Cancer Lett. 2012;318:162–172. doi: 10.1016/j.canlet.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 15.Lo PHY, Xie D, Chan KC, Xu FP, Kuzmin I, Lerman MI, et al. Reduced expression of RASSF1A in esophageal and nasopharyngeal carcinomas significantly correlates with tumor stage. Cancer Lett. 2007;257:199–205. doi: 10.1016/j.canlet.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Rosenthal EL, Matrisian L. Matrix metalloproteinases in head and neck cancer. Head Neck. 2006;28:639–648. doi: 10.1002/hed.20365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen S, Guttridge C, You Z, Zhang Z, Fribley A, Mayo MW, et al. Wnt-1 signaling inhibits apoptosis by activating β-catenin/T cell factor-mediated transcription. J Cell Biol. 2001;152(1):87–96. doi: 10.1083/jcb.152.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruun J, Kolberg M, Nesland JM, Svindland A, Nesbakken A, Lothe RA. Prognostic significance of β-catenin, E-cadherin, and SOX9 in colorectal cancer: result from a large population-representative series. Front Oncol. 2014;4(118):1–16. doi: 10.3389/fonc.2014.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfister DG, Ang KK, Brizel DM, Burtness BA, Cmelak AJ, Colevas AD, et al. Head and neck cancers. J Nat Compr Cancer Netw. 2011;9(6):596–650. doi: 10.6004/jnccn.2011.0053. [DOI] [PubMed] [Google Scholar]
- 20.Galera-Ruiz H, Ríos M, Campora RG, de Miguel M, Carmona MI, Moreno AM, et al. The cadherin–catenin complex in nasopharyngeal carcinoma. Eur Arch Otorhinolaryngol. 2011;268:1335–1341. doi: 10.1007/s00405-010-1464-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osterheld MC, Bian Y, Bosman FT, Benhattar J, Fontolliet C. Beta-catenin expression and its association with prognostic factors in adenocarcinoma developed in Barrett esophagus. Am J Clin Pathol. 2012;117:451–456. doi: 10.1309/1db6-gfvh-ra6w-q07y. [DOI] [PubMed] [Google Scholar]
- 22.Wu J, Lv Q, Qing Lv, Jie He, Zhang H, Mei X, Cui K, et al. MicroRNA-188 supresses G1/S transition by targeting multiple cyclin/CDK complexes. Cell Commun Signal. 2014;12(66):1–13. doi: 10.1186/s12964-014-0066-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oppenheim JJ, Feldman M, Durum SK, Hirano T, Vilcek J, Nicola NA. Cytokine reference: a compendium of cytokines and other mediators of host defence. 1. London: Academic Press; 2000. [Google Scholar]
- 24.Feng X, Ching B, Chen WN. EBV up-regulates cytochrome c through VDAC1 regulations and decrease the release of cytoplasmic Ca2 + in the NPC cell line. Cell Biol Int. 2012;36:733–738. doi: 10.1042/CBI20110368. [DOI] [PubMed] [Google Scholar]
- 25.Lo AKF, Lo K, Tsao SW, Wong HL, Hui JWY, To KF, et al. Epstein–Barr virus infection alters cellular signal cascades in human nasopharyngeal epithelial cell. Neoplasia. 2006;8(3):173–180. doi: 10.1593/neo.05625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshizaki T, Horikawa T, Ren QC, Wakisaka N, Takeshita H, Sheen TS, et al. Induction of interleukin-8 by Epstein–Barr virus latent membran protein-1 and its correlation to angiogenesis in nasopharyngeal carcinoma. Clin Cancer Res. 2001;7:1946–1951. [PubMed] [Google Scholar]
- 27.Man C, Rosa J, Lee LTO, Chow BKC, Lo KW, et al. Latent membran protein 1 supresses RASSF1A expression, disrupts microtubule structures and induces chromosomal aberration in human epithelial cells. Oncogene. 2007;26:3069–3080. doi: 10.1038/sj.onc.1210106. [DOI] [PubMed] [Google Scholar]
- 28.Jiang H, Fan D, Zhou G, Li X, Deng H. Phosphatidylinositol 3-kinase inhibitor (LY294002) induces apoptosis of human nasopharyngeal carcinoma in vitro and in vivo. J Exp Clin Cancer Res. 2010;29(34):1–7. doi: 10.1186/1756-9966-29-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hua Z, Lv Q, We Y, Wong CK, Cai G, Gu D, et al. MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS ONE. 2006;1:116–127. doi: 10.1371/journal.pone.0000116. [DOI] [PMC free article] [PubMed] [Google Scholar]


