Abstract
Artemisinin, also named qinghaosu, is a family of sesquiterpene trioxane lactone originally derived from the sweet wormwood plant (Artemisia annua), which is a traditional Chinese herb that has been universally used as anti-malarial agents for many years. Evidence has accumulated during the past few years which demonstrated the protective effects of artemisinin and its derivatives (artemisinins) in several other diseases beyond malaria, including cancers, autoimmune disorders, inflammatory diseases, viral and other parasite-related infections. Recently, this long-considered anti-malarial agent has been proved to possess anti-oxidant, anti-inflammatory, anti-apoptotic and anti-excitotoxic properties, which make it a potential treatment option for the ocular environment. In this review, we first described the overview of artemisinins, highlighting the activity of artemisinins to other diseases beyond malaria and the mechanisms of these actions. We then emphasized the main points of published results of using artemisinins in targeting ocular disorders, including uveitis, retinoblastoma, retinal neurodegenerative diseases and ocular neovascularization. To conclude, we believe that artemisinins could also be used as a promising therapeutic drug for ocular diseases, especially retinal vascular diseases in the near future.
Keywords: artemisinins, uveitis, retinoblastoma, retinal neurodegenerative diseases, ocular neovascularization
INTRODUCTION
Artemisinin and its derivatives (artemisinins) are isolated from the one ancient Chinese plant Artemisia annua (more commonly known as sweet wormwood), which have been used in traditional Chinese medicine (TCM) for fevers and chills[1]. Following the isolation of the active agent by Dr. You-You Tu's group from the Chinese Academy of TCM in the 1970s, artemisinin-based combination therapies have joined the currently established standard treatments of malarial parasites around the world[2]–[4]. Interestingly, abundant evidences have also demonstrated that artemisinins might also be of therapeutic value for many other diseases beyond malaria, including cancers, autoimmune disorders, inflammatory diseases as well as other infectious conditions[5]. Recently, many ophthalmologists and researchers have also showed their great interest in artemisinins, especially artesunate and dihydroartemisinin (DHA) and their potential protective effects on ocular disorders. Herein, we present an overview of research advances of artemisinins as potential therapeutic methods for ocular diseases, including uveitis, retinoblastoma, retinal neurodegenerative diseases, especially ocular neovascularization (NV). In this review, we also emphasize some important points regarding the potential applications of artemisinins in ocular disorders to provide a platform for additional study.
OVERVIEW OF ARTEMISININS
History and Origins
The medicinal herb Artemisia annua was first recognized by one Chinese physician, Hong Ge (born in the year 283) for its fever-reducing properties[6]. Led by the Chinese project 523 in the 1970s, Dr. You-You Tu's group first successfully isolated artemisinin, a non-toxic extract of Artemisia annua, identified the active component of this extract in 1972 and further identified its stereostucture (sesquiterpene lactones) in 1975[1]. In the 1980-90s, further studies conducted in humans confirmed the recognition of artemisinin-based combination therapies as the first-line option to treat malaria[2]–[4]. This novel anti-malaria therapy has been used universally with great efficacy and safety for a long time and helped Dr. You-You Tu win the 2015 Nobel Prize in Physiology or Medicine for her outstanding achievements[7].
Chemical and Pharmacological Characteristics
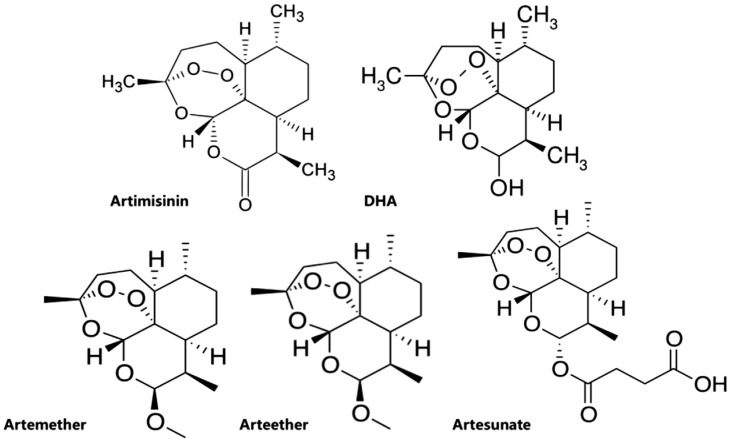
It was Dr. You-You Tu who first clarified the molecule extracted from the herbaceous plant Artemisia annu to be a sesquiterpene lactone endoperoxide by using the combined method of mass spectroscopy, spectrophotometry, X-ray crystallography and polyarithmetic analysis[8]. Those clinically important artemisinins include artesunate, artemether, arteether, and DHA (Figure 1), discovered and developed in 1986[1]. Among which artesunate is the most important analog, which shows a more favorable pharmacological profile because of its greater water-solubility and high oral bioavailability due to the additional hemisuccinate group[9].
Figure 1. Chemical structures of artemisinins.
Beyond Malaria: Activity of Artemisinins to Other Diseases
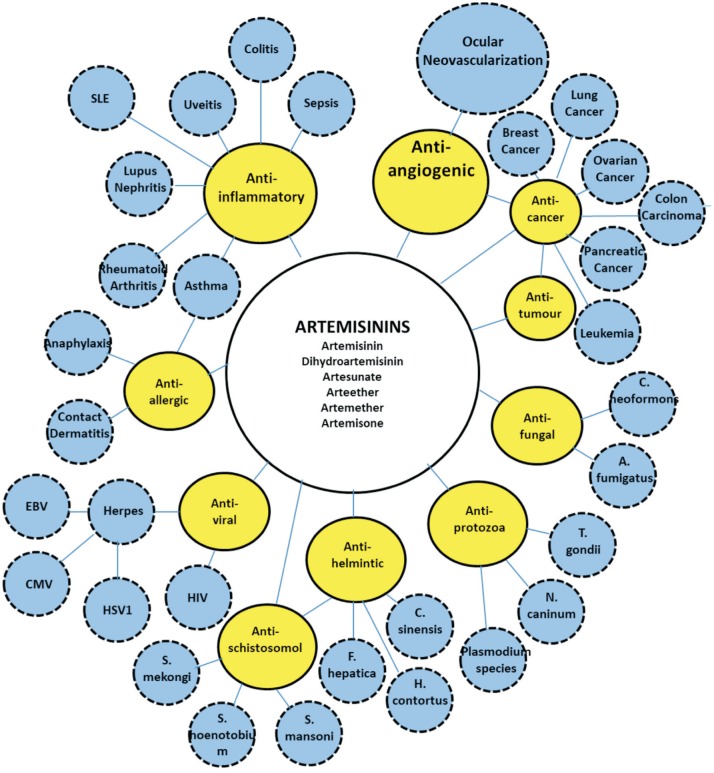
While the efficacy and low toxicity of artemisinins to treat malaria is well-recognized around the world, they have currently been reported to have a great therapeutic value beyond malaria[10]. These capacities include protective functions in non-malaria parasitic infections[11]–[14], anti-viral[15]–[17] and anti-fungal properties[18]–[19], anti-cancer functions[20]–[24], as well as anti-inflammatory[25]–[27] and anti-allergic effects[28]–[29] (Figure 2). Recent results further indicated that artemisinins might also reduce glucose, thus exerting a protective effect on diabetes mellitus[30].
Figure 2. Various biological activities of artemisinins and potential applications in different diseases (Bubble map).
Mechanisms of Actions of Artemisinins
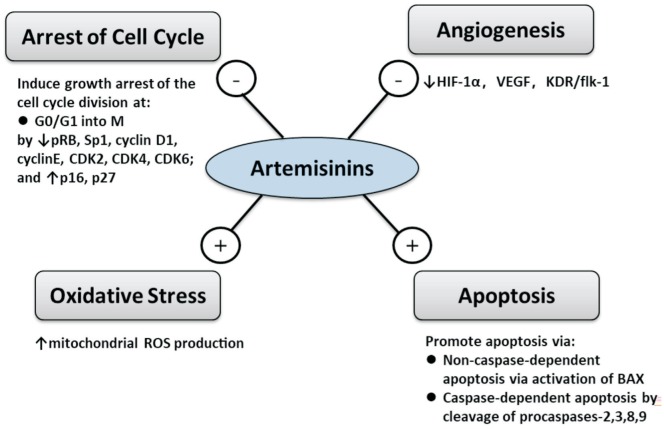
Although artemisinins are long known and effectively used as anti-malaria drugs, their specific biological action is poorly identified and understood. Current in vivo and in vitro studies have proposed numerous possible mechanisms of the actions, which include 1) oxidative stress, 2) induction of apoptosis[31], 3) inhibition of angiogenesis[32]–[33], 4) arrest of cell cycle at G0/G1[34] (Figure 3). As a matter of fact, these functional pathways may overlap in a number of ways.
Figure 3. Overview of mechanisms of actions of artemisinins.
- indicates inhibition and + indicates activation.
Oxidative Stress
Reactive oxygen species (ROS) are the natural byproduct of aerobic metabolism, whose levels can dramatically elevate during times of environmental stress. Studies in various tumor cell lines have proved ROS to have an important role in artemisinins-induced apoptosis[31]. These studies covered neuroblastoma[32], breast cancer[33], T-cell lymphoma[34], embryonal rhabdomyosarcoma cells[35], and glioblastoma[36]. In a recent study on human hepatocellular carcinoma cells, artesunate was shown to be able to induce ROS-dependent apoptosis via Bax-mediated intrinsic pathway[37]. Similarly, DHA was shown to alleviate oxidative stress in bleomycin-induced pulmonary fibrosis[38].
Induction of Apoptosis
Apoptosis, or programmed cell death, is a regulated cellular suicide mechanism involving the degradation of cellular components, which can be initiated via the intrinsic pathway and the extrinsic pathway[39]. Artemisinins could trigger apoptotic cell death through both pathways[40]–[41]. In human colon cancer cell line (HT29), B-cell lymphoma 2 associated X protein (BAX) was proved to be activated by artemisinins, inducing the release of cytochrome C, which led to apoptosis in cancer cells[42]. In human prostate cancer cell line (DU145), cleavage of procaspases 3 and 9 was found to be induced by artesunate, inducing the release of cytochrome C and the subsequent caspase-dependent apoptosis[43]. In human breast cancer cell line (MCF-7), apoptosis was also induced via a caspase-related mechanism under the effect of a semi-synthetic derivative of artemisinin[44].
Inhibition of Angiogenesis
Various models have accumulated mounting evidences, demonstrating the involvement of inhibiting aberrant angiogenesis in the actions of artemisinins[45]–[46]. In mouse embryonic stem cells, artemisinin was shown to be able to reduce the levels of hypoxia inducible factor (HIF)-1α and vascular endothelial growth factor (VEGF), suggesting the mechanism of artemisinin might involve the inhibition of angiogenesis[47]. Artemisinin was also found to be able to significantly reduce lymph-angiogenesis via downregulating the expression of VEGF-C in C57BL/6 mouse Lewis lung carcinoma model[48]. Similarly, in a rat glioma model, artemisinins were shown to have the effect of reducing VEGF and angiogenesis[49]. Moreover, artesunate was proved to be able to suppress osteoclastogenesis and aberrant angiogenesis, thus attenuating anterior cruciate ligament transection (ACLT)-induced osteoarthritis[50].
Arrest of Cell Cycle at G0/G1
Artemisinins have been shown by accumulating current studies to have the potential application in cancer drug development for its action on inducing growth arrest at various stages of cell division cycle[51]–[53]. In prostate cancer cells (LNCaP), phosphorylated retinoblastoma protein (pRB), a mediator cooperating with E2F transcription factors and cyclin-dependent kinases (CDKs) to push forward the cell cycle progression through G1 into S phase was shown to be ablated by artemisinin, inducig G1 cell cycle arrest, thus inhibiting cell division[54]. Willoughby et al[55] has also demonstrated that artemisinin could disrupt specificity protein 1 (Sp1) transcription factor from binding to CDK4 promoter and inhibiting CDK4 gene expression, thus blocking prostate cancer growth and cell cycle progression. Wu et al[56] have further proved the growth inhibition effect of artemisinin in nasopharyngeal carcinoma cell lines by suppressing the level of cyclin D1, cyclin E, CDK2, CDK4, CDK6 and upregulating the inhibitors of cell cycle division (p16, p27).
POTENTIAL APPLICATION IN OCULAR DISEASES
Recently, many ophthalmologists and researchers have noticed the potential protective effects of artemisinins on ocular disorders. Recent findings have shed light on the potential applications of artemisinins as promising therapeutic agents in ocular diseases. In this review, we are going to highlight the main points of published results of using artemisinins in targeting ocular disorders.
Uveitis
Uveitis is the inflammation of the uvea whereas the anti-inflammatory effects of artemisinins have already been recognized in the past few decades[57]. Artesunate has been reported by Li et al[58] to have a protective effect on sepsis mouse model by decreasing serum endotoxin release and toll-like receptors (TLR)4, TLR9 expressions, also suppressing nuclear factor-kappa B (NF-κB) activation. Xu et al[59] also reported that in human rheumatoid arthritis fibroblast-like synoviocytes, artesunate was able to inhibit TNF-α expression and decrease the secretion of pro-inflammatory cytokines. Based on those experimental results, the question of if artesunate could reduce the release of inflammatory cytokines in some type of inflammatory ocular diseases was raised and further investigated. Wang et al[60] studied the protective effect of artesunate by using endotoxin-induced uveitis (EIU) rat model, which has been generally considered as an experimental model for human uveitis[61]. In their study, artesunate of three concentrations (1, 10, 100 mg/kg) were intravenously injected in male Long-Evans rats whereas prednisolone (10 mg/kg) was used as positive control and their results showed that artesunate (10 mg/kg and 100 mg/kg) could suppress infiltrating cells and protein concentration in the aqueous humor, suggesting that artesunate treatment could suppress the inflammation of EIU by inhibiting the production of inflammatory mediators[60]. More future studies will be needed to clearly define the specific cellular mechanisms of the therapeutic effects. The role of artemisinins in modulating ocular inflammatory responses might be of great interest in the future.
Retinoblastoma
In recent years, artemisinins have been shown to exert protective effects in various types of cancer[62]–[66]. Retinoblastoma (RB) is an eye cancer, which is most common among children[67]. Zhao et al[68] tested the anti-neoplastic activity of artesunate against RB to see whether artesunate might be a good candidate to treat RB. Using epithelial retina cell line as normal counterpart, the cytotoxic activity and specificity of artesunate were analyzed in an RB cell line, which showed a dose-dependent manner concerning the cytotoxic activity specific to RB cells, with low toxicity in normal retina cells and high cytotoxicity in RB cells[68]. Their results also demonstrated that artesunate, even at low doses, could block the cell cycle progression at the G1 phase[68]. Artesunate is practically suitable for long-term treatments with few side-effects. Therefore, artesunate could be considered as a promising option for RB treatment. Further randomized studies in vivo need to be done to provide better insights regarding the efficacy as well as efficiency of the novel treatment.
Retinal Neurodegenerative Diseases
Retinal neurodegeneration is a retinopathy which consists in the deterioration of the retina caused by the progressive death of its neuronal cells[69]. There are several reasons for retinal neurodegeneration, including age-related macular degeneration (AMD), diabetic retinopathy (DR), and retinal artery or vein occlusion[69]. Zeng et al[70] studied the neurogenic effects of artemisinin and their findings indicated that artemisinin at low concentration could induce neurite outgrowth as well as promote neuronal differentiation in PC12 cells.
Accordingly, Chong and Zheng[71] demonstrated that artemisinin was able to suppress hydrogen peroxide (H2O2)-induced oxidative stress in D407 retinal pigment epithelium (RPE) cells, which are first damaged in retinal diseases owning to their critical support functions for photoreceptors. The findings of Yan et al[72] also demonstrated that artemisinin could prevent RPE cells from oxidative stress via the MAPK/CREB pathway.
These recent results all shed light on the promising therapeutic value of artemisinin as a candidate drug for the treatment of many retinal neurodegenerative disorders. Though, its specific effects on the retinal neuronal cells need to be further explored.
Ocular Neovascularization
Ocular NV is one of the major causes of blindness among ocular disorders. Substantial evidences have demonstrated that VEGF played an essential part in its pathogenesis[73]. Currently for the treatment of ocular NV, anti-VEGF agents such as ranibizumab and bevacizumab are widely used[74]–[75]. However, these drugs both have a large molecular weight and resistance to these drugs is usually seen in approximately 20%-30% ocular NV patients[76]. Moreover, because of the short aqueous half-life, the recurrence rate is high after anti-VEGF treatments which may also increase the risk of endophthalmitis owning to frequent intravitreal injections[77]. Abundant studies have already demonstrated the anti-angiogenic effects of artemisinins in tumors[78]. The known mechanisms of artemisinins in inhibiting angiogenesis include downregulating several growth factors, inducing apoptosis of vascular endothelial cells, upregulating angiogenesis inhibitors, depleting the levels of the flt-1 and KDR/flk-1-receptors[79]–[80]. In human umbilical vein endothelial cell (HUVEC) lines, artesunate was shown to inhibit angiogenesis through downregulating the levels of the VEGF receptors[81]. Similar protective effects were also investigated in lymphatic endothelial cells and Lewis lung carcinoma cells with the treatment of DHA[82]. In the science of ophthalmology, Cheng et al[83] demonstrated that artesunate could inhibit corneal NV by inducing ROS-dependent apoptosis in animal models. Their results suggested that artesunate could markedly inhibit angiogenesis by specifically inducing apoptosis via an iron/ROS-dependent p38 MAPK-mitochondrial pathway in vascular endothelial cells[83]. Zong et al[84] further investigated the use of artesunate in retinal NV and found that retinal NV could be remarkably inhibited under the effect of artesunate via downregulating the expression of VEGFR2, and PDGFR. Compared to bevacizumab, artesunate could remarkably inhibit retinal NV in rabbits with more durable efficacy. These two published animal evidences indicated the potential role of artesunate as a promising drug candidate to manage ocular NVs. As a newly-discovered anti-angiogenesis drug, artemisinins are worthwhile to be further explored due to a host of advantages.
Compared to the currently used anti-VEGF drugs, the advantages of artesunate are as follows: 1) Small molecule size: artesunate is a 384 Da molecule less than one-hundredth the size of bevacizumab (149 kDa); 2) Safety and low toxicity: artesunate has been widely used for many years as anti-malarial agents, with few adverse side effects and proven safety records; 3) Multi-targets: artesunate was proved to possess not only anti-angiogenetic effects targeting multi-growth factors (VEGF, FGF, HIF-α, and Ang-1), but also anti-inflammatory and anti-apoptotic effects.
Thus, we postulate that artesunate might be a potential novel treatment option for retinal vascular diseases such as AMD, DR, retinal artery or vein occlusion, especially when given intravitreously or being formulated into eye drops.
LIMITATIONS OF ARTEMISININS
The present studies of artemisinins have several limitations. While applying artemisinins for treatments beyond malaria, different research groups have reported inconsistent effective doses even for similar cell lines or animal models. Progress for further clinical trials could be hampered for the lack of a concerted effort to confirm the efficacies of artemisinins in different models. Another limitation is the lack of acute and chronic toxicological studies for acute as well as chronic exposure to artemisinin in ocular diseases, which is necessary for future application in ocular diseases.
CONCLUSION
To date, researches on artemisinins and its applications in ocular diseases are still limited, and much more will need to be studied. Further understanding of the protective activities of artemisinins beyond malaria might lead to improved treatments for ocular disorders.
In this review, we summarized recent studies on artemisinins in treating ocular diseases and we believe that this anti-malaria agent could also be used as a promising therapeutic drug for ocular diseases, especially retinal vascular diseases.
Acknowledgments
Authors' contributions: Lu BW wrote the manuscript; Xie LK participated discussion and provided suggestion.
Conflicts of Interest: Lu BW, None; Xie LK, None.
REFERENCES
- 1.Tu Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat Med. 2011;17(10):1217–1220. doi: 10.1038/nm.2471. [DOI] [PubMed] [Google Scholar]
- 2.Song J, Socheat D, Tan B, Seila S, Xu Y, Ou F, Sokunthea S, Sophorn L, Zhou C, Deng C, Wang Q, Li G. Randomized trials of artemisinin-piperaquine, dihydroartemisinin-piperaquine phosphate and artemether-lumefantrine for the treatment of multi-drug resistant falciparum malaria in Cambodia-Thailand border area. Malar J. 2011;10:231. doi: 10.1186/1475-2875-10-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makowiecki M, Bednarska A, Paciorek M, Kowalska J, Skrzat-Klapaczyńska A, Puła J, Sosińska-Bryła I, Krogulec D, Raczyńska J, Hackiewicz M, Stengiel J, Bursa D, Pihowicz A, Horban A. Usefulness of SOFA score and artesunate-based treatment in severe malaria - a single center study. Przegl Epidemiol. 2018;72(2):215–221. [PubMed] [Google Scholar]
- 4.Mace KE, Arguin PM, Tan KR. Malaria surveillance-United States, 2015. MMWR Surveill Summ. 2018;67(7):1–28. doi: 10.15585/mmwr.ss6707a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raffetin A, Bruneel F, Roussel C, Thellier M, Buffet P, Caumes E, Jauréguiberry S. Use of artesunate in non-malarial indications. Médecine Et Maladies Infect. 2018;48(4):238–249. doi: 10.1016/j.medmal.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 6.van Agtmael MA, Eggelte TA, van Boxtel CJ. Artemisinin drugs in the treatment of malaria: from medicinal herb to registered medication. Trends Pharmacol Sci. 1999;20(5):199–205. doi: 10.1016/s0165-6147(99)01302-4. [DOI] [PubMed] [Google Scholar]
- 7.Chen WJ. Honoring antiparasitics: the 2015 Nobel prize in physiology or medicine. Biomed J. 2016;39(2):93–97. doi: 10.1016/j.bj.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stringham RW, Moore GL, Teager DS, Yue TY. Analysis and isolation of potential artemisinin precursors from waste streams of Artemisia annua extraction. ACS Omega. 2018;3(7):7803–7808. doi: 10.1021/acsomega.8b00974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinheiro LCS, Feitosa LM, Silveira FFD, Boechat N. Current antimalarial therapies and advances in the development of semi-synthetic artemisinin derivatives. An Acad Bras Cienc. 2018;90(1 Suppl 2):1251–1271. doi: 10.1590/0001-3765201820170830. [DOI] [PubMed] [Google Scholar]
- 10.Loo CS, Lam NS, Yu D, Su XZ, Lu F. Artemisinin and its derivatives in treating protozoan infections beyond malaria. Pharmacol Res. 2017;117:192–217. doi: 10.1016/j.phrs.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nibret E, Wink M. Volatile components of four Ethiopian Artemisia species extracts and their in vitro antitrypanosomal and cytotoxic activities. Phytomedicine. 2010;17(5):369–374. doi: 10.1016/j.phymed.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Hencken CP, Jones-Brando L, Bordón C, Stohler R, Mott BT, Yolken R, Posner GH, Woodard LE. Thiazole, oxadiazole, and carboxamide derivatives of artemisinin are highly selective and potent inhibitors of Toxoplasma gondii. J Med Chem. 2010;53(9):3594–3601. doi: 10.1021/jm901857d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh C, Kanchan R, Chaudhary S, Puri SK. Linker-based hemisuccinate derivatives of artemisinin: synthesis and antimalarial assessment against multidrug-resistant Plasmodium yoelii nigeriensis in mice. J Med Chem. 2012;55(3):1117–1126. doi: 10.1021/jm2010699. [DOI] [PubMed] [Google Scholar]
- 14.Ferreira JF, Peaden P, Keiser J. In vitro trematocidal effects of crude alcoholic extracts of Artemisia annua, A. Absinthium, Asimina triloba, and Fumaria officinalis: trematocidal plant alcoholic extracts. Parasitol Res. 2011;109(6):1585–1592. doi: 10.1007/s00436-011-2418-0. [DOI] [PubMed] [Google Scholar]
- 15.Fröhlich T, Reiter C, Saeed MEM, Hutterer C, Hahn F, Leidenberger M, Friedrich O, Kappes B, Marschall M, Efferth T, Tsogoeva SB. Synthesis of thymoquinone-artemisinin hybrids: new potent antileukemia, antiviral, and antimalarial agents. ACS Med Chem Lett. 2018;9(6):534–539. doi: 10.1021/acsmedchemlett.7b00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parvez MK, Arbab AH, Al-Dosari MS, Al-Rehaily AJ. Antiviral natural products against chronic hepatitis B: recent developments. Curr Pharm Des. 2016;22(3):286–293. doi: 10.2174/1381612822666151112152733. [DOI] [PubMed] [Google Scholar]
- 17.Jana S, Iram S, Thomas J, Hayat MQ, Pannecouque C, Dehaen W. Application of the triazolization reaction to afford dihydroartemisinin derivatives with anti-HIV activity. Molecules. 2017;22(2):E303. doi: 10.3390/molecules22020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang CC, Sorrell TC, Chen SC. Pulmonary cryptococcosis. Semin Respir Crit Care Med. 2015;36(5):681–691. doi: 10.1055/s-0035-1562895. [DOI] [PubMed] [Google Scholar]
- 19.Gautam P, Upadhyay SK, Hassan W, Madan T, Sirdeshmukh R, Sundaram CS, Gade WN, Basir SF, Singh Y, Sarma PU. Transcriptomic and proteomic profile of Aspergillus fumigatus on exposure to artemisinin. Mycopathologia. 2011;172(5):331–346. doi: 10.1007/s11046-011-9445-3. [DOI] [PubMed] [Google Scholar]
- 20.Jamalzadeh L, Ghafoori H, Aghamaali M, Sariri R. Induction of apoptosis in human breast cancer MCF-7 cells by a semi-synthetic derivative of artemisinin: a caspase-related mechanism. Iran J Biotechnol. 2017;15(3):157–165. doi: 10.15171/ijb.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li S, Li G, Yang X, Meng Q, Yuan S, He Y, Sun D. Design, synthesis and biological evaluation of artemisinin derivatives containing fluorine atoms as anticancer agents. Bioorg Med Chem Lett. 2018;28(13):2275–2278. doi: 10.1016/j.bmcl.2018.05.035. [DOI] [PubMed] [Google Scholar]
- 22.Yu H, Hou Z, Tian Y, Mou Y, Guo C. Design, synthesis, cytotoxicity and mechanism of novel dihydroartemisinin-coumarin hybrids as potential anti-cancer agents. Eur J Med Chem. 2018;151:434–449. doi: 10.1016/j.ejmech.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Våtsveen TK, Myhre MR, Steen CB, Wälchli S, Lingjærde OC, Bai B, Dillard P, Theodossiou TA, Holien T, Sundan A, Inderberg EM, Smeland EB, Myklebust JH, Oksvold MP. Artesunate shows potent anti-tumor activity in B-cell lymphoma. J Hematol Oncol. 2018;11(1):23. doi: 10.1186/s13045-018-0561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deeken JF, Wang H, Hartley M, Cheema AK, Smaglo B, Hwang JJ, He AR, Weiner LM, Marshall JL, Giaccone G, Liu S, Luecht J, Spiegel JY, Pishvaian MJ. A phase I study of intravenous artesunate in patients with advanced solid tumor malignancies. Cancer Chemother Pharmacol. 2018;81(3):587–596. doi: 10.1007/s00280-018-3533-8. [DOI] [PubMed] [Google Scholar]
- 25.Mu X, Wang C. Artemisinins-a promising new treatment for systemic lupus erythematosus: a descriptive review. Curr Rheumatol Rep. 2018;20(9):55. doi: 10.1007/s11926-018-0764-y. [DOI] [PubMed] [Google Scholar]
- 26.Kuang M, Cen Y, Qin R, Shang S, Zhai Z, Liu C, Pan X, Zhou H. Artesunate attenuates pro-inflammatory cytokine release from macrophages by inhibiting TLR4-mediated autophagic activation via the TRAF6-Beclin1-PI3KC3 pathway. Cell Physiol Biochem. 2018;47(2):475–488. doi: 10.1159/000489982. [DOI] [PubMed] [Google Scholar]
- 27.Jiao J, Yang Y, Liu M, Li J, Cui Y, Yin S, Tao J. Artemisinin and Artemisia annua leaves alleviate Eimeria tenella infection by facilitating apoptosis of host cells and suppressing inflammatory response. Vet Parasitol. 2018;254:172–177. doi: 10.1016/j.vetpar.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 28.Deng Y, Liu Z, Geng Y. Anti-allergic effect of Artemisia extract in rats. Exp Ther Med. 2016;12(2):1130–1134. doi: 10.3892/etm.2016.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Wang B, Luo Y, Bian Y, Wang R. Effect of artemisinin and neurectomy of pterygoid canal in ovalbumin-induced allergic rhinitis mouse model. Allergy Asthma Clin Immunol. 2018;14:22. doi: 10.1186/s13223-018-0249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Casteels T, Frogne T, et al. Artemisinins target GABAA receptor signaling and impair α cell identity. Cell. 2017;168(1-2):86–100.e15. doi: 10.1016/j.cell.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsuda K, Miyamoto L, Hamano S, Morimoto Y, Kangawa Y, Fukue C, Kagawa Y, Horinouchi Y, Xu W, Ikeda Y, Tamaki T, Tsuchiya K. Mechanisms of the pH- and oxygen-dependent oxidation activities of artesunate. Biol Pharm Bull. 2018;41(4):555–563. doi: 10.1248/bpb.b17-00855. [DOI] [PubMed] [Google Scholar]
- 32.Michaelis M, Kleinschmidt MC, Barth S, Rothweiler F, Geiler J, Breitling R, Mayer B, Deubzer H, Witt O, Kreuter J, Doerr HW, Cinatl J, Cinatl J., Jr Anti-cancer effects of artesunate in a panel of chemoresistant neuroblastoma cell lines. Biochem Pharmacol. 2010;79(2):130–136. doi: 10.1016/j.bcp.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 33.Hamacher-Brady A, Stein HA, Turschner S, Toegel I, Mora R, Jennewein N, Efferth T, Eils R, Brady NR. Artesunate activates mitochondrial apoptosis in breast cancer cells via iron-catalyzed lysosomal reactive oxygen species production. J Biol Chem. 2011;286(8):6587–6601. doi: 10.1074/jbc.M110.210047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Q, Wu S, Zhao X, Zhao C, Zhao H, Huo L. Mechanisms of dihydroartemisinin and dihydroartemisinin/holotransferrin cytotoxicity in T-cell lymphoma cells. PLoS One. 2015;10(10):e0137331. doi: 10.1371/journal.pone.0137331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beccafico S, Morozzi G, Marchetti MC, Riccardi C, Sidoni A, Donato R, Sorci G. Artesunate induces ROS- and p38 MAPK-mediated apoptosis and counteracts tumor growth in vivo in embryonal rhabdomyosarcoma cells. Carcinogenesis. 2015;36(9):1071–1083. doi: 10.1093/carcin/bgv098. [DOI] [PubMed] [Google Scholar]
- 36.Berte N, Lokan S, Eich M, Kim E, Kaina B. Artesunate enhances the therapeutic response of glioma cells to temozolomide by inhibition of homologous recombination and senescence. Oncotarget. 2016;7(41):67235–67250. doi: 10.18632/oncotarget.11972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pang Y, Qin G, Wu L, Wang X, Chen T. Artesunate induces ROS-dependent apoptosis via a Bax-mediated intrinsic pathway in Huh-7 and Hep3B cells. Exp Cell Res. 2016;347(2):251–260. doi: 10.1016/j.yexcr.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 38.Yang DX, Qiu J, Zhou HH, Yu Y, Zhou DL, Xu Y, Zhu MZ, Ge XP, Li JM, Lv CJ, Zhang HQ, Yuan WD. Dihydroartemisinin alleviates oxidative stress in bleomycin-induced pulmonary fibrosis. Life Sci. 2018;205:176–183. doi: 10.1016/j.lfs.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 39.Thomas RB, Joy S, Ajayan MS, Paulose CS. Neuroprotective potential of Bacopa monnieri and Bacoside A against dopamine receptor dysfunction in the cerebral cortex of neonatal hypoglycaemic rats. Cell Mol Neurobiol. 2013;33(8):1065–1074. doi: 10.1007/s10571-013-9973-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ho WE, Peh HY, Chan TK, Wong WS. Artemisinins: pharmacological actions beyond anti-malarial. Pharmacol Ther. 2014;142(1):126–139. doi: 10.1016/j.pharmthera.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Gunjan S, Sharma T, Yadav K, Chauhan BS, Singh SK, Siddiqi MI, Tripathi R. Artemisinin derivatives and synthetic trioxane trigger apoptotic cell death in asexual stages of Plasmodium. Front Cell Infect Microbiol. 2018;8:256. doi: 10.3389/fcimb.2018.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riganti C, Doublier S, Viarisio D, Miraglia E, Pescarmona G, Ghigo D, Bosia A. Artemisinin induces doxorubicin resistance in human colon cancer cells via calcium-dependent activation of HIF-1alpha and P-glycoprotein overexpression. Br J Pharmacol. 2009;156(7):1054–1066. doi: 10.1111/j.1476-5381.2009.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakase I, Gallis B, Takatani-Nakase T, Oh S, Lacoste E, Singh NP, Goodlett DR, Tanaka S, Futaki S, Lai H, Sasaki T. Transferrin receptor-dependent cytotoxicity of artemisinin-transferrin conjugates on prostate cancer cells and induction of apoptosis. Cancer Lett. 2009;274(2):290–298. doi: 10.1016/j.canlet.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 44.Zeng Z, Xu J, Zheng W. Artemisinin protects PC12 cells against β-amyloid-induced apoptosis through activation of the ERK1/2 signaling pathway. Redox Biol. 2017;12:625–633. doi: 10.1016/j.redox.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iyer AS, Chapoval SP. Neuroimmune semaphorin 4A in cancer angiogenesis and inflammation: a promoter or a suppressor? Int J Mol Sci. 2018;20(1):E124. doi: 10.3390/ijms20010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei T, Liu J. Anti-angiogenic properties of artemisinin derivatives (Review) Int J Mol Med. 2017;40(4):972–978. doi: 10.3892/ijmm.2017.3085. [DOI] [PubMed] [Google Scholar]
- 47.Wartenberg M, Wolf S, Budde P, Grünheck F, Acker H, Hescheler J, Wartenberg G, Sauer H. The antimalaria agent artemisinin exerts antiangiogenic effects in mouse embryonic stem cell-derived embryoid bodies. Lab Invest. 2003;83(11):1647–1655. doi: 10.1097/01.lab.0000098424.38003.ff. [DOI] [PubMed] [Google Scholar]
- 48.Wang J, Zhang B, Guo Y, Li G, Xie Q, Zhu B, Gao J, Chen Z. Artemisinin inhibits tumor lymphangiogenesis by suppression of vascular endothelial growth factor C. Pharmacology. 2008;82(2):148–155. doi: 10.1159/000148261. [DOI] [PubMed] [Google Scholar]
- 49.Wu ZP, Gao CW, Wu YG, Zhu QS, Yan Chen, Xin Liu, Chuen Liu. Inhibitive effect of artemether on tumor growth and angiogenesis in the rat C6 orthotopic brain gliomas model. Integr Cancer Ther. 2009;8(1):88–92. doi: 10.1177/1534735408330714. [DOI] [PubMed] [Google Scholar]
- 50.Zhao C, Liu Q, Wang K. Artesunate attenuates ACLT-induced osteoarthritis by suppressing osteoclastogenesis and aberrant angiogenesis. Biomed Pharmacother. 2017;96:410–416. doi: 10.1016/j.biopha.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 51.Abd El-Aal NF, Hamza RS, Magdy M. Anti-angiogenic and anti-lymphangiogenic role of praziquantel and artemether in experimental mansoniasis. Acta Parasitol. 2017;62(4):708–716. doi: 10.1515/ap-2017-0085. [DOI] [PubMed] [Google Scholar]
- 52.Im E, Yeo C, Lee HJ, Lee EO. Dihydroartemisinin induced caspase-dependent apoptosis through inhibiting the specificity protein 1 pathway in hepatocellular carcinoma SK-Hep-1 cells. Life Sci. 2018;192:286–292. doi: 10.1016/j.lfs.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 53.Mungun HK, Li S, Zhang Y, Huang S, Jia Z, Ding G, Zhang A. Dihydroartemisinin inhibits indoxyl sulfate (IS)-promoted cell cycle progression in mesangial cells by targeting COX-2/mPGES-1/PGE2 cascade. Am J Transl Res. 2018;10(2):422–431. [PMC free article] [PubMed] [Google Scholar]
- 54.Steely AM, Willoughby JA, Sr, Sundar SN, Aivaliotis VI, Firestone GL. Artemisinin disrupts androgen responsiveness of human prostate cancer cells by stimulating the 26S proteasome-mediated degradation of the androgen receptor protein. Anticancer Drugs. 2017;28(9):1018–1031. doi: 10.1097/CAD.0000000000000547. [DOI] [PubMed] [Google Scholar]
- 55.Willoughby JA, Sr, Sundar SN, Cheung M, Tin AS, Modiano J, Firestone GL. Artemisinin blocks prostate cancer growth and cell cycle progression by disrupting Sp1 interactions with the cyclin-dependent kinase-4 (CDK4) promoter and inhibiting CDK4 gene expression. J Biol Chem. 2009;284(4):2203–2213. doi: 10.1074/jbc.M804491200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu J, Hu D, Yang G, Zhou J, Yang C, Gao Y, Zhu Z. Down-regulation of BMI-1 cooperates with artemisinin on growth inhibition of nasopharyngeal carcinoma cells. J Cell Biochem. 2011;112(7):1938–1948. doi: 10.1002/jcb.23114. [DOI] [PubMed] [Google Scholar]
- 57.Shirahama S, Kaburaki T, Nakahara H, Tanaka R, Takamoto M, Fujino Y, Kawashima H, Aihara M. Epidemiology of uveitis (2013-2015) and changes in the patterns of uveitis (2004-2015) in the central Tokyo area: a retrospective study. BMC Ophthalmol. 2018;18(1):189. doi: 10.1186/s12886-018-0871-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li B, Zhang R, Li J, Zhang L, Ding G, Luo P, He S, Dong Y, Jiang W, Lu Y, Cao H, Zheng J, Zhou H. Antimalarial artesunate protects sepsis model mice against heat-killed Escherichia coli challenge by decreasing TLR4, TLR9 mRNA expressions and transcription factor NF-kappa B activation. Int Immunopharmacol. 2008;8(3):379–389. doi: 10.1016/j.intimp.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 59.Xu H, He Y, Yang X, Liang L, Zhan Z, Ye Y, Yang X, Lian F, Sun L. Anti-malarial agent artesunate inhibits TNF-alpha-induced production of proinflammatory cytokines via inhibition of NF-kappaB and PI3 kinase/Akt signal pathway in human rheumatoid arthritis fibroblast-like synoviocytes. Rheumatology (Oxford) 2007;46(6):920–926. doi: 10.1093/rheumatology/kem014. [DOI] [PubMed] [Google Scholar]
- 60.Wang XQ, Liu HL, Wang GB, Wu PF, Yan T, Xie J, Tang Y, Sun LK, Li C. Effect of artesunate on endotoxin-induced uveitis in rats. Invest Ophthalmol Vis Sci. 2011;52(2):916–919. doi: 10.1167/iovs.10-5892. [DOI] [PubMed] [Google Scholar]
- 61.Sakamoto K, Inukai M, Mori A, Nakahara T. Brilliant Blue G protects against photoreceptor injury in a murine endotoxin-induced uveitis model. Exp Eye Res. 2018;177:45–49. doi: 10.1016/j.exer.2018.07.028. [DOI] [PubMed] [Google Scholar]
- 62.Fröhlich T, Reiter C, Ibrahim MM, Beutel J, Hutterer C, Zeitträger I, Bahsi H, Leidenberger M, Friedrich O, Kappes B, Efferth T, Marschall M, Tsogoeva SB. Synthesis of novel hybrids of quinazoline and artemisinin with high activities against Plasmodium falciparum, human cytomegalovirus, and leukemia cells. ACS Omega. 2017;2(6):2422–2431. doi: 10.1021/acsomega.7b00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Breuer E, Efferth T. Treatment of iron-loaded veterinary sarcoma by Artemisia annua. Nat Prod Bioprospect. 2014;4(2):113–118. doi: 10.1007/s13659-014-0013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li X, Ba Q, Liu Y, Yue Q, Chen P, Li J, Zhang H, Ying H, Ding Q, Song H, Liu H, Zhang R, Wang H. Dihydroartemisinin selectively inhibits PDGFRα-positive ovarian cancer growth and metastasis through inducing degradation of PDGFRα protein. Cell Discov. 2017;3:17042. doi: 10.1038/celldisc.2017.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kumari K, Keshari S, Sengupta D, Sabat SC, Mishra SK. Transcriptome analysis of genes associated with breast cancer cell motility in response to Artemisinin treatment. BMC Cancer. 2017;17(1):858. doi: 10.1186/s12885-017-3863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang T, Hu Y, Wang T, Cai P. Dihydroartemisinin inhibits the viability of cervical cancer cells by upregulating caveolin 1 and mitochondrial carrier homolog 2: Involvement of p53 activation and NAD(P)H: quinone oxidoreductase 1 downregulation. Int J Mol Med. 2017;40(1):21–30. doi: 10.3892/ijmm.2017.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kogachi K, Kim JW, Green S, Jubran R, Berry JL. Lurking below: massive choroidal invasion under a calcified tumor after attempted conservative therapy for retinoblastoma. Ophthalmic Genet. 2018;39(5):653–657. doi: 10.1080/13816810.2018.1513535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao F, Wang H, Kunda P, Chen X, Liu QL, Liu T. Artesunate exerts specific cytotoxicity in retinoblastoma cells via CD71. Oncol Rep. 2013;30(3):1473–1482. doi: 10.3892/or.2013.2574. [DOI] [PubMed] [Google Scholar]
- 69.Arroba AI, Campos-Caro A, Aguilar-Diosdado M, Valverde ÁM. IGF-1, inflammation and retinal degeneration: a close network. Front Aging Neurosci. 2018;10:203. doi: 10.3389/fnagi.2018.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zeng Z, Xu J, Zheng W. Artemisinin protects PC12 cells against β-amyloid-induced apoptosis through activation of the ERK1/2 signaling pathway. Redox Biol. 2017;12:625–633. doi: 10.1016/j.redox.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chong CM, Zheng W. Artemisinin protects human retinal pigment epithelial cells from hydrogen peroxide-induced oxidative damage through activation of ERK/CREB signaling. Redox Biol. 2016;9:50–56. doi: 10.1016/j.redox.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yan F, Wang H, Gao Y, Xu J, Zheng W. Artemisinin protects retinal neuronal cells against oxidative stress and restores rat retinal physiological function from light exposed damage. ACS Chem Neurosci. 2017;8(8):1713–1723. doi: 10.1021/acschemneuro.7b00021. [DOI] [PubMed] [Google Scholar]
- 73.Mesquita J, Castro-de-Sousa JP, Vaz-Pereira S, Neves A, Passarinha LA, Tomaz CT. Evaluation of the growth factors VEGF-a and VEGF-B in the vitreous and serum of patients with macular and retinal vascular diseases. Growth Factors. 2018;36(1-2):48–57. doi: 10.1080/08977194.2018.1477140. [DOI] [PubMed] [Google Scholar]
- 74.Lekha T, Prasad HN, Sarwate RN, Patel M, Karthikeyan S. Intravitreal bevacizumab for choroidal neovascularization associated with angioid streaks: long-term results. Middle East Afr J Ophthalmol. 2017;24(3):136–142. doi: 10.4103/meajo.MEAJO_17_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moreno M, Pow PY, Tabitha TST, Nirmal S, Larsson A, Radhakrishnan K, Nirmal J, Quah ST, Geifman Shochat S, Agrawal R, Venkatraman S. Modulating release of ranibizumab and aflibercept from thiolated chitosan-based hydrogels for potential treatment of ocular neovascularization. Expert Opin Drug Deliv. 2017;14(8):913–925. doi: 10.1080/17425247.2017.1343297. [DOI] [PubMed] [Google Scholar]
- 76.El Alaoui-Lasmaili K, Faivre B. Antiangiogenic therapy: markers of response, “normalization” and resistance. Crit Rev Oncol Hematol. 2018;128:118–129. doi: 10.1016/j.critrevonc.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 77.Borkar DS, Obeid A, Su DC, Storey PP, Gao X, Regillo CD, Kaiser RS, Garg SJ, Hsu J, Wills Post Injection Endophthalmitis (PIE) Study Group Endophthalmitis rates after bilateral same-day intravitreal anti-vascular endothelial growth factor injections. Am J Ophthalmol. 2018;194:1–6. doi: 10.1016/j.ajo.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 78.Kumar M, Dhatwalia SK, Dhawan DK. Role of angiogenic factors of herbal origin in regulation of molecular pathways that control tumor angiogenesis. Tumour Biol. 2016;37(11):14341–14354. doi: 10.1007/s13277-016-5330-5. [DOI] [PubMed] [Google Scholar]
- 79.Verma S, Das P, Kumar VL. Chemoprevention by artesunate in a preclinical model of colorectal cancer involves down regulation of β-catenin, suppression of angiogenesis, cellular proliferation and induction of apoptosis. Chem Biol Interact. 2017;278:84–91. doi: 10.1016/j.cbi.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 80.Zhou HJ, Wang WQ, Wu GD, Lee J, Li A. Artesunate inhibits angiogenesis and downregulates vascular endothelial growth factor expression in chronic myeloid leukemia K562 cells. Vascul Pharmacol. 2007;47(2-3):131–138. doi: 10.1016/j.vph.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 81.Chen HH, Zhou HJ, Wu GD, Lou XE. Inhibitory effects of artesunate on angiogenesis and on expressions of vascular endothelial growth factor and VEGF receptor KDR/flk-1. Pharmacology. 2004;71(1):1–9. doi: 10.1159/000076256. [DOI] [PubMed] [Google Scholar]
- 82.Chen HH, Zhou HJ, Wang WQ, Wu GD. Antimalarial dihydroartemisinin also inhibits angiogenesis. Cancer Chemother Pharmacol. 2004;53(5):423–432. doi: 10.1007/s00280-003-0751-4. [DOI] [PubMed] [Google Scholar]
- 83.Cheng R, Li C, Li C, Wei L, Li L, Zhang Y, Yao Y, Gu X, Cai W, Yang Z, Ma J, Yang X, Gao G. The artemisinin derivative artesunate inhibits corneal neovascularization by inducing ROS-dependent apoptosis in vascular en dothelial cells. Invest Ophthalmol Vis Sci. 2013;54(5):3400–3409. doi: 10.1167/iovs.12-11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zong Y, Yuan Y, Qian X, Huang Z, Yang W, Lin L, Zheng Q, Li Y, He H, Gao Q. Small molecular-sized artesunate attenuates ocular neovascularization via VEGFR2, PKCα, and PDGFR targets. Sci Rep. 2016;6:30843. doi: 10.1038/srep30843. [DOI] [PMC free article] [PubMed] [Google Scholar]