Abstract
Rationale:
The lysergamide lysergic acid diethylamide (LSD) is a prototypical classical hallucinogen with remarkably high potency. LSD remains a popular recreational drug but is also becoming an important research tool for medical and neuroscience studies. Recently, several lysergamides that are close structural analogs of LSD have been sold as recreational drugs, which suggests that further studies are needed to explore the pharmacological properties of these compounds.
Objective:
In this present investigation, another LSD congener, N-ethyl-N-cyclopropyl lysergamide (ECPLA), which to date has not been marketed as a recreational substance, was evaluated for its pharmacological features relative to those previously reported for LSD. The experiments focused on interactions with the 5-HT2A receptor, which is responsible for mediating the psychedelic effects of LSD and other hallucinogens.
Methods:
Competitive binding assays were performed to measure the affinity of ECPLA for 27 monoamine receptors. The ability of ECPLA to activate human 5-HT2 receptor subtypes was assessed using calcium mobilization assays. Head twitch response (HTR) studies were conducted in C57BL/6J mice to determine whether ECPLA activates 5-HT2A receptors in vivo. Two other N-alkyl substituted lysergamides, N-methyl-N-isopropyl lysergamide (MIPLA) and N-methyl-N-propyl lysergamide (LAMPA), were also tested in the HTR paradigm for comparative purposes.
Results:
ECPLA has high affinity for most serotonin receptors, α2-adrenoceptors, and D2-like dopamine receptors. Additionally, ECPLA was found to be a potent, highly efficacious 5-HT2A agonist for Gq-mediated calcium flux. Treatment with ECPLA induced head twitches in mice with a median effective dose (ED50) of 317.2 nmol/kg (IP), which is ~40% of the potency observed previously for LSD. LAMPA (ED50 = 358.3 nmol/kg) was virtually equipotent with ECPLA in the HTR paradigm whereas MIPLA (ED50 = 421.7 nmol/kg) was slightly less potent than ECPLA.
Conclusions:
These findings demonstrate that the pharmacological properties of ECPLA, MIPLA and LAMPA are reminiscent of LSD and other lysergamide hallucinogens.
Keywords: LSD, 5-HT2A receptor, lysergamide, psychedelics, head twitch
INTRODUCTION
(+)-Lysergic acid diethylamide (LSD; Figure 1) is one of the most potent serotonergic hallucinogens, with typical oral doses ranging from 60–200 μg (Shulgin and Shulgin 1997). The activity of LSD is closely tied to the specific structure of its diethylamide moiety, and even minor modifications produce a dramatic reduction of potency. Replacement of the diethylamide group of LSD with another alkylamide group or a cycloalkylamide moiety generally reduces hallucinogenic potency by at least an order of magnitude (Rothlin 1957; Murphree et al. 1958; Abramson 1959; Isbell et al. 1959; Abramson and Rolo 1967). Even N-methyl-N-propyl lysergamide (LAMPA, MPLA or LMP-55), an isomer of LSD with similar hydrophobicity, is markedly less potent (Abramson and Rolo 1967). One exception is the conformationally restricted LSD analog (2′S,4′S)-lysergic acid 2,4-dimethylazetidide (LSZ), which retains high 5-HT2A affinity and potency in rodent behavioral models (Nichols et al. 2002; Brandt et al. 2017a).
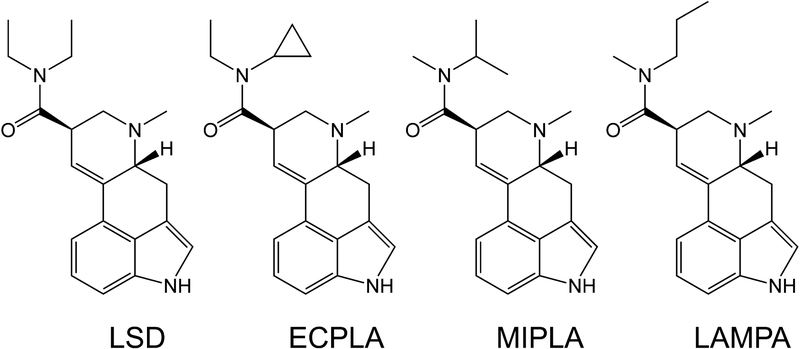
Figure 1.
Chemical structures of lysergic acid diethylamide (LSD), N-ethyl-N-cyclopropyl lysergamide (ECPLA), N-methyl-N-isopropyl lysergamide (MIPLA), and N-methyl-N-propyl lysergamide (LAMPA).
Although very few clinical investigations were carried out with LSD during the past few decades, numerous studies have been published in recent years. Some of these investigations were conducted to assess potential therapeutic applications (Gasser et al. 2014,2015), but most studies were intended to assess the subjective response to LSD (Schmid et al. 2015; Carhart-Harris et al. 2016a; Dolder et al. 2015,2016; Schmid and Liechti 2017) or to evaluate its neurophysiological effects (Carhart-Harris et al. 2016b; Tagliazucchi et al. 2016; Müller et al. 2017a,b; Schmidt et al. 2017). Thus, LSD is rapidly becoming an important tool in medical research.
There has been considerable interest in the pharmacology of LSD due to its high potency. LSD has been found to have high affinity for most serotonin (5-hydroxytryptamine, 5-HT) receptors and also interacts with dopaminergic and adrenergic receptors (Leysen 1989; Peroutka 1994; Marona-Lewicka and Nichols 1995; Watts et al. 1995; Nichols et al. 2002; Rickli et al. 2015). The psychedelic effects of serotonergic hallucinogens are largely mediated by 5-HT2A receptor activation; blockade of the 5-HT2A receptor eliminates the subjective effects produced by LSD and other hallucinogens in humans (Vollenweider et al. 1998; Valle et al. 2016; Barrett et al. 2017; Kraehenmann et al. 2017; Preller et al. 2017). A recent x-ray crystallography study of LSD bound into the 5-HT2B receptor, along with receptor kinetics studies and molecular dynamics simulations, revealed that extracellular loop 2, which connects the top of helix 4 with helix 5, folds over the LSD molecule after it binds, dramatically hindering its dissociation from the receptor (Wacker et al. 2017). This “entrapment” of LSD within the receptor may at least partially account for the high potency and long duration of action of this molecule. Nevertheless, pharmacokinetic studies in humans indicate that the subjective effects of LSD occur in parallel with its presence in the body at effective concentrations (Dolder et al. 2015,2017).
LSD has been a popular recreational drug since the mid-1960s. Although the use of LSD has occurred at relatively stable levels, in recent years several other lysergamides have been distributed as new psychoactive substances (NPS) or “research chemicals” (Brandt et al. 2016,2017a,b,2018). Most of the new lysergamides, including N6-allyl-6-norlysergic acid diethylamide (AL-LAD), N6-ethyl-6-norlysergic acid diethylamide (ETH-LAD), LSZ, and lysergic acid morpholide (LSM-775), were originally synthesized during the course of scientific research (Gogerty and Dille 1957; Hoffman and Nichols 1985; Nichols et al. 2002). Other compounds, however, were specifically developed by NPS vendors as potential recreational drugs. The lysergamides 1-propionyl-LSD (1P-LSD) and 1-propionyl-ETH-LAD (1P-ETH-LAD) were previously unknown in the scientific literature before they were marketed online as psychoactive substances (Brandt et al. 2016,2017b).
According to recent online postings, NPS vendors claimed to have synthesized N-ethyl-N-cyclopropyl lysergamide (ECPLA; Figure 1), a novel lysergamide that is unknown in the scientific literature. Therefore, we have compared the pharmacological properties of ECPLA to those previously reported for LSD. Competitive binding assays were performed to measure the affinity of ECPLA for 27 monoamine receptors. Additionally, activation of 5-HT2 receptor subtypes by ECPLA was assessed using functional assays of Gq-coupled Ca2+-mobilization.
In addition to assessing whether ECPLA is likely to become a new recreational drug, another reason to investigate its pharmacology is to probe the structure-activity relationships governing the interactions between lysergamides and the 5-HT2A receptor. Specifically, the studies were designed to test whether replacing one of the ethyl groups of LSD with a cyclopropyl substituent has a significant effect on 5-HT2A receptor affinity and potency. The potency of LSD is significantly reduced by increasing the steric bulk of one of its amide substituents, as exemplified by N-ethyl-N-isopropyl lysergamide (EIPLA), which had three-fold lower affinity than LSD for 5-HT2A sites labeled with [3H]ketanserin in rat frontal cortex and almost 3-fold lower potency than LSD in the rat drug discrimination paradigm (Huang et al. 1994). However, it is not clear how potency would be affected by a marginal increase in steric bulk. The steric bulk of a cyclopropyl group (with a calculated van der Waals volume of 43.8 Å3) is more comparable to that of an ethyl group (VvdW = 38.9 Å3) compared to an isopropyl group (VvdW = 56.2 Å3; Zhao et al. 2003).
The head twitch response (HTR) is a rapid paroxysmal head movement induced by serotonergic hallucinogens in rats and mice due to 5-HT2A receptor activation (Canal and Morgan 2012; Halberstadt and Geyer 2013). The HTR is commonly used as a behavioral proxy in rodents for human hallucinogenic effects because it can reliably distinguish between hallucinogenic and non-hallucinogenic 5-HT2A receptor agonists (Gonzalez-Maeso et al. 2007). LSD and analogs such as AL-LAD and LSZ induce HTRs in mice with high potency (Corne and Pickering 1967; Halberstadt and Geyer 2013; Brandt et al. 2017a). HTR studies were conducted in C57BL/6J mice to assess whether ECPLA activates 5-HT2A receptors and produces LSD-like behavioral effects in vivo. To compare the potency of ECPLA with other N-alkyl-substituted lysergamides in mice, additional experiments examined the effect of N-methyl-N-isopropyl lysergamide (MIPLA) and LAMPA in the HTR paradigm. These congeners have the same number of carbon atoms in the amide moiety and would have hydrophobicity virtually identical to LSD. In addition, in rats trained to discriminate saline from LSD, MIPLA was only slight less potent than LSD (0.036 mg/kg vs 0.021 mg/kg, respectively). Although MIPLA had 6-fold lower affinity than LSD at [3H]ketanserin-labeled sites in rat forebrain homogenate (28.1 vs. 4.78 nM, respectively), it had about 82% of the affinity of LSD at [125I]DOI-labeled sites (MIPLA Ki = 1.75 nM, LSD Ki = 1.43 nM; Huang et al. 1994). Importantly, MIPLA has been reported to have about one-third the potency of LSD as a psychedelic in man (Shulgin 2016); recent online postings indicate that MIPLA is available as an NPS (Anonymous 2018). By contrast, little is known about the pharmacology of LAMPA. In a study conducted in six hallucinogen-experienced subjects, administration of LAMPA (100 μg p.o.) had no effect in four subjects and produced effects consistent with a threshold dose of LSD in two subjects (Abramson and Rolo 1967).
MATERIALS AND METHODS
Animals
Male C57BL/6J mice (6–8 weeks old) obtained from Jackson Laboratories (Bar Harbor, ME, USA) were housed in a vivarium at the University of California San Diego, an AAALAC-approved animal facility that meets all Federal and State requirements for care and treatment of laboratory animals. Mice were housed up to four per cage in a climate-controlled room on a reverse-light cycle (lights on at 1900 h, off at 0700 h) and were provided with ad libitum access to food and water, except during behavioral testing. Testing was conducted between 1000 and 1800 h. All animal experiments were carried out in accordance with NIH guidelines and were approved by the UCSD animal care committee.
Drugs
N-Ethyl-N-cyclopropyl lysergamide (ECPLA) was obtained from Synex Synthetics BV (Maastricht, The Netherlands) as the hemitartrate (2:1) salt. N-Methyl-N-propyl lysergamide (LAMPA) was obtained from Lipomed Inc. (Cambridge, MA, USA) as the freebase. N-Methyl-N-isopropyl lysergamide (MIPLA) freebase was available from previous studies. Competitive binding and functional assays were performed using ECPLA dissolved in dimethyl sulfoxide. For studies in mice, ECPLA was dissolved in isotonic saline and injected IP at a volume of 5 mL/kg. MIPLA and LAMPA were dissolved in 50 mM tartaric acid (pH ~5) and injected IP at 5 mL/kg.
Binding Studies
A screening at 27 receptor binding sites was performed by the NIMH Psychoactive Drug Screening Program (NIMH PDSP). ECPLA was tested at 10 μM in competition assays against radioactive probe compounds; each primary binding assay was performed in quadruplicate. Sites exhibiting >50% inhibition at 10 μM were tested in secondary assays at the identified receptor using 11 concentrations of ECPLA, measured in triplicate, to generate competition binding isotherms. Ki values were obtained from best-fit IC50 values (derived from nonlinear regression of the binding isotherms) using the Cheng-Prusoff equation (Cheng and Prusoff 1973). The radioligands used were as follows: [3H]8-OH-DPAT (5-HT1A), [3H]GR125743 (5-HT1B/1D), [3H]5-HT (5-HT1E), [3H]ketanserin (5-HT2A), [3H]LSD (5-HT2B/5A/6/7), [3H]mesulergine (5-HT2C), [3H]prazosin (α1A/1B/1D), [3H]rauwolscine (α2A/2B/2C), [125I] pindolol (β1), [3H]CGP12177 (β2, β3), [3H]SCH23390 (D1, D5), [3H]N-methylspiperone (D2/3/4), [3H]pyrilamine (H1), [3H]tiotidine (H2), [3H]α-methylhistamine (H3), and [3H]histamine (H4). The experimental protocols are available from the NIMH PDSP website (Roth 2013).
5-HT2 Receptor Functional Assays
Detailed protocols for the functional assays have been published online (Roth 2013). 5-HT2 functional experiments (measuring Gq-mediated calcium flux) were performed in HEK293 cells stably expressing either human 5-HT2A, 5-HT2B, or 5-HT2C receptors. The day before the assay, cells were seeded into poly-L-lysine-coated black 384-well clear-bottom tissue culture plates with DMEM (Invitrogen, Carlsbad, CA, USA) containing 1% dialyzed fetal bovine serum at a density of ~15,000 cells in 40 μL per well. The next day, a stock solution of ECPLA was diluted in assay buffer (HBSS, 20 mM HEPES, 2.5 mM probenecid, pH 7.4). Media were decanted and replaced with 20 μL per well of assay buffer containing Fluo-4 Direct dye (Invitrogen) and incubated for 1 h at 37°C. Plates were allowed to equilibrate at room temperature and Ca2+ flux was measured using a FLIPRTETRA system (Molecular Devices, Sunnyvale, CA, USA). Plates were read for fluorescence initially for 10 s (1 read per s) to establish a baseline, and then stimulated by the addition of 10 μL/well of drug dilutions or buffer and read for an additional 120 s. Peak fluorescence in each well was normalized to maximum-fold increase over baseline. Data were normalized to the maximum peak fold-over-basal fluorescence produced by 5-HT (100%) and baseline fluorescence (0%). Data were analyzed using the sigmoidal dose-response function of GraphPad Prism 5.0.
Head Twitch Response Studies
The head twitch response (HTR) was assessed using a head-mounted magnet and a magnetometer detection coil (Halberstadt and Geyer 2013,2014; Nichols et al. 2015; Klein et al. 2018). Briefly, mice were anesthetized, a small incision was made in the scalp, and a small neodymium magnet was attached to the dorsal surface of the cranium using dental cement. Following a two-week recovery period, HTR experiments were carried out in a well-lit room with at least 7 days between sessions to avoid carryover effects. Test compounds were injected immediately prior to testing. Mice (n=5–7/group) were injected with drug or vehicle and then HTR activity was recorded in a glass cylinder surrounded by a magnetometer coil for 30 min. Coil voltage was low-pass filtered (2–10 kHz cutoff frequency), amplified, and digitized (20 kHz sampling rate) using a Powerlab/8SP with LabChart v 7.3.2 (ADInstruments, Colorado Springs, CO, USA), then filtered off-line (40–200 Hz band-pass). Head twitches were identified manually based on the following criteria: 1) sinusoidal wavelets; 2) evidence of at least three sequential head movements (usually exhibited as bipolar peaks) with frequency ≥ 40 Hz; 3) amplitude exceeding the level of background noise; 4) duration < 0.15 s; and 5) stable coil voltage immediately preceding and following each response.
Data Analysis
Head twitch counts were analyzed using one-way analyses of variance (ANOVA). Post hoc pairwise comparisons between selected groups were performed using Tukey’s studentized range method. Significance was demonstrated by surpassing an α-level of 0.05. The entire 30-min recordings were examined for head twitches, but in some cases a shorter block of time was used for analysis to accommodate compounds with a brief duration-of-action since potency calculations can be confounded by extended periods of inactivity. Median effective doses (ED50 values) and 95% confidence intervals (95% CI) for HTR dose-response experiments were calculated by nonlinear regression (Prism 7.00, GraphPad Software, San Diego, CA, USA). A Gaussian distribution (Christopoulos et al. 2001) was used to fit biphasic HTR dose-response data:
In these equations, E is the drug effect, Baseline is the response in the control group, Range is the distance from Baseline to the top of the curve, [A] is the dose of the drug, and midA is the logarithm of the dose corresponding to the top of the curve.
RESULTS
Table 1 lists the affinities of ECPLA for 27 serotonergic, adrenergic, dopaminergic, and histaminergic receptors. The binding affinities and selectivity of ECPLA observed for monoaminergic sites are consistent with the values previously reported for LSD (Leysen 1989; Marona-Lewicka and Nichols 1995; Nichols et al. 2002; Rickli et al., 2015). ECPLA was observed to bind nonselectively to 5-HT receptors with affinities in the 1–100 nM range. The sites with the highest affinities were 5-HT1A (Ki = 3.2 nM), 5-HT2B (Ki = 5.3 nM), and 5-HT5A (Ki = 8.6 nM). Using the same conditions, LSD was previously reported to bind to human 5-HT2A receptors (labeled with [3H]ketanserin) and human 5-HT2C receptors (labeled with [3H]mesulergine) with Ki values of 13 nM and 30 nM, respectively (Nichols 2017); compared to LSD, ECPLA has equivalent affinity for the 5-HT2A receptor (Ki = 16.5 nM) but almost 3-fold lower affinity for the 5-HT2C receptor (Ki = 85.7 nM). ECPLA binds to α-adrenoceptors with some preference for α2 subtypes compared to α1A, α1B, and α1D sites. The affinity of ECPLA for α2A and α2B adrenergic sites (Ki values of 144 nM and 62.3 nM, respectively) approximates the reported affinity of LSD for α2-adrenoceptors labeled with [3H]clonidine (Ki = 37–82 nM) in rat cortex (Leysen 1989; Marona-Lewicka and Nichols 1995). In contrast to LSD (Nichols et al. 2002), ECPLA did not bind to β1 or β2 sites with appreciable affinity (<50% displacement at 10 μM), but did interact with β3 sites with affinity in the micromolar range (Ki = 2,415 nM). Similar to LSD (Nichols et al. 2002; Rickli et al. 2015), ECPLA binds to dopamine receptors with some selectivity for D2-like sites (D2, D3, and D4 receptors) compared to D1-like sites (D1 and D5 receptors). ECPLA has negligible affinity for H1, H3, or H4 receptors in these experiments (i.e., <50% displacement at 10 μM), whereas it binds to H2 receptors with a Ki of 890 nM. LSD reportedly binds to H1 receptors with a Ki of 1,100 to 1,500 nM (Nichols et al. 2002; Rickli et al. 2015) but its affinity for other histamine receptors has not been reported.
Table 1.
Receptor binding data for N-ethyl-N-cyclopropyllysergamide (ECPLA).
| Receptor | Speciesa | Radioligand | Ki (nM)b,c |
|---|---|---|---|
| 5-HT1A | Human | [3H]8-OH-DPAT | 3.2 ± 0.5 (3) |
| 5-HT1B | Human | [3H]GR125743 | 42.3 ± 8.7 (3) |
| 5-HT1D | Human | [3H]GR125743 | 12.6 ± 1.9 (3) |
| 5-HT2A | Human | [3H]ketanserin | 16.5 ± 6.0 (3) |
| 5-HT2B | Human | [3H]LSD | 5.3 ± 1.3 (3) |
| 5-HT2c | Human | [3H]mesulergine | 85.7 ± 31.2 (3) |
| 5-HT5A | Human | [3H]LSD | 8.6 ± 4.2 (3) |
| 5-HT6 | Human | [3H]LSD | 21.7 ± 4.4 (3) |
| 5-HT7 | Human | [3H]LSD | 27.3 ± 11.5 (4) |
| α1A | Human | [3H]prazosin | > 10,000 (3) |
| α1B | Human | [3H]prazosin | 1954 ± 152 (3) |
| α1D | Human | [3H]prazosin | > 10,000 (3) |
| α2A | Human | [3H]rauwolscine | 144 ± 64 (3) |
| α2B | Human | [3H]rauwolscine | 62.3 ± 15.1 (3) |
| α2c | Human | [3H]rauwolscine | 438± 118 (3) |
| β1 | Human (heartd) | [125I]pindolol | > 10,000e |
| β2 | Human | [3H]CGP12177 | > 10,000e |
| β3 | Human | [3H]CGP12177 | 2415± 639(4) |
| D1 | Human | [3H]SCH23390 | 502 ± 122 (3) |
| D2 | Human | [3H]N-methylspiperone | 150 ± 32 (3) |
| D3 | Human | [3H]N-methylspiperone | 104 ± 29 (3) |
| D4 | Human | [3H]N-methylspiperone | 92.3 ± 11.9 (3) |
| D5 | Human | [3H]SCH23390 | 1504±386 (4) |
| H1 | Human | [3H]pyrilamine | > 10,000e |
| H2 | Human | [3H]tiotidine | 890 ± 137 (3) |
| H3 | Guinea pig | [3H]α-methylhistamine | > 10,000e |
| H4 | Human | [3H]histamine | > 10,000e |
The experiments were performed using cloned receptors from the species indicated.
Mean ± SEM.
The number of independent determinations (performed in triplicate) are listed in parentheses.
The experiment was performed using tissues natively expressing the receptor.
IC50 > 10 μM in the primary binding assay.
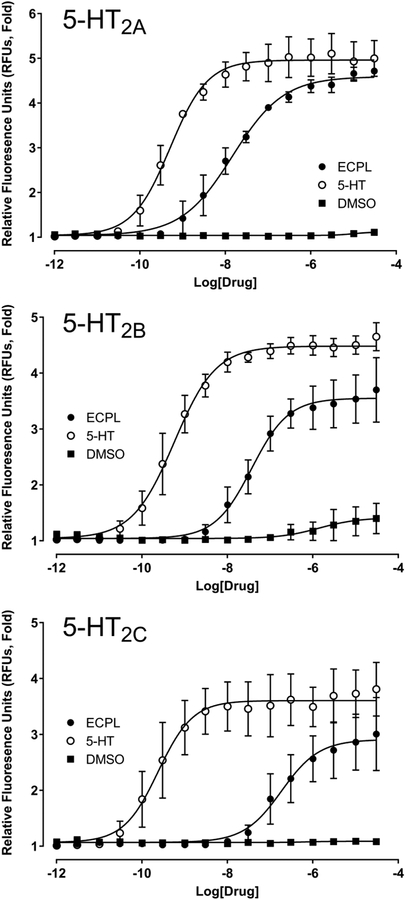
The effect of ECPLA on human 5-HT2A, 5-HT2B, and 5-HT2C receptors was assessed using Gq-mediated Ca2+ mobilization in HEK293 cells (Table 2). As shown in Figure 2, ECPLA acted as a partial agonist at all three 5-HT2 receptor subtypes. ECPLA has the highest potency and efficacy at 5-HT2A receptors (EC50 = 14.6 nM; Emax = 91%) compared to 5-HT2B (EC50 = 36.7 nM; Emax = 73%) and 5-HT2C (EC50 = 183 nM; Emax = 73%) receptors.
Table 2.
Functional activity of N-ethyl-N-cyclopropyllysergamide (ECPLA) at 5-HT2 receptors.
| Receptor | ECPLA | 5-HT | ||
|---|---|---|---|---|
| EC50, nM (pEC50 ± SEM) | Emax % 5-HT | EC50, nM (pEC50 ± SEM) | Emax % 5-HT | |
| h5-HT2A | 14.6 (7.84 ± 0.04) | 91 ± 1 | 0.50 (9.30 ± 0.04) | 100 |
| h5-HT2B | 36.7 (7.44 ± 0.06) | 73 ± 2 | 0.56 (9.25 ± 0.03) | 100 |
| h5-HT2c | 183 (6.74 ± 0.09) | 73 ± 3 | 0.24 (9.63 ± 0.07) | 100 |
Data were acquired with HEK293 cells expressing either human 5-HT2A (h5-HT2A), human 5-HT2B (h5-HT2B), or human 5-HT2C INI (h5-HT2C) receptors. Data represent EC50 and Emax means and standard error of the mean (SEM) from two independent experiments performed in quadruplicate. Emax is defined as percent 5-HT maximum response.
Figure 2.
Agonist potencies of N-ethyl-N-cyclopropyl lysergamide (ECPLA) at 5-HT2 receptor subtypes. Concentration-response curves for ECPLA and dimethylsulfoxide (DMSO) vehicle are shown for human 5-HT2A, 5-HT2B, and 5-HT2C receptors expressed in HEK293 cells. Values are the mean ± SEM of two independent experiments performed in quadruplicate.
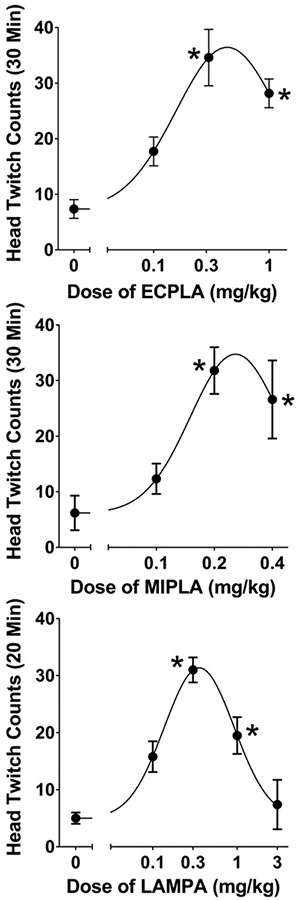
ECPLA produced a significant increase in HTR counts over baseline levels in mice (F(3,20) = 14.92, p<0.0001). Similar to other lysergamides (Halberstadt and Geyer 2013; Brandt et al. 2017a), ECPLA induced head twitches with a biphasic inverted-U-shaped dose-response function, with the peak response occurring at 0.3 mg/kg (p<0.01, Tukey’s test; see Figure 3). The ED50 of ECPLA is 130.2 μg/kg (317.2 nmol/kg) with a 95% CI of 87.5–193.7 μg/kg (213.2–471.9 nmol/kg). In comparison, LSD had an ED50 of 132.8 nmol/kg under equivalent conditions (Halberstadt and Geyer 2013), meaning that ECPLA induces the HTR with 42% of the molar potency of LSD.
Figure 3.
Effect of N-ethyl-N-cyclopropyl lysergamide (ECPLA, top panel), N-methyl-N-isopropyl lysergamide (MIPLA, middle panel), and N-methyl-N-propyl lysergamide (LAMPA, bottom panel) on the head twitch response. Data are presented as group means ± SEM for the first 20-min of the test session (LAMPA) or the entire 30-min test session (ECPLA and MIPLA). *p<0.01, significant difference from the vehicle control group (Tukey’s test).
As shown in Figure 3, MIPLA (F(3,17) = 36.12, p<0.0001) and LAMPA (F(4,23)=14.44, p<0.0001) also induced the HTR in mice with biphasic dose-responses. The ED50 for MIPLA was 136.4 μg/kg (421.7 nmol/kg) with a 95% CI of 116.6–159.5 μg/kg (360.5–493.1 nmol/kg). The ED50 for LAMPA was 115.9 μg/kg (358.3 nmol/kg) with a 95% CI of 78.0–172.2 μg/kg (241.1–532.4 nmol/kg).
DISCUSSION
The present investigation was conducted to examine the pharmacological properties of ECPLA, a novel analog of LSD. Studies assessed the binding of ECPLA to monoamine receptors and its ability to activate 5-HT2 subtypes in vitro. Additional experiments were conducted to determine whether ECPLA induces the HTR in mice. ECPLA bound to 5-HT receptors, adrenergic α2A and α2B receptors, and D2/3/4 dopamine receptors with moderately high affinity, which is reminiscent of the binding profile of LSD. ECPLA was nearly a full agonist for Gq-mediated calcium flux at the 5-HT2A receptor but had lower efficacy and potency at 5-HT2B and 5-HT2C receptors. In addition, the effects of ECPLA in the head twitch paradigm suggest that it is capable of activating 5-HT2A receptors in vivo. In summary, the fact that ECPLA is a 5-HT2A agonist with nanomolar affinity and produces HTRs in mice strongly suggests it will act as an LSD-like (hallucinogenic) agent in humans.
ECPLA and LSD have remarkably similar binding profiles at monoamine receptors. Replacing one of the two ethyl groups present in LSD with a cyclopropyl group apparently has little influence on its ability to interact with serotonergic, dopaminergic, adrenergic, or histaminergic receptors. One notable difference between LSD and ECPLA is that the former compound has moderate affinity for β1 (Ki = 140 nM) and β2 (Ki = 740 nM) receptors (Nichols et al. 2002) whereas the affinity of ECPLA for those receptors is negligible (i.e., <50% displacement at 10 μM). Nevertheless, in humans, the concentration of LSD in cerebrospinal fluid (CSF) probably does not exceed 0.6–0.8 nM after a 200 μg p.o. dose (Dolder et al. 2015), so the interaction between LSD and β-adrenergic receptors is unlikely to contribute to its psychopharmacology. Although the concentration of LSD in CSF is relatively low compared to its reported affinity for 5-HT2A (Ki = 13 nM; Nichols 2017), the competitive binding assays used to determine the Ki are likely to underestimate the affinity of LSD due to its receptor kinetics. LSD has a long residence time at the 5-HT2A receptor; reported t1/2 values range from 20 min (Bennett and Snyder 1975; Burris and Sanders-Bush 1992) to 138.6 min (Wacker et al 2017). Given the slow dissociation of LSD from 5-HT2A, binding may not reach equilibrium during competitive binding assays, resulting in an underestimation of affinity. Indeed, when tested in association and dissociation assays, [3H]LSD binds to the 5-HT2A receptor with KD = 0.33 nM (Wacker et al. 2017), which is consistent with the concentration in CSF.
The binding of LSD to the 5-HT2A receptor is known to be highly sensitive to the steric and conformational properties of the amide substituent (Oberlender et al. 1992; Huang et al. 1994; Monte et al. 1995; Nichols et al. 1996). The conformation of the diethylamide moiety in LSD is optimal for binding because it interacts with a region of the 5-HT2A receptor that has a highly complementary structure (Wacker et al. 2017). ECPLA and LSD exhibited similar affinities for [3H]ketanserin-labeled 5-HT2A sites, indicating the binding site can readily accommodate an amide substituent that is marginally bulkier than ethyl. Although cyclopropyl substitution did not alter 5-HT2A binding affinity relative to LSD, a reduction of agonist potency at the 5-HT2A receptor was observed. ECPLA activates the human 5-HT2A receptor (h5-HT2A) with an EC50 of 14.6 nM. According to Wacker et al. (2017), LSD activates h5-HT2A in the same expression system with an EC50 of 3.61 nM. The difference in 5-HT2A agonist potency between LSD and ECPLA roughly parallels their potencies in the HTR assay, which is consistent with our previous finding that ED50 values in the HTR assay are correlated with potencies for inducing Ca2+ mobilization via the 5-HT2A receptor (Nichols et al. 2015). Therefore, compared to 5-HT2A binding affinity, receptor activation may exhibit a greater sensitivity to changes in the steric bulk of the amide substituent. In addition to increasing steric bulk, cyclopropyl substitution would also alter the conformational mobility of the amide side-chain. Homology modeling suggest that the two ethyl groups in LSD adopt a trans conformation when bound to the 5-HT2A receptor (Wacker et al. 2017); the substituents in ECPLA may adopt a slightly different orientation, potentially reducing agonist potency.
The reduction in in vivo potency produced by replacing one of the N-ethyl groups in LSD with an N-cyclopropyl group closely mirrored the effect of N-isopropyl substitution observed previously with EIPLA. According to Huang et al. (1994), EIPLA produced full substitution in rats trained to discriminate 0.08 mg/kg LSD but was only 36% as potent as the training drug. A similar potency reduction (42%) occurred with ECPLA relative to LSD in the HTR assay. Although many hallucinogens have been tested in both mouse head twitch and rat drug discrimination (DD) studies, it is not clear whether a relationship exists between potencies in these paradigms, making it difficult to compare the relative behavioral potencies of EIPLA and ECPLA. Nevertheless, according to our preliminary studies, there does appear to be a robust and highly significant correlation between rat DD- and mouse HTR-derived ED50 values for phenylalkylamine and tryptamine hallucinogens (Halberstadt et al., unpublished findings), demonstrating that potencies in the HTR and DD paradigms are likely closely related.
MIPLA, the N-methyl-N-isopropyl isomer of LSD, also induced head twitches in mice. On a molar basis, MIPLA was only slightly less potent than ECPLA in the HTR paradigm and has about a third of the potency of LSD. Since MIPLA and ECPLA have nearly comparable potencies in mice, it appears that N-cyclopropyl vs. N-isopropyl substitution does not appreciably alter the behavioral pharmacology of lysergamides. MIPLA was found to fully substitute for LSD in rats, with about half the potency of the training drug (Huang et al. 1994). According to Shulgin, human subjects administered MIPLA at doses of 180–300 μg experienced LSD-like psychedelic effects, making it about two- to threefold less potent than LSD (Shulgin 2016). Hence, there is converging evidence that MIPLA also has behavioral effects comparable to LSD but with reduced potency.
The LSD isomer LAMPA induced head twitches in mice and was virtually equipotent with ECPLA and MIPLA. Although LAMPA is reportedly less potent than LSD in humans (Abramson and Rolo 1967), few details have been published regarding the psychopharmacology of this compound. Based on the HTR data, LAMPA is predicted to act as a hallucinogen in humans with approximately three-fold lower potency than LSD.
The pharmacological and behavioral properties of ECPLA are thus reminiscent of LSD and other lysergamide hallucinogens. In this study, ECPLA acted as a potent and highly efficacious 5-HT2A agonist and induced head twitches in mice with only slightly lower potency than LSD. These findings indicate that ECPLA is likely to produce hallucinogenic effects in humans, although clinical trials are ultimately necessary to understand the human psychopharmacology of ECPLA. Given the pharmacology of ECPLA, it has the potential to be used in a manner similar to other lysergamide hallucinogens. LSD is becoming an important tool in clinical research and the possibility exists that ECPLA could also be used for similar research purposes.
Table 3.
Effect of lysergamides on the head twitch response (HTR).
| compound | Dose (mg/kg) | N | Duration (min) | HTR Counts (Mean ± SEM) | ED50, μg/kg (95% CI) | ED50, nmol/kg (95% CI) |
|---|---|---|---|---|---|---|
| ECPLAa | 30 | 130.2 (87.5–193.7) | 317.2 (213.2–471.9) | |||
| 1 | 6 | 28.2 ± 2.6 * | ||||
| MIPLAb | 30 | 136.4 (116.6–159.5) | 421.7 (360.5–493.1) | |||
| 0.4 | 5 | 26.6 ± 3.1 * | ||||
| LAMPAb | 20 | 115.9 (78.0–172.2) | 358.3 (241.1–532.4) | |||
| 3 | 5 | 7.4 ± 4.3 |
hemitartrate (2:1) salt;
free base.
p<0.01 vs. vehicle control group (Tukey’s test).
ACKNOWLEDGMENTS
These studies were supported by an award from NIDA (R01 DA041336), as well as by the Veteran’s Administration VISN 22 Mental Illness Research, Education, and Clinical Center. Receptor binding data were generously provided by the National Institute of Mental Health’s Psychoactive Drug Screening Program (NIMH PDSP), Contract # HHSN-271-2008-00025-C. The NIMH PDSP is directed by Dr. Bryan Roth at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscol at NIMH, Bethesda, MD, USA.
Funding: This study was funded by NIDA (R01 DA041336)
Footnotes
CONFLICT OF INTEREST
On behalf of all authors, the corresponding author states that there is no conflict of interest.
REFERENCES
- Abramson HA (1959) Lysergic acid diethylamide (LSD-25): XXIX. The response index as a measure of threshold activity of psychotropic drugs in man. J Psychol 48: 65–78. [Google Scholar]
- Abramson HA, Rolo A (1967) Comparison of LSD with methysergide and psilocybin on test subjects In: Abramson HA (ed) The Use of LSD in Psychotherapy and Alcoholism. Bobbs-Merrill Company, Inc., Indianapolis, pp 53–57 [Google Scholar]
- Anonymous (2018) Tripping well grounded: an experience with MIPLA (exp111958). Available online: https://erowid.org/experiences/exp.php?ID=111958 [Accessed: August 21, 2018]
- Barrett FS, Preller KH, Herdener M, Janata P, Vollenweider FX (2017) Serotonin 2A Receptor Signaling Underlies LSD-induced Alteration of the Neural Response to Dynamic Changes in Music. Cereb Cortex: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JP Jr., Snyder SH (1975) Stereospecific binding of D-lysergic acid diethylamide (LSD) to brain membranes: relationship to serotonin receptors. Brain Res 94: 523–44. [DOI] [PubMed] [Google Scholar]
- Brandt SD, Kavanagh PV, Twamley B, Westphal F, Elliott SP, Wallach J, Stratford A, Klein LM, McCorvy JD, Nichols DE, Halberstadt AL (2018) Return of the lysergamides. Part IV: Analytical and pharmacological characterization of lysergic acid morpholide (LSM-775). Drug Test Anal 10: 310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt SD, Kavanagh PV, Westphal F, Elliott SP, Wallach J, Colestock T, Burrow TE, Chapman SJ, Stratford A, Nichols DE, Halberstadt AL (2017a) Return of the lysergamides. Part II: Analytical and behavioural characterization of N6 -allyl-6-norlysergic acid diethylamide (AL-LAD) and (2′S,4′S)-lysergic acid 2,4-dimethylazetidide (LSZ). Drug Test Anal 9: 38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt SD, Kavanagh PV, Westphal F, Elliott SP, Wallach J, Stratford A, Nichols DE, Halberstadt AL (2017b) Return of the lysergamides. Part III: Analytical characterization of N(6) -ethyl-6-norlysergic acid diethylamide (ETH-LAD) and 1-propionyl ETH-LAD (1P-ETH-LAD). Drug Test Anal 9: 1641–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt SD, Kavanagh PV, Westphal F, Stratford A, Elliott SP, Hoang K, Wallach J, Halberstadt AL (2016) Return of the lysergamides. Part I: Analytical and behavioural characterization of 1-propionyl-d-lysergic acid diethylamide (1P-LSD). Drug Test Anal 8: 891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris KD, Sanders-Bush E (1992) Unsurmountable antagonism of brain 5-hydroxytryptamine2 receptors by (+)-lysergic acid diethylamide and bromo-lysergic acid diethylamide. Mol Pharmacol 42: 826–30. [PubMed] [Google Scholar]
- Canal CE, Morgan D (2012) Head-twitch response in rodents induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model. Drug Test Anal 4: 556–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Kaelen M, Bolstridge M, Williams TM, Williams LT, Underwood R, Feilding A, Nutt DJ (2016a) The paradoxical psychological effects of lysergic acid diethylamide (LSD). Psychol Med 46: 1379–90. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Muthukumaraswamy S, Roseman L, Kaelen M, Droog W, Murphy K, Tagliazucchi E, Schenberg EE, Nest T, Orban C, Leech R, Williams LT, Williams TM, Bolstridge M, Sessa B, McGonigle J, Sereno MI, Nichols D, Hellyer PJ, Hobden P, Evans J, Singh KD, Wise RG, Curran HV, Feilding A, Nutt DJ (2016b) Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc Natl Acad Sci U S A 113: 4853–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22: 3099–3108. [DOI] [PubMed] [Google Scholar]
- Christopoulos A, Grant MK, Ayoubzadeh N, Kim ON, Sauerberg P, Jeppesen L, El-Fakahany EE (2001) Synthesis and pharmacological evaluation of dimeric muscarinic acetylcholine receptor agonists. J Pharmacol Exp Ther 298: 1260–8. [PubMed] [Google Scholar]
- Corne SJ, Pickering RW (1967) A possible correlation between drug-induced hallucinations in man and a behavioural response in mice. Psychopharmacologia 11: 65–78. [DOI] [PubMed] [Google Scholar]
- Dolder PC, Schmid Y, Haschke M, Rentsch KM, Liechti ME (2015) Pharmacokinetics and Concentration-Effect Relationship of Oral LSD in Humans. Int J Neuropsychopharmacol 19: pyv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolder PC, Schmid Y, Muller F, Borgwardt S, Liechti ME (2016) LSD Acutely Impairs Fear Recognition and Enhances Emotional Empathy and Sociality. Neuropsychopharmacology 41: 2638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolder PC, Schmid Y, Steuer AE, Kraemer T, Rentsch KM, Hammann F, Liechti ME (2017) Pharmacokinetics and pharmacodynamics of lysergic acid diethylamide in healthy subjects. Clin Pharmacokinet 56: 1219–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser P, Holstein D, Michel Y, Doblin R, Yazar-Klosinski B, Passie T, Brenneisen R (2014) Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J Nerv Ment Dis 202: 513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser P, Kirchner K, Passie T (2015) LSD-assisted psychotherapy for anxiety associated with a life-threatening disease: a qualitative study of acute and sustained subjective effects. J Psychopharmacol 29: 57–68. [DOI] [PubMed] [Google Scholar]
- Gogerty JH, Dille JM (1957) Pharmacology of d-lysergic acid morpholide (LSM). J Pharmacol Exp Ther 120: 340–8. [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA (2007) Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 53: 439–52. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA (2013) Characterization of the head-twitch response induced by hallucinogens in mice: detection of the behavior based on the dynamics of head movement. Psychopharmacology (Berl) 227: 727–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA (2014) Effects of the hallucinogen 2,5-dimethoxy-4-iodophenethylamine (2C-I) and superpotent N-benzyl derivatives on the head twitch response. Neuropharmacology 77: 200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AJ, Nichols DE (1985) Synthesis and LSD-like discriminative stimulus properties in a series of N(6)-alkyl norlysergic acid N,N-diethylamide derivatives. J Med Chem 28: 1252–5. [DOI] [PubMed] [Google Scholar]
- Huang X, Marona-Lewicka D, Pfaff RC, Nichols DE (1994) Drug discrimination and receptor binding studies of N-isopropyl lysergamide derivatives. Pharmacol Biochem Behav 47: 667–73. [DOI] [PubMed] [Google Scholar]
- Isbell H, Miner EJ, Logan CR (1959) Relationships of psychotomimetic to anti-serotonin potencies of congeners of lysergic acid diethylamide (LSD-25). Psychopharmacologia 1: 20–8. [DOI] [PubMed] [Google Scholar]
- Klein LM, Cozzi NV, Daley PF, Brandt SD, Halberstadt AL (2018) Receptor binding profiles and behavioral pharmacology of ring-substituted N,N-diallyltryptamine analogs. Neuropharmacology, in press. doi: 10.1016/j.neuropharm.2018.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraehenmann R, Pokorny D, Vollenweider L, Preller KH, Pokorny T, Seifritz E, Vollenweider FX (2017) Dreamlike effects of LSD on waking imagery in humans depend on serotonin 2A receptor activation. Psychopharmacology (Berl) 234: 2031–46. [DOI] [PubMed] [Google Scholar]
- Leysen JE (1989) Use of 5-HT receptor agonists and antagonists for the characterization of their respective receptor sites In: Boulton AA, Bakrer GB, Juorio AV (eds) Drugs As Tools In Neurotransmitter Research (Neuromethods). Humana Press, Clifton, NJ, pp 299–350 [Google Scholar]
- Marona-Lewicka D, Nichols DE (1995) Complex stimulus properties of LSD: a drug discrimination study with alpha 2-adrenoceptor agonists and antagonists. Psychopharmacology (Berl) 120: 384–91. [DOI] [PubMed] [Google Scholar]
- Monte AP, Marona-Lewicka D, Kanthasamy A, Sanders-Bush E, Nichols DE (1995) Stereoselective LSD-like activity in a series of d-lysergic acid amides of (R)- and (S)-2-aminoalkanes. J Med Chem 38: 958–66. [DOI] [PubMed] [Google Scholar]
- Müller F, Lenz C, Dolder P, Lang U, Schmidt A, Liechti M, Borgwardt S (2017a) Increased thalamic resting-state connectivity as a core driver of LSD-induced hallucinations. Acta Psychiatr Scand 136: 648–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F, Lenz C, Dolder PC, Harder S, Schmid Y, Lang UE, Liechti ME, Borgwardt S (2017b) Acute effects of LSD on amygdala activity during processing of fearful stimuli in healthy subjects. Transl Psychiatry 7: e1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphree HB, de Maar EWJ, Williams HL, Bryan LL (1958) Effects of lysergic acid derivatives on man: antagonism between d-lysergic acid diethylamide and its 2-brom congener. J Pharmacol Exp Ther 122: 55A–56A. [Google Scholar]
- Nichols DE (2017) Chemistry and Structure-Activity Relationships of Psychedelics. Curr Top Behav Neurosci: doi: 10.1007/7854_2017_475. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Frescas S, Marona-Lewicka D, Kurrasch-Orbaugh DM (2002) Lysergamides of isomeric 2,4-dimethylazetidines map the binding orientation of the diethylamide moiety in the potent hallucinogenic agent N,N-diethyllysergamide (LSD). J Med Chem 45: 4344–9. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Monte A, Huang X, Marona-Lewicka D (1996) Stereoselective pharmacological effects of lysergic acid amides possessing chirality in the amide substituent. Behav Brain Res 73: 117–9. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Sassano MF, Halberstadt AL, Klein LM, Brandt SD, Elliott SP, Fiedler WJ (2015) N-Benzyl-5-methoxytryptamines as Potent Serotonin 5-HT2 Receptor Family Agonists and Comparison with a Series of Phenethylamine Analogues. ACS Chem Neurosci 6: 1165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlender R, Pfaff RC, Johnson MP, Huang XM, Nichols DE (1992) Stereoselective LSD-like activity in d-lysergic acid amides of (R)- and (S)-2-aminobutane. J Med Chem 35: 203–11. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ (1994) 5-Hydroxytryptamine receptor interactions of D-lysergic acid diethylamide In: Pletscher A, Ladewig D (eds) 50 Years of LSD Current Status and Perspectives of Hallucinogens. Parthenon Press, New York, pp 19–26 [Google Scholar]
- Preller KH, Herdener M, Pokorny T, Planzer A, Kraehenmann R, Stampfli P, Liechti ME, Seifritz E, Vollenweider FX (2017) The Fabric of Meaning and Subjective Effects in LSD-Induced States Depend on Serotonin 2A Receptor Activation. Curr Biol 27: 451–457. [DOI] [PubMed] [Google Scholar]
- Rickli A, Luethi D, Reinisch J, Buchy D, Hoener MC, Liechti ME (2015) Receptor interaction profiles of novel N-2-methoxybenzyl (NBOMe) derivatives of 2,5-dimethoxy-substituted phenethylamines (2C drugs). Neuropharmacology 99: 546–53. [DOI] [PubMed] [Google Scholar]
- Roth BL (2013) National Institute of Mental Health Psychoactive Drug Screening Program (NIMH PDSP) Assay Protocol Book, Version II. Available online: https://pdspdb.unc.edu/pdspWeb/content/PDSP%20Protocols%20II%202013-03-28.pdf [Accessed: 06 May 2017] [Google Scholar]
- Rothlin E (1957) Pharmacology of lysergic acid and some related compounds. J Pharm Pharmacol 9: 569–587. [DOI] [PubMed] [Google Scholar]
- Schmid Y, Enzler F, Gasser P, Grouzmann E, Preller KH, Vollenweider FX, Brenneisen R, Muller F, Borgwardt S, Liechti ME (2015) Acute Effects of Lysergic Acid Diethylamide in Healthy Subjects. Biol Psychiatry 78: 544–53. [DOI] [PubMed] [Google Scholar]
- Schmid Y, Liechti ME (2017) Long-lasting subjective effects of LSD in normal subjects. Psychopharmacology (Berl) 235: 535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Muller F, Lenz C, Dolder PC, Schmid Y, Zanchi D, Lang UE, Liechti ME, Borgwardt S (2017) Acute LSD effects on response inhibition neural networks. Psychol Med: 1–13. [DOI] [PubMed] [Google Scholar]
- Shulgin A, Shulgin A (1997) TIHKAL: the Continuation. Transform Press, Berkeley [Google Scholar]
- Shulgin AT (2016) Pharmacology Notebook 9. Available online: https://www.erowid.org/library/books_online/shulgin_labbooks/shulgin_pharmacology_notebook9_searchable.pdf [Accessed: January 20, 2018] [Google Scholar]
- Tagliazucchi E, Roseman L, Kaelen M, Orban C, Muthukumaraswamy SD, Murphy K, Laufs H, Leech R, McGonigle J, Crossley N, Bullmore E, Williams T, Bolstridge M, Feilding A, Nutt DJ, Carhart-Harris R (2016) Increased Global Functional Connectivity Correlates with LSD-Induced Ego Dissolution. Curr Biol 26: 1043–50. [DOI] [PubMed] [Google Scholar]
- Valle M, Maqueda AE, Rabella M, Rodriguez-Pujadas A, Antonijoan RM, Romero S, Alonso JF, Mananas MA, Barker S, Friedlander P, Feilding A, Riba J (2016) Inhibition of alpha oscillations through serotonin-2A receptor activation underlies the visual effects of ayahuasca in humans. Eur Neuropsychopharmacol 26: 1161–75. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H, Hell D (1998) Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 9: 3897–902. [DOI] [PubMed] [Google Scholar]
- Wacker D, Wang S, McCorvy JD, Betz RM, Venkatakrishnan AJ, Levit A, Lansu K, Schools ZL, Che T, Nichols DE, Shoichet BK, Dror RO, Roth BL (2017) Crystal Structure of an LSD-Bound Human Serotonin Receptor. Cell 168: 377–389 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts VJ, Lawler CP, Fox DR, Neve KA, Nichols DE, Mailman RB (1995) LSD and structural analogs: pharmacological evaluation at D1 dopamine receptors. Psychopharmacology (Berl) 118: 401–9. [DOI] [PubMed] [Google Scholar]
- Zhao YH, Abraham MH, Zissimos AM (2003) Fast calculation of van der Waals volume as a sum of atomic and bond contributions and its application to drug compounds. J Org Chem 68: 7368–73. [DOI] [PubMed] [Google Scholar]