Abstract
RNA interference (RNAi) is a cellular mechanism for post-transcriptional gene regulation mediated by small interfering RNA (siRNA) and microRNA. siRNA-based therapy holds significant promise for the treatment of a wide-range of arthritic diseases. siRNA selectively suppresses the expression of a gene product and can thus achieve the specificity that is lacking in small molecule inhibitors. The potential use of siRNA-based therapy in arthritis, however, has not progressed to clinical trials despite ample evidence for efficacy in pre-clinical studies. One of the main challenges to clinical translation is the lack of a suitable delivery vehicle to efficiently and safely access diverse pathologies. Moreover, the ideal targets in treatment of arthritides remain elusive given the complexity and heterogeneity of these disease pathogeneses. Herein, we review recent preclinical studies that use RNAi-based drug delivery systems to mitigate inflammation in models of rheumatoid arthritis and osteoarthritis. We discuss a self-assembling peptide-based nanostructure that demonstrates the potential of overcoming many of the critical barriers preventing the translation of this technology to the clinic.
Keywords: RNA interference, drug delivery systems, nanoparticle, rheumatoid arthritis, osteoarthritis
Introduction
RNA interference (RNAi) is an intrinsic cellular mechanism for post-transcriptional control of protein expression in which messenger RNA (mRNA) is targeted for degradation by short double stranded RNA (Figure 1).[1] Tuschl et al. initially proposed that exogenous small interfering RNA (siRNA) could be delivered to exert RNAi.[2] siRNAs are short 21–23 base pair duplex oligonucleotides in which the “antisense” strand is complementary to a target mRNA, and the “sense” strand acts as a bystander. siRNA operates through the native RNAi machinery to assemble the RNA induced silencing complex (RISC). In the RISC, siRNA initiates cleavage of both the sense and antisense strands, based on sequence specificity.[3] This selective degradation of mRNA provides an avenue to decrease the expression of proteins involved in disease pathogenesis.
Figure 1.
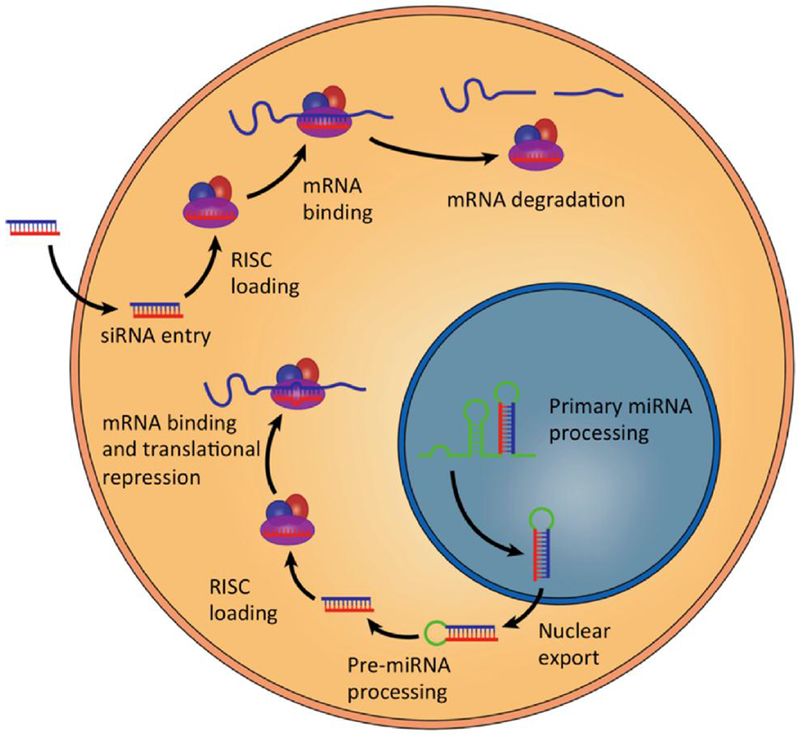
Exogenous siRNA can induce mRNA degradation and gene silencing if delivered into the cytoplasm. (Reproduced with permission from Hou et al. Biotechnology Advances 2015; 33:931-940).
Although the promise of RNA silencing with exogenous siRNA has continued to excite scientists, engineers, and pharma companies since its introduction over two decades ago, only a single product has gained FDA approval: a lipidic complex targeted to the liver galactose receptor now marketed by Alnylam for the treatment of hereditary transthyretin-mediated amyloidosis (Onpattro®).[4–6] Sophisticated molecular modifications of the siRNA itself have both reduced off target effects, and enhanced efficacy.[7, 8] However, because negatively charged siRNA does not cross cell membranes freely, the main hurdle to widespread adoption remains the lack of a suitable delivery vehicle to safely access diverse cell populations after systemic injection.
Traditional classes of delivery agents such as polymers or lipidic nanostructures heretofore have resisted widespread clinical application because they are taken up mostly in the liver and the macrophage phagocytic system (MPS) despite efforts to render them stealthy. What is needed are new approaches for systemic siRNA delivery that avoid the MPS, which would allow sufficient penetration to other molecular targets. Moreover, the problem becomes more complex by the necessity to sequentially breach various physical barriers with sufficient numbers of siRNA to effect silencing. These barriers generally involve: vascular access, traversal of endothelium, cell membrane interactions, cellular uptake, endosomal escape, and cytoplasmic trafficking to the RISC complex. Once in the cytoplasmic compartment, longevity of the exogenous siRNA becomes important for sustained efficacy. If any of these sequential steps fails, the entire process fails.
Comprehensive review articles describing siRNA therapeutics have been published over the last two decades and readers are referred to these for general information on molecular mechanisms of RNAi.[9–11] A more recent review of clinical trials and commercial activity in the siRNA space by Tatiparti et al. also is available.[12] In this review, we highlight recent developments in RNAi applications for the treatment of arthritic conditions and provide updates on peptide-based delivery systems for RNAi.
RNAi applications in rheumatoid arthritis
Rheumatoid arthritis (RA) is a chronic inflammatory arthritis affecting approximately 1% of the general population worldwide, ~1.5 million adults in the United States alone. RA is characterized by inflammation of the synovial lining of diarthrodial joints and an influx of leukocytes through leaky angiogenic blood vessels.[13–15] This synovial proliferation, termed pannus, and cellular influx contribute to the destruction of connective tissues, cartilage, and subchondral bone of the affected joints.
RA is a complex and heterogeneous disease influenced by genetics and environmental factors that shape the immune responses. Insights into these responses have led to the development of a number of “biologics” aimed at inhibiting the action of several inflammatory cytokines, including IL-1, IL-6, and TNF.[16] Despite their effectiveness, continuous systemic administration of biologics may cause significant side effects and heightens the risk of opportunistic infection.[17] Studies have shown that about half of the initial responders either stop responding to a biologic or have to discontinue therapy altogether due to side effects.[18]
siRNA nanotherapy in RA
The use of nanocarriers to deliver therapeutics specifically to the desired sites of inflammation represents a promising and attractive therapeutic approach to RA. In addition to targeted delivery, nanotherapeutics can theoretically lower the drug dose and dose frequency to avoid bystander effects. Many excellent reviews over the years have highlighted the potential of various nanocarriers for the treatment of RA.[19–21] In addition, a recent comprehensive review discussed advances in the applications of siRNA nanotherapeutics for rheumatic conditions, including RA.[22] Strategies highlighted included local application by intra-articular injection, without or with electroporation, hydrodynamic injection, and biocompatible systems that achieved safe and targeted in vivo delivery of siRNA. In this review we will cover studies published since, with a focus on systemic delivery only (Table 1). We will also discuss advances in intra-articular injection as a treatment modality for osteoarthritis (OA) (see below).
Table 1:
Recent siRNA applications for drug delivery in rodent models of RA
| Target | Delivery route | Carrier | Analysis time point | Outcome | Model | Reference |
|---|---|---|---|---|---|---|
| TNF-α | Systemic | Peptide (RVG-9R) | 10 days | Inhibited inflammation; inhibited cartilage and bone erosions | Mouse CAIA | [24] |
| TNF-α | Systemic | Thiolated glycol chitosan polymer | 7 weeks | Abrogated inflammatory cytokine; protected from bone erosion; suppressed early inflammatory arthritis | Mouse CIA | [26] |
| TNF-α | Systemic | Degradable cationic polymer (PDAPEI) | 5 weeks | Reduce severity of inflammation; lessened cartilage damage; inhibited TNF expression | Mouse CIA | [29] |
| TNF-α | Systemic | Folate-PEG-chitosan-DEAE nanoparticle | 10 days | Decreased disease activity; decreased bone erosions and bone metabolism markers | Mouse CAIA | [25] |
| TNF-α | Systemic | Shedd able PEGylated solid-lipid nanoparticle | 8 days | Decreased disease activity; decreased bone loss | Mouse CAIA | [31] |
| NF-κB (p65) | Systemic | Peptide (p5RHH) | 10 days | Decreased disease activity; decreased bone erosions; reduced cartilage damage | Mouse CAIA | [34] |
| NF-κB (p65) | Systemic | Oligopeptide modified micelle | 15 days | Decreased disease activity; decreased inflammatory cytokines | Mouse CIA | [35] |
| NF-κB (p65) + Dexamethasone | Systemic | PCL-PEI/PCL-PEG hybrid polymeric micelle | 7 weeks | Repressed arthritis; preserved cartilage integrity | Mouse CIA | [36] |
| NF-κB (p65) + Methotrexate | Systemic | Folate conjugated liposome-based hybrid carrier | 7 weeks | Suppressed arthritis; reduced expression of cytokines | Mouse CIA | [37] |
| NF-κB (c-Rel) | Systemic | PEG-PLL-PLLeu nanoparticle | 22 days from onset of arthritis | Decreased disease activity; suppressed inflammatory cytokines | Mouse CIA | [38] |
| C5 | Systemic | C5aR1 Ab-protamine | 10 days | Decreased disease activity; decreased inflammation, pannus formation, cartilage and bone damage | Mouse CAIA | [44] |
| MAS P-3 | Systemic | GalNAc-MASP-3 duplex | 10 days | Decreased expression of MASP-3 in the liver; decreased clinical score | Mouse CAIA | [45] |
| ADAM15 | Systemic | Atelocollagen-siRNA complex | 21 days | Decreased arthritis score; reduced histological damage | Mouse CIA | [48] |
| IL-2/IL-15 receptor beta chain | Systemic | PEI or PEI-SPIO nanoparticle | 30 days | Mitigated arthritis manifestation; effect augmented with the incorporation of SPIO | Rat AA | [46], [47] |
| hnRNP A2/B1 | Systemic | Liposome | 60 days (CIA) 10 days (STA) | Decreased incidence and severity of arthritis; decreased production of inflammatory cytokines | Mouse CIA and STA | [49] |
| Notch 1 | Systemic | Thiolated glycol chitosan polymer | 42 days | Slowed down progression of arthritis; mitigated cartilage and bone damage | Mouse CIA | [50] |
| Connexin 43 | IA | Electroporation-assisted siRNA transduction | 28 days | Suppressed arthritis in knee and ankle when siRNA injected into ipsilateral knee | Rat CIA | [51] |
| CCR5 | IA | Electroporation-assisted siRNA transduction | 28 days | Ameliorated arthritis in the knee and ankle when siRNA injected into ipsilateral knee | Rat AA | [52] |
AA = adjuvant arthritis; CIA = collagen-induced arthritis; CAIA = collagen antibody–induced arthritis; IA = intra-articular; STA = serum transfer arthritis
Targeting inflammatory pathways in RA
A number of inflammatory/catabolic molecules and pathways have been targeted to treat RA. Here we highlight some of the key molecules that have gained attention for therapy.
Tumor necrosis factor alpha (TNF-α)-
TNF inhibitors (TNFis), developed as monoclonal antibodies, are currently the best-selling biologics for the treatment of RA. The major adverse effect of TNFis is increased risk of opportunistic infections, especially tuberculosis.[23] With their promise of specificity and potentially lower toxicity, RNAi delivery systems targeting TNF-α have been extensively explored in animal models of RA. Ye et al. used a small peptide, RVG-9R, a 29-amino acid peptide derived from rabies virus glycoprotein fused to 9 arginine residues to silence TNF-α in the collagen antibody-induced arthritis (CAIA) model.[24] When administered systemically, the treatment led to approximately 60% reduction in inflammation when compared to dexamethasone, a steroid that has general immunosuppressive effect. The treatment is preventative, given prior to arthritis development and no toxicity or immune responses (i.e. antibody production against the peptide) were examined. Due to its biocompatibility and low immunogenicity, chitosan (CH) and its derivatives have also been developed to deliver siRNA in vivo. One drawback to CH is poor solubility at physiological pH. A soluble derivative containing diethyl ethylamine (DEAE) was synthesized, conjugated to folic acid for enhanced cellular uptake by folate receptor and used to deliver TNF-α siRNA to mice with CAIA prior to disease development and shown to modestly reduce disease activity while preserving bone structure, as evidenced by decreased bone erosions scores and bone metabolism markers.[25] In addition, self-polymerized thiol-modified siRNA (poly-siRNA) can form stable complexes with biocompatible thiolated glycol CH (tGC) polymers that are readily degraded under reductive conditions in the cell cytosol to monomeric siRNA.[26] These complexes accumulate in inflamed joints and suppresses collagen-induced arthritis (CIA) as efficiently as methotrexate.[26] Strong cationic polymers such as polyethyleneimine (PEI) have been used to deliver siRNA; however, PEI is harder to condense and may be unstable, resulting in early release and degradation of siRNA in serum. Cytotoxicity also limits its application.[27, 28] Low molecular weight PEI (<20 kDa), cross-linked by degradable linkers, shows lower toxicity, self-degrades in an acidic environment (i.e. inflammatory milieu), and significantly suppresses CIA.[29] Complexing TNF-α siRNA with biocompatible cationic lipids such as lecithin, cholesterol and a previously reported acid-sensitive sheddable polyethylene glycol (PEG)[30] led to the development of nanoparticle formulation with minimum burst release (5% of siRNA was released in one-month release study in vitro).[31] The formulation mitigated CAIA by approximately 33% when given prior to disease onset.[31]
Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) –
This is a signaling pathway that controls gene products closely linked to inflammation.[32] The NF-κB family consists of five members: p105 (constitutively processed to p50), p100 (processed to p52 under regulated conditions), p65 (also known as RelA), RelB, and c-Rel.[33] These members form homo- and heterodimers that, in the resting cell, are normally held inactive in the cytoplasm by the association with inhibitors, the IκB proteins. Activation of NF-κB is controlled by the IκB kinase (IKK) complex that phosphorylates IκB proteins and targets them for degradation, releasing the NF-κB subunits for nuclear translocation and transactivation of a multitude of responsive genes, including several inflammatory cytokines. Thus NF-κB pathway plays a crucial role in the inflammatory response of macrophages and lymphocytes in RA. Using the CAIA model and the p5RHH peptide-based nanosystem (described in detail in the section below) that delivered p65 siRNA systemically, we showed that the nanoparticles penetrated through inflamed and leaky vasculature, much like the endothelial permeability and retention (EPR) effect proposed for nanoparticle localization in tumor, and potently suppressed ongoing inflammation in the robust model of K/BxN serum transfer arthritis.[34] This self-assembling, largely non-toxic siRNA delivery platform has promising translational potential for the treatment of RA (and other chronic inflammatory diseases) where repeated dosing may be required. Since this publication, a number of studies have confirmed the utility of targeting NF-κB in inflammatory arthritis models. Delivery of NF-κB siRNA targeting p65 was achieved using block polymers, polymeric micelles, or hybrid nanocarrier.[35–37] Many of these included additional drugs such as dexamethasone or methotrexate loaded onto the particles. Other NF-κB targets have also been explored such as c-Rel and the non-canonical NF-κB-induced kinase (NIK) signaling pathway.[38, 39]
Complement system –
The complement system is an effector arm of the innate immune response and plays a central role in RA development. The complement system comprises three pathways: the classical pathway, the lectin pathway, and the alternative pathway. Activation of all three pathways converges with the cleavage of C3 and C5, generating the anaphylatoxins C3a and C5a. The importance of C5a and its receptor C5aR in preclinical models of arthritis is well delineated.[40–42] However, antagonism of complement C5a-C5aR axis in patients with RA has met with disappointing results in the clinic.[43] In a more recent study, the investigators conjugated protamine to a monoclonal antibody (Ab) directed against C5a receptor 1 (C5aR1) to generate anti-C5aR1 Ab-protamine-C5 siRNA conjugates, taking advantage of the charge-charge interaction between protamine (positively charged) and siRNA (negatively charged).[44] Injection of C5aR1 Ab-protamine-C5 siRNA conjugate in vivo in mice with CAIA led to 83% reduction in CAIA when injected three times, 5 days prior to disease induction (days −5, 0, and 3, in a preventative treatment) while injection with unconjugated components (anti-C5aR1 Ab and C5 siRNA) only mitigated disease by 19%. The same group also targeted mannan-binding lectin-associated serine proteases 3 (MASP-3), a component of the lectin pathway.[45] The investigators hypothesized that silencing liver-derived MASP-3 synthesis would modulate complement activation and attenuate arthritis. Triantennary N-acetylgalactosamine (GalNAc) was conjugated to MASP-3 siRNA (GalNAc-MASP-3-siRNA) to enhance liver uptake and injected into mice with CAIA. GalNAc-MASP-3-siRNA administration did not completely inhibit MASP-3 expression and only delayed onset of arthritis by one day and suppressed disease activity by about 50% if given 10 days prior to arthritis induction. Whether MASP-3 depletion in an established disease has effect remains to be seen.
Miscellaneous targets –
Two separate studies used either PEI/siRNA complex[46] or PEI-superparamagnetic iron oxide (SPIO) nanoparticles[47] to target IL-2/IL-15 receptor beta chain Both approaches reduced the severity of adjuvant arthritis in rats but the effect was augmented by the application of a magnetic field to SPIO-containing nanoparticles.[47] Other targets include a disintegrin and metalloproteinase 15 (ADAM15),[48] heterogeneous nuclear RNP A2/B1,[49] Notch 1,[50] Connexin 43,[51] CCR5,[52] the pore-forming subunit of calcium release-activated calcium (CRAC) channels,[53] and transforming growth factor beta-activated kinase-1 (TAK-1).[54]
Limitations and future directions
Although most studies using siRNA-based therapeutic delivery systems show some efficacy in preclinical animal models of RA, translation to the clinic is far from reality. Several critical issues remain to be worked out, including identification of off-target effects of excess siRNA that can induce type I interferon response.[55] In addition to anti-inflammatory approaches, targeting cell lineages may provide beneficial outcomes. Aberrant T cell regulation is proposed as one of the mechanisms that promote RA.[56] Thus T-cell targeting strategies could offer therapeutic utility. T cell co-stimulation inhibition (Abatacept) is a biologic that is currently doing well in the clinic.[57] Other T cell subsets being explored as targets include Th17 cells that produce IL-17 cytokines (IL-17A and IL-17F), which have been shown to play a critical role in many inflammatory arthritides, including RA and psoriasis/psoriatic arthritis.[58] Humanized monoclonal antibody against IL-17A, however, has mixed effects in RA [59–61] and may worsen inflammatory bowel disease in some instances.[62] Since Th17 cells produce many cytokines other than IL-17 cytokines, targeting Th17 development may lead to improved efficacy against autoimmune arthritides. Thus, silencing the retinoic acid-related orphan receptor gamma t (RORγT), the master transcriptional factor of Th17 lineage may prove superior to blocking a single cytokine.[63] This was recently accomplished in vitro using CD4 aptamer-based delivery of RORγT-siRNA to suppress Th17 differentiation.[64] Aptamers are nucleic acid-based ligands (single stranded DNA or RNA oligonucleotides) that are produced through a process known as systemic evolution of ligands by exponential enrichment (SELEX). [63] SELEX enables the generation of aptamers that bind specifically to a molecule, such as cell-surface receptor, gaining entrance to target cells through receptor-mediated endocytosis. Aptamer-siRNA complex targeting HIV-gp120 inhibits HIV replication in vitro and in vivo,[65, 66] suggesting that this system holds promise as a universal tool for siRNA delivery to specific targets.
RNAi applications in OA and post-traumatic OA
OA is a complex polygenic disease, which is now recognized as a clinical syndrome.[67] It is one of the most common causes of disability in the aging population and its incidence is becoming higher in younger population, especially in association with traumatic knee injuries. Moreover, once reserved for elderly, joint replacement surgeries are becoming more common in the young and active individuals. The true root cause and pathogenesis of primary age-related OA remains incompletely understood. Insights from large-scale genetic studies and information gained from injury-related post-traumatic OA have enabled us to capture some aspects of the disease process. However, a true picture of the pathogenetic pathways is yet far from reality. As the pathogenesis remains to be fully elucidated, treatment options for OA are also limited.
Currently there is no disease modifying OA drug (DMOAD). Despite numerous attempts to devise therapeutic strategies for OA, none has thus far made it to clinic.[68] OA is a chronic pain condition, often associated with structural changes. Although articular cartilage is not innervated, high expression of nerve growth factors in OA joints is associated with pain severity.[69] Hence, the development of therapeutic interventions based on antagonism of nerve growth factors is of great interest. Other treatment modalities include non-steroidal anti-inflammatory drugs and corticosteroids, which provide only a transient relief and can be associated with serious side-effects. In patients where the aforementioned medications are ineffective or contraindicated, opioids are often used.[70] It is estimated that the rate of prescribing opioids for knee OA is about 16%,[71] adding to the opioid epidemic in the USA. Joint replacement surgery is a last resort for patients in whom pain medication or other methods have failed. A number of complications associated with surgery such as chances for infection, osteolysis, need for second or third surgery, high costs as well as extended rehabilitation, make joint replacement a less welcome option.[72] However, with new improvements in techniques and materials, total knee arthroplasty is able to extend the active life and offers a new joint with reduced hospital stays.[73]
Critical barriers to effective OA treatments
A number of other factors contribute to the challenges of developing effective OA therapies.
Genetic complexity –
OA is a complex polygenic disease with multiple risk loci conferring small effects.[74] Moreover, environmental and genetic factors play a key role in disease pathogenesis,[67] with genetic factors accounting for significant variation in OA susceptibility. [75]
Multi-tissue disease –
Mounting evidence suggests that OA is a disease of the whole joint and all tissues within the joint are involved.[76] For instance, in the knee OA, meniscus degeneration, subchondral bone sclerosis, and synovial proliferation and joint degeneration are all consequences of OA. Nonetheless, cartilage degeneration remains a hallmark of end-stage disease.
Incomplete understanding of pathogenesis –
Despite rapid progress in the field of genomics and genetics and emergence of high throughput screening tools, and paradigm shift from the simple “wear and tear” process, there is a clear vacuum in the understanding of the OA pathogenesis. A number of pathways are implicated in OA pathogenesis including Wnt signaling, NF-κB, apoptosis, autophagy, cell cycling, TGF-β, Notch, among others.[77, 78]
Disease heterogeneity –
Even when the spatiotemporal nature of injury is known, it is still unclear how these injuries move the joint in the direction of OA. For example, it was thought that anterior cruciate ligament (ACL) tear destabilizes the joint and mechanical nature of this injury results in OA.[79] However, ACL reconstruction does not improve the biology of the joint and is thought to even increase the risk for future OA.[80, 81] In addition, only about 50% of patients go on to develop post-traumatic OA.[82]
Multifactorial disease –
A number of factors such as age, sex as well as genetic and environmental influences contribute to OA development.[83] Their interaction with the disease process is very complex and some are difficult, if not impossible, to modify. Modification of certain risk factors such as obesity and activity level has shown little effects on OA development.
Survivability of cell-based and cell-engineered biomaterials –
A great deal of effort has been made to identify and test various cell types and biomaterials for an effective cell-based and/or tissue-engineered approach to treat focal cartilage as well as osteochondral defects.[84] However, these approaches have met with a number of challenges.[85] A common problem in tissue engineering strategies for OA is that while the natural tissue has degenerated the underlying cause (inflammation) is not resolved, leaving a slim chance for the engineered construct to survive in the hostile inflammatory or catabolic environment. Nevertheless, we must acknowledge the efforts in this exciting area of research. Biologists, biomechanical engineers and material scientists remain committed to generate a product that is more sustainable, resembles the native tissue and can withstand the catabolic milieu.
Targets in OA
In light of the above discussion, one could perceive that the search for Holy Grail of OA therapy has been disappointing. However, this is not true. We now know a lot more about OA process than before. A number of elegant studies have made significant breakthroughs toward identification of genes and pathways to discern the disease mechanisms. These pathways inform each other and take us one step forward toward better understanding of the disease pathogenesis. Research on epigenetic mechanisms is also on the rise. A variety of highly relevant animal models of OA have been developed. A great deal of work has been done to knockout or mutate individual genes using cutting edge genomic engineering technologies such as the use of CRISPR/Cas9 system to unravel disease pathogenic mechanism and develop novel treatment approaches.[86, 87] In a recent study, investigators explored CRISPR/Cas9-mediated gene editing to treat OA, by targeting Ngf, Il1b, and Mmp13 genes in a mouse model of PTOA. It was demonstrated that Il1b and Mmp13 reduced PTOA progression, while Ngf ablation significantly palliated PTOA-related pain. These findings suggest that CRISPER/Cas9-based gene editing is a useful technology for the identification of promising drug targets and the development of feasible therapeutic strategies for OA treatment.[88]
The focus of OA from a disease of cartilage has changed to a concept of whole joint disease and the concept of orthoregeneration is on the horizon. Available genetic and epigenetic tools and availability of high throughput screening methods, as well as a variety of new materials for tissue engineering has made it all possible to better handle some or most of aspects of the disease. The significance of restoring joint homeostasis has emerged as a conceptually appealing approach and sets new directions for effective OA therapy. Last but not least, therapeutic applications may need to be applied early in the disease process, especially in the case of post-traumatic OA, where molecular changes take place much earlier than clinical manifestation of disease.[89] Table 2 lists a number of key pathways that have been targeted using siRNA.
Table 2:
Key pathways related to OA targeted by siRNAs
siRNA nanotherapy in OA: anti-catabolic and anti-inflammatory
According to clincialtrial.gov there is no ongoing clinical trial for the use of siRNA in OA treatment, although a number of siRNA candidates have reached various stages of clinical trials for the treatment of other conditions.[90] For local delivery of siRNA for OA treatment, a great deal of work still needs to be accomplished before a targeted, sustained-release system that enables spatiotemporal control of gene silencing becomes a reality.[91] RNAi-based therapies for OA have been described elsewhere up to 2012.[22] Here we highlight the studies from 2013-2019 (Table 3).
Table 3:
Recent siRNA applications for drug delivery in rodent models of OA
| Target | Delivery route | Carrier | Analysis time point | Outcome | Model | Reference |
|---|---|---|---|---|---|---|
| Ihh | IA | Lipid nanoparticle | 10 weeks | Chondroprotective; attenuated cartilage degeneration | Rat ACLT | [93] |
| NF-κB | IA | Peptide (p5RHH) | 2 weeks | Reduced synovitis; reduced chondrocyte apoptosis | Mouse joint loading | [96] |
| Yap | IA | None | 8 weeks | Ameliorated OA development; reduced aberrant bone formation; prevented cartilage degradation | Mouse ACLT | [97] |
| Hif-2α | IA | Chondrocyte-homing peptide/PEI | 7 weeks | Reduced cartilage degeneration; Alleviated synovitis | Mouse ACLT, MCLT | [99] |
| MMP-13 ADAMTS-5 | IA | None | 4, 8 weeks | Reduced cartilage degeneration; lowered OA score | DMM | [101] |
| MMP-13 | IA | None | 8 weeks | Reduced OARSI score; delayed cartilage degeneration | DMM | [102] |
| MMP-13 | IA | None | 2 weeks | Reduce cartilage degeneration; decreased OARSI score | DMM | [103] |
ACLT = anterior cruciate ligament transection/tear; IA = intra-articular; MCLT = medial collateral ligament transection; DMM = destabilization of medial meniscus; OARSI = Osteoarthritis Research Society International
Indian hedgehog (Ihh) –
has been implicated in OA progression.[92, 93] While Ihh deletion in mice is lethal, siRNA-mediated ablation in rats has been shown not only to have chondroprotective effects but could ameliorate cartilage degeneration.[94]
NF-κB –
This pathway is most prominent among gene signatures in human OA and rodent OA and plays a key regulatory role for inflammatory signaling and is therefore an important therapeutic target.[95] We have recently demonstrated that siRNA targeting NF-κB improved joint homeostasis, suppressed synovitis and inhibited cartilage degeneration in a mouse model of joint injury.[96]
Yes-associated protein (YAP) –
The role of YAP in OA has just begun to emerge. Levels of YAP are increased in human OA and rodent model of experimental OA.[97] siRNA-mediated knockdown of YAP mitigated OA development by reducing bone formation and preventing cartilage degradation.[97]
Hypoxia induced factor 2 a (Hif2a) –
This molecule acts as a catabolic factor and its overexpression is associated with OA.[98] Inhibition of Hif2a with the use of siRNA nanoparticle complex resulted in mitigation of OA, maintenance of cartilage integrity, and reduction in cartilage degeneration and synovitis.[99]
Matrix degrading enzymes –
Matrix degrading enzymes such as matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTSs) are well-established contributors of cartilage degeneration.[100] In particular, MMP-13 and ADAMTS-5 have emerged as candidate targets for development of OA therapies.[101–103]
siRNA nanotherapy in OA: anabolic and regenerative
siRNA not only can be used to inhibit catabolic genes to reduce the inflammatory milieu in the joint, it can also be used to silence a protein that inhibits tissue regeneration or that results in defective tissue formation in order to improve tissue regeneration process.[104] Here we provide a list of siRNAs that have improved cell function, tissue homeostasis or regeneration of bone and cartilage in vivo (Table 4). The identification of processes and pathways or a set of pathways is an important first step towards a targeted therapy to circumvent the disease progression.
Table 4:
siRNA application for bone and cartilage regeneration
| siRNA target | Tissue | Action | Regenerative effect | Model | Reference |
|---|---|---|---|---|---|
| PHD2 | Bone | Block binding of PHD2 to HIF-1 | Enhanced expression of angiogenic proteins | Sheep periosteal implant | [141] |
| GNAS1 | Bone | Induce expression of transcription factor Cbfa1 | Provoked the production of bone-differentiating proteins | Sheep periosteal implant | [141] |
| SOST | Bone | Silence the expression of SOST | Promoted bone formation | Female mice | [142] |
| HIF2A | Cartilage | Interfere with IL-1β and other catabolic signaling pathways | Restored cartilage homeostasis | Mouse ACLT, MCLT | [99] |
| NFkB | Cartilage | Suppress mTOR activity | Restored cartilage homeostasis | Mouse joint loading | [96] |
ACLT = anterior cruciate ligament transection/tear; IA = intra-articular; MCLT = medial collateral ligament transection; DMM = destabilization of medial meniscus
siRNA nanotherapy in OA: microRNAs
Like siRNAs, microRNAs are also noncoding RNAs with important role in gene regulation. MicroRNAs are endogenous, small (18-24 nucleotide), single-stranded RNAs that regulate gene expression at post-transcriptional level by binding to 3’ untranslated region of target mRNAs.[105] Mounting evidence suggests that microRNAs are implicated in a variety of cell functions such as cell cycle, apoptosis, migration and proliferation.[106] Both microRNAs and siRNAs share a number of similarities. However, their mechanism of action as well as clinical applications are different. For instance, siRNA is highly specific with only one mRNA target, while microRNA has multiple targets. Therefore, the therapeutic applications of siRNA and microRNAs are very distinct.[90] Recent evidence in microRNA research suggests that they play a pivotal role in OA.[107]) The emerging role of microRNAs is evident from studies that compare microRNA expression in both healthy and diseased (OA) cartilage. Depending on the expression pattern of a given microRNA, it is either used as an agomir or antagomir to treat OA. An agomir is a chemically engineered double stranded miRNA that is used to mimic upregulation. In contrast, an antagomir is a chemically modified single stranded miRNA inhibitor that prevents other molecules from binding to desired site on an mRNA molecule. Antagomir is used to silence endogenous miRNAs. It is perfectly complementary to the specific miRNA target that mispairs at the cleavage site of Ago2 (argonaute RNA induced silencing complex catalytic component 2) to inhibit Ago2 cleavage. Here, we have highlighted some of the important studies that utilized microRNAs to mitigate OA (Table 5).
Table 5:
MicroRNAs used to treat OA in rodents using intra-articular delivery
| MicroRNA | Expression | Species | Model | Analysis time point | Outcome | Target gene | Reference |
|---|---|---|---|---|---|---|---|
| miR-140 | Agomir | Rat | ACLT+MMx | 4 weeks, 8 weeks, 12 weeks | Increased cartilage anabolism; reduced cartilage pathology; decreased Mmp13 and Adamts4 expression | Mmp13, Adamts4 | [143] |
| miR-181a-5p | Antisense oligonucleotide | Rat | DMM | 10 weeks | Attenuated cartilage destruction; decreased expression of catabolic, hypertrophic and apoptotic genes; reduced collagen type II breakdown | Mmp13, Col10, Parp, p85, Casp3 | [144] |
| miR-93 | Agomir | Mouse | MMT | 2 weeks | Inhibited levels of Il1b, Tnfa, and Il6; deceased chondrocyte apoptosis | Tlr4, Nfkb | [145] |
| miR-29a | Lentivirus | Mouse | CIA | 8 weeks | Lessened the collagenase aggravation of excessive synovial remodeling reaction; lowered Vegf production and angiogenic activation | Vegf | [146] |
| miR-483-5p | Antagomir | Mouse | DMM | 5 weeks | Decreased cartilage pathology score; marked reduction in Runx2 positive chondrocytes | Matn3 | [147] |
| miR-98 | Antagomir | Rat | ACLT+MMT | 2 weeks | Relieved cartilage degradation; prevented downregulation of Bcl2 in cartilage | Bcl2 | [148] |
| miR-222 | Lentivirus | Mouse | DMM | 8 weeks | Reduced cartilage destruction; decreased Mmp13 levels | Mmp13 | [149] |
miR = microRNA; ACLT = anterior cruciate ligament tear; MMx, total medial meniscectomy; DMM = destabilization of medial meniscus; MMT = medial meniscus tear; CIA = collagen induced arthritis
Summary, limitations and future directions
Available evidence from literature suggests that siRNA-based OA therapies appear to work effectively in treating some aspects of the disease. Moreover, most nanoplatforms are effective in delivering the drug cargo when injected intra-articularly, but they lack specificity for a cell or tissue. So, there is an unmet need for the development of nanoplatforms that will target specific tissue(s) in the joint. In addition to specificity, multiplexing is a characteristic that is not universally available in all available platforms. Multiplexing could increase many fold the effectiveness of treatment as more than one pathway can be targeted simultaneously.
Peptide-based delivery systems for RNAi
A natural starting point for considering peptide nanostructures as oligonucleotide delivery agents begins with the general class of cell penetrating peptides.[108–113] In particular, the HIV-derived Tat peptides and Antennapedia-derived “Pentratin” peptides from Drosophila were among the first described to translocate across cell membranes. The structures and membrane penetrating mechanisms for many of these peptides entail interactions of cationic basic amino acid-rich moieties allowing either direct energy independent membrane translocations or energy requiring uptake by macro- or micro-pinocytosis, or other endocytic mechanisms. Some of these agents have been developed as chemically conjugated peptide-nucleic acid structures that may be susceptible in vivo to proteolysis or toxicity due to high arginine content. Other peptides exert electrostatic interactions with negatively charged siRNA that self-assemble into 50-250 nm particles when mixed in appropriate charge and molar ratios.
Upon internalization into endosomes in particular, the task of release of oligonucleotide cargo and escape from endosomes becomes a critical and time dependent task to avoid persistent sequestration and siRNA degradation. Strategies to achieve endosomolysis have traditionally been based on osmotic agents, fusogenic lipids, and fusogenic peptides.[108] Addition of osmotic endosomolytic agents such as chloroquine create proton buffering effects that induce swelling and rupture of endosomes. Indeed, the use of chloroquine in vitro after cell loading with siRNA constructs is useful for understanding the capacity of novel agents to escape the endosome as full release is achieved after chloroquine.
Self-assembling peptide-based siRNA delivery system
Our own efforts have concentrated on an emerging approach to siRNA delivery: a self-assembling nanostructure comprising of a peptide with intrinsic membrane-disrupting activity that offers an alternative for endosomolysis and siRNA release. Melittin, a 26 amino acid, cationic amphipathic component of bee venom represents an example that has found use in several types of oligonucleotide delivery constructs.[114–117] It has been used in certain types of stable perfluorocarbon nanoparticle (PFC NP) formulations as a potent anti-cancer therapeutic by inducing either necrosis or apoptosis. [118, 119] Melittin assumes a random coil secondary configuration in solution and interacts rapidly with negatively charged cell membranes initially through electrostatic interactions. Subsequently it undergoes a change in secondary structure to alpha helical that facilitates hydrophobic interactions with phospholipid tail moieties in an exothermic process that results in stable membrane insertion. In fluid cell membranes, oligomerization and pore formation can occur, followed by cell lysis that overwhelms native cell membrane repair mechanisms.[120] Although melittin rapidly destroys red blood cells and liposomes,[118, 119, 121] it can be carried safely in PFC NP structures within the surfactant-lipid monolayer surrounding the perfluorocarbon nanostructures because the PFC core is both hydrophobic and lipophobic and remains unaffected by melittin’s pore forming behaviors.[122]
Because melittin acts like a cationic cell penetrating peptide, it was thought to be a potentially interesting candidate for drug and oligonucleotide delivery. However, for in vivo applications, its cell lysis properties need to be controlled until it reaches the target cell where it then might exert endosomolytic behaviors. Initial efforts to block the lytic activity of melittin by several authors utilized acid labile protecting groups in polyconjugated polymeric nanostructures that prevented melittin activation and membrane insertion until released in an acidic endosomal environmental [123–125] This strategy for endosomal escape was adopted for clinical trials against Hepatitis B for siRNA delivery, but proved to cause liver toxicity in related preclinical studies resulting in study abandonment. The platform was modified and subsequently has been restarted by Arrowhead Pharmaceuticals in several trials (e.g., hepatitis, anti-trypsin deficiency) with the use of a subcutaneous delivery approach.
In contrast to the use of masked native melittin for endosomal escape, others have taken advantage of its ability to bind negatively charged nucleotides via electrostatic interactions. A polymerized form of lysine modified melittin and plasmid DNA was described by Chen et al. that operated via thiol oxidation of incorporated cysteine residues to depolymerize and release the melittin in order to effect endosomal escape.[126] In this construct, the binding of DNA itself masked melittin’s lytic activity. However, attempts to tailor the lytic potential of melittin by certain N- or C-terminus truncations abrogated its membrane disruptive and transfective potencies.
A safe and potent alternative format for polymerizing melittin and siRNA has been engineered by Hou et al.[108, 127, 128] Initiation of these efforts began with trials of native melittin as an anti-cancer agents carried in a protective perfluorocarbon nanostructure that sequestered melittin in the outer lipid monolayer until interacting with melanoma cancer cells. [118, 119] The fusogenic potential of melittin proved useful to deliver the peptide to the cell membrane via formation of a hemifusion complex with the cancer cell.[129] This allowed the lipids and associated melittin surrounding the perfluorocarbon core to flow into the membrane leaflet of the cancer cell and enter the cytoplasmic compartment to induce apoptosis and/or necrosis depending on conditions.
Subsequent modifications to melittin along the lines of Chen et al. discussed above were tested to reduce intrinsic lytic capacity by selected truncations.[130, 131] A specific N-terminal truncation of 7 amino acids by Pan et al. resulted in greater than 2 orders of magnitude minimization of cell necrosis such that doses that might be used in vivo should not be hemolytic. This format (so called “peptide 5,” or p5) ultimately was developed as a linker molecule capable of carrying conjugated molecular cargo across cell membranes without cell disruption. However, neither p5 nor native melittin was capable of condensing siRNA into a transfective nanostructure.
Further modifications to p5 were proposed by Hou et al. by adding histidine and arginine moieties to form peptide “p5RHH”, which maintains the original 7 amino acid N-terminus truncation.[127, 128] The added arginines enhance electrostatic interactions between siRNA and the peptide. The uncharged histidines (at neutral pH) permit formation of noncovalent hydrogen bonds between siRNA and the peptide in initial exothermic reactions to enhance the stability, silencing activity, and transfection efficacy of the peptide polyplexes.[132] After uptake by micro-pinocytosis, protenation of the imidazole group of histidines in late endosomal structures upon acidification results in disassembly of the polyplex as pH drops below the pKa of the imidazole group (~6.1) (Figure 2). Coordinated release of the siRNA then permits free p5RHH to interact with the endosomal membrane. The free p5RHH, now in high concentrations in the endosome, elicits endosomolysis without perturbing cell viability according to Hou et al.[127, 128] The overall model accords with that proposed by Chou et al. where a peptide:siRNA polyplex is formed by electrostatic interaction and hydrogen bonding, and disassembly of the nanostructure follows protenation of histidines by overcharging thereby allowing interaction of the peptide with the endosomal membranes. In the case of the p5RHH complex, the modified melittin now greatly facilitates endosomal membrane permeabilization. Subsequent dilution of p5RHH in the cytoplasm and protease activity restrains its intracellular lytic potential.
Figure 2.
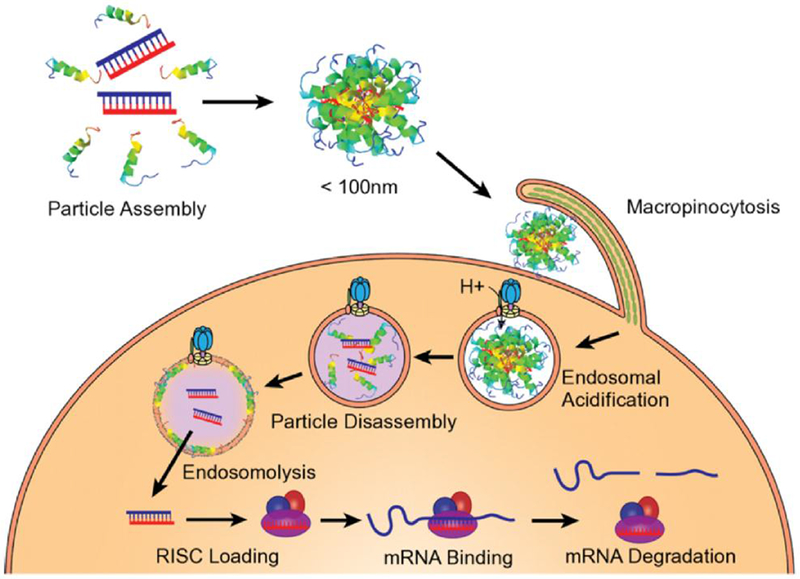
Melittin-derived peptides promote endosomal escape after particle disassembly triggered by endosomal acidification (Reproduced with permission from Hou et al. ACS Nano 2013, 7:8605-15).
The p5RHH nanostructures actually have proven to be more stable in serum, due most likely to coating with albumin which acts as a dis-integrin, helping to avoid uptake by liver.[127, 128] In fact, albumin was demonstrated to be a stabilizer with respect to both particle size and transfection efficiency. In lab-based procedures, a simple mixing procedure at selected ratios of p5RHH and siRNA, followed by albumin coating, creates a transfective 55 nm nanostructure in under 40 minutes. Interestingly, clearance is by the kidney not liver and spleen as the system avoids MPS uptake.[34, 96, 133] The primary mode of deposition is passive “endothelial permeability and retention” in inflamed tissues with leaky vasculature. This approach has proven useful and efficient for delivering the peptide-siRNA nanostructures to other pathologies without the need for molecular targeting. [34, 96, 133, 134]
Summary and future directions
The challenge of delivering siRNA in effective doses to selected pathologies in vivo is well known. Various nanostructures have been used in preclinical studies as siRNA carriers. Careful nanocarrier design is aimed at achieving: 1) high endosomal escapability, 2) specific cell or tissue recognition / homing, and 3) enhanced stability and release (Table 6). We have shown that a variety of peptide-oligonucleotide nanostructures can be formed with the use of our cationic membrane-active melittin-derived peptides that include natural pH sensitive endosomal release mechanisms. The self-assembling nanostructures both prevent destruction of the siRNA in circulation and in endosomes and allow coordinated release of siRNA and endosomal escape. [127, 128] In the RA model we confirmed entry of the nanostructures into the desired compartment (synovial tissue) and subsequent uptake by macrophages following intravenous administration.[34] Due to their size (~55 nm) the peptide-siRNA nanocomplex penetrates through inflamed and leaky vasculature, much like the endothelial permeability and retention (EPR) effect proposed for nanoparticle localization in tumor. A critical barrier to the successful development of OA treatment is the ineffective delivery of therapeutic agents to the resident chondrocytes in cartilage, which is avascular. We show that our peptide-siRNA nanocomplex deeply penetrates human cartilage, suggesting that our approach promises to overcome the obstacles of drug delivery to the highly inaccessible chondrocytes.[96] One thrust of future work is to engineer molecularly targeted peptide-siRNA nanostructures that could confer even more selectively to certain cell types than the conventional endothelial permeability delivery mechanism. The hope is that a broader range of clinical applications will emerge beyond that of liver targeting with polymeric and lipid nanostructures.
Table 6:
Carrier design features to optimize siRNA delivery
| Nanoparticle function | Carrier design | Reference |
|---|---|---|
| Endosomal escapability | Addition of polyethylenimine (PEI) | [29], [36], [46], [47] |
| Polyarginines (RVG-9R) | [24] | |
| Peptides (p5RHH) | [34], [96] | |
| Stability and release | Redox potential responsiveness (glutathione) | [26], [50] |
| Acidic environment (pH) reponsiveness | [29], [32], [34], [96] | |
| Cell and tissue specific recognition | Folate receptor | [25], [37] |
| Monoclonal antibody to cell surface receptors | [44] | |
| Cell-specific ligands | [45] | |
| Tissue specific ligands | [48], [99] | |
| Aptamers | [66] | |
Acknowledgments
The work cited from Author’s laboratories was partially supported by NIH grants R01AR067491, R01HL073646, R01DK102691, R00 AR064837, P30 AR073752. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. All authors have read the journal’s authorship agreement and approved the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest – All authors have read the journal’s policy on conflicts of interests. SAW has equity in Trasir Therapeutics, Inc. The rest declares no conflicts.
References
- [1].Eggleston AK, Eccleston A, Marte B, Lupp C. Regulatory RNA. Nature. 2012;482:321. [DOI] [PubMed] [Google Scholar]
- [2].Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001. ;411:494–8. [DOI] [PubMed] [Google Scholar]
- [3].Sakurai K, Amarzguioui M, Kim DH, Alluin J, Heale B, Song MS, et al. A role for human Dicer in pre-RISC loading of siRNAs. Nucleic Acids Res. 2011;39:1510–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wood H FDA approves patisiran to treat hereditary transthyretin amyloidosis. Nat Rev Neurol. 2018;14:570. [DOI] [PubMed] [Google Scholar]
- [5].Adams D, Hawkins PN, Polydefkis M. Oligonucleotide Drugs for Transthyretin Amyloidosis. N Engl J Med. 2018;379:2086. [DOI] [PubMed] [Google Scholar]
- [6].Kristen AV, Ajroud-Driss S, Conceicao I, Gorevic P, Kyriakides T, Obici L. Patisiran, an RNAi therapeutic for the treatment of hereditary transthyretin-mediated amyloidosis. Neurodegener Dis Manag. 2019;9:5–23. [DOI] [PubMed] [Google Scholar]
- [7].Jackson AL, Linsley PS. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat Rev Drug Discov. 2010;9:57–67. [DOI] [PubMed] [Google Scholar]
- [8].Samuel-Abraham S, Leonard JN. Staying on message: design principles for controlling nonspecific responses to siRNA. FEBS J. 2010;277:4828–36. [DOI] [PubMed] [Google Scholar]
- [9].Castanotto D, Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457:426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tiemann K, Rossi JJ. RNAi-based therapeutics-current status, challenges and prospects. EMBO Mol Med. 2009;1:142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sioud M RNA interference: mechanisms, technical challenges, and therapeutic opportunities. Methods Mol Biol. 2015;1218:1–15. [DOI] [PubMed] [Google Scholar]
- [12].Tatiparti K, Sau S, Kashaw SK, Iyer AK. siRNA Delivery Strategies: A Comprehensive Review of Recent Developments. Nanomaterials (Basel). 2017;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mclnnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–19. [DOI] [PubMed] [Google Scholar]
- [14].Szekanecz Z, Besenyei T, Paragh G, Koch AE. New insights in synovial angiogenesis. Joint Bone Spine. 2009;77:13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Szekanecz Z, Besenyei T, Szentpetery A, Koch AE. Angiogenesis and vasculogenesis in rheumatoid arthritis. Curr Opin Rheumatol. 2010;22:299–306. [DOI] [PubMed] [Google Scholar]
- [16].Agarwal SK. Biologic agents in rheumatoid arthritis: an update for managed care professionals. J Manag Care Pharm. 2011;17:S14–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Singh JA. Infections With Biologics in Rheumatoid Arthritis and Related Conditions: a Scoping Review of Serious or Hospitalized Infections in Observational Studies. Curr Rheumatol Rep. 2016;18:61. [DOI] [PubMed] [Google Scholar]
- [18].Buch MH, Bingham SJ, Bryer D, Emery P. Long-term infliximab treatment in rheumatoid arthritis: subsequent outcome of initial responders. Rheumatology (Oxford). 2007;46:1153–6. [DOI] [PubMed] [Google Scholar]
- [19].Pham CT. Nanotherapeutic approaches for the treatment of rheumatoid arthritis. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3:607–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Prasad LK, O’Mary H, Cui Z. Nanomedicine delivers promising treatments for rheumatoid arthritis. Nanomedicine (Lond). 2015;10:2063–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang Q, Sun X. Recent advances in nanomedicines for the treatment of rheumatoid arthritis. Biomater Sci. 2017;5:1407–20. [DOI] [PubMed] [Google Scholar]
- [22].Apparailly F, Jorgensen C. siRNA-based therapeutic approaches for rheumatic diseases. Nat Rev Rheumatol. 2013;9:56–62. [DOI] [PubMed] [Google Scholar]
- [23].Ai JW, Zhang S, Ruan QL, Yu YQ, Zhang BY, Liu QH, et al. The Risk of Tuberculosis in Patients with Rheumatoid Arthritis Treated with Tumor Necrosis Factor-alpha Antagonist: A Metaanalysis of Both Randomized Controlled Trials and Registry/Cohort Studies. J Rheumatol 2015;42:2229–37. [DOI] [PubMed] [Google Scholar]
- [24].Ye C, Bhan AK, Deshpande V, Shankar P, Manjunath N. Silencing TNF-alpha in macrophages and dendritic cells for arthritis treatment. Scand J Rheumatol. 2013;42:266–9. [DOI] [PubMed] [Google Scholar]
- [25].Shi Q, Rondon-Cavanzo EP, Dalla Picola IP, Tiera MJ, Zhang X, Dai K, et al. In vivo therapeutic efficacy of TNFalpha silencing by folate-PEG-chitosan-DEAE/siRNA nanoparticles in arthritic mice. Int J Nanomedicine. 2018;13:387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lee SJ, Lee A, Hwang SR, Park JS, Jang J, Huh MS, et al. TNF-alpha gene silencing using polymerized siRNA/thiolated glycol chitosan nanoparticles for rheumatoid arthritis. Mol Ther. 2014;22:397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Thomas M, Klibanov AM. Non-viral gene therapy: polycation-mediated DNA delivery. Appl Microbiol Biotechnol. 2003;62:27–34. [DOI] [PubMed] [Google Scholar]
- [28].Lungwitz U, Breunig M, Blunk T, Gopferich A. Polyethylenimine-based non-viral gene delivery systems. Eur J Pharm Biopharm. 2005;60:247–66. [DOI] [PubMed] [Google Scholar]
- [29].Song J, Chen Y, Jiang S, Yang K, Li X, Zhao X, et al. Efficient and Non-Toxic Biological Response Carrier Delivering TNF-alpha shRNA for Gene Silencing in a Murine Model of Rheumatoid Arthritis. Front Immunol. 2016;7:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhu S, Wonganan P, Lansakara PD, O’Mary HL, Li Y, Cui Z. The effect of the acid-sensitivity of 4-(N)-stearoyl gemcitabine-loaded micelles on drug resistance caused by RRM1 overexpression. Biomaterials. 2013;34:2327–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Aldayel AM, O’Mary HL, Valdes SA, Li X, Thakkar SG, Mustafa BE, et al. Lipid nanoparticles with minimum burst release of TNF-alpha siRNA show strong activity against rheumatoid arthritis unresponsive to methotrexate. J Control Release. 2018;283:280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Aggarwal BB, Sung B. NF-kappaB in cancer: a matter of life and death. Cancer Discov. 2011;1:469–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pasparakis M Regulation of tissue homeostasis by NF-kappaB signalling: implications for inflammatory diseases. Nat Rev Immunol. 2009;9:778–88. [DOI] [PubMed] [Google Scholar]
- [34].Zhou HF, Yan H, Pan H, Hou KK, Akk A, Springer LE, et al. Peptide-siRNA nanocomplexes targeting NF-kappaB subunit p65 suppress nascent experimental arthritis. J Clin Invest. 2014;124:4363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kanazawa T, Endo T, Arima N, Ibaraki H, Takashima Y, Seta Y. Systemic delivery of small interfering RNA targeting nuclear factor kappaB in mice with collagen-induced arthritis using arginine-histidine-cysteine based oligopeptide-modified polymer nanomicelles. Int J Pharm. 2016;515:315–23. [DOI] [PubMed] [Google Scholar]
- [36].Wang Q, Jiang H, Li Y, Chen W, Li H, Peng K, et al. Targeting NF-kB signaling with polymeric hybrid micelles that co-deliver siRNA and dexamethasone for arthritis therapy. Biomaterials. 2017;122:10–22. [DOI] [PubMed] [Google Scholar]
- [37].Duan W, Li H. Combination of NF-kB targeted siRNA and methotrexate in a hybrid nanocarrier towards the effective treatment in rheumatoid arthritis. J Nanobiotechnol. 2018;16:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fan T, Zhong F, Liu R, Chen YH, Wang T, Ruan Q. siRNA-mediated c-Rel knockdown ameliorates collagen-induced arthritis in mice. Int Immunopharmacol. 2018;56:9–17. [DOI] [PubMed] [Google Scholar]
- [39].Maracle CX, Kucharzewska P, Helder B, van der Horst C, Correa de Sampaio P, Noort AR, et al. Targeting non-canonical nuclear factor-kappaB signalling attenuates neovascularization in a novel 3D model of rheumatoid arthritis synovial angiogenesis. Rheumatology (Oxford). 2017;56:294–302. [DOI] [PubMed] [Google Scholar]
- [40].Wang Y, Rollins SA, Madri JA, Matis LA. Anti-C5 monoclonal antibody therapy prevents collagen-induced arthritis and ameliorates established disease. Proc Natl Acad Sci U S A. 1995;92:8955–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nandakumar KS, Jansson A, Xu B, Rydell N, Ahooghalandari P, Hellman L, et al. A recombinant vaccine effectively induces c5a-specific neutralizing antibodies and prevents arthritis. PloS One. 2010;5:e13511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Banda NK, Hyatt S, Antonioli AH, White JT, Glogowska M, Takahashi K, et al. Role of C3a receptors, C5a receptors, and complement protein C6 deficiency in collagen antibody-induced arthritis in mice. J Immunol. 2012;188:1469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Vergunst CE, Gerlag DM, Dinant H, Schulz L, Vinkenoog M, Smeets TJ, et al. Blocking the receptor for C5a in patients with rheumatoid arthritis does not reduce synovial inflammation. Rheumatology (Oxford). 2007;46:1773–8. [DOI] [PubMed] [Google Scholar]
- [44].Mehta G, Scheinman RI, Holers VM, Banda NK. A New Approach for the Treatment of Arthritis in Mice with a Novel Conjugate of an Anti-C5aR1 Antibody and C5 Small Interfering RNA. J Immunol. 2015;194:5446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Banda NK, Desai D, Scheinman RI, Pihl R, Sekine H, Fujita T, et al. Targeting of Liver Mannan-Binding Lectin-Associated Serine Protease-3 with RNA Interference Ameliorates Disease in a Mouse Model of Rheumatoid Arthritis. Immunohorizons. 2018;2:274–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhang T, Bai X, Mao X. Systemic delivery of small interfering RNA targeting the interleukin-2/15 receptor beta chain prevents disease progression in experimental arthritis. PloS One. 2013;8:e78619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Duan J, Dong J, Zhang T, Su Z, Ding J, Zhang Y, et al. Polyethyleneimine-functionalized iron oxide nanoparticles for systemic siRNA delivery in experimental arthritis. Nanomedicine (Lond). 2014;9:789–801. [DOI] [PubMed] [Google Scholar]
- [48].Gao J, Zheng W, Wang L, Song B. A disintegrin and metallproteinase 15 knockout decreases migration of fibroblast-like synoviocytes and inflammation in rheumatoid arthritis. Mol Med Rep. 2015;11:4389–96. [DOI] [PubMed] [Google Scholar]
- [49].Herman S, Fischer A, Presumey J, Hoffmann M, Koenders MI, Escriou V, et al. Inhibition of Inflammation and Bone Erosion by RNA Interference-Mediated Silencing of Heterogeneous Nuclear RNP A2/B1 in Two Experimental Models of Rheumatoid Arthritis. Arthritis Rheumatol. 2015;67:2536–46. [DOI] [PubMed] [Google Scholar]
- [50].Kim MJ, Park JS, Lee SJ, Jang J, Park JS, Back SH, et al. Notch1 targeting siRNA delivery nanoparticles for rheumatoid arthritis therapy. J Control Release. 2015;216:140–8. [DOI] [PubMed] [Google Scholar]
- [51].Tsuchida S, Arai Y, Kishida T, Takahashi KA, Honjo K, Terauchi R, et al. Silencing the expression of connexin 43 decreases inflammation and joint destruction in experimental arthritis. J Orthop Res. 2013;31:525–30. [DOI] [PubMed] [Google Scholar]
- [52].Duan H, Yang P, Fang F, Ding S, Xiao W. CCR5 small interfering RNA ameliorated joint inflammation in rats with adjuvant-induced arthritis. Immunology letters. 2014;162:258–63. [DOI] [PubMed] [Google Scholar]
- [53].Liu S, Kiyoi T, Takemasa E, Maeyama K. Intra-articular lentivirus-mediated gene therapy targeting CRACM1 for the treatment of collagen-induced arthritis. J Pharmacol Sci. 2017;133:130–8. [DOI] [PubMed] [Google Scholar]
- [54].Luo X, Chen Y, Lv G, Zhou Z, Chen J, Mo X, et al. Adenovirus-Mediated Small Interfering RNA Targeting TAK1 Ameliorates Joint Inflammation with Collagen-Induced Arthritis in Mice. Inflammation. 2017;40:894–903. [DOI] [PubMed] [Google Scholar]
- [55].Kanasty RL, Whitehead KA, Vegas AJ, Anderson DG. Action and reaction: the biological response to siRNA and its delivery vehicles. Mol Ther. 2012;20:513–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].McInnes IB, Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet. 2017;389:2328–37. [DOI] [PubMed] [Google Scholar]
- [57].Blair HA, Deeks ED. Abatacept: A Review in Rheumatoid Arthritis. Drugs. 2017;77:1221–33. [DOI] [PubMed] [Google Scholar]
- [58].Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. Type 17 T helper cells-origins, features and possible roles in rheumatic disease. Nat Rev Rheumatol. 2009;5:325–31. [DOI] [PubMed] [Google Scholar]
- [59].Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2:52ra72. [DOI] [PubMed] [Google Scholar]
- [60].Patel DD, Lee DM, Kolbinger F, Antoni C. Effect of IL-17A blockade with secukinumab in autoimmune diseases. Ann Rheum Dis. 2013;72 Suppl 2:ii116–23. [DOI] [PubMed] [Google Scholar]
- [61].Genovese MC, Durez P, Richards HB, Supronik J, Dokoupilova E, Mazurov V, et al. Efficacy and safety of secukinumab in patients with rheumatoid arthritis: a phase II, dose-finding, double-blind, randomised, placebo controlled study. Ann Rheum Dis. 2013;72:863–9. [DOI] [PubMed] [Google Scholar]
- [62].Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lin H, Song P, Zhao Y, Xue LJ, Liu Y, Chu CQ. Targeting Th17 Cells with Small Molecules and Small Interference RNA. Mediators Inflamm. 2015;2015:290657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Song P, Chou YK, Zhang X, Meza-Romero R, Yomogida K, Benedek G, et al. CD4 aptamer-RORgammat shRNA chimera inhibits IL-17 synthesis by human CD4(+) T cells. Biochem Biophys Res Commun. 2014;452:1040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Zhou J, Swiderski P, Li H, Zhang J, Neff CP, Akkina R, et al. Selection, characterization and application of new RNA HIV gp 120 aptamers for facile delivery of Dicer substrate siRNAs into HIV infected cells. Nucleic Acids Res. 2009;37:3094–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Neff CP, Zhou J, Remling L, Kuruvilla J, Zhang J, Li H, et al. An aptamer-siRNA chimera suppresses HIV-1 viral loads and protects from helper CD4(+) T cell decline in humanized mice. Sci Transl Med. 2011;3:66ra6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Valdes AM, Spector TD. Genetic epidemiology of hip and knee osteoarthritis. Nat Rev Rheumatol. 2011;7:23–32. [DOI] [PubMed] [Google Scholar]
- [68].Rai MF, Pham CT. Intra-articular drug delivery systems for joint diseases. Curr Opin Pharmacol. 2018;40:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Miller RE, Block JA, Malfait AM. Nerve growth factor blockade for the management of osteoarthritis pain: what can we learn from clinical trials and preclinical models? Curr Opin Rheumatol. 2017;29:110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Thorlund JB, Turkiewicz A, Prieto-Alhambra D, Englund M. Opioid use in knee or hip osteoarthritis: a region-wide population-based cohort study. Osteoarthritis Cartilage. 2019. doi: 10.1016/j.joca.2019.01.005. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [71].DeMik DE, Bedard NA, Dowdle SB, Burnett Ra, McHugh MA, Callaghan JJ. Are We Still Prescribing Opioids for Osteoarthritis? J Arthroplasty. 2017;32:3578–82 e1. [DOI] [PubMed] [Google Scholar]
- [72].Springer BD, Cahue S, Etkin CD, Lewallen DG, McGrory BJ. Infection burden in total hip and knee arthroplasties: an international registry-based perspective. Arthroplast Today. 2017;3:137–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Thobhani S, Scalercio L, Elliott CE, Nossaman BD, Thomas LC, Yuratich D, et al. Novel Regional Techniques for Total Knee Arthroplasty Promote Reduced Hospital Length of Stay: An Analysis of 106 Patients. Ochsner J. 2017;17:233–8. [PMC free article] [PubMed] [Google Scholar]
- [74].Ramos YF, den Hollander W, Bovee JV, Bomer N, van der Breggen R, Lakenberg N, et al. Genes involved in the osteoarthritis process identified through genome wide expression analysis in articular cartilage; the RAAK study. PloS One. 2014;9:e103056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Sandell LJ. Etiology of osteoarthritis: genetics and synovial joint development. Nat Rev Rheumatol. 2012;8:77–89. [DOI] [PubMed] [Google Scholar]
- [76].Poole AR. Osteoarthritis as a whole joint disease. HSS J. 2012;8:4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Saito T, Tanaka S. Molecular mechanisms underlying osteoarthritis development: Notch and NF-kappaB. Arthritis Res Ther. 2017;19:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Goldring MB, Berenbaum F. Emerging targets in osteoarthritis therapy. Curr Opin Pharmacol. 2015;22:51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Fleming BC, Hulstyn MJ, Oksendahl HL, Fadale PD. Ligament Injury, Reconstruction and Osteoarthritis. Curr Opin Orthop. 2005;16:354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Nordenvall R, Bahmanyar S, Adami J, Mattila VM, Fellander-Tsai L. Cruciate ligament reconstruction and risk of knee osteoarthritis: the association between cruciate ligament injury and post-traumatic osteoarthritis. a population based nationwide study in Sweden, 1987-2009. PloS One. 2014;9:e104681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–69. [DOI] [PubMed] [Google Scholar]
- [82].Roos H, Adalberth T, Dahlberg L, Lohmander LS. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthritis Cartilage. 1995;3:261–7. [DOI] [PubMed] [Google Scholar]
- [83].Plotnikoff R, Karunamuni N, Lytvyak E, Penfold C, Schopflocher D, Imayama I, et al. Osteoarthritis prevalence and modifiable factors: a population study. BMC Public Health. 2015;15:1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Diekman BO, Guilak F. Stem cell-based therapies for osteoarthritis: challenges and opportunities. Curr Opin Rheumatol. 2013;25:119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Chahla J, Piuzzi NS, Mitchell JJ, Dean CS, Pascual-Garrido C, LaPrade RF, et al. Intra-Articular Cellular Therapy for Osteoarthritis and Focal Cartilage Defects of the Knee: A Systematic Review of the Literature and Study Quality Analysis. J Bone Joint Surg Am. 2016;98:1511–21. [DOI] [PubMed] [Google Scholar]
- [86].Kim EJ, Kang KH, Ju JH. CRISPR-Cas9: a promising tool for gene editing on induced pluripotent stem cells. Korean J Intern Med. 2017;32:42–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Brunger JM, Zutshi A, Willard VP, Gersbach CA, Guilak F. Genome Engineering of Stem Cells for Autonomously Regulated, Closed-Loop Delivery of Biologic Drugs. Stem Cell Rep. 2017;8:1202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Zhao L, Huang J, Fan Y, Li J, You T, He S, et al. Exploration of CRISPR/Cas9-based gene editing as therapy for osteoarthritis. Ann Rheum Dis. 2019;78:676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Rai MF, Brophy RH, Sandell LJ. Osteoarthritis following meniscus and ligament injury: insights from translational studies and animal models. Curr Opin Rheumatol. 2019;31:70–9. [DOI] [PubMed] [Google Scholar]
- [90].Lam JK, Chow MY, Zhang Y, Leung SW. siRNA Versus miRNA as Therapeutics for Gene Silencing. Mol Ther Nucleic Acids. 2015;4:e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Sarett SM, Nelson CE, Duvall CL. Technologies for controlled, local delivery of siRNA. J Control Release. 2015;218:94–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Zhang C, Wei X, Chen C, Cao K, Li Y, Jiao Q, et al. Indian hedgehog in synovial fluid is a novel marker for early cartilage lesions in human knee joint. Int J Mol Sci. 2014;15:7250–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Zhou J, Chen Q, Lanske B, Fleming BC, Terek R, Wei X, et al. Disrupting the Indian hedgehog signaling pathway in vivo attenuates surgically induced osteoarthritis progression in Col2a1-CreERT2; Ihhfl/fl mice. Arthritis Res Ther. 2014;16:R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Wang S, Wei X, Sun X, Chen C, Zhou J, Zhang G, et al. A novel therapeutic strategy for cartilage diseases based on lipid nanoparticle-RNAi delivery system. Int J Nanomedicine. 2018;13:617–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Olivotto E, Otero M, Marcu KB, Goldring MB. Pathophysiology of osteoarthritis: canonical NF-kappaB/IKKbeta-dependent and kinase-independent effects of IKKalpha in cartilage degradation and chondrocyte differentiation. RMD Open. 2015;1:e000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Yan H, Duan X, Pan H, Holguin N, Rai MF, Akk A, et al. Suppression of NF-kappaB activity via nanoparticle-based siRNA delivery alters early cartilage responses to injury. Proc Natl Acad Sci U S A. 2016;113:E6199–E208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Gong Y, Li SJ, Liu R, Zhan JF, Tan C, Fang YF, et al. Inhibition of YAP with siRNA prevents cartilage degradation and ameliorates osteoarthritis development. J Mol Med (Berl). 2019;97:103–14. [DOI] [PubMed] [Google Scholar]
- [98].Zhang FJ, Luo W, Lei GH. Role of HIF-1alpha and HIF-2alpha in osteoarthritis. Joint Bone Spine. 2015;82:144–7. [DOI] [PubMed] [Google Scholar]
- [99].Pi Y, Zhang X, Shao Z, Zhao F, Hu X, Ao Y. Intra-articular delivery of anti-Hif-2alpha siRNA by chondrocyte-homing nanoparticles to prevent cartilage degeneration in arthritic mice. Gene Ther. 2015;22:439–48. [DOI] [PubMed] [Google Scholar]
- [100].Yang CY, Chanalaris A, Troeberg L. ADAMTS and ADAM metalloproteinases in osteoarthritis - looking beyond the ‘usual suspects’. Osteoarthritis Cartilage. 2017;25:1000–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Hoshi H, Akagi R, Yamaguchi S, Muramatsu Y, Akatsu Y, Yamamoto Y, et al. Effect of inhibiting MMP13 and ADAMTS5 by intra-articular injection of small interfering RNA in a surgically induced osteoarthritis model of mice. Cell Tissue Res. 2017;368:379–87. [DOI] [PubMed] [Google Scholar]
- [102].Nakagawa R, Akagi R, Yamaguchi S, Enomoto T, Sato Y, Kimura S, et al. Single vs. repeated matrix metalloproteinase-13 knockdown with intra-articular short interfering RNA administration in a murine osteoarthritis model. Connect Tissue Res. 2018:1–9. [DOI] [PubMed] [Google Scholar]
- [103].Akagi R, Sasho T, Saito M, Endo J, Yamaguchi S, Muramatsu Y, et al. Effective knock down of matrix metalloproteinase-13 by an intra-articular injection of small interfering RNA (siRNA) in a murine surgically-induced osteoarthritis model. J Orthop Res. 2014;32:1175–80. [DOI] [PubMed] [Google Scholar]
- [104].Mottaghitalab F, Rastegari A, Farokhi M, Dinarvand R, Hosseinkhani H, Ou KL, et al. Prospects of siRNA applications in regenerative medicine. Int J Pharm. 2017;524:312–29. [DOI] [PubMed] [Google Scholar]
- [105].Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. [DOI] [PubMed] [Google Scholar]
- [106].Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Zhang M, Lygrisse K, Wang J. Role of MicroRNA in Osteoarthritis. J Arthritis. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Hou KK, Pan H, Schlesinger PH, Wickline SA. A role for peptides in overcoming endosomal entrapment in siRNA delivery - A focus on melittin. Biotechnol Adv. 2015;33:931–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Lehto T, Ezzat K, Wood MJA, El Andaloussi S. Peptides for nucleic acid delivery. Adv Drug Deliv Rev. 2016;106:172–82. [DOI] [PubMed] [Google Scholar]
- [110].Gopal V Bioinspired peptides as versatile nucleic acid delivery platforms. J Control Release. 2013;167:323–32. [DOI] [PubMed] [Google Scholar]
- [111].Trabulo S, Cardoso AL, Cardoso AM, Morais CM, Jurado AS, Pedroso de Lima MC. Cell-penetrating peptides as nucleic acid delivery systems: from biophysics to biological applications. Curr Pharm Des. 2013;19:2895–923. [DOI] [PubMed] [Google Scholar]
- [112].Bolhassani A Potential efficacy of cell-penetrating peptides for nucleic acid and drug delivery in cancer. Biochim Biophys Acta. 2011;1816:232–46. [DOI] [PubMed] [Google Scholar]
- [113].Kilk K, Langel U. Cellular delivery of peptide nucleic acid by cell-penetrating peptides. Methods Mol Biol. 2005;298:131–41. [DOI] [PubMed] [Google Scholar]
- [114].Dempsey CE. The actions of melittin on membranes. Biochim Biophys Acta. 1990;1031:143–61. [DOI] [PubMed] [Google Scholar]
- [115].Raghuraman H, Chattopadhyay A. Melittin: a membrane-active peptide with diverse functions. Biosci Rep. 2007;27:189–223. [DOI] [PubMed] [Google Scholar]
- [116].Tosteson MT, Tosteson DC. The sting. Melittin forms channels in lipid bilayers. Biophys J. 1981;36:109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Pan H, Soman NR, Schlesinger PH, Lanza GM, Wickline SA. Cytolytic peptide nanoparticles (‘NanoBees’) for cancer therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3:318–27. [DOI] [PubMed] [Google Scholar]
- [118].Soman NR, Baldwin SL, Hu G, Marsh JN, Lanza GM, Heuser JE, et al. Molecularly targeted nanocarriers deliver the cytolytic peptide melittin specifically to tumor cells in mice, reducing tumor growth. J Clin Invest. 2009;119:2830–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Soman NR, Lanza GM, Heuser JM, Schlesinger PH, Wickline SA. Synthesis and characterization of stable fluorocarbon nanostructures as drug delivery vehicles for cytolytic peptides. Nano Lett. 2008;8:1131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Sessa G, Freer JH, Colacicco G, Weissmann G. Interaction of alytic polypeptide, melittin, with lipid membrane systems. J Biol Chem. 1969;244:3575–82. [PubMed] [Google Scholar]
- [121].DeGrado WF, Musso GF, Lieber M, Kaiser ET, Kezdy FJ. Kinetics and mechanism of hemolysis induced by melittin and by a synthetic melittin analogue. Biophys J. 1982;37:329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Lee SJ, Schlesinger PH, Wickline Sa, Lanza GM, Baker NA. Interaction of melittin peptides with perfluorocarbon nanoemulsion particles. J Phys Chem B. 2011;115:15271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Meyer M, Dohmen C, Philipp A, Kiener D, Maiwald G, Scheu C, et al. Synthesis and biological evaluation of a bioresponsive and endosomolytic siRNA-polymer conjugate. Mol Pharm. 2009;6:752–62. [DOI] [PubMed] [Google Scholar]
- [124].Rozema DB, Lewis DL, Wakefield DH, Wong SC, Klein JJ, Roesch PL, et al. Dynamic PolyConjugates for targeted in vivo delivery of siRNA to hepatocytes. Proc Natl Acad Sci U S A. 2007;104:12982–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Wooddell CI, Rozema DB, Hossbach M, John M, Hamilton HL, Chu Q, et al. Hepatocyte-targeted RNAi therapeutics for the treatment of chronic hepatitis B virus infection. Mol Ther. 2013;21:973–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Chen CP, Kim JS, Steenblock E, Liu D, Rice KG. Gene transfer with poly-melittin peptides. Bioconjug Chem. 2006;17:1057–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Hou KK, Pan H, Lanza GM, Wickline SA. Melittin derived peptides for nanoparticle based siRNA transfection. Biomaterials. 2013;34:3110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Hou KK, Pan H, Ratner L, Schlesinger PH, Wickline SA. Mechanisms of nanoparticle-mediated siRNA transfection by melittin-derived peptides. ACS Nano. 2013;7:8605–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Partlow KC, Lanza GM, Wickline SA. Exploiting lipid raft transport with membrane targeted nanoparticles: a strategy for cytosolic drug delivery. Biomaterials. 2008;29:3367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Pan H, Myerson JW, Ivashyna O, Soman NR, Marsh JN, Hood JL, et al. Lipid membrane editing with peptide cargo linkers in cells and synthetic nanostructures. FASEB J. 2010;24:2928–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Pan H, Marsh JN, Christenson ET, Soman NR, Ivashyna O, Lanza GM, et al. Postformulation peptide drug loading of nanostructures. Methods Enzymol. 2012;508:17–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Chou ST, Hom K, Zhang D, Leng Q, Tricoli LJ, Hustedt JM, et al. Enhanced silencing and stabilization of siRNA polyplexes by histidine-mediated hydrogen bonds. Biomaterials. 2014;35:846–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Pan H, Palekar RU, Hou KK, Bacon J, Yan H, Springer LE, et al. Anti-JNK2 peptide-siRNA nanostructures improve plaque endothelium and reduce thrombotic risk in atherosclerotic mice. Int J Nanomedicine. 2018;13:5187–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Mills K, Quinn J, Roach ST, Palisoul M, Nguyen M, Guo L, et al. p5RHH nanoparticle-mediated delivery of AXL siRNA inhibits metastasis of ovarian and uterine cancer cells in mouse xenografts. Sci Rep. 2019; 9:4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Chen LX, Lin L, Wang HJ, Wei XL, Fu X, Zhang JY, et al. Suppression of early experimental osteoarthritis by in vivo delivery of the adenoviral vector-mediated NF-kappaBp65-specific siRNA. Osteoarthritis Cartilage. 2008;16:174–84. [DOI] [PubMed] [Google Scholar]
- [136].Shi J, Chi S, Xue J, Yang J, Li F, Liu X. Emerging Role and Therapeutic Implication of Wnt Signaling Pathways in Autoimmune Diseases. J Immunol Res. 2016;2016:9392132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Snelling SJ, Davidson RK, Swingler TE, Le LT, Barter MJ, Culley KL, et al. Dickkopf-3 is upregulated in osteoarthritis and has a chondroprotective role. Osteoarthritis Cartilage. 2016;24:883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Jones SW, Brockbank SM, Clements KM, Le Good N, Campbell D, Read SJ, et al. Mitogen-activated protein kinase-activated protein kinase 2 (MK2) modulates key biological pathways associated with OA disease pathology. Osteoarthritis Cartilage. 2009;17:124–31. [DOI] [PubMed] [Google Scholar]
- [139].Takebe K, Nishiyama T, Hayashi S, Hashimoto S, Fujishiro T, Kanzaki N, et al. Regulation of p38 MAPK phosphorylation inhibits chondrocyte apoptosis in response to heat stress or mechanical stress. Int J Mol Med. 2011;27:329–35. [DOI] [PubMed] [Google Scholar]
- [140].Tang X, Muhammad H, McLean C, Miotla-Zarebska J, Fleming J, Didangelos A, et al. Connective tissue growth factor contributes to joint homeostasis and osteoarthritis severity by controlling the matrix sequestration and activation of latent TGFbeta. Ann Rheum Dis. 2018;77:1372–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Rios CN, Skoracki RJ, Mathur AB. GNAS1 and PHD2 short-interfering RNA support bone regeneration in vitro and in an in vivo sheep model. Clin Orthop Relat Res. 2012;470:2541–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Basha G, Ordobadi M, Scott WR, Cottle A, Liu Y, Wang H, et al. Lipid Nanoparticle Delivery of siRNA to Osteocytes Leads to Effective Silencing of SOST and Inhibition of Sclerostin In Vivo. Mol Ther Nucleic Acids. 2016;5:e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Si HB, Zeng Y, Liu SY, Zhou ZK, Chen YN, Cheng JQ, et al. Intra-articular injection of microRNA-140 (miRNA-140) alleviates osteoarthritis (OA) progression by modulating extracellular matrix (ECM) homeostasis in rats. Osteoarthritis Cartilage. 2017;25:1698–707. [DOI] [PubMed] [Google Scholar]
- [144].Nakamura A, Rampersaud YR, Nakamura S, Sharma A, Zeng F, Rossomacha E, et al. microRNA-181a-5p antisense oligonucleotides attenuate osteoarthritis in facet and knee joints. Ann Rheum Dis. 2019;78:111–21. [DOI] [PubMed] [Google Scholar]
- [145].Ding Y, Wang L, Zhao Q, Wu Z, Kong L. MicroRNA93 inhibits chondrocyte apoptosis and inflammation in osteoarthritis by targeting the TLR4/NFkappaB signaling pathway. Int J Mol Med. 2019;43:779–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Ko JY, Lee MS, Lian WS, Weng WT, Sun YC, Chen YS, et al. MicroRNA-29a Counteracts Synovitis in Knee Osteoarthritis Pathogenesis by Targeting VEGF. Sci Rep. 2017;7:3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Wang H, Zhang H, Sun Q, Wang Y, Yang J, Yang J, et al. Intra-articular Delivery of Antago-miR-483-5p Inhibits Osteoarthritis by Modulating Matrilin 3 and Tissue Inhibitor of Metalloproteinase 2. Mol Ther. 2017;25:715–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Wang J, Chen L, Jin S, Lin J, Zheng H, Zhang H, et al. MiR-98 promotes chondrocyte apoptosis by decreasing Bcl-2 expression in a rat model of osteoarthritis. Acta Biochim Biophys Sin (Shanghai). 2016;48:923–9. [DOI] [PubMed] [Google Scholar]
- [149].Song J, Jin EH, Kim D, Kim KY, Chun CH, Jin EJ. MicroRNA-222 regulates MMP-13 via targeting HDAC-4 during osteoarthritis pathogenesis. BBA Clin. 2015;3:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]


