Abstract
Hyperglycemia plays crucial roles in vascular disease development, including macrovascular and microvascular diseases from diabetes mellitus (DM). Our previous study demonstrated that Ruellia tuberosa L. (RTL) aqueous and ethanol extracts alleviate hyperglycemia and inhibit insulin resistance in diabetic rats. This study investigated the protective effect of RTL ethanol extract against aorta dysfunction in high‐fat diet (HFD) and streptozotocin (STZ)‐induced type 2 DM (T2DM) rats. Results showed that RTL ethanol extract (100 and 400 mg/kg BW/day) ameliorated serum lipid profiles, including triglyceride, free fatty acid, low‐density lipoprotein cholesterol, very low‐density lipoprotein cholesterol, and high‐density lipoprotein cholesterol levels. It also significantly reduced the level of serum cytokines such as tumor necrosis factor‐α (TNF‐α) and interleukin‐6 in T2DM rats. Additionally, RTL extract decreased endothelin‐1 and endothelial nitric oxide contents, reduced the level of cell adhesion factors, including monocyte chemoattractant protein‐1 and cell adhesion factor vascular cell adhesion molecule‐1, while decreasing content of damage factors, namely tissue factor and von Willebrand factor in aortic tissues of diabetic rats. Equally noteworthy is that RTL extract enhanced the activity of aorta antioxidative enzymes, including superoxidase dismutase and catalase in diabetic rats, suggesting that RTL ethanol extract may ameliorate aorta dysfunction via enhancing aortic antioxidative enzyme activity, which subsequently suppresses aorta endothelial damage‐associated factors in HFD with STZ‐induced T2DM rats.
Keywords: antioxidative enzyme, aorta dysfunction, endothelial damage‐associated factors, Ruellia tuberosa L., type 2 diabetes mellitus
1. INTRODUCTION
Diabetes mellitus (DM) is a chronic metabolic disease commonly known to result in carbohydrate, protein, and lipid metabolism disorders in patients, of whom 95% have type 2 DM (T2DM; ADA, 2015). Insulin resistance (IR) is the hallmark characteristic of T2DM. The pathogenesis of IR is mainly attributed to reduced ability to process glucose as well as decline in insulin sensitivity in peripheral tissue, ultimately leading to hyperinsulinemia and hyperglycemia (King, Park, & Li, 2016). These conditions easily lead to increased oxidative stress as evidenced by chronic inflammatory responses, consequently resulting in several diabetic complications involving macrovascular and microvascular diseases (Al Disi, Anwar, & Eid, 2016; King et al., 2016).
Evidence has shown that vascular endothelial cell dysfunction of the large blood vessels (macrovascular system), including the arteries and aorta, and the capillaries/microvessels (microvascular system) are positively associated with the onset of cardiovascular complications associated with diabetes (Lüscher, Creager, Beckman, & Cosentino, 2003). As one of the most frequently occurring and most observed macrovascular complications from DM, atherosclerosis can lead to premature coronary artery disease, increased risk of cerebrovascular disease, and increased occurrence rate of peripheral vascular disease (Lüscher et al., 2003).
Several risk factors are recognized to be involved in the progression of diabetic vascular complications, including IR or insulin deficiency, hyperglycemia, and dyslipidemia, in which inflammatory responses mediate their pathogenesis (King et al., 2016; Zheng et al., 2013). Zheng et al. (2013) demonstrated that cascades of nuclear factor kappa B (NF‐κB) activate inflammatory factors such as cytokines (e.g., interleukin‐1β [IL‐1β], IL‐6, and tumor necrosis factor‐α [TNF‐α]) and adhesion molecules (e.g., monocyte chemoattractant protein‐1 [MCP‐1] and intracellular cell adhesion molecule‐1 [ICAM‐1]). These factors were observed to be elevated in in vitro high glucose cultured human endothelial cell lines and in vivo in the high‐fat diet (HFD) plus streptozotocin (STZ)‐induced diabetic rats. Long‐term hyperglycemia also disrupts the balance between nitric oxide (NO) and reactive oxygen species (ROS), subsequently resulting in endothelial cell dysfunction (Creager, Luscher, Cosentino, & Beckman, 2003). In addition, lipid accumulation is attributed to the occurrences of IR and hyperglycemia and causes inflammatory responses by activating the NF‐κB transmission pathway (Cavelti‐Weder et al., 2012), subsequently elevating thrombosis and the expression of several factors that mediate vascular endothelial cell damage such as endothelin‐1 (ET‐1), tissue factor (TF), and von Willebrand factor (vWF; Frankel et al., 2008; Holy & Tanner, 2010; Paneni, Beckman, Creager, & Cosentino, 2013).
Ruellia tuberosa L. (RTL) has been used as folk medicine in East Asia for treating diabetes for a couple decades and contains a wide range of pharmacological benefits such as antioxidant, anti‐inflammatory, and anticancer properties (Chothani, Patel, Mishra, & Vaghasiya, 2010; Rajan, Kishor Kumar, Satheesh Kumar, Swathi, & Haritha, 2012). The phytochemicals in RTL have been characterized to encompass three major constituents (i.e., flavonoids, triterpenes, and sterols) besides alkaloids, lignans, betulin, flavone glycoside, syringic acid, coumaric acid, vanillic acid, apigenin, apigenin rutinoside, apigenin glucoside, apigenin‐7‐O‐glucuronide, luteolin, luteolin glucoside, cirsimaritin, cirsimarin, pedalitin, cirsiliol 4‐glucoside, sorbifolin, 3,5‐diglucoside, and indole‐3‐carboxaldehyde (Wulan, Utomo, & Mahdi, 2015). The ethanol leaf extracts of RTL have been reported to improve dyslipidemia in alloxan‐induced type 1 diabetic rats (Ananthakrishnan & Doss, 2010, 2013). We recently demonstrated that aqueous and ethanol extracts from RTL ameliorate blood glucose homeostasis by alleviating IR in skeletal muscles (Ko et al., 2018) and reduce hepatic detoxification function by increasing hepatic antioxidative enzyme activities in HFD and STZ‐induced diabetic rats (Chang et al., 2018). However, there remains a void in the literature regarding how RTL interact with aortic systems in diabetic patients, especially in the T2DM paradigm. The present study aims to investigate the protective potential of RTL against aorta dysfunction and its possible mechanism in a T2DM rat model.
2. MATERIALS AND METHODS
2.1. Preparation of RTL extracts
Ruellia tuberosa L. was gathered from the Herb Light farm, Yi‐Lan County, Taiwan, in May 2014. The RTL stems and leaves were washed, drained, weighed, sliced, and freeze‐dried. Each 1 g of dried material was extracted with 6 ml 95% ethanol (1:6, w/v) at 4°C for 72 hr and filtered through cheese cloth. The filtrate was filtered twice through Whatman No. 1 filter paper and, then, centrifuged at 4,700 g for 20 min. The supernatant was vacuum concentrated using a rotary evaporator at <40°C. The concentrate was freeze‐dried and kept at 80°C until use. The freeze‐dried extract powder appeared brownish green.
2.2. Animal experimental procedures
Forty‐two four‐week‐old male Wistar rats were procured from the National Laboratory Animal Center, Taipei, Taiwan. The room conditions and treatment procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and all protocols were approved by the Institutional Animal Care and Use Committee of National Taiwan Normal University, Taipei, Taiwan (approval no. 103042). The rats were housed in a temperature‐ (22 ± 1°C) and humidity‐controlled (50 ± 20%) room under a 12‐hr light/dark cycle (lights on from 08:00 to 20:00) with free access to food and water. After allowing the rats to adapt to their environment for 1 week, they were fed an HFD (60% calories from fat) for 4 weeks. During the 5th and 6th weeks, STZ (28 and 15 mg/kg body weight, respectively, dissolved in 0.1 M sodium citrate buffer at pH 4.5) was intraperitoneally injected into each HFD rat to induce diabetes. After STZ injection, the rats were supplied with drinking water containing 5% sucrose for 48 hr to reduce early death that might be resulted from insulin discharge from partially injured pancreatic islets. Seventy‐two hours later, the rats were tested for blood sugar. The diabetic rats were then fed HFD for 6 weeks prior to experimental procedures to guarantee the stable phenomena of hyperglycemia. For the experimental design, the rats were divided into five groups of six rats each: normal group, rats fed with normal diet; DM group, diabetic rats fed with HFD (60% calories from fat) as the negative control; DM+Pio group, diabetic rats fed with HFD and orally administered pioglitazone (Pio; 30 mg/kg body weight) daily for 4 weeks as the positive control; and DM+E100 and DM+E400 groups, diabetic rats fed with HFD and orally administered RTL ethanolic extracts (100 or 400 mg/kg body weight, respectively) daily for 4 weeks, respectively. All the rats were sacrificed at the end of the experiment.
2.3. Blood and tissue samples
After the rats were sacrificed, blood samples were collected and allowed to clot for 30 min at room temperature and centrifuged twice at 3,000 g for 20 min each to obtain the serum. The aortic tissue was separated and removed from the heart immediately after death and stored at −80°C until use.
2.4. Serum lipid profiles, cytokines, and cell adhesion factors
Enzyme‐linked immunosorbent assay (ELISA) kits to assess levels of free fatty acid (FFA), total cholesterol, triglyceride, high‐density lipoprotein cholesterol (HDL‐C), low‐density lipoprotein cholesterol (LDL‐C), very low‐density lipoprotein cholesterol (VLDL‐C), IL‐6, and TNF‐α in the rats were purchased from Randox Laboratories (Crumlin Co.). Vascular cell adhesion molecule‐1 (VCAM‐1) and ICAM‐1 were purchased from SunLong Biotech. Biochemical analyses were conducted according to each supplier's protocols.
2.5. Aortic tissue protein preparation and concentration
Bovine serum albumin was used as the standard protein and diluted to a final concentration range of 0–500 μg/ml. The aortic tissue proteins were diluted approximately 100‐fold to bring its concentration within the standard range. The protein concentration was measured at 595 nm using the Bradford method with a Bio‐Rad protein assay kit and a spectrophotometer.
2.6. Aortic antioxidative enzyme activity
Superoxidase dismutase (SOD) and catalase activities were measured as described previously (Chang et al., 2018).
2.7. Aortic cell adhesion factor
Kits for assessing aortic tissue MCP‐1 (Abcam) and VCAM‐1 (SunLong Biotech) were used according to each supplier's protocols.
2.8. Aortic eNOS, ET‐1 and damage factor
Enzyme‐linked immunosorbent assay kits to assess aortic tissue endothelial nitric oxide (eNOS), ET‐1, TF, and vWF were purchased from SunLong Biotech and were used according to the manufacturer's protocols.
2.9. Statistical analyses
Values are presented as the mean ± standard deviation (SD) using SAS v. 9.4 (SAS Institute Inc) and one‐way analysis of variance and Duncan's new multiple range tests. p < .05 was considered statistically significant.
3. RESULTS
3.1. Effect of RTL on lipid profiles in HFD and STZ‐induced T2DM diabetic rats
Table 1 shows that serum TG and VLDL‐C contents in the DM group were significantly higher than those in the normal group (p < .05). Serum TG and VLDL‐C contents in DM+E100 and DM+E400 groups were significantly reduced compared with DM group. Furthermore, serum FFA and LDL‐C levels in DM+E400 group were lower than those in the DM group (p < .05) by 18.07% and 33.33%, respectively. Moreover, HDL‐C level in DM+E400 group was higher than that in the DM group (p < .05) by 21.40%.
Table 1.
Profiles of serum biochemical lipid in high‐fat diet and streptozotocin‐induced type 2 diabetic rats after treating with Ruellia tuberosa L. (RTL) extracts for 4 weeks
| Normal | DM | DM+Pio | DM+E100 | DM+E400 | |
|---|---|---|---|---|---|
| Triglyceride (mg/dl) | 78.0 ± 7.2de | 145.5 ± 26.0a | 60.7 ± 11.1e | 103.7 ± 23.8bc | 125.0 ± 16.6ab |
| Free fatty acid (mmol/L) | 1.11 ± 0.22a | 1.09 ± 0.14a | 0.74 ± 0.08c | 1.06 ± 0.22ab | 0.89 ± 0.11bc |
| Total cholesterol (mg/dl) | 63.3 ± 2.3b | 67.2 ± 7.8ab | 72.2 ± 3.7a | 66.3 ± 7.2ab | 63.7 ± 5.1b |
| High‐density lipoprotein cholesterol (mg/dl) | 53.8 ± 5.6ab | 47.5 ± 5.1b | 54.7 ± 4.5a | 58.0 ± 2.1a | 57.7 ± 4.5a |
| Low‐density lipoprotein cholesterol (mg/dl) | 9.33 ± 0.52ab | 10.00 ± 4.82a | 12.50 ± 1.87a | 8.17 ± 1.60b | 6.67 ± 1.21b |
| Very low‐density lipoprotein cholesterol (mg/dl) | 3.50 ± 0.84b | 5.17 ± 0.75a | 2.83 ± 0.98b | 3.17 ± 0.98b | 1.33 ± 0.52c |
Normal: Normal diet; DM: high‐fat diet (HFD; 60% fat) plus STZ (28 mg/kg body weight, i.p.) induced diabetic rats; DM+Pio: DM rats gavaged with pioglitazone (30 mg/kg body weight) for 4 weeks; DM+E100: DM rats gavaged with RTL ethanol extract (100 mg/kg body weight) for 4 weeks; DM+E400: DM rats gavaged with RTL ethanol extract (400 mg/kg body weight) for 4 weeks. Values were calculated as the mean ± SD, n = 6 for each group. a–c letters = significant differences among all samples tested (p < .05).
3.2. Effect of RTL on eNOS and ET‐1 in aortic tissues of HFD and STZ‐induced diabetic rats
The aorta eNOS and ET‐1 levels in the DM group were higher than those in the normal group and were significantly decreased in the DM+Pio, DM+E100, and DM+E400 groups (p < .05; Figure 1).
Figure 1.
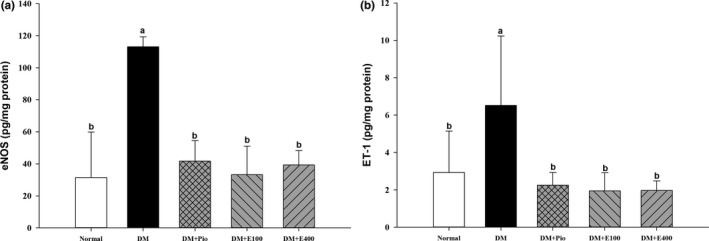
Endothelial nitric oxide synthase (eNOS; a) and endothelin‐1 (ET‐1; b) in aortas of high‐fat diet and streptozotocin‐induced type 2 diabetic rats treated with Ruellia tuberosa L. (RTL) extracts for 4 weeks. Normal: Normal diet; DM: high‐fat diet (HFD; 60% fat) plus STZ (28 mg/kg body weight, i.p.) induced diabetic rats; DM+Pio: DM rats gavaged with pioglitazone (30 mg/kg body weight) for 4 weeks; DM+E100: DM rats gavaged with RTL ethanol extract (100 mg/kg body weight) for 4 weeks; DM+E400: DM rats gavaged with RTL ethanol extract (400 mg/kg body weight) for 4 weeks. Values were calculated as the mean ± SD, n = 6 for each group. a–b letters = significant differences among all samples tested (p < .05)
3.3. Effect of RTL on cell adhesion factors in aortic tissues of HFD and STZ‐induced diabetic rats
Figure 2 shows that MCP‐1 (20.40 ± 0.13 ng/mg protein) and VCAM‐1 (1.07 ± 0.59 ng/mg protein) levels in the aortic tissues of DM group were significantly higher than those in the normal group (p < .05). The aorta MCP‐1 and CAM‐1 levels in the DM+E400 group were decreased (p < .05) by 65.78% and 91.46%, respectively, compared with the DM group.
Figure 2.
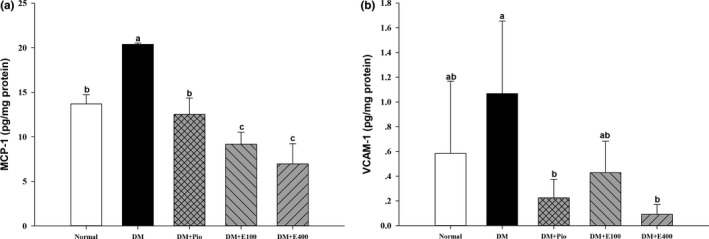
Monocyte chemoattractant protein‐1 (MCP‐1; a) and vascular cell adhesion molecule‐1 (VCAM‐1; b) levels in aortas of high‐fat diet and streptozotocin‐induced type 2 diabetic rats treated with Ruellia tuberosa L. (RTL) extracts for 4 weeks. Normal: Normal diet; DM: high‐fat diet (HFD; 60% fat) plus STZ (28 mg/kg body weight, i.p.) induced diabetic rats; DM+Pio: DM rats gavaged with pioglitazone (30 mg/kg body weight) for 4 weeks; DM+E100: DM rats gavaged with RTL ethanol extract (100 mg/kg body weight) for 4 weeks; DM+E400: DM rats gavaged with RTL ethanol extract (400 mg/kg body weight) for 4 weeks. Values were calculated as the mean ± SD, n = 6 for each group. a–c letters = significant differences among all samples tested (p < .05)
3.4. Effect of RTL on serum cytokines in HFD and STZ‐induced diabetic rats
Table 2 shows that serum IL‐6 and TNF‐α levels were significantly higher in the DM group than in the normal group (p < .05). The serum IL‐6 and TNF‐α levels in the DM+E400 group were lower than that in the DM group (p < .05) by 50.94% and 38.80%, respectively.
Table 2.
Serum cytokines in high‐fat diet and streptozotocin‐induced type 2 diabetic rats after treating with Ruellia tuberosa L. (RTL) extracts for 4 weeks
| Normal | DM | DM+Pio | DM+E100 | DM+E400 | |
|---|---|---|---|---|---|
| Tumor necrosis factor‐α (pg/ml) | 51.5 ± 3.0b | 90.3 ± 7.8a | 45.7 ± 6.8b | 53.1 ± 7.1b | 55.2 ± 7.8b |
| Interleukin‐6 (pg/ml) | 170.3 ± 60.7ab | 190.9 ± 30.5a | 153.4 ± 42.3abc | 127.2 ± 25.4bcd | 93.6 ± 28.8d |
Normal: Normal diet; DM: high‐fat diet (HFD; 60% fat) plus STZ (28 mg/kg body weight, i.p.) induced diabetic rats; DM+Pio: DM rats gavaged with pioglitazone (30 mg/kg body weight) for 4 weeks; DM+E100: DM rats gavaged with RTL ethanol extract (100 mg/kg body weight) for 4 weeks; DM+E400: DM rats gavaged with RTL ethanol extract (400 mg/kg body weight) for 4 weeks. Values were calculated as the mean ± SD, n = 6 for each group. a–c letters = significant differences among all samples tested (p < .05).
3.5. Effect of RTL on damage factors in aortic tissues of HFD and STZ‐induced diabetic rats
The TF and vWF levels in the aortic tissues of DM group were higher than those in the normal group. The aorta TF and vWF levels were significantly decreased in the DM+Pio, DM+E100, and DM+E400 groups compared with those in DM group (p < .05; Figure 3).
Figure 3.
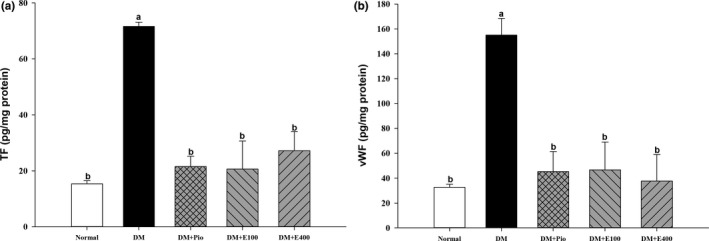
Endothelial tissue factor (TF; a) and von Willebrand factor (vWF; b) in aortas of high‐fat diet and streptozotocin‐induced type 2 diabetic rats treated with Ruellia tuberosa L. (RTL) extracts for 4 weeks. Normal: Normal diet; DM: high‐fat diet (HFD; 60% fat) plus STZ (28 mg/kg body weight, i.p.) induced diabetic rats; DM+Pio: DM rats gavaged with pioglitazone (30 mg/kg body weight) for 4 weeks; DM+E100: DM rats gavaged with RTL ethanol extract (100 mg/kg body weight) for 4 weeks; DM+E400: DM rats gavaged with RTL ethanol extract (400 mg/kg body weight) for 4 weeks. Values were calculated as the mean ± SD, n = 6 for each group. a–b letters = significant differences among all samples tested (p < .05)
3.6. Effect of RTL on SOD and catalase activities in aortic tissues of HFD and STZ‐induced diabetic rats
Superoxidase dismutase activity was significantly lower in the aortic tissues of DM group than that in the normal group (p < .05). However, the aorta SOD activity in the DM+E100 and DM+E400 groups were higher than in the DM group (p < .05) by 174.87% and 146.67%, respectively (Figure 4a). Additionally, catalase activity was also significantly increased in the DM+E400 group compared with the DM group (p < .05; Figure 4b).
Figure 4.
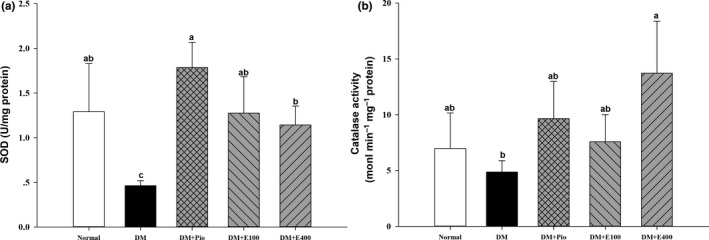
Superoxide dismutase (a) and catalase activity (b) in the aortas of high‐fat diet and streptozotocin‐induced type 2 diabetic rats treated with Ruellia tuberosa L. (RTL) extracts for 4 weeks. Normal: Normal diet; DM: high‐fat diet (HFD; 60% fat) plus STZ (28 mg/kg body weight, i.p.) induced diabetic rats; DM+Pio: DM rats gavaged with pioglitazone (30 mg/kg body weight) for 4 weeks; DM+E100: DM rats gavaged with RTL ethanol extract (100 mg/kg body weight) for 4 weeks; DM+E400: DM rats gavaged with RTL ethanol extract (400 mg/kg body weight) for 4 weeks. Values were calculated as the mean ± SD, n = 6 for each group. a–c letters = significant differences among all samples tested (p < .05)
4. DISCUSSION
Excess fat intake or lipid accumulation plays a crucial role in T2DM and obesity. The serum inflammatory markers and FAA levels reportedly increase in T2DM patients over the levels in healthy subjects (Cavelti‐Weder et al., 2012). Excessive FFAs may bind to toll‐like receptors and initiate NF‐κB signaling pathways to generate proinflammatory cytokines. Moreover, FFA also activates c‐Jun amino‐terminal kinase and protein kinase C (PKC), stimulates insulin receptor substrate‐1 (IRS1) phosphorylation, and inhibits phosphatidylinositol‐4,5‐bisphosphate 3‐kinase and Akt activation, resulting in a reduction in glucose transporter type 4 (GLUT4) expression (Kim, 2012), indicating that lipid accumulation is a factor in the creation of IR and hyperglycemia. In the present study, RTL ameliorated dyslipidemia and reduced serum FFA content, indicating that it may further alleviate inflammation in aortic tissues of diabetic rats.
Pio is a hypoglycemic drug belonging to the thiazolidinedione class that promotes fatty acid intake, fat synthesis, and differentiation through the activation of peroxisome proliferator‐activated receptor‐γ (PPARγ) in adipocytes (Kersten, Desvergne, & Wahli, 2000; Vamecq & Latruffe, 1999). Our observations are consistent with the results of a previous study that RTL ethanol extract at dosages of 500 mg/kg for 30 days decreases blood cholesterol, phospholipids, TG, LDL‐C, and VLDL‐C levels while elevating blood HDL‐C concentration in diabetic rats (Ananthakrishnan & Doss, 2013). It has been speculated that RTL may have a similar effect to Pio, which increases the expression of the lipoprotein lipase gene by activating PPARγ expression.
In the present study, it was found that the ethanol extract from RTL increased SOD and catalase activities in the aortas and that such a phenomenon is similar to that in liver tissues of diabetic rats, as observed in our previous study (Chang et al., 2018). High glucose concentration promotes aorta endothelial PKC activation, activates ET‐1 expression, up‐regulates NADPH oxidase, and subsequently enhances ROS production in the smooth muscle cells of diabetic rats. Excess ROS increases intracellular oxidative stress and initiates the aorta endothelial NF‐κB signaling pathway, further leading to inflammatory responses, and subsequently accelerating the progression of atherosclerotic disease (Paneni et al., 2013). ROS also accelerates the synthesis of glucose metabolites, such as methylglyoxal and hexosamine, and affects the dynamic balance of vascular function, which is the major cause of diabetic vascular disease. Hence, a superior antioxidant system that would scavenge excess free radicals is crucial in maintaining vascular tissue health (Paneni et al., 2013). In addition, the activation of PKC also enhances eNOS expression in the aortic tissue of diabetic rats. This result might be ascribed to the production of oxygen ion () and its downstream product hydrogen peroxide (H2O2) when the vascular tissue is subjected to oxidative stress. Excessive H2O2 results in increase of eNOS expression (Drummond, Cai, Davis, Ramasamy, & Harrison, 2000). When endothelial cells are injured, NADPH oxidation and eNOS decoupling are increased and , peroxynitrite (ONOO−), and eNOS coenzyme tetrahydrobiopterin (BH4) of ONOO− become highly sensitive and easily convertible into trihydrobiopterin (BH3) and dihydrobiopterin, leading to eNOS inactivation. BH3 might convert back to BH4 in the presence of an antioxidant, such as vitamin C (Xia et al., 2016). It is thus postulated that the enhancement of aortic antioxidative ability by RTL is involved in lowing ET‐1 and eNOS expression in aortic endothelial cells of diabetic rats.
Furthermore, this study demonstrated that RTL extract reduced aortic inflammation‐related adhesion molecules, including MCP‐1 and VCAM‐1 expression, which revealed their protective benefits (namely reducing foam cell formation from monocytes), and subsequently alleviating vascular endothelial cells damage in the aortas of diabetic rats. Although the NF‐κB‐induced inflammatory response and histopathological examination of the aortic tissue among all groups showed no significant differences (Appendix S1), the RTL extract significantly decreased serum IL‐6 and TNF‐α level, indicating an anti‐inflammatory potential of RTL in diabetic rats. Such anti‐inflammatory properties of the ethanol extract from RTL were also reported in the egg‐induced immune response rodent model (Alam et al., 2009).
Insulin resistance and hyperglycemia in diabetics may increase the formation of blood agglutination and result in vascular thrombosis (Vazzana, Ranalli, Cuccurullo, & Davì, 2012). In this study, RTL extract inhibited the production of aortic TF, a key factor that triggers a coagulation cascade, by decreasing proinflammatory cytokines. The vWF is a large glycoprotein secreted by the endothelial cells. Increase in vWF expression may subsequently cause vascular platelet aggregation, which is the first step in the formation of thrombosis (Frankel et al., 2008). Our observation indicated that RTL extract might ameliorate aorta endothelial damage by reducing inflammatory cytokines and aortic TF, as well as vWF levels, and would subsequently prevent the conversion of prothrombin into thrombin.
High glucose levels promote PKC activation and oxidative stress, and elevate the expression of adhesion molecules and the production of proinflammatory cytokines, thereby causing vascular damage and increased risk of cardiovascular disease (CVD; Ohkita, Tawa, Kitada, & Matsumura, 2012; Paneni et al., 2013). In this study, we observed that RTL extract exhibited the ability to increase antioxidative enzyme activity, to reduce adhesion factors and proinflammatory cytokines contents, and to alleviate endothelial damage‐associated factors levels in the aortic tissues of diabetic rats. Although RTL reportedly contains various phytochemicals, its constituents are quite complicated and have yet to be elucidated. The active components in RTL extracts are currently being isolated and identified in our Taiwan laboratory.
5. CONCLUSIONS
Vascular endothelial cells damage is attributed to excess oxidative stress and inflammation caused by hyperglycemia. In the present study, ethanol extract from RTL improved dyslipidemia, increased SOD and catalase activity in the aortic tissues of HFD and STZ‐induced T2DM rats. RTL extract also reduced eNOS and ET‐1, and adhesion factors such as MCP‐1 and VCAM‐1, alleviated proinflammatory cytokines such as TNF‐α and IL‐6, and reduced vascular endothelial damage‐associated factors such as TF and vWF in aortic tissues of T2DM rats (Figure 5). These findings suggest that the protective effect of RTL against aorta dysfunction is accomplished by ameliorating dyslipidemia, increasing aortic antioxidative enzyme activity as well as reducing aorta endothelial damage‐associated factors levels, and may possibly alleviate the pathogenesis of other vascular complications in the T2DM paradigm.
Figure 5.
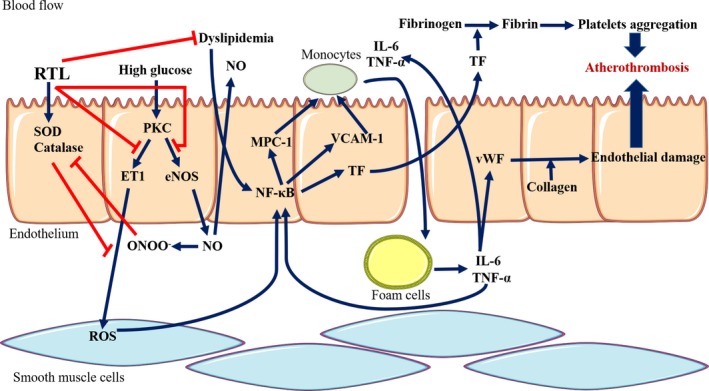
Postulated mechanism of RTL on preventing aorta dysfunction in high‐fat diet and streptozotocin ‐induced type 2 diabetic rats
CONFLICT OF INTEREST
The authors declare that they do not have any conflict of interest.
ETHICAL APPROVAL
The study was conducted in accordance with the ethical guidelines of the Institutional Animal Care and Use Committee of National Taiwan Normal University; Taipei, Taiwan (approval no. 103042).
Supporting information
ACKNOWLEDGMENTS
The authors would like to thank the Ministry of Science and Technology of the Republic of China (ROC), Taiwan, for financially supporting this research under contract No. MOST 104‐2320‐B‐003‐003‐MY3, and partly funded by the Fujian Province Science and Technology Project, China, under contract No. 2018J01290, and the Quanzhou Science and Technology Project, China, under contract No. 2017Z016 and No. 2018Z107. This article was also subsidized by the National Taiwan Normal University (NTNU), Taiwan, R.O.C., and academic funding of the Second Affiliated Hospital of Fujian Medical University (serial No. BSH001).
Ko C‐Y, Lin R‐H, Lo YM, et al. Effect of Ruellia tuberosa L. on aorta endothelial damage‐associated factors in high‐fat diet and streptozotocin‐induced type 2 diabetic rats. Food Sci Nutr. 2019;7:3742–3750. 10.1002/fsn3.1233
Chih‐Yuan Ko and Ru‐Hai Lin contributed equally to this work.
REFERENCES
- Al Disi, S. S. , Anwar, M. A. , & Eid, A. H. (2016). Anti‐hypertensive herbs and their mechanisms of action: Part I. Frontiers in Pharmacology, 6, 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam, M. A. , Subhan, N. , Awal, M. A. , Alam, M. S. , Sarder, M. , Nahar, L. , & Sarker, S. D. (2009). Antinociceptive and anti‐inflammatory properties of Ruellia tuberosa . Pharmaceutical Biology, 47, 209–214. [Google Scholar]
- American Diabetes Association (ADA) (2015). Standards of medical care in diabetes‐2015. Diabetes Care, 38, 1–89. [Google Scholar]
- Ananthakrishnan, M. , & Doss, V. A. (2010). Effect of 50% hydroethanolic leaf extracts of Ruellia tuberosa L. and Dipteracanthus patulus (Jacq.) on non‐enzymic antioxidants and other biochemical parameters in liver, kidney, serum of alloxan induced diabetic Swiss albino rats. Journal of Biomedical Sciences and Research, 2(3), 190–201. [Google Scholar]
- Ananthakrishnan, M. , & Doss, V. A. (2013). Effect of 50% hydro‐ethanolic leaf extracts of Ruellia tuberosa L. and Dipteracanthus patulus (jacq.) on lipid profile in alloxan induced diabetic rats. International Journal of Preventive Medicine, 4(7), 744–747. [PMC free article] [PubMed] [Google Scholar]
- Cavelti‐Weder, C. , Babians‐Brunner, A. , Keller, C. , Stahel, M. A. , Kurz‐Levin, M. , Zayed, H. , … Donath, M. Y. (2012). Effects of gevokizumab on glycemia and inflammatory markers in type 2 diabetes. Diabetes Care, 35, 1654–1662. 10.2337/dc11-2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, W. C. , Huang, D. W. , Chen, J. A. , Chang, Y. F. , Wu, J. S. B. , & Shen, S. C. (2018). Protective effect of Ruellia tuberosa L. extracts against abnormal expression of hepatic detoxification enzymes in diabetic rats. RSC Advances, 8, 21596–21605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chothani, D. L. , Patel, M. B. , Mishra, S. H. , & Vaghasiya, H. U. (2010). Review on Ruellia tuberosa (Cracker plant). Pharmacognosy Journal, 2, 506–512. 10.1016/S0975-3575(10)80040-9 [DOI] [Google Scholar]
- Creager, M. A. , Luscher, T. F. , Cosentino, F. , & Beckman, J. A. (2003). Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Circulation, 108, 1527–1532. 10.1161/01.CIR.0000091257.27563.32 [DOI] [PubMed] [Google Scholar]
- Drummond, G. R. , Cai, H. , Davis, M. E. , Ramasamy, S. , & Harrison, D. G. (2000). Transcriptional and posttranscriptional regulation of endothelial nitric oxidesynthase expression by hydrogen peroxide. Circulation Research, 86, 347–354. 10.1161/01.RES.86.3.347 [DOI] [PubMed] [Google Scholar]
- Frankel, D. S. , Meigs, J. B. , Massaro, J. M. , Wilson, P. W. , O'Donnell, C. J. , D'Agostino, R. B. , & Tofler, G. H. (2008). Von Willebrand factor, type 2 diabetes mellitus, and risk of cardiovascular disease: The Framingham offspring study. Circulation, 118, 2533–2539. 10.1161/CIRCULATIONAHA.108.792986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holy, E. W. , & Tanner, F. C. (2010). Tissue factor in cardiovascular disease pathophysiology and pharmacological intervention. Advances in Pharmacology, 59, 259–292. [DOI] [PubMed] [Google Scholar]
- Kersten, S. , Desvergne, B. , & Wahli, W. (2000). Roles of PPARs in health and disease. Nature, 405, 421–424. 10.1038/35013000 [DOI] [PubMed] [Google Scholar]
- Kim, J. K. (2012). Endothelial nuclear factor kappab in obesity and aging: Is endothelial nuclear factor kappaB a master regulator of inflammation and insulin resistance? Circulation, 125, 1081–1083. [DOI] [PubMed] [Google Scholar]
- King, G. L. , Park, K. , & Li, Q. (2016). Selective insulin resistance and the development of cardiovascular diseases in diabetes: The 2015 Edwin Bierman Award Lecture. Diabetes, 65, 1462–1471. 10.2337/db16-0152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko, C. Y. , Lin, R. H. , Zeng, Y. M. , Chang, W. C. , Huang, D. W. , Wu, J. S. , … Shen, S. C. (2018). Ameliorative effect of Ruellia tuberosa L. on hyperglycemia in type 2 diabetes mellitus and glucose uptake in mouse C2C12 myoblasts. Food Science & Nutrition, 6, 2414–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher, T. F. , Creager, M. A. , Beckman, J. A. , & Cosentino, F. (2003). Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part II. Circulation, 108, 1655–1661. 10.1161/01.CIR.0000089189.70578.E2 [DOI] [PubMed] [Google Scholar]
- Ohkita, M. , Tawa, M. , Kitada, K. , & Matsumura, Y. (2012). Pathophysiological roles of endothelin receptors in cardiovascular diseases. Journal of Pharmacological Sciences, 119, 302–313. 10.1254/jphs.12R01CR [DOI] [PubMed] [Google Scholar]
- Paneni, F. , Beckman, J. A. , Creager, M. A. , & Cosentino, F. (2013). Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. European Heart Journal, 34, 2436–2443. 10.1093/eurheartj/eht149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan, M. , Kishor Kumar, V. K. , Satheesh Kumar, P. S. , Swathi, K. R. , & Haritha, S. (2012). Antidiabetic, antihyperlipidaemic and hepatoprotective activity of methanolic extract of Ruellia tuberosa Linn leaves in normal and alloxan induced diabetic rats. Journal of Chemical and Pharmaceutical Research, 4, 2860–2868. [Google Scholar]
- Vamecq, J. , & Latruffe, N. (1999). Medical significance of peroxisome proliferator‐activated receptors. Lancet, 354, 141–148. 10.1016/S0140-6736(98)10364-1 [DOI] [PubMed] [Google Scholar]
- Vazzana, N. , Ranalli, P. , Cuccurullo, C. , & Davì, G. (2012). Diabetes mellitus and thrombosis. Thrombosis Research, 129, 371–377. 10.1016/j.thromres.2011.11.052 [DOI] [PubMed] [Google Scholar]
- Wulan, D. R. , Utomo, E. P. , & Mahdi, C. (2015). Antidiabetic activity of Ruellia tuberosa L., role of α‐amylase inhibitor: In silico, in vitro, and in vivo approaches. Biochemistry Research International, 2015, 349261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, N. , Horke, S. , Habermeier, A. , Closs, E. I. , Reifenberg, G. , Gericke, A. , … Li, H. (2016). Uncoupling of endothelial nitric oxide synthase in perivascular adipose tissue of diet‐induced obese mice. Arteriosclerosis, Thrombosis, and Vascular Biology, 36, 78–85. 10.1161/ATVBAHA.115.306263 [DOI] [PubMed] [Google Scholar]
- Zheng, X. , Zhu, S. , Chang, S. , Cao, Y. , Dong, J. , Li, J. , … Zhou, Y. (2013). Protective effects of chronic resveratrol treatment on vascular inflammatory injury in streptozotocin‐induced type 2 diabetic rats: Role of NF‐kappa B signaling. European Journal of Pharmacology, 720, 147–157. 10.1016/j.ejphar.2013.10.034 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


