Abstract
Pyruvate kinase M2 is a critical enzyme that regulates cell metabolism and growth under different physiological conditions. In its metabolic role, pyruvate kinase M2 catalyzes the last glycolytic step which converts phosphoenolpyruvate to pyruvate with the generation of ATP. Beyond this metabolic role in glycolysis, PKM2 regulates gene expression in the nucleus, phosphorylates several essential proteins that regulate major cell signaling pathways, and contribute to the redox homeostasis of cancer cells. The expression of PKM2 has been demonstrated to be significantly elevated in several types of cancer, and the overall inflammatory response. The unusual pattern of PKM2 expression inspired scientists to investigate the unrevealed functions of PKM2 and the therapeutic potential of targeting PKM2 in cancer and other disorders. Therefore, the purpose of this review is to discuss the mechanistic and therapeutic potential of targeting PKM2 with the focus on cancer metabolism, redox homeostasis, inflammation, and metabolic disorders. This review highlights and provides insight into the metabolic and non-metabolic functions of PKM2 and its relevant association with health and disease.
Keywords: Pyruvate Kinase M2, Glycolysis, Warburg Effect, Cancer, Redox Homeostasis, Oxidants, Inflammation, Metabolic diseases
Graphical Abstract
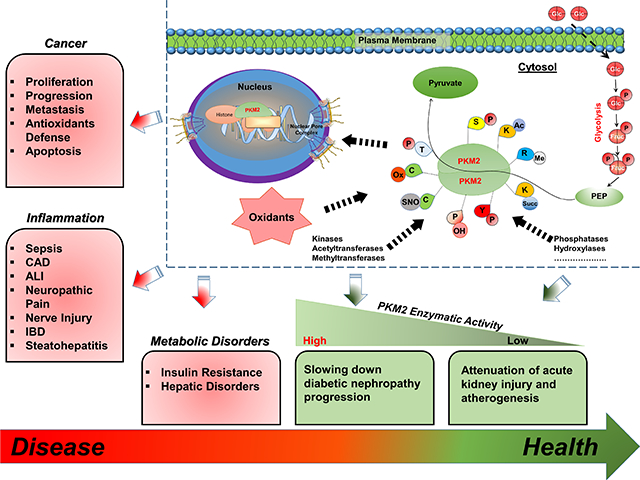
1.1. Introduction
Pyruvate kinase (PK) (EC 2.7.1.40) is a prominent regulatory protein involved in glucose catabolism. Mammalian PK has several isoforms that differ in allosteric regulation and tissue expression, which is likely necessary to meet the tissues’ specific energy demands. For instance, the M2 isoform is expressed in highly proliferative cells, including cancer cells, to favor the accumulation of glycolytic metabolites that serve as building blocks for tumor growth. Additionally, PKM2 is directly involved in the metabolic reprogramming (aerobic glycolysis) associated with cancer [1] and the inflammatory response [2]. Unlike other PK isoforms, PKM2 has been reported to modulate gene expression and function as a kinase that phosphorylates proteins involved in cellular growth and survival [3, 4]. A comprehensive understanding of the role of PKM2 in cancer, inflammatory responses, and metabolic disorders may lead to novel therapeutic approaches to alleviate morbidity and mortality related to these conditions. The purpose of this review is to discuss the enzymatic and non-enzymatic functions of PKM2 and highlight the potential contribution of PKM2 to the pathology of human diseases.
1.2. An Era of Discovery in Glycolysis and Pyruvate Kinases
Glucose is the predominant energy source for most cells and is partially oxidized through glycolysis. Oxidation of glucose through glycolysis produces two molecules of ATP, two molecules of nicotinamide adenine dinucleotide (NADH), and two molecules of pyruvate. Under normal physiological conditions, pyruvate undergoes two fates, and it is either utilized to produce lactate under anaerobic respiration (when oxygen is not available) or entered the tricarboxylic acid (TCA) cycle in the presence of oxygen to provide cells with more ATP. Glycolysis is an oxygen-independent process and consists of 10 steps, three of which are rate-limiting steps that regulate glycolytic flux. These steps are catalyzed by a group of kinases including phosphofructokinase (PFK), hexokinase (HK) or glucokinase (GK), and PK. Through regulation of the glycolytic flux, cells are able to control the levels of metabolic precursors required for many biosynthetic reactions that occur in various cellular compartments [5, 6].
Glycolysis was the first catabolic pathway to be discovered, with the origin of this work dating back to the second half of the 19th century. Interestingly, this early research into this metabolic pathway was financially supported by the alcohol industry due to the significant economic contributions of alcohol fermentation [7]. Early biochemical studies conducted on yeast [8] and muscle [9] identified enzymes and metabolites involved in glycolysis and, in 1940, the complete pathway of glycolysis was revealed for the first time by the Nobel Prize laureates including Otto Fritz Meyerhof for their outstanding accomplishment [7].
Glycolysis is a process that shunts carbohydrates through multiple biochemical reactions to yield energy in the form of ATP and the electron carrier NADH. The rate limiting step for converting phosphoenolpyruvate (PEP) into pyruvate and ATP was first identified in 1934 by Jacob Parnas after conducting an experiment on muscle tissues to inhibit ammonia formation [10]. The history of glycolysis and PK has been extensively reviewed by Barnett [7] and Dayton et al. [11]. However, it is worth noting that although the activity of PK was first described in 1935 by Lehman H, it took approximately ten years for this enzyme to be isolated from muscle [7]. A few years later, multiple isoenzymes of PK were identified by Tanaka et al. [12] in the 1960s. Based on electrophoresis and crystallization procedure, two isoforms of PK were identified. One isoform was isolated from rat liver (type L), while the other was isolated from rat muscle (type M) [12]. In 1968, a third isoenzyme was detected in multiple rat tissues (kidney, spleen, testis, and lung) and was later named PKM2 [13]. However, it was not until 1984, when Josephine Peters confirmed that PKM1 and PKM2 are both transcribed by the same gene [14, 15]. In 1986, Noguchi in collaboration with Tanaka demonstrated the alternative splicing concept of the Pkm gene [16]. Finally, the most significant findings regarding the PK protein is the nuclear localization of the M2 isoform in response to a variety of apoptotic agents (somatostatin and its analogues) [17], as well as the localization of PKM2 into the mitochondria in response to oxidative stress inducers [18]. These findings highlighted novel functions of PKM2 other than its previously identified role in glycolysis.
1.3. PKM2: From Gene to Protein
Pyruvate Kinase M gene is located on Chromosome 15 (in humans), Chromosome 7 (in non-human primates) [19], and Chromosome 9 (in mice) [20] which encodes both the M1 and the M2 isoforms [21–23]. The mouse Pkm2 transcript is 82% identical to the human PKM2, while the non-human primate transcript is 99% identical to the human PKM2. The PKM gene is roughly 32 kb, containing 12 exons and 11 introns. Exons 9 and 10 have equal length and account for the variation in the final transcript. The final mRNA product for PKM2 contains exon 10 but excludes exon 9, which is specific to PKM1 [24]. Interestingly, three spliceosomes; the heterogeneous nuclear ribonucleoproteins A1 [25] and A2 (hnRNPA1, hnRNPA2), and polypyrimidine tract binding protein (PTB), were found to promote the elimination of exon 9 to favor the production of mature Pkm2 RNA [22, 23, 26]. In addition, a recent study has shown that the RNA-binding motif 4 (RBM4) splicing factor negatively regulates PKM2 expression by suppressing the activity of the splicing regulator PTB [27]. On the other hand, the serine/arginine-rich splicing factor 3 (SRSF3) promotes the addition of exon 10 to the mature PKM2 RNA [22, 28, 29]. The final human mRNAs for both isoforms are 1593 base pairs long, and the variation in their mRNA exist within 155 nucleotide residues from 1142 to 1297 [30].
PK is a tetrameric protein with identical subunits. The single PKM2 monomer is made of 531 amino acids and consists of 4 domains: N (43 aa), A (244 aa), B (102 aa) and C (142 aa). However, PKM1 and PKM2 vary from each other in 22 amino acids within a stretch encoded by exons 9 and 10 [31]. The A domain of PKM2 represents the core of the monomer and responsible for mediating the subunits interaction to form a dimer. The tetramer form of PKM2 consists of 2-units that is assembled through the binding of two dimers’ C-subunits. Both the allosteric fructose 1,6-bisphosphate (FBP) binding pocket [31] and the nuclear localization signal sequence (NLS) [32] are located within the C domain. Moreover, the structure of domain C is responsible for the variation observed between the PKM isoforms in regards to the allosteric regulation by FBP. Of note, the activation loop in PKM2 surrounds FBP molecules and closes the allosteric site, while in PKM1 the activation loop is far away from the allosteric site leading to an open conformation of PKM1 allosteric site [31]. However, the active site for PKM2 is located between domains A and B. Domain B regulates the size of the active site by either moving toward or outward of domain A, while the N-terminal domain represents the smallest domain in the PKM2 monomer [31].
1.4. Tissue Distribution of the PK Isoforms
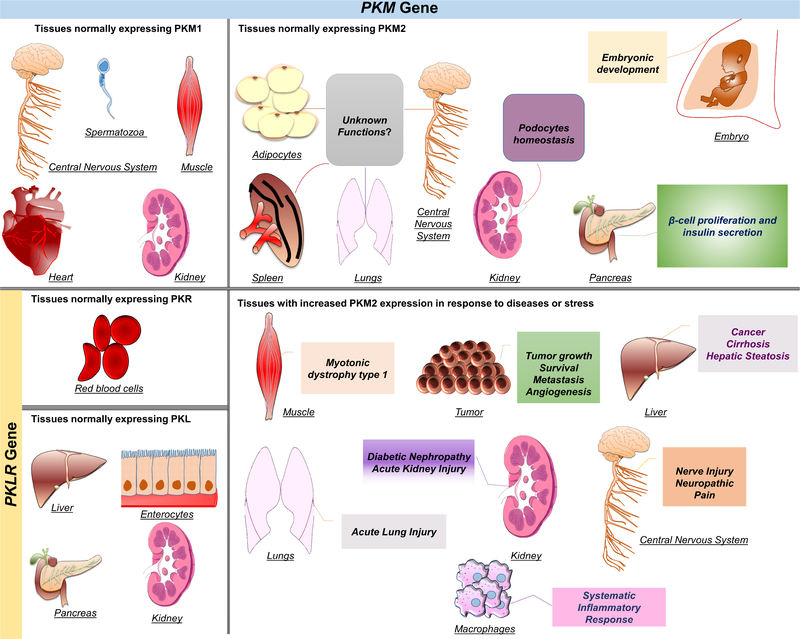
The PK family consists of four isoforms encoded by two separate genes. These isoforms are noted as PKL (liver), PKR (erythrocytes), PKM1 (muscle) and PKM2. The PKLR gene encodes both the L and the R isoforms [33], while the M1 and the M2 are encoded by the Pkm gene [16]. At the protein level, a single tissue can express multiple isoforms. For instance, the kidney predominately expresses PKM2, while the PKL and PKM1 isoforms are expressed at significantly lower levels [34]. The PK isoforms can also be tissue-specific, and they differ in their kinetic properties based on the tissues’ metabolic demands [35]. PKR is the only isoform expressed in erythrocytes [36]. PKL, on the other hand, is the dominant isoform expressed in the liver [34], while PKM1 is the dominant isoform in differentiated skeletal muscle, heart, and brain [34, 37]. Unlike all other isoforms, PKM2 is the only detectable type during the embryonic stages and exists in various differentiated adult tissues [34]. Specifically, PKM2 is the dominant form in the kidneys and exists in multiple adult tissues including the lungs, white and brown adipose, intestines, ovaries, testis [34], and pancreatic islets [38], but it is not exclusively limited to these organs (Figure 1). Notably, the expression of PKM2 is more abundant in highly proliferating cells such as stem and tumor cells [34]. Additionally, PKM2 expression changes during development. For instance, PKM2 is expressed in neonatal skeletal muscle, and its expression decreases during muscle development exhibiting a transition from PKM2 to PKM1 in differentiated muscle [39].
Figure 1. Tissue distribution of PK isoforms and the role of PKM2 in health and disease.
Mammalian pyruvate kinase has four isoforms transcribed by two different genes. PKL and PKR are both products of the PKLR gene, while PKM1 and PKM2 are products of the PKM gene. PKL is predominantly expressed in the liver and at lower levels in the pancreas, kidneys, and enterocytes. PKR is expressed in erythrocytes only. PKM1 is known to be expressed in muscle, mature spermatozoa, central nervous system, heart, and kidneys. PKM2 is the predominant isoform expressed during embryogenesis. It is also expressed in proliferative cells, and several healthy differentiated tissues, including pancreatic islets, adipose tissue, brain, kidneys, lungs, and spleen. However, the precise function of PKM2 in these tissues is largely unexplored. In addition, PKM2 is expressed in most tumors and within the liver under abnormal conditions such as cirrhosis, and hepatic steatosis. In activated macrophages, PKM2 expression is upregulated and contributes to sepsis and other inflammatory disorders. In tumors, PKM2 expression enhances aerobic glycolysis that provides cancer cells with growth advantages and has been associated with poor clinical outcomes.
1.5. PKM2 Enzymatic Function and Regulation
PKM2 exists in four different enzymatic states: an inactive monomer [40], a nearly inactive dimer [40, 41], an inactive T state tetramer, and an active R state tetramer [42]. PKM2 is typically activated by PEP and the upstream glycolytic intermediate FBP or fructose 2,6-bisphosphate (F-2,6-P2). Upon activation, PKM2 catalyzes a critical step in glycolysis by transferring a phosphate group from PEP to ADP, leading to the generation of pyruvate and ATP. During this reaction, PEP and ATP bind to the active site via a mechanism facilitated by the monovalent (K+) and divalent (Mg2+ or Mn2+) cations [22, 43]. This glycolytic reaction is irreversible under normal conditions and plays a critical role in controlling the glycolytic efflux [44]. The conformational switch between the inactive T-state (low enzymatic activity) and the active R-state (high enzymatic activity) during this glycolytic reaction is noteworthy. Furthermore, in addition to the allosteric regulation, several post-translational modifications were reported to influence and determine the transition between the T and R states of PKM2 through manipulating its intramolecular hydrogen bonds [45]. Unlike PKM2, PKM1 is not regulated by FBP and sustain the R-state conformation [44]. In addition, serine is a specific PKM2 activator that binds to each monomer and promotes the formation of the active tetramer [46]. In contrast, PKM2 is allosterically inhibited by ATP, oxalate, alanine, thyroid hormone (T3), and phenylalanine [42, 47, 48]. However, each of these molecules exhibits distinct mechanisms by which they inhibit PKM2 [42]. For example, T3 blocks the tetramer formation by stabilizing the monomer form, while phenylalanine stabilizes the inactive tetramer T-state [42]. This suggests that the inhibition of PKM2 may be additive with no one inhibitor working through the same mechanism.
1.6. PKM2 Post-Transnational Modifications
In addition to the allosteric regulation, PKM2 can be modified by post-translational modifications, which has been extensively reviewed by Prakasam et al. [49]. Major posttranslational modifications include oxidation [50], acetylation [51, 52], methylation [53], and phosphorylation [32, 54–56] (Table 1). These post-translational modifications regulate not only PKM2 activity and stability but also its subcellular localization (Figure 2).
Table 1:
Post-translational modifications of PKM2 and their disease-associated physiological relevance.
| Modification | Specific site | Stimuli/ Source | Regulators | Effects | Proposed Function | Reference |
|---|---|---|---|---|---|---|
| Phosphorylation | S-202 | IGF-1 | AKT1 | Promotes nuclear translocation | Nuclear PKM2 interacts with STAT5 to induce cellular proliferation | [62] |
| S-37 | EGF | ERK1/2 | Nuclear PKM2 induces C-Myc expression to upregulate GLUT1, LADH, and PTB expression | [32] | ||
| T-454 | PIM2 | Increases PKM2 protein stability Reduces PKM2 enzymatic activity |
Promotes PKM2 co-activator activity on HIF-1α and β-catenin Lowers mitochondrial respiration Promotes cancer cell proliferation |
[206] | ||
| T-328 | GSK-3β HSP90 | Increases glycolysis Lowers the apoptotic rate Promotes tumor growth | [55] | |||
| Y-105 | bFGF | FGFR1 ABL JAK2 FLT3 |
Inhibits the formation of the active tetramer Decreases PKM2 enzymatic activity |
Increases cancer cell proliferation, lactate production, and decreases oxidative phosphorylation | [54] | |
| De-phosphorylation | Y-105 | Insulin | PTP1B | Increases PKM2 enzymatic activity | Contributes to glycemic control | [207] |
| Y | Morin (LMW- PTP inhibitor) | LMW-PTP | Inhibits PKM2 nuclear translocation Increases PKM2 enzymatic activity |
Reduces glycolysis and enhances oxidative metabolism | [63] | |
| SUMOylation | K-336 | SUMO1 | Increases PKM2 protein stability | Promotes glycolysis Promotes PKM2 cofactor functions Promotes cancer cells proliferation |
[208] | |
| Acetylation | K-305 | Trichostatin A (HDAC I & II inhibitor) Nicotinamide Glucose | PCAF | Reduces PKM2 enzymatic activity Promotes PKM2 degradation |
Promotes glycolysis Promotes cellular proliferation and tumorigenesis | [52] |
| K-433 | Trichostatin A Nicotinamide | p300 acetyltransferase | Reduces PKM2 enzymatic activity Promotes PKM2 nuclear translocation and kinase activity |
Promotes cell proliferation and tumorigenesis | [51] | |
| Deacetylation | K-433 | Starvation | SIRT6 | Increases PKM2 nuclear export Reduces PKM2 cofactor function |
Reduces cancer cell proliferation and invasiveness | [64] |
| Oxidation | C-358 | Diamide H2O2 | ROS | Promotes the dissociation of the tetramer form to reduce PKM2 enzymatic activity | Lowers ROS production Enhances tumor growth | [50] |
| Glucose | Reduces PKM2 enzymatic activity | Contributes to DN pathogenesis | [204] | |||
| Hydroxylation | P-403 P-408 |
PHD3 | Promotes PKM2 nuclear translocation Promotes HIF-1α transcriptional activity | Promotes the Warburg effect Increases lactate production | [66] | |
| S-nitrosylation | C-423 C-424 |
NO | AKR1A1 (negative regulator) | Inhibits PKM2 tetramer formation | Increases glycolytic metabolites accumulation Promotes antioxidants defense Protects against AKI |
[58] |
| C-358 | eNOS | Reduces PKM2 enzymatic activity Delays atherosclerosis development | Amplifies the antioxidants defense | [59] | ||
| Methylation | R-445 R-447 |
S-Adenosyl-methionine | CARM1 PRMT6 |
Promotes PKM2 tetramer formation to increase PKM2 enzymatic activity | Reduces cellular proliferation | [65] |
| R-445 R-447 R-455 |
S-Adenosyl-methionine | CARM1 | Has little effect on PKM2 enzymatic activity Decreases InsP3R1 & InsP3R3 expression |
Promotes breast cancer cells proliferation Reduces mitochondrial respiration | [53] | |
| Succinylation | K-498 | Suramin (SIRT5 inhibitor) | SIRT5 (negative regulator) | Increases PKM2 activity | Reduces NADPH generation Lowers cellular proliferation and tumor growth | [60] |
| Succinylation | K-311 | succinyl-CoA | SIRT5 (negative regulator) | Decreases PKM2 enzymatic activity Promotes PKM2 nuclear translocation Promotes PKM2 kinase activity |
Promotes pro-inflammatory cytokines Increases the susceptibility to colitis |
[172] |
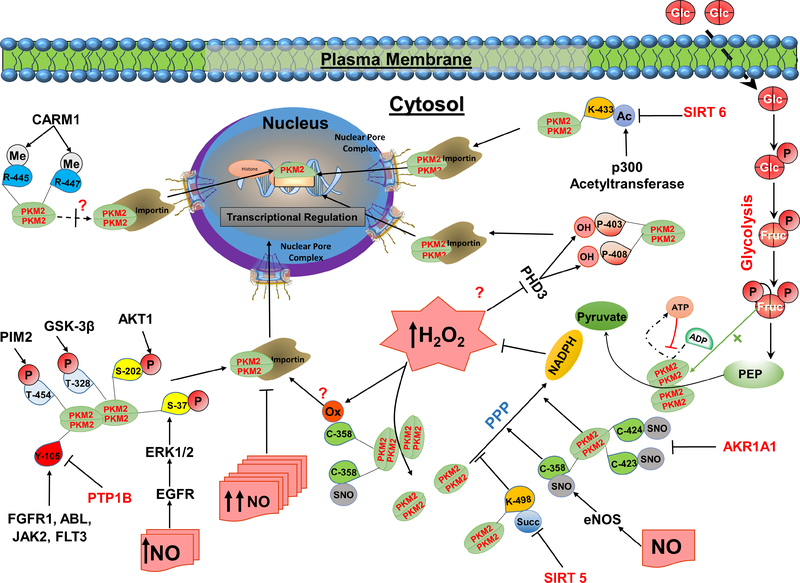
Figure 2. Post-translational regulation of PKM2 and its role in redox homeostasis.
PKM2 catalyzes the generation of pyruvate and ATP from phosphoenolpyruvate (PEP) and ADP. PKM2 enzymatic function is partially controlled by upstream glycolytic metabolites. Fructose 1.6-bisphosphate (FBP) is considered as an allosteric activator that interacts with PKM2 to enhance its active configuration, while ATP binds to the allosteric site to inhibit its catalytic activity. Post-translational modifications of PKM2 by oxidation, hydroxylation, S-nitrosylation, acetylation, methylation, succinylation/desuccinylation, or phosphorylation/dephosphorylation regulate its activity and subcellular localization. In addition, exposure to nitric oxide (NO) and H2O2 reduces its enzymatic activity and drive glycolytic metabolites toward the pentose phosphate pathway (PPP) to generate NADPH and reestablish redox homeostasis. EGFR/ERK2-dependent PKM2 phosphorylation or oxidation of the sulfhydryl group of cysteine-358 (C-358) inhibited PKM2, and it was proposed to promote PKM2 nuclear translocation. Additionally, endothelial NO synthase (eNOS) can directly interact with PKM2 leading to its S-nitrosylation at C-358 and inhibition of its enzymatic activity. Likewise, SIRT5 mediates the desuccinylation of PKM2 at lysine (K-498) leading to a decrease in its enzymatic activity. In contrast, AKAR1A1 inhibits the S-nitrosylation of PKM2 at cysteines-423/424 (C-423/424). The S-nitrosylation of PKM2 at C-423/424 was reported to inhibit PKM2 enzymatic activity. In addition, several kinases were demonstrated to phosphorylate PKM2 at various sites, and differentially regulate its activity and subcellular location. Oncogenic growth factors such as IGF-1, EGFR, and bFGF promote PKM2 phosphorylation in a mechanism mediated by several kinases including AKT1, ERK1/2, and JAK2. Phosphorylation of PKM2 at tyrosine-105 (Y-105) inhibits its catalytic activity by altering the tetramer and the dimer states that promote aerobic glycolysis. Similarly, the phosphorylation of PKM2 at serine-37 (S-37) and threonine-454 (T-454) induces the Warburg effect by enhancing the nuclear accumulation of PKM2. Likewise, acetylation of PKM2 at lysine-433 (K-433) enhances its nuclear localization, while its de-acetylation by SIRT6 causes PKM2 to be exported from the nucleus. In addition, methylation of PKM2 by CARM1 has been reported to be specific to the dimeric form and occurs at several arginine sites including R-445, 447, and 455. Furthermore, PKM2-hydroxylation at prolines-403/408 (P-403/408) by PHD3 was reported to promote its interaction with HIF-1α and its nuclear translocation.
1.6.1. Redox Regulation of PKM2
Various oxidants have been found to, directly and indirectly, modulate PKM2 functions by post-translational modifications at different amino acid sites through distinct mechanisms. A recent study by Li and colleagues demonstrated an important regulatory role for nitric oxide (NO) in cancer metabolism. Exposure of SKOV3 ovary cancer cells to DETA-NONOate (a commonly used NO donor) for 24 hours showed that low physiologic concentrations of NO promotes glycolysis, oxidative defense and cell proliferation. These effects were mediated, in part, by the phosphorylation and translocation of PKM2 to the nucleus in response to the activation of the epidermal growth factor receptor (EGFR) and extracellular signal-regulated protein kinase 2 (ERK2) axis. However, at high NO concentrations, glycolysis and PKM2 translocation were both inhibited, concomitant with increased cell death [57] (Figure 3). In addition, NO has been reported to alter glycolysis by direct inhibition of PKM2 enzymatic activity through S-nitrosylation. In an experimental model of acute kidney injury (AKI), S-nitrosylation of PKM2 at cysteine-423/424 (C-423/424) inhibited the tetramerization of PKM2 and led to the accumulation of glycolytic metabolites [58]. Recently, Siragusa and colleagues reported that active endothelial NO synthase (eNOS) can directly interact with PKM2 leading to the S-nitrosylation and inhibition of PKM2 enzymatic activity, and the subsequent accumulation of reducing equivalents to maintain redox homeostasis in human umbilical vein and mouse pulmonary endothelial cells [59].
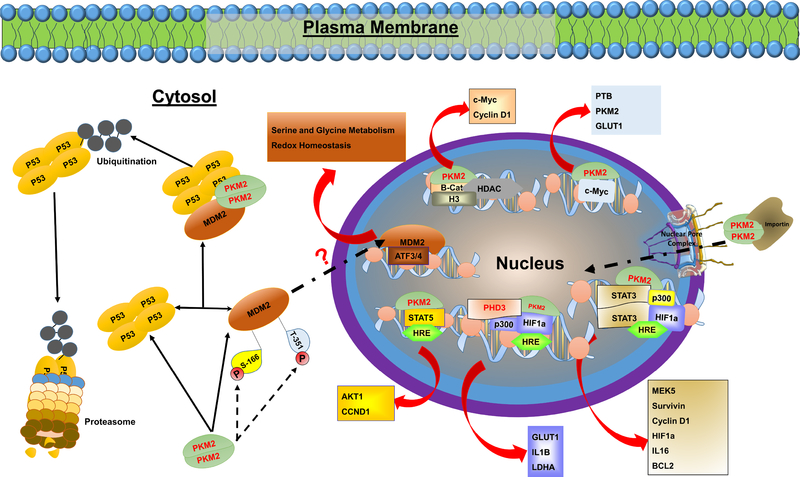
Figure 3. Direct and indirect regulation of gene expression by PKM2.
In addition to its enzymatic role, PKM2 exhibits a role in the regulation of gene expression. In its dimeric form, PKM2 can translocate to the nucleus where it interacts with HIF-1a, c-MYC, STAT3, STAT5, β-Catenin, and many others, to regulate the expression of numerous proteins involved in complex biological and biochemical processes. In addition, PKM2 can directly bind to P53 and MDM2, which leads to P53 ubiquitination and degradation. Recent studies have shown that PKM2-MDM2 interaction leads to increased MDM2 phosphorylation at serine-166 (S-166) and threonine-351 (T-351) sites and prevents its recruitment to nuclear chromatin [205]. However, these findings contradict previous reports showing that MDM2 phosphorylation at S-166 is essential for its nuclear translocation [98] and its role in regulating the expression of genes involved in serine-glycine metabolism, and redox homeostasis [97].
Likewise, the induction of oxidative stress in lung cancer cells through exposure to hydrogen peroxide (H2O2) or diamide remarkably decreased PKM2 enzymatic activity through oxidation of the sulfhydryl group of cysteine-358 (C-358), which promotes the disassociation of the tetramer form [50]. In addition, oxidative stress induced by H2O2 and menadione was demonstrated to increase sirtuin 5 (SIRT5)-mediated desuccinylation of PKM2 at lysine-498 (K-498) and decreases its enzymatic activity [60]. The succinylation of PKM2 at K-498 was reported to alter tumor growth by sensitizing cells to oxidative damage as a result of decreased NADPH levels [60]. In a recent study examining the effects of PKM2 inhibition on human ovarian carcinoma cell survival, the authors demonstrated that shikonin, a liposoluble naphthoquinone isolated from Lithospermum erythrorhizon and a potent inhibitor of PKM2, induced cell death through increased oxidant levels with a concomitant decrease in PKM2 expression. Conversely, treatment with the glutathione precursor and antioxidant; N-acetyl-L-cysteine (NAC) completely abolished the effects of shikonin on PKM2 expression and cell survival [61], emphasizing the role of redox homeostasis in modulating PKM2 activity and functions. Yet, further research is needed to fully understand the mechanisms by which oxidants regulate PKM2 and the subsequent physiological, biochemical, and molecular implications.
1.6.2. Regulation of PKM2 by Phosphorylation
Phosphorylation is one of the major post-translational modifications that either activate or deactivate proteins. PKM2 is phosphorylated at different sites in response to various stimuli (Figure 2). Oncogenic growth factors including insulin growth factor-1 (IGF-1) [62], epidermal growth factor (EGF) [32], and basic fibroblast growth factor (bFGF) [54] promote PKM2 phosphorylation in a mechanism mediated by several kinases including protein kinase B (PKB; a.k.a. AKT), ERK1/2, and Janus kinase 2 (JAK2). Additionally, the phosphorylation of PKM2 at different amino acid sites differentially regulate its activity and subcellular location. For example, the phosphorylation of PKM2 at tyrosine-105 (Y-105) inhibits its catalytic activity by altering the tetramer and dimer states that promote the Warburg effect (aerobic glycolysis) [54]. Similarly, the phosphorylation of PKM2 at serine-37 (S-37) and threonine-454 (T-454) induces the Warburg effect by enhancing PKM2 nuclear accumulation [32, 56]. Generally, PKM2 phosphorylation appears to promote tumorigenesis and decrease mitochondrial function [56]. However, low molecular weight protein tyrosine phosphatase (LMW-PTPs)-mediated dephosphorylation of PKM2 was recently shown to promote PKM2 enzymatic activity and inhibits its nuclear translocation. The dephosphorylation of PKM2 has been correlated with reduced growth and invasiveness in melanoma cells [63].
1.6.3. Other Post-translational Modifications
Aside from the phosphorylation modification of PKM2, it has been reported that the acetylation of PKM2 at lysine-433 (K-433) enhances PKM2 import to the nucleus [51], while its deacetylation by SIRT6 causes the protein to be exported from the nucleus [64]. This nuclear translocation of PKM2 strengthened the interest in identifying potential non-glycolytic functions of PKM2 as reviewed by Alves-Filho et al. [21]. The export of PKM2 from the nucleus has been suggested to decrease its contribution toward tumorigenesis [64]. Specifically, PKM2 nuclear translocation results in increased cancer cell proliferation and migration. In addition, methylation of PKM2 by co-activator-associated arginine methyltransferase 1 (CARM1) has been reported to be specific to the dimeric form at different arginine sites (R-445/447/455), and does not necessarily alter its enzymatic activity [53]. However, in a recent study, methylation of PKM2 at R445/447 was shown to promote PKM2 tetramerization resulting in an increase in its enzymatic activity [65]. The reasons for the differences between the two studies are not clear; however, it is worth noting that in both studies, the interaction of CARM1 and PKM2 appears to play a critical in the metabolic reprogramming of cancer cells and suggest that targeting PKM2 methylation may have therapeutic value. In addition, PKM2-hydroxylation at proline sites 403 and 408 (P-403/408) by prolyl hydroxylase 3 (PHD3) was shown to promote its interaction with hypoxia-inducible factor 1-alpha (HIF-1α) and the transcription of HIF-1α-dependent genes [66] (Figure 2). Taken together, the fact that several post-translational modifications can regulate PKM2 activity, expression, and/or its subcellular localization underscores the importance of this protein and the significance of studying its potential diverse roles in health and disease.
2. PKM2 in Health and Disease
PKM2 exists mainly in the cytosol as a glycolytic enzyme [67]; however, under certain conditions, it can translocate to the nucleus and regulate cell proliferation through altering gene expression. Phosphorylation at S-37 allows PKM2 to bind to the peptidyl-proline isomerase (PIN1) protein, resulting in cis-trans isomerization and the binding of the NLS of PKM2 to importin α5. Once formed, this complex will allow the docking and translocation of PKM2 through the nuclear pore complex (NPC) [32] (Figure 2). The translocation of PKM2 to the nucleus suggests other functions of the enzyme beyond glycolysis [17] (Figure 3). In tumor cells, PKM2 promotes cells survival and proliferation through the phosphorylation of Bub3 and the suppression of cyclin D in order to control the transition between G1 and S phases of the cell cycle [68]. PKM2 has also been reported to regulate HIF-1α activity, leading to an increase in its ability to bind to the hypoxia-responsive elements (HREs) and regulate gene expression [66]. Highlighting the differences between the PKM isoforms and their subcellular localization is essential since the translocation to the nucleus appears to be PKM2 specific. To the best of our knowledge, no prior studies have demonstrated that PKM1 can translocate to the nucleus. In addition to its role in regulating gene expression, the dimeric PKM2 exhibits kinase potential beyond glycolysis, where it also utilizes PEP as a phosphate donor [69, 70]. Recent studies have shown that PKM2 phosphorylates essential proteins involved in cancer pathology and metabolism including histone H3 [4], Bub3 [68], myosin regulatory light chain 2 (MLC-2) [3], signal transducer and activator of transcription 3 (STAT3) [70], and nuclear sterol regulatory element-binding protein 1a (SREB1a) [71].
2.1. PKM2 Roles in Cancer Metabolism
PKM2 plays a crucial role in cancer cell metabolism. In both proliferative and tumor cells, the overall demand for energy is higher. Otto Warburg reported an increase in glucose consumption accompanied by high lactate production in cancer cells. This observation was later called aerobic glycolysis (a.k.a. Warburg effect) due to the production of lactate within the availability of oxygen [72]. Despite the lower production of ATP from aerobic glycolysis compared to the net energy generated from oxidative phosphorylation in the mitochondria, cancer cells favor metabolizing glucose through the Warburg effect to yield ATP at a faster rate in response to the increased demand of energy [5]. PKM2 is considered as a significant regulator of the Warburg effect in cancer cells, and its upregulation is mostly correlated with increased glucose utilization [73] and alterations in the redox balance [74]. This shift in metabolism not only provide cancer cells with ATP but also favors the production of needed intermediates essential for multiple anabolic pathways. For instance, the glycolytic intermediate, dihydroxyacetone phosphate (DHAP), is used for membrane lipid synthesis [75] to promote the production of membranes required for dividing cells, while additional glycolytic metabolites serve as precursors to advance the generation of cellular building blocks (nucleotides and amino acids) [76, 77]. Because oxidative phosphorylation is suppressed in nearly all cancer types [78], citrate first accumulates in the mitochondrial before being extruded into the cytosol, where it is cleaved by ATP-citrate lyase (ACL) into acetyl-CoA and oxaloacetate (OAA). In the cytosol, acetyl-CoA is utilized for histone acetylation and de novo lipogenesis, whereas OAA is converted into pyruvate [79].
2.1.1. Modulation of Tumor Redox Homeostasis by PKM2
Most cancer types at early stages exhibit a significant increase in oxidants compared to normal cells. The increase in oxidants such as H2O2 induces the generation of free hydroxyl radicals mediated through the Fenton reaction, where free hydroxyl ions can attack and oxidize DNA [80], resulting in DNA damage and mutations that contribute to cancer initiation and progression [80, 81]. Oxidants also contribute to the metabolic reprogramming associated with cancer cells by regulating the expression and stability of HIF-1 [82, 83]. HIF-1 is a well-established regulator of PKM2 expression and a major player in cancer cells homeostasis. HIF-1 is composed of two subunits; an inducible alpha-subunit (HIF-1α) and constitutively expressed β-subunit (HIF-1β). The dimerization of both subunits is essential for HIF-1α transcriptional activity in cancer cells. In addition to its role in promoting the transcription of PKM2, HIF-1α also regulates the transcription of other enzymes involved in anaerobic metabolism, including aldolase A (ALDOA). Luo and colleagues demonstrated that this event occurs in response to the direct interaction between PKM2 and HIF-1α, which enhances the ability of HIF-1 to bind to the hypoxia response elements (HRE) and recruit P300 [66] (Figure 3). In addition, a plethora of other proteins and transcription factors involved in angiogenesis, invasion, metastasis, as well as chemical and radiation resistance have also been reported to be regulated by HIF-1α (reviewed in [84–86]).
Several mechanisms for the role of oxidants level in regulating HIF-1α in cancer cells, under both normoxic and hypoxic conditions, have been proposed. These mechanisms of regulation involve major kinases such as phosphoinositide 3-kinase (PI3K), protein kinase C (PKC), and mitogen-activated protein kinase (MAPK) and signaling pathways including the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and the PI3K/AKT pathways [82, 87, 88]. The excessive energy requirement of proliferative cells in a developing tumor leads to hypoxia and stabilization of HIF-1α by inhibiting proteins involved in its proteasomal degradation, particularly the von-Hippel-Lindau (VHL)-ubiquitin ligase complexes and the prolyl hydroxylase domain (PHD)-containing proteins (PHDs). However, under normoxic conditions, proteasome-mediated degradation of HIF-1α occurs via the activation of the von-Hippel-Lindau (VHL)-ubiquitin ligase complexes that recognize two hydroxylated proline sites on HIF-1α 402 and 564 (P-402/564) [89]. Hydroxylation of the two proline sites is mediated by PHDs which were shown to be inhibited under hypoxic conditions by oxidation [90] and nitrosylation [91], in response to increased levels of H2O2 and NO, respectively. Likewise, stabilization of HIF-1α expression can occur in response to oxidation-mediated inhibition of the asparaginyl hydroxylase factor inhibiting HIF-1 (FIH-1) [92]. Furthermore, Jung and colleagues demonstrated that, under hypoxic conditions, the exposure of human prostate carcinoma cells to oxidants such as H2O2 or menadione could lead to the stabilization of HIF-1α through the activation of adenosine monophosphate-activated protein kinase (AMPK). Whereas, inhibition of AMPK abrogates the effects of H2O2 or menadione on the expression of HIF-1α [82]. In addition, oxidants can increase the expression of HIF-1α independent of AMPK activation through activating the PI3K/mammalian target of rapamycin (mTOR) signaling pathway [93].
A growing body of literature supports the role of PKM2 in regulating redox homeostasis and the adaptation of cancer cells to stress via multiple mechanisms. PKM2 has been found to play a major role in the survival of cancer cells by shifting the metabolic flux from glycolysis to the pentose phosphate pathway. In response to oxidative stress inducers, H2O2 mediates the oxidation of PKM2 at C-358, reduces PK enzymatic activity [50], and shifts the glycolytic metabolites toward the pentose phosphate pathway. The pentose phosphate pathway plays a crucial role in supporting cancer cell survival and proliferation through the production of the reduced form of NADPH, needed by glutathione to prevent excessive oxidants accumulation [94] (Figure 4). Likewise, the increase in H2O2 levels enhances SIRT5 and PKM2 interaction leading to the desuccinylation of PKM2 at K-498 residue [60] and the shift of the metabolic flux from glycolysis to the pentose phosphate pathway. On the other hand, enhancing PKM2 enzymatic activity using specific activators such as ML-285 [95] and DASA-10 [50] increases intracellular oxidants accumulation and sensitizes cancer cells to oxidative stress and cell death.
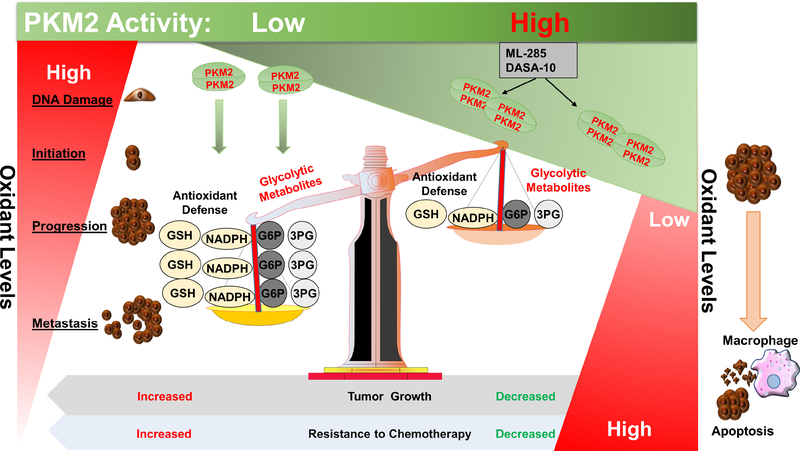
Figure 4. PKM2 regulation of cancer redox homeostasis.
At early stages of cancer, increased oxidant levels play a critical role in promoting cancer initiation and development. Once the tumor is formed, cancer cells adapt a mechanism to reduce oxidant levels and to prevent the cells against oxidant-induced cell death. PKM2 enzymatic activity plays an important role in this antioxidant defense. The increase in oxidant levels leads to a decrease in PKM2 enzymatic activity and accumulation of the glycolytic metabolites; glucose-6-phosphate (G6P) and 3-phosphoglycerate (3PG), which serve as precursors for the pentose phosphate and the serine-glycine biosynthetic pathways, respectively. This result in increased production of reduced equivalents such as NADPH, and glutathione (GSH) to amplify the antioxidant defense and promote tumor growth and resistance to chemotherapy. PKM2 activators such as ML-285 and DASA-10 sensitize cancer cells to oxidant-induced cell death, in part, through a decrease in the levels of glycolytic metabolites and the overall antioxidant defense.
Other potential mechanisms through which PKM2 contributes to the redox balance in cancer cells involve the upregulation of the mouse double minute 2 (MDM2) oncoprotein and the downregulation of the tumor suppressor gene P53. MDM2 is a key player in amino acid metabolism and redox balance, and its expression is upregulated in most cancers [96]. Nuclear translocation of MDM2 in response to a plethora of stimuli leads to the upregulation of genes involved in redox homeostasis, and glycine-serine de novo synthesis [97] (Figure 3). MDM2 nuclear translocation and recruitment to chromatin can be regulated by several kinases, including AKT [98], and PKM2. In addition, MDM2 is also sensitive to cellular redox changes. Treating human non-small cell lung carcinoma cells (H1299) with H2O2 or menadione, enhanced MDM2 recruitment to chromatin. Remarkably, the same study also demonstrated that shikonin-mediated inhibition of PKM2, as well as the knockdown of PKM2, increased the levels of chromatin-bound MDM2. These results were further confirmed by site-directed mutagenesis of serine-166 (S-166) and threonine-351 (T-351) on MDM2 to phosphorylation resistant alanine residues (MDM2-S166A/T351A), which abolished the PKM2-MDM2 interaction and increased the recruitment of MDM2 to chromatin. The authors speculated that PKM2 might inhibit MDM2 recruitment to chromatin mediated by the phosphorylation of MDM2 on S-166 and T-351 [97]. These findings, however, contradict previous reports showing that MDM2 phosphorylation at S-166 is essential for its nuclear translocation [98] and its role in regulating the expression of genes involved in serine-glycine metabolism, and redox homeostasis [97] (Figure 3).
MDM2 is also an important E3 ligase of the tumor suppressor P53 [99], recent studies have shown that PKM2 interaction with MDM2 and the resulting effects on serine biosynthesis and redox homeostasis are independent of P53 [100]. P53 is a transcriptional factor and a tumor suppressor that exhibits pro-apoptotic functions. In many cancer types, P53 is either mutated or deleted. However, in tumors expressing the wild-type, the nuclear translocation of MDM2 leads to P53 cytosolic export and its degradation [101]. In a recent study, PKM2 was demonstrated to regulate P53 activity in a redox dependent manner. Stabilization of the active tetramer form of PKM2 differentially modulates P53 activity by suppressing P53 transcriptional activity and apoptosis in an oxidized environment state, but enhancing them in a low oxidation state [102]. Taken together, these studies highlight the significant role of PKM2 in maintaining or reestablishing redox homeostasis, growth, and survival of cancer cells.
2.1.2. The Role of PKM2 in Cancer Initiation and Proliferation
The decreased in PKM2 expression has been shown to reduce the glycolytic rate and suppress tumor growth in multiple cancer types [103]. The reintroduction of PKM2, but not PKM1, restored both the rate of glycolysis and tumor growth, which indicates a critical role for PKM2 in the metabolic shift towards aerobic glycolysis. However, this altered metabolic state is mostly mediated by the interaction of PKM2 with two major transcription factors, which include HIF-1α [66, 104], and c-Myc [25]. PKM2 activates HIF-1α to enhance glucose uptake and utilization by increasing the expression of glucose transporters (GLUT1, GLUT3) [66, 104, 105], and HK [105]. The activation of HIF-1α increases the expression of lactate dehydrogenase A (LDHA) [66], and pyruvate dehydrogenase kinase 1 (PDK1) [106]. PDK1 acts as a negative regulator that phosphorylates and deactivates pyruvate dehydrogenase (PDH) [107], which results in a diminishment in the production of acetyl-CoA and TCA cycle activity [106]. Finally, c-Myc has been demonstrated to upregulate spliceosomes that demonstrate preference, through alternative splicing, in the production of PKM2 over PKM1 [108]. Taken together, PKM2 regulates a molecular and biochemical network of factors that promote aerobic glycolysis and cell proliferation [32].
The role of PKM2 in cancer progression, proliferation, and metastasis has been thoroughly studied. Deletion of Pkm2 in a xenograft mouse model of NCI-N87 cells resulted in smaller tumors compared to controls [109]. Similarly, administration of the PKM2 activator TEPP-46 (50 mg/kg/twice a day) to mice in a xenograft model of H1299 cells delayed tumor latency and lead to smaller tumors when compared to control mice that received vehicle [110]. These data are consistent with others that show silencing (RNAi), or reduction (pharmacological inhibition) in PKM2, suppressed the proliferation of a wide range of cancer cells, including colorectal [111] and pancreatic [104] cancer cells. Similarly, directly injecting tumors with recombinant PKM2 protein enhanced cell proliferation as judged by the increased expression of the cellular proliferation marker Ki-67 [112]. On the other hand, deletion of Pkm2 in cerebellar granule neuron progenitor (CGNP) cells increased their proliferation rate [113].
Many reports have highlighted the role of PKM2 in tumor-induced angiogenesis. Deletion of PKM2 impairs angiogenesis in pancreatic cancer [104]. Conversely, PKM2 released in the circulation positively correlated with angiogenesis in a xenograft model of colon cancer cells. Similarly, the intraperitoneal administration of a recombinant PKM2 (rPKM2) promoted tumor angiogenesis through the upregulation of cluster of differentiation 31 (CD31), a marker for the presence of endothelial cells and tumor angiogenesis [112]. In this study, rPKM2 also promoted the migration, cell-cell adhesion, and cell-extracellular matrix (ECM) adhesion of endothelial cells. The mechanism underlying the role of PKM2 in angiogenesis appears to be mediated by the translocation of PKM2 to the nucleus and its interaction with HIF-α resulting in increased vascular endothelial growth factor (VEGF) expression and blood vessel formation [104]. On the other hand, deletion of PKM2 in tongue squamous cancer cells lowered the activity of manganese superoxide dismutase (SOD2), H2O2 levels, and attenuated cells migration and invasion. These effects were rescued upon restoring PKM2 expression which caused an increase in H2O2 levels and SOD2 activity, along with increased cells proliferation, migration, and invasion [114]. Together, these studies highlight the importance of PKM2 in tumorigenesis through regulation of the redox homeostasis.
2.1.3. The Role of PKM2 in Cancer Progression and Metastasis
Metastasis is an important feature of cancer pathology and a major cause of mortality among cancer patients. Metastasis is composed of multiple steps, including the migration and invasion of cancer cells to the surrounding tissues [115]. A growing body of evidence points to the potential contribution of PKM2 in cancer cell migration. Inhibition of PKM2 suppresses cell migration in gastric and colorectal cancer cells [109, 116] through modulation of the PI3K, AKT, and the mTOR pathways [109]. Further support comes from the finding that diminished PKM2 expression is associated with less invasive TSCC compared to more invasive TSCC. Likewise, PKM2 knockdown suppresses TSCC metastasis to the lung [114].
The involvement of PKM2 in metastasis is not only limited to cell migration but is also involved in epithelial-mesenchymal transition (EMT). E-cadherin is a major player in EMT, and its downregulation has been shown to promote EMT [117]. Western blot analysis of TSCC cells showed a decrease in E-cadherin and an increase in vimentin (EMT marker) in response to PKM2 overexpression [114]. The suppression of E-cadherin is most likely mediated by the interaction of PKM2 and TGF-β-induced factor homeobox 2 (TGIF2). This interaction facilitates the recruitment of histone deacetylase 3 (HDAC3) to the E-cadherin’s promoter leading to the downregulation of E-cadherin expression [118]. Notably, PKM2 expression and its nuclear accumulation increase upon EMT induction, suggesting an influential role of PKM2 in the induction of EMT [119].
2.1.4. The Clinical Significance of Targeting PKM2 in Cancer Therapy
Several studies have shown that PKM2 levels in circulation is elevated and could be used as a diagnostic indicator for a variety of cancer types [112, 120]. In addition, the overexpression of PKM2 is positively associated with tumor progression due to its role in glycolysis, proliferation [121], and apoptosis [18]. Recent studies highlighted the correlation between PKM2 activity and chemoresistance in cancer [22]. Specifically, pharmaceutical inhibition of PKM2 attenuated cisplatin resistance and decreased tumor growth and metastases in bladder cancer [122]. The correlation between PKM2 expression, tumor progression, apoptosis, and chemoresistance makes PKM2 a promising target for cancer therapy.
Several small molecules have been reported to inhibit PKM2 by targeting the FBP binding site, but these compounds are not specific to PKM2 because other PK isoforms also carry the FBP binding sites [123]. Alternatively, natural compounds such as shikonin display more promising results through selective and specific inhibition of the M2 isoform [124]. A recent study investigated the beneficial effects of shikonin treatment to overcome paclitaxel drug resistant in human ovarian carcinoma cell lines. Co-treatment of shikonin and paclitaxel significantly downregulated PKM2 expression and sensitized ovarian cancer cells to oxidant-induced cell death [61]. Although yet to be confirmed, the pro-apoptotic effects of shikonin could be caused by loss of PKM2 expression, resulting in less oxidant clearance and exacerbated oxidative stress. In addition, shikonin inhibits other proteins with important functions in tumorigenesis such as phosphatase and tensin homolog (PTEN) and protein-tyrosine phosphatase 1B (PTP1B) [125]. Although the exact molecular mechanisms are yet to be determined, PKM2-inhibition by most inhibitors can be partially mitigated by adding FBP. However, studies using vitamins K3 and K5 as PKM2 inhibitors have shown efficient PK inhibition even in the presence of FBP [126], suggesting that these molecules inhibit PKM2 in a mechanism that does not involve its allosteric FBP binding site.
Another potential approach to target PKM2 in cancer therapy is by promoting the formation of the active tetrameric form. For example, ML-265 (a compound that activates PKM2 by binding to the dimer-dimer interface of the PKM2 tetramer) showed a significant reduction of the tumor size in a H1299 mouse xenograft model [127]. Likewise, TEPP-46 activates PKM2 through inducing the tetramer formation and increasing the affinity of PKM2 to PEP, thus stabilizing the active form and inhibiting the conversion of the tetramer to the dimer form. This property of TEPP-46 may enhance its therapeutic potential as compared to other PKM2 inhibitors [128]. Lately, a new class of PKM2 activators derived from 4-hydroxy-thiazolidine-2-thione has been discovered, and these molecules display anti-proliferative effects toward several cancer cell lines [129]. However, further experiments are needed to assess the significance of these activators in vivo.
Cancer cells are characterized by a disturbance between the balance of cellular proliferation and apoptosis. In cancer cells, apoptosis or programmed cell death is reduced, partially due to the imbalance between pro and anti-apoptotic proteins responsible for the apoptotic machinery [130]. Therefore, apoptosis is a potential target for cancer treatment. Small molecules that activate or inhibit PKM2 have been reported to increase apoptosis in multiple cancer cell lines [131, 132]. In terms of inducing apoptosis for cancer treatment, apoptosis is triggered by either an intrinsic or an extrinsic pathway. The intrinsic pathway is regulated by the pro-apoptotic B-cell lymphoma 2 (BCL2) family members, such as BAX, BAD, BIM [133], which act on the mitochondrial membrane to dissipate the proton gradient and ultimately promote the release of cytochrome c and the apoptosis-inducing factor (AIF) to the cytosol. The released cytochrome c binds to apoptotic protease activating factor-1 (APAF-1) together with dATP and procaspase 9. This step is essential for the cleavage and activation of caspase 9, which cleaves and activates caspase 3 [133]. Additionally, apoptosis can be induced by extracellular ligands, such as Fas ligand (FasL, a.k.a. CD95L). FasL triggers apoptosis through binding to its receptor Fas R (CD95) and subsequently recruits Fas associated death domain (FADD) and procaspase 8, resulting in the cleavage and activation of caspase 8 and its downstream signaling [134].
A growing body of evidence points to the potential therapeutic benefits of targeting PKM2 in cancer. PKM2 has been strongly linked to apoptosis in a variety of tumors and cancer cell lines. The knockdown of PKM2 promotes the apoptotic machinery in human non-small lung [135] and colon cancer cells [111]. Additionally, PKM2 inhibition in human gastric cancer cells induced the cleavage and activation of caspase-3, caspase-8, and caspase-9 and promoted apoptotic cell death [132]. Furthermore, the exposure of U87 or U251 human glioblastoma multiform cells to H2O2 induced the translocation of PKM2 to the mitochondria and its interaction with BCL2. PKM2-BCL2 interaction mediates the stabilization of BCL2 through phosphorylation at threonine-69 (T-69) and thereby results in the inhibition of apoptosis [18]. Another study has reported a negative association between PKM2 and BIM (pro-apoptotic protein) in primary liver cancer tissues obtained from human patients [136]. The study also showed upregulation of BIM in hepatic cells in response to PKM2 deficiency [136]. Similarly, the depletion of PKM2 upregulates BAX expression (pro-apoptotic protein) in osteosarcoma cell lines [137]. These studies highlight the vital role of PKM2 in promoting the survival of cancer cells. However, the exact molecular and cellular mechanisms mediating the anti-apoptotic functions of PKM2 in cancer cells remain unveiled, and further research is needed.
Recent advances in the use of MicroRNAs (miRNAs) in cancer research have sparked strong interests in targeting PKM2 with miRNAs. MicroRNAs are non-coding small RNAs fragments that play a vital role in regulating protein expression. They act on messenger RNAs to promote their degradation or to inhibit the translation machinery of a wide range of proteins [138]. The increase in PKM2 expression levels associated with multiple cancers has been linked negatively to multiple miRNAs [139–144]. The identification of miRNAs that suppress the expression of PKM2 could open a new promising era for cancer treatment. For instance, dauricine (an alkaloid compound) has been shown to suppress glycolysis and sensitizes HCC cells to chemotherapy. This beneficial effect of dauricine on HCC is partially mediated by the upregulation of miR-199a, which in turn suppresses the expression of PKM2 [144]. Additionally, the expression of miR-625–5p has been reported to be lower in melanoma tissues compared to control. In melanoma cells, overexpressing miR-625–5p resulted in a significant reduction of PKM2 expression accompanied by lower glycolytic rate and cell proliferation [139]. These findings suggest that targeting PKM2 through its regulatory miRNAs may yield valuable insights into novel and more effective strategies for the treatment of cancer.
Previous research has documented the critical role of PKM2 in cancer progression [70] and treatment [145]. This role of PKM2 in cancer is due, at least in part, to its role in cancer metabolism [1]. However, both cancer and inflammatory disorders share a similar metabolic shift that favors anabolic pathways, which are necessary for cell proliferation and serve as a common theme [146]. The similarities in the metabolic shift between cancer and inflammatory disorders have sparked more interest in characterizing the role of PKM2 in the immune response and certain inflammatory disorders and will be reviewed in the next-coming section.
2.2. PKM2 in Inflammation-Associated Diseases
Inflammation is a complex response system that requires the activation of local leukocytes that lead to the recruitment of the surrounding neutrophils and leukocytes [147]. However, the non-resolving or overactive systematic inflammatory responses (SIRs) can lead to serious health issues, such as sepsis and septic shock [148]. These inflammatory responses require a massive amount of energy to meet the immune cells’ demands [149]. Therefore, the metabolic reprogramming (shift from a resting to an active metabolic state) plays a central role in providing immune cells with not only ATP but also metabolic intermediate molecules needed for the synthesis of pro-inflammatory cytokines [149]. This change in metabolism is similar to that of cancer cells (Warburg effect). Thus, glycolytic enzymes, especially pyruvate kinase M2, play an essential role in this metabolic shift [21].
2.2.1. Sepsis
Previous studies have demonstrated that the activation of toll-like receptors (TLRs) in immune cells during sepsis and septic shock mimics cancer cells’ metabolism and promotes the switch from oxidative phosphorylation to glycolysis and accumulation of TCA cycle intermediates, including succinate and fumarate [150–152]. Notably, the expression of PKM2 has been demonstrated to increase upon lipopolysaccharides (LPS) treatment in activated macrophages and this was attributed to the accumulation of succinate, suggesting a critical role of PKM2 in mediating the inflammatory response during sepsis [153]. Succinate has both autocrine and paracrine functions, and its accumulation in activated immune cells results in the inhibition of PHDs activity and the consequent stabilization of HIF-1α [154–156]. High levels of succinate were found to promote the production of interleukin-1 beta (IL-1β) [154] through PKM2-mediated activation of HIF-1α [153]. In an experimental model of endotoxemia, inhibition of PKM2 using shikonin resulted in a higher survival rate, reduced serum lactate levels and the release of high mobility group box 1 (HMGB1), a well-established mediator of endotoxin-induced lethality [2]. Likewise, genetic ablation of Pkm2 in myeloid cells protected mice from lethal endotoxemia and polymicrobial sepsis through a decrease in IL-1β production, and the inhibition of NOD-like receptor (NLR) family pyrin domain-containing 3 (NLRP3) [157]. Together with other inflammasome components, including absent in melanoma 2 (AIM2), NLRP1, and NLR family CARD domain-containing protein 4 (NLRC4), the NLRP3 complex plays a critical role in the innate immune system and the overall inflammatory response [158, 159]. NLRP3 is positively associated with an increased incidence of multiple inflammatory diseases [160]. The activation of NLRP3 leads to the maturation of two essential proteins in the innate immune system: caspase-1 and IL-1β [161]. Although the mechanisms underlying the activation of NLRP3 are not entirely understood, in a study by Xie and collaborators, PKM2-dependent glycolysis was reported to promote NLRP3 and AIM2 activation in macrophages during sepsis, a process that depends on lactate production. The authors demonstrate that the increase in lactate production promotes the phosphorylation and activation of the eukaryotic translation initiation factor 2 alpha kinase 2 (EIF2AK2) [157]. Once activated, EIF2AK2 physically binds to several components of the NLRP3 complex leading to the assembly and activation of NLRP3 [162]. Collectively, these studies shed light on a novel role for PKM2 in sepsis and identify it as a potential therapeutic target for inflammatory diseases.
2.2.2. Atherosclerosis
The involvement of PKM2 is not limited to sepsis, but it is also involved in the chronic inflammation state seen in atherosclerotic coronary artery diseases (CAD). In patients with atherosclerotic CAD, the elevated levels of cytokines, including IL-6 and IL-1β, induce chronic tissue inflammation, which contributes to the progression of CAD. PKM2 likely increases the production of cytokines through phosphorylating the transcription factor STAT3 [163]. Hyperhomocysteinemia induces atherosclerotic lesion formation in an apolipoprotein E deficient experimental mouse model of CAD. In this model, treatment with shikonin attenuated the hyperhomocysteinemia induced lesion formation, as judged by Oil Red O staining for the aortic roots. More importantly, shikonin treatment decreased the aortic transcription of cytokines [tumor necrosis factor alpha (TNF- α), IL-2, and interferon gamma (IFNγ)], and intracellular adhesion molecule-1 (ICAM-1), vital for lesion formation and development. This beneficial effect of shikonin was attributed to the inhibition of PKM2, which disrupted the metabolic reprogramming required for B cell activation, proliferation, and antibodies production [164]. Recently, inhibition of PKM2 through eNOS-mediated S-nitrosylation, was reported to play a key role in mediating the protective effects of NO against endothelial dysfunction and the development of atherogenesis [59]. Although more in-depth research along these lines is needed, the existing literature points to a novel role for PKM2 in cardiovascular diseases.
2.2.3. Inflammatory Bowel Disease
Inflammatory bowel disease (IBD) is a chronic intestinal inflammatory state which includes Crohn’s disease (CD) and ulcerative colitis (UC) (thoroughly reviewed in [165, 166]). Leukocyte infiltration, increased oxidants production, and elevated cytokines play a major role in the etiology of IBD [167]. Furthermore, there is emerging evidence highlighting the role of PKM2 in the inflammatory response associated with IBD. According to a study by Almousa et al., the level of PKM2 in the serum of IBD patients was approximately 6-fold higher than healthy controls. However, there were no differences in serum PKM2 between CD and UC patients [168]. Although CD and UC are two different conditions, an exacerbated inflammatory response is common between the two conditions and may explain why PKM2 is elevated in both diseases. In the same study, treating intestinal Caco2 cells with LPS increased the expression of PKM2. However, the increase in PKM2 levels was suppressed after treating the cells with flaxseed, a rich source of polyunsaturated fatty acids and soluble fibers with anti-inflammatory properties [168]. Further studies are required to define the contribution of PKM2 to IBD with the focus on leukocyte activation, cytokine production, and redox signaling.
Metabolomic and proteomic studies identified succinylation and sirtuins (SIRTs) as important regulators of the inflammatory responses and redox signaling. Interestingly, the activity of PKM2 was also reported to be regulated by succinylation modification [60, 169]. Succinylation acts as a post-translational covalent modifier through the addition of a succinyl group to a lysine residue. This is a diverse process that is often interconnected or substituted for acetylation reactions [170]. SIRT5 is a protein of the deacetylases family exhibiting weak deacetylase activity [171], and its function remains uncertain. However, recent studies demonstrated that SIRT5 can desuccinylate PKM2 at lysine-311 (K-311) residue, which alters the tetramer to dimer ratio and results in an overall reduction in PKM2 kinase activity and inhibition of its nuclear translocation. Furthermore, this shift in activity through desuccinylation could potentially lead to diverse metabolic alterations regarding PKM2 and its functions. As a result of PKM2 desuccinylation, the expression of IL-1β was lower in LPS-induced macrophages, suggesting an important role of PKM2 post-translational modifications in a model of LPS-induced inflammation. In addition, Sirt5 KO mice are more sensitive to dextrin sulfate sodium (DSS) induced colitis, partially due to the overexpression of IL-1β and the increase in PKM2 dimerization [172]. These studies support the idea that PKM2 may possibly regulate acute inflammatory responses, and the activation of PKM2 may prevent against the onset of ulcerative colitis. Depiction of the significance of the succinylation reactions and how they affect PKM2 activity will be essential for a better understanding of the molecular mechanisms mediating PKM2 roles in immune cells metabolism.
2.3. Neuropathic Pain
PKM2 has also been shown to have a potential role in the inflammatory responses associated with neuropathic pain [173]. In nerve injury, pro-inflammatory cytokines contribute significantly to the etiology of neuropathic pain [174]. These pro-inflammatory cytokines mediate the recruitment of leukocytes to the site of nerve injury, which is critical for initiating neuropathic pain [175]. Wang and collaborators studied the involvement of PKM2 in inflammation and neuropathic pain using an experimental model of chronic constriction injury (CCI) in rats. Of note, PKM2 expression in the spinal cord increased significantly in the CCI in comparison to controls. Specifically, PKM2 expression was higher in neurons, astrocytes, and microglial cells. However, PKM2 deficiency attenuated pain hypersensitivity and decreased TNF-α and IL-1β levels within the spinal cord after CCI [173]. The beneficial effects of PKM2 deficiency in these studies suggest that this protein plays a key role in the pathogenesis of neuropathic pain and its associated diseases and warrant an additional investigation into its site(s) and mechanism(s) of actions.
Previous research has linked PKM2 to chronic inflammation under multiple inflammatory-induced research models. However, chronic inflammation is known to contribute significantly to the pathology of several diseases, including metabolic disorders such as diabetes and diabetic nephropathy [176]. Based on the function of PKM2 and its role in inflammation, glycolysis and regulation of genes involved in the pathogenesis of metabolic diseases (HIF-1α, AKT, GLUT, etc...), it is logical to assume that PKM2 also plays a role in the pathology of metabolic disorders.
2.4. PKM2 and Metabolic Disorders
2.4.1. Insulin Signaling and Regulation
Insulin is an essential hormone that regulates macronutrients metabolism. In cancer cells, it has been elucidated that insulin could favor anabolic pathways and contribute to tumorigenesis by increasing glucose consumption and lactate production. IGF-1, which shares similar activities to insulin but can also be stimulated by insulin, has been positively associated with several cancers [177, 178]. Treating hepatocellular carcinoma (HepG2) cells with insulin significantly increased the expression of PKM2, while the depletion of PKM2 inhibited the effects of insulin on glucose consumption and lactate production [179]. In a different study, insulin stimulation increased the expression of PKM2 mediated through the PI3K/mTOR pathway, which in turn increases the expression of HIF-1α. This upregulation of PKM2 seems to play a crucial role in insulin-induced aerobic glycolysis, as the knockdown of PKM2 partially inhibited glucose uptake and lactate production [180].
On the other hand, HIF-1α has been demonstrated to promote obesity-associated inflammation, inhibits insulin signaling, and promotes angiogenesis in an attempt to lower adipose tissue-hypoxia associated with adipocyte enlargement [181]. The finding that PKM2 could affect HIF-1α expression suggests that PKM2 may regulate the insulin pathway and obesity-associated inflammation [180]. However, further research is needed to better understand the exact metabolic function of PKM2 and its role in insulin signaling under normal physiological conditions. Wang et al. sought to identify the role of PKM2 in beta cells and insulin secretion upon glucose (25 mM) stimulation in a non-human pancreatic cell line (NIT-1) [182]. The main finding of the study demonstrated that PKM2 expression decreased when cells were exposed to high glucose conditions. This decline of PKM2 expression suggested a potential contribution of PKM2 in insulin secretion. The authors subsequently indicated that PKM2 overexpression leads to an increase in beta cell proliferation and a significant reduction in apoptosis. As a result of these cellular changes in response to PKM2 overexpression, the authors observed a significant increase in insulin secretion which is most likely mediated through the Wnt/catenin beta 1 (Wnt/CTNNB1) pathway. This study provided new insight into the role of PKM2 in beta islets and suggested the use of PKM2 as a potential therapeutic target in treating diabetes [182]. Yet, further research on the role of PKM2 on insulin synthesis and overall glucose homeostasis is required.
2.4.2. Shikonin Enhances Glucose Tolerance
Obesity is a serious health issue and a risk factor for multiple metabolic disorders such as type 2 diabetes. Type 2 diabetes often results from disruptions in glucose homeostasis, partially due to the development of insulin resistance in major metabolic tissues such as the liver and adipose tissue. In previous studies, we determined the effects of shikonin on glucose tolerance and adiposity in mice fed either regular chow or high-fat diets [183], and we reported a reduction in weight gain and resistance to HFD-induced glucose intolerance in shikonin-treated animals. Furthermore, shikonin treatment attenuated HFD-induced hepatic dyslipidemia, enhanced hepatic insulin signaling in both chow and HFD-fed mice. These data suggest that PKM2 is a key player in glucose homeostasis and adiposity [183]. However, as indicated above, shikonin does not only inhibit PKM2 but also inhibits other signaling molecules such as PTEN and PTP1B [125]. Therefore, future studies utilizing a genetic deletion of PKM2 may further elucidate the role of PKM2 in these metabolic disorders.
2.4.3. Hepatic Disorders
Insulin plays a significant role in regulating the metabolic phenotype of the liver, especially as it relates to glucose homeostasis. Depletion of insulin in the liver results in hyperglycemia and altered glucose metabolism [184]. The involvement of PKM2 in liver metabolism was recently investigated by Chen and colleagues who reported a significant increase in PKM2 expression in the liver of insulin-resistant animals. The overexpression of PKM2, in vitro, resulted in higher lipid accumulation in hepatic cancer cells (HepG2) treated with palmitate. However, PKM2 deficiency in these cells enhanced the activation of both AKT and glycogen synthase kinase 3 beta (GSK3β) upon treatment with palmitate and insulin. Mechanistically, these effects appear to be mediated, at least in part, by the activation of STAT 3, as the overexpression of PKM2 increased the phosphorylation and subsequent activation of STAT3 [185]. Previous reports have shown that activation of the STAT3 pathway may result in hepatic insulin resistance via the suppressor of cytokine signaling 3 (SOCS3) protein [186, 187].
Aside from its role in hepatic insulin resistance, increased PKM2 expression was also shown to be associated with obesity and steatohepatitis [188]. PKM2 levels were higher in hepatic tissues obtained from non-alcoholic steatohepatitis mice, and this increase in PKM2 expression seems to be specific to Kupffer cells [188]. Moreover, recent studies have identified a novel role of PKM2 in lipid metabolism and cell proliferation of cancer cells through regulating the activity of sterol regulatory element binding proteins 1a (SREBP-1a). SREBPs are a class of transcription factors that tightly regulate lipid synthesis [189]. Zhao and colleagues demonstrated a direct nuclear interaction between PKM2 and SREBP-1a in hepatocellular carcinoma HepG2 cells. This interaction enables PKM2 to phosphorylate SREBP-1 at threonine-59 (T-59) and increases its stability, which in turn enhances lipid synthesis and cell proliferation [71]. These findings point to a possible contribution of PKM2 to the etiology of multiple metabolic disorders and urge the need for further investigations of the roles of PKM2 in tissues and cells other than tumor cells.
Metabolic disorders affecting the liver are characterized by increased insulin resistance and chronic inflammatory state that disrupt the redox balance. Oxidants are well known for their role in mediating and propagating inflammatory responses [190]. In alcoholic and non-alcoholic steatohepatitis, the transition from acute inflammation towards a chronic inflammatory state with a persistent increase in oxidative stress induces severe liver damage that is mediated through HIF-1α [191]. In a recent study, treatment with digoxin, a cardiac glycoside drug with potent effects on chronic heart failure, attenuated hepatic oxidants production in mice injected with LPS or fed HFD. These beneficial effects were mediated, at least in part, through the suppression of PKM2-promoted HIF-1α transactivation [192]. In theory, these findings could potentially provide a rational basis for targeting PKM2 for the treatment of alcoholic and non-alcoholic steatohepatitis and warrant further investigation.
2.4.4. PKM2 Role in Renal Diseases
Renal diseases are a worldwide public health problem and reaching epidemic proportions. In the United States, renal diseases are the ninth leading cause of death, accounting for 1.8% of deaths in men and women [193]. Despite the efforts of the federal and states governments to monitor and improve the detection, management, and prevention of renal diseases, emerging evidence indicates that the prevalence of both AKI and chronic kidney diseases (CKD), is still on the rise. Hence, early detection and timely treatment are critical for the treatment of both types of renal diseases. Diabetes, obesity, and high blood pressure are among the main causes of CKD. Approximately 14.8% of US adults are estimated to have CKD. 36% of adults with diabetes and 31.2% of adults with high blood pressure have CKD [194]. Other risk factors for CKD include cardiovascular diseases, obesity, smoking, high cholesterol, lupus, and family history of CKD [195]. CKD causes progressive and irreversible damage to the kidneys’ structure and function [196, 197]. AKI, on the other hand, is more commonly reversible than CKD. AKI is characterized by a sudden episode of kidney failure and impairment in the glomerular filtration (GF) capacity leading to proteinuria and increased serum creatinine levels [198]. Risk factors for AKI include a plethora of stressors, such as sepsis and metabolic associated disorders.
Remarkably, both diabetes and hypertension are the primary risk factors for AKI, suggesting that the molecular mechanisms of both types of renal injuries may share common signal transducers. Indeed, mounting evidence indicates that AKI and CKD are closely intertwined and possibly promote one another as both lead to increased proteinuria and a decline in the glomerular filtration rate (GFR) and nephrotoxicity [199]. Moreover, a growing body of literature provides evidence that PKM2 may serve as a biomarker for nephrotoxicity justifying the increased interest in unveiling the potential role of PKM2 in renal diseases. In a recent study, cisplatin-induced nephrotoxicity in rats led to increased urinary PKM2 levels [200] concomitant with increased lactate excretion and altered levels of amino acids, glucose, and TCA intermediates in the urine [201]. Consistent with these findings, renal tubular HK-2 cells treated with cisplatin, but also other nephrotoxic agents such as cyclosporine A, CdCl2, or HgCl2 exhibited an increase in PKM2 secretion in the conditioned media [202]. These effects were specific to the kidney cells, as neither liver nor breast cancer cells excreted PKM2 in response to cisplatin treatment [202]. Additionally, PKM2 excretion into the media coincided with the appearance of apoptotic markers and was blocked in the presence of Z-VAD-FMK, a pan-caspase and apoptosis inhibitor [202]. Taken together, these studies identify PKM2 as a significant contributor to renal function and possibly a novel marker of nephrotoxicity. This hypothesis is further strengthened by the findings that renal PKM2 activity and expression are significantly altered in patients with diabetic nephropathy (DN). DN is a serious health condition resulting from disturbances in glucose homeostasis. Specifically, DN is the result of hyperglycemia, and it is a classical complication of diabetes [203]. In most severe cases, DN leads to end-stage renal disease (ESRD) and is characterized by nephrotoxicity and alterations in podocyte function [203]. Both PKM2 expression and activity were shown to be lower in DN patients compared to diabetic patients without DN [204]. In addition, PKM2 activity was reduced in cultured podocytes upon glucose exposure. This reduction in PKM2 activity is possibly due to its oxidation at C-358 residue, leading to the inhibition of the tetramer formation. Importantly, specific deletion of PKM2 in podocytes increases albuminuria and induces apoptosis in an STZ-induced diabetes model. Furthermore, while depletion of PKM2 caused mitochondrial dysfunction, its activation by TEPP-46 rescued these effects. Based on these findings, targeting PKM2 may be a novel strategy for preventing or treating renal diseases [204].
3. Conclusion and Perspective
Despite the robust evidence for the critical role of PKM2 in cancer, inflammation, and metabolic disorders, most of the studies investigated the potential therapeutic role of PKM2 were conducted in cell and animal models. This raises a question whether the manipulation of PKM2 in humans will lead to beneficial effects similar to what have been observed in animal studies. Additionally, most of the inhibitory compounds used to manipulate PKM2 were not specific to the M2 isoform. In addition, they may inhibit other PK isoforms that are essential for healthy physiology, which may result in toxicity for untargeted organs. Furthermore, these compounds are not tissue-specific, which may lead to the inhibition of PKM2 in healthy organs, possibly resulting in undesirable clinical outcomes. Therefore, further studies are required to illustrate the clinical significance of PKM2 activators and inhibitors and their short and long-term health effects.
Highlights.
Pyruvate kinase M2 is a critical enzyme that regulates cell metabolism and growth under different physiological conditions.
Despite the robust evidence for the critical role of PKM2 in cancer, little is known about its potential roles in inflammation and metabolic diseases. A growing body of literature suggests that this protein may be crucial for the homeostasis of normal healthy tissues.
PKM2 inhibition in humans may lead to beneficial effects similar to those observed in animal studies. However, most of the used compounds were not specific to the PKM2 isoform and may inhibit other pyruvate kinase isoforms that are essential for maintaining homeostasis in healthy tissues.
There is a need to evaluate the effects of PKM2 inhibitors on healthy tissue prior to their use in cancer therapy.
Acknowledgements
This work was supported by the National Institute of Health (R00DK100736) to A.B. MQ is supported by the Saudi Arabia Cultural Mission.
Abbreviations
- ACL
ATP-citrate lyase
- AIF
apoptosis-inducing factor
- AIM2
absent in melanoma 2
- AKI
acute kidney injuries
- AKR1A1
aldo-keto reductase family 1 member A1
- ALDOA
aldolase A
- AMPK
adenosine monophosphate-activated protein kinase
- Apaf-1
apoptotic protease activating factor-1
- ATP
adenosine triphosphate
- BAD
BCL2-associated agonist of cell death
- BAX
BCL2-associated X protein
- Bcl2
B-cell lymphoma 2
- bFGF
basic fibroblast growth factor
- BIM
BCL-2-like protein 11
- CAD
coronary artery diseases
- CARM
co-activator-associated arginine methyltransferase
- Caspase
cysteine-aspartic acid protease
- CCI
chronic constriction injury
- CD
Crohn’s disease
- CD31
cluster of differentiation 31
- CGNP
cerebellar granule neuron progenitor
- COX
cyclooxygenase
- DHAP
dihydroxyacetone phosphate
- DN
diabetic nephropathy
- DSS
dextrin sulfate sodium
- EGF
epidermal growth factor
- EIF2AK2
eukaryotic translation initiation factor 2 alpha kinase 2
- EMT
epithelial-mesenchymal transition
- eNOS
endothelial NO synthase
- ER stress
endoplasmic reticulum stress
- ERK
extracellular signal-regulated protein kinase
- ESRD
end-stage renal disease
- FADD
Fas associated death domain
- FBP
fructose 1,6-bisphosphate
- FIH-1
asparaginyl hydroxylase factor inhibiting HIF-1
- GF
glomerular filtration
- GFR
glomerular filtration rate
- GK
glucokinase
- GLUT
glucose transporter
- H2O2
hydrogen peroxide
- HDAC
histone deacetylase
- HFD
high fat diet
- HIF-1α
hypoxia-inducible factor 1-alpha
- HK
hexokinase
- HMGB
high mobility group box
- hnRNPA
nuclear ribonucleoproteins A
- IBD
Inflammatory bowel disease
- ICAM
intracellular adhesion molecule
- IFNγ
Interferon gamma
- IGF
Insulin growth factor
- IL-1β
interleukin-1 beta
- JAK
Janus kinase
- LDHA
lactate dehydrogenase A
- LMW-PTPs
low molecular weight protein tyrosine phosphatase
- LPS
lipopolysaccharides
- MAPK
mitogen-activated protein kinase
- MDM2
mouse double minute 2
- MLC-2
myosin regulatory light chain 2
- mTOR
the mammalian target of rapamycin
- NAC
N-acetyl-L-cysteine
- NADH
nicotinamide adenine dinucleotide
- NADPH
nicotinamide adenine dinucleotide phosphate
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NLRC4
CARD domain-containing protein 4
- NLRP
node like receptor
- NLS
nuclear localization signal sequence
- NO
nitric oxide, OAA: oxaloacetate
- NPC
nuclear pore complex
- PDK
pyruvate dehydrogenase kinase
- PEP
phosphoenolpyruvate
- PFK
phosphofructokinase
- PHD
pyruvate dehydrogenase
- PHDs
prolyl hydroxylase domain-containing proteins
- PHGDH
phosphoglycerate dehydrogenase
- PI3K
phosphoinositide 3-kinase
- PIN1
peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
- PK
pyruvate kinase
- PKB
protein kinase B
- PKC
protein kinase C
- PKM
pyruvate kinase M
- PSAT1
phosphoserine aminotransferase
- PTB
polypyrimidine tract binding protein
- PTEN
phosphatase and tensin homolog
- PTP
protein-tyrosine phosphatase
- RBM4
RNA-binding motif
- ROS
reactive oxygen species
- SIR
systematic inflammatory responses
- SIRT
sirtuins
- SOCS3
suppressor of cytokine signaling 3
- SOD2
superoxide dismutase
- SREBP
sterol regulatory element binding proteins
- SRSF3
serine/arginine-rich splicing factor 3
- STAT
signal transducer and activator of transcription
- TCA
Tricarboxylic acid
- TGIF2
TGF-β-induced factor homeobox 2
- TLR
toll-like receptor
- TNF-α
tumor necrosis factor alpha
- UC
ulcerative colitis
- VEGF
vascular endothelial growth factor
Footnotes
Conflict of interest: The authors declare that there is no conflict of interest regarding the publication of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC, The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth, Nature 452(7184) (2008) 230–3. [DOI] [PubMed] [Google Scholar]
- [2].Yang L, Xie M, Yang M, Yu Y, Zhu S, Hou W, Kang R, Lotze MT, Billiar TR, Wang H, Cao L, Tang D, PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis, Nature communications 5 (2014) 4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jiang Y, Wang Y, Wang T, Hawke DH, Zheng Y, Li X, Zhou Q, Majumder S, Bi E, Liu DX, Huang S, Lu Z, PKM2 phosphorylates MLC2 and regulates cytokinesis of tumour cells, Nature communications 5 (2014) 5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yang W, Xia Y, Hawke D, Li X, Liang J, Xing D, Aldape K, Hunter T, Alfred Yung WK, Lu Z, PKM2 phosphorylates histone H3 and promotes gene transcription and tumorigenesis, Cell 150(4) (2012) 685–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lunt SY, Vander Heiden MG, Aerobic glycolysis: meeting the metabolic requirements of cell proliferation, Annual review of cell and developmental biology 27 (2011) 441–64. [DOI] [PubMed] [Google Scholar]
- [6].Jeremy M Berg JLT, and Lubert Stryer, Biochemistry, 5th edition, New York: W H Freeman; 2002. [Google Scholar]
- [7].Barnett JA, A history of research on yeasts 5: the fermentation pathway, Yeast (Chichester, England) 20(6) (2003) 509–43. [DOI] [PubMed] [Google Scholar]
- [8].Suomalainen H, Arhimo E, Hexosediphosphoric Acid in Living Yeast, Science (New York, N. Y.) 105(2714) (1947) 17–8. [DOI] [PubMed] [Google Scholar]
- [9].Colowick SP, Kalckar HM, The role of myokinase in transphosphorylations I. The enzymatic phosphorylation of hexoses by adenyl pyrophosphate, J. Biol. Chem. 148(1) (1943) 117–126. [Google Scholar]
- [10].Cori CF, Some highlights of the early period of bioenergetics, Molecular and cellular biochemistry 5(1–2) (1974) 47–53. [DOI] [PubMed] [Google Scholar]
- [11].Dayton TL, Jacks T, Vander Heiden MG, PKM2, cancer metabolism, and the road ahead, EMBO reports 17(12) (2016) 1721–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tanaka T, Harano Y, Sue F, Morimura H, Crystallization, characterization and metabolic regulation of two types of pyruvate kinase isolated from rat tissues, Journal of biochemistry 62(1) (1967) 71–91. [DOI] [PubMed] [Google Scholar]
- [13].Susor WA, Rutter WJ, Some distinctive properties of pyruvate kinase purified from rat liver, Biochemical and biophysical research communications 30(1) (1968) 14–20. [DOI] [PubMed] [Google Scholar]
- [14].Peters J, Nash HR, Eicher EM, Bulfield G, Polymorphism of kidney pyruvate kinase in the mouse is determined by a gene, Pk-3, on chromosome 9, Biochemical genetics 19(7–8) (1981) 757–69. [DOI] [PubMed] [Google Scholar]
- [15].Peters J, Andrews SJ, The Pk-3 gene determines both the heart, M1, and the kidney, M2, pyruvate kinase isozymes in the mouse; and a simple electrophoretic method for separating phosphoglucomutase-3, Biochemical genetics 22(11–12) (1984) 1047–63. [DOI] [PubMed] [Google Scholar]
- [16].Noguchi T, Inoue H, Tanaka T, The M1- and M2-type isozymes of rat pyruvate kinase are produced from the same gene by alternative RNA splicing, The Journal of biological chemistry 261(29) (1986) 13807–12. [PubMed] [Google Scholar]
- [17].Stetak A, Veress R, Ovadi J, Csermely P, Keri G, Ullrich A, Nuclear translocation of the tumor marker pyruvate kinase M2 induces programmed cell death, Cancer research 67(4) (2007) 1602–8. [DOI] [PubMed] [Google Scholar]
- [18].Liang J, Cao R, Wang X, Zhang Y, Wang P, Gao H, Li C, Yang F, Zeng R, Wei P, Li D, Li W, Yang W, Mitochondrial PKM2 regulates oxidative stress-induced apoptosis by stabilizing Bcl2, Cell research 27(3) (2017) 329–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Benson DA, Cavanaugh M, Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW, GenBank, Nucleic acids research 45(D1) (2017) D37–D42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Johnson FM, Chasalow F, Anderson G, Macdougal P, Hendren RW, Lewis SE, A variation in mouse kidney pyruvate kinase activity determined by a mutant gene on chromosome 9, Genetical research 37(2) (1981) 123–31. [DOI] [PubMed] [Google Scholar]
- [21].Alves-Filho JC, Palsson-McDermott EM, Pyruvate Kinase M2: A Potential Target for Regulating Inflammation, Front Immunol 7 (2016) 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Israelsen WJ, Vander Heiden MG, Pyruvate kinase: Function, regulation and role in cancer, Seminars in cell & developmental biology 43 (2015) 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yang W, Lu Z, Pyruvate kinase M2 at a glance, Journal of cell science 128(9) (2015) 1655–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Takenaka M, Noguchi T, Sadahiro S, Hirai H, Yamada K, Matsuda T, Imai E, Tanaka T, Isolation and characterization of the human pyruvate kinase M gene, European journal of biochemistry 198(1) (1991) 101–6. [DOI] [PubMed] [Google Scholar]
- [25].Luan W, Wang Y, Chen X, Shi Y, Wang J, Zhang J, Qian J, Li R, Tao T, Wei W, Hu Q, Liu N, You Y, PKM2 promotes glucose metabolism and cell growth in gliomas through a mechanism involving a let-7a/c-Myc/hnRNPA1 feedback loop, Oncotarget 6(15) (2015) 13006–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Clower CV, Chatterjee D, Wang Z, Cantley LC, Vander Heiden MG, Krainer AR, The alternative splicing repressors hnRNP A1/A2 and PTB influence pyruvate kinase isoform expression and cell metabolism, Proceedings of the National Academy of Sciences of the United States of America 107(5) (2010) 1894–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Su CH, Hung KY, Hung SC, Tarn WY, RBM4 Regulates Neuronal Differentiation of Mesenchymal Stem Cells by Modulating Alternative Splicing of Pyruvate Kinase M, Molecular and cellular biology 37(3) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang Z, Chatterjee D, Jeon HY, Akerman M, Vander Heiden MG, Cantley LC, Krainer AR, Exon-centric regulation of pyruvate kinase M alternative splicing via mutually exclusive exons, Journal of molecular cell biology 4(2) (2012) 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kuranaga Y, Sugito N, Shinohara H, Tsujino T, Taniguchi K, Komura K, Ito Y, Soga T, Akao Y, SRSF3, a Splicer of the PKM Gene, Regulates Cell Growth and Maintenance of Cancer-Specific Energy Metabolism in Colon Cancer Cells, International journal of molecular sciences 19(10) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Geer LY, Marchler-Bauer A, Geer RC, Han L, He J, He S, Liu C, Shi W, Bryant SH, The NCBI BioSystems database, Nucleic acids research 38(Database issue) (2010) D492–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dombrauckas JD, Santarsiero BD, Mesecar AD, Structural basis for tumor pyruvate kinase M2 allosteric regulation and catalysis, Biochemistry 44(27) (2005) 9417–29. [DOI] [PubMed] [Google Scholar]
- [32].Yang W, Zheng Y, Xia Y, Ji H, Chen X, Guo F, Lyssiotis CA, Aldape K, Cantley LC, Lu Z, ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect, Nature cell biology 14(12) (2012) 1295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tani K, Fujii H, Tsutsumi H, Sukegawa J, Toyoshima K, Yoshida MC, Noguchi T, Tanaka T, Miwa S, Human liver type pyruvate kinase: cDNA cloning and chromosomal assignment, Biochemical and biophysical research communications 143(2) (1987) 431–8. [DOI] [PubMed] [Google Scholar]
- [34].Imamura K, Tanaka T, Multimolecular forms of pyruvate kinase from rat and other mammalian tissues. I. Electrophoretic studies, Journal of biochemistry 71(6) (1972) 1043–51. [DOI] [PubMed] [Google Scholar]
- [35].Cardenas JM, Dyson RD, Mammalian pyruvate kinase hybrid isozymes: tissue distribution and physiological significance, The Journal of experimental zoology 204(3) (1978) 361–7. [DOI] [PubMed] [Google Scholar]
- [36].Muirhead H, Isoenzymes of Pyruvate Kinase, Biochemical Society Transactions 18 (1990) 193–196. [DOI] [PubMed] [Google Scholar]
- [37].de Luis O, del Mazo J, Gene expression of mouse M1 and M2 pyruvate kinase isoenzymes correlates with differential poly[A] tract extension of their mRNAs during the development of spermatogenesis, Biochimica et biophysica acta 1396(3) (1998) 294–305. [DOI] [PubMed] [Google Scholar]
- [38].MacDonald MJ, Chang CM, Pancreatic islets contain the M2 isoenzyme of pyruvate kinase. Its phosphorylation has no effect on enzyme activity, Molecular and cellular biochemistry 68(2) (1985) 115–20. [DOI] [PubMed] [Google Scholar]
- [39].Osterman J, Fritz PJ, Wuntch T, Pyruvate kinase isozymes from rat tissues. Developmental studies, The Journal of biological chemistry 248(3) (1973) 1011–8. [PubMed] [Google Scholar]
- [40].Wong N, Ojo D, Yan J, Tang D, PKM2 contributes to cancer metabolism, Cancer letters 356(2 Pt A) (2015) 184–91. [DOI] [PubMed] [Google Scholar]
- [41].Yan M, Chakravarthy S, Tokuda JM, Pollack L, Bowman GD, Lee YS, Succinyl-5-aminoimidazole-4-carboxamide-1-ribose 5’-Phosphate (SAICAR) Activates Pyruvate Kinase Isoform M2 (PKM2) in Its Dimeric Form, Biochemistry 55(33) (2016) 4731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Morgan HP, O’Reilly FJ, Wear MA, O’Neill JR, Fothergill-Gilmore LA, Hupp T, Walkinshaw MD, M2 pyruvate kinase provides a mechanism for nutrient sensing and regulation of cell proliferation, Proceedings of the National Academy of Sciences of the United States of America 110(15) (2013) 5881–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gupta RK, Oesterling RM, Dual divalent cation requirement for activation of pyruvate kinase; essential roles of both enzyme- and nucleotide-bound metal ions, Biochemistry 15(13) (1976) 2881–7. [DOI] [PubMed] [Google Scholar]
- [44].Valentini G, Chiarelli L, Fortin R, Speranza ML, Galizzi A, Mattevi A, The allosteric regulation of pyruvate kinase, The Journal of biological chemistry 275(24) (2000) 18145–52. [DOI] [PubMed] [Google Scholar]
- [45].Wang P, Sun C, Zhu T, Xu Y, Structural insight into mechanisms for dynamic regulation of PKM2, Protein & cell 6(4) (2015) 275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chaneton B, Hillmann P, Zheng L, Martin ACL, Maddocks ODK, Chokkathukalam A, Coyle JE, Jankevics A, Holding FP, Vousden KH, Frezza C, O’Reilly M, Gottlieb E, Serine is a natural ligand and allosteric activator of pyruvate kinase M2, Nature 491(7424) (2012) 458–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yuan M, McNae IW, Chen Y, Blackburn EA, Wear MA, Michels PAM, Fothergill-Gilmore LA, Hupp T, Walkinshaw MD, An allostatic mechanism for M2 pyruvate kinase as an amino-acid sensor, The Biochemical journal 475(10) (2018) 1821–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Li Q, Qi X, Jia W, 3,3’,5-triiodothyroxine inhibits apoptosis and oxidative stress by the PKM2/PKM1 ratio during oxygen-glucose deprivation/reperfusion AC16 and HCM-a cells: T3 inhibits apoptosis and oxidative stress by PKM2/PKM1 ratio, Biochemical and biophysical research communications 475(1) (2016) 51–6. [DOI] [PubMed] [Google Scholar]
- [49].Prakasam G, Iqbal MA, Bamezai RNK, Mazurek S, Posttranslational Modifications of Pyruvate Kinase M2: Tweaks that Benefit Cancer, Front Oncol 8 (2018) 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang JK, Shen M, Bellinger G, Sasaki AT, Locasale JW, Auld DS, Thomas CJ, Vander Heiden MG, Cantley LC, Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses, Science (New York, N.Y.) 334(6060) (2011) 1278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lv L, Xu YP, Zhao D, Li FL, Wang W, Sasaki N, Jiang Y, Zhou X, Li TT, Guan KL, Lei QY, Xiong Y, Mitogenic and oncogenic stimulation of K433 acetylation promotes PKM2 protein kinase activity and nuclear localization, Molecular cell 52(3) (2013) 340–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lv L, Li D, Zhao D, Lin R, Chu Y, Zhang H, Zha Z, Liu Y, Li Z, Xu Y, Wang G, Huang Y, Xiong Y, Guan KL, Lei QY, Acetylation targets the M2 isoform of pyruvate kinase for degradation through chaperone-mediated autophagy and promotes tumor growth, Molecular cell 42(6) (2011) 719–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Liu F, Ma F, Wang Y, Hao L, Zeng H, Jia C, Wang Y, Liu P, Ong IM, Li B, Chen G, Jiang J, Gong S, Li L, Xu W, PKM2 methylation by CARM1 activates aerobic glycolysis to promote tumorigenesis, Nature cell biology 19(11) (2017) 1358–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hitosugi T, Kang S, Vander Heiden MG, Chung TW, Elf S, Lythgoe K, Dong S, Lonial S, Wang X, Chen GZ, Xie J, Gu TL, Polakiewicz RD, Roesel JL, Boggon TJ, Khuri FR, Gilliland DG, Cantley LC, Kaufman J, Chen J, Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth, Science signaling 2(97) (2009) ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Xu Q, Tu J, Dou C, Zhang J, Yang L, Liu X, Lei K, Liu Z, Wang Y, Li L, Bao H, Wang J, Tu K, HSP90 promotes cell glycolysis, proliferation and inhibits apoptosis by regulating PKM2 abundance via Thr-328 phosphorylation in hepatocellular carcinoma, Molecular cancer 16(1) (2017) 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Yu Z, Huang L, Qiao P, Jiang A, Wang L, Yang T, Tang S, Zhang W, Ren C, PKM2 Thr454 phosphorylation increases its nuclear translocation and promotes xenograft tumor growth in A549 human lung cancer cells, Biochemical and biophysical research communications 473(4) (2016) 953–958. [DOI] [PubMed] [Google Scholar]
- [57].Li L, Zhu L, Hao B, Gao W, Wang Q, Li K, Wang M, Huang M, Liu Z, Yang Q, Li X, Zhong Z, Huang W, Xiao G, Xu Y, Yao K, Liu Q, iNOS-derived nitric oxide promotes glycolysis by inducing pyruvate kinase M2 nuclear translocation in ovarian cancer, Oncotarget 8(20) (2017) 33047–33063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zhou HL, Zhang R, Anand P, Stomberski CT, Qian Z, Hausladen A, Wang L, Rhee EP, Parikh SM, Karumanchi SA, Stamler JS, Metabolic reprogramming by the S-nitroso-CoA reductase system protects against kidney injury, Nature 565(7737) (2019) 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Siragusa M, Thole J, Bibli SI, Luck B, Loot AE, de Silva K, Wittig I, Heidler J, Stingl H, Randriamboavonjy V, Kohlstedt K, Brune B, Weigert A, Fisslthaler B, Fleming I, Nitric oxide maintains endothelial redox homeostasis through PKM2 inhibition, The EMBO journal (2019) e100938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Xiangyun Y, Xiaomin N, Linping G, Yunhua X, Ziming L, Yongfeng Y, Zhiwei C, Shun L, Desuccinylation of pyruvate kinase M2 by SIRT5 contributes to antioxidant response and tumor growth, Oncotarget 8(4) (2017) 6984–6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wang Z, Yin J, Li M, Shen J, Xiao Z, Zhao Y, Huang C, Zhang H, Zhang Z, Cho CH, Wu X, Combination of shikonin with paclitaxel overcomes multidrug resistance in human ovarian carcinoma cells in a P-gp-independent manner through enhanced ROS generation, Chinese medicine 14 (2019) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Park YS, Kim DJ, Koo H, Jang SH, You YM, Cho JH, Yang SJ, Yu ES, Jung Y, Lee DC, Kim JA, Park ZY, Park KC, Yeom YI, AKT-induced PKM2 phosphorylation signals for IGF-1-stimulated cancer cell growth, Oncotarget 7(30) (2016) 48155–48167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lori G, Gamberi T, Paoli P, Caselli A, Pranzini E, Marzocchini R, Modesti A, Raugei G, LMW-PTP modulates glucose metabolism in cancer cells, Biochim Biophys Acta Gen Subj 1862(12) (2018) 2533–2544. [DOI] [PubMed] [Google Scholar]
- [64].Bhardwaj A, Das S, SIRT6 deacetylates PKM2 to suppress its nuclear localization and oncogenic functions, Proceedings of the National Academy of Sciences of the United States of America 113(5) (2016) E538–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Abeywardana T, Oh M, Jiang L, Yang Y, Kong M, Song J, Yang Y, CARM1 suppresses de novo serine synthesis by promoting PKM2 activity, The Journal of biological chemistry 293(39) (2018) 15290–15303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Luo W, Hu H, Chang R, Zhong J, Knabel M, O’Meally R, Cole RN, Pandey A, Semenza GL, Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1, Cell 145(5) (2011) 732–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Chen Z, Wang Z, Guo W, Zhang Z, Zhao F, Zhao Y, Jia D, Ding J, Wang H, Yao M, He X, TRIM35 Interacts with pyruvate kinase isoform M2 to suppress the Warburg effect and tumorigenicity in hepatocellular carcinoma, Oncogene 34(30) (2015) 3946–56. [DOI] [PubMed] [Google Scholar]
- [68].Jiang Y, Li X, Yang W, Hawke DH, Zheng Y, Xia Y, Aldape K, Wei C, Guo F, Chen Y, Lu Z, PKM2 regulates chromosome segregation and mitosis progression of tumor cells, Molecular cell 53(1) (2014) 75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ignacak J, Stachurska MB, The dual activity of pyruvate kinase type M2 from chromatin extracts of neoplastic cells, Comparative biochemistry and physiology. Part B, Biochemistry & molecular biology 134(3) (2003) 425–33. [DOI] [PubMed] [Google Scholar]
- [70].Gao X, Wang H, Yang JJ, Liu X, Liu ZR, Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase, Molecular cell 45(5) (2012) 598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Zhao X, Zhao L, Yang H, Li J, Min X, Yang F, Liu J, Huang G, Pyruvate kinase M2 interacts with nuclear sterol regulatory element-binding protein 1a and thereby activates lipogenesis and cell proliferation in hepatocellular carcinoma, The Journal of biological chemistry 293(17) (2018) 6623–6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Warburg O, On the origin of cancer cells, Science (New York, N.Y.) 123(3191) (1956) 309–14. [DOI] [PubMed] [Google Scholar]
- [73].Luo W, Semenza GL, Emerging roles of PKM2 in cell metabolism and cancer progression, Trends in endocrinology and metabolism: TEM 23(11) (2012) 560–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Gwangwa MV, Joubert AM, Visagie MH, Crosstalk between the Warburg effect, redox regulation and autophagy induction in tumourigenesis, Cellular & molecular biology letters 23 (2018) 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Dean JM, Lodhi IJ, Structural and functional roles of ether lipids, Protein & cell 9(2) (2018) 196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Fisher AB, Huber GA, Furia L, Bassett D, Rabinowitz JL, Evidence for lipid synthesis by the dihydroxyacetone phosphate pathway in rabbit lung subcellular fractions, The Journal of laboratory and clinical medicine 87(6) (1976) 1033–40. [PubMed] [Google Scholar]
- [77].Zhang Y, Yang JM, Altered energy metabolism in cancer: a unique opportunity for therapeutic intervention, Cancer biology & therapy 14(2) (2013) 81–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Ashton TM, McKenna WG, Kunz-Schughart LA, Higgins GS, Oxidative Phosphorylation as an Emerging Target in Cancer Therapy, Clinical cancer research : an official journal of the American Association for Cancer Research 24(11) (2018) 2482–2490. [DOI] [PubMed] [Google Scholar]
- [79].Icard P, Poulain L, Lincet H, Understanding the central role of citrate in the metabolism of cancer cells, Biochimica et biophysica acta 1825(1) (2012) 111–6. [DOI] [PubMed] [Google Scholar]
- [80].Liou GY, Storz P, Reactive oxygen species in cancer, Free radical research 44(5) (2010) 479–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Waris G, Ahsan H, Reactive oxygen species: role in the development of cancer and various chronic conditions, Journal of carcinogenesis 5 (2006) 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Jung SN, Yang WK, Kim J, Kim HS, Kim EJ, Yun H, Park H, Kim SS, Choe W, Kang I, Ha J, Reactive oxygen species stabilize hypoxia-inducible factor-1 alpha protein and stimulate transcriptional activity via AMP-activated protein kinase in DU145 human prostate cancer cells, Carcinogenesis 29(4) (2008) 713–21. [DOI] [PubMed] [Google Scholar]
- [83].Movafagh S, Crook S, Vo K, Regulation of hypoxia-inducible factor-1a by reactive oxygen species: new developments in an old debate, Journal of cellular biochemistry 116(5) (2015) 696–703. [DOI] [PubMed] [Google Scholar]
- [84].Masoud GN, Li W, HIF-1alpha pathway: role, regulation and intervention for cancer therapy, Acta Pharm Sin B 5(5) (2015) 378–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Speer RE, Karuppagounder SS, Basso M, Sleiman SF, Kumar A, Brand D, Smirnova N, Gazaryan I, Khim SJ, Ratan RR, Hypoxia-inducible factor prolyl hydroxylases as targets for neuroprotection by “antioxidant” metal chelators: From ferroptosis to stroke, Free radical biology & medicine 62 (2013) 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ziello JE, Jovin IS, Huang Y, Hypoxia-Inducible Factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia, Yale J Biol Med 80(2) (2007) 51–60. [PMC free article] [PubMed] [Google Scholar]
- [87].Koshikawa N, Hayashi J, Nakagawara A, Takenaga K, Reactive oxygen species-generating mitochondrial DNA mutation up-regulates hypoxia-inducible factor-1alpha gene transcription via phosphatidylinositol 3-kinase-Akt/protein kinase C/histone deacetylase pathway, The Journal of biological chemistry 284(48) (2009) 33185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kasuno K, Takabuchi S, Fukuda K, Kizaka-Kondoh S, Yodoi J, Adachi T, Semenza GL, Hirota K, Nitric oxide induces hypoxia-inducible factor 1 activation that is dependent on MAPK and phosphatidylinositol 3-kinase signaling, The Journal of biological chemistry 279(4) (2004) 2550–8. [DOI] [PubMed] [Google Scholar]
- [89].Chan DA, Sutphin PD, Yen SE, Giaccia AJ, Coordinate regulation of the oxygen-dependent degradation domains of hypoxia-inducible factor 1 alpha, Molecular and cellular biology 25(15) (2005) 6415–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Lee G, Won HS, Lee YM, Choi JW, Oh TI, Jang JH, Choi DK, Lim BO, Kim YJ, Park JW, Puigserver P, Lim JH, Oxidative Dimerization of PHD2 is Responsible for its Inactivation and Contributes to Metabolic Reprogramming via HIF-1alpha Activation, Scientific reports 6 (2016) 18928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Chowdhury R, Flashman E, Mecinovic J, Kramer HB, Kessler BM, Frapart YM, Boucher JL, Clifton IJ, McDonough MA, Schofield CJ, Studies on the reaction of nitric oxide with the hypoxia-inducible factor prolyl hydroxylase domain 2 (EGLN1), Journal of molecular biology 410(2) (2011) 268–79. [DOI] [PubMed] [Google Scholar]
- [92].Masson N, Singleton RS, Sekirnik R, Trudgian DC, Ambrose LJ, Miranda MX, Tian YM, Kessler BM, Schofield CJ, Ratcliffe PJ, The FIH hydroxylase is a cellular peroxide sensor that modulates HIF transcriptional activity, EMBO Rep 13(3) (2012) 251–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Hwang JT, Lee M, Jung SN, Lee HJ, Kang I, Kim SS, Ha J, AMP-activated protein kinase activity is required for vanadate-induced hypoxia-inducible factor 1alpha expression in DU145 cells, Carcinogenesis 25(12) (2004) 2497–507. [DOI] [PubMed] [Google Scholar]
- [94].Jin L, Zhou Y, Crucial role of the pentose phosphate pathway in malignant tumors, Oncol Lett 17(5) (2019) 4213–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Brimacombe KR, Anastasiou D, Hong BS, Tempel W, Dimov S, Veith H, Auld DS, Vander Heiden MG, Thomas CJ, Park HW, Shen M, Cantley LC, Boxer MB, ML285 affects reactive oxygen species’ inhibition of pyruvate kinase M2, Probe Reports from the NIH Molecular Libraries Program, Bethesda (MD), 2010. [PubMed] [Google Scholar]
- [96].Zhao Y, Yu H, Hu W, The regulation of MDM2 oncogene and its impact on human cancers, Acta biochimica et biophysica Sinica 46(3) (2014) 180–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Riscal R, Schrepfer E, Arena G, Cisse MY, Bellvert F, Heuillet M, Rambow F, Bonneil E, Sabourdy F, Vincent C, Ait-Arsa I, Levade T, Thibaut P, Marine JC, Portais JC, Sarry JE, Le Cam L, Linares LK, Chromatin-Bound MDM2 Regulates Serine Metabolism and Redox Homeostasis Independently of p53, Molecular cell 62(6) (2016) 890–902. [DOI] [PubMed] [Google Scholar]
- [98].Mayo LD, Donner DB, A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus, Proceedings of the National Academy of Sciences of the United States of America 98(20) (2001) 11598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Du W, Jiang P, Li N, Mei Y, Wang X, Wen L, Yang X, Wu M, Suppression of p53 activity by Siva1, Cell death and differentiation 16(11) (2009) 1493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Arena G, Riscal R, Linares LK, Le Cam L, MDM2 controls gene expression independently of p53 in both normal and cancer cells, Cell death and differentiation 25(9) (2018) 1533–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Ozaki T, Nakagawara A, Role of p53 in Cell Death and Human Cancers, Cancers 3(1) (2011) 994–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Saleme B, Gurtu V, Zhang Y, Kinnaird A, Boukouris AE, Gopal K, Ussher JR, Sutendra G, Tissue-specific regulation of p53 by PKM2 is redox dependent and provides a therapeutic target for anthracycline-induced cardiotoxicity, Science translational medicine 11(478) (2019). [DOI] [PubMed] [Google Scholar]
- [103].Tamada M, Suematsu M, Saya H, Pyruvate kinase M2: multiple faces for conferring benefits on cancer cells, Clinical cancer research : an official journal of the American Association for Cancer Research 18(20) (2012) 5554–61. [DOI] [PubMed] [Google Scholar]
- [104].Azoitei N, Becher A, Steinestel K, Rouhi A, Diepold K, Genze F, Simmet T, Seufferlein T, PKM2 promotes tumor angiogenesis by regulating HIF-lalpha through NF-kappaB activation, Molecular cancer 15 (2016) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL, Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha, Genes & development 12(2) (1998) 149–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Kim JW, Tchernyshyov I, Semenza GL, Dang CV, HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia, Cell metabolism 3(3) (2006) 177–85. [DOI] [PubMed] [Google Scholar]
- [107].Gogvadze V, Orrenius S, Zhivotovsky B, Mitochondria in cancer cells: what is so special about them?, Trends in cell biology 18(4) (2008) 165–73. [DOI] [PubMed] [Google Scholar]
- [108].David CJ, Chen M, Assanah M, Canoll P, Manley JL, HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer, Nature 463(7279) (2010) 364–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Wang C, Jiang J, Ji J, Cai Q, Chen X, Yu Y, Zhu Z, Zhang J, PKM2 promotes cell migration and inhibits autophagy by mediating PI3K/AKT activation and contributes to the malignant development of gastric cancer, Scientific reports 7(1) (2017) 2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Anastasiou D, Yu Y, Israelsen WJ, Jiang JK, Boxer MB, Hong BS, Tempel W, Dimov S, Shen M, Jha A, Yang H, Mattaini KR, Metallo CM, Fiske BP, Courtney KD, Malstrom S, Khan TM, Kung C, Skoumbourdis AP, Veith H, Southall N, Walsh MJ, Brimacombe KR, Leister W, Lunt SY, Johnson ZR, Yen KE, Kunii K, Davidson SM, Christofk HR, Austin CP, Inglese J, Harris MH, Asara JM, Stephanopoulos G, Salituro FG, Jin S, Dang L, Auld DS, Park HW, Cantley LC, Thomas CJ, Vander Heiden MG, Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis, Nature chemical biology 8(10) (2012) 839–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Ao R, Guan L, Wang Y, Wang JN, Effects of PKM2 Gene Silencing on the Proliferation and Apoptosis of Colorectal Cancer LS-147T and SW620 Cells, Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology 42(5) (2017) 1769–1778. [DOI] [PubMed] [Google Scholar]
- [112].Li L, Zhang Y, Qiao J, Yang JJ, Liu ZR, Pyruvate kinase M2 in blood circulation facilitates tumor growth by promoting angiogenesis, The Journal of biological chemistry 289(37) (2014) 25812–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Tech K, Tikunov AP, Farooq H, Morrissy AS, Meidinger J, Fish T, Green SC, Liu H, Li Y, Mungall AJ, Moore RA, Ma Y, Jones SJM, Marra MA, Vander Heiden MG, Taylor MD, Macdonald JM, Gershon TR, Pyruvate Kinase Inhibits Proliferation during Postnatal Cerebellar Neurogenesis and Suppresses Medulloblastoma Formation, Cancer research 77(12) (2017) 3217–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Wang W, He Q, Sun J, Liu Z, Zhao L, Lu Z, Zhou X, Wang A, Pyruvate kinase M2 deregulation enhances the metastatic potential of tongue squamous cell carcinoma, Oncotarget 8(40) (2017) 68252–68262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Clark AG, Vignjevic DM, Modes of cancer cell invasion and the role of the microenvironment, Current opinion in cell biology 36 (2015) 13–22. [DOI] [PubMed] [Google Scholar]
- [116].Zhou CF, Li XB, Sun H, Zhang B, Han YS, Jiang Y, Zhuang QL, Fang J, Wu GH, Pyruvate kinase type M2 is upregulated in colorectal cancer and promotes proliferation and migration of colon cancer cells, IUBMB life 64(9) (2012) 775–82. [DOI] [PubMed] [Google Scholar]
- [117].Kalluri R, Weinberg RA, The basics of epithelial-mesenchymal transition, J Clin Invest 119(6) (2009) 1420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Renart J, Carrasco-Ramirez P, Fernandez-Munoz B, Martin-Villar E, Montero L, Yurrita MM, Quintanilla M, New insights into the role of podoplanin in epithelial-mesenchymal transition, International review of cell and molecular biology 317 (2015) 185–239. [DOI] [PubMed] [Google Scholar]
- [119].Hamabe A, Konno M, Tanuma N, Shima H, Tsunekuni K, Kawamoto K, Nishida N, Koseki J, Mimori K, Gotoh N, Yamamoto H, Doki Y, Mori M, Ishii H, Role of pyruvate kinase M2 in transcriptional regulation leading to epithelial-mesenchymal transition, Proceedings of the National Academy of Sciences of the United States of America 111(43) (2014) 15526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Weinberger R, Appel B, Stein A, Metz Y, Neheman A, Barak M, The pyruvate kinase isoenzyme M2 (Tu M2-PK) as a tumour marker for renal cell carcinoma, European journal of cancer care 16(4) (2007) 333–7. [DOI] [PubMed] [Google Scholar]
- [121].Elbers JR, van Unnik JA, Rijksen G, van Oirschot BA, Roholl PJ, Oosting J, Staal GE, Pyruvate kinase activity and isozyme composition in normal fibrous tissue and fibroblastic proliferations, Cancer 67(10) (1991) 2552–9. [DOI] [PubMed] [Google Scholar]
- [122].Wang X, Zhang F, Wu XR, Inhibition of Pyruvate Kinase M2 Markedly Reduces Chemoresistance of Advanced Bladder Cancer to Cisplatin, Scientific reports 7 (2017) 45983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Vander Heiden MG, Christofk HR, Schuman E, Subtelny AO, Sharfi H, Harlow EE, Xian J, Cantley LC, Identification of small molecule inhibitors of pyruvate kinase M2, Biochemical pharmacology 79(8) (2010) 1118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Chen J, Xie J, Jiang Z, Wang B, Wang Y, Hu X, Shikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase-M2, Oncogene 30(42) (2011) 4297–306. [DOI] [PubMed] [Google Scholar]
- [125].Nigorikawa K, Yoshikawa K, Sasaki T, Iida E, Tsukamoto M, Murakami H, Maehama T, Hazeki K, Hazeki O, A naphthoquinone derivative, shikonin, has insulin-like actions by inhibiting both phosphatase and tensin homolog deleted on chromosome 10 and tyrosine phosphatases, Molecular pharmacology 70(3) (2006) 1143–9. [DOI] [PubMed] [Google Scholar]
- [126].Chen J, Jiang Z, Wang B, Wang Y, Hu X, Vitamin K(3) and K(5) are inhibitors of tumor pyruvate kinase M2, Cancer letters 316(2) (2012) 204–10. [DOI] [PubMed] [Google Scholar]
- [127].Jiang J, Walsh MJ, Brimacombe KR, Anastasiou D, Yu Y, Israelsen WJ, Hong BS, Tempel W, Dimov S, Veith H, Yang H, Kung C, Yen KE, Dang L, Salituro F, Auld DS, Park HW, Vander Heiden MG, Thomas CJ, Shen M, Boxer MB, ML265: A potent PKM2 activator induces tetramerization and reduces tumor formation and size in a mouse xenograft model, Probe Reports from the NIH Molecular Libraries Program, Bethesda (MD), 2010. [PubMed] [Google Scholar]
- [128].Jiang JK, Boxer MB, Vander Heiden MG, Shen M, Skoumbourdis AP, Southall N, Veith H, Leister W, Austin CP, Park HW, Inglese J, Cantley LC, Auld DS, Thomas CJ, Evaluation of thieno[3,2-b]pyrrole[3,2-d]pyridazinones as activators of the tumor cell specific M2 isoform of pyruvate kinase, Bioorganic & medicinal chemistry letters 20(11) (2010) 3387–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Li R, Ning X, Zhou S, Lin Z, Wu X, Chen H, Bai X, Wang X, Ge Z, Li R, Yin Y, Discovery and structure-activity relationship of novel 4-hydroxy-thiazolidine-2-thione derivatives as tumor cell specific pyruvate kinase M2 activators, European journal of medicinal chemistry 143 (2018) 48–65. [DOI] [PubMed] [Google Scholar]
- [130].Wong RS, Apoptosis in cancer: from pathogenesis to treatment, Journal of experimental & clinical cancer research : CR 30 (2011) 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Li RZ, Fan XX, Shi DF, Zhu GY, Wang YW, Luo LX, Pan HD, Yao XJ, Leung EL, Liu L, Identification of a new pyruvate kinase M2 isoform (PKM2) activator for the treatment of non-small-cell lung cancer (NSCLC), Chemical biology & drug design (2018). [DOI] [PubMed] [Google Scholar]
- [132].Hou Y, Xu J, Liu X, Xia X, Li N, Bi X, Shikonin induces apoptosis in the human gastric cancer cells HGC-27 through mitochondria-mediated pathway, Pharmacognosy magazine 11(42) (2015) 250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Siddiqui WA, Ahad A, Ahsan H, The mystery of BCL2 family: Bcl-2 proteins and apoptosis: an update, Archives of toxicology 89(3) (2015) 289–317. [DOI] [PubMed] [Google Scholar]
- [134].Elmore S, Apoptosis: a review of programmed cell death, Toxicologic pathology 35(4) (2007) 495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Yuan S, Qiao T, Zhuang X, Chen W, Xing N, Zhang Q, Knockdown of the M2 Isoform of Pyruvate Kinase (PKM2) with shRNA Enhances the Effect of Docetaxel in Human NSCLC Cell Lines In Vitro, Yonsei medical journal 57(6) (2016) 1312–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Hu W, Lu SX, Li M, Zhang C, Liu LL, Fu J, Jin JT, Luo RZ, Zhang CZ, Yun JP, Pyruvate kinase M2 prevents apoptosis via modulating Bim stability and associates with poor outcome in hepatocellular carcinoma, Oncotarget 6(9) (2015) 6570–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Yuan Q, Yu H, Chen J, Song X, Sun L, Knockdown of pyruvate kinase type M2 suppresses tumor survival and invasion in osteosarcoma cells both in vitro and in vivo, Experimental cell research 362(1) (2018) 209–216. [DOI] [PubMed] [Google Scholar]
- [138].Wahid F, Shehzad A, Khan T, Kim YY, MicroRNAs: synthesis, mechanism, function, and recent clinical trials, Biochimica et biophysica acta 1803(11) (2010) 1231–43. [DOI] [PubMed] [Google Scholar]
- [139].Zhang H, Feng C, Zhang M, Zeng A, Si L, Yu N, Bai M, miR-625–5p/PKM2 negatively regulates melanoma glycolysis state, Journal of cellular biochemistry 120(3) (2019) 2964–2972. [DOI] [PubMed] [Google Scholar]
- [140].Zheng X, Zhou Y, Chen W, Chen L, Lu J, He F, Li X, Zhao L, Ginsenoside 20(S)-Rg3 Prevents PKM2-Targeting miR-324–5p from H19 Sponging to Antagonize the Warburg Effect in Ovarian Cancer Cells, Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology 51(3) (2018) 1340–1353. [DOI] [PubMed] [Google Scholar]
- [141].Chen T, Li Y, Cao W, Liu Y, miR-491–5p inhibits osteosarcoma cell proliferation by targeting PKM2, Oncology letters 16(5) (2018) 6472–6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Cui S, Yang X, Zhang L, Zhao Y, Yan W, LncRNA MAFG-AS1 promotes the progression of colorectal cancer by sponging miR-147b and activation of NDUFA4, Biochemical and biophysical research communications 506(1) (2018) 251–258. [DOI] [PubMed] [Google Scholar]
- [143].Xu Q, Dou C, Liu X, Yang L, Ni C, Wang J, Guo Y, Yang W, Tong X, Huang D, Oviductus ranae protein hydrolysate (ORPH) inhibits the growth, metastasis and glycolysis of HCC by targeting miR-491–5p/PKM2 axis, Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 107 (2018) 1692–1704. [DOI] [PubMed] [Google Scholar]
- [144].Li W, Qiu Y, Hao J, Zhao C, Deng X, Shu G, Dauricine upregulates the chemosensitivity of hepatocellular carcinoma cells: Role of repressing glycolysis via miR- 199a:HK2/PKM2 modulation, Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 121 (2018) 156–165. [DOI] [PubMed] [Google Scholar]
- [145].Prakasam G, Singh RK, Iqbal MA, Saini SK, Tiku AB, Bamezai RNK, Pyruvate kinase M knockdown-induced signaling via AMP-activated protein kinase promotes mitochondrial biogenesis, autophagy, and cancer cell survival, The Journal of biological chemistry 292(37) (2017) 15561–15576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Gaber T, Strehl C, Buttgereit F, Metabolic regulation of inflammation, Nature reviews. Rheumatology 13(5) (2017) 267–279. [DOI] [PubMed] [Google Scholar]
- [147].von Andrian UH, Chambers JD, McEvoy LM, Bargatze RF, Arfors KE, Butcher EC, Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte beta 2 integrins in vivo, Proceedings of the National Academy of Sciences of the United States of America 88(17) (1991) 7538–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Martin GS, Sepsis, severe sepsis and septic shock: changes in incidence, pathogens and outcomes, Expert review of anti-infective therapy 10(6) (2012) 701–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Fox CJ, Hammerman PS, Thompson CB, Fuel feeds function: energy metabolism and the T-cell response, Nature reviews. Immunology 5(11) (2005) 844–52. [DOI] [PubMed] [Google Scholar]
- [150].Everts B, Amiel E, Huang SC, Smith AM, Chang CH, Lam WY, Redmann V, Freitas TC, Blagih J, van der Windt GJ, Artyomov MN, Jones RG, Pearce EL, Pearce EJ, TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKvarepsilon supports the anabolic demands of dendritic cell activation, Nature immunology 15(4) (2014) 323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Rodriguez-Prados JC, Traves PG, Cuenca J, Rico D, Aragones J, Martin-Sanz P, Cascante M, Bosca L, Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation, J Immunol 185(1) (2010) 605–14. [DOI] [PubMed] [Google Scholar]
- [152].Mills EL, Kelly B, Logan A, Costa ASH, Varma M, Bryant CE, Tourlomousis P, Dabritz JHM, Gottlieb E, Latorre I, Corr SC, McManus G, Ryan D, Jacobs HT, Szibor M, Xavier RJ, Braun T, Frezza C, Murphy MP, O’Neill LA, Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages, Cell 167(2) (2016) 457–470 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Palsson-McDermott EM, Curtis AM, Goel G, Lauterbach MA, Sheedy FJ, Gleeson LE, van den Bosch MW, Quinn SR, Domingo-Fernandez R, Johnston DG, Jiang JK, Israelsen WJ, Keane J, Thomas C, Clish C, Vander Heiden M, Xavier RJ, O’Neill LA, Pyruvate kinase M2 regulates Hif-1alpha activity and IL-1beta induction and is a critical determinant of the warburg effect in LPS-activated macrophages, Cell metabolism 21(1) (2015) 65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, Zheng L, Gardet A, Tong Z, Jany SS, Corr SC, Haneklaus M, Caffrey BE, Pierce K, Walmsley S, Beasley FC, Cummins E, Nizet V, Whyte M, Taylor CT, Lin H, Masters SL, Gottlieb E, Kelly VP, Clish C, Auron PE, Xavier RJ, O’Neill LA, Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha, Nature 496(7444) (2013) 238–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Patil NK, Bohannon JK, Hernandez A, Patil TK, Sherwood ER, Regulation of leukocyte function by citric acid cycle intermediates, Journal of leukocyte biology 106(1) (2019) 105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E, Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase, Cancer cell 7(1) (2005) 77–85. [DOI] [PubMed] [Google Scholar]
- [157].Xie M, Yu Y, Kang R, Zhu S, Yang L, Zeng L, Sun X, Yang M, Billiar TR, Wang H, Cao L, Jiang J, Tang D, PKM2-dependent glycolysis promotes NLRP3 and AIM2 inflammasome activation, Nature communications 7 (2016) 13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD, Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome, Nature genetics 29(3) (2001) 301–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Segovia JA, Chang TH, Winter VT, Coalson JJ, Cagle MP, Pandranki L, Bose S, Baseman JB, Kannan TR, NLRP3 Is a Critical Regulator of Inflammation and Innate Immune Cell Response during Mycoplasma pneumoniae Infection, Infection and immunity 86(1) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Lee S, Nakahira K, Dalli J, Siempos II, Norris PC, Colas RA, Moon JS, Shinohara M, Hisata S, Howrylak JA, Suh GY, Ryter SW, Serhan CN, Choi AMK, NLRP3 Inflammasome Deficiency Protects against Microbial Sepsis via Increased Lipoxin B4 Synthesis, American journal of respiratory and critical care medicine 196(6) (2017) 713–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J, NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder, Immunity 20(3) (2004) 319–25. [DOI] [PubMed] [Google Scholar]
- [162].Lu B, Nakamura T, Inouye K, Li J, Tang Y, Lundback P, Valdes-Ferrer SI, Olofsson PS, Kalb T, Roth J, Zou Y, Erlandsson-Harris H, Yang H, Ting JP, Wang H, Andersson U, Antoine DJ, Chavan SS, Hotamisligil GS, Tracey KJ, Novel role of PKR in inflammasome activation and HMGB1 release, Nature 488(7413) (2012) 670–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Shirai T, Nazarewicz RR, Wallis BB, Yanes RE, Watanabe R, Hilhorst M, Tian L, Harrison DG, Giacomini JC, Assimes TL, Goronzy JJ, Weyand CM, The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease, The Journal of experimental medicine 213(3) (2016) 337–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Deng J, Lu S, Liu H, Liu B, Jiang C, Xu Q, Feng J, Wang X, Homocysteine Activates B Cells via Regulating PKM2-Dependent Metabolic Reprogramming, Journal of immunology (Baltimore, Md. : 1950) 198(1) (2017) 170–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Hanic M, Trbojevic-Akmacic I, Lauc G, Inflammatory bowel disease - glycomics perspective, Biochimica et biophysica acta. General subjects (2019). [DOI] [PubMed] [Google Scholar]
- [166].Kim DH, Cheon JH, Pathogenesis of Inflammatory Bowel Disease and Recent Advances in Biologic Therapies, Immune Netw 17(1) (2017) 25–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Biasi F, Leonarduzzi G, Oteiza PI, Poli G, Inflammatory bowel disease: mechanisms, redox considerations, and therapeutic targets, Antioxidants & redox signaling 19(14) (2013) 1711–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Almousa AA, Morris M, Fowler S, Jones J, Alcorn J, Elevation of serum pyruvate kinase M2 (PKM2) in IBD and its relationship to IBD indices, Clinical biochemistry (2017). [DOI] [PubMed] [Google Scholar]
- [169].Zhang N, Gao R, Yang J, Zhu Y, Zhang Z, Xu X, Wang J, Liu X, Li Z, Li Z, Gong D, Li J, Bi J, Kong C, Quantitative Global Proteome and Lysine Succinylome Analyses Reveal the Effects of Energy Metabolism in Renal Cell Carcinoma, Proteomics 18(19) (2018) e1800001. [DOI] [PubMed] [Google Scholar]
- [170].Weinert BT, Scholz C, Wagner SA, Iesmantavicius V, Su D, Daniel JA, Choudhary C, Lysine succinylation is a frequently occurring modification in prokaryotes and eukaryotes and extensively overlaps with acetylation, Cell reports 4(4) (2013) 842–51. [DOI] [PubMed] [Google Scholar]
- [171].Du J, Zhou Y, Su X, Yu JJ, Khan S, Jiang H, Kim J, Woo J, Kim JH, Choi BH, He B, Chen W, Zhang S, Cerione RA, Auwerx J, Hao Q, Lin H, Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase, Science (New York, N.Y.) 334(6057) (2011) 806–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Wang F, Wang K, Xu W, Zhao S, Ye D, Wang Y, Xu Y, Zhou L, Chu Y, Zhang C, Qin X, Yang P, Yu H, SIRT5 Desuccinylates and Activates Pyruvate Kinase M2 to Block Macrophage IL-1beta Production and to Prevent DSS-Induced Colitis in Mice, Cell reports 19(11) (2017) 2331–2344. [DOI] [PubMed] [Google Scholar]
- [173].Wang B, Liu S, Fan B, Xu X, Chen Y, Lu R, Xu Z, Liu X, PKM2 is involved in neuropathic pain by regulating ERK and STAT3 activation in rat spinal cord, The journal of headache and pain 19(1) (2018) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Zhou L, Hu Y, Li C, Yan Y, Ao L, Yu B, Fang W, Liu J, Li Y, Levo-corydalmine alleviates vincristine-induced neuropathic pain in mice by inhibiting an NF-kappa B-dependent CXCL1/CXCR2 signaling pathway, Neuropharmacology 135 (2018) 34–47. [DOI] [PubMed] [Google Scholar]
- [175].Kiguchi N, Kobayashi D, Saika F, Matsuzaki S, Kishioka S, Pharmacological Regulation of Neuropathic Pain Driven by Inflammatory Macrophages, Int J Mol Sci 18(11) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Duran-Salgado MB, Rubio-Guerra AF, Diabetic nephropathy and inflammation, World journal of diabetes 5(3) (2014) 393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Boguszewski CL, Boguszewski M, Growth Hormone’s Links to Cancer, Endocrine reviews (2018). [DOI] [PubMed] [Google Scholar]
- [178].Sachdev D, Yee D, Disrupting insulin-like growth factor signaling as a potential cancer therapy, Molecular cancer therapeutics 6(1) (2007) 1–12. [DOI] [PubMed] [Google Scholar]
- [179].Li Q, Liu X, Yin Y, Zheng JT, Jiang CF, Wang J, Shen H, Li CY, Wang M, Liu LZ, Jiang BH, Insulin regulates glucose consumption and lactate production through reactive oxygen species and pyruvate kinase M2, Oxidative medicine and cellular longevity 2014 (2014) 504953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Iqbal MA, Siddiqui FA, Gupta V, Chattopadhyay S, Gopinath P, Kumar B, Manvati S, Chaman N, Bamezai RN, Insulin enhances metabolic capacities of cancer cells by dual regulation of glycolytic enzyme pyruvate kinase M2, Molecular cancer 12 (2013) 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Ban JJ, Ruthenborg RJ, Cho KW, Kim JW, Regulation of obesity and insulin resistance by hypoxia-inducible factors, Hypoxia (Auckland, N.Z.) 2 (2014) 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Wang S, Yang Z, Gao Y, Li Q, Su Y, Wang Y, Zhang Y, Man H, Liu H, Pyruvate kinase, muscle isoform 2 promotes proliferation and insulin secretion of pancreatic beta-cells via activating Wnt/CTNNB1 signaling, International journal of clinical and experimental pathology 8(11) (2015) 14441–8. [PMC free article] [PubMed] [Google Scholar]
- [183].Bettaieb A, Hosein E, Chahed S, Abdulaziz A, Kucera HR, Gaikwad NW, Haj FG, Decreased adiposity and enhanced glucose tolerance in shikonin treated mice, Obesity (Silver Spring, Md: 23(11) (2015) 2269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Rui L, Energy metabolism in the liver, Comprehensive Physiology 4(1) (2014) 177–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Chen L, Tang Z, Wang X, Ma H, Shan D, Cui S, PKM2 aggravates palmitate-induced insulin resistance in HepG2 cells via STAT3 pathway, Biochemical and biophysical research communications 492(1) (2017) 109–115. [DOI] [PubMed] [Google Scholar]
- [186].Wunderlich CM, Hovelmeyer N, Wunderlich FT, Mechanisms of chronic JAK-STAT3-SOCS3 signaling in obesity, Jak-stat 2(2) (2013) e23878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Torisu T, Sato N, Yoshiga D, Kobayashi T, Yoshioka T, Mori H, Iida M, Yoshimura A, The dual function of hepatic SOCS3 in insulin resistance in vivo, Genes Cells 12(2) (2007) 143–54. [DOI] [PubMed] [Google Scholar]
- [188].Meoli L, Gupta NK, Saeidi N, Panciotti CA, Biddinger SB, Corey KE, Stylopoulos N, Nonalcoholic fatty liver disease and gastric bypass surgery regulate serum and hepatic levels of pyruvate kinase isoenzyme M2, American journal of physiology. Endocrinology and metabolism 315(4) (2018) E613–e621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Eberle D, Hegarty B, Bossard P, Ferre P, Foufelle F, SREBP transcription factors: master regulators of lipid homeostasis, Biochimie 86(11) (2004) 839–48. [DOI] [PubMed] [Google Scholar]
- [190].Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB, Reactive oxygen species in inflammation and tissue injury, Antioxidants & redox signaling 20(7) (2014) 1126–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Ju C, Colgan SP, Eltzschig HK, Hypoxia-inducible factors as molecular targets for liver diseases, Journal of molecular medicine (Berlin, Germany) 94(6) (2016) 613–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Ouyang X, Han SN, Zhang JY, Dioletis E, Nemeth BT, Pacher P, Feng D, Bataller R, Cabezas J, Starkel P, Caballeria J, Pongratz RL, Cai SY, Schnabl B, Hoque R, Chen Y, Yang WH, Garcia-Martinez I, Wang FS, Gao B, Torok NJ, Kibbey RG, Mehal WZ, Digoxin Suppresses Pyruvate Kinase M2-Promoted HIF-lalpha Transactivation in Steatohepatitis, Cell metabolism 27(2) (2018) 339–350.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Heron M, Deaths: Leading Causes for 2016, Natl Vital Stat Rep 67(6) (2018) 1–77. [PubMed] [Google Scholar]
- [194].C.C.f.D.C.a. Prevention, Behavioral Risk Factors Surveillance System (BRFSS), Accessed January 31, 2019 https://www.cdc.gov/brfss/index.html.
- [195].Glassock R, Delanaye P, El Nahas M, An Age-Calibrated Classification of Chronic Kidney Disease, JAMA : the journal of the American Medical Association 314(6) (2015) 559–60. [DOI] [PubMed] [Google Scholar]
- [196].Staples A, Wong C, Risk factors for progression of chronic kidney disease, Curr Opin Pediatr 22(2) (2010) 161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Eddy AA, Progression in chronic kidney disease, Advances in chronic kidney disease 12(4) (2005) 353–65. [DOI] [PubMed] [Google Scholar]
- [198].Glodowski SD, Wagener G, New insights into the mechanisms of acute kidney injury in the intensive care unit, J Clin Anesth 27(2) (2015) 175–80. [DOI] [PubMed] [Google Scholar]
- [199].Hsu RK, Hsu CY, The Role of Acute Kidney Injury in Chronic Kidney Disease, Seminars in nephrology 36(4) (2016) 283–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Cheon JH, Kim SY, Son JY, Kang YR, An JH, Kwon JH, Song HS, Moon A, Lee BM, Kim HS, Pyruvate Kinase M2: A Novel Biomarker for the Early Detection of Acute Kidney Injury, Toxicol Res 32(1) (2016) 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Won AJ, Kim S, Kim YG, Kim KB, Choi WS, Kacew S, Kim KS, Jung JH, Lee BM, Kim S, Kim HS, Discovery of urinary metabolomic biomarkers for early detection of acute kidney injury, Molecular bioSystems 12(1) (2016) 133–44. [DOI] [PubMed] [Google Scholar]
- [202].Kim SY, Sohn SJ, Won AJ, Kim HS, Moon A, Identification of noninvasive biomarkers for nephrotoxicity using HK-2 human kidney epithelial cells, Toxicological sciences : an official journal of the Society of Toxicology 140(2) (2014) 247–58. [DOI] [PubMed] [Google Scholar]
- [203].Schena FP, Gesualdo L, Pathogenetic mechanisms of diabetic nephropathy, Journal of the American Society of Nephrology : JASN 16 Suppl 1 (2005) S30–3. [DOI] [PubMed] [Google Scholar]
- [204].Qi W, Keenan HA, Li Q, Ishikado A, Kannt A, Sadowski T, Yorek MA, Wu IH, Lockhart S, Coppey LJ, Pfenninger A, Liew CW, Qiang G, Burkart AM, Hastings S, Pober D, Cahill C, Niewczas MA, Israelsen WJ, Tinsley L, Stillman IE, Amenta PS, Feener EP, Vander Heiden MG, Stanton RC, King GL, Pyruvate kinase M2 activation may protect against the progression of diabetic glomerular pathology and mitochondrial dysfunction, Nature medicine 23(6) (2017) 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Riscal R, Le Cam L, Linares LK, Chromatin-bound MDM2, a new player in metabolism, Mol Cell Oncol 3(5) (2016) e1210560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Yu Z, Zhao X, Huang L, Zhang T, Yang F, Xie L, Song S, Miao P, Zhao L, Sun X, Liu J, Huang G, Proviral insertion in murine lymphomas 2 (PIM2) oncogene phosphorylates pyruvate kinase M2 (PKM2) and promotes glycolysis in cancer cells, The Journal of biological chemistry 288(49) (2013) 35406–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Bettaieb A, Bakke J, Nagata N, Matsuo K, Xi Y, Liu S, AbouBechara D, Melhem R, Stanhope K, Cummings B, Graham J, Bremer A, Zhang S, Lyssiotis CA, Zhang ZY, Cantley LC, Havel PJ, Haj FG, Protein tyrosine phosphatase 1B regulates pyruvate kinase M2 tyrosine phosphorylation, The Journal of biological chemistry 288(24) (2013) 17360–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].An S, Huang L, Miao P, Shi L, Shen M, Zhao X, Liu J, Huang G, Small ubiquitin-like modifier 1 modification of pyruvate kinase M2 promotes aerobic glycolysis and cell proliferation in A549 human lung cancer cells, OncoTargets and therapy 11 (2018) 2097–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]