Abstract
AIM
To detect the possible role of interleukin (IL)-26 in diabetic retinopathy (DR) patients.
METHODS
Subjects were divided into diabetes without retinopathy (DWR) group (n=20), non-proliferative diabetic retinopathy (NPDR) group (n=20), proliferative diabetic retinopathy (PDR) group (n=20) and normal control group (n=20). The protein expression of IL-26 in the serum and vitreous fluid were measured by enzyme-linked immunosorbent assay (ELISA). The mRNA change of IL-26 in peripheral blood mononuclear cells (PBMCs) was assessed by real-time polymerase chain reaction.
RESULTS
The serum expression of IL-26 in PDR group was significantly elevated compared with the normal control group, DWR group and NPDR group. The vitreous fluid concentration of IL-26 in PDR patients (without anti-VEGF therapy) was also higher compared to normal controls. However, no obvious significance was found concerning the expression of IL-26 in vitreous fluid between PDR after anti-VEGF therapy and normal controls. In PDR group, the mRNA level of IL-26 significantly increased compared with the normal controls and DWR patients in the PBMCs.
CONCLUSION
Protein and mRNA expression of IL-26 are increased in serum, vitreous fluid and PBMCs in PDR patients, suggesting that IL-26 may be associated with the pathogenesis of PDR.
Keywords: interleukin-26, serum, vitreous fluid, peripheral blood mononuclear cells, proliferative diabetic retinopathy
INTRODUCTION
Diabetic retinopathy (DR), related to inadequate glycemic control among diabetes patients, has become a usual sight-threatening disease[1]. It has been reported that increased polyol pathway, oxidative stress, advanced glycation end-products (AGEs), the renin-angiotensin-aldosterone system (RAAS) and inflammation contributed to the occurrence and development of DR[2]–[6]. Recently, there is plenty of evidence suggesting that low-grade inflammation and immune responses play critical roles in DR[6].
Th17 cells, differentiated from CD4+ T-helper cells[7], are pivotal in the pathogenesis of autoimmune diseases and produce inflammatory cytokines in coordination with special cells into the target organ to induce tissue inflammation. Recent studies have focused on the role of Th17 cells and related inflammatory cytokines [interleukin (IL)-17A, IL-17F, IL-21, IL-22] in DR. It has been reported that disturbances in Th17 cells and IL-17, IL-22 in serum and peripheral blood mononuclear cells (PBMCs) are possibly associated with DR[8]–[9]. Takeuchi et al[10] demonstrated that IL-17A, IL-17F, IL-21, IL-22 were overexpressed in vitreous fluid of proliferative diabetic retinopathy (PDR) patients.
IL-26, another cytokine produced by Th17 cells, is a part of the IL-10 cytokine family, which includes IL-19, IL-20, IL-22, IL-24 and type III interferons (IFN-λ), namely IL-28A, IL-28B and IL-29[11]–[13]. After it has been secreted, IL-26 binds to special receptor complex, constituted by IL-20R1/IL-10R2, and induces the secretion of inflammatory cytokines, including IL-1β, IL-8, tumor necrosis factor (TNF)-α and granulocyte-macrophage colony-stimulating factor, through activating STAT3 and STAT1 signaling[14]. Besides, IL-26 can bind to extracellular DNA and generate the secretion of IL-6 and IL-1β by human monocytes in a stimulator of IFN genes- and inflammasome-dependent manner[15]. It has been reported that IL-26 participate in the onset and development of multiple chronic inflammatory and autoimmune-related diseases, such as rheumatoid arthritis, chronic graft-versus-host disease, inflammatory bowel disease and chronic hepatitis C virus (HCV), and increased concentration of IL-26 could be detected[7],[16]–[18]. However, there is no study about the effect of IL-26 in DR. Therefore, we explored whether IL-26 was associated with the development of DR.
SUBJECTS AND METHODS
Ethical Approval
This study got the approvement by the Clinical Ethical Research Committee of the First Affiliated Hospital of Chongqing Medical University. All procedures referred to the tenets of Helsinki declaration and informed consents were signed from patients.
Subjects
Sixty type 2 diabetes mellitus (T2DM) patients (31 men and 29 women, average age =59y) were recruited in this study. Patients were divided into three groups: group 1, T2DM patients without DR (DWR, n=20); group 2, T2DM patients with non-proliferative diabetic retinopathy (NPDR, n=20); group 3, T2DM patients with PDR (n=20). The above division was based on the international classification standard of DR according to fundus photography and fluorescein angiography. Normal controls (n=20, including 11 men and 9 women, mean age =60y) were recruited. The patients who had a chronic systemic disease (such as hematological or autoimmune disease), dialysis, cancer, ocular disorders or previous intraocular surgery were excluded. Serum was gained from blood samples after centrifugation and stored at -80°C. The PBMCs were segregated from heparinized blood by Ficoll-Hypaque density-gradient centrifugation. Vitreous body was extracted by 1 mL needle inserted into vitreous cavity via manual suction from PDR patients (n=20) before anti-vascular endothelial growth factor (VEGF) treatment or a pars plana vitrectomy after anti-VEGF treatment. As getting vitreous via manual suction was difficult, most samples were obtained by a pars plana vitrectomy after anti-VEGF therapy (n=14). Twenty patients who were diagnosed with idiopathic macular epiretinal membrane (IMEM, n=8), idiopathic macula hole (MH, n=12) and had undergone vitrectomy were recruited as the control group. Subjects with diabetes, hypertension, hematological disease, or renal insufficiency with dialysis were excluded. Vitrectomy samples were reserved at -80°C after centrifugation. The control group was matched for age and gender with the diabetes mellitus patients.
Enzyme-Linked Immunosorbent Assay
The concentration of IL-26 in the serum and vitreous from DWR, NPDR, PDR patients and normal controls were measured by human ELISA kits (Yuanye, China) followed the manufacturer's instructions. A microplate reader (Molecular Devices, Sunnyvale, CA, USA) was applied to read the absorbance at 450 nm. The minimum detectable concentrations by this assay was 10 pg/mL.
Real-time Quantitative RT-PCR
RNA was abstracted from the PBMCs with Trizol Reagent (Takara, Japan) complied with the manufacturer's instruction. cDNA was synthesized using Superscript III Reverse Transcriptase (Takara, Japan) and then the synthesized first-strand of cDNA was measured by real-time quantitative PCR analysis with SYBR Green labeling method. Quantitative PCR was conducted by an Applied Biosystems 7500 Fast Real-Time PCR System (Foster City, CA). To investigate IL-26 expression, we used the following primer sequences: β-actin, forward 5′-AGG GAA ATC GTG CGT GAC-3′, reverse 5′-CGC TCA TTG CCG ATA GTG-3′; human IL-26, forward 5′-CAATTGCAAGGCTGCAAGAA-3′, reverse 5′-TCTCTAGCTGATGAAGCACAGGAA-3′. The real-time PCR reaction was followed by the guidelines of the SYBRH Premix Ex TaqTM kit (Takara, Japan). The relative gene expression of IL-26 were performed by the 2−ΔΔCT method for analyses.
Statistical Analysis
Statistical analysis was carried out using SPSS 19.0. Graphs were made by Prism version 5 (GraphPad Software Inc., La Jolla, CA, USA). All data were presented as mean±standard deviation (SD). Statistical comparisons among the normal control group, DWR group, NPDR group and PDR group about serum and mRNA concertration were performed using one-way analysis of variance (ANOVA) followed by the Student-Newman-Keuls (SNK) test. Data for the vitreous fluid level of IL-26 was analyzed using independent samples t-test after performing the Kolmogorov-Smirnov test. P<0.05 was thought to be reached the statistical significance.
RESULTS
Clinical Characteristic of all Participants
As Table 1 showed, there were no significant difference was found referring to age, gender and blood pressure between all participants. Fasting plasma glucose (FPG) and HbA1c of diabetes patients were higher compared with normal controls.
Table 1. Clinical and laboratory features of the PDR patient and the control groups.
| Parameters | Controls | DWR | NPDR | PDR | P |
| Gender (M/F) | 11/9 | 13/7 | 8/12 | 10/10 | |
| Age (y) | 60±10 | 65±11 | 57±10 | 56±9 | 0.056 |
| Systolic BP (mm Hg) | 125±14 | 135±12 | 132±25 | 134±15 | 0.388 |
| Diastolic BP (mm Hg) | 75±7 | 81±9 | 78±9 | 80±9 | 0.266 |
| HbA1c (%) | 5.4±0.27 | 7.0±1.4 | 7.9±1.2 | 8.6±1.5 | <0.001 |
| FPG (mmol/L) | 5.17±0.17 | 8.32±0.37 | 9.12±0.91 | 10.01±1.29 | <0.001 |
| No. of patients receiving anti-VEGF (after/before vitreous sampling) | 14/6 |
BP: Blood pressure; FPG: Fasting plasmaglucose; DWR: Diabetic without retinopathy; NPDR: Non-proliferative diabetic retinopathy; PDR: Proliferative diabetic retinopathy.
n=20
Increased Protein Expression of IL-26 in Serum and Vitreous Fluid of PDR Patients
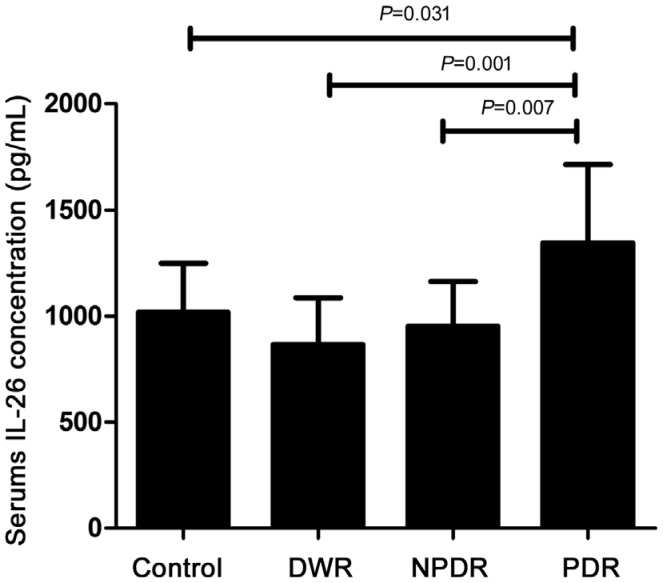
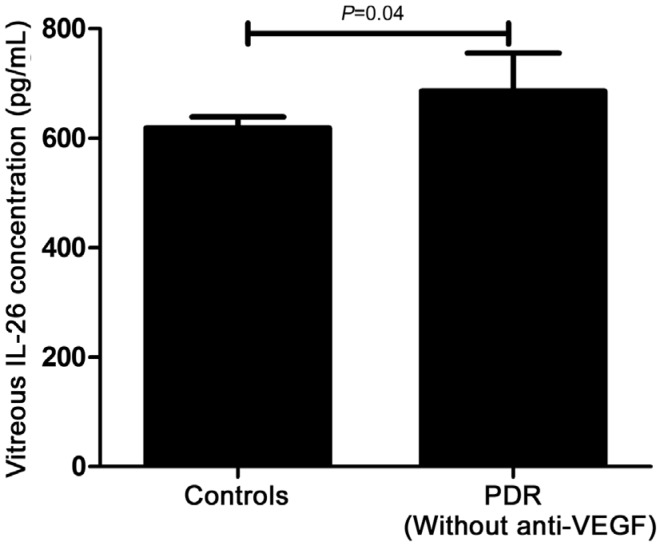
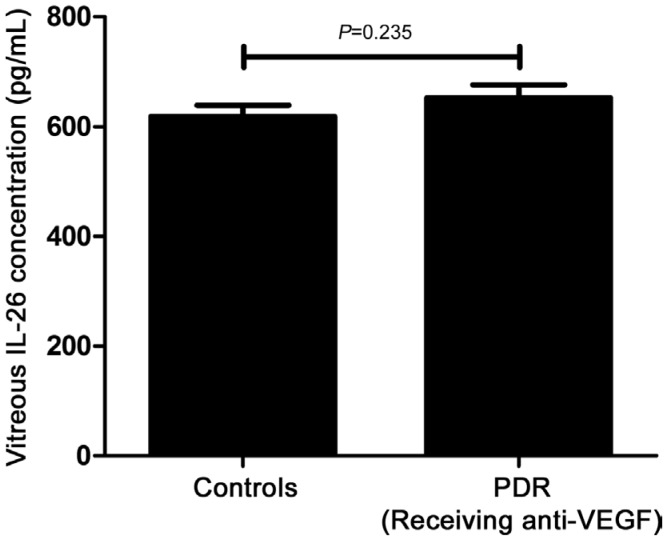
As Table 2 showed, serum concentration of IL-26 in PDR patients was 1343.75±370.41 pg/mL, which was significantly increased compared with the normal controls (P=0.031), DWR (P=0.01) and NPDR patients (P=0.007, Figure 1). However, there wasn't any difference found among healthy controls, DWR and NPDR patients in this study. Furthermore, we also explored the protein concentration of IL-26 in vitreous fluid. Just as shown in Table 3, as many patients had been received anti-VEGF treatment, to exclude the influence of anti-VEGF drugs on the expression of IL-26, we respectively compared the patients who had received anti-VEGF treatment (n=14) and did not have anti-VEGF treatment (n=6) with the controls. We found that the concentration of IL-26 in vitreous body from PDR patients without receiving anti-VEGF therapy was remarkably elevated as compared to healthy controls (P=0.04, Figure 2). However, the statistical difference could not be found between the patients who had received anti-VEGF treatment and the controls (P=0.235, Figure 3).
Table 2. Serum concentrations of IL-26.
| Serum concentration | Controls | DWR | NPDR | PDR |
| IL-26 (pg/mL) | 1017.86 | 865.95 | 951.31 | 1343.75 |
| Range | 762.50-1498.5a | 553.00-1288.50c | 567.50-1251.00b | 908.00-2411.50 |
Dunnett test, LSD test, SNK t-test were performed to analyze differences between groups. aP<0.05, bP<0.01, cP<0.001.
n=20
Figure 1. IL-26 protein concentration in serum in normal controls (n=20), DWR (n=20), NPDR (n=20) and PDR patients (n=20).
Table 3. Vitreous concentrations of IL-26.
| Vitreous concentration | Controls, n=20 | PDR |
|
| Without anti-VEGF, n=6 | Receiving anti-VEGF, n=14 | ||
| IL-26 (pg/mL) | 618.69 | 686.00a | 666.85 |
| Range | 563.0-645.5 | 618.50-822.50a | 625.50-681.50 |
Independent samples t-test were performed to analyze differences between groups. aP<0.05 vs controls.
Figure 2. IL-26 protein concentration in vitreous fluid in normal controls (n=20) and PDR patients (n=6, without anti-VEGF therapy).
Figure 3. IL-26 protein concentration in vitreous fluid in normal controls (n=20) and PDR patients (n=14, receiving anti-VEGF therapy).
Increased Gene Expression of IL-26 in PBMCs of PDR Patients
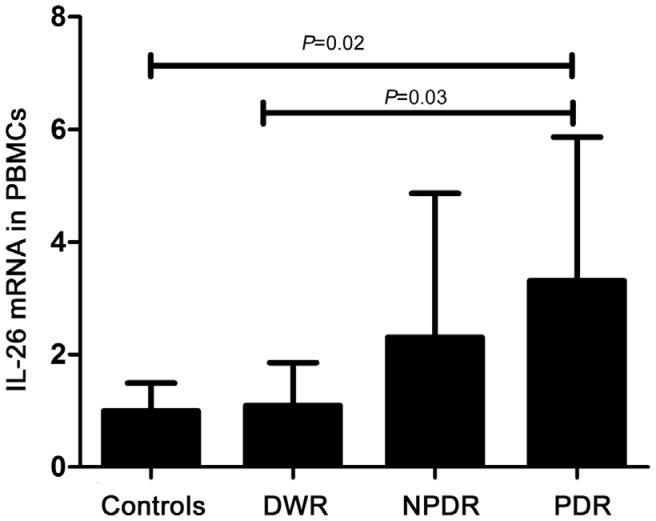
We further assayed the concertration of IL-26 mRNA in PBMCs (Figure 4). The IL-26 mRNA expression in PDR patients was remarkablely increased than that in healthy controls and DWR patients (P<0.05). However, no obivious difference was observed among the normal controls, DWR and NPDR patients, or between the NPDR patients and PDR patients.
Figure 4. IL-26 mRNA expression in PBMCs from healthy controls (n=20), DWR (n=20), NPDR (n=20) and PDR patients (n=20).
DISCUSSION
Our study showed that PDR patients had an increased expression of IL-26 in serum and PBMCs. In the meantime, we also found that IL-26 protein expression in vitreous fluid from PDR patients without receiving intravitreal anti-VEGF therapy significantly elevated. These data mentioned above indicate that the increased expression of IL-26 may contribute to the pathogenesis of PDR.
Our study displayed an increased protein level of IL-26 in the serum of PDR patients as compared to normal controls, DWR and NPDR patients. Our results are in consistent with the previous studies reported in other autoimmune and chronic inflammatory diseases. Miot et al[17] found that the serum expression of IL-26 in HCV was higher than that in controls, and chronic HCV infection relates to inflammation. Lopalco et al[19] reported the serum expression of IL-26 in Behçet's disease (BD) patients with mucocutaneous manifestations plus ocular involvement was significantly higher than that in the subgroup with only mucocutaneous involvement. Collectively, these results certify that IL-26 is involved in the pathogenesis of various autoimmune and chronic inflammatory disorders.
Inflammatory cytokines aggregate in special tissue and form a proinflammatory microenvironment. Furthermore, we detected the protein concentration of IL-26 in vitreous fluid and found that the concertration of IL-26 was higher in vitreous fluid of PDR patients without anti-VEGF therapy than that in normal controls. Our findings agreed with the findings reported by Kaabach et al[20] and Corvaisier et al[7], which showed IL-26 was highly expressed in the cerebrospinal fluid of neuro-BD patients and in the synovial fluids in rheumatoid arthritis patients. Moreover, we found that the concentration of IL-26 in the vitreous fluid was downregulated after intravitreal injection of anti-VEGF therapy, which indicates that anti-VEGF therapy may directly inhibit the expression of IL-26. However, it needs further study to verify the effect of anti-VEGF on the concentration of IL-26 in vitro experiments.
Next, we designed an experiment to measure the gene expression of IL-26 in PBMCs. We found that there was an elevated expression of IL-26 mRNA in PDR patients compared with normal controls and DWR patients. This result is in accordance with the protein expression of IL-26 in serum. However, the exact mechanism about how to regulate the expression of IL-26 in PBMCs in DR deserves further investigation.
Acknowledgments
Foundation: Supported by the National Natural Science Foundation of China (No.81870643).
Conflicts of Interest: Wang P, None; Wang WY, None; Zhang XD, None.
REFERENCES
- 1.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 2.Lorenzi M. The polyol pathway as a mechanism for diabetic retinopathy: attractive, elusive, and resilient. Exp Diabetes Res. 2007;2007:61038. doi: 10.1155/2007/61038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ran RJ, Du LP, Zhang XD, Chen XL, Fang YH, Li YY, Tian HY. Elevated hydrogen sulfide levels in vitreous body and plasma in patients with proliferative diabetic retinopathy. Retina. 2014;34(10):2003–2009. doi: 10.1097/IAE.0000000000000184. [DOI] [PubMed] [Google Scholar]
- 4.Stitt AW, Jenkins AJ, Cooper ME. Advanced glycation end products and diabetic complications. Expert Opin Investig Drugs. 2002;11(9):1205–1223. doi: 10.1517/13543784.11.9.1205. [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson-Berka JL. Angiotensin and diabetic retinopathy. Int J Biochem Cell Biol. 2006;38(5-6):752–765. doi: 10.1016/j.biocel.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Tomić M, Ljubić S, Kastelan S. The role of inflammation and endothelial dysfunction in the pathogenesis of diabetic retinopathy. Coll Antropol. 2013;37(Suppl 1):51–57. [PubMed] [Google Scholar]
- 7.Corvaisier M, Delneste Y, Jeanvoine H, Preisser L, Blanchard S, Garo E, Hoppe E, Barré B, Audran M, Bouvard B, Saint-André JP, Jeannin P. IL-26 is overexpressed in rheumatoid arthritis and induces proinflammatory cytokine production and Th17 cell generation. PLoS Biol. 2012;10(9):e1001395. doi: 10.1371/journal.pbio.1001395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H, Ren XR, Liao NY, Wen F. Th17 cell frequency and IL-17A concentrations in peripheral blood mononuclear cells and vitreous fluid from patients with diabetic retinopathy. J Int Med Res. 2016;44(6):1403–1413. doi: 10.1177/0300060516672369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H, Wen F, Zhang XZ, Su SB. Expression of T-helper-associated cytokines in patients with type 2 diabetes mellitus with retinopathy. Mol Vis. 2012;18:219–226. [PMC free article] [PubMed] [Google Scholar]
- 10.Takeuchi M, Sato T, Tanaka A, Muraoka T, Taguchi M, Sakurai Y, Karasawa Y, Ito M. Elevated levels of cytokines associated with Th2 and Th17 cells in vitreous fluid of proliferative diabetic retinopathy patients. PLoS One. 2015;10(9):e0137358. doi: 10.1371/journal.pone.0137358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fickenscher H, Hör S, Küpers H, Knappe A, Wittmann S, Sticht H. The interleukin-10 family of cytokines. Trends Immunol. 2002;23(2):89–96. doi: 10.1016/s1471-4906(01)02149-4. [DOI] [PubMed] [Google Scholar]
- 12.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 13.Sabat R. IL-10 family of cytokines. Cytokine Growth Factor Rev. 2010;21(5):315–324. doi: 10.1016/j.cytogfr.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 14.You W, Tang QY, Zhang CY, Wu JD, Gu CR, Wu ZS, Li XC. IL-26 promotes the proliferation and survival of human gastric cancer cells by regulating the balance of STAT1 and STAT3 activation. PLoS One. 2013;8(5):e63588. doi: 10.1371/journal.pone.0063588. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Poli C, Augusto JF, Dauvé J, Adam C, Preisser L, Larochette V, Pignon P, Savina A, Blanchard S, Subra JF, Chevailler A, Procaccio V, Croué A, Créminon C, Morel A, Delneste Y, Fickenscher H, Jeannin P. IL-26 confers proinflammatory properties to extracellular DNA. J Immunol. 2017;198(9):3650–3661. doi: 10.4049/jimmunol.1600594. [DOI] [PubMed] [Google Scholar]
- 16.Dambacher J, Beigel F, Zitzmann K, De Toni EN, Göke B, Diepolder HM, Auernhammer CJ, Brand S. The role of the novel Th17 cytokine IL-26 in intestinal inflammation. Gut. 2009;58(9):1207–1217. doi: 10.1136/gut.2007.130112. [DOI] [PubMed] [Google Scholar]
- 17.Miot C, Beaumont E, Duluc D, et al. IL-26 is overexpressed in chronically HCV-infected patients and enhances TRAIL-mediated cytotoxicity and interferon production by human NK cells. Gut. 2015;64(9):1466–1475. doi: 10.1136/gutjnl-2013-306604. [DOI] [PubMed] [Google Scholar]
- 18.Ohnuma K, Hatano R, Aune TM, Otsuka H, Iwata S, Dang NH, Yamada T, Morimoto C. Regulation of pulmonary graft-versus-host disease by IL-26+CD26+CD4 T lymphocytes. J Immunol. 2015;194(8):3697–3712. doi: 10.4049/jimmunol.1402785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopalco G, Lucherini OM, Lopalco A, Venerito V, Fabiani C, Frediani B, Galeazzi M, Lapadula G, Cantarini L, Iannone F. Cytokine signatures in mucocutaneous and ocular behçet's disease. Front Immunol. 2017;8:200. doi: 10.3389/fimmu.2017.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaabachi W, Bouali E, Berraïes A, Dhifallh IB, Hamdi B, Hamzaoui K, Hamzaoui A. Interleukin-26 is overexpressed in Behçet's disease and enhances Th17 related-cytokines. Immunol Lett. 2017;190:177–184. doi: 10.1016/j.imlet.2017.08.008. [DOI] [PubMed] [Google Scholar]