Abstract
Vascular access for hemodialysis has a long and rich history. This article highlights major innovations and milestones in the history of angioaccess for hemodialysis. Advances in achievement of lasting hemodialysis access, swift access transition, immediate and sustaining access to vascular space built the momentum at different turning points of access history and shaped the current practice of vascular access strategy. In the present era, absent of large-scale clinical trials to validate practice, the ever-changing demographic and comorbidity makeup of the dialysis population pushes against stereotypical angioaccess goals. The future of hemodialysis vascular access would benefit from proper randomized clinical trials and acclimatization to clinical contexts.
1 |. INTRODUCTION
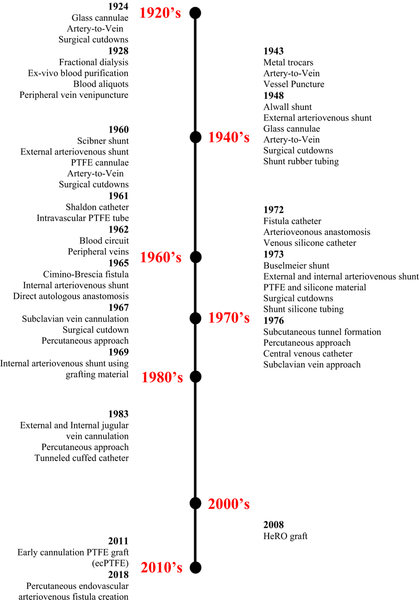
Almost one century ago, the understanding of biochemical phenomena of diffusion and ultrafiltration, coupled with the technical invention of hemodialysis machine, demarcated the beginning of a long path filled with hurdles and successes to attain a durable vascular access. Over time, the means of achieving access to large blood vessels capable of providing consistent and rapid extracorporeal blood flow followed a journey of radical transformation. This article provides a view at the landscape of vascular access for hemodialysis and explores future frontiers. To provide a historical perspective, we have constructed a time line of hemodialysis angioaccess to include important milestones, some of which marked paradigm shifts in vascular access strategy (Figure 1).
FIGURE 1.
History of hemodialysis vascular access
2 |. THE PERIOD OF PROVISIONAL VASCULAR ACCESS (1924–1965)
The first attempt at hemodialysis in humans, launched by Georg Haas, took place in 1924. At that time, vascular access consisted of glass cannulae inserted by surgical cut-down of two separate blood vessels, an artery, and a vein.1 Blood purification was unsuccessful, largely due to technical difficulties related to the dialysis machine. As a result, Haas resorted to a substitute method which he called “fractionated dialysis” whereby instead of circulating blood outside the body, circa 400 mL of blood was withdrawn from the patient, heparinized, circulated for 30 minutes through the dialyzer, reintroduced in the blood stream, and repeated the procedure nine times. By the end of 1928, Haas discontinued his work.
The field of blood purification remained at a standstill until 1943, when a more efficient dialysis machine was designed and introduced by Willem Kolff. By this time, medical vascular access insertion changed from surgical cut-down to venipuncture using metal trocars. Access to vascular space for hemodialysis continued to involve simultaneous arterial and venous cannulation, usually of upper and lower extremity (ie, radial artery, cephalic vein, femoral artery, and femoral vein). The ongoing nature of repetitive cannulation of peripheral vasculature with each dialysis session caused rapid exhaustion of available vessels. Therefore, dialysis delivery and patient survival were not possible beyond a few months due to eventual in-ability to gain entry into the vascular space.2
In 1948, Nils Alwall developed the concept of a bypass that connected venous and arterial glass cannulae with rubber tubing to form a continuous shunt and maintain access patency between dialyses sessions. Because the materials used at that time induced clotting after a few uses, this technique was abandoned within 2 years.3 Nevertheless, it was Alwall’s concept of creating an arteriovenous shunt that laid the foundation of future methodology for lasting vascular access.
3 |. THE ESTABLISHMENT OF LONG-LASTING VASCULAR ACCESS (1966–1989)
3.1 |. The external arteriovenous shunt
By mid-1950s, the use of polytetrafluoroethylene (PTFE)-based materials in the medical field spurred improvement of the external arteriovenous shunt originally developed by Nils Alwall. The experience with PTFE tubing in cardiac surgery had revealed major medical advantages of this material, such as improved biocompatibility and low thrombogenicity.4 In 1960, Wayne E. Quinton, David Dillard, and Belding H. Scribner used PTFE (aka Teflon) tubing to insert access in an artery and adjacent vein, one end burrowed subcutaneously, and opposite, external end of the tubes connected through a U-shaped plastic tube.5 This device, publicized as the Scribner shunt, represented a remarkable incremental progress in the history of hemodialysis vascular access, being the first mechanism to provide rapid and effective access to vascular space for long-term hemodialysis.
Surgical cut-down for Scribner shunt placement usually involved the radial artery and cephalic vein or the posterior tibial artery and the greater saphenous vein at the ankle. The cannulae were customized (diameter and curvature) at the time of insertion by gently heating the PTFE tubing, and the external shunt was mounted to an arm plate connected with metal fittings. To increase flexibility, a later model adapted silicone segments inserted between the PTFE vascular tips and the extracutaneous shunt, eliminating the need for the arm plate.6 The design carried high risk of external piece dislodgement, for which patients carried bulldog clamps and restricted use of the associated extremity. With frequent occurrences of infection and clotting and average life span of 6 months, patients necessitated several shunts in the upper and lower extremities. Distal limb ischemia was common, owing to distal artery ligation or thrombosis at the end of the shunt use.7
In an attempt to decrease the risk of external shunt dislodgement, Buselmeier et al modified the Scribner shunt in 1973 by using a smaller U-shaped silastic tube attached to PTFE arterial and venous tips. The U-shaped tube had two PTFE-plugged outlets that communicated to the outside of the body, and the device could be implanted subcutaneously, leaving only the ports protruding through the skin. In spite of these improvements, the Buselmeier version of the external shunt did not gain popularity as other methods of vascular access were being developed.8
3.2 |. Transition from vascular cannulas to intravascular catheters
Progress in vascular cannulation continued alongside that of shunt development. In 1961, Stanley Shaldon introduced PTFE tubing implants (ie, catheters) in femoral artery and femoral vein by percutaneous puncture, with wires used as catheter guides (Seldinger technique).9 This approach evolved into retention of catheters between dialysis sessions by injecting heparin directly into the tubing and temporary clamping. To limit the added risks of arterial cannulation, dialysis angioaccess shifted to vein only cannulation, single-lumen venous catheter using one vein and double-pump hemodialysis machine, or double-lumen dialysis catheter inserted in a single peripheral vein, most commonly femoral vein.
In 1962, James Cimino proposed direct use of peripheral veins by venipuncture in the arm. He created intermittent pressure in the arm with a tourniquet applied 10–15 cm above the needle, used a blood pump to obtain blood supply to the dialyzer at a blood flow of 150–400 mL/min, and returned the blood through a needle inserted in a different peripheral vein.10 Because this technique worked mainly in patients with volume overload and for a temporary period of time, it was soon abandoned.
3.3 |. Direct arteriovenous anastomosis
In 1965, Kenneth Appell performed the first internal, autologous arteriovenous shunt as a subcutaneous anastomosis between the radial artery and the cephalic vein, a procedure proposed by James E. Cimino and Michael J. Brescia. The results of the first procedures of direct arteriovenous anastomosis, performed on 16 patients, were published in 1966, and the technique was dubbed arteriovenous fistula.11 Originally described as side-to-side anastomosis between the radial artery and the cephalic vein, it was then adapted to include end-to-side, end-to-end anastomoses, and other locations. At that time, early fistula failure was reported in 5%−12% of cases.12–14 At its inception, dialysis was performed through the fistula the day after surgery using a tourniquet to produce venous engorgement. Its reliability, infrequent infection, and thrombosis, as well as its easy maintenance, made it the ideal option in patients who had suitable vessels. It was later noted that with time, the vessels became more prominent and thick-walled, making venipuncture even easier.
In the early 1970s, James J. Cole and colleagues explored the idea of fistula catheter. With this hybrid access, an arteriovenous anastomosis was created, followed by placement of a small-length silicone catheter in the venous outflow attached with a polyethylene terephthalate (PETE, aka Dacron) cuff at the percutaneous catheter exit site. The rationale for this technique was that the catheter would salvage even fistulas not able to develop and afford ease of cannulation while maintaining a vigorous blood flow provided by the arteriovenous anastomosis.15 This vascular access mechanism did not gain popularity and was rapidly retired.
3.4 |. Internal prosthetic arteriovenous shunt
After the introduction of the arteriovenous fistula technique at a large scale, the procedure was deemed challenging and not many centers were able to perform this surgery. The failure to achieve good venous outflow and difficulty in mastering successful cannulation became an issue. In light of these challenges, the search for alternatives to autologous arteriovenous anastomosis continued.
In 1969, the idea of subcutaneous insertion of a conduit between an arterial and venous vessel was introduced. The types of conduits explored included biological (autogenous, homologous, or xenograft) and synthetic materials. Of the biological graft materials, the saphenous vein autogenous graft was most commonly used primarily because of its long-standing utilization for vascular reconstruction.16 Unfortunately, when used for dialysis access, it was unable to tolerate repeated cannulation, and early occlusion often occurred.17 A series of attempts at using homologous vessels (umbilical or saphenous veins)18,19 or xenograft conduits (bovine carotid artery, mesenteric vein, or ureteric graft)20 took place, all of which delivered poor clinical results.
In the end, two types of prosthetic conduits dominated the field of vascular surgery: PETE and PTFE grafts. The filamentous velour PETE graft had the theoretical advantage of increased durability for recurrent cannulation. In clinical application, however, the PETE fabric often frayed and became structurally unstable.21 Expanded PTFE (ePTFE) material (which is soft, flexible, and porous PTFE) was used in the late 1970s as a subcutaneous conduit for vascular access, and soon became the most popular graft material.22 The first report on the clinical use of ePTFE prosthesis for peripheral arteriovenous access came in 1978. From that time, ePTFE was the most common graft material used for dialysis vascular access as it caused less infection and aneurysm formation compared to other prosthetic materials or grafts.23
3.5 |. Central vein cannulation with subcutaneous catheter
Subclavian vein cannulation via surgical cut-down was introduced in 1967, and the percutaneous technique of subclavian catheter insertion continued to be improved in the 1970s.24 Relative to femoral catheters which at that time were the primary means of temporary vascular access catheterization, the indwelling subclavian central catheters were found to be better suited for longer periods of dialysis in patients awaiting maturation of a fistula.25 As a result, in 1970s, the use of subclavian catheters began to supersede that of femoral catheters as temporary access for hemodialysis.
In 1976, the formation of subcutaneous tunnels to secure catheters and the placement of the catheter tips in the right atrium to reduce pain and thrombotic complications were described with long-term use of silicone catheters for parenteral nutrition and chemotherapy.24,26 The new design incorporated a PETE cuff at the skin exit site that provided catheter anchorage in the percutaneous tunnel. The methodology of central vein catheterization with placement of a tunneled, PETE-cuffed silicone catheter was adapted to patients on hemodialysis in the late 1970s and early 1980s.
At the same time, the technique for cannulation of external and internal jugular vein was being described. The first report in 1983 describes the experience of internal jugular vein tunneled catheters placed by means of a cut-down, maneuvered for tip placement in the superior portion of the right atrium for long-term use in hemodialysis vascular access.27 The technique evolved to percutaneous approach, use of peel-away sheath introducer, and use of image intensifier for accurate positioning. Later reports emerged that the risk of stenosis and thrombosis associated with subclavian vein cannulation substantially exceeded those seen with jugular vein catheterization.28 From that time, jugular vein access with tunneled, cuffed catheters became the most widespread form of central venous catheterization for hemodialysis.
4 |. POSTCENTRAL VEIN CATHETERIZATION ERA (1990S-PRESENT)
Since the introduction of central venous catheterization, catheter design has evolved, with constant modifications in an attempt to optimize blood flow delivery, decrease the rate of thrombosis, increase biocompatibility, increase resistance to occlusion, strengthen resistance to antiseptic agents, and reduce the rate of catheter collapse, kinking, or break.29 Changes in catheter design included placement of two individual catheters side by side, or placement of a single double-lumen catheter with varying side hole, or tip design (step tip, split tip, symmetric tip, or curved tip). More recently, heparin-or antibiotic-coated catheters and self-centering superior vena cava catheters have been added to the armamentarium.30
Owing to the number of effective vascular access strategies, patient life span on hemodialysis rose and the predicament of obtaining access to vascular space has resurfaced. For patients who exhaust peripheral venous access sites for traditional fistulas or grafts, an alternative to tunneled central venous catheters was introduced in 2008 with the Hemodialysis Reliable Outflow (HeRO) graft. This access is comprised of two elements, a graft anastomosed to the ipsilateral brachial artery and tunneled subcutaneously, and a catheter placed percutaneously into the right atrium through the subclavian or internal jugular vein, tunneled subcutaneously and attached to the graft through a titanium connector, bypassing regions of central vascular stenosis or occlusion.31 The device provides a continuous subcutaneous shunt from the upper arm artery to the right atrium, used as an external catheter-sparing access option in patients with severely compromised central veins. There is, however, a high complication rate, including high incidence of steal syndrome in women (25%), high thrombosis rate, and low rates of primary (11%) and secondary patency (32%) at 12 months.32,33
Modifications of the PTFE prosthetic material have also been initiated, among the most recent being PTFE graft material that allow early cannulation (ecPTFE).34 The ecPTFE grafts have a tri-layer construction, comprised of an inner layer of heparinized ePTFE, an outer layer of standard ePTFE, and a central elastomeric layer. The central layer gives the graft unique “low-bleed” properties and permits early cannulation, reducing the time to achieve hemostasis. Several studies have shown that these materials permit prompt cannulation. Standard practice with ePTFE grafts has been to avoid cannulation for 2–4 weeks following placement, but new generation grafts (Flixene, Avflo, Rapidax and Acuseal grafts) can accommodate cannulation within 72 hours of insertion and catheter avoidance in the majority of patients; patency and bacteremia rates were comparable to standard ePTFE grafts.35,36
More recently, two devices have received approval from the U.S. Food and Drug Administration for endovascular creation of upper extremity arteriovenous fistula, which hold high promise as an alternative to open surgery.37,38 The everlinQ endovascular arteriovenous fistula system uses a thermal resistance anastomosis device, developed to create proximal radial artery to perforating vein fistulas with a side-to-side anastomosis with minimal vessel trauma once the blood vessels have been approximated via magnets. Ellipsys vascular system uses a deep communicating vein and a novel catheter to capture the radial artery and create an arteriovenous anastomosis with thermal energy. The initial results with both systems demonstrated encouraging outcomes with high technical success rates, low re-intervention, and failure rates and good usability for hemodialysis.38–41 If these results are replicated on a larger scale, endovascular arteriovenous fistula creation may hold the key to better success for hemodialysis angioaccess practice.
4.1 |. The chronological landscape of hemodialysis vascular access practice
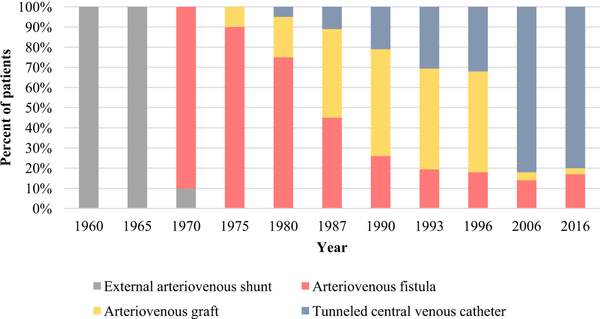
The hurdles of arteriovenous fistula placement and development compete with the use of central vein catheters for long-term chronic hemodialysis.27,42 The ease of insertion, the ability to be used in virtually any patient, and the suitability of immediate use propelled the catheters to becoming the most common type of vascular access used for hemodialysis (Figure 2). In 1993, 9.7% of patients on hemodialysis were using a tunneled cuffed catheter 30 days after the initiation of dialysis, 17% were using a fistula, 47% were using a graft, and the remaining were using a nontunneled catheter.43 In 1996 and 60 days after the initiation of dialysis, these numbers changed to 15%, 18%, and 50%, respectively.44 In 2006, at hemodialysis initiation, 82% used a tunneled central venous catheter, 14% used a fistula, and 4% used a graft.45 In 2016, at hemodialysis initiation, 80% used a tunneled central venous catheter, 17% used a fistula, and 3% a graft; 90 days after dialysis initiation, 69% of the patients were still using a catheter (Figure 2).46
FIGURE 2.
Trends in the practice of hemodialysis vascular access
Notably, the shift to catheter dominance predated the first vascular access guidelines.47 Therefore, accusations previously cast at fistula-first vascular guidelines for generating the perpetual rise in catheter use seem unwarranted. To a good extent, the culpability may simply rest on having the availability of such an utterly convenient method of access to vascular space conferred by the catheters.
4.2 |. “Temporary” versus “permanent” hemodialysis angioaccess
Historically, the byname of “permanent” given to the hemodialysis arteriovenous access was logical at the time, as the arteriovenous shunts saved the patients from repetitive vascular cannulations and made chronic dialysis possible. From a different perspective, this epithet seems to not be most suitable. Figure 2 displays a snapshot distribution of vascular access used for hemodialysis. The observation that in 2016, a much lower proportion of patients used catheters 90 days after initiating hemodialysis (69% compared with 80% on day 1 of hemodialysis) suggests—at face value—a successful conversion to a permanent vascular access; however, a more valuable metric for angioaccess resides in a longitudinal context.
In one study of 4532 incident patients initiated on long-term hemodialysis between 1996 and 2004, the first vascular access used for dialysis was a catheter in 69%, and arteriovenous graft in 18%, and an arteriovenous fistula in 13%.48 After a median follow-up of 1 year, 20% of patients who initiated hemodialysis with an arteriovenous access converted to using a central venous catheter (median time to conversion 62–84 days); of these, only 55% converted back to arteriovenous access (median time to conversion 44–71 days). Of patients who initiated hemodialysis with a central venous catheter, 45% converted to using an arteriovenous access (median time to conversion 66–105 days); of these, 30% converted back to a catheter (median time to conversion 58–70 days).48 In a recent study, we analyzed longitudinal transitions between catheter-based and arteriovenous access-based angioaccess outcomes in 391 patients initiating chronic hemodialysis with a tunneled central venous catheter between 2012 and 2013 and calculated the proportion of dialysis sessions delivered via an arteriovenous access across all hemodialysis sessions observed.49 After a mean follow-up period of 2.8 years, 83% of the patients converted from using a catheter to arteriovenous access; of these, 31% returned to using a catheter followed by a 58% re-conversion rate to using an arteriovenous access. Annual per-patient vascular access transition rates were 2.02 (SD 0.09) hemodialysis periods using a catheter and 0.54 (SD 0.03) hemodialysis periods using an arteriovenous access. Overall, of the patients who converted from catheter-based to arteriovenous access-based hemodialysis, only 52% received dialysis via the arteriovenous access for ≥80% of dialysis treatments.49 Another study, done in Canada, evaluated 1091 patients initiated on hemodialysis between 2004 and 2012 who received at least one arteriovenous fistula creation attempt.50 During the first year following hemodialysis initiation, catheter-free fistula use for ≥90% of hemodialysis treatments was achieved in only 31% of patients who received one arteriovenous fistula and 11% of patients who received two fistulas.50
Collectively, these data show that angioaccess conversion from central venous catheter to arteriovenous access does not ensure that most of the hemodialysis treatments will be delivered via an arteriovenous access during the patient life span. In fact, longitudinal studies have now uncovered a lack of “permanency” of arteriovenous access, unmasking a substantial gap between cross-sectional figures, expectations, and reality.
5 |. THE FUTURE OF VASCULAR ACCESS PRACTICE
To this date, the arteriovenous fistula has remained widely acknowledged as the most effective access for hemodialysis, leading as the preferred, first-line vascular access approach; arteriovenous grafts are considered second-line approach. Ever since 1990s, clinical guidelines have encouraged attempts to place an arteriovenous fistula whenever possible in hemodialysis patients.47 Incentives—or disincentives—designed to reinforce the guideline crystallized provider efforts to achieve predefined vascular access practice metrics.51,52 Despite these efforts, the overall conformity of vascular practices lagged behind expectations.53
The gap between valid guideline recommendations and delivery of care may be due to numerous barriers. Timely placement and development of functional fistulas for hemodialysis remain difficult logistical problems along with their high failure rate. Clinical practice guidelines are largely conceived as tools that standardize health professionals’ decisions rather than foster customized care or patient involvement in decision making. An emerging body of literature shows that the association between patient survival and vascular access used at dialysis initiation is a surrogate of better health status in those having undergone creation of a fistula or using a fistula.54–56 In several studies, the survival advantage associated with removal of the catheter was similar whether patients converted to fistula or graft.48,55,57 The relative benefit of fistulas may vary depending on the patient population, modulated by age and comorbidities, such that the benefits of arteriovenous fistula over those of arteriovenous graft dissipate in older patients.57
Getting a definitive answer as to whether a causal relationship exists between the type of vascular access elected for hemodialysis and patient survival requires large, multicenter, randomized clinical trials. A pilot trial is presently randomizing patients ≥65 years old with incident end-stage kidney disease and no prior arteriovenous access surgery, initiated on hemodialysis with a central venous catheter, to a strategy of fistula-first or graft-first vascular access placement (). This pilot study will yield valuable data related to participant recruitment, study dropout, and the conduct of study assessments to inform operational requirements for a large-scale trial.58 Another trial is currently enrolling patients ≥70 years of age with stage 4 or stage 5 chronic kidney disease, expected to undergo hemodialysis within 6 months of presentation, or with end-stage kidney disease on hemodialysis and failure of previous arteriovenous access, to be randomized into receiving an arteriovenous fistula or graft ().
Pending the results of these and other trials, the time seems right to adapt clinical practice such that the professional’s perspective as care provider and the patients’ characteristics and preferences are considered in the decision-making process.
6 |. CONCLUSIONS
The time line of hemodialysis angioaccess reveals steady progress punctuated by paradigm shifts in clinical strategy of vascular access selection. The successes in meeting the vascular access needs for hemodialysis patients have come not just from isolated scientific triumphs but also from efficient translation of new biochemical materials discoveries into medical application. To this date, each individual’s navigation of vascular access remains challenging. Access conversion from catheter to arteriovenous fistula or graft often does not denote subsequent successful access use for a meaningful amount of time. Medical regulations on vascular access practice ought to have some flexibility to accommodate patient specific needs and circumstances and facilitate patient involvement in clinical decision making. We anticipate that progress will continue, and we hope that a time line crafted three decades from now will reveal novel therapies and new paradigms that push our understanding of dialysis and vascular access to a level unimaginable today.
REFERENCES
- 1.Wizemann V, Ritz E. Georg Haas: a forgotten pioneer of haemodialysis. Nephrology. 1998;4(4):229–234. [Google Scholar]
- 2.Kolff WJ. First clinical experience with the artificial kidney. Ann Intern Med. 1965;62:608–619. [DOI] [PubMed] [Google Scholar]
- 3.Alwall N, Bergsten BW, Gedda P, et al. On the artificial kidney; the technique in animal experiments. Acta Med Scand. 1949;132(4):392–411. [PubMed] [Google Scholar]
- 4.Merendino KA, Girvin GW, Thomas GI. The clinical use of Teflon tubes as a vascular substitute for major arteries. Surgery. 1958;43(6):959–968. [PubMed] [Google Scholar]
- 5.Quinton W, Dillard D, Scribner BH. Cannulation of blood vessels for prolonged hemodialysis. Trans Am Soc Artif Intern Organs. 1960;6:104–113. [PubMed] [Google Scholar]
- 6.Ramirez O, Swartz C, Onesti G, Mailloux L, Brest AN. The winged in line shunt. Trans Am Soc Artif Intern Organs. 1966;12:220–221. [PubMed] [Google Scholar]
- 7.Nolph KD. External shunts and internal fistulas. Ann Intern Med. 1971;74(6):1008–1009. [DOI] [PubMed] [Google Scholar]
- 8.Buselmeier TJ, Kjellstrand CM, Simmons RL, et al. A totally new subcutaneous prosthetic arterio-venous shunt. Trans Am Soc Artif Intern Organs. 1973;19:25–32. [DOI] [PubMed] [Google Scholar]
- 9.Shaldon S Hemodialysis by percutaneous catheterization of femoral artery and vein with regional heparinization. Lancet. 1961;2:857–859. [Google Scholar]
- 10.Cimino JE, Brescia MJ, Aboody R. Simple venipuncture for hemodialysis. N Engl J Med. 1962;267:608–609. [DOI] [PubMed] [Google Scholar]
- 11.Brescia MJ, Cimino JE, Appel K, Hurwich BJ. Chronic hemodialysis using venipuncture and a surgically created arteriovenous fistula. N Engl J Med. 1966;275(20):1089–1092. [DOI] [PubMed] [Google Scholar]
- 12.Lindfors O, Paldanius R. Experience with arteriovenous fistulas CIMINO in chronic hemodialysis. A report on forty-five patients. Scand J Urol Nephrol. 1976;10(1):80–83. [DOI] [PubMed] [Google Scholar]
- 13.Paruk S, Koenig M, Levitt S, Hardy MA. Arteriovenous fistulas for hemodialysis in 100 consecutive patients. Am J Surg. 1976;131(5):552–555. [DOI] [PubMed] [Google Scholar]
- 14.Kinnaert P, Vereerstraeten P, Toussaint C, Van Geertruyden J. Nine years’ experience with internal arteriovenous fistulas for haemodialysis: a study of some factors influencing the results. Br J Surg. 1977;64(4):242–246. [DOI] [PubMed] [Google Scholar]
- 15.Cole JJ, Dennis MB Jr, Hickman RO, Coglon T, Jensen WM, Scribner BH. Preliminary studies with the fistula catheter—a new vascular access prosthesis. Trans Am Soc Artif Intern Organs. 1972;18:448–451, 456. [DOI] [PubMed] [Google Scholar]
- 16.May J, Tiller D, Johnson J, Stewart J, Sheil AG. Saphenous-vein arteriovenous fistula in regular dialysis treatment. N Engl J Med. 1969;280(14):770. [DOI] [PubMed] [Google Scholar]
- 17.Girardet RE, Hackett RE, Goodwin NJ, Friedman EA. Thirteen months experience with the saphenous vein graft arteriovenous fistula for maintenance hemodialysis. Trans Am Soc Artif Intern Organs. 1970;16:285–291. [PubMed] [Google Scholar]
- 18.Dardik H, Ibrahim IM,Dardik I. Arteriovenous fistulas constructed with modified human umbilical cord vein graft. Arch Surg. 1976;111(1):60–62. [DOI] [PubMed] [Google Scholar]
- 19.Vegeto A, Berardinelli L, Malan E. Use of homologous vein grafts for chronic hemodialysis. J Cardiovasc Surg (Torino). 1977;18(5):501–504. [PubMed] [Google Scholar]
- 20.Brems J, Castaneda M, Garvin PJ. A five-year experience with the bovine heterograft for vascular access. Arch Surg. 1986;121(8):941–944. [DOI] [PubMed] [Google Scholar]
- 21.Sauvage LR, Berger K, Beilin LB, Smith JC, Wood SJ, Mansfield PB. Presence of endothelium in an axillary-femoral graft of knitted Dacron with an external velour surface. Ann Surg. 1975;182(6):749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elliott MP, Gazzaniga AB, Thomas JM, Haiduc NJ, Rosen SM. Use of expanded polytetrafluoroethylene grafts for vascular access in hemodialysis: laboratory and clinical evaluation. Am Surg. 1977;43(7):455–459. [PubMed] [Google Scholar]
- 23.Haimov M Clinical experience with the expanded polytetrafluoroethylene vascular prosthesis. Angiology. 1978;29(1):1–6. [DOI] [PubMed] [Google Scholar]
- 24.Hickman RO, Buckner CD, Clift RA, Sanders JE, Stewart P, Thomas ED. A modified right atrial catheter for access to the venous system in marrow transplant recipients. Surg Gynecol Obstet. 1979;148(6):871–875. [PubMed] [Google Scholar]
- 25.Kjellstrand CM, Merino GE, Mauer SM, Casali R, Buselmeier TJ. Complications of percutaneous femoral vein catheterizations for hemodialysis. Clin Nephrol. 1975;4(1):37–40. [PubMed] [Google Scholar]
- 26.Heimbach DM, Ivey TD. Technique for placement of a permanent home hyperalimentation catheter. Surg Gynecol Obstet. 1976;143:634–636. [PubMed] [Google Scholar]
- 27.McGonigle DJ, Schrock LG, Hickman RO. Experience using central venous access for long-term hemodialysis. A new concept. Am J Surg. 1983;145(5):571–573. [DOI] [PubMed] [Google Scholar]
- 28.Schwab SJ, Quarles LD, Middleton JP, Cohan RH, Saeed M, Dennis VW. Hemodialysis-associated subclavian vein stenosis. Kidney Int. 1988;33(6):1156–1159. [DOI] [PubMed] [Google Scholar]
- 29.Ash SR. Advances in tunneled central venous catheters for dialysis: design and performance. Semin Dial. 2008;21(6):504–515. [DOI] [PubMed] [Google Scholar]
- 30.Niyyar VD, Chan MR. Interventional nephrology: catheter dysfunction—prevention and troubleshooting. Clin J Am Soc Nephrol. 2013;8(7):1234–1243. [DOI] [PubMed] [Google Scholar]
- 31.Katzman HE, McLafferty RB, Ross JR, Glickman MH, Peden EK, Lawson JH. Initial experience and outcome of a new hemodialysis access device for catheter-dependent patients. J Vasc Surg. 2009;50(3):600–607, 607.e601. [DOI] [PubMed] [Google Scholar]
- 32.Wallace JR, Chaer RA, Dillavou ED. Report on the hemodialysis reliable outflow (HeRO) experience in dialysis patients with central venous occlusions. J Vasc Surg. 2013;58(3):742–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis KL, Gurley JC, Davenport DL, Xenos ES. The use of HeRo catheter in catheter-dependent dialysis patients with superior vena cava occlusion. J Vasc Access. 2016;17(2):138142. [DOI] [PubMed] [Google Scholar]
- 34.Schild AF, Schuman ES, Noicely K, et al. Early cannulation prosthetic graft (Flixene) for arteriovenous access. J Vasc Access. 2011;12(3):248–252. [DOI] [PubMed] [Google Scholar]
- 35.Aitken EL, Jackson AJ, Kingsmore DB. Early cannulation prosthetic graft (Acuseal) for arteriovenous access: a useful option to provide a personal vascular access solution. J Vasc Access. 2014;15(6):481–485. [DOI] [PubMed] [Google Scholar]
- 36.Al Shakarchi J, Houston G, Inston N. Early cannulation grafts for haemodialysis: a systematic review. J Vasc Access. 2015;16(6): 493–497. [DOI] [PubMed] [Google Scholar]
- 37.Radosa CG, Radosa JC, Weiss N, et al. Endovascular creation of an arteriovenous fistula (endoAVF) for hemodialysis access: first results. Cardiovasc Intervent Radiol. 2017;40(10):1545–1551. [DOI] [PubMed] [Google Scholar]
- 38.Lok CE, Rajan DK, Clement J, et al. Endovascular proximal forearm arteriovenous fistula for hemodialysis access: results of the prospective, multicenter novel endovascular access trial (NEAT). Am J Kidney Dis. 2017;70(4):486–497. [DOI] [PubMed] [Google Scholar]
- 39.Hull JE, Jennings WC, Cooper RI, Waheed U, Schaefer ME, Narayan R. The pivotal multicenter trial of ultrasound-guided percutaneous arteriovenous fistula creation for hemodialysis access. J Vasc Interv Radiol. 2018;29(2):149–158.e145. [DOI] [PubMed] [Google Scholar]
- 40.Mallios A, Jennings WC, Boura B, Costanzo A, Bourquelot P, Combes M. Early results of percutaneous arteriovenous fistula creation with the Ellipsys Vascular Access System. J Vasc Surg. 2018;68(4):1150–1156. [DOI] [PubMed] [Google Scholar]
- 41.Hebibi H, Achiche J, Franco G, Rottembourg J. Clinical hemodialysis experience with percutaneous arteriovenous fistulas created using the Ellipsys® vascular access system. Hemodial Int; 2019;23:167–172. International Symposium on Home Hemodialysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwab SJ, Beathard G. The hemodialysis catheter conundrum: hate living with them, but can’t live without them. Kidney Int. 1999;56(1):1–17. [DOI] [PubMed] [Google Scholar]
- 43.USRDS. Annal Data Report. Dialysis Morbidity and Mortality. Chapter IV. 1996. https://www.usrds.org/download/1996/ch04.pdf1996:45-66. Accessed February 26, 2019.
- 44.USRDS. Annual Data Report, Dialysis Morbidity and Mortality. Chapter IV. 1997. https://www.usrds.org/download/1997/ch04.pdf. Accessed February 26, 2019.
- 45.USRDS. Annual Data Report. End-stage Renal Disease (ESRD) in the United States. Volume 2. Chapter 3. Patient Characteristics. 2008. https://www.usrds.org/2008/pdf/V203_2008.pdf. Accessed February 26, 2019.
- 46.USRDS. Annual Data Report. End-stage Renal Disease (ESRD) in the United States. Chapter 3. Vascular Access. 2018. https://www.usrds.org/2018/view/v2_03.aspx. Accessed February 26, 2019.
- 47.Schwab S, Besarab A, Beathard G, et al. National kidney foundation DOQI clinical practice guidelines for hemodialysis vascular access Working group. Am J Kidney Dis. 1997;30(Suppl 3):S154–S196. [Google Scholar]
- 48.Bradbury BD, Chen F, Furniss A, et al. Conversion of vascular access type among incident hemodialysis patients: description and association with mortality. Am J Kidney Dis. 2009;53(5):804–814. [DOI] [PubMed] [Google Scholar]
- 49.Murea M, Brown WM, Divers J, et al. Vascular access placement order and outcomes in hemodialysis patients: a longitudinal study. Am J Nephrol. 2017;46(4):268–275. [DOI] [PubMed] [Google Scholar]
- 50.Kamar F, Quinn RR, Oliver MJ, et al. Outcomes of the first and second hemodialysis fistula: a cohort study. Am J Kidney Dis. 2019;73(1):62–71. [DOI] [PubMed] [Google Scholar]
- 51.Himmelfarb J, Berns A, Szczech L, Wesson D. Cost, quality, and value: the changing political economy of dialysis care. J Am Soc Nephrol. 2007;18(7):2021–2027. [DOI] [PubMed] [Google Scholar]
- 52.Fishbane S, Hazzan A. Meeting the 2012 QIP (Quality Incentive Program) clinical measures: strategies for dialysis centers. Am J Kidney Dis. 2012;60(5 Suppl 1):S5–S13; quiz S14–17. [DOI] [PubMed] [Google Scholar]
- 53.Ethier J, Mendelssohn DC, Elder SJ, et al. Vascular access use and outcomes: an international perspective from the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant. 2008;23(10):3219–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alencar de Pinho N, Coscas R, Metzger M, et al. Vascular access conversion and patient outcome after hemodialysis initiation with a nonfunctional arteriovenous access: a prospective registry-based study. BMC Nephrol. 2017;18(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuo TH, Chaer RA, Dillavou ED, Leers SA, Makaroun MS. Patients started on hemodialysis with tunneled dialysis catheter have similar survival after arteriovenous fistula and arteriovenous graft creation. J Vasc Surg. 2015;1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown RS, Patibandla BK, Goldfarb-Rumyantzev AS. The survival benefit of “fistula first, catheter last” in hemodialysis is primarily due to patient factors. J Am Soc Nephrol. 2017;28(2):645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DeSilva RN, Patibandla BK, Vin Y, et al. Fistula first is not always the best strategy for the elderly. J Am Soc Nephrol. 2013;24(8):1297–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murea M, Geary RL, Edwards MS, et al. A randomized pilot study comparing graft-first to fistula-first strategies in older patients with incident end-stage kidney disease: clinical rationale and study design. Contemp Clin Trials Commun. 2019;14:100357. [DOI] [PMC free article] [PubMed] [Google Scholar]