Abstract
Trichloroethene (TCE) exposure is associated with the development of various autoimmune diseases (ADs), including autoimmune hepatitis (AIH) and systemic lupus erythematosus (SLE), potentially through the generation of excessive reactive oxygen and nitrogen species (RONS; oxidative stress). However, the mechanisms by which oxidative stress contributes to these TCE-mediated ADs are not fully understood, and are the focus of current investigation. Female MRL+/+ mice were treated with TCE along with or without antioxidant N-acetylcysteine (NAC) for 6 weeks (TCE, 10 mmol/kg, i.p., every 4th day; NAC, 250 mg/kg/day via drinking water). TCE-treated mice had elevated antinuclear antibodies (ANA) and 4-hydroxynonenal (HNE)-specific circulating immune complexes, suggesting the association of TCE-induced oxidative stress with autoimmune response. In addition, TCE exposure led to prominent lobular inflammation with sinusoid dilation, increased sinusoidal cellularity and increased staining for proliferating cell nuclear antigen (PCNA), confirming inflammatory and hepatocellular cell proliferation. Importantly, TCE exposure resulted in the activation of hepatic inflammasome (NLRP3 and caspase-1) and up-regulation of pro-inflammatory cytokine IL-1β, and these changes were attenuated by NAC supplementation. TCE treatment also led to dysregulation of hepatic immune response as evident from markedly increased hepatic lymphocyte infiltration (especially B cells) and imbalance between Tregs (decreased) and Th17 cells (increased). Interestingly, TCE-mediated dysregulation of various hepatic and splenic immune cells was also effectively attenuated by NAC. Taken together, our findings provide evidence for TCE-mediated inflammasome activation, infiltration of various immune cells, and skewed balance of Treg and Th17 cells in the liver. The attenuation of TCE-mediated hepatic inflammasome activation and immune responses by NAC further supports a critical role of oxidative stress in TCE-mediated inflammation and autoimmunity. These novel findings could help in designing therapeutic strategies for such ADs.
Keywords: TCE, oxidative stress, inflammasome, inflammation, autoimmunity
1. Introduction
Trichloroethene (trichloroethylene, TCE) is a chlorinated organic solvent which is widely used as cleaning and degreasing agent, and is considered a ubiquitous environmental pollutant [1, 2]. TCE exposure in humans is associated with the pathogenesis of autoimmune diseases (ADs), including systemic lupus erythematosus (SLE) and autoimmune hepatitis (AIH) [3–5]. Previous studies have shown that TCE and its oxidative metabolites can elicit autoimmune responses in experimental animals [1, 6–8]. Among factors contributing to TCE-mediated ADs, oxidative stress (OS) is considered as one of the major effector mechanisms. Reactive oxygen species (ROS)-mediated protein modifications (carboxylation and nitration) and generation of lipid peroxidation-derived aldehydes [i.e., 4-hydroxynonenal (HNE) and malondialdehyde (MDA)] could further cause endogenous macromolecule modifications, leading to the formation of neoantigens, and perturbation in immune responses, ultimately resulting in the breakdown of self-tolerance [9–12]. Although TCE-mediated autoimmunity is attributed to OS and/or OS-mediated protein modifications, the cellular, inflammatory and molecular mechanisms by which TCE mediates autoimmune responses, especially the mechanisms by which OS contributes to ADs is not well known.
Inflammasomes are intracellular multiprotein complexes. Upon sensing danger signals by NOD-like receptors (NLRs), NLRP3 recruits pro-caspase1 and adapter ASC (apoptosis-associated speck-like protein containing CARD), resulting in the activation of effector protease caspase-1 [13], which triggers the maturation and release of interleukin (IL)-1β and IL-18 [14]. NLRP3 inflammasome activation has been shown to play an important role in the pathogenesis of IL-1β-mediated autoimmune hepatitis through ROS production [15]. Furthermore, the proinflammatory cytokine IL-1β has been reported to mediate T cell differentiation into Th17 cells, promoting inflammation and autoimmunity [16, 17]. NLRP3 inflammasome is significantly activated by immune complex deposition in organs, and is critical factor for the development of SLE [18]. However, status of NLRP3 inflammasome activation in TCE-mediated autoimmunity is not known, but could represent as an important link in the understanding of OS-mediated immune responses.
Our previous studies have shown that TCE exposure is also associated with increased formation of reactive nitrogen species (RNS; nitrosative stress) and iNOS induction in both livers and peripheral blood [19]. Furthermore, iNOS-null MRL+/+ mice were relatively resistant to TCE-mediated autoimmune responses, evidenced from reduced autoantibodies [20]. Pharmacological inhibition of iNOS has also been shown to prevent lupus-like renal disease in MRL/lpr mice by down-regulating the inflammatory responses [21]. However, the molecular mechanism by which iNOS regulates TCE-mediated autoimmunity remains unclear, and is also a subject of investigation here, specifically to establish its role in TCE-induced inflammasome activation.
Dysregulation of immune tolerance is critical in driving autoimmunity by modulating both innate and adaptive immune cells [22]. Natural killer (NK) cells are innate immune cells which can promote autoimmunity by increasing inflammatory cytokine production and activating antibody-secreting B cells [23]. Dendritic cells (DCs), the professional antigen-presenting cells (APCs), promote the Th1 and Th17 differentiation while suppress regulatory T Cells (Tregs), leading to autoimmunity [24–26]. Both CD4+ and cytotoxic CD8+ T cells can functionally contribute to SLE disease activity through cytokine production [27, 28]. In previous studies, immune responses to TCE exposure were extensively focused on the functional changes in CD4+ T cells in lymphoid organs [29, 30]. However, TCE-mediated hepatic immune responses, especially the contribution of innate and adaptive immune cells, and the role of OS in the initiation of immune dysregulation are currently not known.
Even though TCE-mediated autoimmunity is attributed to OS and/or OS-mediated protein modifications [10], it remains to be established if TCE activates inflammasome and contributes to hepatic immune cell dysregulation via OS-mediated mechanisms. Therefore, using MRL+/+ mice, this study was primarily focused on establishing (1) the potential of TCE in causing inflammasome activation, (2) hepatic immune dysregulation, especially imbalance between Tregs and Th17 cells, and (3) if antioxidant N-acetylcysteine (NAC) supplementation provides protection against inflammasome activation and restores immune homeostasis by boosting Treg expansion.
2. Materials and methods
2.1. Animals and treatments
Five-week old female MRL+/+ mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were maintained under specific pathogen-free facility and acclimatized for one week prior to any treatment. All experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of the University of Texas Medical Branch. Mice were randomly divided into four groups (n=6 per group), and designated as control (CON), trichloroethene (TCE), NAC or TCE + NAC groups. TCE (10 mmol/kg) in corn oil was administered intraperitoneally every 4th day, whereas NAC (250 mg/kg/day) was given through drinking water [31–33]. The control mice received equal volume of corn oil only. MRL+/+ mice were used in this study because these mice spontaneously develop various autoantibodies after 9 months and SLE disease late in the second year of life [3, 30]. This slow development of the disease allows evaluation of specific effects of TCE or other agents on inducing/exacerbating the disease in relatively young animals. Furthermore, chronic exposure to TCE in these mice also leads to induction of AIH [5, 34]. Thus, MRL+/+ mice are excellent choice to study both AIH and SLE induction in response to toxicants. Female mice were chosen for this study due to higher susceptibility and prevalence of ADs in females [35, 36]. Also, the choice of dose and duration of TCE and/or NAC exposure was based on earlier studies [3, 19, 32, 33, 35, 37]. After 6 weeks of various treatments, all the animals were euthanized and major organs were weighed, frozen in liquid nitrogen, and stored at −80 °C for further analyses. Sera obtained from blood samples were stored in small aliquots at −80 °C until further analysis.
2.2. Isolation of lymphocytes from liver tissues
Intrahepatic lymphocytes (IHLs) were isolated as we described earlier [38]. Briefly, the liver was perfused with PBS, minced and digested with RPMI 1640 containing 0.05% collagenase IV (Roche, Indianapolis, IN) at 37 °C for 30 min. After digestion, cell suspensions were passed through 70 μm cell strainers, followed by a centrifugation over a 30/70% discontinuous Percoll density gradient (Sigma, St. Louis, MO) at 400 g room temperature (RT) for 30 min. The cells were collected from the interphase, washed, and resuspended in complete RPMI 1640 containing 10% FBS. The total number of IHLs per liver was counted. The relative percentages of immune cell populations were analyzed by flow cytometry, and the absolute numbers of these lymphocyte subpopulations per liver were calculated according to their percentages and the total IHL numbers in each liver.
2.3. Flow cytometry
For surface staining, cells were first incubated with FcγR blocker (CD16/32), followed by fluorochrome-labeled antibodies (Abs). For intracellular staining, cells were stimulated by phorbol myristate acetate (50 ng/ml) and ionomycin (750 ng/ml) for 5 hrs. After incubation, cells were stained for surface markers first, then fixed by using Foxp3/transcription factor staining set followed by intracellular staining. The specific antibodies and their corresponding isotype controls were purchased from Biolegend (San Diego, CA) and eBioscience (Waltham, MA). The following Abs were used in combinations: PE-Cy7 anti-mouse CD3, Pacific Blue anti-mouse CD4, APC-Cy7 anti-mouse CD8, PE anti-mouse CD44, FITC anti-mouse CD62L, APC anti-mouse CD11b, APC-Cy7 anti-mouse CD11c, AF700 anti-mouse CD19, Percp-cy5.5 anti-mouse CD45R/B220, APC anti-mouse IL-17 Abs, and PE anti-mouse Foxp3 (BD Pharmingen, San Jose, CA). Flow cytometric experiments were performed using an LSRII Fortessa (Becton Dickinson, San Jose, CA), and analyzed by using FlowJo software 10.0 (TreeStar, Ashland, OR).
2.4. Determination of antinuclear antibodies (ANA) and HNE-protein adduct-specific circulating immune complexes (CICs) in the sera
The ANA levels in the sera were determined by mouse antinuclear antibody ELISA kit according to the manufacturer’s instructions (Alpha Diagnostic Int’l, San Antonio, TX). Lipid peroxidation product HNE-specific immune complexes in the sera were analyzed according to our method published earlier [11]. Briefly, 96-well plates were pre-coated with rabbit anti-HNE-protein adduct antibodies overnight (at 4°C). Diluted serum samples were incubated at RT for 2 hrs. Rabbit anti-mouse IgG-HRP was added and incubated at RT for 1 hr. After TMB substrate and stop solution, OD was read at 450 nm on a Biotek Microplate spectrophotometer (Winooski, VT).
2.5. Quantitative reverse transcriptase PCR (qRT-PCR) analyses
Total RNA was extracted from liver tissues using Trizol Reagent (Sigma, St. Louis, MO) and treated with DNase I (Qiagen, Hilden, Germany). cDNA was synthesized with the iScript reverse transcription supermix (Bio-Rad, Hercules, CA). qRT-PCR was performed using iTaq universal SYBR green supermix kit (Bio-Rad, Hercules, CA) on a Bio-Rad CFX96 real time PCR machine. The mRNA expression of selected genes related to inflammasome activation was analyzed. The primer sequences for the genes analyzed are listed below:
NLRP1: F5’AGTAATCTGGAGGGGTTGGAC3’, R5’GTTGGCAGCCAGGGTATATCA3’; NLRP3: F5’ATGCTGCTTCGACATCTCCT3’,R5’AACCAATGCGAGATCCTGAC3’; ASC: F5’CTTGTCAGGGGATGAACTCAAAA3’, R5’GCCATACGACTCCAGATAGTAGC3’; Caspase-1: F 5’AGATGGCACATTTCCAGGAC3’, R 5’GATCCTCCAGCAGCAACTTC3’; IL-1β: F 5’CAGGCAGGCAGTATCACTCA3’, R 5’AGGCCACAGGTATTTTGTCG3’; IL-18: F5’ GACTCTTGCGTCAACTTCAAGG3’, R 5’CAGGCTGTCTTTTGTCAACGA3’; Mouse glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the housekeeping gene.
2.6. Western blot analysis
Total liver protein was homogenized in RIPA buffer including 1% protease inhibitor cocktail (Sigma-Aldrich) and protein concentration in the lysates was determined by Bio-Rad Protein Assay method (Bio-Rad). Twenty μg of protein per sample was loaded onto a 12% Novex Tris-Glycine Gel and subsequently transferred to a PVDF membrane. The membrane was blotted with primary antibodies at 4°C overnight. Antibody detection was accomplished using horseradish peroxidase conjugated secondary antibodies and visualized with ECL [39].
2.7. Histological examination and immunohistochemistry
Liver tissue specimens were fixed in 10% buffered formalin, dehydrated and paraffin embedded. Paraffin sections (5 μM thick) were stained with hematoxylin-eosin [3], and then blindly analyzed to evaluate the cellular and structural changes in the liver. For immunohistochemical staining, sections were de-paraffinized, rehydrated and subjected to antigen retrieval. Anti-PCNA antibody (1:5000 dilution, Cell Signaling Technology, Danvers, MA) was added to the slides and incubated at 4 °C overnight. The slides were washed and incubated with biotinylated anti-rabbit Ig followed by avidin-conjugated HRP as detection enzyme. Substrate (DAB) was added to the slides to visualize the PCNA intensity. Nuclear counter-staining was done with hematoxylin.
2.8. Statistical analysis
Statistical analysis was performed using GraphPad Prism software 7.0 (GraphPad, La Jolla, CA). One-way analysis of variance (ANOVA) followed by Tukey-Kramer multiple comparison test was done to determine statistical significance between any two groups. The p values <0.05 were considered to be statistically significant. * p < 0.05; ** p < 0.01.
3. Results
3.1. TCE exposure induced autoimmune response and oxidative stress
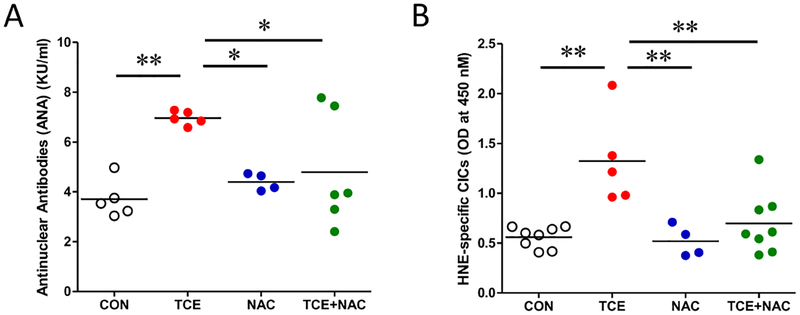
Using female MRL+/+ mice, we have established the potential of TCE in inducing an autoimmune response [3, 20]. To further evaluate and verify the potential of TCE in inducing an autoimmune response and oxidative stress, we determined the autoimmune response and oxidative stress marker in MRL+/+ mice exposed to TCE with or without antioxidant (NAC) supplementation. Consistent with our previous studies [31–33], TCE exposure in MRL+/+ mice resulted in significantly increased ANA level (Fig. 1A). We and others have shown that lipid peroxidation-derived reactive aldehydes and their protein adducts (i.e., HNE- and MDA-protein adducts) are important mediators of autoimmune responses leading to SLE [9, 10, 40]. Here, for the first time, we report significantly higher levels of HNE-specific CICs in the serum following TCE exposure (Fig. 1B). Increased formation of these immune complexes and their deposition in the organs could trigger inflammatory response and thus, could contribute to disease pathogenesis [41]. More importantly, the levels of both ANA and HNE-specific CICs were effectively attenuated by NAC supplementation (Fig. 1A and B). Together, these data suggest that TCE-mediated autoimmunity was associated with oxidative stress and antioxidant dramatically attenuated this autoimmune response.
Fig. 1. TCE exposure induced autoimmune response and oxidative stress in MRL+/+ mice, which were attenuated by NAC supplementation.
Mice were treated with TCE w/wo NAC supplementation, and designated as CON, TCE, NAC or TCE+NAC groups. Using sera from these mice, we determined: (A) Antinuclear antibodies, (B) HNE-specific CICs. Each symbol represents an individual mouse, and horizontal lines indicate the mean. *p<0.05; **p<0.01.
3.2. TCE induced inflammasome activation via NLRP3/Caspase-1/IL-1β pathway
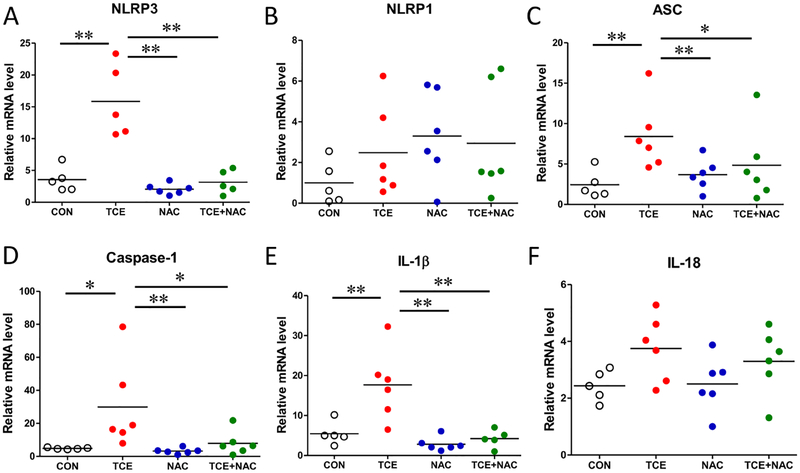
Since inflammasome activation is linked to acute and chronic liver injury [14], we examined the potential of TCE in inducing inflammasome activation and the contribution of oxidative stress in this process. We measured the mRNA expression of several inflammasome complex proteins and other related factors (i.e. NLRP1, NLRP3, ASC, and caspase-1) and downstream cytokines (IL-1β and IL-18) in the liver tissue. As apparent from Fig. 2, TCE exposure led to significantly increased mRNA levels of NLRP3, ASC and caspase-1. However, TCE exposure did not cause any noticeable change in the mRNA level of NLRP1. The pro-inflammatory cytokine IL-1β level was significantly higher in the TCE-exposed livers compared with those in the livers of control mice, but no significant difference was observed in IL-18 levels between the two groups. Importantly, NAC supplementation remarkably inhibited the TCE-induced inflammasome activation, evidenced by significantly reduced expression of inflammasome NLRP3 and IL-1β in the liver tissues of mice treated with TCE+NAC (Fig. 2).
Fig. 2. Up-regulation of inflammasome-related genes in the livers of TCE-exposed mice.
Total RNA was extracted from liver tissues and relative mRNA expressions of NLRP3 (A), NLRP1 (B), ASC (C), caspase-1 (D), IL-1β (E) and IL-18 (F) were measured by RT-PCR. Each symbol represents an individual mouse, and horizontal lines indicate the mean. *p<0.05; **p<0.01.
3.3. TCE-induced inflammasome activation is mediated via oxidative stress and iNOS
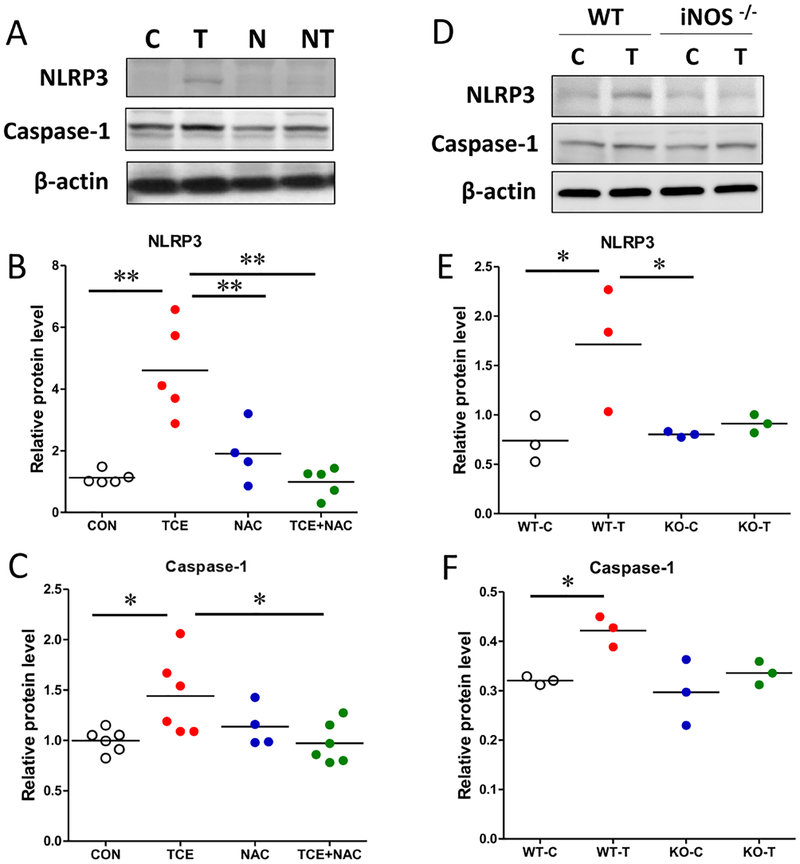
Excessive redox activation and/or oxidative stress are known as NLRP3 inflammasome activating triggers [42]. Since TCE is known to cause oxidative stress and inflammatory response [33, 35], it was of interest to evaluate the potential of antioxidant NAC on TCE-mediated inflammasome response. TCE-mediated inflammasome activation in the liver (increased NLRP3 and caspase-1) was markedly attenuated in mice also given NAC (Fig. 3 A–C). To provide further evidence that OS/iNOS is pivotal for TCE-induced inflammasome activation, we determined inflammasome NLRP3 and caspase-1 levels in the liver samples of wild-type (WT) and iNOS−/− MRL+/+ mice treated with or without TCE for 6 weeks previously [19]. Consistent with our earlier presented results, we observed significantly increased inflammasome activation in TCE-exposed WT mice as indicated by elevated NLRP3 and caspase-1 levels. Expectedly, there was no significant difference in the inflammasome activation between control and TCE-treated iNOS−/− mice (Fig. 3 D–F), further supporting the contribution of OS/iNOS in this process.
Fig. 3. TCE-induced inflammasome activation is mediated via oxidative stress/iNOS.
Protein lysates were subjected to SDS-PAGE and blotted with indicated antibodies. (A) Representative Western blot for NLRP3 and caspase-1 of liver tissues from CON, TCE, NAC or TCE+NAC treated mice. (B, C) Protein band density of caspase-1 and NLRP3 was quantified and normalized to a respective loading control protein (β-actin). (D) Representative Western blot of liver tissues from WT and iNOS−/− MRL+/+ mice exposed to TCE, designated as WT-C, WT-T, KO-C and KO-T. (E, F) Protein band density of caspase-1 and NLRP3 was digitally quantified. Each symbol represents an individual mouse, and horizontal lines indicate the mean. *p<0.05; **p<0.01.
3.4. TCE exposure induced B cell activation in the spleen and liver
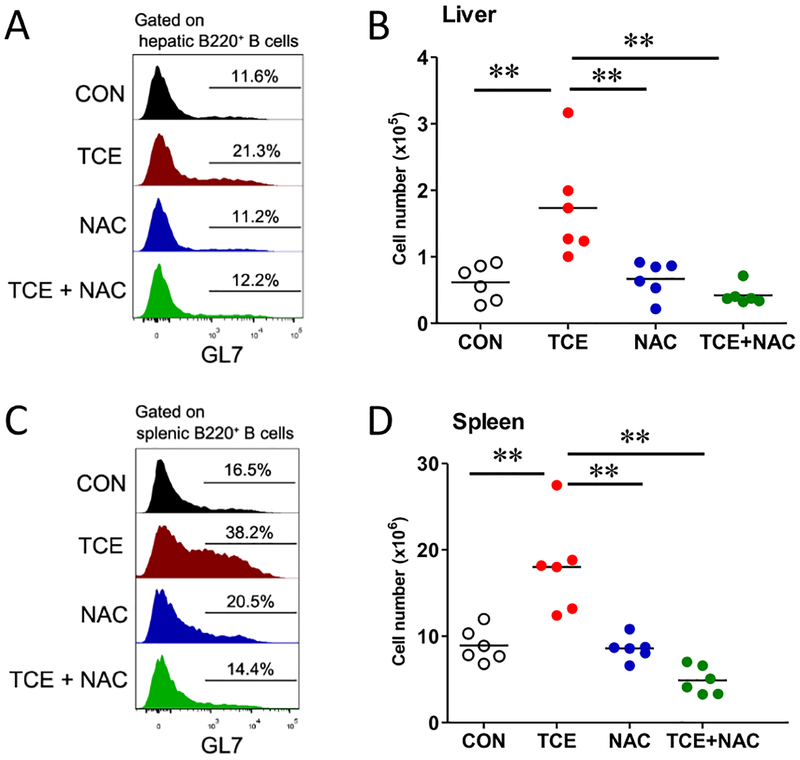
Autoreactive B cell activation and differentiation into plasma cells contribute to the pathogenesis of SLE, and B cell maturation and differentiation mainly occur in the germinal center [43–45]. In this study, TCE exposure significantly induced B cell activation with increased GL7+B220+ B cells in both the spleen and liver (Fig. 4), and this effect was also effectively attenuated by NAC supplementation (Fig. 4). This result is also consistent with our observation showing that NAC supplementation inhibited TCE-induced ANA formation (Fig. 1). The current data suggest that B cell activation plays an important role in TCE-mediated autoimmune response, and more importantly, oxidative stress is a key mediator of B cell activation since NAC effectively blocked TCE-induced B cell activation.
Fig. 4. TCE exposure led to B cell activation in the liver and spleen, which was attenuated by NAC treatment.
Flow cytometric analysis of GL7+B220+ B cells from liver and spleen of TCE-exposed mice. Histogram of GL7 expression on B cells from liver (A) and spleen (C). Cell counts of the indicated cell populations in liver (B) and spleen (D). Each symbol represents an individual mouse, and horizontal lines indicate the mean. *p<0.05; **p<0.01.
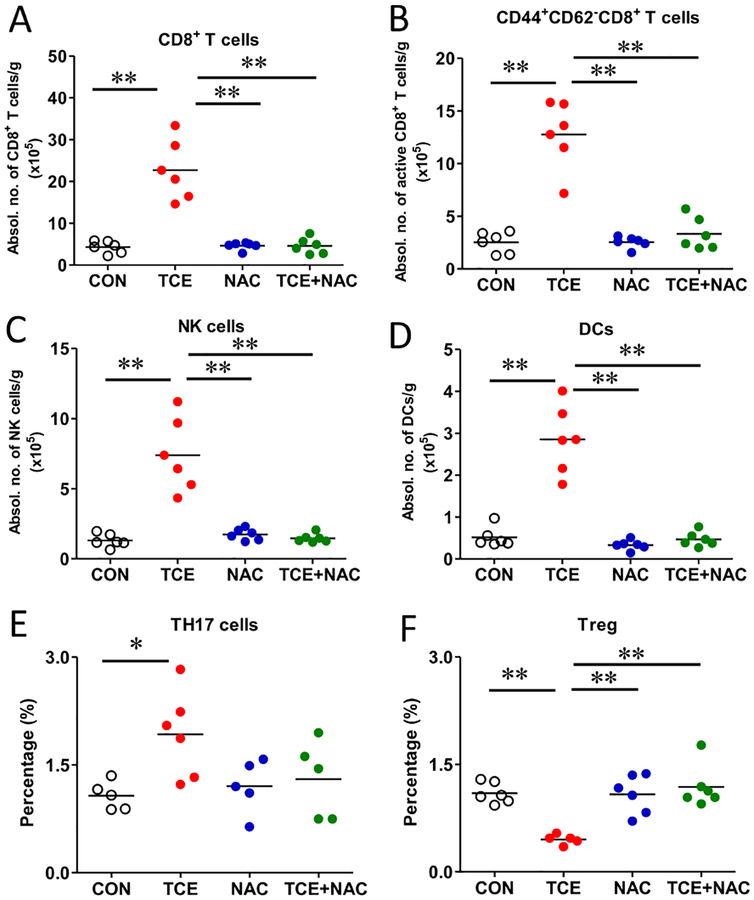
3.5. TCE induced hepatic immune dysregulation which was ameliorated by NAC
SLE is a multifactorial disease characterized with dysregulation of T cells, B cells and myeloid lineage cells [46, 47]. To determine the potential role of different immune cells in TCE-mediated autoimmune responses, especially their early responses leading to pathogenesis of AIH, we first analyzed the number and activation of hepatic T cells by flow cytometry. Our results revealed that the population and absolute number of CD8+ T cells and CD44+CD62L− CD8 effector T cells were significantly increased followed TCE exposure (Fig. 5 A and B). On the other hand, there were no significant changes in the population of CD4+ T cells or CD44+CD62L− CD4 effector T cells (data not shown). To further address whether hepatic Th17 cells and Tregs are involved in the pathogenesis of TCE-mediated autoimmunity, we determined the expression of IL-17 as a signature cytokine of Th17 and Foxp3 as a nuclear marker for Treg cells [48]. As apparent from Fig. 5, the frequency of Th17 cells was increased, whereas the proportion of Treg cells was decreased in the TCE-treated group, providing evidence that TCE exposure skewed the balance of Treg and Th17 cells in the liver. Moreover, the percentage of IL-17 from γδ T cells did not show any appreciable change (data not included), suggesting that Th17 cells are the major source of TCE-induced IL-17 production.
Fig. 5. TCE-mediated hepatic immune dysregulation and its amelioration by NAC.
Intrahepatic lymphocytes from liver were isolated and analyzed by flow cytometry. Hepatic cell counts of CD8+ T cells (A); activated CD44+CD62L−CD8+ T cells (B); NK cells (C); CD11b+ CD11c+ DCs (D) and frequencies of Th17 and Treg in the liver (E, F). Each symbol represents an individual mouse, and horizontal lines indicate the mean. *p<0.05; **p<0.01.
The specific role of innate immune cells in the pathogenesis of TCE-mediated liver immune dysregulation is not known. DCs are the major antigen-presenting cells, linking innate and adaptive immune responses in the liver [49]. We observed significantly increased number of CD11b+CD11c+ cells after TCE exposure (Fig. 5D), suggesting that TCE, its metabolites and their protein adducts may promote DC antigen presenting activity. NK cells are the main effector innate immune cells, and their activation leads to cytotoxic cytokine production and cell death in liver diseases [50, 51]. Our data showed that the absolute number of NK cells was significantly increased in TCE group, and NAC supplementation potently inhibited TCE-induced NK cell infiltration in the liver (Fig. 5C). Collectively, our results demonstrate that TCE exposure leads to hepatic immune cell expansion and activation, and NAC ameliorated immune dysregulation, most likely by inhibiting oxidative stress and activation of NLRP3 inflammasome.
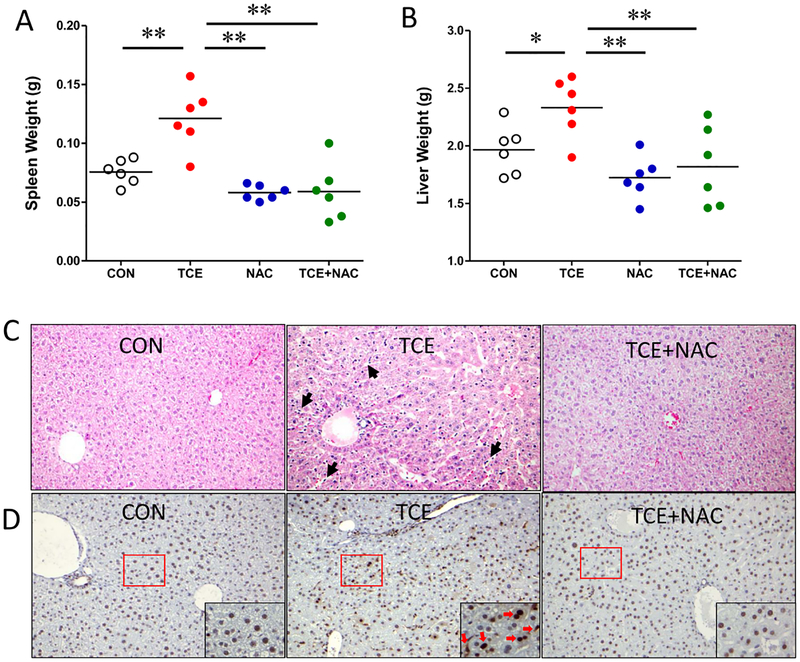
3.6. Attenuation of TCE-induced hepatic morphological changes by NAC
Chronic exposure to TCE is associated with AIH in MRL+/+ mice [5, 34], which is also evident from our observation of increased hepatic immune cell infiltration and their activation in this study (Fig. 5). In addition, TCE exposure led to significantly enlarged spleen and liver (Fig. 6A–B). Accordingly, histological features after TCE exposure w/wo NAC treatment were evaluated. Liver sections of TCE-exposed mice showed prominent lobular inflammation with sinusoidal dilatation and increased cellularity (Fig. 6C). Furthermore, immunohistochemical staining of PCNA was increased, indicative of proliferation of immune cells and hepatocytes following TCE exposure (Fig. 6D). Remarkably, NAC supplementation alleviated TCE-induced pathological changes (Fig. 6).
Fig. 6. Restoration of TCE-induced hepatic histological changes by NAC.
(A-B) Spleen and liver weights. Each symbol represents an individual mouse, and horizontal lines indicate the mean. (C) Representative liver sections with H&E. Arrow indicates the sinusoidal dilation and hyper-cellularity in TCE treated group. (D) Immunohistochemical staining for PCNA highlights cellular proliferation.
4. Discussion
TCE is a ubiquitous environmental toxicant that is associated with several ADs, including SLE and AIH [3, 29]. These devastating diseases are characterized by excessive production of autoantibodies against self-antigens, immune dysregulation, increased immune complex formation and ultimately tissue damages [47]. Previous studies from our laboratory established the potential of TCE to induce an autoimmune response in experimental animals [3]. Studies form our laboratory have also established a causative role of oxidative stress in TCE-mediated autoimmunity [10, 31]. Furthermore, mechanistic studies have provided evidence for the role of TCE metabolite dichloacetyl chloride [52], TCE metabolite-adducted proteins [53], and lipid peroxidation-derived reactive aldehydes (HNE and MDA) and their protein conjugates in the induction of autoimmunity/SLE [9, 11]. Despite extensive evaluation of TCE-induced autoimmunity, early mechanisms contributing to the disease pathogenesis of either AIH or SLE remain elusive. Using female MRL+/+ mice, here we provide evidence that TCE exposure causes increased activation of inflammasome and caspase-1, dysregulation of hepatic immune response and induction of autoimmunity. Furthermore, NAC supplementation attenuated these responses, suggesting that OS-induced inflammasome activation is essential for immune dysregulation and TCE-mediated autoimmune responses.
Inflammasomes are multiprotein complex that triggers caspase-1-mediated maturation of IL-1β and IL-18 cytokines [13]. Previously, it has been established that NLRP3 inflammasome activation and IL-1β secretion from macrophages contribute to ConA-induced AIH, and genetic or pharmacological inhibition of inflammasome activation alleviates disease activity [15]. This led us to define the role of NLRP3 in TCE-mediated pathogenic events that could potentially contribute to AIH. Our current data provides evidence that TCE exposure leads to inflammasome activation as evident from increased protein and mRNA expression of NLRP3 and caspase-1 in the liver. Additionally, TCE-mediated inflammasome activation also led to increased production of proinflammatory cytokine IL-1β. IL-1β induces the chemotaxis of leukocytes, and its release is a critical step in inflammation via induction of other inflammatory cytokines [54]. Also, IL-1β can act on lymphocytes by upregulating IL-2 receptor expression, prolonging survival of T cells and enhancing antibody production via B cell proliferation [55]. Interestingly, inflammasome is activated by mitochondrial ROS, which is regulated by depletion of glutathione [56]. Previous studies demonstrated that TCE exposure leads to significant reduction in glutathione level, and NAC, a precursor of GSH, attenuated TCE-induced glutathione depletion [33]. In the present study, NAC alleviated TCE-induced inflammasome activation and hepatic morphological changes, suggesting that ROS acts as a link between inflammasome activation and glutathione depletion.
Studies have shown that nitrotyrosine (NT), a biomarker of nitrosative modification of proteins, is enhanced in ADs, including SLE [57, 58]. Oxidative/nitrosative stress could trigger inflammasome activation through peroxynitrite formation [59]. In fact, nitrosative stress contributes to hypoadiponectinemia-induced NLRP3 inflammasome activation in diabetic mellitus [60]. In our earlier studies, iNOS null MRL+/+ mice [19] and NAC treatment [31] provided protection against TCE-mediated autoimmunity, evidenced by reduced NT levels and various autoimmune markers [19, 33]. Here, for the first time, we provide evidence that TCE-mediated NLRP3 inflammasome activation is attenuated in iNOS null MRL+/+ mice and also in mice given NAC treatment, suggesting that increased oxidative/nitrosative stress plays a causative role in TCE-induced activation of NLRP3 inflammasome in MRL+/+ mice.
ADs are chronic inflammatory diseases characterized by dysregulated T and B cells, autoantibody production and tissue damages, which ultimately lead to the disease [28, 44, 61]. Numerous studies have established the importance of intracellular glutathione in the modulation of lymphocyte activities, linking glutathione depletion to lymphocyte hyperactivation, and its restoration leading to attenuated SLE disease activity via expansion of Tregs [62]. In SLE, CD4+ T cells are known to have altered cellular function including hyperactivation, mitochondrial abnormalities and mTOR activation [46, 63]. TCE inhalation is associated with decreased circulating CD4+ T cells in human [64], while TCE exposure via drinking water leads to activation of splenic CD4+ T cells toward Th1 phenotype [29]. Earlier we have shown that IL-17 is a key mediator of TCE-mediated autoimmunity and NAC supplementation remarkably suppressed IL-17 production from the splenic lymphocytes [30, 31]. Tregs are critically involved in the limitation of chronic inflammation and development of tolerance, and an imbalance between Th17 and Treg cells is generally considered as a new paradigm in the pathogenesis of ADs [48]. In this study, TCE exposure resulted in the reduction of hepatic immunosuppressive Tregs and increased Th17 cells, and NAC supplementation alleviated TCE-mediated imbalance between Tregs and Th17 cells, further supporting a key role of ROS in this process. T helper cells are essential for the differentiation of autoantibody-producing B cells, and CD8+ T cells mediate tissue damage in ADs [65]. The major contribution of CD8+ T cells to AD rely on its cytotoxic responses and effector cytokines, leading to breakdown to self-tolerance [66]. The increased activated CD8+ T cell population following TCE treatment and their reduced hepatic infiltration after NAC treatment, suggest that CD8+ T cells could be effector in TCE-mediated autoimmunity and oxidative stress is key modulator of its function. TCE exposure in this study also led to B cell activation in both spleen and liver, evidenced by significantly increased number of GL7+B220+ cells, and this effect was also effectively attenuated by NAC supplementation. What is more remarkable is that NAC supplementation also attenuated the production of autoantibodies (ANA), suggesting that TCE-induced oxidative stress is contributing to B cell activation and autoantibody production. Inflammasome activation in dendritic cells, monocytes/macrophages and neutrophils is known to be a contributing factor in SLE [67–69]. Our data clearly demonstrated that NAC not only downregulated TCE-induced inflammasome activation, but also dampened TCE-mediated hepatic immune cells infiltration including DCs and NKs. This anti-inflammatory effect of NAC is consistent with previous reports showing that inhibition of the NLRP3 inflammasome ameliorates lupus nephritis by inhibiting NF-κB in SLE murine models [70–72]. In this study, NAC supplementation also effectively blunted the TCE-induced immune dysregulation in the liver. Collectively, these results provide a new molecular mechanism for the protective role of NAC in ADs by blocking inflammasome activation and immune cell activation.
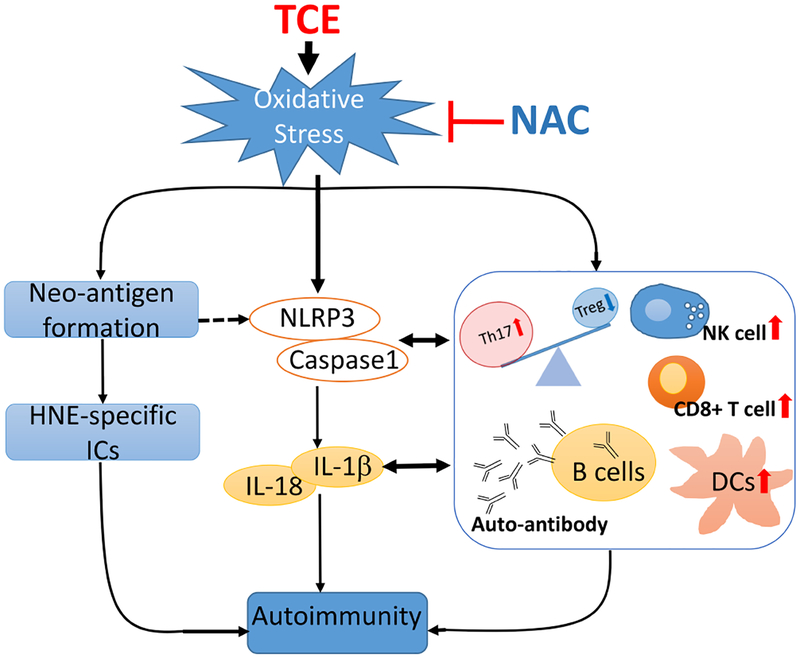
In conclusion, our data show that TCE exposure causes oxidative stress, inflammasome activation and hepatic immune cell dysregulation, leading to autoimmunity in MRL+/+ mice. Inhibition of oxidative stress by NAC decreased inflammasome activation and IL-1β production, leading to suppression of Th17 cells, which suggests that redox signaling as a critical regulator of inflammasome and downstream immune cell dysregulation (Fig. 7). Further studies on the contribution of specific immune cell types, mechanisms by which ROS and inflammasome regulate ADs, and development of agents to specifically inhibit NLRP3 activation could lead to identification of pivotal and novel therapeutic targets of the ADs.
Fig. 7. Plausible pathways contributing to TCE-mediated autoimmunity.
TCE exposure causes oxidative stress, inflammasome activation, IL-1β induction and hepatic immune cell dysregulation, leading to autoimmunity in MRL+/+ mice. NAC supplementation provides protection by alleviating TCE-induced oxidative modifications and inflammasome activation.
Highlights.
TCE exposure led to oxidative stress and hepatic inflammasome NLRP3 activation.
TCE induced hepatic CD8+ T cells, NK cells, dendritic cells, and B cell activation.
TCE exposure was associated with skewed Treg/Th17 ratio in the liver.
NAC attenuated TCE-induced inflammasome activation and autoimmune response.
Acknowledgements
This work was supported by RO1 grants [ES016302 and ES026887] from the National Institute of Environmental Health Sciences (NIEHS), NIH, and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Chiu WA, Jinot J, Scott CS, Makris SL, Cooper GS, Dzubow RC, Bale AS, Evans MV, Guyton KZ, Keshava N, Lipscomb JC, Barone S Jr., Fox JF, Gwinn MR, Schaum J, Caldwell JC, Human health effects of trichloroethylene: key findings and scientific issues, Environ Health Perspect 121(3) (2013) 303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dumas O, Despreaux T, Perros F, Lau E, Andujar P, Humbert M, Montani D, Descatha A, Respiratory effects of trichloroethylene, Respir Med 134 (2018) 47–53. [DOI] [PubMed] [Google Scholar]
- [3].Khan MF, Kaphalia BS, Prabhakar BS, Kanz MF, Ansari GAS, Trichloroethene-induced autoimmune-response in Female MRL+/+ Mice, Toxicol Appl Pharm 134(1) (1995) 155–160. [DOI] [PubMed] [Google Scholar]
- [4].Khan MF, Wang G, Environmental Agents, Oxidative Stress and Autoimmunity, Curr Opin Toxicol 7 (2018) 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gilbert KM, Reisfeld B, Zurlinden TJ, Kreps MN, Erickson SW, Blossom SJ, Modeling toxicodynamic effects of trichloroethylene on liver in mouse model of autoimmune hepatitis, Toxicol Appl Pharmacol 279(3) (2014) 284–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cooper GS, Makris SL, Nietert PJ, Jinot J, Evidence of autoimmune-related effects of trichloroethylene exposure from studies in mice and humans, Environ Health Perspect 117(5) (2009) 696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lash LH, Parker JC, Scott CS, Modes of action of trichloroethylene for kidney tumorigenesis, Environ Health Perspect 108 Suppl 2 (2000) 225–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Salama MM, El-Naggar DA, Abdel-Rahman RH, Elhak SAG, Toxic effects of trichloroethylene on rat neuroprogenitor cells, Front Pharmacol 9 (2018) 741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Frostegard J, Svenungsson E, Wu RH, Gunnarsson I, Lundberg IE, Klareskog L, Horkko S, Witztum JL, Lipid peroxidation is enhanced in patients with systemic lupus erythematosus and is associated with arterial and renal disease manifestations, Arthritis Rheum 52(1) (2005) 192–200. [DOI] [PubMed] [Google Scholar]
- [10].Wang G, König R, Ansari GAS, Khan MF, Lipid peroxidation-derived aldehyde-protein adducts contribute to trichloroethene-mediated autoimmunity via activation of CD4(+) T cells, Free Radical Bio Med 44(7) (2008) 1475–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang G, Li H, Khan MF, Differential oxidative modification of proteins in MRL+/+ and MRL/lpr mice: Increased formation of lipid peroxidation-derived aldehyde-protein adducts may contribute to accelerated onset of autoimmune response, Free Radical Res 46(12) (2012) 1472–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Grune T, Michel P, Sitte N, Eggert W, Albrecht-Nebe H, Esterbauer H, Siems WG, Increased levels of 4-hydroxynonenal modified proteins in plasma of children with autoimmune diseases, Free Radic Biol Med 23(3) (1997) 357–60. [DOI] [PubMed] [Google Scholar]
- [13].Broz P, Dixit VM, Inflammasomes: mechanism of assembly, regulation and signalling, Nat Rev Immunol 16(7) (2016) 407–20. [DOI] [PubMed] [Google Scholar]
- [14].Wree A, Marra F, The inflammasome in liver disease, J Hepatol 65(5) (2016) 1055–1056. [DOI] [PubMed] [Google Scholar]
- [15].Luan J, Zhang X, Wang S, Li Y, Fan J, Chen W, Zai W, Wang S, Wang Y, Chen M, Meng G, Ju D, NOD-like receptor protein 3 inflammasome-dependent IL-1 beta accelerated ConA-induced hepatitis, Front Immunol 9 (2018) 758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chung Y, Chang SH, Martinez GJ, Yang XXO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C, Critical regulation of early Th17 cell differentiation by interleukin-1 signaling, Immunity 30(4) (2009) 576–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sha Y, Markovic-Plese S, Activated IL-1RI signaling pathway induces Th17 cell differentiation via interferon regulatory factor 4 signaling in patients with relapsing-remitting multiple sclerosis, Front Immunol 7 (2016) 543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kahlenberg JM, Kaplan MJ, The inflammasome and lupus: another innate immune mechanism contributing to disease pathogenesis?, Curr Opin Rheumatol 26(5) (2014) 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang G, Wakamiya M, Wang J, Ansari GA, Khan MF, iNOS null MRL+/+ mice show attenuation of trichloroethene-mediated autoimmunity: contribution of reactive nitrogen species and lipid-derived reactive aldehydes, Free Radic Biol Med 89 (2015) 770–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang G, Cai P, Ansari GA, Khan MF, Oxidative and nitrosative stress in trichloroethene-mediated autoimmune response, Toxicology 229(3) (2007) 186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Reilly CM, Farrelly LW, Viti D, Redmond ST, Hutchison F, Ruiz P, Manning P, Connor J, Gilkeson GS, Modulation of renal disease in MRL/lpr mice by pharmacologic inhibition of inducible nitric oxide synthase, Kidney Int 61(3) (2002) 839–46. [DOI] [PubMed] [Google Scholar]
- [22].Choi J, Kim ST, Craft J, The pathogenesis of systemic lupus erythematosus-an update, Curr Opin Immunol 24(6) (2012) 651–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Shahrabi S, Zayeri ZD, Ansari N, Hadad EH, Rajaei E, Flip-flops of natural killer cells in autoimmune diseases versus cancers: Immunologic axis, J Cell Physiol (2019). [DOI] [PubMed] [Google Scholar]
- [24].Nistala K, Wedderburn LR, Th17 and regulatory T cells: rebalancing pro- and anti-inflammatory forces in autoimmune arthritis, Rheumatology (Oxford) 48(6) (2009) 602–6. [DOI] [PubMed] [Google Scholar]
- [25].Grant CR, Liberal R, Holder BS, Cardone J, Ma Y, Robson SC, Mieli-Vergani G, Vergani D, Longhi MS, Dysfunctional CD39(POS) regulatory T cells and aberrant control of T-helper type 17 cells in autoimmune hepatitis, Hepatology 59(3) (2014) 1007–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Liu J, Cao X, Regulatory dendritic cells in autoimmunity: A comprehensive review, J Autoimmun 63 (2015) 1–12. [DOI] [PubMed] [Google Scholar]
- [27].Blanco P, Pitard V, Viallard JF, Taupin JL, Pellegrin JL, Moreau JF, Increase in activated CD8+ T lymphocytes expressing perforin and granzyme B correlates with disease activity in patients with systemic lupus erythematosus, Arthritis Rheum 52(1) (2005) 201–11. [DOI] [PubMed] [Google Scholar]
- [28].Suarez-Fueyo A, Bradley SJ, Tsokos GC, T cells in systemic lupus erythematosus, Curr Opin Immunol 43 (2016) 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Griffin JM, Gilbert KM, Lamps LW, Pumford NR, CD4(+) T-cell activation and induction of autoimmune hepatitis following trichloroethylene treatment in MRL+/+ mice, Toxicol Sci 57(2) (2000) 345–52. [DOI] [PubMed] [Google Scholar]
- [30].Wang G, Wang J, Fan X, Ansari GA, Khan MF, Protein adducts of malondialdehyde and 4-hydroxynonenal contribute to trichloroethene-mediated autoimmunity via activating Th17 cells: dose- and time-response studies in female MRL+/+ mice, Toxicology 292(2–3) (2012) 113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wang G, Wang J, Ma H, Ansari GA, Khan MF, N-Acetylcysteine protects against trichloroethene-mediated autoimmunity by attenuating oxidative stress, Toxicol Appl Pharmacol 273(1) (2013) 189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wang G, Ma H, Wang J, Khan MF, Contribution of poly(ADP-ribose)polymerase-1 activation and apoptosis in trichloroethene-mediated autoimmunity, Toxicol Appl Pharmacol 362 (2019) 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wang G, Wang J, Luo X, Ansari GA, Khan MF, Nitrosative stress and nitrated proteins in trichloroethene-mediated autoimmunity, PLoS One 9(6) (2014) e98660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kondraganti S, König R, Boor PJ, Khan S, Kaphalia BS, Khan MF, Ansari GAS, Mechanistic evaluation of trichloroethene-mediated autoimmune hepatitis-like disease in female MRL+/+ mice, The Open Toxicology Journal 5 (2012) 10. [Google Scholar]
- [35].Khan MF, Wu X, Ansari GA, Anti-malondialdehyde antibodies in MRL+/+ mice treated with trichloroethene and dichloroacetyl chloride: possible role of lipid peroxidation in autoimmunity, Toxicol Appl Pharmacol 170(2) (2001) 88–92. [DOI] [PubMed] [Google Scholar]
- [36].Schwinge D, Schramm C, Sex-related factors in autoimmune liver diseases, Semin Immunopathol 41(2) (2019) 165–175. [DOI] [PubMed] [Google Scholar]
- [37].Suwannaroj S, Lagoo A, Keisler D, McMurray RW, Antioxidants suppress mortality in the female NZB × NZW F1 mouse model of systemic lupus erythematosus (SLE), Lupus 10(4) (2001) 258–65. [DOI] [PubMed] [Google Scholar]
- [38].Liang Y, Yi P, Yuan DMK, Jie Z, Kwota Z, Soong L, Cong Y, Sun J, IL-33 induces immunosuppressive neutrophils via a type 2 innate lymphoid cell/IL-13/STAT6 axis and protects the liver against injury in LCMV infection-induced viral hepatitis, Cell Mol Immunol 16(2) (2019)126–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wang H, Wang G, Ansari GAS, Khan MF, Trichloroethene metabolite dichloroacetyl chloride induces apoptosis and compromises phagocytosis in Kupffer Cells: Activation of inflammasome and MAPKs, PLoS One 13(12) (2018) e0210200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Otaki N, Chikazawa M, Nagae R, Shimozu Y, Shibata T, Ito S, Takasaki Y, Fujii J, Uchida K, Identification of a lipid peroxidation product as the source of oxidation-specific epitopes recognized by anti-DNA autoantibodies, J Biol Chem 285(44) (2010) 33834–33842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zharkova O, Celhar T, Cravens PD, Satterthwaite AB, Fairhurst AM, Davis LS, Pathways leading to an immunological disease: systemic lupus erythematosus, Rheumatology (Oxford) 56(suppl_1) (2017) i55–i66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhou R, Yazdi AS, Menu P, Tschopp J, A role for mitochondria in NLRP3 inflammasome activation, Nature 469(7329) (2011) 221–5. [DOI] [PubMed] [Google Scholar]
- [43].Nashi E, Wang Y, Diamond B, The role of B cells in lupus pathogenesis, Int J Biochem Cell Biol 42(4) (2010) 543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Dorner T, Giesecke C, Lipsky PE, Mechanisms of B cell autoimmunity in SLE, Arthritis Res Ther 13(5) (2011) 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Malkiel S, Barlev AN, Atisha-Fregoso Y, Suurmond J, Diamond B, Plasma cell differentiation pathways in systemic lupus erythematosus, Front Immunol 9 (2018) 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Morel L, Immunometabolism in systemic lupus erythematosus, Nat Rev Rheumatol 13(5) (2017) 280–290. [DOI] [PubMed] [Google Scholar]
- [47].Tsokos GC, Lo MS, Costa Reis P, Sullivan KE, New insights into the immunopathogenesis of systemic lupus erythematosus, Nat Rev Rheumatol 12(12) (2016) 716–730. [DOI] [PubMed] [Google Scholar]
- [48].Lee GR, The Balance of Th17 versus Treg cells in autoimmunity, Int J Mol Sci 19(3) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lau AH, Thomson AW, Dendritic cells and immune regulation in the liver, Gut 52(2) (2003) 307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hudspeth K, Pontarini E, Tentorio P, Cimino M, Donadon M, Torzilli G, Lugli E, Della Bella S, Gershwin ME, Mavilio D, The role of natural killer cells in autoimmune liver disease: A comprehensive review, J Autoimmun 46 (2013) 55–65. [DOI] [PubMed] [Google Scholar]
- [51].Tian ZG, Chen YY, Gao B, Natural killer cells in liver disease, Hepatology 57(4) (2013) 1654–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Khan MF, Kaphalia BS, Ansari GA, Time-dependent autoimmune response of dichloroacetyl chloride in female MRL +/+ mice, Immunopharmacol Immunotoxicol 19(2) (1997) 265–77. [DOI] [PubMed] [Google Scholar]
- [53].Cai P, Konig R, Khan MF, Qiu S, Kaphalia BS, Ansari GA, Autoimmune response in MRL+/+ mice following treatment with dichloroacetyl chloride or dichloroacetic anhydride, Toxicol Appl Pharmacol 216(2) (2006) 248–55. [DOI] [PubMed] [Google Scholar]
- [54].Eskan MA, Benakanakere MR, Rose BG, Zhang P, Zhao J, Stathopoulou P, Fujioka D, Kinane DF, Interleukin-1beta modulates proinflammatory cytokine production in human epithelial cells, Infect Immun 76(5) (2008) 2080–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Shen HH, Yang YX, Meng X, Luo XY, Li XM, Shuai ZW, Ye DQ, Pan HF, NLRP3: A promising therapeutic target for autoimmune diseases, Autoimmun Rev 17(7) (2018) 694–702. [DOI] [PubMed] [Google Scholar]
- [56].Kim SR, Kim DI, Kim SH, Lee H, Lee KS, Cho SH, Lee YC, NLRP3 inflammasome activation by mitochondrial ROS in bronchial epithelial cells is required for allergic inflammation, Cell Death Dis 5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ahsan H, 3-Nitrotyrosine: A biomarker of nitrogen free radical species modified proteins in systemic autoimmunogenic conditions, Hum Immunol 74(10) (2013) 1392–9. [DOI] [PubMed] [Google Scholar]
- [58].Wang G, Pierangeli SS, Papalardo E, Ansari GA, Khan MF, Markers of oxidative and nitrosative stress in systemic lupus erythematosus: correlation with disease activity, Arthritis Rheum 62(7) (2010) 2064–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Bellezza I, Grottelli S, Costanzi E, Scarpelli P, Pigna E, Morozzi G, Mezzasoma L, Peirce MJ, Moresi V, Adamo S, Minelli A, Peroxynitrite activates the NLRP3 inflammasome cascade in SOD1(G93A) mouse model of amyotrophic lateral sclerosis, Mol Neurobiol 55(3) (2018) 2350–2361. [DOI] [PubMed] [Google Scholar]
- [60].Zhang J, Xia L, Zhang F, Zhu D, Xin C, Wang H, Zhang F, Guo X, Lee Y, Zhang L, Wang S, Guo X, Huang C, Gao F, Liu Y, Tao L, A novel mechanism of diabetic vascular endothelial dysfunction: Hypoadiponectinemia-induced NLRP3 inflammasome activation, Biochim Biophys Acta Mol Basis Dis 1863(6) (2017) 1556–1567. [DOI] [PubMed] [Google Scholar]
- [61].Webb GJ, Hirschfield GM, Krawitt EL, Gershwin ME, Cellular and molecular mechanisms of autoimmune hepatitis, Annu Rev Pathol 13 (2018) 247–292. [DOI] [PubMed] [Google Scholar]
- [62].Lai ZW, Hanczko R, Bonilla E, Caza TN, Clair B, Bartos A, Miklossy G, Jimah J, Doherty E, Tily H, Francis L, Garcia R, Dawood M, Yu J, Ramos I, Coman I, Faraone SV, Phillips PE, Perl A, N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: a randomized, double-blind, placebo-controlled trial, Arthritis Rheum 64(9) (2012) 2937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Perl A, Oxidative stress in the pathology and treatment of systemic lupus erythematosus, Nat Rev Rheumatol 9(11) (2013) 674–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hosgood HD 3rd, Zhang L, Tang X, Vermeulen R, Qiu C, Shen M, Smith MT, Ge Y, Ji Z, Xiong J, He J, Reiss B, Liu S, Xie Y, Guo W, Galvan N, Li L, Hao Z, Rothman N, Huang H, Lan Q, Decreased numbers of CD4(+) naive and effector memory T cells, and CD8(+) naive T cells, are associated with trichloroethylene exposure, Front Oncol 1 (2011) 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Mak A, Kow NY, The pathology of T cells in systemic lupus erythematosus, J Immunol Res 2014 (2014) 419029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Gravano DM, Hoyer KK, Promotion and prevention of autoimmune disease by CD8+ T cells, J Autoimmun 45 (2013) 68–79. [DOI] [PubMed] [Google Scholar]
- [67].Zhang H, Fu R, Guo C, Huang Y, Wang H, Wang S, Zhao J, Yang N, Anti-dsDNA antibodies bind to TLR4 and activate NLRP3 inflammasome in lupus monocytes/macrophages, J Transl Med 14(1) (2016) 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Tucci M, Quatraro C, Lombardi L, Pellegrino C, Dammacco F, Silvestris F, Glomerular accumulation of plasmacytoid dendritic cells in active lupus nephritis: role of interleukin-18, Arthritis Rheum 58(1) (2008) 251–62. [DOI] [PubMed] [Google Scholar]
- [69].Yu Y, Su K, Neutrophil extracellular traps and systemic lupus erythematosus, J Clin Cell Immunol 4 (2013). 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Tsai PY, Ka SM, Chang JM, Chen HC, Shui HA, Li CY, Hua KF, Chang WL, Huang JJ, Yang SS, Chen A, Epigallocatechin-3-gallate prevents lupus nephritis development in mice via enhancing the Nrf2 antioxidant pathway and inhibiting NLRP3 inflammasome activation, Free Radic Biol Med 51(3) (2011) 744–54. [DOI] [PubMed] [Google Scholar]
- [71].Li X, Wang M, Hong H, Luo C, Liu Z, Yang R, Sophocarpine attenuates murine lupus nephritis via inhibiting NLRP3 inflammasome and NF-kappaB activation, Immunol Res 66(4) (2018) 521–527. [DOI] [PubMed] [Google Scholar]
- [72].Zhao J, Zhang H, Huang Y, Wang H, Wang S, Zhao C, Liang Y, Yang N, Bay11–7082 attenuates murine lupus nephritis via inhibiting NLRP3 inflammasome and NF-kappaB activation, Int Immunopharmacol 17(1) (2013) 116–22. [DOI] [PubMed] [Google Scholar]