Abstract
Cancer is a significant health concern worldwide and its clinical treatment presents many challenges. Consequently, much research effort has focused on the development of new anticancer drugs to combat this disease. One area of exploration, in particular, has been in the therapeutic application of RNA interference (RNAi). Although RNAi appears to be an attractive therapeutic tool for the treatment of cancer, one of the primary obstacles towards its pervasive use in the clinic has been cell/tissue type-specific cytosolic delivery of therapeutic small interfering RNA (siRNA) molecules. Consequently, varied drug delivery platforms have been developed and widely explored for siRNA delivery. Among these candidate drug delivery systems, peptides have shown great promise as siRNA carriers due to their varied physiochemical properties and functions, simple formulations, and flexibility in design. In this review, we will focus on distinguishing between the different classes of peptide carriers based on their functions, as well as summarize and discuss the various design strategies and advancements that have been made in circumventing the barriers to siRNA delivery for cancer treatment. Resolution of these challenges by peptide carriers will accelerate the translation of RNAi-based therapies to the clinic.
INTRODUCTION
In recent years, the discovery of new treatment avenues in the fight against cancer has expanded rapidly. Treatment professionals are increasingly adding to their therapeutic options and are no longer limited to more rudimental, broad-spectrum therapies of past decades. One of the more recent areas of exploration in cancer treatment has been in the therapeutic application of RNA interference (RNAi).
RNAi is a highly conserved post-transcriptional gene regulatory mechanism triggered by small, non-coding double-stranded RNA molecules that can specifically silence gene expression by inducing targeted mRNA degradation.1,2 The discovery in 2001 that the introduction of chemically synthesized small interfering RNAs (siRNAs) into cultured mammalian cells could efficiently induce sequence-specific inhibition of gene expression,3 made evident the therapeutic potential of harnessing RNAi as a means to specifically target and silence disease-causing genes. Indeed, one year later, the first in vivo demonstration of the therapeutic potential of RNAi was reported through the effective siRNA targeting of a sequence from hepatitis C virus in transgene-expressing mice.4 Since then, there have been many studies confirming and expanding on the therapeutic potential of RNAi, including various clinical trials, culminating with the recent US Food and Drug Administration’s approval of the world’s first siRNA drug, Alnylam Pharmaceuticals’ ONPATTRO® (Patisiran).5–7 Inclusive of these studies, are encouraging results obtained from preclinical animal cancer models and early phase clinical trials of human cancer patients that warrant the further development and advancement of RNAi-based anticancer therapies.5–7
Despite this progress, there are still many clinical challenges associated with RNAi therapy; including its use in cancer treatment. Perhaps the most important challenge to its clinical application is cell/tissue type-specific cytosolic delivery of therapeutic siRNAs.8 Naked siRNAs undergo rapid renal clearance, are subject to degradation by endogenous RNases, and can be recognized by the innate immune system.5,9–12 Moreover, due to their hydrophilicity, negative charge, and large molecular weight, siRNA molecules cannot readily cross the cell membrane.13,14 Furthermore, since, most highly charged macromolecules enter cells via endocytosis,8 siRNAs are subject to endosomal entrapment and rapid degradation during endosome-lysosome trafficking,8,14 thereby diminishing their ability to reach the site of RNAi action in the cytosol15 and consequently limiting their silencing effectiveness. Therefore, there is a need for optimized siRNA drug delivery platforms in order to facilitate nuclease resistance, cell/tissue-type specific targeting, cellular internalization, endosomal escape, and release of the siRNA drug from the delivery platform in order to maximize the silencing efficiency of the siRNA within the cytoplasmic compartment of the diseased cell.
Varied drug delivery platforms have been developed and widely investigated for siRNA delivery, including both viral and non-viral vectors.16 Regarding the non-viral vectors, these delivery platforms have encompassed such technologies as lipid-based vectors, organic/inorganic nanovectors, nanogels, and peptide carriers.16 Peptides, in particular, have received specific attention because they show great promise as siRNA carriers, based on the diversity of their physiochemical properties and functions. The unique amino acid sequences within each specific peptide can confer different electrostatic charges, 3D structures, and physiochemical characteristics that translate into the peptides exhibiting various biological functions including, siRNA complexation, cell/tissue-type targeting, cell permeability, and endosome disruption.
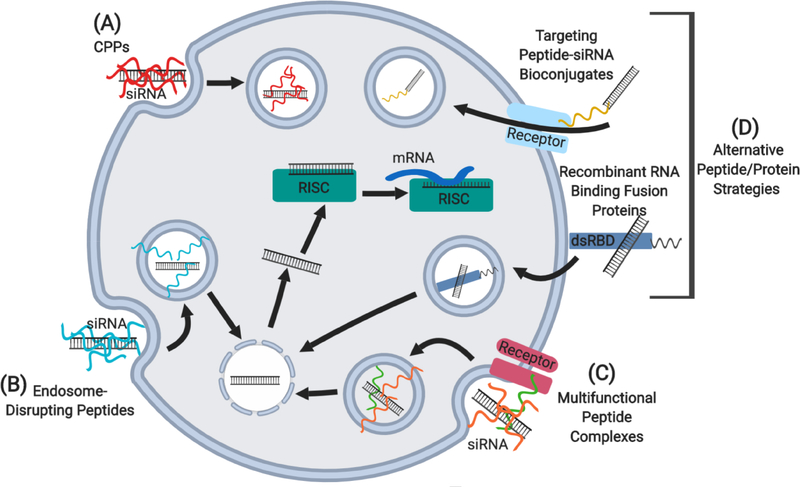
Based on function, several classes of peptides have shown potential to overcome siRNA efficacy-limiting barriers, including cell-penetrating peptides, endosome-disrupting peptides, non-covalent multifunctional peptide complexes, and several alternative peptide/protein strategies, such as peptide-siRNA bioconjugates and recombinant RNA binding fusion proteins (see Figure 1). In this context, the aim of this review will be to focus on the versatility of peptide carrier functions and the specific design strategies they employ in overcoming the barriers to siRNA delivery for cancer treatment. Of note, this review will focus on the peptides themselves, as the siRNA drug carriers and not as adjuvants of more complex siRNA drug-delivery platforms.
Fig 1.
Schematic representation of peptide-mediated siRNA delivery strategies into cells. (A) Cell-penetrating peptides (CPPs), including cationic, amphipathic, and targeting/homing peptides. (B) Endosome-disrupting peptides, including fusogenic, pH-sensitive amphipathic, and proton buffering peptides. (C) Multifunctional peptide complexes. (D) Alternative peptide/protein strategies, including targeting/homing peptide-siRNA bioconjugates and recombinant RNA binding fusion proteins. dsRBD, double-stranded RNA binding domain; RISC, RNA-induced silencing complex.
CELL-PENETRATING PEPTIDES
Cell-penetrating peptides (CPPs), also known as protein transduction domains, represent a diverse class of short peptides (typically 4–40 amino acid residues in length17), that can penetrate the plasma membrane of a cell and facilitate the delivery of various cargoes.18 Greater than 100 CPPs have been identified with varied amino acid sequences, lengths, physiochemical features, origins, mechanisms of cellular uptake, and function.8 Based on these parameters, several methods have been proposed to classify CPPs, however, no set criteria currently exists for CPP classification.19 Regardless, in terms of physiochemical features, CPPs are often categorized into cationic, amphipathic, or hydrophobic subgroups.16,20
Because CPPs represent promising delivery vehicles for a vast range of biologically functional cargoes with therapeutic potential, including siRNAs, the study of how they enter cells and access the cytoplasm are areas of active investigation. In fact, multiple factors appear to dictate the cellular internalization routes of CPPs with the route of choice being dependent on the physiochemical properties of the peptide, temperature, the extracellular concentration of the peptide and its cargo, the size of the cargo, and the properties of the plasma membrane (e.g. lipid composition and protein content).21–23 Currently, the three main CPP uptake modes into cells, include endocytosis (an energy-dependent process), direct crossing of the plasma membrane via transient pore formation, referred to as direct translocation (an energy-independent process), and internalization through the formation of a temporary membrane structure (i.e. inverted micelles).19 More recently, however, the transient pore formation concept associated with direct translocation has been challenged with evidence that CPPs enter cells instead by inducing membrane multilamellarity and fusion.24 Nevertheless, the main route of CPP cell entry (with/without cargo) appears to involve endocytic pathways, including macropinocytosis and clathrin/caveolae-mediated endocytosis.25
CPPs can deliver biologically active nucleic acids via either covalent linkages or non-covalent complex formations, with the latter approach possible due to CPPs typically having a high positively-charged residue density that enables the peptides to interact with the negatively-charged backbone of nucleic acids.18,26 Regarding siRNAs, both CPP carrying strategies have been employed in delivering siRNAs into cells/tissues,27 however, the non-covalent approach appears to be more suitable for siRNA delivery, as it exhibits several advantages over covalent conjugation strategies.28,29 For instance, complexation avoids the steric hindrance problem of siRNA loading into the RNA-induced silencing complex (RISC), as terminal modifications of siRNAs with bulky chemical groups, such as peptides, can impair RNAi activity.8,30 An exception to this problem is the use of cleavable linkers (e.g. disulfide bonds),8,31 but these can still produce unwanted side effects, such as the premature reduction of the disulfide bonds and release of the siRNAs from the CPPs prior to reaching the target cell.32 Moreover, the formulation of non-covalent complexes are simple and no chemical modifications of the siRNAs are needed to protect them from nuclease degradation within blood, as CPPs and siRNAs are known to form highly stable complexes.17,26
Cationic CPPs, which contain one or more stretches of highly dense positive charged regions typically derived from basic amino acids (i.e. arginine and/or lysine) are highly hydrophilic, exhibit high cell membrane permeability, can interact with siRNAs via electrostatic interactions, and promote efficient siRNA condensation.8,19,32 The first-ever CPP discovered that exhibited cell-penetrating ability was the TAT peptide (RKKRRQRRR) from the HIV-1 TAT protein in 1989.33 Subsequent research into cationic CPPs, in particular oligoarginine peptides, demonstrated that arginine residues confer more efficient CPP internalization than lysine residues, due to arginine containing a guanidine group that displays a stronger affinity for negatively-charged phospholipids within the plasma membrane of cells.8,12,19,34 Furthermore, membrane permeability was shown to be dependent on oligoarginine peptide length, with peptides shorter than six arginine residues unable to translocate across cell membranes.19,35 Conversely, increasing the number of arginine residues, thereby lengthening the oligoarginine peptides, was found to enhance translocation.19,34,35
In the context of RNAi-mediated cancer therapeutics, cationic CPPs, such as pentadecaarginine peptide (R15), have been demonstrated to mediate the delivery of siRNAs into cells both in vitro and in vivo36 (see Table 1). More specifically, the in vitro studies showed that complexation of R15 with siRNAs designed to target luciferase exhibited a 50% reduction of luciferase-specific expression 24 hours post-transfection of COS-7 cells stably expressing luciferase.36 More importantly, in vivo therapeutic analyses demonstrated that intratumoral administration of R15 in complex with siRNAs designed to target the HER-2 oncogene (siHER-2) every three days (at a 4 μg siRNA dosage) for a period of 17 days inhibited tumor growth in nude mice bearing human ovarian cancer (SKOV-3) xenografts by approximately 80%, with no reported toxicities, compared to control treatment groups.36 Moreover, significant HER-2 protein silencing was observed in the harvested tumor tissues treated with the R15+siHER-2 complex, albeit no quantitative densitometry was performed.36 Taken together, these results demonstrated the potential for an oligoarginine peptide-mediated siRNA delivery system for therapeutic application against cancer.
Table 1.
Peptide-mediated siRNA delivery strategies for cancer therapy.
| Category | Subgroup | Peptide name | Peptide sequencea | siRNA gene target | Cancer type(s) | Model | Administration route | % Silencing / siRNA Dosage | Refsb |
|---|---|---|---|---|---|---|---|---|---|
| CPPs | Cationic peptides | R15 | RRRRRRRRRRRRRRR | HER-2 | Ovarian cancer | Cells; Xenograft tumor | Intratumoral | ~85%(P; NQ) / 4 μg | 36 |
| Amphipathic peptides | MPG-8 | βAFLGWLGAWGTMGWSPKKKRK-cysteamide / (+Cholesterol) | Cyclin B1 | Prostate & lung cancer | Cells; Xenograft tumor | Intratumoral | 58%(mRNA) / 1 μg 82%(mRNA) / 5 μg |
40 | |
| Intravenous | 60%(P) / 5 μg 80%(P) / 10 μg |
||||||||
| RICK | kwllrwlsrllrwlarwlg (retro-inverso of CADY-K) | Luciferase; Cyclin B1 | Brain cancer | Cells | N/A | N/A | 41 | ||
| Targeting/homing proteins/peptides | F5-P | anti-HER-2 ScFv Ab - RSQSRSRYYRQRQRSRRRRRRS (truncated form of protamine) - H6 (tag) | PLK1 | Breast cancer | Cells; Xenograft tumor | Intravenous | ~75%(mRNA) / 40 μg | 46 | |
| TP-LyP-1 | myristate - GWTLNSAGYLLGKINLKALAALAKKIL - GGGG - cyclic(CGNKRTRGC) | ID4 | Ovarian cancer | Cells; Xenograft tumor | Intravenous | 80%(mRNA) / 20 μg | 50 | ||
| Intraperitoneal | 90%(mRNA) / 20 μg | ||||||||
| TAT-A1 | WFLLTM - RKKRRQRRR | GAPDH | Liver cancer | Cells | N/A | N/A | 52 | ||
| Endosome-disrupting peptides | Fusogenic peptides | 599 | GLFEAIEGFIENGWEGMIDGWYGGGGrrrrrrrrrK-biotin | CIP2A | Oral cancer | Cells; Xenograft tumor | Intratumoral | ~40%(mRNA); ~83%(P) / 5 μg and 2.5 μg | 56,57 |
| pH-sensitive amphipathic peptides | KALA | WEAKLAKALAKALAKHLAKALAKALKACEA | VEGF | Prostate cancer | Cells | N/A | N/A | 60 | |
| Endoporter | LHKLLHHLLHHLHKLLHHLHHLLHKL | Ssb | Cervical cancer | Cells | N/A | N/A | 58 | ||
| Proton buffering peptides | H3K4b | branched KHHHKHHHKHHHKHHHK | Raf-1 | Skin cancer (melanoma) | Xenograft tumor | Intratumoral | ~50%(P; NQ) / 4 μg | 61 | |
| H3K(+H)4b | branched KHHHKHHHKHHHHKHHHK | Raf-1 | Skin cancer (melanoma) | Cells; Xenograft tumor | Intravenous | ~50%(P; NQ) / 50 μg | 62 | ||
| Multifunctional peptide complexes | Synthetic peptides | I4R-9r(A1*):sHGP-9r | CRKRLDRNC-GG-rrarrrrrr : RGWEVLKYWWNLLQY-GG-rrrrrrrrr | GADPH; Luciferase | Colon cancer | Cells; Xenograft tumor | Intratumoral | ~58%(P) / 40 μg | 63 |
| GE11R9:599 | YHWYGYTPQNVIGGGGrrrrrrrrrK-biotin : GLFEAIEGFIENGWEGMIDGWYGGGGrrrrrrrrrK-biotin | CIP2A | Oral cancer | Cells; Xenograft tumor | Intravenous | ~50%(mRNA) / 10 μg | 64 | ||
| myr-R9-LyP-1:E9 | myristate - rrrrrrrrr - GGGGK - cyclic (CGNKRTRGC) : GLFEAIEGFIENGWEGMIDGWYGGGGEEEEEEEEE | Stat3; c-Myc | Breast cancer | Cells | N/A | N/A | 70 | ||
| PF14:TG1 | Stearyl-AGYLLGKLLOOLAAAALOOLL-NH2 : EEEEEEXTFFYGGSRGKRNNFKTEEY-NH2 | Luciferase | Brain cancer | Cells | N/A | N/A | 17 | ||
| Alternative Peptide/Protein Strategies | Targeting/Homing peptide-siRNA bioconjugates | cRGD (monovalent) | cyclic(RGDfk) - linker - siRNA | EGFR | Brain cancer | Cells; Xenograft tumor | Intravenous | ~50%(mRNA; P) / ~70 μg | 75 |
| biRGD (bivalent) | [cyclic(RGDfk)-Ahx]2 - E - PEG-MAL - linker - siRNA | PIK3CB | Brain cancer | Cells; Xenograft tumor | Intravenous | ~50%(mRNA; P) / ~15 μg | 76 | ||
| Recombinant RNA binding fusion proteins | TatU1A-Alexa | RKKRRQRRR - U1A (RNA binding domain) - Alexa Fluor 546 | EGFP; EGFR (shRNA) | Skin cancer (epidermoid) | Cells | N/A | N/A | 78 | |
| PTD-DRBD | [RKKRRQRRR]3 - [dsRBD of PKR protein] | GFP; GAPDH; Luciferase | Lung cancer | Cells; Transgenic ROSA26 | Intranasal | ~50%(P) / ~10 μg | 77 | ||
| dsRBD-His18 | [dsRBD of PKR protein] - H18 | GFP | Prostate cancer | Cells | N/A | N/A | 79 | ||
Protease-resistant D-amino acids are indicated by small letters.
Ahx, 6-Aminocaproic acid; CPPs, cell-penetrating peptides; dsRBD, double-stranded RNA binding domain; N/A, not applicable; NQ, not quantitated (an approximation); O, Ornithine; P, protein; PEG-MAL, 8-amino-3,6-dioxaoctanoic acid and β-maleimidopropionic acid; Refs, references; scFv Ab, single-chain fragment variable antibody; shRNA, short hairpin RNA; X, 6-aminohexanoic acid linker.
Based on physiochemical features, another subgroup of CPPs that have been extensively studied in their application as siRNA carriers are the amphipathic peptides. These CPPs are chimeric or fused peptides derived from different sources, which contain both hydrophilic (cationic or anionic in charge) and hydrophobic segments within their sequences, with amphipathicity determined by either the primary or secondary structure.8,19,37 In terms of siRNA drug delivery, the hydrophilic region binds and condenses the siRNA cargo and the hydrophobic region helps stabilize the complex through intermolecular hydrophobic interactions.8 Additionally, the amphipathic property of these CPPs allows for cell membrane penetration by first mediating an interaction with the polar head groups of phospholipids and second facilitating membrane fusion and crossing of the complex through the hydrophobic lipid bilayer into the cytosol.8,37
MPG-8, a derivative and improved variant of the peptide carrier MPG,38 is an example of an amphipathic peptide, which contains a hydrophobic region derived from HIV glycoprotein 41 fused to a hydrophilic (cationic) nuclear localization signal of SV40 large T antigen, that has been demonstrated to hold promise in the therapeutic administration of siRNAs. More specifically, MPG-8 complexation with siRNAs designed to target cyclin B1 (sicycB1), a key regulator of cell cycle entry into and progression through mitosis,39 was reported to reduce cyclin B1 protein and mRNA levels by ~80–90% in cultured cells, with a consequent induction of G2-cell cycle arrest and impairment of cancer cell proliferation.40 Upon intratumoral injection of the MPG-8+sicycB1 complex every three days in vivo, a 75% reduction in tumor growth was observed and tumor growth was completely prevented, using 1 μg and 5 μg of siRNA, respectively, approximately fifty days post-treatment of human prostate carcinoma cell (PC3)-xenografted mice.40 Interestingly, functionalization of the MPG-8 carrier with cholesterol resulted in higher siRNA serum stability and better siRNA tissue biodistribution in vivo.40 Subsequent systemic administration of human prostate carcinoma cell (PC3)-xenografted mice with the cholesterol-functionalized MPG-8+sicycB1 complex every three days, further demonstrated a 60% and 92% inhibition in tumor growth with 5 μg and 10 μg of siRNAs, respectively, fifty days post-treatment.40 The reduction of tumor sizes were directly correlated to knockdown of cyclin B1 protein levels by 60% and 80% in animals treated with 5 μg and 10 μg of siRNA, respectively.40 Moreover, similar results were observed in human lung cancer (SCK3-HER-2) xenografted mice, where a 70% reduction in tumor growth was achieved after 20 days treatment with 10 μg of sicycB1 in complex with the cholesterol-functionalized MPG-8 carrier.40 Further analysis of these mice, also showed a higher degree of survivability compared to control treatments.40 Taken together, these findings inferred that the MPG-8 peptide-based carrier system constituted a promising technology for systemic administration of siRNAs in a cancer therapeutic context.
A potential limitation in the development of CPP carriers for in vivo applications is their likely degradation by extra/intra-cellular proteases. Consequently, peptide design strategies have been developed in an attempt to confer protease resistance to these carriers. One recent example of an amphipathic CPP that was modified to be proteolytically resistant and further demonstrated to successfully deliver siRNAs to cancer cells in vitro was the RICK peptide.41 RICK was designed as a retro-inverso form of the CADY-K peptide,41 which is a shorter version of the amphipathic peptide CADY42 that comprises hydrophobic tryptophan and hydrophilic arginine residues and that was previously shown to display a two-fold higher luciferase knockdown efficiency than CADY.43 Of note, the term “retro-inverso” refers to peptides consisting of proteolytically-resistant D-amino acids in the reverse sequence of the parent peptide that comprises naturally occurring L-isoforms. More specifically, in studies involving a human glioblastoma cell line (U87MG), RICK peptide was found to exhibit similar efficacies in siRNA cellular delivery and gene silencing (~75% knockdown of overexpressed luciferase and ~80% of endogenous cyclin B1), compared to its parent homologue, CADY-K, but with the advantage of improved resistance to proteolytic conditions (i.e. pre-incubation with trypsin or serum).41 These findings were significant because the proteolytic stability offered by RICK could potentially prevent unwanted siRNA degradation in vivo and thereby prolong the longevity of the complex in blood circulation, which would of course be therapeutically beneficial.
Although the previous two subgroups of CPPs, described above, were very effective at delivering siRNAs into potentially any cell/tissue type, their main disadvantage was that they could not selectively deliver the siRNA cargo into cells/tissues, which could be detrimental in terms of therapeutics, especially if the CPP+siRNA complex cannot distinguish between normal and diseased cells/tissues, as this could potentially lead to unwanted cellular toxicities. Moreover, the CPP+siRNA complex was thus highly reliant on the gene targeting specificity of the siRNA cargo, which could also be problematic, due to siRNA off-targeting effects.44 Consequently, varied strategies have focused on developing targeting/homing CPPs through the addition of targeting moieties and/or sequences.
In one particular study, a recombinant fusion protein consisting of an anti-HER-2 single-chain fragmented variable antibody linked to a truncated peptide form of cationic protamine (F5-P) was found to selectively deliver siRNAs designed to target PLK1 (siPLK1), a kinase that promotes cell division,45 and inhibit the growth of HER-2(+) breast cancer cells and primary human tumors in an orthotopic breast cancer mouse model.46 In particular, treatment of cells with the F5-P+siPLK1 complex demonstrated selective targeting to HER-2 (+) breast cancer cell lines and primary human cancer cells in vitro, with a ~70% silencing effect on PLK1 mRNA expression and a consequent reduction in proliferation.46 Moreover, treatment of these cells with the complex induced apoptosis, but did not trigger an interferon response.46 Subsequent studies using a mouse model of breast cancer found that intravenous injection of the 5F-P+siPLK1 complex concentrated in orthotopic HER- 2 (+) breast cancer tumor xenografts and persisted for at least 72 hours, whereas no targeting of the complex was observed for HER-2 (−) tumors.46 More importantly, in assessing the therapeutic potential of the complex, treatment of HER-2 (+) breast cancer tumors via intravenous administration of the F5-P+siPLK1 complex (twice weekly for four consecutive weeks using 40 μg of siPLK1 per injection) showed a significant ~75% reduction in PLK1 mRNA expression with a consequent impairment of tumor growth (>80%) in HER-2 (+) breast cancer tumor xenografts, but not in HER-2 (−) tumors seven weeks post-treatment.46 Furthermore, administration of the F5-P+siPLK1 complex suppressed HER-2 (+) breast tumor metastasis and prolonged survival with no evidence of toxicities.46 Interestingly, because cancer is a multi-genic and multi-factor disease,47,48 a popular strategy to potentially combat it using RNAi therapeutics has been to use combinations of multi-target siRNAs within a single payload to confer a synergistic therapeutic effect.48 Consequently, to test the feasibility of such an approach, this study proceeded in further complexing F5-P with a siRNA cocktail targeting three genes that promote tumor proliferation at different cell cycle stages, including PLK1, and found that it had a greater anti-tumor effect than siPLK1 on its own.46 This result was similar to an earlier report published by Song et al., which likewise found that an anti-HIV-1 envelope Fab antibody fragment-protamine fusion protein non-covalently complexed to a different cocktail of siRNAs could target HIV-1 envelope-expressing tumors specifically and enhance the suppression of their growth when compared to complexes containing each of the siRNAs alone.49 The significance of these findings were that they demonstrated the versatility in the approaches that siRNAs could be used as cancer therapeutic agents in complexation with peptide/protein carriers. One limitation regarding the F5-P study, however, was that it did not evaluate whether repeated doses of the complex would induce an immune response that might interfere with its therapeutic effectiveness.46 Regardless, this study provided strong preclinical support for the therapeutic potential of a targetable antibody-CPP fusion protein in the delivery of siRNAs for the treatment of HER-2 (+) breast cancers.
Another approach in the design of targetable CPPs has involved the use of smaller chemically synthesized CPP conjugates that comprise a targeting/homing amino acid sequence. For example, in an effort to design a tumor-specific CPP siRNA carrier, the non-targeting peptide, transportan, was conjugated to the p32-targeting cyclic nonapeptide LyP-1 to form a cancer cell-selective CPP, named TP-Lyp-1.50 The targeting peptide LyP-1 was selected because it binds to p32, a mitochondrial protein whose cell surface expression is elevated in a wide range of tumor types.51 A hydrophobic myristate was also added to facilitate interactions with membrane lipids.50 Upon treatment of cultured cells in vitro, the TP-Lyp-1 peptide was found to enhance the uptake of siRNAs and mediate specific reporter gene silencing (~40%) compared to a control non-targeting peptide complex.50 Additionally, treatment of ovarian cancer cell lines with TP-Lyp-1 in complex with siRNAs designed to target the ID4 oncogene (siID4), induced ID4 protein silencing with a concomitant decrease in cell viability and an increased rate of apoptosis.50 More importantly, in subsequent in vivo experimentations, TP-Lyp-1 in complex with siRNAs increased the targeting capacity of siRNAs to tumors threefold compared to the control non-targeting peptide.50 Furthermore, upon repeated intravenous or intraperitoneal injections (every 3 days for 25 days) of TP-Lyp-1 in complex with 20 μg of siID4, an 80–90% decrease in ID4 mRNA levels with a consequent 82–87% suppression of tumor growth was observed 45 days post-initial treatment.50 Notably, intraperitoneal treatment of TP-Lyp-1 in complex with 100 μg of siID4 also resulted in an 80% survival rate, 80 days post-initial tumor generation compared to a 0–20% survival rate for the control treatments.50
Other examples of cancer cell/tissue type-targeting/homing CPP siRNA carriers include the TAT- A1 peptide that comprises the non-targeting cationic peptide TAT fused to the vascular endothelial growth factor receptor-1 targeting peptide A1.52 In in vitro cell uptake assays, the TAT-A1 peptide was found to enhance the intracellular delivery efficiency of siRNAs by ~30% compared to the parent peptide TAT and it could also induce gene silencing efficiencies comparable to a commercial transfection agent.52
ENDOSOME-DISRUPTING PEPTIDES
A common hurdle to CPP-mediated siRNA delivery is endosomal entrapment.14 Because most CPP mediated delivery strategies enter cells via endocytosis, most often the siRNA cargo remains entrapped, and unless it can escape into the cytosol it will be rendered biologically inactive and hence therapeutically ineffective. One problem stems from the fact that CPPs in general do not exhibit endosomolytic properties. Consequently, numerous peptide design strategies have been developed to confer endosome-disrupting properties to peptide carriers to enable them to mediate siRNA escape from endocytic vesicles into the cytosol.
One particular subgroup that has been developed to facilitate the intracellular delivery and endosomal escape of siRNAs are fusogenic peptides. Fusogenic peptides are generally derived from proteins with endosome-disruptive fusion peptide domain sequences, such as the HA2 subunit of the influenza virus hemagglutinin protein that mediates destabilization of the host cell endosomal membrane in an acidification-dependent manner upon entry of the virus into cells via receptor mediated endocytosis.53 The functional activities of these peptides are pH-dependent, where exposure to acidic environments, such as in endocytic and lysosomal vesicles, they adopt an amphipathic helix structure that causes them to fuse with and disrupt the membranes enabling the release of any associated cargo from these compartments.8,25,54,55
In our studies, we designed a chimeric peptide, termed 599, that combined an influenza virus-derived fusogenic peptide sequence, INF-7, that has a more potent membrane-destabilizing property than the parent HA2 peptide, with cationic cell-penetrating nona(D-arginine) residues, to mediate complexation, stability, cellular entry, and endosomal escape of siRNAs. In particular, we found that the 599 peptide when complexed to siRNAs designed to target the CIP2A oncoprotein (siCIP2A), it could promote significant ~60–85% silencing of CIP2A mRNA levels, with a concomitant ~40% decrease in oral cancer cell growth and cell invasiveness in vitro.56 Of greater relevance regarding siRNA-based therapeutics was that in our subsequent in vivo studies, the 599 peptide was found to protect siRNAs from degradation upon intratumoral injection up to a minimum of 72 hours post-treatment and significantly impaired tumor growth 8 days post initial treatment using an orthotopic oral cancer (CAL 27) mouse model.57 More specifically, intratumoral injection of three doses of 599 peptide complexed to siCIP2A every 72 hours, where, 5 μg of siRNA was used in the first two doses and 2.5 μg in the third dose, resulted in a significant decrease in both weight (~50% decrease) and volume (~60% decrease) of the excised tumors compared to control treatments.57 Moreover, analyses of the tumor tissues exhibiting tumor growth inhibition confirmed significant silencing of CIP2A at both the mRNA (~40% reduction) and protein (~83% reduction) levels with no apparent toxicities associated with the treatment or induction of the interferon response.57 Together, these data suggested that the 599 peptide carrier, comprising fusogenic and CPP sequences, was a clinically effective mediator of RNAi-based cancer therapeutics.
Another subgroup of endosome-disruptive peptides that have been studied as carriers of siRNAs, include the KALA and Endoporter peptides, which can be categorized as pH-sensitive amphipathic peptides. These peptides are synthetic and designed to function similarly to fusogenic peptides, in that they can adopt amphipathic helical structures under acidic conditions triggering endosomal membrane disruption.58,59 In a study using KALA, poly[ethylene glycol] (PEG)-conjugated siRNAs (linked via a disulfide bridge) designed to target the vascular endothelial growth factor (siVEGF) were complexed with KALA to form polyelectrolyte complex micelles.60 Upon treatment of a human prostate carcinoma cell line (PC-3) with the KALA+(PEG)-siVEGF complex, ~80% VEGF silencing was observed compared to control treatments in vitro.60 Similarly, Endoporter, which is a histidine-rich amphipathic peptide, whose amphipathic helix formation is dependent on the histidine response to pH changes, when complexed to siRNAs was also found to enhance the cytosolic delivery of its siRNA cargo through endsosome escape in vitro.58 More specifically, Endoporter in complex with siRNAs designed to target Sjögren’s syndrome antigen B (Ssb) was found to mediate cell entry and undergo α-helical structure formation within the acidic environment of endosomes, thereby promoting endosomal escape of the siRNA cargo into the cytosol with a consequent ~75% knockdown of Ssb mRNA levels in HeLa cells in vitro.58
Proton buffering peptides, such as branched histidine-lysine peptide polymers, are another type of endosome-disrupting peptide subgroup that have been utilized as carriers of siRNAs, but that function to disrupt endosomes via the proton sponge effect.8 The histidine residue, because of its excellent buffering capacity at pH 6 is very effective at absorbing protons and therefore when incorporated into histidine-rich polypeptides, upon their subsequent accumulation within acidic endosomal vesicles can contribute to an increase in osmotic pressure across their membranes causing the membranes to swell and/or rupture, thereby releasing any associated cargo into the cytosol.8,25 The branched histidine-lysine peptide polymer, H3K4b, is an example of a proton buffering peptide that was shown to be an effective carrier of siRNAs designed to target Raf-1 (siRaf-1), a key regulator of tumor growth and angiogenesis, by its ability to reduce tumor growth of melanoma tumor (MDA-MB-435) xenografts.61 In particular, three intratumoral injections of H3K4b in complex with 4 μg of siRaf-1 were found to silence Raf-1 protein levels, reduce blood vessel density, and impair tumor growth by 50-60% compared to control treatments 15 days post-treatment.61 Furthermore, multiple intravenous doses of an analog of H3K4b, termed H3K(+H)4b, in complex with 50 μg of siRaf-1 was found to reduce xenograft melanoma tumor sizes by 50% in comparison to control treatments 30 days post initial injection.62 Thus, taken together, these data provide good evidence that proton buffering peptides also appear to be capable and effective siRNA carriers in the treatment of cancer.
NON-COVALENT MULTIFUNCTIONAL PEPTIDE COMPLEXES
Another emerging strategy in overcoming the varied siRNA delivery barriers in the treatment of cancer is to combine multiple classes of peptides within a single nanoparticle complex, so as to multi- functionalize the complex and facilitate its ability to non-covalently encapsulate siRNAs, target specific cells/tissues, induce endosomal escape, and release siRNAs from the complex to exert their biological activity. One of the first examples in the utilization of this type of strategy was published in a report by Jun et al., where they demonstrated that a multicomponent peptide-woven nanocomplex they designed, which comprised an interleukin-4 receptor-targeting peptide (I4R) and an endosomolytic CPP (sHGP), both with polyarginine cores, could mediate gene silencing in vitro and in vivo.63 Although, this study shed some insight into the importance of the 3D structure of the multi-peptide complex in releasing the siRNA cargo, unfortunately, the in vivo tumor-targeting capability of the multicomponent peptide-woven nanocomplex was never evaluated. In fact, the route of administration tested was intratumoral and the in vivo gene silencing effect was not reported to be statistically significant compared to the control treatments, thus, making it difficult to interpret the effectiveness of this multicomponent peptide-woven nanocomplex design in mediating siRNA delivery.
Regarding our own recent studies, although the endosome-disruptive 599 peptide appeared to be an effective delivery vehicle,56,57 its limitation was that it was not cell/tissue-specific and because systemic delivery remains the standard method of administration of drugs for the treatment of solid tumors, targeted delivery was a necessity. Consequently, we developed a dual peptide-mediated carrier approach to enable targeted delivery of siRNAs to cancer cells via systemic delivery.64 More specifically, because the epidermal growth factor receptor (EGFR) is frequently overexpressed in oral cancer cells,65 we designed a second peptide, termed GE11R9, that comprised a non-mitogenic EGFR-targeting peptide sequence,66 which when co-complexed with the 599 peptide synergistically mediated the effective targeting and delivery of siCIP2As into EGFR-overexpressing oral cancer cells (CAL 27) and the induction of CIP2A mRNA (~60%) and protein silencing in vitro.64 Of note, siRNA complexation with GE11R9 peptide alone did show preferred siRNA accumulation in EGFR overexpressing oral cancer cells; however, GE11R9 was unable to deliver bioactive siRNAs, evidenced by the lack of gene silencing and the apparent endosomal entrapment of siRNAs, hence the need for the 599 peptide.64 Further examination upon systemic administration of the dual peptide carrier in complex with 10 μg of siCIP2A to mice bearing orthotopic oral cancer (CAL 27) tumors, demonstrated that it could increase targeted delivery of siRNAs to tumor tissues and significantly enhance CIP2A mRNA silencing (~50%) unlike complexes of siCIP2As with 599 peptide alone.64 Despite the promising ability of the dual peptide-mediated carrier technology to enhance the delivery of bioactive siRNAs to the targeted tumor tissues, one drawback we observed was the rapid clearance of the complex from systemic circulation,64 thus potentially limiting the therapeutic effectiveness of the administered siRNA cargo. The most likely cause for this rapid clearance was the cationic property of the dual peptide+siRNA complex,64 as it is well documented that cationic nanoparticles attract negatively-charged serum proteins, which lead to aggregation and rapid plasma clearance.67,68 Hence, one future approach to enhance the systemic circulation of the dual peptide+siRNA carrier could be to develop an electrostatic shielding component, through the use of ionic counterparts and/or hydrophilic uncharged polymers.69
In other studies, similar multi-functional approaches have been recently developed for therapeutic siRNA delivery in the treatment of both breast cancer and glioblastomas.17,70 More specifically, in a study by Bjorge et al., the researchers designed a multi-functional complex, comprising two peptides, a myristoylated p32-targeting LyP-1 peptide sequence with a polyarginine core (myr-R9-LyP-1) and an endosome-disruptive INF-7 fusogenic sequence with a polyglutamate core (E9) for delivery of siRNAs to breast cancer cells in vitro.70 The addition of myristate at the amino-terminus of myr-R9- LyP-1 was to facilitate the formation of small particles and assist with the delivery of the complex across cell membranes, as previously described, whereas the positively-charged arginine-rich segment was incorporated to allow binding via electrostatic interactions with negatively charged siRNAs and the plasma membrane.70 Moreover, the endosomolytic peptide E9 was synthesized to contain nine negatively-charged glutamate residues to facilitate electrostatic interactions with the cationic myr-R9-LyP-1 peptide in complex with siRNAs.70 Upon complexation of the two peptides with siRNAs designed to target the Stat3 or c-Myc oncogenes,71,72 they observed between 50–70% silencing of these proteins in cultured breast cancer cells (MDA-MB-231), with a consequent ~74% reduction in anchorage independent growth upon co-mixing of the two siRNAs in complex with the multi-functional peptide carrier.70 Although enhancement of gene silencing was observed upon co-complexation of the endosome-disruptive E9 peptide with the myr-R9-LyP-1 peptide, no evidence was provided for the targeting specificity of the multi-functional peptide carrier to breast cancer cells. Nonetheless, in the study on the development of therapeutic siRNA delivery in the treatment of glioblastomas, Srimanee et al. similarly tested a multi-functional peptide complex approach in the targeting of siRNAs to improve glioblastoma-targeted specificity and gene silencing efficiency.17 However, in their case, they did observe that co-complexation of the CPP, PepFect 14 (PF14; which is speculated to also have endosome-disruptive abilities17,73) with a LRP-1 receptor-targeting peptide containing a polyglutamate core (TG1) could more effectively transfect and promote gene silencing in glioblastoma cells (U87MG), which are known to overexpress the LRP-1 receptor on their cell surface74, compared to HeLa cells, which do not express this receptor.17 Moreover, the PF14 peptide in complex with TG1 could induce 70% downregulation of reporter protein expression at a low siRNA concentration and a two-fold higher gene silencing efficiency compared to complexes of siRNAs with PF14 alone.17 Interestingly, covalent conjugate peptide constructs of the PF14 and TG1 sequences in complex with siRNAs were reported to be ineffective at inducing gene silencing compared to their non-covalent peptide complex counterpart,17 thus, highlighting the latter design strategy as a more promising form of carrier for cancer-targeted siRNA delivery.
CONCLUSIONS, ALTERNATIVE STRATEGIES, AND FUTURE PERSPECTIVES
Peptides have been shown to be promising carriers for effective siRNA delivery; however, of the 20, recruiting, completed, or terminated clinical trials using siRNAs in the treatment of cancer (www.ClinicalTrials.gov), not one has made use of peptides as a delivery mechanism, with most, including the recently approved ONPATTRO®, employing lipid nanoparticles. Despite the relatively large level of research detailing the potential benefits of peptide-mediated siRNA delivery, there has yet to be any advancement in clinical data from human cancer patients. It appears most of the field is focused on optimizing peptide carrier designs for cellular uptake and establishing proofs of principle through the use of reporter genes, as opposed to actual targeting of oncogenes and exploring the consequent gene silencing effects on tumor growth in animal cancer models. Therefore, there needs to be a greater push for more cancer-relevant preclinical studies, as peptide carriers show great promise due to their diverse functionalities and flexibility in design.
Although this review primarily focused on non-covalent formulations, covalent peptide-siRNA bioconjugate designs, incorporating targetable peptides, represent an alternative design strategy in combating cancer. While covalent formulations come with their own hurdles, there exists the potential benefit of being able to produce and use clearly defined and reproducible molecules for cancer therapeutics. For example, He et al. developed a promising anti-tumor conjugate comprising a chemically-stabilized EGFR siRNA (siEGFR) and a cyclic arginine-glycine-aspartate (cRGD) peptide, which selectively binds to αvβ3 integrins, for the treatment of glioblastomas.75 In particular, intravenous administration of the cRGD-siEGFR bioconjugate to glioblastoma (U87MG) tumor-bearing nude mice led to significant 50% inhibition of tumor growth and ~50% reduction of EGFR expression in tumor tissues.75 Furthermore, in a subsequent study, using a peptide-siRNA bioconjugate comprising a bivalent cRGD (biRGD) peptide, which was found to enhance uptake into glioblastoma cells compared to the monomeric cRGD peptide, when conjugated to chemically-modified PIK3CB siRNA, it substantially slowed glioblastoma growth, while silencing PIK3CB expression by ~80% and ~50%, respectively.76 While both of the results from the studies showed promise in using a targeting peptide-siRNA bioconjugate approach for treatment of cancer, it routinely took either relatively high concentrations of these bioconjugates and/or the use of chemically modified siRNAs to achieve significant knockdown and impaired tumor growth.
Finally, another emerging strategy for the delivery of siRNAs employs the generation of recombinant RNA binding fusion proteins. In this approach, RNA binding domains (e.g. U1A or double-stranded RNA binding domains) from RNA binding proteins have been constructed as recombinant chimeric proteins that also comprise CPP residues, endosome-disruptive peptide sequences, and/or chemical moieties to facilitate binding, cell/tissue-type specific targeting, cell permeability, endosome escape, and bioavailability of the siRNA cargo.77–79 The concept behind this strategy was to limit the positive charges of the complexes, which is typically high for CPP complexes, so as to avoid non-specific interactions with negatively-charged serum proteins that can then lead to aggregation and rapid clearance of the siRNA cargo from blood.
Since their initial discovery 30 years ago, the research field on peptide carriers has greatly evolved and shows promise in overcoming the barriers to siRNA delivery for cancer treatment. Although there are still many challenges to its therapeutic application in humans and the need for more pre-clinical research involving animal cancer models, peptide-mediated siRNA delivery strategies present numerous advantages, to other delivery technologies, such as adaptability, simple formulations, and inexpensive drug synthesis/production. Moreover, the therapeutic effectiveness of these carriers upon intratumoral and systemic administration, clearly demonstrate their clinical potential in the treatment of a wide variety of cancer types, with the former administration route possibly being a viable option in the treatment of tumors that are more easily accessible, such as oral and skin cancers. By treating locally, one could shrink tumor sizes sufficiently to aid surgeons in their subsequent removal, while minimizing toxicity and immune activation. Nonetheless, with new insights into cellular uptake and endosome escape mechanisms of the peptide+siRNA complexes,21 their predicted structural features for complex assembly and siRNA release,63,80 and the factors influencing their structural formation and disassembly,29 together, these will lead to advancements in peptide carrier designs for siRNA delivery in the treatment of cancer and help accelerate their translation to the clinic.
ACKNOWLEDGMENTS
This work was supported by the National Institute of Dental and Craniofacial Research (NIDCR) grant R21DE027231 (A.J.). The authors have read the journal’s policy on disclosure of potential conflicts of interest and have none to declare. All authors have read the journal’s authorship statement, and have reviewed and approved the manuscript.
Abbreviations
- CPPs
cell-penetrating peptides
- dsRBD
double-stranded RNA binding domain
- EGFR
epidermal growth factor receptor
- I4R
interleukin-4 receptor
- PEG
poly[ethylene glycol]
- RISC
RNA-induced silencing complex
- RNAi
RNA interference
- siRNA
small interfering RNA
- Ssb
Sjögren’s syndrome antigen B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE and Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998; 391: 806–11. [DOI] [PubMed] [Google Scholar]
- 2.Rana TM. Illuminating the silence: understanding the structure and function of small RNAs. Nat Rev Mol Cell Biol 2007; 8: 23–36. [DOI] [PubMed] [Google Scholar]
- 3.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K and Tuschl T. Duplexes of 21 - nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001; 411: 494–8. [DOI] [PubMed] [Google Scholar]
- 4.McCaffrey AP, Meuse L, Pham TT, Conklin DS, Hannon GJ and Kay MA. RNA interference in adult mice. Nature 2002; 418: 38–9. [DOI] [PubMed] [Google Scholar]
- 5.Das M, Musetti S and Huang L. RNA Interference-Based Cancer Drugs: The Roadblocks, and the “Delivery” of the Promise. Nucleic Acid Ther 2019; 29: 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bobbin ML and Rossi JJ. RNA Interference (RNAi)-Based Therapeutics: Delivering on the Promise? Annu Rev Pharmacol Toxicol 2016; 56: 103–22. [DOI] [PubMed] [Google Scholar]
- 7.Zuckerman JE and Davis ME. Clinical experiences with systemically administered siRNA-based therapeutics in cancer. Nat Rev Drug Discov 2015; 14: 843–56. [DOI] [PubMed] [Google Scholar]
- 8.Tai W and Gao X. Functional peptides for siRNA delivery. Adv Drug Deliv Rev 2017; 110–111: 157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou J and Rossi JJ. Mechanisms and barriers to RNAi delivery. Advanced Delivery and Therapeutic Applications of RNAi 2013: 3–17. [Google Scholar]
- 10.Whitehead KA, Langer R and Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov 2009; 8: 129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gavrilov K and Saltzman WM. Therapeutic siRNA: principles, challenges, and strategies. Yale J Biol Med 2012; 85: 187–200. [PMC free article] [PubMed] [Google Scholar]
- 12.Meng Z and Lu M. RNA Interference-Induced Innate Immunity, Off-Target Effect, or Immune Adjuvant? Front Immunol 2017; 8: 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Lu Z, Wientjes MG and Au JL. Delivery of siRNA therapeutics: barriers and carriers. AAPS J 2010; 12: 492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dominska M and Dykxhoorn DM. Breaking down the barriers: siRNA delivery and endosome escape. J Cell Sci 2010; 123: 1183–9. [DOI] [PubMed] [Google Scholar]
- 15.Zeng Y and Cullen BR. RNA interference in human cells is restricted to the cytoplasm. RNA 2002; 8: 855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marquez AR, Madu CO and Lu L. An overview of various carriers for siRNA delivery. Oncomedicine 2018; 3: 48–58. [Google Scholar]
- 17.Srimanee A, Arvanitidou M, Kim K, Hallbrink M and Langel U. Cell-penetrating peptides for siRNA delivery to glioblastomas. Peptides 2018; 104: 62–69. [DOI] [PubMed] [Google Scholar]
- 18.Lehto T, Ezzat K, Wood MJA and El Andaloussi S. Peptides for nucleic acid delivery. Adv Drug Deliv Rev 2016; 106: 172–82. [DOI] [PubMed] [Google Scholar]
- 19.Singh T, Murthy ASN, Yang HJ and Im J. Versatility of cell-penetrating peptides for intracellular delivery of siRNA. Drug Deliv 2018; 25: 1996–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bechara C and Sagan S. Cell-penetrating peptides: 20 years later, where do we stand? FEBS Lett 2013; 587: 1693–702. [DOI] [PubMed] [Google Scholar]
- 21.Zeller S, Choi CS, Uchil PD, et al. Attachment of cell-binding ligands to arginine-rich cell- penetrating peptides enables cytosolic translocation of complexed siRNA. Chem Biol 2015; 22: 50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saalik P, Niinep A, Pae J, et al. Penetration without cells: membrane translocation of cell- penetrating peptides in the model giant plasma membrane vesicles. J Control Release 2011; 153: 117–25. [DOI] [PubMed] [Google Scholar]
- 23.Pae J, Saalik P, Liivamagi L, et al. Translocation of cell-penetrating peptides across the plasma membrane is controlled by cholesterol and microenvironment created by membranous proteins. J Control Release 2014; 192: 103–13. [DOI] [PubMed] [Google Scholar]
- 24.Allolio C, Magarkar A, Jurkiewicz P, et al. Arginine-rich cell-penetrating peptides induce membrane multilamellarity and subsequently enter via formation of a fusion pore. Proc Natl Acad Sci U S A 2018; 115: 11923–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McClorey G and Banerjee S. Cell-Penetrating Peptides to Enhance Delivery of Oligonucleotide- Based Therapeutics. Biomedicines 2018; 6: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deshayes S, Morris M, Heitz F and Divita G. Delivery of proteins and nucleic acids using a noncovalent peptide-based strategy. Adv Drug Deliv Rev 2008; 60: 537–47. [DOI] [PubMed] [Google Scholar]
- 27.Huang YW, Lee HJ, Tolliver LM and Aronstam RS. Delivery of nucleic acids and nanomaterials by cell-penetrating peptides: opportunities and challenges. Biomed Res Int 2015; 2015: 834079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Figueiredo IR, Freire JM, Flores L, Veiga AS and Castanho M. Cell-penetrating peptides: A tool for effective delivery in gene-targeted therapies. IUBMB Life 2014; 66: 182–94. [DOI] [PubMed] [Google Scholar]
- 29.Parnaste L, Arukuusk P, Langel K, Tenson T and Langel U. The Formation of Nanoparticles between Small Interfering RNA and Amphipathic Cell-Penetrating Peptides. Mol Ther Nucleic Acids 2017; 7: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh N, Agrawal A, Leung AK, Sharp PA and Bhatia SN. Effect of nanoparticle conjugation on gene silencing by RNA interference. J Am Chem Soc 2010; 132: 8241–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davidson TJ, Harel S, Arboleda VA, et al. Highly efficient small interfering RNA delivery to primary mammalian neurons induces MicroRNA-like effects before mRNA degradation. J Neurosci 2004; 24: 10040–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lundberg P, El-Andaloussi S, Sutlu T, Johansson H and Langel U. Delivery of short interfering RNA using endosomolytic cell-penetrating peptides. FASEB J 2007; 21: 2664–71. [DOI] [PubMed] [Google Scholar]
- 33.Green M, Ishino M and Loewenstein PM. Mutational analysis of HIV-1 Tat minimal domain peptides: identification of trans-dominant mutants that suppress HIV-LTR-driven gene expression. Cell 1989; 58: 215–23. [DOI] [PubMed] [Google Scholar]
- 34.Tunnemann G, Ter-Avetisyan G, Martin RM, Stockl M, Herrmann A and Cardoso MC. Live-cell analysis of cell penetration ability and toxicity of oligo-arginines. J Pept Sci 2008; 14: 469–76. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell DJ, Kim DT, Steinman L, Fathman CG and Rothbard JB. Polyarginine enters cells more efficiently than other polycationic homopolymers. J Pept Res 2000; 56: 318–25. [DOI] [PubMed] [Google Scholar]
- 36.Kim SW, Kim NY, Choi YB, Park SH, Yang JM and Shin S. RNA interference in vitro and in vivo using an arginine peptide/siRNA complex system. J Control Release 2010; 143: 335–43. [DOI] [PubMed] [Google Scholar]
- 37.Fernandez-Carneado J, Kogan MJ, Pujals S and Giralt E. Amphipathic peptides and drug delivery. Biopolymers 2004; 76: 196–203. [DOI] [PubMed] [Google Scholar]
- 38.Morris MC, Vidal P, Chaloin L, Heitz F and Divita G. A new peptide vector for efficient delivery of oligonucleotides into mammalian cells. Nucleic Acids Res 1997; 25: 2730–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morgan DO. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol 1997; 13: 261–91. [DOI] [PubMed] [Google Scholar]
- 40.Crombez L, Morris MC, Dufort S, et al. Targeting cyclin B1 through peptide-based delivery of siRNA prevents tumour growth. Nucleic Acids Res 2009; 37: 4559–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaissiere A, Aldrian G, Konate K, et al. A retro-inverso cell-penetrating peptide for siRNA delivery. J Nanobiotechnology 2017; 15: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crombez L, Aldrian-Herrada G, Konate K, et al. A new potent secondary amphipathic cell- penetrating peptide for siRNA delivery into mammalian cells. Mol Ther 2009; 17: 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Konate K, Lindberg MF, Vaissiere A, et al. Optimisation of vectorisation property: A comparative study for a secondary amphipathic peptide. Int J Pharm 2016; 509: 71–84. [DOI] [PubMed] [Google Scholar]
- 44.Jackson AL and Linsley PS. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat Rev Drug Discov 2010; 9: 57–67. [DOI] [PubMed] [Google Scholar]
- 45.Eckerdt F, Yuan J and Strebhardt K. Polo-like kinases and oncogenesis. Oncogene 2005; 24: 267–76. [DOI] [PubMed] [Google Scholar]
- 46.Yao YD, Sun TM, Huang SY, et al. Targeted delivery of PLK1-siRNA by ScFv suppresses Her2+ breast cancer growth and metastasis. Sci Transl Med 2012; 4: 130ra48. [DOI] [PubMed] [Google Scholar]
- 47.Zimmermann GR, Lehar J and Keith CT. Multi-target therapeutics: when the whole is greater than the sum of the parts. Drug Discov Today 2007; 12: 34–42. [DOI] [PubMed] [Google Scholar]
- 48.Li T, Wu M, Zhu YY, Chen J and Chen L. Development of RNA interference-based therapeutics and application of multi-target small interfering RNAs. Nucleic Acid Ther 2014; 24: 302–12. [DOI] [PubMed] [Google Scholar]
- 49.Song E, Zhu P, Lee SK, et al. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat Biotechnol 2005; 23: 709–17. [DOI] [PubMed] [Google Scholar]
- 50.Ren Y, Cheung HW, von Maltzhan G, et al. Targeted tumor-penetrating siRNA nanocomplexes for credentialing the ovarian cancer oncogene ID4. Sci Transl Med 2012; 4: 147ra12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fogal V, Zhang L, Krajewski S and Ruoslahti E. Mitochondrial/cell-surface protein p32/gC1qR as a molecular target in tumor cells and tumor stroma. Cancer Res 2008; 68: 7210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fang B, Jiang L, Zhang M and Ren FZ. A novel cell-penetrating peptide TAT-A1 delivers siRNA into tumor cells selectively. Biochimie 2013; 95: 251–7. [DOI] [PubMed] [Google Scholar]
- 53.Stegmann T Membrane fusion mechanisms: the influenza hemagglutinin paradigm and its implications for intracellular fusion. Traffic 2000; 1: 598–604. [DOI] [PubMed] [Google Scholar]
- 54.Luneberg J, Martin I, Nussler F, Ruysschaert JM and Herrmann A. Structure and topology of the influenza virus fusion peptide in lipid bilayers. J Biol Chem 1995; 270: 27606–14. [DOI] [PubMed] [Google Scholar]
- 55.Plank C, Oberhauser B, Mechtler K, Koch C and Wagner E. The influence of endosome- disruptive peptides on gene transfer using synthetic virus-like gene transfer systems. J Biol Chem 1994; 269: 12918–24. [PubMed] [Google Scholar]
- 56.Cantini L, Attaway CC, Butler B, Andino LM, Sokolosky ML and Jakymiw A. Fusogenic- oligoarginine peptide-mediated delivery of siRNAs targeting the CIP2A oncogene into oral cancer cells. PLoS One 2013; 8: e73348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alexander-Bryant AA, Dumitriu A, Attaway CC, Yu H and Jakymiw A. Fusogenic-oligoarginine peptide-mediated silencing of the CIP2A oncogene suppresses oral cancer tumor growth in vivo. J Control Release 2015; 218: 72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bartz R, Fan H, Zhang J, et al. Effective siRNA delivery and target mRNA degradation using an amphipathic peptide to facilitate pH-dependent endosomal escape. Biochem J 2011; 435: 475–87. [DOI] [PubMed] [Google Scholar]
- 59.Wyman TB, Nicol F, Zelphati O, Scaria PV, Plank C and Szoka FC Jr. Design, synthesis, and characterization of a cationic peptide that binds to nucleic acids and permeabilizes bilayers. Biochemistry 1997; 36: 3008–17. [DOI] [PubMed] [Google Scholar]
- 60.Lee SH, Kim SH and Park TG. Intracellular siRNA delivery system using polyelectrolyte complex micelles prepared from VEGF siRNA-PEG conjugate and cationic fusogenic peptide. Biochem Biophys Res Commun 2007; 357: 511–6. [DOI] [PubMed] [Google Scholar]
- 61.Leng Q and Mixson AJ. Small interfering RNA targeting Raf-1 inhibits tumor growth in vitro and in vivo. Cancer Gene Ther 2005; 12: 682–90. [DOI] [PubMed] [Google Scholar]
- 62.Leng Q, Scaria P, Lu P, Woodle MC and Mixson AJ. Systemic delivery of HK Raf-1 siRNA polyplexes inhibits MDA-MB-435 xenografts. Cancer Gene Ther 2008; 15: 485–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jun E, Kim S, Kim JH, et al. Design of a multicomponent peptide-woven nanocomplex for delivery of siRNA. PLoS One 2015; 10: e0118310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alexander-Bryant AA, Zhang H, Attaway CC, et al. Dual peptide-mediated targeted delivery of bioactive siRNAs to oral cancer cells in vivo. Oral Oncol 2017; 72: 123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laimer K, Spizzo G, Gastl G, et al. High EGFR expression predicts poor prognosis in patients with squamous cell carcinoma of the oral cavity and oropharynx: a TMA-based immunohistochemical analysis. Oral Oncol 2007; 43: 193–8. [DOI] [PubMed] [Google Scholar]
- 66.Li Z, Zhao R, Wu X, et al. Identification and characterization of a novel peptide ligand of epidermal growth factor receptor for targeted delivery of therapeutics. FaSeb J 2005; 19: 1978–85. [DOI] [PubMed] [Google Scholar]
- 67.Shukla RS, Qin B and Cheng K. Peptides used in the delivery of small noncoding RNA. Mol Pharm 2014; 11: 3395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chertok B, David AE, Moffat BA and Yang VC. Substantiating in vivo magnetic brain tumor targeting of cationic iron oxide nanocarriers via adsorptive surface masking. Biomaterials 2009; 30: 6780–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raucher D and Ryu JS. Cell-penetrating peptides: strategies for anticancer treatment. Trends Mol Med 2015; 21: 560–70. [DOI] [PubMed] [Google Scholar]
- 70.Bjorge JD, Pang A and Fujita DJ. Delivery of gene targeting siRNAs to breast cancer cells using a multifunctional peptide complex that promotes both targeted delivery and endosomal release. PLoS One 2017; 12: e0180578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Levy DE and Inghirami G. STAT3: a multifaceted oncogene. Proc Natl Acad Sci U S A 2006; 103: 10151–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dang CV. MYC on the path to cancer. Cell 2012; 149: 22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Regberg J, Vasconcelos L, Madani F, Langel U and Hallbrink M. pH-responsive PepFect cell- penetrating peptides. Int J Pharm 2016; 501: 32–8. [DOI] [PubMed] [Google Scholar]
- 74.Gonias SL and Campana WM. LDL receptor-related protein-1 : a regulator of inflammation in atherosclerosis, cancer, and injury to the nervous system. Am J Pathol 2014; 184: 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.He S, Cen B, Liao L, et al. A tumor-targeting cRGD-EGFR siRNA conjugate and its anti-tumor effect on glioblastoma in vitro and in vivo. Drug Deliv 2017; 24: 471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cen B, Wei Y, Huang W, et al. An Efficient Bivalent Cyclic RGD-PIK3CB siRNA Conjugate for Specific Targeted Therapy against Glioblastoma In Vitro and In Vivo. Mol Ther Nucleic Acids 2018; 13: 220–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eguchi A, Meade BR, Chang YC, et al. Efficient siRNA delivery into primary cells by a peptide transduction domain-dsRNA binding domain fusion protein. Nat Biotechnol 2009; 27: 567–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Endoh T, Sisido M and Ohtsuki T. Cellular siRNA delivery mediated by a cell-permeant RNA- binding protein and photoinduced RNA interference. Bioconjug Chem 2008; 19: 1017–24. [DOI] [PubMed] [Google Scholar]
- 79.Liu HY and Gao X. A universal protein tag for delivery of SiRNA-aptamer chimeras. Sci Rep 2013; 3: 3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rathnayake PV, Gunathunge BG, Wimalasiri PN, Karunaratne DN and Ranatunga RJ. Trends in the Binding of Cell Penetrating Peptides to siRNA: A Molecular Docking Study. J Biophys 2017; 2017: 1059216. [DOI] [PMC free article] [PubMed] [Google Scholar]