Abstract
Nonalcoholic fatty liver disease is a major public health burden. Although many features of nonalcoholic fatty liver disease pathogenesis are known, the specific mechanisms and susceptibilities that determine an individual’s risk of developing nonalcoholic steatohepatitis versus isolated steatosis are not well delineated. The predominant and defining histologic and imaging characteristic of nonalcoholic fatty liver disease is the accumulation of lipids. Dysregulation of lipid homeostasis in hepatocytes leads to transient generation or accumulation of toxic lipids that result in endoplasmic reticulum (ER) stress with inflammation, hepatocellular damage, and apoptosis. ER stress activates the unfolded protein response (UPR) which is classically viewed as an adaptive pathway to maintain protein folding homeostasis. Recent studies have uncovered the contribution of the UPR sensors in the regulation of hepatic steatosis and in the cellular response to lipotoxic stress. Interestingly, the UPR sensors can be directly activated by toxic lipids, independently of the accumulation of misfolded proteins, termed lipotoxic and proteotoxic stress, respectively. The dual function of the UPR sensors in protein and lipid homeostasis suggests that these two types of stress are interconnected likely due to the central role of the ER in protein folding and trafficking and lipid biosynthesis and trafficking, such that perturbations in either impact the function of the ER and activate the UPR sensors in an effort to restore homeostasis. The precise molecular similarities and differences between proteotoxic and lipotoxic ER stress are beginning to be understood. Herein, we provide an overview of the mechanisms involved in the activation and cross-talk between the UPR sensors, hepatic lipid metabolism, and lipotoxic stress, and discuss the possible therapeutic potential of targeting the UPR in nonalcoholic fatty liver disease.
Keywords: nonalcoholic steatohepatitis, lipotoxicity, endoplasmic reticulum stress, palmitate, sphingolipids
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) is currently the leading cause of chronic liver disease and estimated to affect approximately 30% of the U.S population (Satapathy & Sanyal, 2015; Younossi, et al., 2018). NAFLD is a heterogeneous disease ranging from simple steatosis to nonalcoholic steatohepatitis (NASH) with or without hepatic fibrosis. Its prevalence in specific groups, such as patients with type 2 diabetes or morbid obesity is even higher, occurring in roughly 60 and 80% of these individuals, respectively (Amor & Perea, 2019; Blond, et al., 2017; Younossi, et al., 2018). NAFLD is characterized by the accumulation of various lipid species within hepatocytes (Marra & Svegliati-Baroni, 2018). The lipids that can activate and induce progressive liver injury are defined as toxic lipids in NASH pathogenesis. In general, the saturated fatty acids (SFAs) such as palmitate, ceramides, lysophosphatidylcholine (LPC), and free cholesterol are directly cytotoxic, whereas the monounsaturated free fatty acids, such as oleate and palmitoleate, may protect from SFA-induced toxicity (Akazawa, et al., 2010; Hirsova, Ibrahim, Gores, & Malhi, 2016; Malhi, Barreyro, Isomoto, Bronk, & Gores, 2007; Malhi, Bronk, Werneburg, & Gores, 2006). The consequent hepatocellular damage characterized by inflammation and hepatocellular apoptosis is linked to dysfunction of the endoplasmic reticulum (ER), resulting from toxic lipids termed as lipotoxic ER stress or lipid bilayer stress (Pagliassotti, 2012; Volmer, van der Ploeg, & Ron, 2013). Lipotoxic ER stress appears to follow a continuum that progresses from non-lethal or sub-lethal responses to ER stress-induced cell death. Lipotoxic ER stress may also be proinflammatory as we have recently demonstrated that lipotoxic sublethal ER stress response leads to the release of proinflammatory extracellular vesicles (Kakazu, Mauer, Yin, & Malhi, 2016). Steatosis and ER stress are also features of alcoholic hepatitis and ER stress is now accepted as an important mechanism in alcoholic hepatitis pathogenesis and progression (Joshi-Barve, Kirpich, Cave, Marsano, & McClain, 2015; Masouminia, et al., 2016). The role of ER stress in alcoholic hepatitis is reviewed elsewhere in detail (Ji, 2014; Joshi-Barve, et al., 2015; Masouminia, et al., 2016). This review focuses on the UPR in lipid metabolism and NAFLD. We review the signaling pathways mediated by the canonical UPR, how the UPR sensors contribute to and respond to perturbation in hepatic lipid homeostasis under ER stress or independently, and lipotoxic ER stress. Lastly, we discuss the therapeutic potential of targeting the UPR in NAFLD.
2. Unfolded Protein Response
The ER is an essential subcellular compartment responsible for the synthesis and folding of proteins that traffic through the secretory pathway in the cell. Protein folding is sensitive to alterations in ER homeostasis including Ca2+ levels, energy and nutrient availability, as well as the protein-folding load in the ER (S. Wang & Kaufman, 2014) (Walter & Ron, 2011). Perturbations in these pathways interfere with protein folding in the ER leading to proteotoxic ER stress, which in turn activates the UPR. The UPR is initially an adaptive signaling pathway which aims to elicit global cellular changes such as attenuation of translation and activate specific pathways of protein folding and degradation to restore ER homeostasis (S. Wang & Kaufman, 2012). When ER homeostasis cannot be restored ER stress-induced apoptosis occurs. Canonically, the UPR is activated via the luminal domains of three principal transmembrane sensors: inositol-requiring enzyme (IRE)-1α, protein kinase RNA-like ER kinase (PERK), and activating transcription factor (ATF)-6α.
A. IRE1α pathway
The IRE1α pathway is the most conserved arm of the UPR. IRE1α is a ubiquitously expressed transmembrane protein that possesses dual enzymatic activities of serine/threonine kinase and endoribonuclease (RNase) on its cytosolic domain (Lebeaupin, Vallee, Hazari, et al., 2018). In the physiologic state, the luminal domain of IRE1α is bound by the ER chaperone binding immunoglobulin protein (BiP) and remains inactive. In response to the accumulation of unfolded proteins, BiP releases IRE1α allowing its activation by dimerization and autophosphorylation via kinase activity, inducing a conformational change that activates the RNase activity. Alternatively, unfolded proteins may directly bind to the luminal domain of IRE1α, inducing allosteric changes that trigger its activation (Karagoz, et al., 2017). Activated IRE1α induces unconventional (cytosolic) excision of 26 nucleotides from the mRNA encoding X-box binding protein 1 (XBP1) to generate spliced XBP1. Spliced XBP1 is a transcription factor which upregulates the expression of several ER chaperones to increase protein folding capacity and also ER-associated degradation (ERAD). In irremediable ER stress, IRE1α RNase activity becomes promiscuous, leading to degradation of many other ER-bound mRNAs and microRNAs through regulated IRE1α-dependent decay (RIDD) (D. Han, et al., 2009; Hollien, et al., 2009; Hollien & Weissman, 2006). Enhanced RIDD leads to pro-apoptotic signaling by rapid decay of select micro-RNAs that normally repress the pro-apoptotic pathway (Upton, et al., 2012). Activated IRE1α also recruits tumor necrosis factor receptor-associated factor 2 leading to activation of the c-Jun N-terminal kinase (JNK) via apoptosis-signal-regulating kinase 1 and the activation of nuclear factor κ B (NFκB) (Hu, Han, Couvillon, Kaufman, & Exton, 2006; Tam, Mercado, Hoffmann, & Niwa, 2012; Urano, et al., 2000). JNK promotes apoptosis via Bcl-2 homology (BH) 3-only proteins and the Bax/Bak-dependent mitochondrial apoptotic machinery.
B. PERK pathway
PERK is a transmembrane protein with an N-terminal stress-sensing domain and a cytosolic kinase domain. Similar to IRE1α, the luminal domain of inactive PERK is bound to BiP (Bertolotti, Zhang, Hendershot, Harding, & Ron, 2000). PERK activation relies on dimerization and tetramerization mediated via its luminal domain leading to auto-phosphorylation upon the sensing of unfolded proteins (Carrara, Prischi, Nowak, & Ali, 2015). After activation, PERK phosphorylates eukaryotic translation initiation factor 2α (eIF2α) (N. Donnelly, Gorman, Gupta, & Samali, 2013). To attenuate overall protein overload, phosphorylated eIF2α holds back mRNA translation preventing 80S ribosome assembly, except for select mRNAs including the transcription factor ATF4 (Blais, et al., 2004; Harding, Zhang, & Ron, 1999). ATF4 then activates the transcription of specific UPR target genes, such as CCAAT-enhancer-binding protein homologous protein (CHOP) and the growth arrest and DNA damage-inducible protein (GADD34). CHOP is a transcription factor that triggers cell death by inducing genes involved in apoptosis in the presence of ER stress (Nishitoh, 2012) and may also promote ER stress-induced cell death by premature translational recovery (J. Han, et al., 2013). In contrast, GADD34 forms a feedback loop by dephosphorylation of eIF2α and recovery from translational inhibition in the UPR (Novoa, Zeng, Harding, & Ron, 2001).
C. ATF6α pathway
ATF6α is a type II ER transmembrane protein with a cytosolic bZip transcription factor domain and specialized in the regulation of ER quality control proteins (Adachi, et al., 2008). Upon proteotoxic ER stress, ATF6α is packed into coat protein complex II vesicles and transported to the Golgi (Schindler & Schekman, 2009). In the Golgi apparatus it is cleaved by site-1 protease (S1P) and site-2 protease (S2P), releasing a 50 kDa cytosolic fragment that migrates to the nucleus, and is referred to as ATF6N or ATF6F. Though the activation of ATF6α in the Golgi is similar to SREBPs (discussed below), the proximal pathways that mediate translocation of ATF6α or SREBPs to the Golgi are distinct. ATF6α is activated by the accumulation of unfolded proteins and SREBPs are regulated by sterols (Haze, Yoshida, Yanagi, Yura, & Mori, 1999; Horton, Goldstein, & Brown, 2002). Cleaved ATF6α activates UPR target genes including ERAD, phospholipid synthesis, and cooperatively the transcription of XBP1 target genes via direct binding to the ER stress response element (Bommiasamy, et al., 2009; Ho, Xu, & Thibault, 2018; Wu, et al., 2007; Yoshida, Matsui, Yamamoto, Okada, & Mori, 2001). Enforced expression of cleaved ATF6α can also upregulate cholesterol synthesis (Maruyama, Kamoshida, Shimizu, Inoue, & Sato, 2013).
3. Hepatic lipid Metabolism
Lipids are part of the cell structure, and are involved in essential functions such as cellular homeostasis, cell-to-cell communication and regulation of inflammation. The liver plays a predominant role in regulating lipid homeostasis via the major lipid metabolic pathways, including de novo lipogenesis which includes fatty acid (FA) synthesis, triglyceride (TG) synthesis and storage, synthesis of complex lipids such as cholesterol, ceramide and phospholipid, lipoprotein synthesis and secretion, lipolysis and FA oxidation (Simha & Garg, 2006; S. Wang & Kaufman, 2014). Many of these functions are housed in the ER, especially de novo ceramide biosynthesis, cholesterol synthesis, lipid droplet formation and export of TG as very low density lipoprotein (VLDL) (Fagone & Jackowski, 2009).
A. De Novo Lipogenesis
Hepatic de novo lipogenesis is regulated by ER membrane-bound transcription factors, the sterol regulatory element binding proteins (SREBPs), SREBP-1a, SREBP-1c, and SREBP-2, of which SREBP-1c and SREBP-2 are predominantly expressed in the liver (Brown & Goldstein, 1997; Eberle, Hegarty, Bossard, Ferre, & Foufelle, 2004; Horton, et al., 2002). SREBP-1c controls FA and TG biosynthesis, while SREBP-2 controls cholesterol metabolism and low density lipoprotein receptor expression (Hinz, Giebel, & Campos-Ortega, 1994; Yokoyama, et al., 1993; Zhou & Liu, 2014). SREBP-1c and SREBP-2 expression can be regulated transcriptionally and post-transcriptionally, comprehensively reviewed elsewhere (Horton, et al., 2002). Post-transcriptional processing of SREBPs is suppressed by sterols. SREBP-1c is embedded in the ER membranes bound to the protein SREBP cleavage-activating protein (SCAP). SCAP interacts with the insulin-induced gene (INSIG) protein which retains the complex SREBP-1c/SCAP in the ER. Similar to canonical regulation by sterols and insulin, under ER stress, SCAP dissociates from INSIG and the complex SCAP/SREBP-1c is transferred to the Golgi apparatus in coat protein II vesicles (Kammoun, et al., 2009). SREBP-1c undergoes regulated intramembrane proteolysis by S1P and S2P to release a cytosolic transcription factor that traffics to the nucleus to induce the expression of genes involved in de novo FA synthesis including acetyl-CoA carboxylase (ACC), FA synthase, the long-chain elongase and stearolyl-CoA desaturase (SCD) (Brown & Goldstein, 1997; Foufelle & Ferre, 2002; Postic & Girard, 2008; S. Wang & Kaufman, 2014). Cleavage of SREBP-2 occurs in a manner similar to SREBP-1c via dissociation of INSIG from SREBP-2/SCAP leading to transcriptional upregulation of cholesterol biosynthetic genes (Dong, Tang, & Chen, 2012; Sakakura, et al., 2001). SREBP-1c and SREBP-2
B. Hepatic lipid storage in the form of TGs
The hallmark of NAFLD is the accumulation of fat in the hepatocytes, in the form of lipid droplets containing TG (Marra & Svegliati-Baroni, 2018). TGs are synthesized from FAs and glycerol by a group of ER-localized acyltransferase enzymes including glycerol-3-phosphate acyltransferase, acylglycerolphosphate acyltransferase, monoacylglycerol acyltransferase or diacylcglycerol acyltransferase (Gimeno & Cao, 2008; Q. Liu, Siloto, Lehner, Stone, & Weselake, 2012). The majority of TG produced by the ER further drives the synthesis of lipid droplets, which originate from the ER membrane and form phospholipid monolayer enclosed droplet structures containing a high abundance of hydrophobic TGs, cholesterol ester and droplet structural proteins. Lipid droplets composed of TGs and cholesterol esters serve as a storage reservoir to prevent toxic accumulation of free FAs and cholesterol (Olofsson, et al., 2008; Reue, 2011). In addition, membrane phospholipids including phosphatidylcholine or phosphatidylethanolamine are synthesized via ER-localized enzymes, including the diacylglycerol choline/ethanolaminephosphotransferases (Fagone & Jackowski, 2009; Lagace & Ridgway, 2013).
C. Export of TG as VLDL
TGs are secreted from the liver in the form of VLDL through the fusion of apolipoprotein B-100 (Cohen & Fisher, 2013). VLDL synthesis is initiated by co-translational translocation of apolipoprotein B (ApoB) into the ER lumen for assembly with bulk neutral lipids, especially TGs (Tiwari & Siddiqi, 2012). The microsomal TG transfer protein (MTP) protein complex, composed of MTP and protein disulfide isomerase 1, is thought to play a critical role in facilitating the accretion of TGs into the ER lumen for ApoB lipidation and prevent intrahepatic TG accumulation (Hussain, Shi, & Dreizen, 2003). VLDL assembly and secretion are complex processes involving an array of protein and lipid factors (Rutledge, Su, & Adeli, 2010). A significant portion of ER chaperone proteins were found to be associated with ApoB, which could either promote ApoB folding and lipidation or mediate ApoB co-translational or post-translational degradation (Olofsson & Boren, 2012). Overproduction of hepatic VLDL particles can cause dyslipidemia, which is frequently observed in NAFLD and tightly associated with an increased risk of cardiovascular disease in obese patients with type 2 diabetes and NAFLD (Hassing, et al., 2012). Thus, the ER in hepatocytes not only plays an important role in intrahepatic lipid homeostasis, but is also tightly linked to circulating lipid homeostasis.
4. Unfolded protein response and hepatic lipid metabolism
Protein misfolding in the ER with associated ER stress causes hepatic steatosis, which is observed in mice with genetic ablation of the UPR sensors (Rutkowski, et al., 2008). ER stress-induced hepatic steatosis is multifactorial and can occur via the regulation of lipogenesis, VLDL secretion, and FA oxidation. Not only is steatosis observed in response to ER stress, recent studies have demonstrated that the UPR sensors directly play a role in the regulation of lipid metabolism, and lipotoxicity can activate an ER stress response, as discussed below. Overall, the interplay between UPR sensors and hepatic lipid metabolism is complex and likely only partially understood.
A. IRE1α/XBP1 pathway
IRE1α is a key regulator of hepatic lipid homeostasis through repressing hepatic lipid accumulation and maintaining lipoprotein secretion. Under ER stress with chemical inducing agent, mice with hepatocyte-specific deletion of IRE1α developed hepatic steatosis and increased expression of several transcriptional genes involved in lipid metabolism, including CCAAT/enhancer-binding protein (C/EBP) β, C/EBPδ, peroxisome proliferator-activated receptor (PPAR) γ, and enzymes involved in TG biosynthesis pointing to increased de novo lipogenesis (Zhang, et al., 2011). On the other hand, in the genetic absence of Ire1α in hepatocytes, under basal conditions there was reduced protein disulfide isomerase, which acts with MTP to promote the delivery of neutral lipids to the smooth ER lumen for VLDL assembly (S. Wang, et al., 2012). Thus, hepatocyte-specific Ire1α deletion did not alter lipogenesis, but rather, specifically impaired VLDL assembly and secretion, leading to steatosis (S. Wang, et al., 2012; Zhang, et al., 2011). In addition, experimental manipulation of XBP1, a downstream transcription factor of IRE1α, highlighted a critical role for IRE1α in lipid metabolism. Hepatic deletion of Xbp1 decreased de novo hepatic lipogenesis, leading to reduced serum TG, cholesterol, and free FAs by regulation of lipogenic genes including diacylglycerol acyltransferase 2, Scd1, and Acc2 (Lee, Scapa, Cohen, & Glimcher, 2008). Later studies demonstrated that Xbp1 deletion triggers feedback hyperactivation of IRE1α, inducing RIDD of cytosolic mRNAs encoding lipid metabolism functions, thereby reducing plasma TG and cholesterol in these mice (So, et al., 2012). IRE1α is also a target for inflammatory signaling as recently demonstrated via inducible nitric oxide synthase mediated nitrosylation of IRE1α which results in a reduction in IRE1α mediated Xbp1 splicing and impaired glucose homeostasis (L. Yang, et al., 2015), and impaired degradation of microRNAs which repress PPARα and the deacetylase sirtuin 1, thus decreasing FA oxidation and lipolysis (J. M. Wang, et al., 2018). Elevated plasma VLDL is a component of the dyslipidemia commonly observed in obesity-associated NAFLD. It is possible that IRE1α activation leads to increased rates of VLDL secretion in obesity, and thus contributes to dyslipidemia. These studies place IRE1α in a central position in mitigating hepatic steatosis via repression of lipolysis and export of VLDL.
On the other hand, there is evidence that constraining IRE1α activity in hepatocytes and in mice impairs inflammasome activation, liver injury, inflammation and hepatocyte apoptosis (Lebeaupin, Vallee, Rousseau, et al., 2018). Recently, it has been demonstrated that IRE1α activation leads to the release of proinflammatory extracellular vesicles from hepatocytes (Kakazu, et al., 2016). Thus inhibition of proinflammatory extracellular vesicle release may be a possible explanation for why inhibition of IRE1α activation impairs hepatic inflammation. Altogether, observations on the role of IRE1α in regulation of lipid metabolism point toward IRE1α-mediated mitigation of steatosis which would suggest that IRE1α activation might improve hepatic steatosis. However, IRE1α also promotes inflammation. We propose that the predominant output of IRE1α activation in the subset of NAFLD that develop progressive hepatic injury and inflammation (NASH) is proinflammatory. The determinants of IRE1α fates suggest a role for Bax Inhibitor 1 (BI-1), a negative regulator of IRE1α activation, and the magnitude of RIDD. For example, absence of BI-1 worsens NASH, potentially by hyperactivation of IRE1α with resultant persistent XBP1 processing and greater RIDD, with activation of the inflammasome, inflammation, and liver injury.
B. PERK-eIF2α pathway
The PERK pathway also regulates hepatic lipid metabolism. Antipsychotic drug-induced PERK and eIF2α phosphorylation results in increased hepatic steatosis through activation of SREBP-1c and SREBP-2 (Lauressergues, et al., 2012). The eIF2α phosphorylation resistant knockin mutant mice also showed exacerbation of lipid droplet coat proteins in response to pharmacologic ER stress (Rutkowski, et al., 2008). Transgenic mice with compromised eIF2α phosphorylation by overexpressing GADD34, eIF2α specific phosphatase, are protected from high-fat diet (HFD)-induced hepatic steatosis (Oyadomari, Harding, Zhang, Oyadomari, & Ron, 2008). ATF4, the downstream effector of the PERK/eIF2α pathway, is involved in lipid metabolism. Atf4−/− mice displayed high-carbohydrate diet induced suppression of SCD1 expression which was protective for hepatic steatosis (H. Li, et al., 2011). Atf4 depletion also attenuated lipid accumulation accompanied by a significant reduction in hepatic expression of SREBP-1c, ACC, and FA synthase (G. Xiao, et al., 2013).
CHOP is involved in the disruption of FA oxidation and lipoprotein secretion through suppression of C/EBPα (Rutkowski, et al., 2008). Under pharmacologic ER stress, deletion of Chop in mice showed less suppressed expression of transcriptional regulators, including C/EBPα, PPARα, SREBP-1, and PPAR gamma coactivator 1α, compared with wild-type mice. However, though Chop−/− mice exhibit greater adiposity basally, they showed reduced expression of lipogenic genes with less lipid accumulation than wild-type mice upon human immunodeficiency virus protease inhibitor (Y. Wang, et al., 2013). Therefore, it is necessary to elucidate the exact molecular and cellular mechanisms underlying CHOP-mediated dysregulation of hepatic lipid metabolism.
C. ATF6α pathway
Transcriptional induction of mammalian ER quality control proteins is mediated by combined action of ATF6α and XBP1 (Yamamoto, et al., 2007). ATF6α stimulates hepatic FA oxidation possibly through interaction with PPARα. The PPARα/retinoid X receptor heterodimer may serve as the key functional regulator transducing ATF6α signaling to the transcription of genes involving hepatic FA oxidation, such as carnitine palmitoyltransferase 1A, medium-chain acyl-coenzyme A dehydrogenase, and fibroblast growth factor 21(FGF21), via the PPAR response element sequence (Chen, et al., 2016). Cleaved ATF6α binds to sterol regulatory response element-bound SREBP-2 and recruits histone deacetylase 1, thus inhibiting sterol regulatory response element-mediated transcriptional activation and activate genes involved in UPR (Zeng, et al., 2004). Atf6α knockout mice accumulated lipids in the liver, due to blockage of β-oxidation of FAs and the suppression of VLDL formation (DeZwaan-McCabe, et al., 2017; Yamamoto, et al., 2010). When fed a HFD, Atf6α−/− mice developed hepatic steatosis and glucose intolerance in association with increased expression of SREBP-1c (Usui, et al., 2012).
5. Lipotoxic ER stress
Lipotoxicity is defined as dysregulation of the lipid environment and/or intracellular composition leading to the accumulation or transient generation of toxic lipids, which may result in hepatocyte injury or death (Malhi & Gores, 2008). Toxic lipid classes include the following; the SFA palmitate, sphingolipids including C16:0 ceramide, the phospholipid LPC, and free cholesterol (Musso, Cassader, Paschetta, & Gambino, 2018). ER stress is one manifestation of lipotoxicity due to these lipid classes, whereas the monounsaturated free FAs, such as oleate and palmitoleate, may protect from SFA-induced toxicity (Fig 1) (Akazawa, et al., 2010; Malhi, et al., 2007; Malhi, et al., 2006).
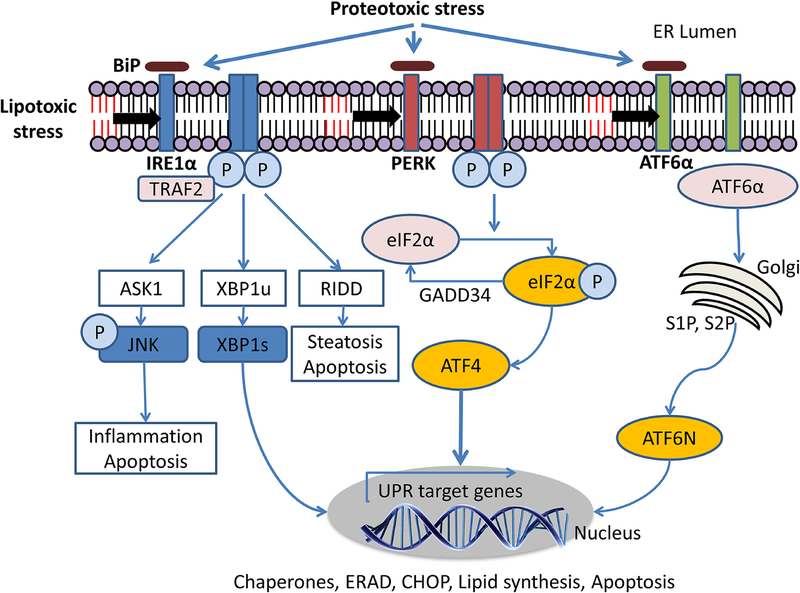
Figure 1. Proteotoxic and lipotoxic unfolded protein response signaling.
Proteotoxic stress due to the accumulation of misfolded or unfolded proteins activates the three transmembrane ER stress sensors (IRE1α, PERK, ATF6) by releasing them from BiP binding or by direct biding of the unfolded proteins to the luminal domain of the UPR sensors. Lipotoxic ER stress due to increased membrane saturation or sphingolipid accumulation is sensed by the transmembrane domains of the UPR sensors.Activated IRE1α induces splicing of XBP1. Spliced XBP1 transcriptionally upregulates genes that encode protein folding machinery, ERAD genes, and lipid synthesis pathways. PERK phosphorylates eIF2α which suppress mRNA translation leading to attenuation of protein synthesis. ATF4 is selectively translated and upregulates several transcriptional targets including CHOP. CHOP-dependent apoptosis occurs under unresolved ER stress. ATF6α translocates from the ER to the Golgi complex and cleaved by S1P and S2P. Cleaved ATF6α, termed ATF6N, transcriptionally upregulates UPR target genes. Abbreviation: IRE1α, inositol-requiring enzyme 1; PERK, PKR-like endoplasmic reticulum kinase; ATF6α, activating transcription factor 6; TRAF2, Tumor necrosis factor receptor-associated factor 2; ASK1, apoptosis-signal-regulating kinase 1; JNK, c-Jun N-terminal kinase; XBP1u, X-box binding protein 1; XBP1s, spliced X-box binding protein 1; RIDD, regulated IRE1α-dependent decay; eIF2α, eukaryotic translation initiation factor 2α; GADD34, growth arrest and DNA damage-inducible protein; ATF4, activating transcription factor 4; UPR, unfolded protein response; ERAD, endoplasmic reticulum-associated degradation; CHOP, CCAAT-enhancer-binding protein homologous protein; ATF6N, cleaved ATF6; BiP, binding immunoglobulin protein; S1P, site 1 protease; S2P, site 2 protease.
A. Palmitate
Palmitate in hepatocytes is converted to palmitoyl-CoA and under excess conditions can be incorporated in to various lipids (Figure 2). Palmitate can induce the accumulation of ceramide from de novo synthesis at the ER, increase LPC formation via phospholipase A2 (PLA2) action on phosphatidylcholine (PC), which in turn is derived from diacylglycerol (Hirsova, et al., 2016). Excess palmitate can also lead to the accumulation of di-saturated glycerolipids in the ER which trigger sustained IRE1α and PERK activation (Ariyama, Kono, Matsuda, Inoue, & Arai, 2010; Volmer, et al., 2013). The increased availability of palmitate by upregulating ER localized glycerol-3-phosphate acyltransferase can incorporate this FA into membrane lipids (Piccolis, et al., 2019). Additionally, in hepatocytes, palmitate can increase the de novo biosynthesis of saturated phospholipids contributing to palmitate-induced lipotoxic ER stress (Leamy, et al., 2014). Therefore, homeostatic pathways that maintain membrane lipid saturation during palmitate stress are important to prevent lipotoxicity. Palmitate-induced ER stress can result in apoptosis, such that knockdown of PERK gene expression significantly inhibited palmitate-induced apoptosis (Cao, et al., 2012). Attenuation of ER stress by BiP overexpression, suppressed palmitate-induced cell death through regulation of IRE1α, phosphorylated eIF2α and CHOP (Gu, et al., 2010). Downstream of persistent UPR activation, palmitate-mediated hepatocyte lipoapoptosis was triggered by JNK activation and CHOP- and JNK-dependent upregulation of the potent proapoptotic BH3-only protein p53 upregulated modulator of apoptosis (PUMA) (Cazanave, et al., 2010; Kakisaka, et al., 2012).
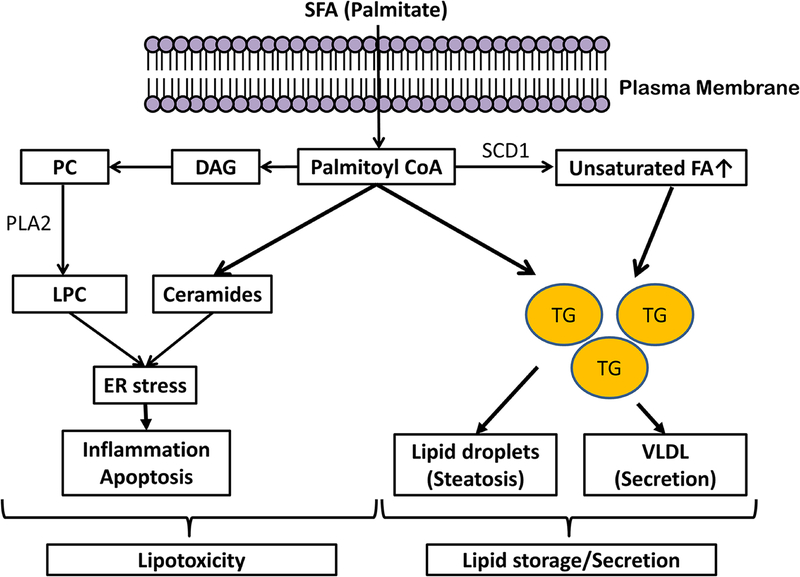
Figure 2. Palmitate-induced lipotoxicity.
Excess palmitate availability to the hepatocyte, derived predominantly from adipose tissue lipolysis in insulin resistance, leads to lipid accumulation and palmitate lipotoxicity. To maintain lipid homeostasis, fatty acid disposal in the liver occurs through the formation of triglyceride which is then stored temporarily as lipid droplets (steatosis) or secreted as VLDL. In hepatocytes, conversion of free fatty acids to triglycerides protects the cells from lipotoxicity; whereas higher levels of SFAs such as palmitate, are lipotoxic. Palmitate can be directly or indirectly metabolized into other lipid classes including ceramides and LPC which contribute to palmitate-induced ER stress. The generation of lipotoxic metabolites of fatty acids typically occurs in parallel with the accumulation of triglyceride droplets (steatosis), resulting in the hallmark features recognized as nonalcoholic steatohepatitis where steatosis and hepatocellular injury are present together. Abbreviation: SFA, saturated fatty acid; DAG, diacylglycerol; PC, phosphatidylcholine; PLA2, phospholipase A2; LPC, lysophosphatidyl choline; SCD1, stearolyl-CoA desaturase; TG, triglyceride; VLDL, very low density protein; ER, endoplasmic reticulum.
B. Ceramides
Ceramides are members of the sphingolipid family including sphingosine and sphingosine 1-phosphate (SP1P) and are integral to the structure of the lipid bilayer that makes up cell membranes (Hannun & Obeid, 2008; Pagadala, Kasumov, McCullough, Zein, & Kirwan, 2012). Ceramides can be synthesized de novo from serine and palmitate by the sequential action of 3 ER resident enzymes (serine palmitoyltransferase, ceramide synthase, and dihydroceramide desaturase) or generated from hydrolysis of plasma membrane sphingomyelin into ceramide and phosphocholine by the enzyme sphingomyelinase (Musso, et al., 2018). Sphingolipids are necessary to maintain lipid homeostasis and prevent ER stress, although it is unclear how alterations in sphingolipid biosynthesis are sensed by the IRE1α and PERK (Breslow, 2013). ATF6α can be activated by specific sphingolipids which are recognized by a motif in its transmembrane domain (Tam, et al., 2018). The deletion of Orm1 or Orm2 in yeast, which negatively regulate serine palmitoyltransferase complex in sphingolipid synthesis, led to lipid-mediated UPR activation (Futerman & Riezman, 2005; S. Han, Lone, Schneiter, & Chang, 2010; Ho, et al., 2018; Jonikas, et al., 2009; M. Liu, Huang, Polu, Schneiter, & Chang, 2012). In addition, the accumulation of C16 ceramide induced ER stress by the disturbance in Ca2+ homeostasis and led to cell death through the activation of the PERK/ATF4 and ATF6α arms of the UPR, resulting in the induction of CHOP expression (Aflaki, et al., 2012; Epstein, et al., 2012; Pettus, Chalfant, & Hannun, 2002).
Ceramides also play a critical role in insulin resistance and regulation of hepatic steatosis. Although the mechanism by which ceramides induce hepatic insulin resistance is not completely understood, ceramide-mediated insulin resistance may be induced through atypical protein kinase C activation and associated lipogenic and lipid uptake processes, while simultaneously impairing Akt-mediated regulation of hepatic glucose output (Ribaux & Iynedjian, 2003; Summers, 2006; G. Yang, et al., 2009). Recent studies show that inhibition of dihydroceramide desaturase 1 in the liver or the adipose tissue was sufficient to restore insulin sensitivity in high fat fed mice. These studies not only identified a potential therapeutic target for the treatment of obesity-associated insulin resistance, but also suggest that ceramides may mediate organ-to-organ crosstalk as deletion of the enzyme in liver alone or adipose tissue alone restored insulin sensitivity (Chaurasia, et al., 2019). Experimental models show that ceramide promotes protein kinase C ζ activation as a mediator of SREBP-1c driven lipogenesis and CD36-mediated lipid uptake in the liver (Xia, et al., 2015). In HFD mouse, overexpression of sphingosine kinase 1 which promotes the conversion of ceramide to SP1P ameliorates insulin resistance (Bruce, et al., 2012). The accumulation of SP1P upon depletion of SP1P-phosphohydrolase triggered ER stress and autophagy (Lepine, et al., 2011). Hepatic ceramide accumulation is increased in NAFLD and correlated with disease progression (Gorden, et al., 2015). Furthermore, blocking de novo ceramide synthesis reduced fat accumulation and hepatic TG content (G. Yang, et al., 2009). Ceramides may also promote inflammation in NASH as palmitate-induced the release of pro-inflammatory extracellular vesicles in an IRE1α/XBP1-dependent manner via the transcriptional activation of the de novo ceramide synthesis pathway (Kakazu, et al., 2016). Though ceramide accumulation in the ER is implicated in pancreatic β-cell death (J. Han & Kaufman, 2016), inhibition of de novo ceramide synthesis did not prevent palmitate-induced ER stress and apoptosis in hepatocytes (Wei, Wang, Topczewski, & Pagliassotti, 2006). Altogether, these data suggest a role for sphingolipid metabolism in maintenance of ER homeostasis and in insulin resistance, hepatic steatosis as well as formation of lipotoxic extracellular vesicles.
C. Lysophosphatidylcholine
LPC is a major component of cell membrane bilayers, lipid droplet envelop monolayers, and VLDL (Neuschwander-Tetri, 2010; Wiesner, Leidl, Boettcher, Schmitz, & Liebisch, 2009). LPC is another important phospholipid mediator of SFA induced lipotoxicity in NASH (M. S. Han, et al., 2008). LPC is generated from PC intracellularly by PLA2 or extracellularly by plasma lecithin-cholesterol acyltransferase (Musso, Gambino, & Cassader, 2009). PLA2 inhibition decreased intracellular LPC and palmitate-induced apoptosis (K. L. Donnelly, et al., 2005; Kakisaka, et al., 2012). An additional important mechanism of lipotoxicity is the depletion of membrane PC caused by PLA2 activation (Z. Li, et al., 2006). PC is the most abundant phospholipid and is essential for cell membrane integrity, and is an important feedback inhibitor of SREBP-1c-mediated lipogenesis (Walker, et al., 2011). Hepatocytes have a high demand for PC, which is used for the production and secretion of VLDL. Hepatic PC depletion and perturbation of hepatocyte membrane integrity result in extracellular release of lipotoxic lipids, inflammation, and hepatocyte apoptosis. Similar to palmitate, LPC induces ER stress including phosphorylation of eIF2α and subsequent increased CHOP expression and JNK activation, resulting in the induction of the BH3-only protein PUMA. Increased PUMA causes subsequent Bax activation, caspase 3/7 activation, and apoptosis (Kakisaka, et al., 2012).
D. Interconnectedness of lipotoxic and proteotoxic ER stress
The dual function of the ER in protein and lipid homeostasis suggests that proteotoxic and lipotoxic stress are interconnected. ER stress triggered by disequilibrium in the lipid bilayer is evidently distinct from proteotoxic ER stress, which is triggered by the accumulation of unfolded or misfolded proteins in the ER lumen. Furthermore, activation of the UPR sensors in lipotoxic stress relies on their transmembrane domains whereas misfolded proteins are sensed by their luminal domains. Both IRE1α and PERK can be activated via their transmembrane domains upon increases in membrane lipid saturation (Volmer, et al., 2013). Interestingly, ATF6α can be activated by specific sphingolipids, dihydroceramide and dihydrosphingosine, which are recognized by a motif in its transmembrane domain (Tam, et al., 2018). However, in spite of these differences, the relationship between lipotoxic ER stress and proteotoxic ER stress is likely bidirectional. SFA accumulation not only disrupts phospholipid homeostasis at the ER membrane, but also impacts on the morphology and integrity of the ER, thereby leading to disturbed ER proteostasis (Borradaile, et al., 2006). In addition, treatment with the chemical chaperone 4-phenylbutyric acid (4-PBA), which binds ER misfolded proteins, stabilized lipid-induced UPR, suggesting that lipotoxicity induced proteotoxic stress (Pineau, et al., 2009). Thus, it is likely that the impairment in protoestasis secondary to lipotoxic ER stress is a consequence of the disruption of normal ER structure and function. Furthermore, lipotoxic UPR induced proteome-remodeling mitigated the detrimental effects of lipotoxic lipid bilayer stress (Thibault, et al., 2012). Conversely, proteotoxic UPR activates ER expansion which requires lipid biosynthesis and ER stress impacts cellular lipid homeostasis, as discussed above. The comprehensive signaling outputs of the UPR retain some similarities but also demonstrate some differences between lipotoxic and proteotoxic ER stress. For example, sphingolipid-induced activation of ATF6α preferentially activated ER lipid biosynthetic genes over ER chaperones and protein-folding genes (Tam, et al., 2018). In Caenorhabditis elegans models lipid disequilibrium from impaired phosphatidylcholine synthesis resulted in activation of an ER stress response with a transcriptional profile quite distinct from proteotoxic ER stress (Fun & Thibault, 2019). One distinct pathway is autophagy which is activated in an IRE1α/XBP-1 dependent manner and may be protective against lipotoxic ER stress (Koh, Wang, Beaudoin-Chabot, & Thibault, 2018). Lipotoxic ER stress may also contribute to proteotoxic ER stress by degradation of ER proteins, as recently demonstrated in a Saccharomyces cerevisiae lipotoxic ER stress model (Shyu, et al., 2019). Thus lipotoxic and proteotoxic ER stress are interconnected and further studies are needed to understand this relationship especially in hepatocytes in the context of fatty liver disease.
6. Therapeutic potential targeting ER stress and UPR in NAFLD
ER stress is a pathogenic feature of NAFLD (Bechmann, et al., 2012; Malhi & Kaufman, 2011); thus targeting the specific UPR signaling pathways to attenuate ER stress and UPR activation may provide opportunities for developing new therapeutic strategies to treat NAFLD. Several compounds have been considered for the treatment of various diseases via their effects on the UPR signaling pathway (Table 1). However, further preclinical development and clinical trials are needed to validate their therapeutic potential in NAFLD.
Table 1.
Targeting the UPR in NAFLD
| Molecule | Target | Mechanism | Ref. |
|---|---|---|---|
| 4-PBA | Chemical chaperone | Stabilize misfolded proteins | (U. Ozcan, et al., 2006) |
| TUDCA | Chemical chaperone | Stabilize misfolded proteins | (Lebeaupin, et al., 2015) |
| Salubrinal | PERK | Prevent eIF2α dephosphorylation | (Kuo, et al., 2012) |
| GSK2606414 | PERK | PERK-induced JNK activation | (Win, et al., 2015) |
| FGF21 | PERK | Reduce eIF2α-ATF4-CHOP signaling | (Jiang, et al., 2014) |
| STF-083010 | IRE1α | Inhibits RNase activity | (Lebeaupin, Vallee, Rousseau, et al., 2018) |
| 4u8C | IRE1α | Inhibits RNase activity | (Tufanli, et al., 2017) |
| Toyocamycin | IRE1α | Block XBP1 processing | (Takahara, et al., 2017) |
A. PERK-eIF2α-ATF4 Signaling
PERK signaling reduces protein translation by phosphorylated eIF2α and attenuates ER stress levels by reducing protein misfolding overload. Salubrinal is a small molecule that inhibits dephosphorylation of eIF2α and protected cells from ER stress-induced apoptosis (Boyce, et al., 2005; Hetz, Chevet, & Harding, 2013). It also inhibited free FA-induced-apoptosis and NFκB activation (Kuo, et al., 2012). The PERK inhibitor, GSK2606414, was shown to attenuate palmitic acid–induced JNK activation and cell death in primary mouse hepatocytes (Win, et al., 2015). FGF21 was recently identified to play important roles in UPR signaling (Lin, et al., 2015; Shimizu, Morimoto, Maruyama, Inoue, & Sato, 2015). Administration of recombinant FGF21 alleviated tunicamycin-induced hepatic steatosis, in parallel with reduction of eIF2α-ATF4-CHOP signaling (Jiang, et al., 2014). Guanabenz is a α2-adrenergic receptor agonist approved for hypertension. It also binds to GADD34 and selectively inhibits GADD34-mediated dephosphorylation of eIF2α and exerted a cytoprotective effect against ER stress by prolonging UPR signaling (Tsaytler, Harding, Ron, & Bertolotti, 2011). As optimal eIF2α phosphorylation is protective in several metabolic pathologies, including β cell failure, guanabenz may be a promising candidate for the treatment of NAFLD.
B. IRE1α Signaling
Activated IRE1α induces processing of XBP1 and RNA degradation through RIDD via its RNAse activity. Salicylaldimine analogs, such as STF-083010 and 4μ8C were shown to covalently attach to a lysine residue and to inhibit the RNase activity of IRE1α (Mimura, et al., 2012; Papandreou, et al., 2011; Sanches, et al., 2014; Volkmann, et al., 2011). Many such salicylaldehyde derivatives block both XBP1 processing and RIDD without affecting the kinase activity of IRE1α (Cross, et al., 2012; Sanches, et al., 2014; Volkmann, et al., 2011). STF-083010 or 4μ8C showed improvement of NAFLD in HFD-fed mice (Lebeaupin, Vallee, Rousseau, et al., 2018). Toyocamycin was recently reported to attenuate the activation of XBP1, possibly by inducing a conformational change in IRE1α (Ri, et al., 2012). Toyocamycin ameliorated hepatic steatosis and liver injury caused by HFD-induced NASH (Takahara, et al., 2017).
C. Chemical chaperons
Chemical chaperones modulate ER stress by attenuating protein misfolding and aggregation and stabilizing folding intermediates. Chemical chaperones reduced ER stress, improved insulin sensitivity and glucose homeostasis, and reversed leptin resistance in the liver of an obese mouse model (Hetz, et al., 2013; L. Ozcan, et al., 2009; U. Ozcan, et al., 2006). Tauroursodeoxycholic acid (TUDCA) and 4-PBA are chemical chaperones that have been shown to reduce ER stress by facilitating proper protein folding and trafficking (Henkel, 2018; Vilatoba, et al., 2005; Xie, et al., 2002). Treatment with 4-PBA improved glucose tolerance in insulin-resistant patients and TUDCA partially restored insulin sensitivity in liver and muscle, but not adipose tissue in obese patients (Kars, et al., 2010; C. Xiao, Giacca, & Lewis, 2011). Mitigating ER stress using these chemical chaperones in mouse models with steatotic livers reduces hepatic lipid accumulation and hepatic fibrogenesis (Jimenez-Castro, et al., 2012; Namisaki, et al., 2016; U. Ozcan, et al., 2006). Unfortunately, the efficacy of these chaperones in NAFLD has been less impressive. Several randomized trials using ursodeoxycholic acid have failed to improve the overall histology in patients with NASH in comparison with placebo (Dufour, et al., 2006; Leuschner, et al., 2010; Lindor, et al., 2004). Whether other chemical chaperones may be effective remains to be determined.
D. Additional consideration on the therapeutic modulation of the UPR
BiP is an essential chaperone involved in the activation of PERK and IRE1α and translocation of ATF6α. BiP inducer X was identified as a modulator to induce BiP expression in the neural system and kidney (Nakanishi, et al., 2013; Prachasilchai, et al., 2009). Studies on the use of BiP inducer X in NAFLD remain to be explored. CHOP is another UPR target. Inhibitors of p38 mitogen-activated protein kinase inhibit the phosphorylation of CHOP, which is critical for its function as a transcription factor (X. Z. Wang & Ron, 1996). However, not only is there a shortage of CHOP-specific inhibitors, but there is a lack of research on the effectiveness of CHOP inhibitors in NAFLD. Glucagon-like peptide-1 analogues that are currently used in the treatment of type 2 diabetes reduce hepatic steatosis and insulin resistance in mouse models of fatty liver disease and reduce ER stress and fat accumulation in cultured human hepatocytes (Ding, Saxena, Lin, Gupta, & Anania, 2006; Sharma, Mells, Fu, Saxena, & Anania, 2011). The safety and efficacy of the long acting glucagon-like peptide-1 analogue, liraglutide, has been reported in patients with NASH (Armstrong, et al., 2016). However, whether this improvement involves a reduction of ER stress remains to be proven.
7. Conclusions
NASH is a complex disease that is modulated by numerous mechanisms including metabolic, genetic, and environmental factors. Although steatosis is a fundamental characteristic of NAFLD, the specific signaling mechanisms that lead to inflammation and progressive injury in NASH are not completely delineated. ER stress is considered a key factor in steatosis, such that hepatic lipid accumulation is both a cause and a consequence of ER stress, thus creating a positive feedback loop which may promote the development of hepatic steatosis. We further propose that the selective lipotoxic activation or inactivation of UPR sensors and their downstream signaling molecules may play a role in determining the outcomes of isolated steatosis versus progressive NASH. This concept needs further experimental testing to identify which of these signaling pathways offers interesting targets to therapeutically inhibit NASH progression. Lastly, the inter-connectedness of proteotoxic and lipotoxic ER stress is beginning to be defined, as are their unique characteristics, which will have relevance to disease pathogenesis and therapeutic targeting.
Acknowledgments
Grant Support: This work is partially supported by the NIH grant DK111378 and the Mayo Foundation.
Abbreviations
- ACC
acetyl-CoA carboxylase
- ATF
activating transcription factor
- ApoB
apolipoprotein B
- BI-I
Bax Inhibitor 1
- BH
Bcl-2 homology
- BiP
binding immunoglobulin protein
- C/EBP
CCAAT/enhancer-binding protein
- CHOP
CCAAT-enhancer-binding protein homologous protein
- ER
endoplasmic reticulum
- ERAD
ER-associated degradation
- eIF2α
eukaryotic translation initiation factor 2α
- FA
fatty acid
- FGF21
fibroblast growth factor 21
- GADD34
growth arrest and DNA damage-inducible protein
- HFD
high fat diet
- IRE
inositol-requiring enzyme
- INSIG
insulin-induced gene
- JNK
c-Jun N-terminal kinase
- LPC
lysophosphatidylcholine
- MTP
microsomal TG transfer protein
- NAFLD
Nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- 4-PBA
4-phenylbutyric acid
- PC
phosphatidylcholine
- PERK
protein kinase RNA-like ER kinase
- PLA2
phospholipase A2
- PPAR
peroxisome proliferator-activated receptor
- PUMA
p53 upregulated modulator of apoptosis
- RIDD
regulated IRE1α-dependent decay
- RNase
endoribonuclease
- SCAP
SREBP cleavage-activating protein
- SCD
stearolyl-CoA desaturase
- SFA
saturated fatty acid
- S1P
site 1 protease
- S2P
site 2 protease
- SP1P
sphingosine 1-phosphate
- SREBP
sterol regulatory element binding proteins
- TG
triglyceride
- TUDCA
tauroursodeoxycholic acid
- UPR
unfolded protein response
- VLDL
very low density lipoprotein
- XBP1
X-box binding protein 1
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adachi Y, Yamamoto K, Okada T, Yoshida H, Harada A, & Mori K (2008). ATF6 is a transcription factor specializing in the regulation of quality control proteins in the endoplasmic reticulum. Cell Struct Funct, 33, 75–89. [DOI] [PubMed] [Google Scholar]
- Aflaki E, Doddapattar P, Radovic B, Povoden S, Kolb D, Vujic N, Wegscheider M, Koefeler H, Hornemann T, Graier WF, Malli R, Madeo F, & Kratky D (2012). C16 ceramide is crucial for triacylglycerol-induced apoptosis in macrophages. Cell Death Dis, 3, e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akazawa Y, Cazanave S, Mott JL, Elmi N, Bronk SF, Kohno S, Charlton MR, & Gores GJ (2010). Palmitoleate attenuates palmitate-induced Bim and PUMA up-regulation and hepatocyte lipoapoptosis. J Hepatol, 52, 586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amor AJ, & Perea V (2019). Dyslipidemia in nonalcoholic fatty liver disease. Curr Opin Endocrinol Diabetes Obes, 26, 103–108. [DOI] [PubMed] [Google Scholar]
- Ariyama H, Kono N, Matsuda S, Inoue T, & Arai H (2010). Decrease in membrane phospholipid unsaturation induces unfolded protein response. J Biol Chem, 285, 22027–22035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K, Abouda G, Aldersley MA, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hubscher SG, & Newsome PN (2016). Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet, 387, 679–690. [DOI] [PubMed] [Google Scholar]
- Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, & Canbay A (2012). The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol, 56, 952–964. [DOI] [PubMed] [Google Scholar]
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, & Ron D (2000). Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol, 2, 326–332. [DOI] [PubMed] [Google Scholar]
- Blais JD, Filipenko V, Bi M, Harding HP, Ron D, Koumenis C, Wouters BG, & Bell JC (2004). Activating transcription factor 4 is translationally regulated by hypoxic stress. Mol Cell Biol, 24, 7469–7482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blond E, Disse E, Cuerq C, Drai J, Valette PJ, Laville M, Thivolet C, Simon C, & Caussy C (2017). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease in severely obese people: do they lead to over-referral? Diabetologia, 60, 1218–1222. [DOI] [PubMed] [Google Scholar]
- Bommiasamy H, Back SH, Fagone P, Lee K, Meshinchi S, Vink E, Sriburi R, Frank M, Jackowski S, Kaufman RJ, & Brewer JW (2009). ATF6alpha induces XBP1-independent expansion of the endoplasmic reticulum. J Cell Sci, 122, 1626–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, & Schaffer JE (2006). Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res, 47, 2726–2737. [DOI] [PubMed] [Google Scholar]
- Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, & Yuan J (2005). A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science, 307, 935–939. [DOI] [PubMed] [Google Scholar]
- Breslow DK (2013). Sphingolipid homeostasis in the endoplasmic reticulum and beyond. Cold Spring Harb Perspect Biol, 5, a013326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, & Goldstein JL (1997). The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell, 89, 331–340. [DOI] [PubMed] [Google Scholar]
- Bruce CR, Risis S, Babb JR, Yang C, Kowalski GM, Selathurai A, Lee-Young RS, Weir JM, Yoshioka K, Takuwa Y, Meikle PJ, Pitson SM, & Febbraio MA (2012). Overexpression of sphingosine kinase 1 prevents ceramide accumulation and ameliorates muscle insulin resistance in high-fat diet-fed mice. Diabetes, 61, 3148–3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Dai DL, Yao L, Yu HH, Ning B, Zhang Q, Chen J, Cheng WH, Shen W, & Yang ZX (2012). Saturated fatty acid induction of endoplasmic reticulum stress and apoptosis in human liver cells via the PERK/ATF4/CHOP signaling pathway. Mol Cell Biochem, 364, 115–129. [DOI] [PubMed] [Google Scholar]
- Carrara M, Prischi F, Nowak PR, & Ali MM (2015). Crystal structures reveal transient PERK luminal domain tetramerization in endoplasmic reticulum stress signaling. Embo j, 34, 1589–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazanave SC, Elmi NA, Akazawa Y, Bronk SF, Mott JL, & Gores GJ (2010). CHOP and AP-1 cooperatively mediate PUMA expression during lipoapoptosis. Am J Physiol Gastrointest Liver Physiol, 299, G236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurasia B, Tippetts TS, Mayoral Monibas R, Liu J, Li Y, Wang L, Wilkerson JL, Sweeney CR, Pereira RF, Sumida DH, Maschek JA, Cox JE, Kaddai V, Lancaster GI, Siddique MM, Poss A, Pearson M, Satapati S, Zhou H, McLaren DG, Previs SF, Chen Y, Qian Y, Petrov A, Wu M, Shen X, Yao J, Nunes CN, Howard AD, Wang L, Erion MD, Rutter J, Holland WL, Kelley DE, & Summers SA (2019). Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science, 365, 386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhang F, Gong Q, Cui A, Zhuo S, Hu Z, Han Y, Gao J, Sun Y, Liu Z, Yang Z, Le Y, Gao X, Dong LQ, Gao X, & Li Y (2016). Hepatic ATF6 Increases Fatty Acid Oxidation to Attenuate Hepatic Steatosis in Mice Through Peroxisome Proliferator-Activated Receptor alpha. Diabetes, 65, 1904–1915. [DOI] [PubMed] [Google Scholar]
- Cohen DE, & Fisher EA (2013). Lipoprotein metabolism, dyslipidemia, and nonalcoholic fatty liver disease. Semin Liver Dis, 33, 380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross BC, Bond PJ, Sadowski PG, Jha BK, Zak J, Goodman JM, Silverman RH, Neubert TA, Baxendale IR, Ron D, & Harding HP (2012). The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. Proc Natl Acad Sci U S A, 109, E869–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeZwaan-McCabe D, Sheldon RD, Gorecki MC, Guo DF, Gansemer ER, Kaufman RJ, Rahmouni K, Gillum MP, Taylor EB, Teesch LM, & Rutkowski DT (2017). ER Stress Inhibits Liver Fatty Acid Oxidation while Unmitigated Stress Leads to Anorexia-Induced Lipolysis and Both Liver and Kidney Steatosis. Cell Rep, 19, 1794–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Saxena NK, Lin S, Gupta NA, & Anania FA (2006). Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology, 43, 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XY, Tang SQ, & Chen JD (2012). Dual functions of Insig proteins in cholesterol homeostasis. Lipids Health Dis, 11, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, & Parks EJ (2005). Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest, 115, 1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly N, Gorman AM, Gupta S, & Samali A (2013). The eIF2alpha kinases: their structures and functions. Cell Mol Life Sci, 70, 3493–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour JF, Oneta CM, Gonvers JJ, Bihl F, Cerny A, Cereda JM, Zala JF, Helbling B, Steuerwald M, & Zimmermann A (2006). Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin e in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol, 4, 1537–1543. [DOI] [PubMed] [Google Scholar]
- Eberle D, Hegarty B, Bossard P, Ferre P, & Foufelle F (2004). SREBP transcription factors: master regulators of lipid homeostasis. Biochimie, 86, 839–848. [DOI] [PubMed] [Google Scholar]
- Epstein S, Kirkpatrick CL, Castillon GA, Muniz M, Riezman I, David FP, Wollheim CB, & Riezman H (2012). Activation of the unfolded protein response pathway causes ceramide accumulation in yeast and INS-1E insulinoma cells. J Lipid Res, 53, 412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagone P, & Jackowski S (2009). Membrane phospholipid synthesis and endoplasmic reticulum function. J Lipid Res, 50 Suppl, S311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foufelle F, & Ferre P (2002). New perspectives in the regulation of hepatic glycolytic and lipogenic genes by insulin and glucose: a role for the transcription factor sterol regulatory element binding protein-1c. Biochem J, 366, 377–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fun XH, & Thibault G (2019). Lipid bilayer stress and proteotoxic stress-induced unfolded protein response deploy divergent transcriptional and non-transcriptional programmes. Biochim Biophys Acta Mol Cell Biol Lipids. [DOI] [PubMed] [Google Scholar]
- Futerman AH, & Riezman H (2005). The ins and outs of sphingolipid synthesis. Trends Cell Biol, 15, 312–318. [DOI] [PubMed] [Google Scholar]
- Gimeno RE, & Cao J (2008). Thematic review series: glycerolipids. Mammalian glycerol-3-phosphate acyltransferases: new genes for an old activity. J Lipid Res, 49, 2079–2088. [DOI] [PubMed] [Google Scholar]
- Gorden DL, Myers DS, Ivanova PT, Fahy E, Maurya MR, Gupta S, Min J, Spann NJ, McDonald JG, Kelly SL, Duan J, Sullards MC, Leiker TJ, Barkley RM, Quehenberger O, Armando AM, Milne SB, Mathews TP, Armstrong MD, Li C, Melvin WV, Clements RH, Washington MK, Mendonsa AM, Witztum JL, Guan Z, Glass CK, Murphy RC, Dennis EA, Merrill AH Jr., Russell DW, Subramaniam S, & Brown HA (2015). Biomarkers of NAFLD progression: a lipidomics approach to an epidemic. J Lipid Res, 56, 722–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Li K, Laybutt DR, He ML, Zhao HL, Chan JC, & Xu G (2010). Bip overexpression, but not CHOP inhibition, attenuates fatty-acid-induced endoplasmic reticulum stress and apoptosis in HepG2 liver cells. Life Sci, 87, 724–732. [DOI] [PubMed] [Google Scholar]
- Han D, Lerner AG, Vande Walle L, Upton JP, Xu W, Hagen A, Backes BJ, Oakes SA, & Papa FR (2009). IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell, 138, 562–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Back SH, Hur J, Lin YH, Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M, Kilberg MS, Sartor MA, & Kaufman RJ (2013). ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol, 15, 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, & Kaufman RJ (2016). The role of ER stress in lipid metabolism and lipotoxicity. J Lipid Res, 57, 1329–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MS, Park SY, Shinzawa K, Kim S, Chung KW, Lee JH, Kwon CH, Lee KW, Lee JH, Park CK, Chung WJ, Hwang JS, Yan JJ, Song DK, Tsujimoto Y, & Lee MS (2008). Lysophosphatidylcholine as a death effector in the lipoapoptosis of hepatocytes. J Lipid Res, 49, 84–97. [DOI] [PubMed] [Google Scholar]
- Han S, Lone MA, Schneiter R, & Chang A (2010). Orm1 and Orm2 are conserved endoplasmic reticulum membrane proteins regulating lipid homeostasis and protein quality control. Proc Natl Acad Sci U S A, 107, 5851–5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun YA, & Obeid LM (2008). Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol, 9, 139–150. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, & Ron D (1999). Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature, 397, 271–274. [DOI] [PubMed] [Google Scholar]
- Hassing HC, Surendran RP, Mooij HL, Stroes ES, Nieuwdorp M, & Dallinga-Thie GM (2012). Pathophysiology of hypertriglyceridemia. Biochim Biophys Acta, 1821, 826–832. [DOI] [PubMed] [Google Scholar]
- Haze K, Yoshida H, Yanagi H, Yura T, & Mori K (1999). Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell, 10, 3787–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel AS (2018). Unfolded Protein Response Sensors in Hepatic Lipid Metabolism and Nonalcoholic Fatty Liver Disease. Semin Liver Dis, 38, 320–332. [DOI] [PubMed] [Google Scholar]
- Hetz C, Chevet E, & Harding HP (2013). Targeting the unfolded protein response in disease. Nat Rev Drug Discov, 12, 703–719. [DOI] [PubMed] [Google Scholar]
- Hinz U, Giebel B, & Campos-Ortega JA (1994). The basic-helix-loop-helix domain of Drosophila lethal of scute protein is sufficient for proneural function and activates neurogenic genes. Cell, 76, 77–87. [DOI] [PubMed] [Google Scholar]
- Hirsova P, Ibrahim SH, Gores GJ, & Malhi H (2016). Lipotoxic lethal and sublethal stress signaling in hepatocytes: relevance to NASH pathogenesis. J Lipid Res, 57, 1758–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho N, Xu C, & Thibault G (2018). From the unfolded protein response to metabolic diseases - lipids under the spotlight. J Cell Sci, 131. [DOI] [PubMed] [Google Scholar]
- Hollien J, Lin JH, Li H, Stevens N, Walter P, & Weissman JS (2009). Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol, 186, 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollien J, & Weissman JS (2006). Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science, 313, 104–107. [DOI] [PubMed] [Google Scholar]
- Horton JD, Goldstein JL, & Brown MS (2002). SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest, 109, 1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, Han Z, Couvillon AD, Kaufman RJ, & Exton JH (2006). Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol, 26, 3071–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain MM, Shi J, & Dreizen P (2003). Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J Lipid Res, 44, 22–32. [DOI] [PubMed] [Google Scholar]
- Ji C (2014). New Insights into the Pathogenesis of Alcohol-Induced ER Stress and Liver Diseases. Int J Hepatol, 2014, 513787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Yan C, Fang QC, Shao ML, Zhang YL, Liu Y, Deng YP, Shan B, Liu JQ, Li HT, Yang L, Zhou J, Dai Z, Liu Y, & Jia WP (2014). Fibroblast growth factor 21 is regulated by the IRE1alpha-XBP1 branch of the unfolded protein response and counteracts endoplasmic reticulum stress-induced hepatic steatosis. J Biol Chem, 289, 29751–29765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Castro MB, Elias-Miro M, Mendes-Braz M, Lemoine A, Rimola A, Rodes J, Casillas-Ramirez A, & Peralta C (2012). Tauroursodeoxycholic acid affects PPARgamma and TLR4 in Steatotic liver transplantation. Am J Transplant, 12, 3257–3271. [DOI] [PubMed] [Google Scholar]
- Jonikas MC, Collins SR, Denic V, Oh E, Quan EM, Schmid V, Weibezahn J, Schwappach B, Walter P, Weissman JS, & Schuldiner M (2009). Comprehensive characterization of genes required for protein folding in the endoplasmic reticulum. Science, 323, 1693–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi-Barve S, Kirpich I, Cave MC, Marsano LS, & McClain CJ (2015). Alcoholic, Nonalcoholic, and Toxicant-Associated Steatohepatitis: Mechanistic Similarities and Differences. Cell Mol Gastroenterol Hepatol, 1, 356–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakazu E, Mauer AS, Yin M, & Malhi H (2016). Hepatocytes release ceramide-enriched pro-inflammatory extracellular vesicles in an IRE1alpha-dependent manner. J Lipid Res, 57, 233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakisaka K, Cazanave SC, Fingas CD, Guicciardi ME, Bronk SF, Werneburg NW, Mott JL, & Gores GJ (2012). Mechanisms of lysophosphatidylcholine-induced hepatocyte lipoapoptosis. Am J Physiol Gastrointest Liver Physiol, 302, G77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferre P, & Foufelle F (2009). GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest, 119, 1201–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagoz GE, Acosta-Alvear D, Nguyen HT, Lee CP, Chu F, & Walter P (2017). An unfolded protein-induced conformational switch activates mammalian IRE1. Elife, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kars M, Yang L, Gregor MF, Mohammed BS, Pietka TA, Finck BN, Patterson BW, Horton JD, Mittendorfer B, Hotamisligil GS, & Klein S (2010). Tauroursodeoxycholic Acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes, 59, 1899–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh JH, Wang L, Beaudoin-Chabot C, & Thibault G (2018). Lipid bilayer stress-activated IRE-1 modulates autophagy during endoplasmic reticulum stress. J Cell Sci, 131, jcs217992. [DOI] [PubMed] [Google Scholar]
- Kuo TF, Tatsukawa H, Matsuura T, Nagatsuma K, Hirose S, & Kojima S (2012). Free fatty acids induce transglutaminase 2-dependent apoptosis in hepatocytes via ER stressstimulated PERK pathways. J Cell Physiol, 227, 1130–1137. [DOI] [PubMed] [Google Scholar]
- Lagace TA, & Ridgway ND (2013). The role of phospholipids in the biological activity and structure of the endoplasmic reticulum. Biochim Biophys Acta, 1833, 2499–2510. [DOI] [PubMed] [Google Scholar]
- Lauressergues E, Bert E, Duriez P, Hum D, Majd Z, Staels B, & Cussac D (2012). Does endoplasmic reticulum stress participate in APD-induced hepatic metabolic dysregulation? Neuropharmacology, 62, 784–796. [DOI] [PubMed] [Google Scholar]
- Leamy AK, Egnatchik RA, Shiota M, Ivanova PT, Myers DS, Brown HA, & Young JD (2014). Enhanced synthesis of saturated phospholipids is associated with ER stress and lipotoxicity in palmitate treated hepatic cells. J Lipid Res, 55, 1478–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeaupin C, Proics E, de Bieville CH, Rousseau D, Bonnafous S, Patouraux S, Adam G, Lavallard VJ, Rovere C, Le Thuc O, Saint-Paul MC, Anty R, Schneck AS, Iannelli A, Gugenheim J, Tran A, Gual P, & Bailly-Maitre B (2015). ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell Death Dis, 6, e1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeaupin C, Vallee D, Hazari Y, Hetz C, Chevet E, & Bailly-Maitre B (2018). Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J Hepatol, 69, 927–947. [DOI] [PubMed] [Google Scholar]
- Lebeaupin C, Vallee D, Rousseau D, Patouraux S, Bonnafous S, Adam G, Luciano F, Luci C, Anty R, Iannelli A, Marchetti S, Kroemer G, Lacas-Gervais S, Tran A, Gual P, & Bailly-Maitre B (2018). Bax inhibitor-1 protects from nonalcoholic steatohepatitis by limiting inositol-requiring enzyme 1 alpha signaling in mice. Hepatology, 68, 515–532. [DOI] [PubMed] [Google Scholar]
- Lee AH, Scapa EF, Cohen DE, & Glimcher LH (2008). Regulation of hepatic lipogenesis by the transcription factor XBP1. Science, 320, 1492–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepine S, Allegood JC, Park M, Dent P, Milstien S, & Spiegel S (2011). Sphingosine-1-phosphate phosphohydrolase-1 regulates ER stress-induced autophagy. Cell Death Differ, 18, 350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuschner UF, Lindenthal B, Herrmann G, Arnold JC, Rossle M, Cordes HJ, Zeuzem S, Hein J, & Berg T (2010). High-dose ursodeoxycholic acid therapy for nonalcoholic steatohepatitis: a double-blind, randomized, placebo-controlled trial. Hepatology, 52, 472–479. [DOI] [PubMed] [Google Scholar]
- Li H, Meng Q, Xiao F, Chen S, Du Y, Yu J, Wang C, & Guo F (2011). ATF4 deficiency protects mice from high-carbohydrate-diet-induced liver steatosis. Biochem J, 438, 283–289. [DOI] [PubMed] [Google Scholar]
- Li Z, Agellon LB, Allen TM, Umeda M, Jewell L, Mason A, & Vance DE (2006). The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab, 3, 321–331. [DOI] [PubMed] [Google Scholar]
- Lin Z, Pan X, Wu F, Ye D, Zhang Y, Wang Y, Jin L, Lian Q, Huang Y, Ding H, Triggle C, Wang K, Li X, & Xu A (2015). Fibroblast growth factor 21 prevents atherosclerosis by suppression of hepatic sterol regulatory element-binding protein-2 and induction of adiponectin in mice. Circulation, 131, 1861–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P, Lymp JF, Burgart L, & Colin P (2004). Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology, 39, 770–778. [DOI] [PubMed] [Google Scholar]
- Liu M, Huang C, Polu SR, Schneiter R, & Chang A (2012). Regulation of sphingolipid synthesis through Orm1 and Orm2 in yeast. J Cell Sci, 125, 2428–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Siloto RM, Lehner R, Stone SJ, & Weselake RJ (2012). Acyl-CoA:diacylglycerol acyltransferase: molecular biology, biochemistry and biotechnology. Prog Lipid Res, 51, 350–377. [DOI] [PubMed] [Google Scholar]
- Malhi H, Barreyro FJ, Isomoto H, Bronk SF, & Gores GJ (2007). Free fatty acids sensitise hepatocytes to TRAIL mediated cytotoxicity. Gut, 56, 1124–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi H, Bronk SF, Werneburg NW, & Gores GJ (2006). Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem, 281, 12093–12101. [DOI] [PubMed] [Google Scholar]
- Malhi H, & Gores GJ (2008). Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin Liver Dis, 28, 360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi H, & Kaufman RJ (2011). Endoplasmic reticulum stress in liver disease. J Hepatol, 54, 795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra F, & Svegliati-Baroni G (2018). Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol, 68, 280–295. [DOI] [PubMed] [Google Scholar]
- Maruyama R, Kamoshida Y, Shimizu M, Inoue J, & Sato R (2013). ATF6alpha stimulates cholesterogenic gene expression and de novo cholesterol synthesis. Biosci Biotechnol Biochem, 77, 1734–1738. [DOI] [PubMed] [Google Scholar]
- Masouminia M, Samadzadeh S, Ebaee A, French BA, Tillman B, & French SW (2016). Alcoholic steatohepatitis (ASH) causes more UPR-ER stress than non-alcoholic steatohepatitis (NASH). Exp Mol Pathol, 101, 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura N, Fulciniti M, Gorgun G, Tai YT, Cirstea D, Santo L, Hu Y, Fabre C, Minami J, Ohguchi H, Kiziltepe T, Ikeda H, Kawano Y, French M, Blumenthal M, Tam V, Kertesz NL, Malyankar UM, Hokenson M, Pham T, Zeng Q, Patterson JB, Richardson PG, Munshi NC, & Anderson KC (2012). Blockade of XBP1 splicing by inhibition of IRE1alpha is a promising therapeutic option in multiple myeloma. Blood, 119, 5772–5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso G, Cassader M, Paschetta E, & Gambino R (2018). Bioactive Lipid Species and Metabolic Pathways in Progression and Resolution of Nonalcoholic Steatohepatitis. Gastroenterology, 155, 282–302.e288. [DOI] [PubMed] [Google Scholar]
- Musso G, Gambino R, & Cassader M (2009). Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD). Prog Lipid Res, 48, 1–26. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Shimazawa M, Sugitani S, Kudo T, Imai S, Inokuchi Y, Tsuruma K, & Hara H (2013). Role of endoplasmic reticulum stress in light-induced photoreceptor degeneration in mice. J Neurochem, 125, 111–124. [DOI] [PubMed] [Google Scholar]
- Namisaki T, Noguchi R, Moriya K, Kitade M, Aihara Y, Douhara A, Nishimura N, Takeda K, Okura Y, Kawaratani H, Takaya H, Seki K, & Yoshiji H (2016). Beneficial effects of combined ursodeoxycholic acid and angiotensin-II type 1 receptor blocker on hepatic fibrogenesis in a rat model of nonalcoholic steatohepatitis. J Gastroenterol, 51, 162–172. [DOI] [PubMed] [Google Scholar]
- Neuschwander-Tetri BA (2010). Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology, 52, 774–788. [DOI] [PubMed] [Google Scholar]
- Nishitoh H (2012). CHOP is a multifunctional transcription factor in the ER stress response. J Biochem, 151, 217–219. [DOI] [PubMed] [Google Scholar]
- Novoa I, Zeng H, Harding HP, & Ron D (2001). Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol, 153, 1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson SO, & Boren J (2012). Apolipoprotein B secretory regulation by degradation. Arterioscler Thromb Vasc Biol, 32, 1334–1338. [DOI] [PubMed] [Google Scholar]
- Olofsson SO, Bostrom P, Andersson L, Rutberg M, Levin M, Perman J, & Boren J (2008). Triglyceride containing lipid droplets and lipid droplet-associated proteins. Curr Opin Lipidol, 19, 441–447. [DOI] [PubMed] [Google Scholar]
- Oyadomari S, Harding HP, Zhang Y, Oyadomari M, & Ron D (2008). Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab, 7, 520–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Myers MG Jr., & Ozcan U (2009). Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab, 9, 35–51. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, & Hotamisligil GS (2006). Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science, 313, 1137–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagadala M, Kasumov T, McCullough AJ, Zein NN, & Kirwan JP (2012). Role of ceramides in nonalcoholic fatty liver disease. Trends Endocrinol Metab, 23, 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliassotti MJ (2012). Endoplasmic reticulum stress in nonalcoholic fatty liver disease. Annu Rev Nutr, 32, 17–33. [DOI] [PubMed] [Google Scholar]
- Papandreou I, Denko NC, Olson M, Van Melckebeke H, Lust S, Tam A, Solow-Cordero DE, Bouley DM, Offner F, Niwa M, & Koong AC (2011). Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood, 117, 1311–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettus BJ, Chalfant CE, & Hannun YA (2002). Ceramide in apoptosis: an overview and current perspectives. Biochim Biophys Acta, 1585, 114–125. [DOI] [PubMed] [Google Scholar]
- Piccolis M, Bond LM, Kampmann M, Pulimeno P, Chitraju C, Jayson CBK, Vaites LP, Boland S, Lai ZW, Gabriel KR, Elliott SD, Paulo JA, Harper JW, Weissman JS, Walther TC, & Farese RV Jr. (2019). Probing the Global Cellular Responses to Lipotoxicity Caused by Saturated Fatty Acids. Mol Cell, 74, 32–44.e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineau L, Colas J, Dupont S, Beney L, Fleurat-Lessard P, Berjeaud JM, Berges T, & Ferreira T (2009). Lipid-induced ER stress: synergistic effects of sterols and saturated fatty acids. Traffic, 10, 673–690. [DOI] [PubMed] [Google Scholar]
- Postic C, & Girard J (2008). The role of the lipogenic pathway in the development of hepatic steatosis. Diabetes Metab, 34, 643–648. [DOI] [PubMed] [Google Scholar]
- Prachasilchai W, Sonoda H, Yokota-Ikeda N, Ito K, Kudo T, Imaizumi K, & Ikeda M (2009). The protective effect of a newly developed molecular chaperone-inducer against mouse ischemic acute kidney injury. J Pharmacol Sci, 109, 311–314. [DOI] [PubMed] [Google Scholar]
- Reue K (2011). A thematic review series: lipid droplet storage and metabolism: from yeast to man. J Lipid Res, 52, 1865–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ri M, Tashiro E, Oikawa D, Shinjo S, Tokuda M, Yokouchi Y, Narita T, Masaki A, Ito A, Ding J, Kusumoto S, Ishida T, Komatsu H, Shiotsu Y, Ueda R, Iwawaki T, Imoto M, & Iida S (2012). Identification of Toyocamycin, an agent cytotoxic for multiple myeloma cells, as a potent inhibitor of ER stress-induced XBP1 mRNA splicing. Blood Cancer J, 2, e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribaux PG, & Iynedjian PB (2003). Analysis of the role of protein kinase B (cAKT) in insulin-dependent induction of glucokinase and sterol regulatory element-binding protein 1 (SREBP1) mRNAs in hepatocytes. Biochem J, 376, 697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski DT, Wu J, Back SH, Callaghan MU, Ferris SP, Iqbal J, Clark R, Miao H, Hassler JR, Fornek J, Katze MG, Hussain MM, Song B, Swathirajan J, Wang J, Yau GD, & Kaufman RJ (2008). UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell, 15, 829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge AC, Su Q, & Adeli K (2010). Apolipoprotein B100 biogenesis: a complex array of intracellular mechanisms regulating folding, stability, and lipoprotein assembly. Biochem Cell Biol, 88, 251–267. [DOI] [PubMed] [Google Scholar]
- Sakakura Y, Shimano H, Sone H, Takahashi A, Inoue N, Toyoshima H, Suzuki S, & Yamada N (2001). Sterol regulatory element-binding proteins induce an entire pathway of cholesterol synthesis. Biochem Biophys Res Commun, 286, 176–183. [DOI] [PubMed] [Google Scholar]
- Sanches M, Duffy NM, Talukdar M, Thevakumaran N, Chiovitti D, Canny MD, Lee K, Kurinov I, Uehling D, Al-awar R, Poda G, Prakesch M, Wilson B, Tam V, Schweitzer C, Toro A, Lucas JL, Vuga D, Lehmann L, Durocher D, Zeng Q, Patterson JB, & Sicheri F (2014). Structure and mechanism of action of the hydroxy-aryl-aldehyde class of IRE1 endoribonuclease inhibitors. Nat Commun, 5, 4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satapathy SK, & Sanyal AJ (2015). Epidemiology and Natural History of Nonalcoholic Fatty Liver Disease. Semin Liver Dis, 35, 221–235. [DOI] [PubMed] [Google Scholar]
- Schindler AJ, & Schekman R (2009). In vitro reconstitution of ER-stress induced ATF6 transport in COPII vesicles. Proc Natl Acad Sci U S A, 106, 17775–17780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Mells JE, Fu PP, Saxena NK, & Anania FA (2011). GLP-1 analogs reduce hepatocyte steatosis and improve survival by enhancing the unfolded protein response and promoting macroautophagy. PLoS One, 6, e25269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu M, Morimoto H, Maruyama R, Inoue J, & Sato R (2015). Selective Regulation of FGF19 and FGF21 Expression by Cellular and Nutritional Stress. J Nutr Sci Vitaminol (Tokyo), 61, 154–160. [DOI] [PubMed] [Google Scholar]
- Shyu P Jr., Ng BSH, Ho N, Chaw R, Seah YL, Marvalim C, & Thibault G (2019). Membrane phospholipid alteration causes chronic ER stress through early degradation of homeostatic ER-resident proteins. Sci Rep, 9, 8637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simha V, & Garg A (2006). Lipodystrophy: lessons in lipid and energy metabolism. Curr Opin Lipidol, 17, 162–169. [DOI] [PubMed] [Google Scholar]
- So JS, Hur KY, Tarrio M, Ruda V, Frank-Kamenetsky M, Fitzgerald K, Koteliansky V, Lichtman AH, Iwawaki T, Glimcher LH, & Lee AH (2012). Silencing of lipid metabolism genes through IRE1alpha-mediated mRNA decay lowers plasma lipids in mice. Cell Metab, 16, 487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers SA (2006). Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res, 45, 42–72. [DOI] [PubMed] [Google Scholar]
- Takahara I, Akazawa Y, Tabuchi M, Matsuda K, Miyaaki H, Kido Y, Kanda Y, Taura N, Ohnita K, Takeshima F, Sakai Y, Eguchi S, Nakashima M, & Nakao K (2017). Toyocamycin attenuates free fatty acid-induced hepatic steatosis and apoptosis in cultured hepatocytes and ameliorates nonalcoholic fatty liver disease in mice. PLoS One, 12, e0170591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam AB, Mercado EL, Hoffmann A, & Niwa M (2012). ER stress activates NF-kappaB by integrating functions of basal IKK activity, IRE1 and PERK. PLoS One, 7, e45078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam AB, Roberts LS, Chandra V, Rivera IG, Nomura DK, Forbes DJ, & Niwa M (2018). The UPR Activator ATF6 Responds to Proteotoxic and Lipotoxic Stress by Distinct Mechanisms. Dev Cell, 46, 327–343.e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault G, Shui G, Kim W, McAlister GC, Ismail N, Gygi SP, Wenk MR, & Ng DT (2012). The membrane stress response buffers lethal effects of lipid disequilibrium by reprogramming the protein homeostasis network. Mol Cell, 48, 16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari S, & Siddiqi SA (2012). Intracellular trafficking and secretion of VLDL. Arterioscler Thromb Vasc Biol, 32, 1079–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaytler P, Harding HP, Ron D, & Bertolotti A (2011). Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science, 332, 91–94. [DOI] [PubMed] [Google Scholar]
- Tufanli O, Telkoparan Akillilar P, Acosta-Alvear D, Kocaturk B, Onat UI, Hamid SM, Cimen I, Walter P, Weber C, & Erbay E (2017). Targeting IRE1 with small molecules counteracts progression of atherosclerosis. Proc Natl Acad Sci U S A, 114, E1395–e1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton JP, Wang L, Han D, Wang ES, Huskey NE, Lim L, Truitt M, McManus MT, Ruggero D, Goga A, Papa FR, & Oakes SA (2012). IRE1alpha cleaves select microRNAs during ER stress to derepress translation of proapoptotic Caspase-2. Science, 338, 818–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, & Ron D (2000). Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science, 287, 664–666. [DOI] [PubMed] [Google Scholar]
- Usui M, Yamaguchi S, Tanji Y, Tominaga R, Ishigaki Y, Fukumoto M, Katagiri H, Mori K, Oka Y, & Ishihara H (2012). Atf6alpha-null mice are glucose intolerant due to pancreatic beta-cell failure on a high-fat diet but partially resistant to diet-induced insulin resistance. Metabolism, 61, 1118–1128. [DOI] [PubMed] [Google Scholar]
- Vilatoba M, Eckstein C, Bilbao G, Smyth CA, Jenkins S, Thompson JA, Eckhoff DE, & Contreras JL (2005). Sodium 4-phenylbutyrate protects against liver ischemia reperfusion injury by inhibition of endoplasmic reticulum-stress mediated apoptosis. Surgery, 138, 342–351. [DOI] [PubMed] [Google Scholar]
- Volkmann K, Lucas JL, Vuga D, Wang X, Brumm D, Stiles C, Kriebel D, Der-Sarkissian A, Krishnan K, Schweitzer C, Liu Z, Malyankar UM, Chiovitti D, Canny M, Durocher D, Sicheri F, & Patterson JB (2011). Potent and selective inhibitors of the inositol-requiring enzyme 1 endoribonuclease. J Biol Chem, 286, 12743–12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volmer R, van der Ploeg K, & Ron D (2013). Membrane lipid saturation activates endoplasmic reticulum unfolded protein response transducers through their transmembrane domains. Proc Natl Acad Sci U S A, 110, 4628–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AK, Jacobs RL, Watts JL, Rottiers V, Jiang K, Finnegan DM, Shioda T, Hansen M, Yang F, Niebergall LJ, Vance DE, Tzoneva M, Hart AC, & Naar AM (2011). A conserved SREBP-1/phosphatidylcholine feedback circuit regulates lipogenesis in metazoans. Cell, 147, 840–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P, & Ron D (2011). The unfolded protein response: from stress pathway to homeostatic regulation. Science, 334, 1081–1086. [DOI] [PubMed] [Google Scholar]
- Wang JM, Qiu Y, Yang Z, Kim H, Qian Q, Sun Q, Zhang C, Yin L, Fang D, Back SH, Kaufman RJ, Yang L, & Zhang K (2018). IRE1alpha prevents hepatic steatosis by processing and promoting the degradation of select microRNAs. Sci Signal, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Chen Z, Lam V, Han J, Hassler J, Finck BN, Davidson NO, & Kaufman RJ (2012). IRE1alpha-XBP1s induces PDI expression to increase MTP activity for hepatic VLDL assembly and lipid homeostasis. Cell Metab, 16, 473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, & Kaufman RJ (2012). The impact of the unfolded protein response on human disease. J Cell Biol, 197, 857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, & Kaufman RJ (2014). How does protein misfolding in the endoplasmic reticulum affect lipid metabolism in the liver? Curr Opin Lipidol, 25, 125–132. [DOI] [PubMed] [Google Scholar]
- Wang XZ, & Ron D (1996). Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP Kinase. Science, 272, 1347–1349. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang L, Wu X, Gurley EC, Kennedy E, Hylemon PB, Pandak WM, Sanyal AJ, & Zhou H (2013). The role of CCAAT enhancer-binding protein homologous protein in human immunodeficiency virus protease-inhibitor-induced hepatic lipotoxicity in mice. Hepatology, 57, 1005–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Wang D, Topczewski F, & Pagliassotti MJ (2006). Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab, 291, E275–281. [DOI] [PubMed] [Google Scholar]
- Wiesner P, Leidl K, Boettcher A, Schmitz G, & Liebisch G (2009). Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. J Lipid Res, 50, 574–585. [DOI] [PubMed] [Google Scholar]
- Win S, Than TA, Le BH, Garcia-Ruiz C, Fernandez-Checa JC, & Kaplowitz N (2015). Sab (Sh3bp5) dependence of JNK mediated inhibition of mitochondrial respiration in palmitic acid induced hepatocyte lipotoxicity. J Hepatol, 62, 1367–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Rutkowski DT, Dubois M, Swathirajan J, Saunders T, Wang J, Song B, Yau GD, & Kaufman RJ (2007). ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell, 13, 351–364. [DOI] [PubMed] [Google Scholar]
- Xia JY, Holland WL, Kusminski CM, Sun K, Sharma AX, Pearson MJ, Sifuentes AJ, McDonald JG, Gordillo R, & Scherer PE (2015). Targeted Induction of Ceramide Degradation Leads to Improved Systemic Metabolism and Reduced Hepatic Steatosis. Cell Metab, 22, 266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Giacca A, & Lewis GF (2011). Sodium phenylbutyrate, a drug with known capacity to reduce endoplasmic reticulum stress, partially alleviates lipid-induced insulin resistance and beta-cell dysfunction in humans. Diabetes, 60, 918–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G, Zhang T, Yu S, Lee S, Calabuig-Navarro V, Yamauchi J, Ringquist S, & Dong HH (2013). ATF4 protein deficiency protects against high fructose-induced hypertriglyceridemia in mice. J Biol Chem, 288, 25350–25361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Khaoustov VI, Chung CC, Sohn J, Krishnan B, Lewis DE, & Yoffe B (2002). Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation. Hepatology, 36, 592–601. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, Harada A, & Mori K (2007). Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell, 13, 365–376. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Takahara K, Oyadomari S, Okada T, Sato T, Harada A, & Mori K (2010). Induction of liver steatosis and lipid droplet formation in ATF6alpha-knockout mice burdened with pharmacological endoplasmic reticulum stress. Mol Biol Cell, 21, 2975–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Badeanlou L, Bielawski J, Roberts AJ, Hannun YA, & Samad F (2009). Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am J Physiol Endocrinol Metab, 297, E211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Calay ES, Fan J, Arduini A, Kunz RC, Gygi SP, Yalcin A, Fu S, & Hotamisligil GS (2015). METABOLISM. S-Nitrosylation links obesity-associated inflammation to endoplasmic reticulum dysfunction. Science, 349, 500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama C, Wang X, Briggs MR, Admon A, Wu J, Hua X, Goldstein JL, & Brown MS (1993). SREBP-1, a basic-helix-loop-helix-leucine zipper protein that controls transcription of the low density lipoprotein receptor gene. Cell, 75, 187–197. [PubMed] [Google Scholar]
- Yoshida H, Matsui T, Yamamoto A, Okada T, & Mori K (2001). XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell, 107, 881–891. [DOI] [PubMed] [Google Scholar]
- Younossi Z, Tacke F, Arrese M, Sharma BC, Mostafa I, Bugianesi E, Wong VW, Yilmaz Y, George J, Fan J, & Vos MB (2018). Global Perspectives on Non-alcoholic Fatty Liver Disease and Non-alcoholic Steatohepatitis. Hepatology. [DOI] [PubMed] [Google Scholar]
- Zeng L, Lu M, Mori K, Luo S, Lee AS, Zhu Y, & Shyy JY (2004). ATF6 modulates SREBP2-mediated lipogenesis. Embo j, 23, 950–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Wang S, Malhotra J, Hassler JR, Back SH, Wang G, Chang L, Xu W, Miao H, Leonardi R, Chen YE, Jackowski S, & Kaufman RJ (2011). The unfolded protein response transducer IRE1alpha prevents ER stress-induced hepatic steatosis. Embo j, 30, 1357–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, & Liu R (2014). ER stress and hepatic lipid metabolism. Front Genet, 5, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]